Summary
Fanconi anaemia (FA), dyskeratosis congenita (DC), Diamond-Blackfan anaemia (DBA), and Shwachman-Diamond syndrome (SDS) comprise major inherited bone marrow failure syndromes (IBMFS). Adverse events include severe bone marrow failure (BMF), myelodysplastic syndrome (MDS), acute myeloid leukaemia (AML), and solid tumours (ST). The natural history of FA is well characterised; hazard rates in the other syndromes have not yet been quantified. An open cohort was established at the National Cancer Institute (NCI) in 2002. Patients enrolled prior to December, 2007 were followed up to December, 2008. Diagnoses were confirmed with standard tests. Age-associated risks of adverse events were calculated. Most patients in each syndrome survived to young adulthood. Patients with FA had earlier onset of cancers, need for stem cell transplant, and death; followed by DC; DBA and SDS were mildest. While FA and DC patients had markedly increased risks of cancer, AML and MDS, there were no cases of leukaemia in DBA or SDS patients. The NCI cohort provides the first direct quantitative comparison of timing and magnitude of cancer risk in the IBMFS. The findings demonstrate that both FA and DC are major cancer susceptibility syndromes. The IBMFS, historically considered paediatric disorders, have important management implications for physicians treating adult patients.
Keywords: bone marrow failure, Fanconi anaemia, dyskeratosis congenita, leukaemia, solid tumours
Patients with inherited bone marrow failure syndromes (IBMFS) comprise a substantial subset of individuals with bone marrow failure (BMF), myelodysplastic syndrome (MDS), acute myeloid leukaemia (AML), and syndrome-specific solid tumours (ST). While we cannot currently determine the proportion of all individuals developing these outcomes that can be attributed to inherited syndromes, we can, for the first time, directly compare the age at onset, cumulative incidence, and types of these outcomes in patients with several of the most common IBMFS. Single-centre cohorts indicated that the majority of patients with Fanconi Anaemia [FA, Mendelian Inheritance in Man (MIM) 227650] develop early-onset bone marrow failure, while AML, head and neck squamous cell carcinomas (HNSCC) and gynaecological SCC increase in frequency with age (Kutler et al, 2003a; Rosenberg et al, 2003, 2007; Tamary et al, 2009). Our recent literature review suggested that patients with dyskeratosis congenita (DC, MIM 30500, 127550, 224230) are at risk of similar adverse outcomes as patients with FA, but did not address the hazard rates of these events in DC (Alter et al, 2009). In contrast, patients with Diamond-Blackfan Anaemia (DBA, MIM 106550), Shwachman-Diamond syndrome (SDS, MIM 260400) and severe congenital neutropenia (SCN, MIM 202700) are primarily at risk of leukaemia (Rosenberg et al, 2006; Alter, 2007; Vlachos et al, 2008; Alter et al, 2009), and the age-specific rates of these events have not been examined.
To better understand the age dependence of the long-term outcomes for patients with an IBMFS, we established a retrospective/prospective cohort study at the National Cancer Institute (NCI), the ‘NCI IBMFS Cohort,’ focusing on patients with confirmed diagnoses of specific IBMFS. This cohort provides the first single centre direct comparison of the major IBMFS. We now quantify for the first time that patients with FA have the earliest and highest risks, and also that patients with DC have risks that are nearly as high but which occur at older ages. In contrast, patients with DBA or SDS may have lower and later risks than suggested by literature case reports.
Methods
The NCI IBMFS cohort is an open retrospective/prospective cohort, established in January 2002, with approval from the NCI Institutional Review Board. Data reported here include individuals enrolled prior to December, 2007, with follow-up through to December, 2008. The protocol, NCI 02-C-0052 [NCT00056121] (http://www.marrowfailure.cancer.gov), was advertised by mailing to paediatric haematologists/oncologists, medical geneticists, and IBMFS family support groups. Voluntary enrollment by the family contact (usually a parent or proband; a proxy was used for deceased patients) began with a telephone interview. Discussion at a team meeting determined whether the proband met the criteria for the suspected syndrome or needed further testing. A Family History Questionnaire provided medical information about relatives. Written informed consent and medical record release forms were signed. Individual Information Questionnaires (medical history, cancer risk factors, etc.) were sent to the proband (or proxy) and first-degree relatives. Biannual follow-up was obtained on all participants. Cancer diagnoses were confirmed by medical record review. All participants were enrolled in the ‘Field Cohort.’ Those who visited the National Institutes of Health (NIH) Warren G. Magnuson Clinical Center were reassigned to the ‘Clinic Cohort.’ Families in the Clinic Cohort visited the NIH for 5 d, for thorough review of medical histories and physical examinations by haematologists and multiple subspecialists, as well as aetiologically-focused laboratory tests.
Participants were assigned to a specific syndrome according to standard criteria and confirmed by syndrome-specific tests where available (Alter, 2003). FA was diagnosed by abnormal chromosome breakage in peripheral blood lymphocytes, using both diepoxybutane and mitomycin C (Cervenka et al, 1981; Auerbach et al, 1989). Skin fibroblasts were analysed when lymphocytes were normal but FA remained highly suspect (seeking evidence for haematopoietic mosaicism) (Alter et al, 2005). FA complementation group analyses were performed using retroviral correction (Chandra et al, 2005).
The clinical diagnosis of DC was made in individuals with components of the diagnostic triad (nail dystrophy, reticular pigmentation, and oral leucoplakia), or those with at least one other typical physical finding (Vulliamy et al, 2006), in association with marrow failure. We expanded the inclusion criteria to patients with marrow failure, any of the above physical parameters, and blood leucocyte subset telomere lengths below the first percentile of normal-for-age (Alter et al, 2007a). We also classified as ‘DC’ probands and healthy family members who had pathogenic mutations in known DC genes, such as DKC1, TERC, TERT, and TINF2, including those with none of the typical physical findings (Savage & Alter, 2009).
The diagnosis of DBA was made in those with macrocytic pure red cell aplasia, and supported by finding increased red cell adenosine deaminase (Glader & Backer, 1988). Patients with SDS had neutropenia and exocrine pancreatic insufficiency, confirmed by detection of sub-normal levels of serum pancreatic trypsinogen and isoamylase (Ip et al, 2002).
All living affected individuals were specifically screened for all of the major IBMFS; genotyping was performed when possible (Ameziane et al, 2008; Moghrabi et al, 2009). Affected individuals who had not received a transplant had bone marrow aspirations, biopsies and cytogenetic studies. Individuals who could not be classified as having a specific IBMFS were designated as ‘Others.’ Categories of ‘DC-like,’ ‘FA-like,’ and ‘SDS-like’ were used for individuals whose features initially suggested DC, FA, or SDS but who failed to meet diagnostic criteria. Severe bone marrow failure was defined as impaired haematopoiesis sufficiently severe to lead to bone marrow transplant (BMT) or death (Rosenberg et al, 2003); MDS required severe pancytopenia and dyspoietic morphology, with or without a cytogenetic clone (Alter et al, 2000).
Analyses used Microsoft Excel 11.0 (Microsoft, Redmond, WA, USA), Stata 10.1 (StataCorp, College Station, TX, USA), and MATLAB 2008b software (The MathWorks, Natick, MA, USA). The Kaplan-Meier product limit estimator was used to calculate actuarial survival probabilities by age and cumulative incidences in the absence of competing risks; subjects were censored at death (Kaplan & Meier, 1958). Subgroup survivals were compared using the log-rank test for equality of survivor functions. Cause-specific hazards and cumulative incidence curves accounting for competing risks were calculated as described previously (Rosenberg et al, 2003). The observed number of cancers was compared with the expected number (O/E ratio), based on general population incidence data from the Surveillance, Epidemiology, and End Results (SEER) Program, adjusting for age, sex, race, and birth cohort (Ries et al, 2008). Sex ratios were examined using the binomial test of comparison with a male:female ratio of 1:1. Statistical tests were 2-sided, and P-values ≤0·05 were considered significant.
Results
FA, DC, DBA, and SDS were the major rare syndromes in our cohort (Table I); a small number of patients had thrombocytopenia absent radii (TAR, MIM 274000), SCN, or other conditions. More than 40% of the affected subjects were seen at the Clinical Center. A total of 248 affected individuals from 184 families were enrolled through 2007. After extensive investigation, 42 persons (17%) from 35 families were determined to lack sufficient evidence for any of the specified syndromes; final classification awaits discovery of new IBMFS genes. Excesses of females with FA and males with DC were statistically significant. We accrued 5205 person-years of observation (1384, 1195, 1449 and 274 person-years for FA, DC, DBA, SDS, respectively, and 1 803 for other diagnoses). The male:female ratio in FA was 0·5:1, and in DC was 2·8:1, both significantly skewed from the expectation of 1:1 for a primarily autosomal recessive disorder (FA), or a disorder with a large component of autosomal dominant and recessive patients (DC). These aberrations may be due to the relatively small numbers of patients in these categories. Most participants were non-Hispanic whites. More than 60% of the patients in each group were alive at the time of analysis; the highest crude death rates were approximately 40% in those with FA and DC. Leukaemia was documented in patients with FA, DC, and TAR; MDS in FA and DC; and solid tumours in FA, DC, and DBA. Severe bone marrow failure occurred in 32% of FA and 22% of DC patients, and severe anaemia (leading to transplant or death from complications) in 6% of DBA. BMT was provided in 30% of the FA and 20% of the DC patients, both prior to (14 FA, 5 DC patients) and following enrollment (6 FA and 7 DC patients); indications were severe BMF, leukaemia, or MDS.
Table I.
Participants in the NCI IBMFS cohort.
| Feature | FA | DC | DBA | SDS | TAR | SCN | Other | Total |
|---|---|---|---|---|---|---|---|---|
| N Families | 51 | 32 | 41 | 17 | 4 | 4 | 35 | 184 |
| N Affecteds | 66 | 50 | 63 | 17 | 5 | 5 | 42 | 248 |
| Person-Years | 1384 | 1195 | 1449 | 274 | 98 | 81 | 724 | 5205 |
| Birth Years | 1945–03 | 1930–05 | 1921–05 | 1962–05 | 1961–05 | 1976–02 | 1950–06 | 1921–06 |
| Male:Female (ratio) | 22:44 (0·5:1*) | 37:13 (2·8:1*) | 37:26 (1·4:1) | 8:9 (0·9:1) | 2:3 (0·7:1) | 2:3 (0·7:1) | 25:17 (1·5:1) | 133:115 (1·2:1) |
| Race White (%) | 50 (76) | 36 (72) | 48 (76) | 12 (71) | 5 (100) | 4 (80) | 25 (60) | 180 (73) |
| Ethnicity Non-Hispanic (%) | 51 (77) | 39 (78) | 44 (70) | 12 (71) | 5 (100) | 2 (40) | 22 (52) | 175 (71) |
| N in CC (%) | 31 (47) | 32 (64) | 24 (38) | 7 (42) | 3 (60) | 0 | 8 (19) | 105 (42) |
| N Alive (%) | 41 (62) | 32 (64) | 58 (92) | 17 (100) | 5 (100) | 5 (100) | 41 (98) | 199 (80) |
| Age Alive (years), median (range) | 20 (5–54) | 18 (6–52) | 16 (4–59) | 14 (3–46) | 17 (4–48) | 12 (7–33) | 11 (2–59) | 14 (2–59) |
| N Deceased (%) | 25 (38) | 18 (36) | 5 (8) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 49 (20) |
| Age Deceased (years), median (range) | 21 (4–43) | 29 (1–63) | 33 (13–70) | – | – | – | 35 | 26 (1–70) |
| N with any cancer (%) | 15 (23) | 7 (14) | 3 (5) | 0 | 1 (20) | 0 | 3 (7) | 29 (12) |
| N with leukaemia (%) | 4 (6) | 2 (4) | 0 | 0 | 1 (20) | 0 | 0 | 6 (2) |
| N with solid tumour (%) | 11 (17) | 5 (10) | 4 (6) | 0 | 0 | 0 | 0 | 20 (8) |
| N with any BMT (%) | 20 (30) | 12 (24) | 2 (3) | 0 | 0 | 0 | 0 | 34 (14) |
| N with BMT for BMF (%) | 14 (21) | 4 (8) | 2 (3) | 0 | 0 | 0 | 0 | 20 (8) |
| N with death from BMF, no BMT (%) | 7 (11) | 7 (14) | 2 (3) | 0 | 0 | 0 | 0 | 16 (6) |
| N with MDS (%) | 8 (12) | 5 (10) | 0 | 0 | 0 | 0 | 0 | 13 (5) |
FA, Fanconi anaemia; DC, Dyskeratosis congenita; DBA, Diamond-Blackfan anaemia; SDS, Shwachman-Diamond syndrome; TAR, thrombocytopenia absent radii; SCN, severe congenital neutropenia; N, number; CC, Clinic Cohort; ST, solid tumour; BMT, bone marrow transplantation; BMF, severe bone marrow failure.
‘Other’ includes: 2 FA-like, 10 DC-like, 14 SDS-like, 5 neutropenia, 4 aplastic anaemia, 1 acute lymphocytic leukaemia, 1 Dubowitz syndrome, 1 adult with head and neck squamous cell carcinoma, 1 myelodysplastic syndrome (MDS), 1 Rothman Thompson syndrome, 2 thrombocytopenia.
MDS is not included in Cancer. Race and ethnicity were unknown or missing for 20% and 24%, respectively.
Person-Years are calculated from birth, not from study entry.
Excess of females in FA, P = 0·005, and males in DC, P = 0·0005.
Thirty-two (13%) of the affected individuals in the study had died from their syndrome prior to being enrolled by their proxy. These included primarily siblings in the FA cohort, and siblings or parents in the DC and DBA cohorts. There were 16 patients with FA, of whom two had died from AML, five from bone marrow failure, two from MDS, three from cancer (tongue, brain, vulva) and four following BMT (two for AML, two for bone marrow failure). Among 11 DC patients, three died from bone marrow failure, three had cancer (cervical, tongue, and lymphoma), and five died following transplantation (one AML, four bone marrow failure); three of the 11 had pulmonary fibrosis. Two patients with DBA died from cancer (lung and colon), one died following transplantation, and one died from complications of transfusion-related iron overload.
Tumours and leukaemia were reported in patients with FA, DC, and DBA, but not SDS (Table II), including four cases of AML in FA and two in DC, and 12, five, and three solid tumours which occurred in patients with FA, DC and DBA, respectively, who had not been transplanted. The tumours were primarily HNSCC and gynaecological SCC. In addition, one FA patient developed independent primary SCC of the vulva, tongue, and scalp, starting 13 years after transplantation for marrow failure. Five FA and four DC patients had multiple cancers, or tumours plus MDS. Twelve of the malignancies in FA were prevalent, three were incidental, and four of the five who were alive with prevalent cancers at enrollment progressed or recurred during follow-up. All seven of the cancers in DC were prevalent, two of these patients were alive at enrollment; one with AML subsequently died following bone marrow transplantation, and one with HNSCC died from bone marrow failure.
Table II.
Cancers in patients in the NCI IBMFS cohort.
| Cancer | Ages (years), median (range) | Observed | Expected | O/E* | 95% CI |
|---|---|---|---|---|---|
| Fanconi anaemia | |||||
| All Sites | 29 (4–44) | 16 | 0·42 | 39* | 22–63 |
| Solid Tumours | 30 (4–44) | 12 | 0·32 | 37* | 19–65 |
| Specific malignancies | |||||
| HNSCC | 35 (29–44) | 5 | 0·01 | 831* | 268–1940 |
| Leukaemia (3 AML, 1 AMoL) | 19 (16–27) | 4 | 0·01 | 311* | 84–797 |
| Vulva | 24, 29, 31 | 3 | 0 | 2098* | 461–6129 |
| Brain | 4, 5 | 2 | 0·03 | 64* | 7–230 |
| Oesophagus | 35 | 1 | 0 | 3520* | 46–19586 |
| Breast | 30 | 1 | 0·07 | 14 | 0·2–76 |
| MDS | 18 (6–42) | 8 | 0 | 4910* | 2114–9676 |
| Skin SCC | 33, 38 | 2 | NA | NA | NA |
| Skin BCC | 31 | 1 | NA | NA | NA |
| Dyskeratosis congenita | |||||
| All Sites | 37 (25–44) | 7 | 0·6 | 11* | 4–23 |
| Solid Tumours | 37 (25–42) | 5 | 0·5 | 8* | 2–20 |
| Specific malignancies | |||||
| HNSCC | 25, 25, 42 | 3 | 0 | 1154* | 232–3372 |
| AML | 28, 44 | 2 | 0·01 | 196* | 22–707 |
| Cervix | 37 | 1 | 0·02 | 43 | 0·6–236 |
| Non-Hodgkin lymphoma | 42 | 1 | 0·03 | 34 | 0·5–191 |
| MDS | 35 (19–61) | 5 | 0 | 2663* | 858–6215 |
| Skin BCC | 29 | 1 | NA | NA | NA |
| Diamond-Blackfan anaemia | |||||
| All Sites | 56 (41–70) | 3 | 1 | 2·9 | 0·6–8·6 |
| Solid Tumours | 56 (41–70) | 3 | 1 | 3·5 | 0·7–10·3 |
| Specific malignancies | |||||
| Colon | 56 | 1 | 0·05 | 22 | 0·3–123 |
| Lung | 56, 70 | 2 | 0·09 | 23* | 3–81 |
| Skin BCC | 41 | 1 | NA | NA | NA |
NA, not available, because SEER does not track nonmelanoma skin cancer; HNSCC, head and neck squamous cell carcinoma; AML, acute myeloid leukaemia; AMoL, acute monocytic leukaemia; SCC, squamous cell carcinoma; BCC, basal cell carcinoma; MDS, myelodysplastic syndrome.
All cancers in the table were in patients who had not received a transplant. Observed includes all cancers recorded in SEER, including multiple cancers in the same patient, but excluding skin SCC or BCC. For all cancer types other than MDS, expected cancer incidence rates were calculated from SEER 9 Registry rates 1973–05 for white/black/other, after adjustment for age, sex, and birth cohort. Time at risk before 1973 used 1973–74 rates, and time at risk after 2005 used 2000–05 rates. For MDS, expected rates were calculated from SEER 9 Registry rates for 2001–05.
There were no cancers in SDS or SCN. One patient with TAR had AML, 1 with an FA-like phenotype had a nasopharyngeal Burkitt lymphoma, 1 DC-like had an SCC of the finger with metastases, and 1 ‘other’ had ALL. The O/E data for DC were published elsewhere, and are included here to emphasise the similarity with FA (Alter et al, 2009).
Bold, P < 0·05 that true O/E ratio >1·0 (exact 2-sided tests).
Despite small absolute numbers of cancers, the O/E ratios were extremely high, due to the very young age at diagnosis for cancers usually seen in older adults (Table II). The O/E (fold increase) for all sites combined was significantly elevated in FA [39-fold, 95% confidence interval (CI) 22–63] and DC (11-fold, 95% CI 4–23). The respective O/E for tumours were 37-fold (95% CI 19–65) and eight-fold (95% CI 2–20). The substantially increased HNSCC and AML risks were of similar magnitude in FA and DC, 831 and 311 in FA versus 1154 and 196 in DC. Elevated O/E ratios, which were unique to FA, included vulvar cancer (2098-fold) and brain tumours (64-fold). The similar cancer types and elevated O/E ratios in FA and DC were distinct from those in DBA. The two cases of lung cancer in DBA patients had a significant O/E (23-fold), but were in people in one family who both smoked, and thus might have a non-DBA aetiology. MDS had extremely high O/E in FA and DC, at 4910-fold and 2663-fold, respectively. No MDS, AML, or solid tumours occurred in SDS. In addition, the crude rate of MDS in DBA was significantly lower than in FA (exact P = 0·003). Although other comparisons were not significant, the statistical power was limited.
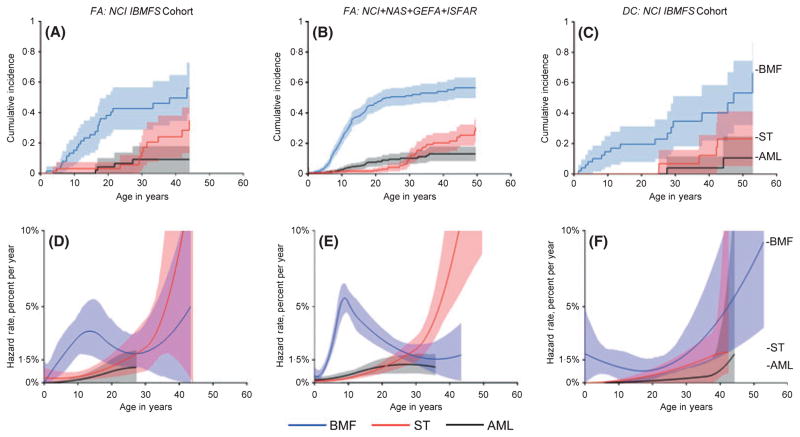
Cause-specific hazards and cumulative incidence curves are shown in Fig 1 for the major adverse events in the NCI FA and DC cohorts, and for combined data from other FA cohorts from which we have primary data (Rosenberg et al, 2003, 2007; Tamary et al, 2009). The bone marrow failure hazard rate peaked in childhood by age 10 years at c.5% per year in all FA cohorts, decreased in early adulthood, and then appeared to rise again after 30 years of age. The marrow failure hazard rate in the NCI DC cohort was constant at c.1% per year throughout childhood, and then increased dramatically throughout adulthood (with substantial uncertainty in these point estimates). Despite these differences in age-at–onset, the cumulative incidence of severe bone marrow failure was c.50% by age 50 years in both FA and DC.
Fig 1.
Cumulative incidence and annual hazard rate of competing adverse events by age in patients with Fanconi Anaemia (FA) or Dyskeratosis congenita (DC). Adverse events are severe bone marrow failure (BMF, blue), leukaemia (AML, black), or solid tumours (ST, red). A and D, NCI FA cohort, N = 66. B and E, combined results of the NCI FA cohort with three previously published cohorts: NAS, North American Survey, N = 145; GEFA, German Fanconi Anaemia Registry, N = 181; ISFAR, Israeli Fanconi Anaemia Registry, N = 66 (Rosenberg et al, 2003, 2007; Tamary et al, 2009). C and F, NCI DC cohort, N = 50. A–C, cumulative incidence by age (cumulative percentage experiencing each event as initial cause of failure) and 95% confidence intervals (CI) (shaded areas). D–F, annual hazard rates (incidence rate per year among subjects who are still susceptible) and 95% CI (shaded areas).
The hazard rates for AML were very low during childhood in FA and DC, increasing during teen and early adult years in FA, and at older ages in DC. The cumulative incidences of AML were c.10% by 50 years of age in both syndromes. The hazard rate for solid tumours in FA rose slowly initially, and then increased sharply, reaching c.10% by age 40 years; the hazard rate in DC was similar to FA until age 30 years, with limited follow-up available thereafter. The cumulative incidence of solid tumours was 20–30% in both syndromes by 50 years of age.
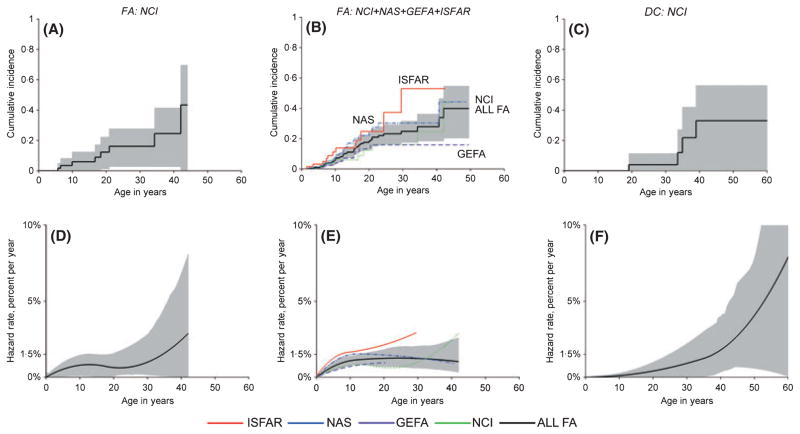
MDS is not a competing risk for bone marrow failure or AML, and was thus analysed separately (Fig 2). The FA-related MDS annual hazard rate was c.1%. The hazard rate for MDS appeared to increase more quickly with age in DC versus FA. The cumulative incidence of MDS in FA was 40% by age 50 years, and around 30% in DC. Despite wide confidence intervals due to small numbers, these data suggest that the age-specific bone marrow failure and MDS hazard rates differed between FA and DC, although the cumulative incidences were similar by 50 years of age (Figs 1 and 2, respectively).
Fig 2.
Cumulative incidence and annual hazard rate of myelodysplastic syndrome by age in patients with Fanconi Anaemia (FA) or Dyskeratosis congenita (DC). A and D, NCI FA cohort. B and E, combined results of the NCI FA cohort with the three previously published cohorts. C and F, NCI DC cohort. A–C, cumulative incidence by age and 95% CIs. D–F, annual hazard rates and 95% confidence intervals. B and E, red, ISFAR; blue, NAS; purple, GEFA; green NCI; black, combined FA.
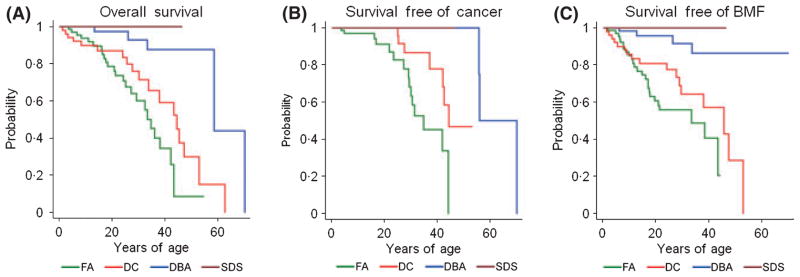
Figure 3 compares the overall, cancer-free, and bone marrow failure-free survival rates among the four major IBMFS. The median overall survival was 33 years of age in FA, 43 in DC, 58 in DBA, and not reached in SDS. Despite the relatively small numbers of cases in these categories, which could have led to these results by chance, the differences were highly statistically significant (P < 0·001). Cancer developed at a younger age in FA, with DC older and DBA oldest; the respective medians were 34, 44, and 55 years, also highly significant (P < 0·001). The time free from severe bone marrow failure was not significantly different in FA and DC (respective median ages of 33 and 45 years, P = 0·08), and significantly shorter than in DBA or SDS (P < 0·001). Patients with FA had the earliest onset of all the major complications, followed by DC, while DBA and SDS developed adverse events at relatively older ages.
Fig 3.
Survival curves according to syndrome. A, probability of overall survival. B, probability of survival free of cancer. C, probability of survival free of severe bone marrow failure. Green, Fanconi anaemia; red, dyskeratosis congenita; blue, Diamond-Blackfan anaemia; maroon, Shwachman-Diamond syndrome. Numbers in each syndrome are provided in Table I.
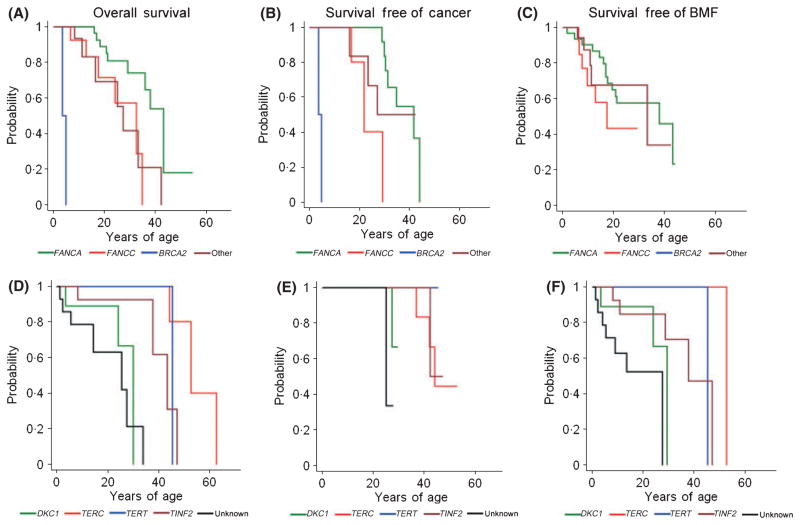
Our cohort provides the first age-specific correlations between specific genotypes and outcomes, as shown in Table III and Fig 4. The two patients with BRCA2 (FANCD1) had very short survival (Alter et al, 2007b), while those with FANCA survived the longest (median c.40 years); FANCC and Other/unknown c.30 years (P < 0·01). Cancer was diagnosed at very young ages in BRCA2 (FANCD1); FANCC and Other/unknown had median ages of c.30 years, while the median for FANCA was >40 years (P < 0·001 for FANCA vs. FANCC). The age at development of severe bone marrow failure was slightly earlier in those with FANCC than FANCA. These genotype/outcome associations in FA are similar to those reported previously from the International Fanconi Anaemia Registry (IFAR) (Kutler et al, 2003b).
Table III.
Outcomes according to genotype.
| Feature
|
FA
|
DC
|
DBA
|
SDS
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | FANCA | FANCC | BRCA2 (FANCD1) | Other/Unk | DKC1 | TERC | TERT | TINF2 | Unk | RPS19 | Unk | SBDS | Unk |
| N (%)* | 31 (57%) | 14 (26%) | 2 (4%) | 19 | 9 (18%) | 8 (16%) | 4 (8%) | 15 (30%) | 14 | 17 (27%) | 49 | 16 (94%) | 1 |
| Male:Female | 12:19 | 3:11 | 1:1 | 6:13 | 9:0 | 2:6 | 3:1 | 12:3 | 11:3 | 10:7 | 27:19 | 7:9 | 1:0 |
| Person-Years | 822 | 254 | 9 | 300 | 167 | 343 | 125 | 372 | 188 | 409 | 1039 | 258 | 15 |
| Death | 10 | 6 | 2 | 7 | 3 | 3 | 1 | 4 | 7 | 0 | 5 | 0 | 0 |
| N, BMT | 10 | 5 | 0 | 3 | 2 | 0 | 0 | 4 | 4 | 1 | 1 | 0 | 0 |
| N, BMF | 14 | 6 | 0 | 5 | 3 | 1 | 1 | 5 | 7 | 1 | 3 | 16 | 0 |
| N, AML | 0 | 2 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| N, ST | 7 | 1 | 2 | 1 | 0 | 2 | 0 | 1 | 2 | 0 | 3 | 0 | 0 |
| N, None | 10 | 5 | 0 | 11 | 5 | 4 | 3 | 9 | 5 | 16 | 40 | 0 | 1 |
Abbreviations defined in legend to Table I.
DNA was available for 59 of the 66 patients with FA; 57% were FANCA and 26% were FANCC (4 IVS4 + 4A>T, 5 had one exon 14 allele, and 8 had at least one 322delG allele). Thirty-six of the 50 patients with DC had mutations in known genes: DKC1 18%,, TERC 16%, TERT 8%, and TINF2 30%; the latter includes eight members of the family in which TINF2 was first identified (Savage et al, 2008).
Unk, unknown, either because known genes were not mutated, or no material was available (usually the patient had died).
FA Other: 1 FANCF, 1 FANCG, 2 BRIP1 (FANCJ), 2 with 2 bands of FANCD2 protein, 1 no mutations in known genes, 7 dead with no material, and 5 in progress. DC Other: 14 without mutations in the known genes. DBA Other: 3 RPS24, 1 RPL11, and 45 unknown. SDS Other: 1 with wild type SBDS.
% of the 54 with FA where mutations have been sought, and all those with DC, DBA, and SDS.
Death = vital status, not a first event. BMT is for any reason. BMF, AML, and ST are first events. BMF is severe, defined as bone marrow transplantation for, or death from, aplastic anaemia.
Fig 4.
Survival curves according to genotype within FA and DC. A and D, probability of overall survival. B and E, probability of survival free of cancer. C and F, probability of survival free of severe bone marrow failure. A–C, FA genotypes. Green, FANCA; red, FANCC; blue, BRCA2 (FANCD1); maroon, Other or unknown. D–F, DC genotypes. Green, DKC1; red, TERC; blue, TERT; maroon, TINF2; black, unknown. Numbers in each genotype are provided in Table III.
For DC, the Unknown and DKC1 genotypes had the shortest overall survival (median ages 25 and 30 years) compared with TERT and TINF2 (c.45 years), and TERC (>50 years); these trends were significant (P < 0·05). Cancer developed at an older age in those with TERC and TINF2 mutations (P < 0·01), although the numbers in each genotype were small. Bone marrow failure appeared earliest in those with Unknown or DKC1 mutations, followed by mutated TINF2, TERT, and TERC (P < 0·001).
Discussion
The NCI IBMFS cohort provides the first quantitative and age-specific comparison of survival and risks of severe bone marrow failure and cancer in the major IBMFS. Our data are strongest for FA and DC. In this study, we noted both surprising similarities and differences. In FA, the highest risks were for MDS, and squamous cell cancers of the oesophagus, vulva and head/neck, followed by AML; in DC, the most frequent adverse events – MDS, HNSCC, and AML – overlapped with those for FA. Although FA was unique in displaying an early childhood peak in the hazard rate of severe bone marrow failure, the corresponding cumulative incidences climbed to around 50% by 50 years of age in both FA and DC. Similarly, in both syndromes, the cumulative incidence reached 20–30% for solid tumours (age 50 years), and 10% for AML (age 30–40 years). In contrast, we did not observe AML in either DBA or SDS. In DBA, the O/E ratio for AML was zero (95% CI, 0 to 181). Our 63 DBA patients permitted detection of an O/E ratio >7, suggesting that the rate of AML is significantly lower in DBA than in FA, and also lower than might be inferred from DBA case reports (Vlachos et al, 2008). The lack of AML in our small SDS cohort may be due to insufficient statistical power, because cases have been reported in another SDS cohort (Rosenberg et al, 2006).
The experience of DC patients enrolled in our study is striking in suggesting that DC closely resembles FA in both the rates and types of severe adverse events. While the statistical uncertainties in the FA and DC point estimates were similarly large, due to the comparatively small numbers of patients and events, the qualitative results are statistically significant and clinically important. Our prospective data, together with our synthesis of DC-related cancers reported in literature cases (Alter et al, 2009), strongly suggest that FA and DC have very similar – albeit temporally different – neoplastic risks, perhaps related to abnormal DNA repair via defects in the FA or telomere biology pathways.
Our cohort also provides the first survival data in DC, with a median age of 42 years, which is 10 years older than the median of 33 in FA in our cohort (P = 0·08). In our comparison of the IBMFS syndromes, patients with FA had the shortest overall survival, and earliest marrow failure and cancer, followed in order by DC, DBA and SDS. In FA, the overall survival did not differ by gender, while in DC females (the minority) had a longer survival overall than males (data not shown), This may in part explain the better overall survival of patients with DC compared with those with FA. However, patients with FA developed cancer earlier than those with DC, independent of gender. We also identified specific deleterious genotypes that appear to produce adverse events later in life (FANCA in FA; TERC and TERT in DC). These individuals may also lack characteristic physical findings; hence, they are more likely to reach adulthood, when the first syndrome-related manifestation may be cancer without the risk factors typical of the general population.
A key strength of our multi-syndrome study design is that it enables the first direct comparison of all of the IBMFS in a single cohort, evaluated by the same investigators, specialty consultants, laboratory tests, and outcome analyses. Also, possible syndrome misclassification should be minimal, because all participants were examined by the same team using a standardised protocol, carefully-specified diagnostic criteria and highly sensitive and specific tests.
There are several possible limitations inherent in our design. Biased referral or volunteerism among probands with prevalent cancer is a genuine concern, given our cancer-specific focus. However, the similarity between the NCI FA Cohort and other FA cohorts not based at cancer centres (described below), argues against a ‘cancer’ bias in our series. Families with mild features may be under-represented, which would produce over-estimation of O/E ratios. Left truncation due to survival bias could produce an under-estimation of early risks. Our cohort is more likely to have enrolled adults than those based at children’s hospitals.
Despite these potential limitations, the data in the NCI FA cohort were remarkably consistent with other FA cohorts described by us and others, including the North American Survey (NAS) (Rosenberg et al, 2003) and German Fanconi Anaemia Registry (GEFA) (Rosenberg et al, 2007) (national) and Israeli (national and retrospective) (Tamary et al, 2010) and the IFAR (prospective) (Kutler et al, 2003b). Indeed, the high cause-specific hazards with similar age-associated contours, and cumulative incidence of bone marrow failure, AML, solid tumours, and MDS were remarkably concordant among all FA cohorts, as were the types of neoplasms, and the very large O/E ratios. This consistency strongly suggests that the design of the NCI IBMFS cohort would lead to valid data in the non-FA disorders, since the recruitment methods and follow-up were the same. Of course, this suggestion cannot be verified until cohorts of patients with DC and other IBMFS from sites other than the NCI are analysed; however, we are very encouraged by the similarity of the findings for FA.
Assuming that our results are generalizable, a key observation is that more than 80% of patients in each syndrome survived past 20 years of age. This highlights the importance of considering an IBMFS diagnosis in non-paediatric patients with bone marrow failure, AML, MDS, or syndrome-specific solid tumours, occurring at younger-than-usual ages in the absence of conventional risk factors. Although these syndromes have historically been considered within the purview of paediatricians, the majority of patients are likely to survive into, or first be recognised as having a syndrome, during adulthood. Therefore, it would be valuable for physicians to know that syndrome-specific diagnostic tests exist, understand the limitations of these tests, and refer appropriate patients to geneticists for evaluation, including testing for syndrome-specific germline mutations. Family history may be uninformative in recessive disorders or patients with de novo mutations and thus a negative family history should not deter consideration of an IBMFS, when the likely aetiology is not otherwise clear.
Recognition of a genetic syndrome such as FA or DC has major implications for management, prognosis, and counselling of the affected individuals and their families, as well as in the selection of unaffected (possibly asymptomatic) related donors for stem cell transplantation. Improvements in molecular diagnostics and in syndrome-related survival due to bone marrow transplantation for marrow failure and more effective therapy of childhood leukaemia have created the need for providers of adult health care to incorporate new genomic concepts and information into their clinical practice. The IBMFS are no longer the exclusive domain of paediatricians.
Acknowledgments
We are grateful to all the patients who participated in the National Cancer Institute inherited bone marrow failure syndromes cohort, to the physicians who referred the patients, and to our colleagues in the Clinical Genetics Branch of the NCI, and the subspecialty clinics at the National Institutes of Health for their evaluations of the patients. Study management was provided by Sara Glashofer of Westat (Rockville, MD), through NIH contracts N02-CP-11019, N02-CP-65504, and N02-CP-65501. Observed-to-expected ratios were provided by Jeremy Miller at Information Management Systems (Silver Spring, MD), through NIH contract N02-CP-31003. This work was supported in part by the Intramural Program of the National Institutes of Health and the National Cancer Institute.
Footnotes
Conflict-of-interest
All authors declare no competing financial interests.
Author contribution
All authors contributed to the design of the cohort and the development of the survey instruments. B.P.A., N.G., and S.A.S. personally examined many of participants. L.L. provided nursing support. A.C. and J.A.P. provided genetic counselling support. B.P.A., N.G., S.A.S., and P.S.R. analysed the data. P.S.R. and B.P.A. performed the statistical analyses and wrote the paper; all authors revised and checked the final version of the paper.
References
- Alter BP. Inherited bone marrow failure syndromes. In: Nathan DG, Orkin SH, Look AT, Ginsburg D, editors. Nathan and Oski’s Hematology of Infancy and Childhood. 6. WB Saunders; Philadelphia, PA: 2003. pp. 280–365. [Google Scholar]
- Alter BP. Diagnosis, genetics, and management of inherited bone marrow failure syndromes. Hematology American Society of Hematology Education Program. 2007;2007:29–39. doi: 10.1182/asheducation-2007.1.29. [DOI] [PubMed] [Google Scholar]
- Alter BP, Caruso JP, Drachtman RA, Uchida T, Elghetany MT. Fanconi’s anemia: myelodysplasia as a predictor of outcome. Cancer Genetics and Cytogenetics. 2000;117:125–131. doi: 10.1016/s0165-4608(99)00159-4. [DOI] [PubMed] [Google Scholar]
- Alter BP, Joenje H, Oostra AB, Pals G. Fanconi anemia: adult head and neck cancer and hematopoietic mosaicism. Archives of Otolaryngology – Head and Neck Surgery. 2005;131:635–639. doi: 10.1001/archotol.131.7.635. [DOI] [PubMed] [Google Scholar]
- Alter BP, Baerlocher GM, Savage SA, Chanock SJ, Weksler BB, Willner JP, Peters JA, Giri N, Lansdorp PM. Very short telomere length by flow FISH identifies patients with dyskeratosis congenita. Blood. 2007a;110:1439–1447. doi: 10.1182/blood-2007-02-075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter BP, Rosenberg PS, Brody LC. Clinical and molecular features associated with biallelic mutations in FANCD1/BRCA2. Journal of Medical Genetics. 2007b;44:1–9. doi: 10.1136/jmg.2006.043257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in dyskeratosis congenita. Blood. 2009;113:6549–6557. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameziane N, Errami A, Leveille F, Fontaine C, de Vries Y, van Spaendonk RM, de Winter JP, Pals G, Joenje H. Genetic subtyping of Fanconi anemia by comprehensive mutation screening. Human Mutation. 2008;29:159–166. doi: 10.1002/humu.20625. [DOI] [PubMed] [Google Scholar]
- Auerbach AD, Ghosh R, Pollio PC, Min Z. Diepoxybutane (DEB) test for prenatal and postnatal diagnosis of Fanconi anemia. In: Schroeder TM, Auerbach AD, Obe G, editors. Fanconi Anemia Clinical Cytogenetic and Experimental Aspects. Springer-Verlag; Heidelberg: 1989. pp. 71–82. [Google Scholar]
- Cervenka J, Arthur D, Yasis C. Mitomycin C test for diagnostic differentiation of idiopathic aplastic anemia and Fanconi anemia. Pediatrics. 1981;67:119–127. [PubMed] [Google Scholar]
- Chandra S, Levran O, Jurickova I, Maas C, Kapur R, Schindler D, Henry R, Milton K, Batish SD, Cancelas JA, Hanenberg H, Auerbach AD, Williams DA. A rapid method for retrovirus-mediated identification of complementation groups in Fanconi anemia patients. Molecular Therapy. 2005;12:976–984. doi: 10.1016/j.ymthe.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Glader BE, Backer K. Elevated red cell adenosine deaminase activity: a marker of disordered erythropoiesis in Diamond-Blackfan anaemia and other haematologic diseases. British Journal of Haematology. 1988;68:165–168. doi: 10.1111/j.1365-2141.1988.tb06184.x. [DOI] [PubMed] [Google Scholar]
- Ip WF, Dupuis A, Ellis L, Beharry S, Morrison J, Stormon MO, Corey M, Rommens JM, Durie PR. Serum pancreatic enzymes define the pancreatic phenotype in patients with Shwachman-Diamond syndrome. Journal of Pediatrics. 2002;141:259– 265. doi: 10.1067/mpd.2002.125849. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- Kutler DI, Auerbach AD, Satagopan J, Giampietro PF, Batish SD, Huvos AG, Goberdham A, Shah JP, Singh B. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Archives of Otolaryngology – Head and Neck Surgery. 2003a;129:106–112. doi: 10.1001/archotol.129.1.106. [DOI] [PubMed] [Google Scholar]
- Kutler DI, Singh B, Satagopan J, Batish SD, Berwick M, Giampietro PF, Hanenberg H, Auerbach AD. A 20-year perspective on the International Fanconi Anemia Registry (IFAR) Blood. 2003b;101:1249–1256. doi: 10.1182/blood-2002-07-2170. [DOI] [PubMed] [Google Scholar]
- Moghrabi NN, Johnson MA, Yoshitomi MJ, Zhu X, Al-Dhalimy MJ, Olson SB, Grompe M, Richards CS. Validation of Fanconi anemia complementation Group A assignment using molecular analysis. Genetics in Medicine. 2009;11:183–192. doi: 10.1097/GIM.0b013e318193ba67. [DOI] [PubMed] [Google Scholar]
- Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, Mariotto A, Miller BA, Feuer EJ, Altekruse SF, Lewis DR, Clegg L, Eisner MP, Reid A, Reichman M, Edwards BK. SEER Cancer Statistics Review, 1975–2005. 2008 http://seer.cancer.gov/csr/1975_2005/
- Rosenberg PS, Greene MH, Alter BP. Cancer incidence in persons with Fanconi anemia. Blood. 2003;101:822–826. doi: 10.1182/blood-2002-05-1498. [DOI] [PubMed] [Google Scholar]
- Rosenberg PS, Alter BP, Bolyard AA, Bonilla MA, Boxer LA, Cham B, Fier C, Freedman M, Kannourakis G, Kinsey S, Schwinzer B, Zeidler C, Welte K, Dale DC. The incidence of leukemia and mortality from sepsis in patients with severe congenital neutropenia receiving long-term G-CSF therapy. Blood. 2006;107:4628–4635. doi: 10.1182/blood-2005-11-4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg PS, Alter BP, Ebell W. Cancer Risks in Fanconi Anemia: experience of the German Fanconi Anemia (GEFA) Registry. Haematologica. 2007;93:511–517. doi: 10.3324/haematol.12234. [DOI] [PubMed] [Google Scholar]
- Savage SA, Alter BP. Dyskeratosis congenita. Hematology/Oncology Clinics of North America. 2009;23:215–231. doi: 10.1016/j.hoc.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage SA, Giri N, Baerlocher GM, Orr N, Lansdorp PM, Alter BP. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. American Journal of Human Genetics. 2008;82:501–509. doi: 10.1016/j.ajhg.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamary H, Nishri D, Yacobovich J, Zilber R, Aviner S, Stepensky P, Vilk-Ravel S, Bitan M, Kaplinsky C, Ben Barak A, Kapelusnik J, Koren A, Levin C, Yaniv I, Rosenberg PS, Alter BP. Frequency and natural history of inherited bone marrow failure syndromes: the Israeli inherited bone marrow failure registry. Blood. 2009;112:382–383. doi: 10.3324/haematol.2009.018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamary H, Nishri D, Yacobovich J, Zilber R, Dgany O, Krasnov T, Aviner S, Stepensky P, Vilk-Ravel S, Bitan M, Kaplinsky C, Ben Barak A, Ronit Elhasid R, Kapelusnik J, Koren A, Levin C, Atias D, Laor R, Yaniv I, Rosenberg PS, Alter BP. Frequency and natural history of inherited bone marrow failure syndromes: the Israeli inherited bone marrow failure registry. Haematologica. 2010 doi: 10.3324/haematol.2009.018119. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachos A, Ball S, Dahl N, Alter BP, Sheth S, Ramenghi U, Meerpohl J, Karlsson S, Liu JM, Leblanc T, Paley C, Kang EM, Leder EJ, Atsidaftos E, Shimamura A, Bessler M, Glader B, Lipton JM. Diagnosing and treating Diamond Blackfan anaemia: results of an international clinical consensus conference. British Journal of Haematology. 2008;142:859–876. doi: 10.1111/j.1365-2141.2008.07269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulliamy TJ, Marrone A, Knight SW, Walne A, Mason PJ, Dokal I. Mutations in dyskeratosis congenita: their impact on telomere length and the diversity of clinical presentation. Blood. 2006;107:2680–2685. doi: 10.1182/blood-2005-07-2622. [DOI] [PubMed] [Google Scholar]