Abstract
Objective
To estimate the utility of first-trimester 3D placental volume and vascular flow indices in the prediction of adverse pregnancy outcomes.
Methods
A prospective cohort study including women with singleton pregnancies seen between 11 – 14 weeks’ as part of a screening program for aneuploidy. Placental volume and vascularization indices were obtained using 3D power Doppler imaging and the VOCAL technique. Placental volume (PV), Vascularization Index (VI), Flow Index (FI) and Vascularization Flow Index (VFI) were calculated. The adverse pregnancy outcomes investigated include preeclampsia (PE), gestational hypertension (GH) and small for gestational age (SGA). The predictive ability of each variable was evaluated using receiver-operating characteristic (ROC) curves.
Results
Of 388 women included, PE was seen in 30 (7.7%), GH in 37 (9.0%) and SGA in 31 (8.0%). Placental volume was not significantly different between the pregnancies with adverse outcomes and those without. The mean values of the VI and VFI were significantly lower in the pregnancies that developed PE but not in GH or SGA. The area under the ROC curve for the prediction of PE was 0.71, 0.69 and 0.70 for VI, FI and VFI, respectively.
Conclusion
The study confirms lower 3D power Doppler vascular flow indices in pregnancies that develop PE. The discriminatory ability of using these indices alone for predicting PE appears modest.
Keywords: Placental volume, Vascular flow indices, 3D power Doppler, preeclampsia, small for gestational age
Introduction
Placental maldevelopment plays a pivotal role in the pathogenesis of most pregnancy complications. Improvements in three-dimensional (3D) ultrasound technology have made it possible to evaluate the placenta volume as well as its vascularization status using Power Doppler (1–6). The Doppler parameters derived from 3D interrogation of the placenta are different include the Vascularization index (VI), the flow index (FI) and the vascularization flow index (VFI).
Several small studies have suggested that parameters derived from 3D-power Doppler evaluation of the placenta in the first trimester can predict adverse pregnancy outcomes including preeclampsia (PE) and fetal growth restriction (7–10). While the findings from these studies are promising, the methodologies used and the definitions of abnormal indices have varied. For example, Noguchi et al studied the vascularization indices using placental sonobiopsy in 208 normal versus 13 growth restricted fetuses. In that study, they found significantly lower VI, FI and VFI in most of the small for gestational age (SGA) pregnancies (8). Rizzo et al studied 27 pregnancies with (SGA) compared with 57 of normal birth weight (10). The latter study found a significant difference in the mean values of VI, FI and VFI, specifically in the SGA group with low pregnancy associated plasma protein-A (PAPP-A).
Given the significant contribution of PE, GH and SGA pregnancies to perinatal morbidity and mortality, efforts at identifying reliable prediction models to identify those at risk are critical. The objective of the present study is to estimate the utility of first-trimester 3D placental volume and vascular flow indices in the prediction of adverse pregnancy outcomes.
Materials and Methods
This is a prospective cohort study including women with singleton pregnancies seen between 11 – 14 weeks’ at the Washington University Divison of Ultrasound and Genetics as part of a screening program for aneuploidy. We excluded any cases with fetal anomalies or chromosomal anomalies. The study is approved by the Institutional Review Board at Washington University in St. Louis and each patient participating in the study gave a signed and approved informed consent form.
Acquisition of the images used for the determination of placental volume and vascularization indices were obtained at the time of the first-trimester visit. All 3D scans were performed by experienced sonographers. The methods for obtaining placental volume and vascularization indices have previously been described (8–13).Briefly, Voluson 730 Expert ultrasound machines (GE Medical Systems, Milwaukee, WI, USA) equipped with a 4–8 MHz transducer were used to acquire all images. The same pre-established instrument power settings were used in all cases (angio mode: cent; smooth: 4/5; FRQ: low; quality: 16; density: 6; enhance: 16; balance: GO150; filter: 2; actual power: 2 dB; pulse repetition frequency: 0.9). The entire view of the placenta was identified by 2D-ultrasound, the volume box was adjusted to scan the entire placenta, and the ultrasound images were stored on a removable hard disk for subsequent analysis. The angle of volume acquisition varied from 45° to 90°, and the duration of image acquisition lasted was 10 to 15 seconds. For posterior and laterally located placentas a slight lateral inclination of the transducer was used to acquire the images. Each image was recovered from the disk in succession for processing. Evaluation of the entire placenta was performed using the rotational technique in the VOCAL™ program included in the 4DView computer software (GE Medical Systems, Milwaukee, WI, USA). This involved rotating the image at 30° intervals and outlining the contour of the placenta six times. The placental volume was then calculated and the VI, FI, and VFI were obtained from 4-D power Doppler histograms.
The primary outcome for the study was the ability of 3D-placental volume and vascular flow indices to predict PE, GHand SGA.
Preeclampsia and other hypertensive disorders were defined using guidelines of the American College of Obstetricians and Gynecology (14) and by the criteria proposed by the National High Blood Pressure Education Program Working Group Report in Pregnancy (15). Gestational hypertension was defined as elevated blood pressure (BP) without proteinuria detected for the first time in mid-pregnancy (15). Mild preeclampsia was defined as: BP >140/90 after 20 weeks gestation in a woman with a previously normal BP and proteinuria of ≥300mg in a 24-hour urine sample or at least 1+ on urine dipstick. Severe preeclampsia was defined using the presence of any of the following criteria in patients with preeclampsia: BP ≥160/110 on two or more occasions more at least 6 hours apart; proteinuria of at least 5g or 3+ on urine dipstick on 2 samples randomly taken at least 4 hours apart; elevated liver enzymes; visual disturbance, headache or other neurological disturbances; persistent right upper quadrant or epigastric pain; oliguria with <500ml of urine in 24 hours; oligohydramnios and fetal growth restriction.
Small for gestational age was defined as fetal weight <10th percentile for gestational age using the growth chart by Alexander et al (16).
Normal distribution of the data was confirmed with the Kolmogorov-Smirnov test and log transformed when necessary. We had previously demonstrated good reproducibility of these measurements in our population (12). Comparisons of the 3D-placental volume and vascular indices between women who developed any of the adverse pregnancy outcomes and those with normal outcomes were performed using the student t test. Logistic regression analysis was used to determine if the placenta volume and vascularization indices had a significant contribution to the prediction of PE, GH or SGA. The performance of the variables in a screening paradigm was determined using receiver operator characteristic (ROC) curves, with the area under the curve (AUC) being of primary interest.
Statistical analyses were performed using STATA version 10.0 (Stata Corp., College Station, TX). Tests with p values < 0.05 were considered significant.
Results
There were 405 women enrolled over an 18 month period. Of these patients, 2 experienced a spontaneous miscarriage, and 6 were lost to follow-up. In 9 women, the 3D Doppler evaluation could not be performed due to technical reasons. Over the 18 month period of the study, there were no cases with chromosomal abnormality or fetal abnormality enrolled.
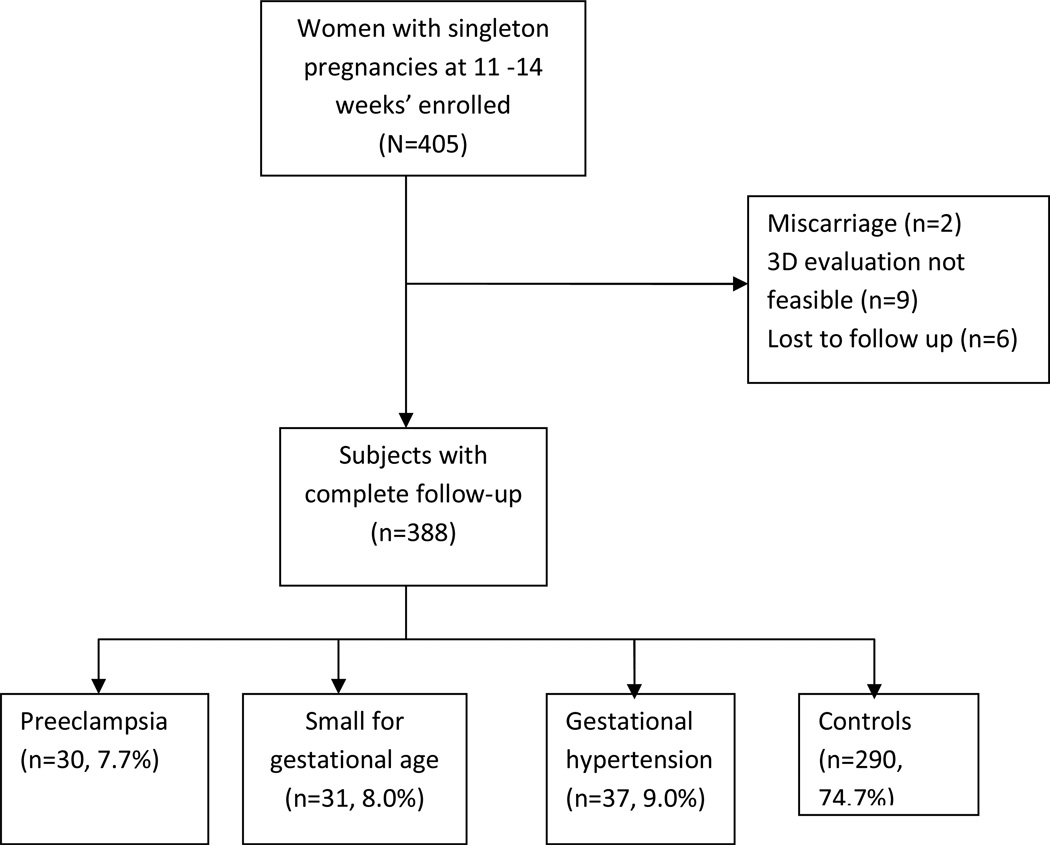
Complete pregnancy outcome information was available for the remaining 388 women. PE was seen in 30 (7.7%), GH in 37 (9.0%) and SGA in 31 (8.0%) patients (Figure 1). Of the pregnancies with PE, 6 (20%) required delivery under 34 weeks’ gestation. The demographic characteristics of the study population are shown in Table 1. The placental volume and vascular indices were similar between the smokers and non-smokers. For example, the mean VI and standard deviation among the smokers was 17.8±10.6 compared with 15.6± 9.8 in the non-smokers, p=0.25. The placental volume and vascular indices were normally distributed using Kolmogorov-Smirnov test; therefore, log transformation was not needed.
Figure 1.
Flow chart of study subjects
Table 1.
Demographics and outcomes of study population (SD= standard deviation)
| N =388 | % | |
|---|---|---|
| Mean age (±SD) | 31.6 (±5.6) | |
| Gravidity, median (range) | 2 (1–10) | |
| Parity, median (range) | 1 (0–5) | |
| Race | ||
| White | 226 | 58.2 |
| African American | 103 | 26.6 |
| Asian | 37 | 9.5 |
| Hispanic | 8 | 2.1 |
| Others | 14 | 3.6 |
| Smoking | 34 | 8.8 |
| Current BMI, mean (±SD) | 28.4 (±7.5) | |
| Chronic hypertension | 34 | 8.8 |
| Pregestational diabetes | 28 | 7.2 |
| Placental location | ||
| Anterior | 200 | 51.5 |
| Posterior | 180 | 46.4 |
| Fundal | 6 | 1.5 |
| Lateral | 2 | 0.6 |
| Placental volume(cm3), mean (±SD) | 45.1 (±18.9) | |
| VI, mean (±SD) | 15.8 (±9.9) | |
| FI, mean (±SD) | 43.2 (±9.0) | |
| VFI, mean (±SD) | 7.2 (±5.4) | |
| Gestational age at delivery in weeks, mean (±SD) | 38.0 ±3.8 | - |
| Birth weight (g), mean ±SD | 3247.1 (±654.8) | |
| Preeclampsia | 30 | 7.7 |
| Gestational Hypertension | 37 | 9.5 |
| Small for gestational age | 318.0 | 8.0 |
The mean values of placenta volumes were lower in the pregnancies with GH and SGA compared with unaffected pregnancies, but these were not statistically significant (Table 2). When converted to placental quotients (PQ) (placental volume/crown rump length), the median volume was significantly lower in the pregnancies with GH (p=0.03).
Table 2.
Placental volume and vascular flow indices in pregnancies with adverse outcomes
| Preeclampsia | Gestational hypertension | Small for Gestational age | ||||
|---|---|---|---|---|---|---|
| Mean (±SD) | p-value | Mean (±SD) | p-value | Mean (±SD) | p-value | |
| Placental Volume(cm3) | ||||||
| Normal | 45.2 (18.8) | 45.9 (19.3) | 45.9 (19.0) | |||
| Affected | 47.7 (21.6) | 0.56 | 40.9 (15.6) | 0.09 | 40.6 (18.2) | 0.14 |
| Placental quotient | ||||||
| Normal | 0.8 (0.6) | 0.80 (0.60) | 0.80 (0.60) | |||
| Affected | 0.8 (0.3) | 0.89 | 0.70 (0.19) | 0.03 | 0.64 (0.29) | 0.06 |
| VI | ||||||
| Normal | 16.0 (10.1) | 15.8 (10.1) | 15.9 (10.0) | |||
| Affected | 13.0 (7.0) | 0.04 | 15.8 (7.4) | 0.99 | 14.4 (8.5) | 0.35 |
| FI | ||||||
| Normal | 43.4 (9.0) | 43.2 (9.0) | 43.2 (8.8) | |||
| Affected | 40.3 (7.9) | 0.05 | 43.2 (8.7) | 0.98 | 43.5 (10.5) | 0.85 |
| VFI | ||||||
| Normal | 7.4(5.6) | 7.3 (5.6) | 7.3 (5.5) | |||
| Affected | 5.4 (3.2) | 0.004 | 7.0 (4.0) | 0.72 | 6.7 (4.7) | 0.54 |
SD = standard deviation. VI= vascularization index; FI= flow index; vfi=vascularization flow index;
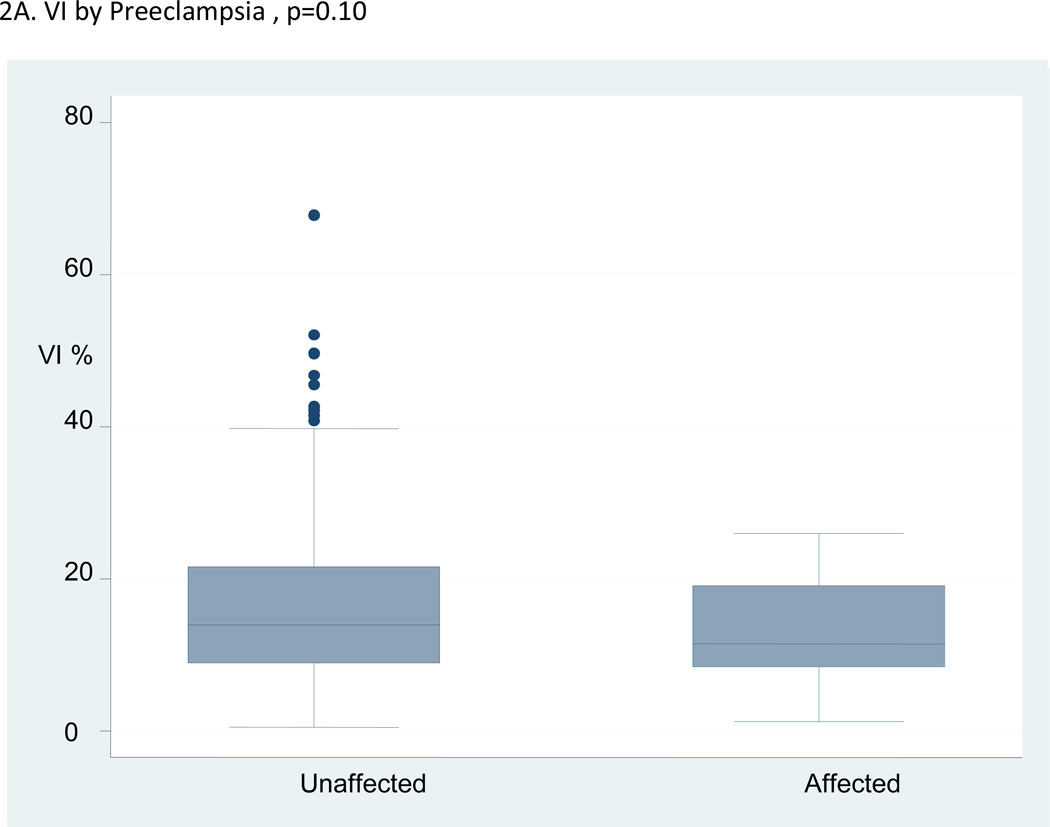
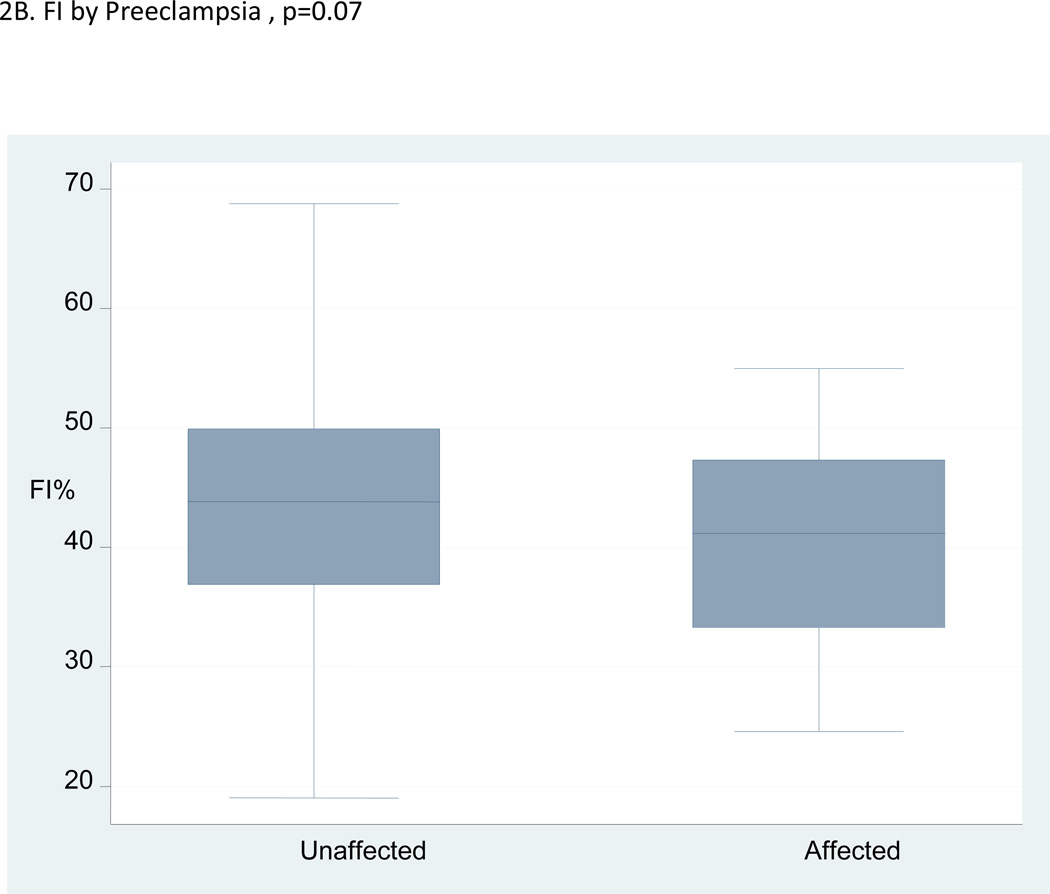
The mean values of the VI and VFI were significantly lower in the pregnancies that developed preeclampsia compared with the unaffected pregnancies, but the placenta volumes were not. The median, interquartile range and range of VI, FI and VFI in the pregnancies with preeclampsia compared to unaffected pregnancies are shown in Figures 2A–C. The figures illustrate a high degree of overlap of the median values in the pregnancies affected by preeclampsia compared with those that were unaffected.
Figure 2.
A–C:Box and whisker plots for vascularization index (VI), flow index (FI) and vascularization flow index (VFI) in women who developed preeclampsia versus unaffected women.
The AUC for the logistic regression models using the placental volume, PQ, VI, FI and VFI in predicting PE, GH and SGA are shown in Table 3. These models are adjusted for maternal BMI and placental location (entered as posterior location: yes or no) as suggested in previous studies (9, 16). The best models for predicting PE were with using VI and VFI. These were however, not significantly different from using FI as demonstrated by the overlapping confidence intervals in Table 2. The regression equations for the prediction of PE are: Y= −4.2828 - 0.0336 × VI + 0.0792 × BMI −0.0587 if placental location is posterior, R2=0.07, p=0.003; y= −3.9307 – 0.01577 × FI + 0.0744 × BMI −0.1356 if placental location is posterior, R2=,0.06, p=0.007; and Y= −4.1601 – 0.07334 × VFI + 0.0750 × BMI −0.1012 if placental location is posterior, R2 = 0.07, p=0.002; for VI, FI and VFI, respectively.
Table 3.
Screening Efficiency from the receiver operating characteristic (ROC)curves of Placental volume and vascular indices for adverse pregnancy outcomes (Models are adjusted for maternal BMI and placental location).
| Preeclampsia AUC | Gestational hypertension |
Small for gestational age |
|
|---|---|---|---|
| Area under ROC curve (95% CI) |
Area under ROC curve (95% CI) |
Area under ROC curve (95% CI) |
|
| Placenta volume (cm3) | 0.67 (0.57–0.76) | 0.65 (0.55–0.74) | 0.59 (0.49–0.69) |
| Placental quotient | 0.67 (0.57–0.76) | 0.64 (0.54–0.73) | 0.62 (0.52–0.71) |
| VI | 0.71 (0.61–0.80) | 0.59 (0.49–0.69) | 0.60 (0.50–0.70) |
| FI | 0.69 (0.59–0.78) | 0.60 (0.50–0.70) | 0.57 (0.47–0.67) |
| VFI | 0.70 (0.60–0.79) | 0.59 (0.49–0.69) | 0.58 (0.48–0.68) |
ROC – receiver operator characteristic. VI= vascularization index; FI= flow index; vfi=vascularization flow index; AUC= area under ROC curve
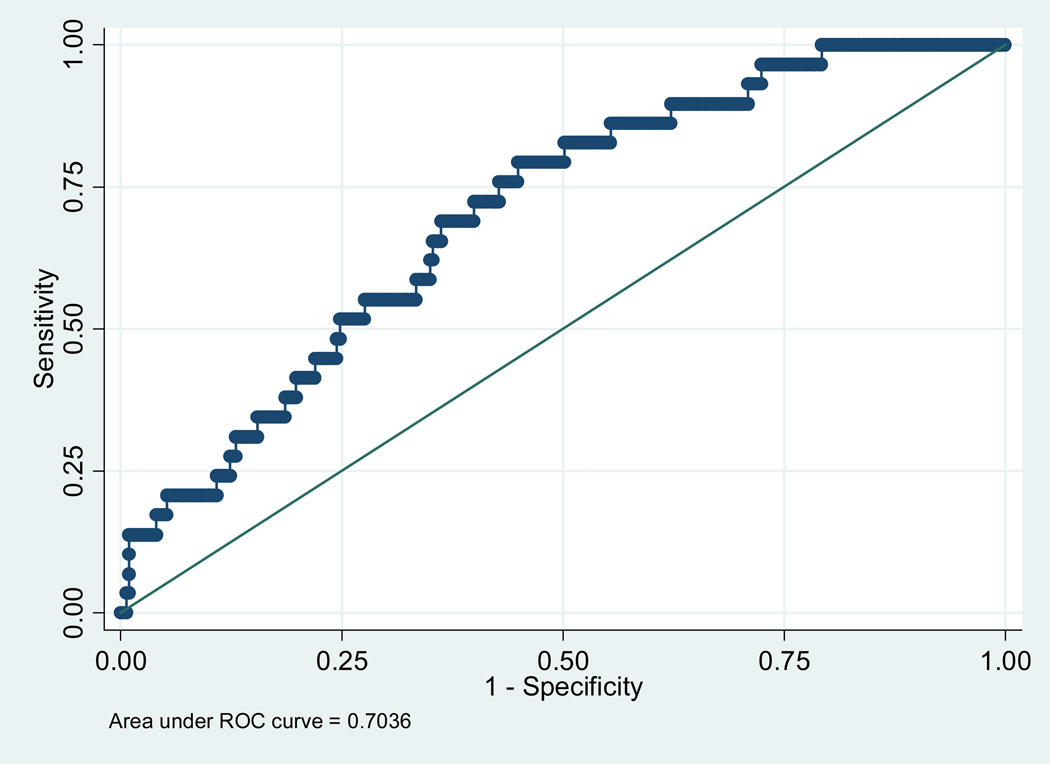
The detection rates and false positive rates for PE using VI and VFI are: 44.8% and 22.6%; and 44.8% and 22.1%, respectively. For a fixed false positive rate of 10%, the detection rate using either VI or VFI is 22%. The regression equations for the prediction models for other adverse outcomes (SGA and GH) are available from the authors. Figure 3 is an example of the ROC curve for predicting PE using VFI.
Figure 3.
Receiver operating characteristic curve for predicting preeclampsia using VFI (after adjusting for placental location and maternal BMI)
We performed a sub-analysis categorized by early Preeclampsia and also by the presence of SGA and Preeclampsia. The results are shown in Table 4. The only significant difference was seen in the lower FI in those with both Preeclampsia and SGA. We did develop a prediction model for these cases due to the small event rates.
Table 4.
Subgroup analysis of mean placental volume and vascularization indices in cases with early onset preeclampsia (delivered under 34 weeks) and those with both preeclampsia and small for gestational age (SGA).
| Affected (mean± SD) |
Normal | p-value | |
|---|---|---|---|
| Preeclampsia delivered <34 weeks (n=6) | |||
| Placental Volume | 40.1±24.0 | 45.2±18.8 | 0.62 |
| VI | 11.6±7.9 | 16.0±10.1 | 0.23 |
| FI | 39.8±11.6 | 43.4±9.0 | 0.48 |
| VFI | 5.2±4.5 | 7.4±5.6 | 0.62 |
| Preeclampsia and SGA (n=6) | |||
| Placental Volume | 46.7±10.7 | 45.7 ±18.7 | 0.83 |
| VI | 15.1±10.2 | 16.2±10.2 | 0.81 |
| FI | 33.6±5.8 | 43.2±8.9 | <.01 |
| VFI | 5.3±3.9 | 7.4±5.6 | 0.25 |
VI= vascularization index; FI= flow index; vfi=vascularization flow index; SD =standard deviation
Discussion
In this study, we found that the mean vascular indices of first-trimester placentas were lower in pregnancies that subsequently developed PE compared with unaffected pregnancies. In addition, those pregnancies with both PE and SGA had a significantly lower FI compared with normal controls. The prediction models for PE using these indices were, however, associated with only modest discriminatory ability as shown by the values of the AUC of about 70%. In addition, our study showed that the mean first-trimester placental volumes in these pregnancies that developed PE were not significantly different compared to unaffected gestations. The latter finding was different from the finding in pregnancies that developed GH and SGA where the mean placenta volumes were smaller than the normal controls. The prediction models for GH and SGA using placental volume were not as promising as those using the vascular indices for PE.
Our findings are not as robust as previous studies that suggested better prediction of PE and SGA using placental volumes and vascularization indices (8, 10, 17,). Differences in methodology of placental volume acquisition, machine settings, and sample sizes of the studies may contribute to the different results. For example, Dar et al using recently reported AUC ranging from 77.6% to 79.6% for PE prediction using spherical volume biopsy of the placenta. The authors admitted to not using a similar setting in each machine used for the study; however, it is known that machine settings can influence the quantitative values obtained from 3D power Doppler interrogation of the placenta (18, 19). The reports from Noguchi et al and Rizzo et al used smaller sample sizes (8, 10). Similar to our finding, Rizzo et al also found no significant difference in the placental volumes of the SGA group (10).
In this study we also found that maternal smoking did not affect the placental vascular indices. We therefore did not adjust for smoking in the prediction model. This is contrary to the finding by Rizzo et al who found lower values of the vascular flow indices among women who smoked over 10 cigarettes per day (20). In the current study we did not have the information on the number of cigarettes smoked by our patients and therefore could not evaluate any potential dose response effect.
Despite the statistically significant differences in mean values observed in our study, the detection rates for PE using VI, FI and VFI were poor and the false positive rates high. This finding suggests that these indices may need to be combined with other reliable maternal characteristics; biochemical markers and biophysical properties become reliable predictors of PE. This is the subject of many ongoing investigations.
An important finding from the current study is that placental volume may be different depending on the pregnancy complication being studied. In particular, we found the placental volumes to be lower in the pregnancies with GH and SGA compared with PE. This is consistent with studies that have demonstrated different morphologic characteristics of the placenta in pregnancies with growth restriction compared with PE (21). However, as a screening tool, placental volume was associated with poor predictive efficiency even in SGA and GH pregnancies in the current study.
Our study is not with out limitations, including the relative small sample size; however, it is still one of the largest series evaluating 3D power Doppler of the placenta in the literature. The incidence of PE and GH in our population is higher than would be expected in a general population. This may reflect the high-risk characteristics of our study demographic, being a major regional referral Center. Despite the significant differences in mean values of VI and VFI observed, the median values are very close and, as shown in figure 2, there is considerable overlap in the range of values between normal pregnancies and those with PE. These findings may be responsible for the modest predictive ability of PE. The findings cannot be attributed to inter-observer variability among our sonographers, as we had conducted a rigorous training of our technicians which is supported by our prior reliability study (12). Although the settings of our machines were fixed, it is still possible that when suboptimal volumes were seen, the sonographer may have adjusted the settings. This latter point illustrates a need for ongoing quality assurance, similar to those used for nuchal translucency training if 3D power Doppler is to be introduced as a screening tool for PE or other adverse pregnancy outcomes. Finally, the findings from the study may suggest that the VOCAL technique needs optimization to obtain more reliable results. Studies comparing the VOCAL technique to other methods of evaluating vascular flow such as the use of fractional moving blood volume (FMBV) with Matlab (The MathWorks, Natick, MA, USA) are needed (22). This technique has demonstrated promise in evaluating vascular flow in other fetal organs.
In conclusion, our study found the vascular flow indices obtained using 3D power Doppler in the first-trimester to be significantly lower in pregnancies that subsequently developed PE. The screening efficiency of the indices was, however, modest. This suggests the need for future studies combining this modality with other screening tools to develop a more reliable prediction model.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hafner E, Philipp T, Schuchter K, Dillinger-Paller B, Philipp K, Bauer P. Second-trimester measurements of placental volume by three-dimensional ultrasound to predict small-for gestational- age infants. Ultrasound Obstet Gynecol. 1998;12:97–102. doi: 10.1046/j.1469-0705.1998.12020097.x. [DOI] [PubMed] [Google Scholar]
- 2.Pairleitner H, Steiner H, Hasenoehrl G, Staudach A. Three-dimensional power Doppler sonography: imaging and quantifying blood flow and vascularization. Ultrasound Obstet Gynecol. 1999;14:139–143. doi: 10.1046/j.1469-0705.1999.14020139.x. [DOI] [PubMed] [Google Scholar]
- 3.Hafner T, Kurjak A, Funduk-Kurjak B, Bekavac I. Assessment of early chorionic circulation by three-dimensional power Doppler. J Perinat Med. 2002;30(1):33–39. doi: 10.1515/JPM.2002.005. [DOI] [PubMed] [Google Scholar]
- 4.Matijevic R, Kurjak A. The assessment of placental blood vessels by three-dimensional power Doppler ultrasound. J Perinat Med. 2002;30:26–32. doi: 10.1515/JPM.2002.004. [DOI] [PubMed] [Google Scholar]
- 5.Pretorius DH, Nelson TR, Baergen RN, Pai E, Cantrell C. Imaging of placental vasculature using three-dimensional ultrasound and color power Doppler: a preliminary study. Ultrasound Obstet Gynecol. 1998;12:45–49. doi: 10.1046/j.1469-0705.1998.12010045.x. [DOI] [PubMed] [Google Scholar]
- 6.Guimarães Filho HA, da Costa LL, Araújo Júnior E, Nardozza LM, Nowak PM, Moron AF, Mattar R, Pires CR. Placenta: angiogenesis and vascular assessment through three-dimensional power Doppler ultrasonography. Arch Gynecol Obstet. 2008;277:195–200. doi: 10.1007/s00404-007-0453-y. [DOI] [PubMed] [Google Scholar]
- 7.Hafner E, Schuchter K, van Leeuwen M, et al. Three-dimensional sonographic volumetry of the placenta and the fetus between weeks 15 and 17 of gestation. Ultrasound Obstet Gynecol. 2001;18:116. doi: 10.1046/j.1469-0705.2001.00489.x. [DOI] [PubMed] [Google Scholar]
- 8.Noguchi J, Hata K, Tanaka H, Hata T. Placental vascular sonobiopsy using three-dimensional power Doppler ultrasound in normal and growth restricted fetuses. Placenta. 2009;30:391–397. doi: 10.1016/j.placenta.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Hafner E, Metzenbauer M, Stümpflen I, Waldhör T, Philipp K. First trimester placental and myometrial blood perfusion measured by 3D power Doppler in normal and unfavourable outcome pregnancies. Placenta. 2010 Sep;31(9):756–763. doi: 10.1016/j.placenta.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Rizzo G, Capponi A, Pietrolucci ME, Capece A, Arduini D. First-trimester placental volume and vascularization measured by 3-dimensional power Doppler sonography in pregnancies with low serum pregnancy-associated plasma protein a levels. J Ultrasound Med. 2009 Dec;28(12):1615–1622. doi: 10.7863/jum.2009.28.12.1615. [DOI] [PubMed] [Google Scholar]
- 11.Alcazar JL. Three-dimensional power Doppler derived vascular indices: what are we measuring and how are we doing it? Ultrasound Obstet Gynecol. 2008;32:485–487. doi: 10.1002/uog.6144. [DOI] [PubMed] [Google Scholar]
- 12.Huster KM, Haas K, Schoenborn J, McVean D, Odibo AO. Reproducibility of placental volume and vasculature indices obtained by 3-dimensional power Doppler sonography. J Ultrasound Med. 2010 Jun;29(6):911–916. doi: 10.7863/jum.2010.29.6.911. [DOI] [PubMed] [Google Scholar]
- 13.Tuuli MG, Houser M, Odibo L, Huster K, Macones GA, Odibo AO. Validation of placental vascular sonobiopsy for obtaining representative placental vascular indices by three-dimensional power Doppler ultrasonography. Placenta. 2010 Mar;31(3):192–196. doi: 10.1016/j.placenta.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 14.American College of Obstetricians and Gynecologists. Diagnosis and management of preeclampsia and eclampsia. Washington, DC: The College; 2002. ACOG practice bulletin no. 33. [Google Scholar]
- 15.Report of the National High Blood Pressure Education Program Working Group. Report on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 16.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan MA. United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–171. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 17.Dar P, Gebb J, Reimers L, Bernstein PS, Chazotte C, Merkatz IR. First-trimester 3-dimensional power Doppler of the uteroplacental circulation space: a potential screening method for preeclampsia. Am J Obstet Gynecol. 2010 Sep;203(3):238.e1–238.e7. doi: 10.1016/j.ajog.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Raine-Fenning NJ, Nordin NM, Ramnarine KV, Campbell BK, Clewes JS, Perkins A, Johnson IR. Evaluation of the effect of machine settings on quantitative three-dimensional power Doppler angiography: an in-vitro flow phantom experiment. Ultrasound Obstet Gynecol. 2008 Sep;32(4):551–559. doi: 10.1002/uog.6138. [DOI] [PubMed] [Google Scholar]
- 19.Jones NW, Hutchinson ES, Brownbill P, Crocker IP, Eccles D, Bugg GJ, Raine-Fenning NJ. In vitro dual perfusion of human placental lobules as a flow phantom to investigate the relationship between fetoplacental flow and quantitative 3D power doppler angiography. Placenta. 2009 Feb;30(2):130–135. doi: 10.1016/j.placenta.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Rizzo G, Capponi A, Pietrolucci ME, Arduini D. Effects of maternal cigarette smoking on placental volume and vascularization measured by 3-dimensional power Doppler ultrasonography at 11+0 to 13+6 weeks of gestation. Am J Obstet Gynecol. 2009 Apr;200(4):415.e1–415.e5. doi: 10.1016/j.ajog.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 21.Mayhew TM. A stereological perspective on placental morphology in normal and complicated pregnancies. J Anat. 2009 Jul;215(1):77–90. doi: 10.1111/j.1469-7580.2008.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez-Andrade E, Jansson T, Figueroa-Diesel H, Rangel-Nava H, Acosta-Rojas R, Gratacós E. Evaluation of fetal regional cerebral blood perfusion using power Doppler ultrasound and the estimation of fractional moving blood volume. Ultrasound Obstet Gynecol. 2007 May;29(5):556–561. doi: 10.1002/uog.4005. [DOI] [PubMed] [Google Scholar]