Abstract
Objectives
This study investigated the short- and long-term outcome of children with pulmonary arterial hypertension (PAH) treated with inhaled iloprost.
Background
Inhaled iloprost has been approved for the treatment of adults with PAH, but little is known about the effects in children with PAH.
Methods
We evaluated the acute effects of inhaled iloprost on hemodynamic status and lung function and the response to long-term therapy in 22 children (range 4.5 to 17.7 years) with PAH (idiopathic, n = 12; congenital heart disease, n = 10). Cardiac catheterization, standard lung function testing before and after iloprost inhalation, 6-min walk test, World Health Organization functional class, and hemodynamic parameters were monitored.
Results
Acute administration of inhaled iloprost lowered mean pulmonary artery pressure equivalent to the response to inhaled nitric oxide with oxygen. Acute iloprost inhalation reduced forced expiratory volume in 1 s and mid-volume forced expiratory flow by 5% and 10%, respectively, consistent with acute bronchoconstriction. At 6 months, functional class improved in 35%, decreased in 15%, and remained unchanged in 50% of children. Sixty-four percent of patients continued receiving long-term iloprost therapy, 36% stopped iloprost, due to lower airway reactivity, clinical deterioration, or death. In 9 patients on chronic intravenous prostanoids, 8 transitioned from intravenous prostanoids to inhaled iloprost, which continued during follow-up.
Conclusions
Inhaled iloprost caused sustained functional improvement in some children with PAH, although inhaled iloprost occasionally induced bronchoconstriction. Most patients tolerated the transition from intravenous to inhaled prostanoid therapy. Clinical deterioration, side effects, and poor compliance, owing to the frequency of treatments, could limit chronic treatment in children.
Pulmonary arterial hypertension (PAH) is characterized by elevation of pulmonary artery pressure (PAP) and pulmonary vascular resistance (PVR), which can lead to progressive right heart failure and death (1–4). Pulmonary arterial hypertension occurs in diverse clinical settings, such as in association with congenital heart disease, chronic lung disease, connective tissue disease, liver disease, and anorexigen use, or could be idiopathic or familial (5,6). Before the development of intravenous (IV) prostacyclin as a chronic therapy for PAH, the National Health Registry estimated that the median survival after diagnosis was 2.8 years for adults and 0.8 years for children with idiopathic PAH (7). Over the past decade, significant advances in the pharmacologic treatment of PAH have improved survival; however, there remains no cure (8–12). On the basis of advances in vascular biology and known pathogenic mechanisms underlying PAH, 3 general classes of therapeutic agents have been developed and are currently available for the treatment of PAH. These include: prostacyclin analogues (epoprostenol, treprostinil, and iloprost), endothelin receptor antagonists (bosentan and ambrisentan), and phosphodiesterase inhibitors (sildenafil) (3,13–24).
Until recently, chronic treatment with prostacyclin analogues has required IV or subcutaneous administration, with each approach limited by such problems as line infections, thrombosis, or site pain. Previous studies of inhaled iloprost have been performed in adult patients. In 1 large multicenter, randomized, placebo-controlled trial of iloprost therapy for 3 months, a larger percentage of patients on iloprost demonstrated the combined end point of at least a 10% improvement in the 6-min walk (6MW) distance and improvement in World Health Organization (WHO) functional class, with no deterioration or death versus patients on placebo (16).
Most recently, inhaled iloprost has been studied in patients who remain symptomatic (WHO functional class III or IV) on bosentan therapy (25). In this multicenter, randomized, controlled trial, 67 patients with PAH were randomized to receive inhaled iloprost or placebo. There were significant improvements in 6MW distance, WHO functional class, time to clinical worsening, and postinhalation mean PAP and PVR. Combination therapy appeared safe and well tolerated. Although extensively studied in adults with PAH, little is known about efficacy of iloprost in children, especially with regard to long-term therapy (16,17,25–30). Therefore, to determine the potential role for inhaled iloprost therapy in children with PAH, we retrospectively evaluated the acute and chronic effects of inhaled iloprost in children with PAH.
Methods
Study population
To evaluate the safety, tolerability, and clinical effects of inhaled iloprost in children with PAH, we reviewed data from all children with PAH who were treated with inhaled iloprost before August 2006 at 5 pediatric pulmonary hypertension clinics. Each institution received institutional review board approval or exempt status.
Patient selection and approach to dosage and treatment
Indications for initiation of iloprost therapy included newly diagnosed PAH (n = 3), perceived inadequate response to prior therapy or refusal to initiate IV prostanoid therapy (n = 10), and transition from IV or subcutaneous prostanoids (n = 9; 3 of 9 transitioned for recurrent central line infections; 1 of 9 for site pain; 5 of 9 per patient request for noninvasive therapy). After insurance approval, iloprost (Ventavis, Actelion Inc., South San Francisco, California) was administered by inhalation with the Prodose AAD (Profile Therapeutics PLC, West Sussex, United Kingdom) or Ineb Adaptive Aerosol Delivery System (Respironics Inc., Murrysville, Pennsylvania) delivery devices. The initial dose of inhaled iloprost was 2.5 μg (with the exception of 1 patient initiated at 0.625 μg). Iloprost therapy was initiated during hospital stay or in the clinic setting, depending upon patient stability. Patients with side effects or difficulty tolerating the duration of each treatment were maintained on the initial dose for several weeks or months. Those with minimal to no side effects were increased to 5 μg and, if tolerated, maintained at that dose for chronic therapy. Some patients were increased to a dose of 7.5 μg/dose. The dose of iloprost could be reduced if adverse effects were noted, with plans to increase the dose again as tolerated. The frequency of dosing was initiated at 5 to 9 inhalations daily but titrated individually according to severity of illness, tolerability, and patient compliance.
Patients transitioning from IV epoprostenol or IV treprostinil to iloprost were hospitalized for 24 to 96 h in a pediatric intensive care unit for monitoring at the time of initiation of inhaled iloprost therapy. Transition methods varied, but in general, patients were initiated at 2.5 μg iloprost while remaining on their baseline dose of IV prostanoid therapy. Two to 3 h later, iloprost 5 μg was administered and repeated every 2.5 to 3 h while awake, during which time the IV prostanoid therapy dose was decreased. One patient increased to 7.5 μg/dose every 3 h after 24 h. The rate of IV prostanoid weaning varied from 10% reductions every 6 h to an initial 50% reduction followed by 10% serial dose reductions after 24 h. One patient transitioned from subcutaneous treprostinil over a 4-day period with frequent clinic visits. Reduction of prostanoid dose occurred primarily during the day to ensure accurate assessment of symptoms and side effects related to severe PAH, including concerns for the risk of acute pulmonary hypertensive crisis.
Acute assessments
Before the initiation of iloprost therapy, assessments included: cardiac catheterization (n = 22), pulmonary function tests (n = 13), 6MW distance (n = 13), and assessment of WHO functional class (n = 22) on the basis of physician judgment and age of the patient. Cardiac catheterization at baseline included acute vasoreactivity testing to assess the hemodynamic response of inhaled iloprost (5 μg) and inhaled nitric oxide (NO) (40 ppm) with oxygen (n = 8). Baseline pulmonary function measurements, spirometry and lung volumes, were performed with standard methods before and after inhalation of iloprost to study acute changes in lung function. Study parameters measured included forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, mid-volume forced expiratory flow (FEF25–75), total lung capacity (TLC), residual volume (RV), and RV/TLC ratio. These measurements were performed with a SensorMedics Vmax22 (SensorMedics Corporation, Yorba Linda, California) system.
The 6MW test was performed in children 8 years of age and older. Patients were instructed to walk at their own pace but to walk as far as possible in 6 min. Patients were allowed to stop during the walk if needed for symptoms and resume again when able. The test was performed in a covered corridor and the patient did not receive encouragement. At completion of the 6 min, the total distance covered was measured and recorded. The functional class was assigned on the basis of standard WHO definitions.
The acute response to inhaled iloprost and comparisons with the effects of inhaled NO were studied by cardiac catheterization in 8 patients. Candidates for studies of inhaled iloprost were patients who were undergoing cardiac catheterization for clinical care, independent of this study protocol, and consent was obtained before study. Patients were newly diagnosed (n = 3) or continued on chronic therapy before catheterization. At cardiac catheterization, patients were sedated with fentanyl and midazolam. Arterial and venous access was obtained by the femoral approach via standard techniques. Cardiac output was measured in triplicate by thermodilution technique or by the Fick equation with measured oxygen consumption. Study measurements included mean PAP, pulmonary capillary wedge pressure, mean aortic/systemic arterial pressure, right atrial pressure, cardiac index, pulmonary vascular resistance (PVR) index, systemic vascular resistance (SVR) index, and PVR/SVR ratio. Pulmonary and systemic vascular resistances were indexed for body surface area and expressed as PVR index and SVR index, respectively, in Wood units (U × m2).
After obtaining baseline hemodynamic measurements, the acute response to inhaled NO was measured during administration of NO at 40 ppm by facemask All patients were allowed to equilibrate in each given condition for at least 10 min before the hemodynamic responses were measured. After NO administration, patients were returned to baseline status, and then iloprost was administered with the Prodose AAD or Ineb delivery devices at a dose of 5 μg. To achieve delivery with sedated patients in a supine position, a PARI mask set (PARI Respiratory Inc., Midlothian, Virginia) with Y-piece expiratory valve was connected to corrugate tubing and attached to the mouthpiece of the delivery device. Sealing of the mask on the patient's face allowed for appropriate triggering of the device.
Chronic therapy
Outpatient dose titration of iloprost was based upon a perceived balance between prostanoid side effects and pulmonary hypertension symptoms. Biweekly or weekly phone contact with the patient continued until their first follow-up visit. Follow-up visits occurred from 2 weeks to 6 months after initiation of iloprost, depending on clinical course. Evaluations at the follow-up visits included physical examination, echocardiogram, 6MW test, and WHO functional class. Repeat cardiac catheterizations were preformed in some patients (n = 12; 3 clinical worsening, 9 scheduled follow-up) on the basis of the investigator's clinical judgment. All adverse events were assessed during phone and clinic appointments and recorded.
Data analysis
Data analyzed included: hemodynamic parameters during acute vasoreactivity testing with inhaled NO followed by inhaled iloprost, exercise capacity as measured by the 6MW test, functional assessment according to the WHO classification, pulmonary function tests before and after iloprost inhalation measuring forced expiratory volume of air in 1 s (FEV1.0), and mid-volume forced expiratory flow rate (FEF25%–75%). Data are expressed as median and range for some of the descriptions of the study population or as mean ± SD. Analysis for statistically significant differences was performed with paired t test or with repeated measures analysis of variance with Tukey-Kramer multiple comparisons post hoc test.
Results
Patient population
We studied the effects of inhaled iloprost in 22 pediatric PAH patients (12 male, 10 female), with a median age of 11.5 years (range 4.5 to 17.7 years) and median body weight of 35.6 kg (range 15 to 73 kg) (Table 1). Pulmonary arterial hypertension was idiopathic or familial in 12 patients and associated with congenital heart disease in 10 patients, which included the following diagnoses: unrepaired heart disease in 1 (atrial septal defect) and surgically repaired disease in 9 patients (atrial septal defect; ventricular septal defect; ventricular septal defect with patent ductus arteriosus; ventricular septal defect with coarctation of the aorta; D-transposition of the great arteries repaired by the arterial switch procedure; patent ductus arteriosus with left pulmonary artery stenosis and congenital diaphragmatic hernia; patent ductus arteriosus; partial atrioventricular canal; and double-outlet right ventricle with patent ductus arteriosus and coarctation of the aorta).
Table 1. Baseline Characteristics.
| Patient # | Gender | Diagnosis | Weight (kg) |
Age (yrs) |
Ventavis Duration (yrs) |
PAPm (mm Hg) |
PCWPm (mm Hg) |
AOPm (mm Hg) |
RAPm (mm Hg) |
CI (l/min/m2) |
PVRI (U × m2) |
PVR/SVR | WHO Class | 6MW (m) |
PH Medications at Transition |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | IPAH | 15 | 5 | 1.1 | 47 | 11 | 76 | 8 | 3.3 | 10.9 | 0.5 | 3 | NA | BOS, SIL |
| 2 | M | IPAH | 16 | 6 | 7.9 | 73 | 8 | 67 | 5 | 5.6 | 25.0 | 2.2 | 4 | 120 | CCB |
| 3 | F | CHD/repaired | 22 | 7 | 0.9 | 37 | 8 | 50 | 4 | 4.6 | 6.0 | 0.6 | 3 | NA | BOS, SIL |
| 4 | M | IPAH | 32 | 8 | 5.2 | 66 | 6 | 57 | 6 | 2.6 | 23.0 | 1.2 | 4 | 100 | |
| 5 | M | CHD/repaired | 28 | 9 | 1.1 | 76 | 5 | 81 | 6 | 3.2 | 18.7 | 0.9 | 3 | 446 | BOS, SIL |
| 6 | M | IPAH | 33 | 10 | 1.0 | 62 | 8 | 66 | 4 | 2.4 | 12.9 | 0.9 | 2 | 492 | BOS, SIL |
| 7 | M | IPAH | 38 | 10 | 1.1 | 42 | 11 | 84 | 2 | 3.0 | 9.0 | 0.4 | 2 | 480 | IV EPO, CCB, BOS, SIL |
| 8 | M | FPAH | 27 | 11 | 0.9 | 28 | 10 | 78 | 8 | 4.7 | 3.9 | 0.3 | 2 | 462 | IV EPO, BOS |
| 9 | M | IPAH | 32 | 11 | 1.7 | 84 | 12 | 69 | 9 | 2.9 | 25.1 | 1.4 | 3 | 423 | BOS |
| 10 | M | IPAH | 33 | 11 | 0.1 | 70 | 6 | 67 | 6 | 2.9 | 22.4 | 1.1 | 3 | 582 | IV TRE, SIL |
| 11 | F | CHD/repaired | 41 | 11 | 0.1 | 70 | 7 | 72 | 6 | 1.6 | 15.8 | 0.9 | 2 | 482 | BOS, SIL |
| 12 | M | IPAH | 56 | 12 | 5.6 | 71 | 13 | 69 | 8 | 4.8 | 37.0 | 2.9 | 3 | 475 | |
| 13 | M | CHD/repaired | 33 | 13 | 0.7 | 65 | 9 | 70 | 4 | 3.5 | 16.9 | 0.9 | 2 | 583 | IV TRE, BOS, SIL |
| 14 | F | CHD | 28 | 13 | 1.1 | 83 | 12 | 68 | 7 | 3.4 | 26.1 | 1.5 | 3 | 435 | IV TRE, SIL |
| 15 | F | CHD/Repaired | 73 | 13 | 0.2 | 82 | 10 | 87 | 13 | 3.3 | 21.8 | 1.0 | 3 | 360 | SIL, CCB |
| 16 | M | CHD/repaired | 58 | 14 | 1.1 | 60 | 6 | 67 | 6 | 3.7 | 12.7 | 0.8 | 3 | 494 | SQ TRE, SIL |
| 17 | F | CHD/repaired | 46 | 14 | 1.0 | 60 | 7 | 84 | 7 | 2.8 | 35.3 | 1.3 | 2 | 542 | IV EPO, SIL |
| 18 | F | CHD/repaired | 44 | 16 | 0.5 | 77 | 12 | 68 | 12 | 1.9 | 34.0 | 1.2 | 3 | 434 | BOS, SIL |
| 19 | F | IPAH | 44 | 16 | 0.1 | 55 | 8 | 70 | 6 | 2.8 | 14.7 | 0.7 | 3 | 534 | BOS, SIL |
| 20 | F | CHD/repaired | 46 | 17 | 0.9 | 52 | 8 | 62 | 5 | 4.7 | 9.3 | 0.8 | 3 | 300 | IV TRE, SIL |
| 21 | F | FPAH | 64 | 17 | 0.9 | 82 | 8 | 75 | 15 | 1.4 | 54.9 | 1.2 | 4 | 278 | |
| 22 | M | IPAH | 65 | 18 | 0.6 | 70 | 3 | 64 | 3 | 2.1 | 22.7 | 0.8 | 2 | 469 | IV TRE, SIL |
| Mean ± SD | 40 ± 16 | 12 ± 4 | 1.5 ± 2.0 | 64 ± 15 | 9 ± 3 | 70 ± 9 | 7 ± 3 | 3.2 ± 1.1 | 20.8 ± 11.9 | 1.1 ± 0.6 | 2.8 ± 0.7 | 425 ± 133 |
6MW = 6-min walk (test); AOPm = mean arterial pressure; BOS = bosentan; CCB = calcium channel blocker; CHD = congenital heart disease; CI = cardiac index; EPO = epoprostenol; FPAH = familial pulmonary arterial hypertension; IPAH = idiopathic pulmonary arterial hypertension; IV = intravenous; PAPm = mean pulmonary artery pressure; PCWPm = mean pulmonary capillary wedge pressure; PH = pulmonary hypertension; PVR = pulmonary vascular resistance; PVRI = pulmonary vascular resistance index; RAPm = mean right atrial pressure; SIL = sildenafil; SQ = subcutaneous; SVR = systemic vascular resistance; TRE = treprostinil; WHO = World Health Organization.
Because a recent publication reported that anatomical airway obstruction might be present in as many as 25% of children with PAH (31), previous chest tomographies were reviewed, if available. Of 17 patients, 1 showed evidence of upper airway obstruction caused by tonsillar hypertrophy (tonsillectomy performed) and another showed compression of left main bronchus by the aorta. Fifty-three percent showed pulmonary artery enlargement and 18% had ground glass appearance.
At the time of iloprost initiation, 19 patients were treated with at least 1 other PAH therapy (Table 1). Concomitant therapies included IV treprostinil (n = 5), IV epoprostenol (n = 3), subcutaneous treprostinil (n = 1), sildenafil (n = 16), bosentan (n = 11), and calcium channel blockers (n = 3) (Table 1). Seven patients had concomitant PAH therapies added for inadequate response to therapy (Patients #2, #4, #9, #12, #17, #21, and #22) at a median time of 2.4 months after the initiation of iloprost. Bosentan doses were consistent with those recommended in the pediatric pharmacokinetic trial (32). The median sildenafil dose was 40 mg three times a day (3.5 mg/kg/dose) and did not change significantly during the study period. In 2 patients (Patients #3 and #21), concomitant PAH therapy at initiation was discontinued after iloprost initiation at a median time of 3 months.
Acute hemodynamic and pulmonary effects of inhaled iloprost
Cardiac catheterization was performed in all patients before the initiation of iloprost (n = 22) (Table 1). Baseline hemodynamic status demonstrated significant PAH (i.e., mean PAP 64 ± 15 mm Hg [mean ± SD; median 68 mm Hg, range 28 to 84 mm Hg]) and a pulmonary to systemic vascular resistance index ratio of 1.1 ± 0.6. Of these 22 patients, 8 (Patients #2, #5, #6, #7, #11, #13, #19, and #21) had acute pulmonary vasoreactivity testing with inhaled NO and iloprost (Table 2). Reduction in the mean PAP by inhaled NO and iloprost were similar (Fig. 1). Inhaled NO (40 ppm) reduced mean PAP from 66 ± 13 mm Hg at baseline to 58 ± 18 mm Hg (p < 0.05 vs. baseline), representing a reduction of 12% for the entire study group. The reduction in mean PAP after acute inhalation of iloprost (57 ± 19 mm Hg; p < 0.05 vs. baseline) was similar to the level achieved with inhaled NO therapy. Neither inhaled NO nor inhaled iloprost significantly lowered PVR. The PVR/ SVR ratio fell from 1.0 ± 0.5 at baseline to 0.8 ± 0.4 (p < 0.05) after iloprost inhalation. There were no significant changes from baseline values for cardiac index, pulmonary capillary wedge pressure, or right atrial pressure, during the acute treatment with either NO or iloprost. Two patients (Patients #6 and #7) had a decrease in mean PAP of at least 20% in response to inhaled NO and inhaled iloprost.
Table 2. Acute Hemodynamic Effects of Inhaled Iloprost.
| Baseline (n = 8) |
iNO With 100% O2 (n = 8) |
Inhaled Iloprost (n = 8) |
|
|---|---|---|---|
| RAPm (mm Hg) | 6 ± 4 | 6 ± 3 | 6 ± 3 |
| SAPm (mm Hg) | 73 ± 6 | 73 ± 6 | 75 ± 7 |
| PCWPm (mm Hg) | 8 ± 2 | 8 ± 2 | 8 ± 3 |
| CI (l/min/m2) | 2.9 ± 1.3 | 2.8 ± 1.3 | 3.1 ± 1.4 |
| PVRI (U × m2) | 21 ± 14 | 18 ± 17 | 17 ± 15 |
| PVRI/SVRI | 1.0 ± 0.5 | 0.9 ± 0.7 | 0.8 ± 0.4* |
Values mean ± SD. Analysis with repeated measures analysis of variance with Tukey-Kramer multiple comparisons post hoc test.
p < 0.05 vs. baseline.
iNO = inhaled nitric oxide; CI = cardiac index; PCWPm = mean pulmonary capillary wedge pressure; PVRI = pulmonary vascular resistance index; RAPm = mean right atrial pressure; SAPm = mean systemic arterial pressure; SVRI = systemic vascular resistance index.
Figure 1. Acute Inhalation of Iloprost Lowered Mean PAP Equivalent to the Response to 40 ppm Inhaled NO.
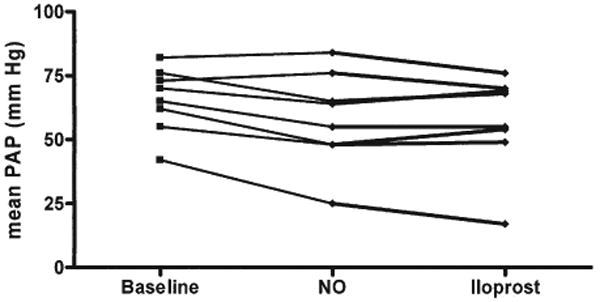
Inhaled nitric oxide (NO) (40 ppm) reduced mean pulmonary artery pressure (PAP) from 66 ± 13 mm Hg at baseline to 58 ± 18 mm Hg (p < 0.05 vs. baseline); n = 8. The reduction in mean PAP after acute inhalation of iloprost was similar to the level achieved with inhaled NO therapy (57 ± 19 mm Hg; p < 0.05 vs. baseline).
The acute effects of inhaled iloprost were also assessed by pulmonary function tests before the initiation of chronic iloprost therapy in 13 patients. Baseline FEV1 (expressed as % predicted) was 84 ± 16% (range 56% to 119%), which decreased to 79 ± 15% after a single inhalation of iloprost (p = 0.02) (Fig. 2). At baseline, mean FEF25–75 was 82% of predicted (range 32% to 119%). After iloprost inhalation, mean FEF25–75 decreased to 72 ± 29% (p = 0.03) (Fig. 3). In 5 of 13 (38%) patients, FEF25–75 decreased by more than 15% (range −53% to −17%). Two of these patients did not receive chronic iloprost therapy, owing to symptomatic lower airway obstruction.
Figure 2. The Acute Effects of Inhaled Iloprost Were Assessed by Pulmonary Function Tests in 13 Patients.
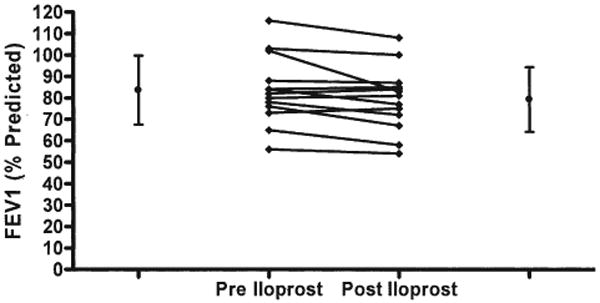
Baseline forced expiratory volume in 1 s (FEV1) (expressed as % predicted) was 84% (range 56% to 119%) and decreased after a single inhalation of iloprost to 79% (range −18% to +3%; p = 0.02).
Figure 3. Acute Effects of Inhaled Iloprost Were Assessed by Pulmonary Function Tests in 13 Patients.
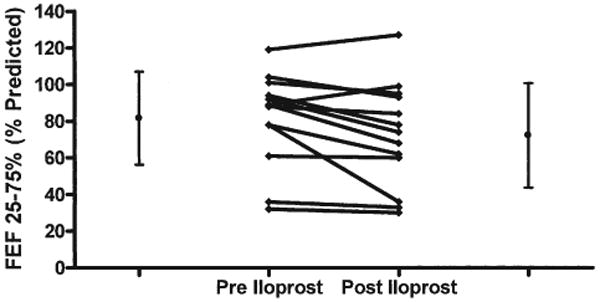
At baseline, mean mid-volume forced expiratory flow (FEF25%–75%) was 82% of predicted (range 32% to 119%). After iloprost inhalation, mean FEF25%–75% decreased to 72% of predicted (p = 0.03). In 5 of 14 (38%) patients, FEF25%–75% decreased by more than 15% (range −53% to −17%).
Physiologic effects of chronic iloprost therapy
The median duration of iloprost therapy was 0.9 years (range 0.1 to 7.9 years). At initiation (n = 22) the median dose of iloprost was 5 μg (range 0.63 to 10 μg), with a frequency of 6 times daily (range 4 to 9) and total daily dose of 30 μg/day (range 3.75 to 50 μg/day) (Table 3). At 6 months, 18 patients continued on iloprost therapy; the median dose was 5 μg (range 2.5 to 10 μg), with a median frequency of 6 times daily and a total daily dose of 30 μg/day (range 13.75 to 50 μg/day). Twelve patients remained on therapy for 12 months or longer. At one year, the median dose was 5 μg (range 5 to 10 μg), with a median frequency of 6 times daily and total daily dose of 30 μg/day (range 25 to 50 μg/day).
Table 3. Iloprost Dosing.
| Initiation (n = 22) |
6 Months (n = 18) |
12 Months (n = 12) |
|
|---|---|---|---|
| Dose (μg) | 5 ± 2 | 5 ± 1.9 | 5 ± 2 |
| Range | (0.63–10) | (2.5–10) | (5–10) |
| ≤2.5/5.0/ >5.0 | 8/12/2 | 2/13/3 | 0/10/2 |
|
| |||
| Frequency (/day) | 6 ± 1 | 6 ± 0.7 | 6 ± 0.7 |
| Range | (4–9) | (4–7) | (5–7) |
|
| |||
| Total daily dose (μg) | 30 ± 13 | 30 ± 10 | 30 ± 8 |
| Range | (3.75–50) | (13.75–50) | (25–50) |
Follow-up cardiac catheterizations were performed in 12 patients (Patients #1, #2, #3, #4, #5, #6, #8, #9, #12, #16, #18, and #21) to assess the response to long-term iloprost therapy at trough before iloprost inhalation at a mean follow-up period of 10 months (range 3 to 24 months) (Table 4). In comparison with baseline hemodynamic status, there were no differences in mean PAP, cardiac index, PVR index, or PVR/SVR ratio. In a subgroup of patients (n = 7) receiving inhaled NO during the initial and follow-up cardiac catheterizations, the acute response to inhaled NO did not change, despite chronic iloprost therapy.
Table 4. Chronic Hemodynamic Effects of Inhaled Iloprost.
| Baseline (n = 12) |
Median Follow-Up 10 Months (Range 3-24 Months) (n = 12) |
|
|---|---|---|
| PAPm (mm Hg) | 64 ± 18 | 64 ± 13 |
| RAPm (mm Hg) | 8 ± 3 | 6 ± 2 |
| SAPm (mm Hg) | 69 ± 9 | 68 ± 7 |
| PCWP (mm Hg) | 9 ± 3 | 7 ± 2 |
| CI (l/min/m2) | 3.4 ± 1.3 | 3.6 ± 1.4 |
| PVRI (U × m2) | 22 ± 15 | 19 ± 7 |
| PVR/SVR | 1.2 ± 0.7 | 1.1 ± 0.4 |
Values mean ± SD. Analysis with repeated measures analysis of variance with Tukey-Kramer multiple comparisons post hoc test. p < 0.05 vs. baseline.
PAPm = mean pulmonary artery pressure; other abbreviations as in Table 2.
The 6MW tests were obtained for 13 of 22 patients at baseline and after 6 months (Fig. 4). Overall, there was no change in 6MW distance from baseline (n = 13; 397 m) to 6 months (n = 13; 428 m); however, 6MW distance did increase by >10% in 5, was unchanged in 7, and decreased by >10% in 1 child. By comparison, the mean 6MW distance was 355 m with an average improvement of 30 m in 12 weeks in the adult trial of iloprost as an add on therapy to bosentan (25).
Figure 4. 6MW Tests Were Obtained for 13 of 22 Patients at Baseline and After 6 Months.
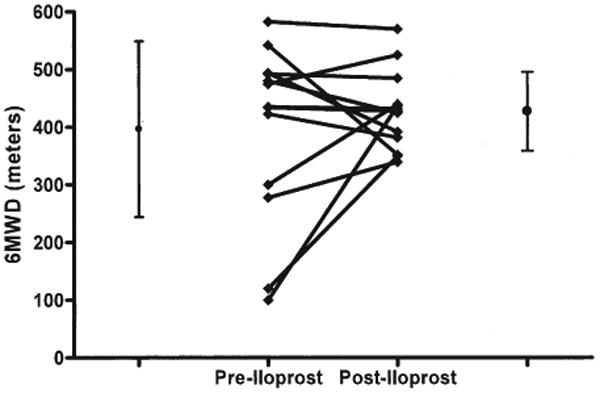
Overall, there was no change in 6-min walk distance (6MWD) from baseline (397 m) to 6 months (428 m); however, 6MWD did increase by >10% in 5, was unchanged in 7, and decreased by >10% in 1 child.
The median WHO functional class of the 22 patients at baseline was class III. Among the 20 patients that remained on therapy at 6 months, WHO class improved in 7 patients, remained unchanged in 10 patients, and worsened in 3 patients (Fig. 5). Of these 20 patients, 13 were receiving iloprost therapy for 12 months or longer. During the second 6-month period, the functional class improved in 2 patients, declined in 3 patients, and remained unchanged in 8 patients.
Figure 5. Of 22 Patients, 20 Remained on Therapy at 6 Months.
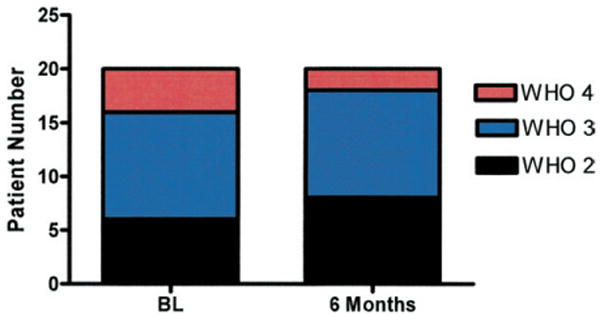
World Health Organization (WHO) class improved in 7 patients, remained unchanged in 10 patients, and worsened in 3 patients. BL = baseline.
Safety and tolerability
The most common side effects reported were headache (36%), cough (23%), and dizziness (14%), which generally improved within several days of initiation. Two patients experienced syncope during the study period, which could have been related to noncompli-ance with recommended frequency. Although 5 patients (23%) were initiated at 7 to 9 treatments daily, within 9 months, all patients remaining on iloprost reported 5 to 6 treatments daily, owing to the time required for treatments.
Of the 22 patients, 6 (27%) had marked deterioration during the study period (Fig. 6). Two deaths occurred, and 4 patients were transitioned to IV prostanoid therapy. Two patients (Patients #15 and #22) died 2 and 6 months, respectively, after initiation of inhaled therapy and refused IV prostanoid therapy. Four patients were transitioned to IV prostanoid therapy from inhaled iloprost for clinical deterioration (Patients #2, #3, #5, and #21).
Figure 6. Of the 22 Patients Treated With Iloprost, 9 Were Transitioned From IV Prostanoids With 8 Tolerating the Transition.
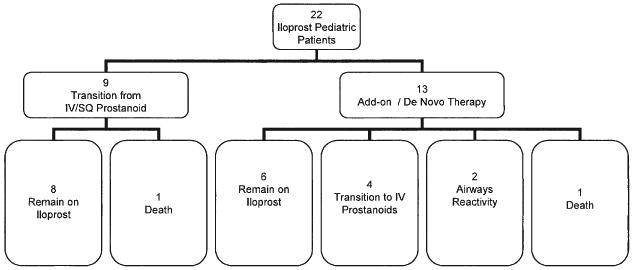
In 13 children, iloprost was used as add-on or de novo therapy. Of the 13 patients, 6 remain on therapy and 4 required transition to intravenous (IV) prostanoids. Two patients discontinued iloprost, owing to airways reactivity, and 1 patient died. SQ = subcutaneous.
In 9 patients on chronic IV prostanoids, 8 tolerated the transition from IV prostanoids to inhaled iloprost therapy (Fig. 6). One patient had a moderate fall in systemic arterial oxygen saturations through an unrepaired atrial septal defect but elected to remain on inhaled iloprost, owing to recurrent and severe central venous line infections. Among the transition patients, 1 death occurred 7 months after the transition (owing to worsening PAH).
Two patients without a prior history of lung disease discontinued initial therapy, owing to persistent cough and dyspnea (Fig. 6). In Patient #11, inhaled iloprost was discontinued after a single dose of 2.5 μg. In this patient, predicted FEV1 decreased by 18% and FEF25–75 decreased by 53%. A repeat trial of 2.5 μg during cardiac catheterization produced audible wheezing, respiratory distress, and a decrease in room air oxygen saturation from 95% to 88%. In Patient #19, iloprost was discontinued after 48 h (dose, 2.5 μg 6 times daily), owing to complaints of tingling in the chest and shortness of breath, accompanied by a decrease in room air oxygen saturation from 100% to 89% after iloprost inhalation. Pre-treatment spirometry showed a decrease in predicted FEV1 of 12% and a decrease in FEF25–75 of 20% after inhalation of iloprost.
Two patients became symptomatic with signs of lower airways obstruction several months after initiation of iloprost. One patient (Patient #20) had congenital scoliosis and a history of wheezing. Both patients were initiated on chronic inhaled corticosteroids and beta-agonist agents and subsequently tolerated chronic iloprost therapy.
Discussion
Although inhaled iloprost therapy is currently approved for the treatment of PAH in adults, little is known about the effects of inhaled iloprost in children. In this open-labeled observational study, we examined the acute and chronic effects of inhaled iloprost in 22 children with idiopathic or familial PAH or PAH associated with congenital heart disease. We report that: 1) the acute pulmonary vasodilator response to inhaled iloprost is equivalent to the effects of inhaled NO; 2) acute inhalation of iloprost induces acute intrathoracic airways obstruction in some children, as demonstrated by cough and reductions in FEV1.0 and FEF25–75 by pulmonary function tests; 3) the addition of inhaled iloprost therapy reduces the need for IV prostanoid therapy in some patients; and 4) some patients may clinically deteriorate during chronic inhaled iloprost therapy requiring rescue therapy with IV prostanoids.
These data must be interpreted with caution with regard to recommendations for treatment. Further studies are required before such recommendations might be made. However, the physicians treating these patients seemed to use iloprost in several settings. In general, iloprost was not used as de novo or monotherapy, unless other therapies were not available. Most children had a perceived “inadequate response” to other PAH agents and required additional therapy. Inhaled iloprost was chosen over IV therapy in those with a perceived inadequate response to oral therapy, mostly owing to patient preference. Because some children deteriorated while receiving inhaled iloprost therapy, prognostic predictors of the response to iloprost are needed. Children with severe disease warrant close monitoring; 5 of 6 children who died or required IV prostanoid rescue were WHO class III to IV with a 6MW distance ≤450 m.
It is interesting that most of the children who were transitioned from IV therapy to inhaled iloprost tolerated the transition without marked clinical worsening. It is likely that these children had been treated with IV prostanoids for sufficient time to obtain clinical benefit, as has been previously shown (33). Iloprost in addition to other background therapy, such as bosentan or sildenafil, was able to maintain this effect, but long-term follow-up is required. Owing to the presence of frequent central venous line infections in some patients, removal of the central venous line and apparent maintenance of efficacy on an inhaled therapy was beneficial. The transition from IV epoprostenol or treprostinil to inhaled iloprost in most patients was tolerated without clinical worsening. One patient had decreased systemic arterial oxygen saturations, and in another child, clinical deterioration and death occurred after transition to iloprost. Thus, close clinical monitoring is critical, as is patient education regarding the potential for deterioration and need for re-initiation of parental prostanoid therapy.
This study did not evaluate a dose-response effect of iloprost. In most of our patients, the final dose of iloprost was similar to that used in adult patients, and side effects were similar. However, some younger children only tolerated a dose of 2.5 μg/treatment. Furthermore, some children decreased the number of treatments per day on their own. This might have been tolerated, owing to the presence of background therapy.
In 2 children, iloprost was discontinued almost immediately, owing to apparent airway reactivity; neither patient had a history of lung disease. In these 2 patients, decreases in percent predicted FEV1 by >12% and in percent predicted FEF25–75 by >20% upon inhalation of iloprost were documented. Two additional patients also had late onset of airway reactivity with initiation of iloprost, which improved with albuterol. However, because delivery of inhaled medication is dependent on pulmonary function, iloprost-induced lower airway obstruction could potentially affect drug delivery. It is likely that iloprost intolerance due to airway reactivity could be predicted via spirometric measurements obtained before and after inhalation at the onset of therapy; this requires further study. It might also be possible to ameliorate this effect with daily fluticasone/salmeterol inhalation therapy, as suggested by the clinical course of Patients #17 and #20; this also requires further study. Pulmonary function testing before and after inhaled iloprost should be routinely considered in pediatric patients with PAH before initiation of chronic iloprost therapy and at an interval several months after initiation or with any airway symptoms.
The mechanism for airway reactivity after inhalation of iloprost is unclear from our study. Although some individuals were symptomatic after iloprost and experienced reduced lung function, others were not. Intrathoracic airways obstruction with airway reactivity has been reported recently in pediatric patients with PAH (34). The drop in airflow after inhalation of iloprost could be caused by constriction of bronchial smooth muscle due to the chemical impact of iloprost or its carrier. However, an increase in pulmonary arterial blood flow in the medium-to-small pulmonary arteries could have a compressive effect on the neighboring small bronchi and bronchioles. Furthermore, although airway obstruction with response to bronchodilator is a hallmark of asthma, there is reason to believe that these individuals are not typical asthma patients. It is uncommon for them to have histories of frank wheezing or episodes of significant dyspnea. Of the patients demonstrating airway reactivity, none showed evidence of anatomical airway obstruction on chest computed tomography. Our data suggest a careful, individually tailored approach with lung function testing for children with PAH receiving iloprost treatment.
Previous investigators have suggested that evaluation of acute pulmonary vasoreactivity with inhaled NO and with inhaled iloprost in children with PAH and congenital heart disease could reflect the degree of vasodilatory capacity of the pulmonary vessels and thus the likelihood of response to long-term therapy (17,35–38). Evaluation of vasoreactivity at the initiation of iloprost therapy might prove to be a useful indicator of the likelihood of clinical response; however, a large series of patients will be needed to evaluate this.
Conclusions
This series describes the safety and efficacy of inhaled iloprost in pediatric patients with PAH. Most children tolerated combination therapy with either an endothelin receptor antagonist and/or phosphodiesterase inhibitor. Importantly, inhaled iloprost appears to induce bronchoconstriction in some children, which could limit its use but would also be prevented in some cases with inhaled steroid pre-treatment. Transition from IV or subcutaneous prostanoid therapy to inhaled iloprost may be possible in some patients under close observation, but long-term follow-up is necessary. It can be hoped that the benefits of inhaled iloprost that have been documented in adults with PAH will be similarly present in children with PAH, but further studies are necessary.
Acknowledgments
Supported in part by grant M01-RR00069 from the General Clinical Research Center branch of the National Center for Research Resources, National Institutes of Health. Dr. Ivy is a consultant for Actelion, Gilead, and United Therapeutics. Dr. Abman serves as a scientific advisor for INO Therapeutics. Aimee Doran is a consultant for Actelion, Gilead, and United Therapeutics. Dr. Barst is a consultant for Actelion, Gilead, United Therapeutics, INO Therapeutics, Biogen Idec, Pfizer, and Lilly.
Abbreviations and Acronyms
- 6MW
6-min walk
- FEV1
forced expiratory volume in 1 s
- FEF25–75
mid-volume forced expiratory flow
- FVC
forced vital capacity
- IV
intravenous
- NO
nitric oxide
- PAH
pulmonary arterial hypertension
- PAP
pulmonary arterial pressure
- PVR
pulmonary vascular resistance
- TLC
total lung capacity
- WHO
World Health Organization
References
- 1.Galie N, Torbicki A, Barst R, et al. Guidelines on diagnosis and treatment of pulmonary arterial hypertension. The Task Force on Diagnosis and Treatment of Pulmonary Arterial Hypertension of the European Society of Cardiology. Eur Heart J. 2004;25:2243–78. doi: 10.1016/j.ehj.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Rashid A, Ivy D. Pulmonary hypertension in children. Curr Paediatr. 2006;16:237–47. [Google Scholar]
- 3.Berman Rosenzweig EB, Barst RJ. Pulmonary arterial hypertension: a comprehensive review of pharmacological treatment. Treat Respir Med. 2006;5:117–27. doi: 10.2165/00151829-200605020-00005. [DOI] [PubMed] [Google Scholar]
- 4.Beghetti M. Pulmonary hypertension associated with congenital heart disease. Rev Mal Respir. 2006;23 4:49–59. [PubMed] [Google Scholar]
- 5.Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:40S–7S. doi: 10.1016/j.jacc.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 6.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351:1425–36. doi: 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- 7.D'Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–9. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 8.Badesch DB, Abman SH, Simonneau G, et al. Medical therapy for pulmonary arterial hypertension: updated ACCP evidence-based clinical practice guidelines. Chest. 2007;131:1917–28. doi: 10.1378/chest.06-2674. [DOI] [PubMed] [Google Scholar]
- 9.Yung D, Widlitz AC, Rosenzweig EB, Kerstein D, Maislin G, Barst RJ. Outcomes in children with idiopathic pulmonary arterial hypertension. Circulation. 2004;110:660–5. doi: 10.1161/01.CIR.0000138104.83366.E9. [DOI] [PubMed] [Google Scholar]
- 10.Rosenzweig EB, Ivy DD, Widlitz A, et al. Effects of long-term bosentan in children with pulmonary arterial hypertension. J Am Coll Cardiol. 2005;46:697–704. doi: 10.1016/j.jacc.2005.01.066. [DOI] [PubMed] [Google Scholar]
- 11.Sitbon O, McLaughlin VV, Badesch DB, et al. Survival in patients with class III idiopathic pulmonary arterial hypertension treated with first line oral bosentan compared with an historical cohort of patients started on intravenous epoprostenol. Thorax. 2005;60:1025–30. doi: 10.1136/thx.2005.040618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLaughlin VV. Survival in patients with pulmonary arterial hypertension treated with first-line bosentan. Eur J Clin Invest. 2006;36 3:10–5. doi: 10.1111/j.1365-2362.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 13.Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40:780–8. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation. 2002;106:1477–82. doi: 10.1161/01.cir.0000029100.82385.58. [DOI] [PubMed] [Google Scholar]
- 15.Simonneau G, Barst RJ, Galie N, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165:800–4. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
- 16.Olschewski H, Simonneau G, Galie N, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347:322–9. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 17.Rimensberger PC, Spahr-Schopfer I, Berner M, et al. Inhaled nitric oxide versus aerosolized iloprost in secondary pulmonary hypertension in children with congenital heart disease: vasodilator capacity and cellular mechanisms. Circulation. 2001;103:544–8. doi: 10.1161/01.cir.103.4.544. [DOI] [PubMed] [Google Scholar]
- 18.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 19.Sitbon O, Beghetti M, Petit J, et al. Bosentan for the treatment of pulmonary arterial hypertension associated with congenital heart defects. Eur J Clin Invest. 2006;36 3:25–31. doi: 10.1111/j.1365-2362.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- 20.Simpson CM, Penny DJ, Cochrane AD, et al. Preliminary experience with bosentan as initial therapy in childhood idiopathic pulmonary arterial hypertension. J Heart Lung Transplant. 2006;25:469–73. doi: 10.1016/j.healun.2005.11.438. [DOI] [PubMed] [Google Scholar]
- 21.Maiya S, Hislop AA, Flynn Y, Haworth SG. Response to bosentan in children with pulmonary hypertension. Heart. 2006;92:664–70. doi: 10.1136/hrt.2005.072314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galie N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–57. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 23.Humpl T, Reyes JT, Holtby H, Stephens D, Adatia I. Beneficial effect of oral sildenafil therapy on childhood pulmonary arterial hypertension: twelve-month clinical trial of a single-drug, open-label, pilot study. Circulation. 2005;111:3274–80. doi: 10.1161/CIRCULATIONAHA.104.473371. [DOI] [PubMed] [Google Scholar]
- 24.Mukhopadhyay S, Sharma M, Ramakrishnan S, et al. Phosphodiesterase-5 inhibitor in Eisenmenger syndrome: a preliminary observational study. Circulation. 2006;114:1807–10. doi: 10.1161/CIRCULATIONAHA.105.603001. [DOI] [PubMed] [Google Scholar]
- 25.McLaughlin VV, Oudiz RJ, Frost A, et al. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;174:1257–63. doi: 10.1164/rccm.200603-358OC. [DOI] [PubMed] [Google Scholar]
- 26.Hoeper MM, Leuchte H, Halank M, et al. Combining inhaled iloprost with bosentan in patients with idiopathic pulmonary arterial hypertension. Eur Respir J. 2006;28:691–4. doi: 10.1183/09031936.06.00057906. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann R, Kreuder J, Michel-Behnke I, Voelkel NF, Schranz D. Pulmonary flow reseve in children with idiopathic pulmonary arterial hypertension: implications for diagnosis and therapy. Eur J Med Res. 2006;11:208–13. [PubMed] [Google Scholar]
- 28.Ghofrani HA, Rose F, Schermuly RT, et al. Oral sildenafil as long-term adjunct therapy to inhaled iloprost in severe pulmonary arterial hypertension. J Am Coll Cardiol. 2003;42:158–64. doi: 10.1016/s0735-1097(03)00555-2. [DOI] [PubMed] [Google Scholar]
- 29.Theodoraki K, Rellia P, Thanopoulos A, et al. Inhaled iloprost controls pulmonary hypertension after cardiopulmonary bypass. Can J Anaesth. 2002;49:963–7. doi: 10.1007/BF03016884. [DOI] [PubMed] [Google Scholar]
- 30.Beghetti M, Berner M, Rimensberger PC. Long term inhalation of iloprost in a child with primary pulmonary hypertension: an alternative to continuous infusion. Heart. 2001;86:E10. doi: 10.1136/heart.86.3.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaudry G, MacDonald C, Adatia I, et al. CT of the chest in the evaluation of idiopathic pulmonary arterial hypertension in children. Pediatr Radiol. 2007;37:345–50. doi: 10.1007/s00247-007-0410-8. [DOI] [PubMed] [Google Scholar]
- 32.Barst RJ, Ivy D, Dingemanse J, et al. Pharmacokinetics, safety, and efficacy of bosentan in pediatric patients with pulmonary arterial hypertension. Clin Pharmacol Ther. 2003;73:372–82. doi: 10.1016/s0009-9236(03)00005-5. [DOI] [PubMed] [Google Scholar]
- 33.Ivy DD, Doran A, Claussen L, Bingaman D, Yetman A. Weaning and discontinuation of epoprostenol in children with idiopathic pulmonary arterial hypertension receiving concomitant bosentan. Am J Cardiol. 2004;93:943–6. doi: 10.1016/j.amjcard.2003.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rastogi D, Ngai P, Barst RJ, et al. Lower airway obstruction, bronchial hyperresponsiveness, and primary pulmonary hypertension in children. Pediatr Pulmonol. 2004;37:50–5. doi: 10.1002/ppul.10363. [DOI] [PubMed] [Google Scholar]
- 35.Roberts JD, Jr, Lang P, Bigatello LM, Vlahakes GJ, Zapol WM. Inhaled nitric oxide in congenital heart disease. Circulation. 1993;87:447–53. doi: 10.1161/01.cir.87.2.447. [DOI] [PubMed] [Google Scholar]
- 36.Ivy DD, Griebel JL, Kinsella JP, Abman SH. Acute hemodynamic effects of pulsed delivery of low flow nasal nitric oxide in children with pulmonary hypertension. J Pediatr. 1998;133:453–6. doi: 10.1016/s0022-3476(98)70287-2. [DOI] [PubMed] [Google Scholar]
- 37.Atz AM, Adatia I, Lock JE, Wessel DL. Combined effects of nitric oxide and oxygen during acute pulmonary vasodilator testing. J Am Coll Cardiol. 1999;33:813–9. doi: 10.1016/s0735-1097(98)00668-8. [DOI] [PubMed] [Google Scholar]
- 38.Hoeper MM, Olschewski H, Ghofrani HA, et al. A comparison of the acute hemodynamic effects of inhaled nitric oxide and aerosolized iloprost in primary pulmonary hypertension. German PPH study group. J Am Coll Cardiol. 2000;35:176–82. doi: 10.1016/s0735-1097(99)00494-5. [DOI] [PubMed] [Google Scholar]


