SUMMARY
Secondary bacterial infection is a common sequela to viral infection and is associated with increased lethality and morbidity. However, the underlying mechanisms remain poorly understood. We show that the TLR3/MDA5 agonist poly I:C or viral infection dramatically augments signaling via the NLRs Nod1 and Nod2 and enhances the production of pro-inflammatory cytokines. Enhanced Nod1 and Nod2 signaling by poly I:C required the TLR3/MDA5 adaptors TRIF and IPS-1, and was mediated by type I IFNs. Mechanistically, poly I:C or IFN-β induced the expression of Nod1, Nod2, and the Nod-signaling adaptor RIP2. Systemic administration of poly I:C or IFN-β, or infection with murine norovirus-1, promoted inflammation and lethality in mice superinfected with E. coli, which was independent of bacterial burden, but attenuated in the absence of Nod1/Nod2 or RIP2. Thus, crosstalk between type I IFNs and Nod1/Nod2 signaling promotes bacterial recognition, but induces harmful effects in the virally infected host.
INTRODUCTION
Bacterial superinfection in the context of ongoing viral infection is a relatively common event that can be associated with increased morbidity and mortality. For instance, influenza viral infection increases the susceptibility to several respiratory bacterial pathogens (Beadling and Slifka, 2004; Brundage, 2006). Similarly, varicella has been reported to predispose human to severe streptococcal and staphylococcal infection (Barnes et al., 1996; Zerr et al., 1999). Consistent with the human studies, infection of mice with multiple viruses increases the sensitivity and lethality to bacterial products including lipopolysaccharide (LPS) (Doughty et al., 2001; Fejer et al., 2005; Nansen and Randrup Thomsen, 2001). The mechanism whereby viral infections enhance and aggravate bacterial superinfection is poorly understood, but it is likely to involve multiple factors including local destruction of antibacterial barriers at epithelial surfaces, suppression of anti-bacterial immunity, induction of apoptosis in immune cells and sensitization to LPS (Doughty et al., 2001; Fejer et al., 2005; Herold et al., 2008; Jamieson et al., 2010; Nansen and Randrup Thomsen, 2001; Navarini et al., 2006).
Detection of viruses and bacteria by host cells is mediated by the recognition of conserved and unique microbial structures by pattern-recognition molecules, such as the Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), and RIG-like helicases (Akira et al., 2006; Kanneganti et al., 2007). TLRs mediate bacterial recognition of several molecules including LPS and microbial nucleic acids at the cell surface or by endosomes (Akira et al., 2006). In contrast, NLRs and RIG-like helicases induce innate immune responses through cytosolic sensing of bacterial and viral components (Kanneganti et al., 2007; Ting and Davis, 2005). Two NLR family members, Nod1 and Nod2, are activated by molecules produced during the synthesis and/or degradation of bacterial peptidoglycan (PGN) (Chamaillard et al., 2003; Girardin et al., 2003a; Girardin et al., 2003b; Inohara et al., 2003). Nod1 activation is triggered by γ-D-glutamyl-meso-diaminopimelic acid (meso-DAP), which is unique to PGN structures from all Gram-negative bacteria and certain Gram-positive bacteria, whereas Nod2 is activated by muramyl dipeptide (MDP), a PGN motif present in all Gram-negative and -positive bacteria (Chamaillard et al., 2003; Girardin et al., 2003a; Girardin et al., 2003b; Inohara et al., 2003). Once activated, Nod1 and Nod2 induce transcription of immune response genes through the NF-κB transcription factor and the mitogen-activated protein kinase (MAPK) signaling pathways (Chamaillard et al., 2003; Girardin et al., 2003a; Girardin et al., 2003b; Inohara et al., 2003; Kobayashi et al., 2005). In contrast, the RIG-like helicases RIG-I and melanoma differentiation-associated gene 5 (MDA5) recognize short double-stranded (ds) RNAs with a 5′ triphosphate end, and long dsRNAs such as polyinosinic polycytidylic acid (poly I:C), respectively (Kato et al., 2006; Yoneyama et al., 2004). Upon activation, RIG-I and MDA-5 induce type I IFNs via IFN-β promoter stimulator-1 (IPS-1), also known as MAVS/Cardif/VISA (Kawai et al., 2005). IPS-1 subsequently activates two IκB kinase (IKK) related kinases, IKK-i, and TANK-binding kinase 1 (TBK1). These kinases phosphorylate IFN-regulatory factor (IRF) 3 and IRF7, which initiate the transcription of type I IFNs (Kawai et al., 2005).
The interaction between microorganisms and host cells during microbial infection triggers immune responses that include the production of pro-inflammatory molecules. Although pro-inflammatory cytokines are critical for bacterial killing and activation of adaptive immunity, excessive amounts of certain cytokines such as TNF-α produced in response to infection are harmful to the host and can lead to septic shock and multi-organ failure (Cook et al., 2004; Danner et al., 1991). During infection with viruses, TLR and RIG-I-like receptor activation induce production of type I IFNs which can potentiate the inflammatory response to TLR ligands including LPS (Doughty et al., 2001; Nansen and Randrup Thomsen, 2001). In addition, viral infection can augment or repress the immune response to bacteria (Navarini et al., 2006). However, the innate signaling pathways activated in response to secondary infection with bacteria that promote lethality in virally infected hosts remain poorly understood. We provide evidence that viral infection enhances the response to bacteria through Nod1 and Nod2 signaling pathways. The enhanced activation of Nod1 and Nod2 induced by secondary bacterial infection was mediated by type I IFNs and contributed to TNF-α production and lethality in virally infected mice.
RESULTS
Poly I:C augments Nod2 activation via TRIF- and IPS-1-dependent signaling pathways in macrophages
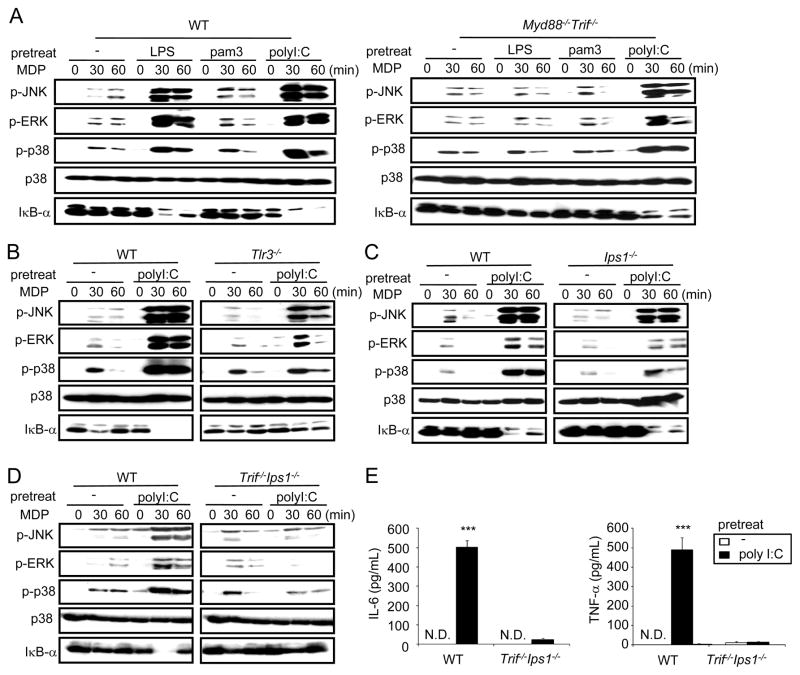
To examine a possible link between viral infection and Nod2 signaling, mouse bone marrow-derived macrophages were pre-stimulated with poly I:C, a synthetic dsRNA analog that mimics viral infection, and 24 h later with MDP, a Nod2 microbial activator. As a control, macrophages were stimulated with lipopolysaccharide (TLR4 agonist) or N-palmitoyl-S-[2,3-bis(palmitoyloxy)-propyl]-(R)-cysteinyl-(lysyl)3-lysine (pam3CSK4) (TLR2 agonist) followed by MDP. Stimulation with MDP induced JNK, ERK, and p38 phosphorylation as well as IκB-α degradation which was greatly enhanced in cells pre-treated with LPS and poly I:C, but little or not at all with the TLR2 agonist (Figure 1A). As expected, the enhancement of Nod2-dependent signaling induced by LPS was abrogated in Myd88−/−Trif−/− macrophages, the two adaptors that mediate all TLR signaling (Figure 1A). In contrast, MAPK and NF-κB activation induced by MDP in macrophages pre-stimulated with poly I:C was partially reduced in Myd88−/−Trif−/− macrophages (Figure 1A). Poly I:C is known to induce signaling through TLR3-TRIF and MDA5-IPS-1 signaling pathways (Kumar et al., 2008). In line with this, augmentation of MDP-induced signaling was reduced in Tlr3−/− and Ips1−/− macrophages and abrogated in Trif−/−Ips1−/− macrophages (Figure 1, B–D). Consistently, TNF-α and IL-6 secretion was induced by MDP in macrophages pre-stimulated with poly I:C, but not in untreated macrophages, and the secretion of these cytokines was abolished in Trif−/−Ips1−/− cells (Figure 1E). Unlike the response to MDP, production of TNF-α induced by pam3CSK4 stimulation was unimpaired in Nod2−/− or Trif−/−Ips1−/− macrophages (Figure S1). These results indicate that poly I:C enhances Nod2 signaling via TRIF- and IPS-1, enabling the macrophages to secrete cytokines in response to MDP.
Figure 1. Poly I:C augments Nod2 activation via TRIF- and IPS-1-dependent signaling pathways in macrophages.
(A–D) BMDMs from WT and indicated mutant mice were left untreated (−) or pretreated with LPS (A), pam3CSK4 (A), or poly I:C (A–D) for 24 h and then restimulated with MDP. Cell extracts were collected at the indicated times and assessed for MAPK and NF-κB activation using phosphospecific antibodies. (E) BMDMs from WT and Trif−/−Ips1−/− mice were treated with poly I:C or left untreated for 24 h. The macrophages were then re-stimulated with MDP. Cell-free supernatants were analyzed by ELISA for production of IL-6 and TNF-α. (*** p < 0.001, compared with untreated and poly I:C-treated or WT and mutant macrophage cultures). N.D. denotes not detected. Results are representative of 2 or 3 independent experiments. Data are expressed as mean ± S.D.
Enhancement of Nod2 signaling by poly I:C and the chemotherapeutic molecule DMXAA is mediated by type I IFNs
Type I IFNs are induced by poly I:C via TRIF- and IPS-1-dependent signaling (Kumar et al., 2008). To determine whether type-I IFN production is important in augmenting Nod2 activation, WT and mutant macrophages deficient in the type I IFN receptor (IFNAR1) were pre-stimulated with poly I:C and then MDP. We found that poly I:C-mediated enhancement of JNK, ERK, and p38 phosphorylation as well as IκB-α degradation in response to MDP was greatly inhibited in the absence of IFNAR1 (Figure 2A). Consistently, MDP stimulation induced IL-6 and TNF-α in macrophages pre-stimulated with poly I:C or DMXAA, a chemotherapeutic drug in clinical trials that activates the IRF-3 and type-I IFNs (Roberts et al., 2007), but not in the absence of pre-stimulation (Figure 2B and 2D). Notably, the production of TNF-α and IL-6 induced by MDP was abrogated in Ifnar1−/− or Nod2−/− macrophages (Figure 2B and 2D). In addition, pre-treatment with DMXAA or IFN-β augmented MAPK and NF-κB activation in response to MDP which was abolished in macrophages lacking IFNAR1 (Figure 2C). In contrast, the production of TNF-α induced by DMXAA and IFN-β required IFNAR1, but not TRIF/IPS-1 (Figure S2), which is consistent with the fact that these two molecules act downstream of the TRIF/IPS-1 adaptors to induce type I IFNs. These results indicate that Nod2 signaling is enhanced by poly I:C and the chemotherapeutic molecule DMXAA through the production of type I IFNs.
Figure 2. Enhancement of Nod2 signaling by poly I:C and the chemotherapeutic molecule DMXAA is mediated via type-I IFNs.
(A and C) BMDMs from WT and Ifnar1−/− mice were left untreated (control) or pretreated with poly I:C (A), DMXAA, or IFN-β (C) for 24 h and then restimulated with MDP. Cell extracts were collected at the indicated times and assessed for MAPK and NF-κB activation using phosphospecific antibodies. (B and D) BMDMs from WT, Ifnar1−/− (B), or Nod2−/− mice (D) were treated with poly I:C, DMXAA, or left untreated for 24 h and then re-stimulated with MDP. Cell-free supernatants were analyzed for production of IL-6 and TNF-α (*** p < 0.001, compared with WT and mutant macrophage cultures). N.D. denotes not detected. Results are representative of 3 independent experiments. Data are expressed as mean ± S.D.
Type I IFN signaling induces expression of Nod1, Nod2, and RIP2
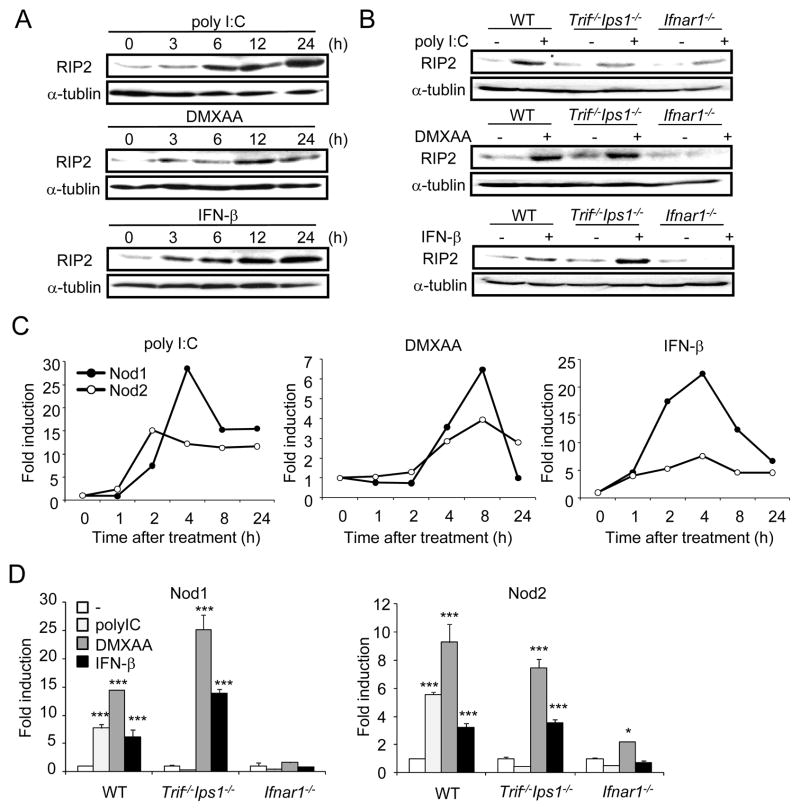
RIP2 is the essential adaptor that mediates gene induction in response to Nod1 and Nod2 agonists (Park et al., 2007). To assess whether the expression of RIP2 is regulated by type I IFN production, macrophages were stimulated with poly I:C, DMXAA, or IFN-β and the amount of RIP2 in cell extracts was determined by immunoblotting. All three stimuli enhanced RIP2 production which was first detected by 3 hrs and further increased by 12–24 h of stimulation (Figure 3A). Furthermore, induction of RIP2 in response to poly I:C was abrogated in Trif−/−Ips1−/− or Ifnar1−/− macrophages (Figure 3B). In contrast, enhanced RIP2 production in response to DMXAA and IFN-β required IFNAR1, but not TRIF/IPS-1 (Figure 3B), which is consistent the results shown in Figure S2. In addition, stimulation of macrophages with poly I:C, DMXAA, and IFN-β induced the expression of Nod1 and Nod2 mRNA (Figure 3C). The induction of Nod1 and Nod2 by poly I:C, DMXAA, and IFN-β was impaired in Ifnar1−/−macrophages (Figure 3D). In contrast, enhanced Nod1 and Nod2 expression by DMXAA and IFN-β was unimpaired in Trif−/−Ips1−/− macrophages (Figure 3D), in line with the Rip2 results shown in Figure 3B. These results indicate that Type I IFNs induce expression of Nod1 and Nod2 as well as RIP2, the mediator of Nod1 and Nod2 signaling.
Figure 3. Type-I IFN signaling induces expression of RIP2.
(A) BMDMs from WT mice were treated with poly I:C, DMXAA, or IFN-β for the indicated times, and cell extracts were assessed for RIP2 and α-tubulin levels by immunoblotting. (B) BMDMs from WT, Trif−/−Ips1−/−, and Ifnar1−/− mice were treated with poly I:C, DMXAA, or IFN-β for 24 h, and cell extracts were assessed for RIP2 and α-tubulin levels by immunoblotting. (C) BMDMs from WT mice were treated with poly I:C, DMXAA, or IFN-β for the indicated times, and total RNA was isolated to analyze for Nod1 and Nod2 expression. Gene expression was normalized to that of β-actin. (D) BMDMs from WT, Trif−/−Ips1−/−, and Ifnar1−/− mice were treated with poly I:C, DMXAA, or IFN-β for 8 h, and total RNA was isolated to analyze expression of Nod1 and Nod2. * p < 0.05, *** p < 0.001, comparison between untreated and treated macrophage cultures. Results are representative of 2 or 3 independent experiments.
Viral infection augments Nod1 and Nod2 signaling
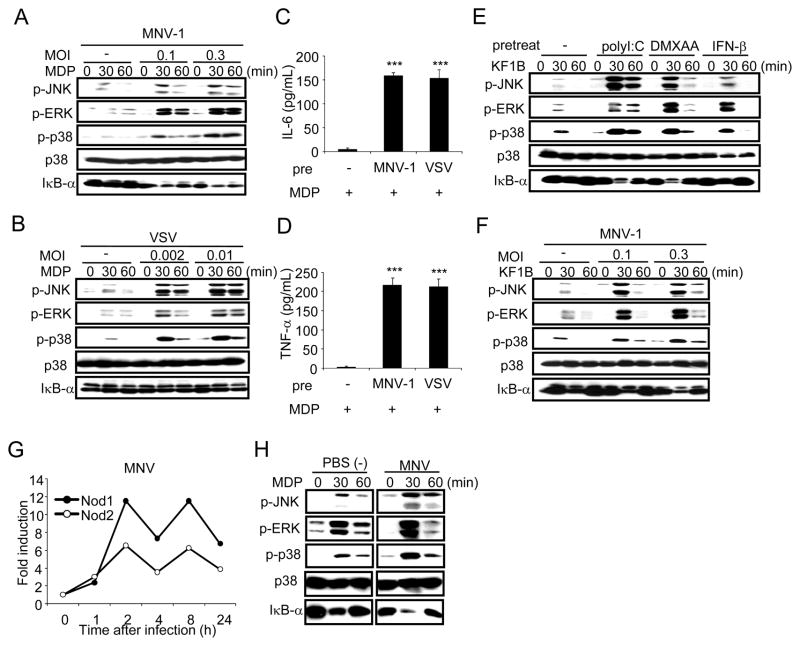
We next determined whether infection of macrophages with viruses augments Nod2 signaling. Macrophages were infected with murine norovirus-1 (MNV-1) or vesicular stomatitis virus (VSV) for 24 h and then stimulated with MDP. Viral infection enhanced JNK, ERK, and p38 phosphorylation in response to MDP when compared to that observed in uninfected cells (Figure 4A and 4B). Consistently, MDP induced IL-6 and TNF-α production in macrophages previously infected with MNV-1 or VSV, but not in uninfected cells (Figure 4C and 4D). In addition, infection with MNV-1 or stimulation with poly I:C, DMXAA, or IFN-β augmented MAPK and NF-κB activation induced by KF1B, a synthetic molecule containing the iE-DAP dipeptide that activates Nod1 (Figure 4E and 4F). Consistent with Figure 3C, infection of macrophages with MNV-1 induced the expression of Nod1 and Nod2 (Figure 4G). To determine whether viral infection affects Nod2 signaling in macrophages in vivo, mice were injected i. p. with thioglycollate and 3 days later, the mice were infected i. p. with MNV-1 or PBS as a control, and peritoneal macrophages were purified for analysis ex-vivo 24 h later. Exposure of macrophages to MNV-1 in vivo enhanced the activation of JNK, ERK, p38, and IκB-α-degradation in response to MDP when compared to macrophages derived from uninfected mice (Figure 4H). Collectively, these results indicate that viral infection enhances Nod1 and Nod2 signaling in macrophages.
Figure 4. Viral infection augments Nod1 and Nod2 signaling.
(A and B) BMDMs from WT mice were mock-infected (−) or infected with MNV-1 at MOIs of 0.1 and 0.3 (A) or VSV at MOIs of 0.002 and 0.01 (B) for 24 h and then restimulated with MDP. Cell extracts were collected at the indicated times and assessed for MAPK and NF-κB activation using phosphospecific antibodies. (C and D) BMDMs from WT mice were mock-infected (−) or infected with MNV-1 at MOI of 0.3 or VSV at MOI of 0.01 for 24 h. The macrophages were then restimulated with MDP. Cell-free supernatants were analyzed for IL-6 (C) and TNF-α (D) production. (*** p < 0.001, compared with mock-infected and infected cultures). (E and F) BMDMs from WT mice were left untreated (−) or treated with poly I:C, DMXAA, or IFN-β (E) or infected with MNV-1 (F) for 24 hr and then restimulated with the Nod1 ligand KF1B. Cell extracts were collected at the indicated times and assessed for MAPK and NF-κB activation using phosphospecific antibodies. (G) BMDMs from WT mice were infected with MNV-1 for the indicated times, and total RNA was isolated to analyze for the expression of Nod1 and Nod2. (H) WT mice were injected i.p. with thioglycollate for 3 days and then treated with PBS(−) or infected with MNV-1 (1 × 106 PFU/mouse) i. p. Elicited peritoneal macrophages were isolated 24 h after MNV-1 infection and stimulated with MDP ex-vivo. Cell extracts were collected at the indicated time and assessed for MAPK and NF-κB activation using phosphospecific antibodies. Data are expressed as mean ± S.D. Results are representative of 3 independent experiments.
Nod1 and Nod2 contribute to bacteria-induced TNF-α production and NF-κB activation in macrophages infected with MNV-1
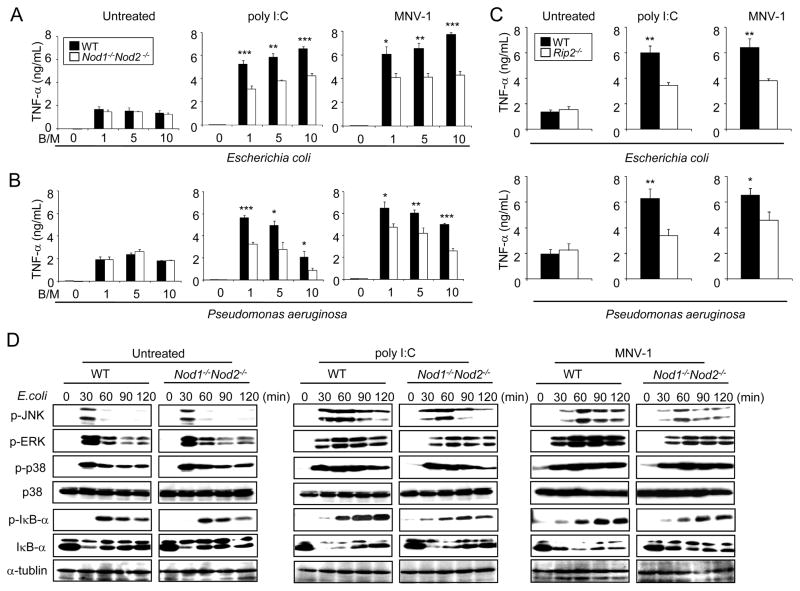
We next determined the role of Nod1 and Nod2 in mediating the inflammatory response induced by Gram-negative bacteria in macrophages treated with poly I:C or infected with MNV-1. Production of TNF-α and IL-6 in response to E. coli or P. aeruginosa was independent of Nod1 and Nod2 in unstimulated macrophages (Figure 5A and 5B and Figure S3). Notably, pre-treatment with poly I:C or infection with MNV-1 augmented the production of TNF-α and IL-6 induced by infection with E. coli or P. aeruginosa (Figure 5A and 5B and Figure S3). Importantly, the enhancement of TNF-α and IL-6 was significantly attenuated in Nod1−/−Nod2−/− macrophages (Figure 5A and 5B and Figure S3) and Rip2−/− macrophages (Figure 5C). In agreement with these results, Iκ-Bα phosphorylation and degradation induced by infection with E. coli was comparable in untreated macrophages from WT and Nod1−/−Nod2−/− mice (Figure 5D), but reduced in Nod1−/−Nod2−/− macrophages when the cells were previously treated with poly I:C or infected with MNV-1 (Figure 5D). In addition, JNK and ERK phosphorylation was diminished in Nod1−/−Nod2−/− macrophages treated with poly I:C or infected with MNV-1 when compared to WT cells (Figure 5D). These results indicate that Nod1 and Nod2 contributes to bacteria-induced TNF-α production and NF-κB activation in macrophages infected with MNV-1 followed by Gram-negative bacteria infection.
Figure 5. Nod1 and Nod2 contribute to bacteria-induced TNF-α production and NF-κB activation in macrophages infected with MNV-1.
(A and B) BMDMs from WT and Nod1−/−Nod2−/− mice were left untreated, treated with poly I:C, or infected with MNV-1 for 24 h and then infected with live E. coli (A) or P. aeruginosa (B) at a bacteria/macrophage ratio (B/M) of 1, 5, and 10. Cell-free supernatants were analyzed for production of TNF-α (* p < 0.05, ** p < 0.01, *** p < 0.001, compared with WT and mutant macrophages cultures). (C) BMDMs from WT and Rip2−/− mice were left untreated, treated with poly I:C, or infected with MNV-1 for 24 h and then infected with live E. coli or P. aeruginosa at a bacterial/macrophage ratio (B/M) of 1. Cell free supernatants were analyzed for production of TNF-α (* p < 0.05, ** p < 0.01, compared with WT and mutant macrophages cultures). (D) BMDMs from WT and Nod1/Nod2−/− mice were left untreated or treated with poly I:C or infected with MNV-1 for 24 h and then infected with E. coli at bacteria/macrophage ratio of 10 for the indicated times. Cell extracts were assessed for MAPK, NF-κB activation and α-tubulin levels by immunoblotting. Data are expressed as mean ± S.D. Results are representative of 3 independent experiments
Nod1 and Nod2 signaling potentiate TNF-α production and lethality induced by secondary bacterial infection in mice treated with poly I:C or infected with MNV-1
We next examined the role of Nod1 and Nod2 in regulating the susceptibility of virus-infected mice to bacteria. To test this, we first treated groups of WT and Nod1−/−Nod2−/− mice with PBS or poly I:C i. p. for 24 h, then infected the mice with E. coli and monitored mouse survival. We found that a dose of 3 × 108 CFU of E. coli induced lethality in 100% of the mice previously injected i. p. with poly I: C (Figure S4A). Using this dose of E. coli, all WT and mutant mice that were treated with PBS survived the infection (Figure 6A). In contrast, 100% of the WT mice treated with poly I:C succumbed to E. coli infection whereas ~ 30% of the Nod1−/−Nod2−/− mice survived (Figure 6A). The increased mortality induced by poly I:C administration was associated with increased amounts of TNF-α in the serum of WT and Nod1−/−Nod2−/− mice (Figure 6B). Importantly, there was reduced production of TNF-α in Nod1−/−Nod2−/− mice compared to WT mice in animals treated with poly I:C and infected with E. coli (Figure 6B). We also found that intraperitoneal administration of poly I: C promoted lethality in mice infected i. n. with P. aeruginosa (Figure 6C and Figure S4B). As it was found in the E. coli model, lethality was significantly attenuated in Nod1−/−Nod2−/− mice which correlated with reduced TNF-α production in the lung (Figure 6D).
Figure 6. Murine norovirus infection promotes bacteria-induced TNF-α production and lethality in mice via Nod1 and Nod2.
(A and B) WT, and Nod1−/−Nod2−/− mice were injected i.p. with PBS (n=5) or poly I:C (200 μg/mouse, n=10) and then infected with E. coli 3 × 108 CFU/mouse i. p. 24 h after poly I:C administration. Lethality was monitored for 5 days after infection (A). At 3 h post-infection, the levels of TNF-α in serum were assessed by ELISA (n=5 or 10) (B). (C and D) WT, and Nod1−/−Nod2−/− mice were injected with PBS or poly I:C (200 μg/mouse) i.p. and then infected with P. aeruginosa 2.5 × 107 CFU/mouse i. n. 24 h after poly I:C administration. Lethality was monitored for 5 days after infection (n=10–11) (C). At 3 hrpost-infection, the level of TNF-α in lung homogenates was assessed by ELISA (n=6) (D) (E–I) WT, Nod1−/−Nod2−/−, and Rip2−/− mice were mock-infected or infected orally with MNV-1 (1 × 107 PFU/mouse) and then infected with E. coli 3 × 108 CFU/mouse i. p. 24 h after the MNV-1 infection. Lethality was monitored for 5 days after E. coli infection (n=9–10) (E). Bacterial loads in lung (F) and blood (G) samples were determined at 8 h post-infection by plating (n=7–8). The short black horizontal lines show the mean values for the groups. Each symbol represents one animal. N.S. denotes not significant. At 3 h post-infection, serum TNF-α levels were assessed by ELISA (n=9–10). N.S. denotes not significant (H). 1 h before E. coli infection, WT mice were injected i.p. with control rat IgG or anti-TNF-α rat IgG and lethality was monitored for 5 days after infection (n=10) (I). Data are expressed as mean ± S.D. Results are representative of 2 or 3 independent experiments.
To test more directly the role of Nod1 and Nod2 signaling in the context of viral infection, WT, Nod1−/−Nod2−/−, or Rip2−/− mice were infected with MNV-1 orally, the normal route of infection, and then challenged with E. coli. All mock-infected mice, regardless of genotype, survived the infection with E. coli (Figure 6E). Consistent with the results observed with poly I:C, ~ 30% of the Nod1−/−Nod2−/− or Rip2−/− mice infected with MNV-1 survived, whereas 100% of the WT mice succumbed after infection with E. coli (Figure 6E). Similar results were obtained when the mice were infected with MNV-1 via the i. p. route (Figure S4C and S4D). The increased survival of Nod1−/−Nod2−/− was not explained by altered IFN-β production because infection of dendritic cells derived from the bone marrow of WT, Nod1−/−, Nod2−/−, or Nod1−/−Nod2−/− mice with MNV-1 induced comparable amounts of IFN-β (Figure S4E). Furthermore, oral infection of WT, Nod1−/−, Nod2−/−, or Nod1−/−Nod2−/− mice with MNV-1 induced comparable levels of IFN-β in the serum (Figure S4F). Thus, Nod1 and Nod2 did not regulate the production of IFN-β in response to MNV-1 infection. The effect on lethality induced by secondary infection with E. coli in WT and Nod1−/−Nod2−/− mice was not correlated with different bacterial loads in lung, blood (Figure 6F and 6G), or spleen (Figure S4G). As it was observed after poly I:C administration, infection with MNV-1 augmented the production of TNF-α in the serum which was associated with increased lethality in E. coli-infected mice (Figure 6H). Furthermore, increased survival of Nod1−/−Nod2−/− or Rip2−/− mice was correlated with reduced amounts of TNF-α in the serum of mutant animals when compared to WT mice (Figure 6H), but not with IL-6 production (Figure S4H). To determine whether TNF-α plays a role in the increased lethality of WT mice, we injected the mice with a blocking anti-TNF-α or control antibody. Administration of the anti-TNF-α antibody significantly delayed the death of the mice induced by infection with MNV-1 and E. coli when compared to mice treated with control antibody (Figure 6I). Thus, infection with MNV-1 increases lethality induced by secondary infection with E. coli and this is mediated, in part, by TNF-α production.
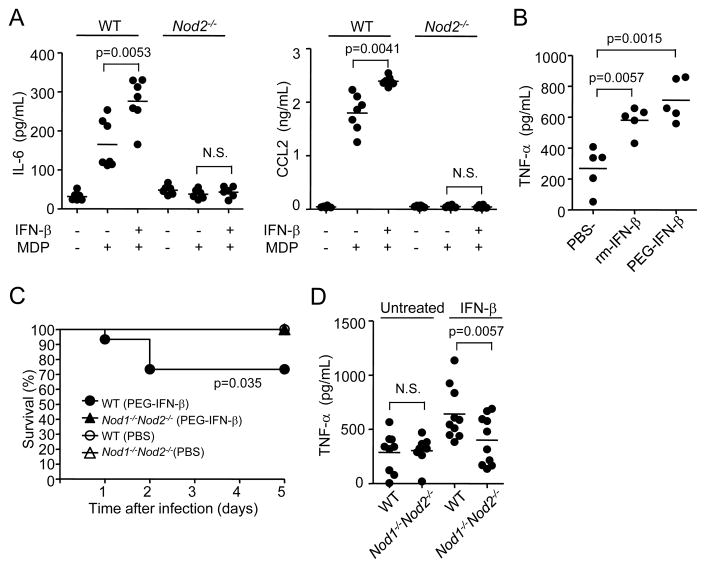
IFN-β enhances MDP-induced cytokine production in vivo and promotes bacteria-induced lethality via Nod1 and Nod2
We next assessed whether administration of IFN-β was sufficient to enhance Nod2 signaling in vivo. To determine this, WT mice were given murine PEGylated IFN-β or PBS i. p. and 18 h later the mice were stimulated with MDP, the Nod2 agonist. Administration of IFN-β enhanced the serum levels of IL-6 and CCL2, two molecules that are induced by MDP stimulation in vivo (Kim et al., 2008; Magalhaes et al., 2008), in WT but not in Nod2−/− mice (Figure 7A). Furthermore, serum TNF-α production in response to E. coli infection was enhanced by pre-treatment with unmodified or PEGylated murine IFN-β (Figure 7B). To determine whether administration of IFN-β promotes mouse lethality, WT and Nod1−/−Nod2−/− mice were given PEGylated IFN-β or PBS i. p. and 18 h later the mice were infected with E. coli and monitored for survival. We found that 30% of the WT mice succumbed to bacterial infection when the mice were given IFN-β whereas no lethality was observed in PBS treated mice or IFN-β-treated Nod1−/−Nod2−/− animals (Figure 7C). The increased mortality induced by IFN-β administration was associated with increased amounts of TNF-α in the serum of WT mice (Figure 7D). These results indicate that IFN-β is sufficient to enhance Nod2 signaling and to promote bacteria-induced lethality in vivo. Furthermore, IFN-β induced lethality in mice infected with E. coli required Nod1 and Nod2.
Figure 7. IFN-β enhances MDP-induced cytokine production in vivo and promotes bacteria-induced lethality via Nod1 and Nod2.
(A) WT (n=7), and Nod2−/− (n=6) mice were injected with PBS or PEGylated murine IFN-β (5 × 103 U/mouse) i.p. and 18 h later injected with MDP (300 μg/mouse). 3 h later, the levels of IL-6 and CCL2 in serum were assessed by ELISA. (B) WT mice (n=5/group) were injected with PBS or PEGylated murine IFN-β (5 × 103 U/mouse) i. p. or with unmodified murine IFN-β (2.5 × 104 U/mouse, twice separated by 3 h) s.c. and 18 h later infected with 3 × 108 CFU of E. coli/mouse. 3 h post-injection with E. coli, the level of TNF-α in serum was assessed by ELISA. (C) WT, and Nod1−/−Nod2−/− mice were injected with PBS (n=15) or PEGylated murine IFN-β (5 × 103 U/mouse, n=15) i.p. and 18 h later infected with 3 × 108 CFU of E. coli/mouse i. p. Mouse survival was monitored for 5 days after infection. (D) 3 h post-infection, the level of TNF-α in serum was assessed by ELISA (n=9 or 10).
DISCUSSION
Viral infections are potent stimuli for type I IFN production which is induced via the recognition of viral RNA by TLR3, TLR7 and RIG-I helicases (Takeuchi and Akira, 2010). Our results indicate that type I IFNs elicited by enteric or systemic infection with the MNV-1 virus augment the response to Nod1/Nod2 stimulation and these coordinated signaling events contribute to lethality associated with secondary E. coli infection. The protection afforded by deficiency in Nod1/Nod2 or RIP2 against E. coli infection in mice treated with poly I:C, IFN-β, or infected with MNV-1 correlated with reduced TNF-α levels. However, the inhibition of mouse lethality induced by TNF-α blockade was partial, indicating that other pro-inflammatory mediators induced by enhanced Nod1/Nod2 signaling are also critical for lethality. The latter results are consistent with observations indicating that various molecules including TNF-α, IL-1β, IFN-γ, and MIF produced during bacterial sepsis contribute to lethality (Bernhagen et al., 1993; Beutler et al., 1985; Car et al., 1994; Fantuzzi et al., 1996). The role of pro-inflammatory molecules such as TNF-α in bacterial infection is complex in that they contribute to host defense, but also promote organ damage and lethality particularly in the presence of high numbers of bacteria (Beutler et al., 1985). Thus, these studies suggest that over stimulation of Nod1/Nod2 by E. coli promotes the overproduction of harmful pro-inflammatory molecules such as TNF-α that mediate lethality in virally infected mice.
Several lines of evidence support our conclusions that viral infection promoted by type I IFNs increase innate recognition of bacteria and lethality. First, enhancement of MDP-induced NF-κB and MAPK activation by poly I:C was abolished in macrophages deficient in IFNAR1 or innate immune receptors that are required for IFN-α/β production. Second, stimulation with IFN-β or DMXAA, a synthetic molecule that activates IRF3, was sufficient to enhance Nod1 and Nod2 signaling in vitro and in vivo. Third, type I IFNs induced the expression of Nod1, Nod2, and RIP2, the essential kinase that is required for Nod1 and Nod2 signaling. Fourth, Nod1−/−Nod2−/− mice infected with MNV-1 virus or stimulated with poly I:C were partially protected from E. coli infection. Although Nod1−/−Nod2−/− mice infected with MNV-1 exhibited reduced susceptibility to E. coli infection, the mutant mice were more susceptible than animals that were not stimulated with poly I:C or infected with the virus. Furthermore, the enhancement of TNF-α production by poly I:C or MNV-1 in macrophages infected with Gram-negative bacteria was reduced, but not abolished in Nod1−/−Nod2−/− macrophages. Thus, Nod1 and Nod2 contribute to the inflammatory response and lethality induced by bacteria in virally-infected mice, but additional host factors are involved. Possible candidates or the latter are TLRs given that type I IFNs enhance the sensitivity to LPS and susceptibility to LPS-induced mortality in mice (Doughty et al., 2001; Nansen and Randrup Thomsen, 2001). Thus, the production of IFN-α/β is not only critical for the control of viral replication, but also potentiate TLR and Nod1/Nod2 signaling pathways which are involved in the recognition of bacteria and mediate the production of harmful cytokines that mediate lethality. Unlike the effect of poly I:C or MNV-1 infection, we only observed 30% lethality induced by bacterial infection in mice pre-treated with IFN-β. One possibility is that sustained production of high levels of IFN-β is needed for 100% lethality and administration of exogenous IFN-β could not fully mimic endogenous production of IFN-β induced by poly I:C or MNV-1 infection. An alternative, non-excluding possibility, is that IFN-β is sufficient to promote lethality, but the partial effect reflects the additional contribution of signaling pathway(s) such as NF-κB that are induced by poly I:C or MNV-1 infection.
The biological reason for coupling type I IFNs induced during viral infection to bacterial recognition is unclear. One possibility is that it serves to boost the immune response of the virally infected host to bacterial infection and this may be helpful when the host is superinfected with bacterial pathogens. The enhancement of Nod1 and Nod2 signaling by type I IFNs is associated with increased NF-κB and MAPK activation and thus it is expected to increase the immune response to bacteria and production of anti-bacterial molecules. Consistent with this, we found enhanced production of TNF-α in macrophages and mice pretreated with poly I:C or infected with MNV-1 in response to bacteria which was attenuated in the absence of Nod1/Nod2 or RIP2. However, type I IFN-mediated enhancement of Nod1/Nod2 signaling is also expected to augment the potentially harmful effect mediated by TNF-α and other pro-inflammatory molecules in the setting of systemic bacterial infection. The latter may be particularly relevant in the context of viruses that induce systemic production of type IFNs and superinfection with high number of bacteria that induces high levels of TNF-α and other harmful pro-inflammatory cytokines. Another, non-excluding, possibility is that the coupling IFN-α/β production and innate signaling pathways involved in bacterial recognition primarily serves to enhance the immune response to viruses, but not to bacteria, since Nod2 have been also implicated in viral recognition (Sabbah et al., 2009). However, we found that Nod1 and Nod2 are not required for IFN-β production in response to MNV-1 infection in vivo. Another possibility is that the crosstalk between Type I IFN and Nod1/Nod2 signaling is more relevant to bacterial infection or interactions between two types of bacteria. For example, many Gram-negative bacteria induce Type I IFNs via TLR4-TRIF signaling and this will enhance Nod1/Nod2 signaling and production of inflammatory molecules in response to bacteria.
In humans, the outcome of bacterial sepsis correlates with the magnitude of the inflammatory response (Casey et al., 1993; Sunden-Cullberg et al., 2005). The factors that regulate the strength of the immune response to bacteria in humans are poorly understood, but are likely to include genetic variation and age as well as environmental factors. The current work focused on MNV-1, a virus that models human infection by noroviruses which are now recognized as being the most common cause of epidemics of gastroenteritis and an important cause of sporadic gastroenteritis in both children and adults (Glass et al., 2009). Although the production of type I IFNs in response to norovirus infection in humans has not been studied, high levels of IFN-α/β are found in individuals with certain viral infections such as that resulting from adenovirus and rotavirus (Gendrel et al., 1999). Thus, it is possible that the cross talk between type I IFNs and signaling pathways activated in response to bacterial superinfection may contribute to morbidity and mortality in human populations.
EXPERIMENTAL PROCEDURES
Mice
C57BL/6 mice were generated through breeding in our animal facility from breeders purchased from Jackson Laboratories (Bar Harbor, ME). Nod1−/−, Nod2−/−, Nod1−/−Nod2−/−, and Rip2−/− in C57BL/6 background have been described (Kim et al., 2008). Ifnar1−/− mice were obtained from Mariana Kaplan, the University of Michigan. Myd88−/−Trif−/−, Tlr3−/−, Ips1−/−, and Trif−/−Ips1−/− mice have been described (Kumar et al., 2008). The animal studies were conducted under approved protocols by the University of Michigan Committee on Use and Care of Animals.
Reagents and Bacterial infection
Poly I:C and pam3CSK4 (pam3, Invivogen), synthetic MDP (Bachem), Ultrapure LPS from E. coli O55:B5 and DMXAA (Sigma), and mouse IFN-β (PBL Interferon Source) were used at the indicated concentrations. For in vivo experiments, unmodified (3.5 × 107 U/mL) and PEGylated murine IFN-β (1.6 × 106 U/mL) were provided by BiogenIdec Inc., Cambridge, MA (Pepinsky et al., 2001). Synthetic Nod1 ligand, KF1B (Hasegawa et al., 2007), was a gift of Dr. K. Fukase, Osaka University. E. coli strain K-12 was a gift of M.O’Riordan, the University of Michigan. Pseudomonas aeruginosa strain PAK was a gift of Stephen Lory, Harvard University. Single colonies were inoculated into Luria-Bertani (LB) medium and grown overnight at 37°C with shaking. On the day of the infection, a 1/10 dilution of the overnight culture was prepared and allowed to grow at 37°C with shaking to A600= 0.5, which corresponds to 2 × 109 CFU/mL. Bacteria were diluted to the desired concentration and used to infect macrophages at different bacterial/macrophage ratios. After 60 min of infection at 37° C, the macrophages were washed twice with PBS and IMDM containing 50 μg/mL of gentamycin, which was added to limit the growth of extracellular bacteria. The plaque-purified murine norovirus-1 (MNV-1) clone (GV/MNV1/2002/USA) MNV-1.CW3 (Thackray et al., 2007) was used at passage 6 for all infections. MNV-1 was passaged on RAW 264.7 cells and concentrated as described (Chachu et al., 2008; Wobus et al., 2004). Vesicular stomatitis virus (VSV), strain Orsay (ATCC VR-1421), was propagated in chinese hamster ovary (CHO) cells.
Preparation and stimulation of murine macrophages
BMDM were prepared as described previously (Celada et al., 1984). Briefly, the femors and tibias from 6–12 week old mice were dissected and the bone marrow flushed out. Bone marrow cells were cultured with IMDM media supplemented with 30% L929 supernatant containing macrophage stimulating factor, glutamine, sodium pyruvate, 10% heat-inactivated fetal bovine serum (Gibco-BRL), and antibiotics for 5 days. Macrophages were cultured in 48-well plates at a concentration of 2 × 105/well or in 6-well plates at a concentration of 2 × 106 cells/well. The day after the plating, cells were treated with poly I:C (100 μg/mL), LPS (100 ng/mL), pam3CSK4 (1 μg/mL), DMXAA (10 μg/mL), IFN-β (2 × 102 units/mL) or infected with MNV-1 (MOI=0.1 and 0.3 ), and VSV (MOI=0.002 and 0.01) for 24 hrs and then treated with MDP (10 μg/mL), KF1B (10 μg/mL), LPS (100 ng/mL), pam3CSK4 (pam3, 1 μg/mL), poly I:C (100 μg/mL), or infected with E. coli or P. aeruginosa. Peritoneal macrophages were elicited by intraperitoneal injection of thioglycollate for 3 days. Thioglycollate-elicited peritoneal macrophages (PMs) were harvested by peritoneal lavage with PBS, and adherent cells were enriched by plastic adherence for 2 hr. 1 day before isolating PMs, mice were injected PBS (−) or infected with MNV i.p. PMs were then treated with MDP (10 μg/mL).
Measurement of cytokines
Mouse cytokines were measured in culture supernatants using enzyme-linked immunoabsorbent assay (ELISA) kits from R&D Systems.
Immunoblotting
Cells were lysed in buffer containing 1% NP-40 supplemented with complete protease inhibitor cocktail (Roche, Mannheim, Germany) and 2 mM dithiothreitol. Lysate proteins were separated by SDS-PAGE and transferred to PVDF membranes by electro-blotting (Biorad). Membranes were immunoblotted with antibodies for mouse IκB-α, phospho-IκB-α, p38, phospho-p38, phospho-ERK, phospho-JNK (Cell Signaling Technology, Beverly, MA), and RIP2 (Enzo Life Sciences International, Inc. Plymouth Meeting, PA ).
cDNA synthesis and real-time RT-PCR
Total RNA was extracted from cultured macrophages after stimulation with poly I:C (100 μg/mL), DMXAA (10 μg/mL), IFN-β (2 × 102 units/mL), or infection with MNV-1 (MOI= 0.1) for the indicated periods, using E.Z.N.A Total RNA Kit I (OMEGA Bio-Tek. Inc.) according to the manufacturer’s instructions. cDNA was synthesized using High Capacity RNA-to-cDNA Kit (Applied Biosystems) according to manufacturer’s instructions. Real-time PCR was performed using SYBR Green master mix (Applied Biosystems) at the University of Michigan Comprehensive Cancer Center Affymetrix and Microarray Core Facility (University of Michigan, Ann Arbor). The PCR conditions were as follows: initial denaturation for 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C. The primers sequence was: Nod1 forward, GCCGAAGATGCAGAGATTGT; Nod1 reverse, CAGACACCTCCTCGCCTTT; Nod2 forward, AACTGTCCAACAATGGCATCAC; Nod2 reverse, TTCCCTCGAAGCCAAACCT; β-actin forward, AGAGGGAAATCGTGCGTGGAC; and β-actin reverse, CAATAGTGATGACCTGGCCGT. Nod1 or Nod2 to β-actin relative expression was calculated using the 2(−Ct) method.
Mouse stimulation and infection
Mice were treated with poly I:C (200 μg/mouse), PEG-mIFN-β (5 × 103U/mouse) i. p. or rmIFN-β(2.5 × 104 U/mouse) twice s. c., or infected with MNV-1 (1 × 107 PFU/mouse orally or 1 × 106 PFU/mouse i. p.) and 18–24 h later injected with PBS or MDP (300 μg/mouse) i.p., or infected with 3 × 108 CFU of E. coli i. p. or P. aeruginosa 2.5 × 107 i.n. Cytokine levels in serum or lung homogenates were determined 3 h post-infection. The survival rate of infected mice was monitored for 5 days after challenge with E. coli or P. aeruginosa.
Statistical analysis
Statistically significant differences between two groups were determined by the two-tailed t-test with unequal variance (Aspin-Welch’s t-test). Bacterial counts of infected mice were analyzed by Man-Whitney U test. The survival rate of infected mice was analyzed using the log rank test. Differences were considered significant when p values were <0.05.
Supplementary Material
HIGHLIGHTS.
Viral infection augments Nod1 and Nod2 activation via the production of Type I IFNs
Type I IFN signaling induces expression of Nod1, Nod2, and RIP2
Nod1/Nod2 potentiate susceptibility to bacterial infection in virally-infected mice
IFN-β enhances bacteria-induced lethality via Nod1 and Nod2
Acknowledgments
The authors are grateful to Richard Flavell for Nod2 deficient mice, Biogen for providing purified unmodified and PEGylated murine IFN-β, Joel Whitfield from the Cellular Immunology Core Facility of the University of Michigan Cancer Center for ELISA assays, and Sharon Koonse for animal husbandry. We are grateful to Michael Shaw and Grace Chen for critical review of the manuscript and Nick Lukacs, Adolfo Garcia-Sastre, Koichi Fukase, Mary O’Riordan, and Stephen Lory for reagents or bacterial/viral strains. This work was supported by NIH grant R01 DK61707. Y.-G. Kim was supported by training funds from the University of Michigan Comprehensive Cancer Center.
Nonstandard abbreviations used
- BMDM
bone marrow derived macrophages
- MDP
muramyl dipeptide
- meso-DAP
meso-diaminopimelic acid
- NLR
nucleotide-binding oligomerization domain-like receptors
- MNV-1
murine norovirus-1
- VSV
Vesicular stomatitis virus
Footnotes
CONFLICT OF INTEREST:
Darren P. Baker is employed by Biogen Idec. Biogen Idec provided unmodified and PEGylated murine IFN-β for these studies at no cost and did not provide funds for these studies, nor was Biogen Idec involved in the study design, data analysis or any decisions relating to manuscript preparation or submission.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Barnes AJ, Johnson AS, Shelly MP, Orton CI. Bacterial superinfection in chickenpox….but it can occur at any age. BMJ. 1996;313:1145. doi: 10.1136/bmj.313.7065.1145a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadling C, Slifka MK. How do viral infections predispose patients to bacterial infections? Curr Opin Infect Dis. 2004;17:185–191. doi: 10.1097/00001432-200406000-00003. [DOI] [PubMed] [Google Scholar]
- Bernhagen J, Calandra T, Mitchell RA, Martin SB, Tracey KJ, Voelter W, Manogue KR, Cerami A, Bucala R. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Brundage JF. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect Dis. 2006;6:303–312. doi: 10.1016/S1473-3099(06)70466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Car BD, Eng VM, Schnyder B, Ozmen L, Huang S, Gallay P, Heumann D, Aguet M, Ryffel B. Interferon gamma receptor deficient mice are resistant to endotoxic shock. J Exp Med. 1994;179:1437–1444. doi: 10.1084/jem.179.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119:771–778. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- Celada A, Gray PW, Rinderknecht E, Schreiber RD. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J Exp Med. 1984;160:55–74. doi: 10.1084/jem.160.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachu KA, Strong DW, LoBue AD, Wobus CE, Baric RS, Virgin HWt. Antibody is critical for the clearance of murine norovirus infection. J Virol. 2008;82:6610–6617. doi: 10.1128/JVI.00141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, Kawasaki A, Fukase K, Kusumoto S, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–707. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004;5:975–979. doi: 10.1038/ni1116. [DOI] [PubMed] [Google Scholar]
- Danner RL, Elin RJ, Hosseini JM, Wesley RA, Reilly JM, Parillo JE. Endotoxemia in human septic shock. Chest. 1991;99:169–175. doi: 10.1378/chest.99.1.169. [DOI] [PubMed] [Google Scholar]
- Doughty L, Nguyen K, Durbin J, Biron C. A role for IFN-alpha beta in virus infection-induced sensitization to endotoxin. J Immunol. 2001;166:2658–2664. doi: 10.4049/jimmunol.166.4.2658. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G, Zheng H, Faggioni R, Benigni F, Ghezzi P, Sipe JD, Shaw AR, Dinarello CA. Effect of endotoxin in IL-1 beta-deficient mice. J Immunol. 1996;157:291–296. [PubMed] [Google Scholar]
- Fejer G, Szalay K, Gyory I, Fejes M, Kusz E, Nedieanu S, Pali T, Schmidt T, Siklodi B, Lazar G, Jr, et al. Adenovirus infection dramatically augments lipopolysaccharide-induced TNF production and sensitizes to lethal shock. J Immunol. 2005;175:1498–1506. doi: 10.4049/jimmunol.175.3.1498. [DOI] [PubMed] [Google Scholar]
- Gendrel D, Raymond J, Coste J, Moulin F, Lorrot M, Guerin S, Ravilly S, Lefevre H, Royer C, Lacombe C, et al. Comparison of procalcitonin with C-reactive protein, interleukin 6 and interferon-alpha for differentiation of bacterial vs viral infections. Pediatr Infect Dis J. 1999;18:875–881. doi: 10.1097/00006454-199910000-00008. [DOI] [PubMed] [Google Scholar]
- Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jehanno M, Viala J, Tedin K, Taha MK, Labigne A, Zahringer U, et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003a;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003b;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- Glass RI, Parashar UD, Estes MK. Norovirus gastroenteritis. N Engl J Med. 2009;361:1776–1785. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Kawasaki A, Yang K, Fujimoto Y, Masumoto J, Breukink E, Nunez G, Fukase K, Inohara N. A role of lipophilic peptidoglycan-related molecules in induction of Nod1-mediated immune responses. J Biol Chem. 2007;282:11757–11764. doi: 10.1074/jbc.M700846200. [DOI] [PubMed] [Google Scholar]
- Herold S, Steinmueller M, von Wulffen W, Cakarova L, Pinto R, Pleschka S, Mack M, Kuziel WA, Corazza N, Brunner T, et al. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Exp Med. 2008;205:3065–3077. doi: 10.1084/jem.20080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- Jamieson AM, Yu S, Annicelli CH, Medzhitov R. Influenza virus-induced glucocorticoids compromise innate host defense against a secondary bacterial infection. Cell Host Microbe. 2010;7:103–114. doi: 10.1016/j.chom.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- Kim YG, Park JH, Shaw MH, Franchi L, Inohara N, Nunez G. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity. 2008;28:246–257. doi: 10.1016/j.immuni.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- Kumar H, Koyama S, Ishii KJ, Kawai T, Akira S. Cutting edge: cooperation of IPS-1- and TRIF-dependent pathways in poly IC-enhanced antibody production and cytotoxic T cell responses. J Immunol. 2008;180:683–687. doi: 10.4049/jimmunol.180.2.683. [DOI] [PubMed] [Google Scholar]
- Magalhaes JG, Fritz JH, Le Bourhis L, Sellge G, Travassos LH, Selvanantham T, Girardin SE, Gommerman JL, Philpott DJ. Nod2-dependent Th2 polarization of antigen-specific immunity. J Immunol. 2008;181:7925–7935. doi: 10.4049/jimmunol.181.11.7925. [DOI] [PubMed] [Google Scholar]
- Nansen A, Randrup Thomsen A. Viral infection causes rapid sensitization to lipopolysaccharide: central role of IFN-alpha beta. J Immunol. 2001;166:982–988. doi: 10.4049/jimmunol.166.2.982. [DOI] [PubMed] [Google Scholar]
- Navarini AA, Recher M, Lang KS, Georgiev P, Meury S, Bergthaler A, Flatz L, Bille J, Landmann R, Odermatt B, et al. Increased susceptibility to bacterial superinfection as a consequence of innate antiviral responses. Proc Natl Acad Sci U S A. 2006;103:15535–15539. doi: 10.1073/pnas.0607325103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Kim YG, McDonald C, Kanneganti TD, Hasegawa M, Body-Malapel M, Inohara N, Nunez G. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J Immunol. 2007;178:2380–2386. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- Pepinsky RB, LePage DJ, Gill A, Chakraborty A, Vaidyanathan S, Green M, Baker DP, Whalley E, Hochman PS, Martin P. Improved pharmacokinetic properties of a polyethylene glycol-modified form of interferon-beta-1a with preserved in vitro bioactivity. J Pharmacol Exp Ther. 2001;297:1059–1066. [PubMed] [Google Scholar]
- Roberts ZJ, Goutagny N, Perera PY, Kato H, Kumar H, Kawai T, Akira S, Savan R, van Echo D, Fitzgerald KA, et al. The chemotherapeutic agent DMXAA potently and specifically activates the TBK1-IRF-3 signaling axis. J Exp Med. 2007;204:1559–1569. doi: 10.1084/jem.20061845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah A, Chang TH, Harnack R, Frohlich V, Tominaga K, Dube PH, Xiang Y, Bose S. Activation of innate immune antiviral responses by Nod2. Nat Immunol. 2009;10:1073–1080. doi: 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunden-Cullberg J, Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, Lee ML, Andersson J, Tokics L, Treutiger CJ. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005;33:564–573. doi: 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Thackray LB, Wobus CE, Chachu KA, Liu B, Alegre ER, Henderson KS, Kelley ST, Virgin HWt. Murine noroviruses comprising a single genogroup exhibit biological diversity despite limited sequence divergence. J Virol. 2007;81:10460–10473. doi: 10.1128/JVI.00783-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting JP, Davis BK. CATERPILLER: a novel gene family important in immunity, cell death, and diseases. Annu Rev Immunol. 2005;23:387–414. doi: 10.1146/annurev.immunol.23.021704.115616. [DOI] [PubMed] [Google Scholar]
- Wobus CE, Karst SM, Thackray LB, Chang KO, Sosnovtsev SV, Belliot G, Krug A, Mackenzie JM, Green KY, Virgin HW. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2004;2:e432. doi: 10.1371/journal.pbio.0020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Zerr DM, Alexander ER, Duchin JS, Koutsky LA, Rubens CE. A case-control study of necrotizing fasciitis during primary varicella. Pediatrics. 1999;103:783–790. doi: 10.1542/peds.103.4.783. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.