Abstract
Renal cell cancer (RCC) has an increasing incidence internationally and is a disease for which there have been limited therapeutic options until recently. The last decade has seen a vastly improved understanding of the biological and clinical factors that predict the outcome of this disease. We now understand some of the different molecular underpinnings of renal clear cell carcinoma by mutation or silencing of the von Hippel Lindau (VHL) gene and subsequent deregulated proliferation and angiogenesis. Survival in advanced disease is predicted by factors (performance status, anemia, hypercalcemia, and serum lactate dehydrogenase, time from diagnosis to recurrence) incorporated into the Memorial Sloan Kettering Cancer Center (MSKCC) criteria (also referred to as ‘Motzer’ criteria). These criteria allow classification of patients with RCC into good, intermediate and poor risk categories with median overall survivals of 22 months, 12 months and 5.4 months, respectively. Predicated upon these advances, six new targeted drugs (sorafenib, sunitinib, temsirolimus, everolimus, bevacizumab and pazopanib) have been tested in well-designed phase III trials, selected or stratified for MSKCC risk criteria, with positive results. All of these new drugs act at least in part through vascular endothelial growth factor (VEGF) mediated pathways with other potential therapeutic impact on platelet-derived growth factor (PDGF), raf kinase and mammalian target of rapamycin (mTOR) pathways. Importantly, data from each of these trials show a consistent doubling of progression-free survival (PFS) over prior standard of care treatments. In addition, sorafenib, sunitinib and temsirolimus, have demonstrated significant overall survival (OS) benefits as well; further follow-up is required to determine whether the disease control exhibited by everolimus and pazopanib will translate into a survival advantage. These drugs are generally well tolerated, as demonstrated by quality-of-life improvement in clinical trials, and result in clinical benefit for in excess of 70% of patients treated. They have challenged the traditional outcomes of clinical trial design by achieving their benefits with relatively few radiographic responses, but high rates of disease stability. The unique side-effect profile coupled with the chronicity of therapy requires increased vigilance to maximize exposure to the drugs while maintaining quality of life and minimizing toxicity. This review focuses on the background, clinical development and practical use of these new drugs in RCC.
Keywords: renal cell carcinoma, bevacizumab, everolimus, sorafenib, sunitinib, temsirolimus
Introduction
The incidence of renal cell cancer (RCC) is rising [Decastro and Mckiernan, 2008; Mathew et al. 2002; Chow et al. 1999]. The precise reason for this is unclear but may relate to the higher prevalence of risk factors such as obesity, hypertension and tobacco abuse and the broader use of imaging technology in many populations. While still an uncommon disease, RCC presents a unique paradigm for cancer therapeutics because of relatively limited genetic heterogeneity compared to other cancers. This relative homogeneity has allowed the delineation of different pathological subgroups of RCC: clear versus non-clear cell. In addition, it has meant molecular characterization of tumors that will eventually allow integration of tumor genetics into clinical nomograms that predict response to specific agents and ideally survival. Detailed understanding of the RCC clinical-pathological-molecular phenotype has already guided therapeutic development, and has the potential to foster individualized therapy algorithms. From a practical cancer therapy standpoint, six novel targeted agents in the last 3 years have been found to double progression-free survival (PFS), with three of these drugs demonstrating significant overall survival (OS) benefit. In doing so RCC has served as a vehicle for five new oncology drugs. Although these successes may have begun the transformation of RCC into a chronic condition, cure for patients with advanced RCC is rare, and remains the goal of ongoing investigations.
The clinical molecular biology of renal cell cancer
From a molecular perspective, cancer is characterized by a number of hallmark molecular changes [Hanahan and Weinberg, 2000]. In common with many other malignancies, RCC has an anomalous or aberrant function on a number of key cellular pathways and paracrine/autocrine regulatory loops, including: attenuated immune surveillance, angiogenesis, signal transduction, cell-cycle regulation, apoptosis, extracellular matrix modulation, and regulation of transcription. Factors and clinical implications for these alterations are summarized in Table 1. Of all these, clear cell RCC is uniquely dependent upon two factors: dyskinetic angiogenesis and immune dysfunction.
Table 1.
Summary of molecular aberrancy in renal cell cancer (RCC).
| Specific factor | Effect | Outcome or clinical correlated | References | |
|---|---|---|---|---|
| Angiogenesis | VHL gene and protein | Dysregulated hypoxia inducible factor production | Increased VEGF and PDGFß with resultant classic angiogenic RCC phenotype | [Kaelin, 2009; George and Kaelin, 2003] |
| VHL mutation | Loss of VHL protein production | Not prognostic but associated with disease stage may predict response to VEGF TKI therapy | [Choueiri et al. 2008; Brauch et al. 2000] | |
| HIF 1α and 2α | Increased levels lead to increased VEGF, EGF and PDGFß; HIF2 appears responsible for inducing classic renal clear cell phenotype | High HIF levels in tumor tissues are required for response to sunitinib | [Kaelin, 2009; Patel et al. 2008] | |
| VEGF | Increased expression of VEGF and its receptor. | Increased stage | [Slaton et al. 2001; Dosquet et al. 1997; Takahashi et al. 1994; Brown et al. 1993] | |
| Increased VEGF | Poorer prognosis in some series | [Jacobsen et al. 2000]. | ||
| Attenuated immune surveillance | T-cell immunity | Diminished activation, proliferation, and cytotoxic effect of T cells | Suppression of the hosts response to tumor; utilized in immunotherapeutic approaches | [Li and Verma, 2002; Finke et al. 2001; Kim et al. 1999; Uzzo et al. 1999a, 1999b; Grumont et al. 1998; Mcdonald et al. 1997] |
| NF-kappaB | Broad immune dysfunction, interrupting cytokine-modulated coordination of B-lymphocytes, natural killer cells and neutrophils | Currently of questionable clinical or therapeutic significance but NF-kappaB remains a target of interest for drug development | [Li and Verma, 2002; Grumont et al. 1998; Mcdonald et al. 1997] | |
| Altered regulation of transcription | Several including PTEN, akt, mTOR and S6 kinase | Aberration of the mTOR/akt/PTEN pathway | Increased cellular proliferation through dysregulated action of cyclins and c-myc as well as through hypoxia inducible factors important in the regulation of angiogenesis | [Hara et al. 2005; Atkins et al. 2004; Majumder et al. 2004; Brugarolas et al. 2003; Horiguchi et al. 2003] |
| Expression of a variety of pathway components as predictive of survival in RCC | [Pantuck et al. 2007] | |||
| Signal transduction | EGF and EGF receptor-1 | Over-expression | Higher tumor grade and inferior survival | [Hofmockel et al. 1997; Stumm et al. 1996; Uhlman et al. 1995; Gomella et al. 1990; Sargent et al. 1989] |
| Cell cycle regulation | Cyclin A over expression, p27Kip1 loss and increased pRB expression | Adversely prognostic | [Migita et al. 2002; Haitel et al. 2001; Aaltomaa et al. 1999; Hedberg et al. 1999] | |
| Apoptosis | p53 | Frequent mutation or aberration | Inferior survival | [Sionov and Haupt, 1999; Oda et al. 1995; Reiter et al. 1993] |
| Bcl-2 | Frequent over expressed proto-oncogene | Inferior survival | [Gobé et al. 2002; Uchida et al. 2002; Haitel et al. 2001; Huang et al. 1999; Uhlman et al. 1994] | |
| Extracellular matrix modulation | MMPs and their tissue inhibitors | Over expression of MMPs and/or TIMPs | Common in non-clear cell cancer and predict inferior survival | [Kallakury et al. 2001; Slaton et al. 2001] |
EGF, epidermal growth factor; HIF, hypoxia inducible factors; MMPs, matrix metalloproteinase; mTOR, mammalian target of rapamycin; PDGF, platelet-derived growth factor; TIMPs, tissue inhibitor of metalloproteinases; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor; VHL, Von Hippel Lindau.
The angiogenic phenotype is crucial in both tumor progression and metastasis [Fidler and Ellis, 1994]. The key factor involved in signaling for angiogenesis in nearly all human tumors is vascular endothelial growth factor (VEGF) [Dvorak et al. 1995; Senger et al. 1993] (Figure 1). VEGF and its receptors are critical for healthy blood vessel formation [Carmeliet et al. 1996; Ferrara et al. 1996; Fong et al. 1995; Shalaby et al. 1995]; increased expression of VEGF receptors on endothelial cells within the tumor vasculature suggests it is also important in tumor angiogenesis [Chan et al. 1998; Leung et al. 1997; Cheng et al. 1996]. VEGF is crucial for the development of tumor masses exceeding a diameter of 3–5 mm [Kim et al. 1993].
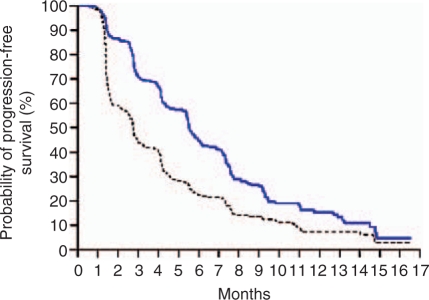
Figure 1.
Sorafenib (blue line) versus placebo (black line) TARGET study progression-free survival curves for second-line good- and intermediate-risk renal cell cancer. After Escudier et al. [Escudier et al. 2007]. With permission, New England Medical Society.
As noted above, increased expression of VEGF and its receptors is common and prognostic in RCC [Slaton et al. 2001; Dosquet et al. 1997; Takahashi et al. 1994; Brown et al. 1993]; the mechanism of VEGF dysregulation is linked to the Von-Hippel Lindau (VHL) gene. There is a high frequency of VHL gene abnormalities in both familial and sporadic cases of RCC [Maher and Kaelin, 1997; Prowse et al. 1997; Gnarra et al. 1996a]. In sporadic RCC of clear cell type, mutations of VHL are found in 75% of the tumors [Na et al. 2003; Vogelzang and Stadler, 1998]. Another 10–25% of patients have hypermethylation of the VHL promoter region, which leads to suppressed mRNA and protein production, and loss of VHL activity. VHL protein (VHLp) acts as a component of ubiquitin E3 ligase and those VHL mutations commonly seen in RCC result in loss of this ligase activity [Hansen et al. 2002; Hon et al. 2002; Clifford et al. 2001; Iwai et al. 1999; Duan et al. 1995]. When tissue oxygen levels are normal, this ligase activity and other actions of wild-type VHLp down-regulate VEGF expression through proteolytic, post-translational control of hypoxia-inducible factors (HIFs) 1 and 2 [Clifford and maher, 2001; Gunningham et al. 2001; Krieg et al. 2000; Gnarra et al. 1996b; Iliopoulos et al. 1996]. In conditions of hypoxia or VHLp dysfunction, HIFs accumulate to concentrations that increase the expression of various molecular growth factors, including VEGF and platelet-derived growth factor (PDGF) [Mack et al. 2003; Maxwell et al. 1999; Thrash-Bingham and Tartof, 1999; Iliopoulos et al. 1996]. These growth factors bind to specific tyrosine kinase receptors (TKRs) on the surface of endothelial cells and vascular pericytes respectively, resulting in cell migration, proliferation, survival, and tumor angiogenesis [Wykoff et al. 2004]. HIFs may, under certain conditions, also regulate epidermal growth factor (EGF), which along with VEGF stimulates proliferation of epithelial renal cancer cells [Gunaratnam et al. 2003]. To summarize, the vast majority of patients with clear cell RCC have tumors in which VHL is not functional, leading to increased angiogenesis.
Tumor angiogenesis is also stimulated through the PI3K (phosphatidylinositol-3 kinase) – AKT – mammalian target of rapamycin (mTOR) pathway and agents inhibiting the activity of this pathway may be expected to have activity in RCC and other cancers [Del Bufalo et al. 2006; Majumder et al. 2004; Brugarolas et al. 2003; Kenerson et al. 2002]. mTOR regulates a number of molecular and cellular functions including proliferation through c-myc and cyclin dependent kinases and angiogenesis through the regulation of HIF [Del Bufalo et al. 2006; Kenerson et al. 2002; Blancher et al. 2001]. This pathway is up-regulated in a significant proportion of patients with poor-risk RCC according to Memorial Sloan Kettering Cancer Center (MSKCC) criteria, making its components attractive targets in this group of patients [Hara et al. 2005; Atkins et al. 2004; Horiguchi et al. 2003].
Immune dysfunction
Deficits in tumor-directed T-cell immunity as well as cytokine-mediated effects on dendritic cells are characteristic of RCC [Li and Verma, 2002; Finke et al. 2001; Uzzo et al. 1999a, b; Kim et al. 1999; Grumont et al. 1998; Mcdonald et al. 1997; Li et al. 1994]. Factors which have been implicated in this immune dysfunction include nuclear factor kappa-β (NF-κB), which has a central role in coordinating the expression of a wide variety of genes that control immune responses in both T and B lymphocytes, natural killer cells and neutrophils [Li and Verma, 2002; Grumont et al. 1998; Mcdonald et al. 1997]. Agents that are immunostimulatory, such as interleukin-2 and interferon-α, have documented utility in RCC, although the exact mechanisms by which they exert their actions have been difficult to elucidate. One possible mechanism involves VEGF. Elevated VEGF-levels in tumor-bearing patients not only inhibit dendritic cell (DC) development from hematopoietic precursors but also decrease DC migration into tumors [Ohm et al. 2003; Gabrilovich et al. 1998; Oyama et al. 1998]. In mouse tumor models, the infusion of anti-VEGF antibodies results in a dramatic increase in the effectiveness of immunotherapy employing antigen-pulsed DCs [Li et al. 2002; Niethammer et al. 2002; Gabrilovich et al. 1999]. This suggests that therapies impacting VEGF, and perhaps other parts of the VEGF pathway, may help to reverse the immune dysfunction characteristic of RCC. This hypothesis remains to be tested in the clinical setting.
Recent analysis demonstrates significant clinical, pathological and molecular characteristics for non-clear RCC compared to clear cell RCC [Tamaskar et al. 2007; David et al. 2006; Ronnen et al. 2006; Upton et al. 2005; Dulaimi et al. 2004; Amin et al. 2002; Krishnan and Truong, 2002; Motzer et al. 2002]. The distinguishing molecular characteristics of non-clear RCC have recently been reviewed [Furge et al. 2007].
Advances in understanding of disease outcome based on clinical factors
For many years clinicians have recognized the heterogeneity of survival outcome in RCC. Recently revised pathological classification separates RCC into clear cell and non-clear cell types, the latter consisting of patients with papillary, chromophobe, oncocytoma and collecting duct tumors [Amin et al. 2002]. Each of these tumor types has a characteristic molecular profile [Takahashi et al. 2003] (see reviews) and a different clinical response to therapy [Ronnen et al. 2006]. Most particularly, it is important to note that only clear cell cancers respond to immunotherapy with cytokines while non-clear cases do not [Ronnen et al. 2006].
Major recent advances have been made in predicting outcomes for patients with advanced RCC, led by the Groupe Francais d’Immunotherapie, University of California Los Angles (UCLA), University of Padova and MSKCC [Bensalah et al. 2008; Escudier et al. 2007a; Karakiewicz et al. 2007; Patard et al. 2004a,b; Negrier et al. 2002; Zisman et al. 2001; Motzer et al. 2000, 1999]. Motzer and colleagues examined the survival rates for patients treated with interferon-α in a series of trials at MSKCC, and found that performance status, anemia, hypocalcaemia, elevated serum lactate dehydrogenase and duration from initial diagnosis to treatment for advanced or recurrent disease were predictive. In some models, the patients who had not undergone nephrectomy had a poorer outcome. Patients with none of these factors had a median OS of 22 months (good risk – around 20% of cases), 1 or 2 of these factors, a median survival of 11.9 months (intermediate risk – 65% of cases) and 3 or more of these factors 5.4 months (poor risk – 15% of cases) [Motzer et al. 2004].
The MSK or ‘Motzer classification system for advanced RCC has now been validated in a series of scenarios with excellent predictive value and subsequent trials have used this classification system to select and stratify patients for treatment. The first evidence of the importance of this approach came in the Groupe Francais d’Immunotherapie PERCY Quattro trial where intermediate risk patients were selected for supportive care with medroxyprogesterone acetate (MPA) or three combinations of low-dose chronic cytokine therapy [Negrier et al. 2007]. The study demonstrated no survival benefit to any of the cytokine regimens over supportive care centered on MPA. Recent reports suggest that the factors comprising the MSK score predict outcome for untreated patients and will likely be incorporated into nomograms to predict survival in patients treated with the newer targeted therapies [Motzer et al. 2008a; Mekhail et al. 2005], although alternative models are also under development. For instance, a Cox proportional hazards model using patients from nine trials of targeted therapy at the Cleveland Clinic identified a set of five different predictive variables: time from diagnosis to treatment, baseline neutrophil and platelet counts, ECOG performance status, and corrected serum calcium [Choueiri et al. 2007].
Before targeted agents – a brief history of renal cell cancer treatment
Early trials in advanced RCC focused on cytotoxic chemotherapy and hormonal treatments [Yagoda et al. 1995]. Cytotoxic therapy was rarely beneficial and does not appear to impact positively when added to cytokines or other agents [Ryan et al. 2002; Dutcher et al. 2000; Yagoda et al. 1995]. On the other hand, hormonal therapy with progestins (MPA or megestrol acetate) proved useful for ameliorating the symptoms of cancer-cachexia syndrome and, in the absence of specific anticancer effects in RCC, still has an important place in supportive care and palliation [Turner et al. 2007; Simons et al. 1996].
In the early 1980s, it became clear that patients with RCC had rare but major responses to immunomodulatory therapy with the human cytokines, interleukin-2 and interferon-α [Dutcher et al. 2001; Negrier et al. 2000a]. Subsequent trials demonstrated the ability of high-dose interleukin-2 to produce complete remissions in up to 15% of patients, with long-term durability in 80% of those who attained complete remission [Rosenberg et al. 1994]. Based on a series of phase II studies, the United States Federal Drug Agency (FDA) approved high-dose interleukin-2 for RCC [Rosenberg et al., 1994]. However, the use of high-dose interleukin-2 comes at the cost of significant acute toxicity, which is entirely reversible, but nevertheless requires in-patient admission with access to intensive care facilities. Only patients with excellent performance status and no cardiorespiratory comorbities are suitable candidates because of the toxicity profile [Gitlitz et al. 2001; Fisher et al. 2000, 1988; Textor et al. 1987]. Regardless, given that high-dose interleukin-2 remains the only curative treatment for RCC, it remains an important treatment consideration, and continues to be offered in specialized centers to carefully selected patients. Work is underway in an attempt to better delineate patients likely to benefit from high-dose interleukin-2. Preliminary work suggested that overexpression of carbonic anhydrase IX in renal cancer tumor tissue increased the odds ratio of response to HD-IL2 by a factor of approximately three [Atkins et al. 2005]. The high-dose interleukin-2 ‘SELECT’ trial for metastatic RCC is ongoing within the Cytokine Working Group with the aim of prospectively validating carbonic anhydrase IX expression and search for other markers of response with the potential to exclude patients who are very unlikely to benefit from this therapy [Mcdermott et al. 2009].
Given the limited applicability of high-dose interleukin-2, outpatient subcutaneous cytokine regimens were developed using either interleukin-2, interferon-α or a combination of both [Figlin et al. 1992; Rosenberg et al. 1989; Fisher et al. 1988]. A series of trials were undertaken with these combinations. Importantly, cytokines were superior to therapy with the cytotoxic agents such as vinblastine or paclitaxel [Yagoda et al. 1995; Walpole et al. 1993]. Other studies suggested delay in disease progression for combined low-dose therapy compared to single agent treatment, although this never translated to OS benefit [Negrier et al. 2000b, 1998]. The addition of low-dose interferon-α to interleukin-2 was not advantageous [Atkins et al. 1993] nor was infusion of tumor-infiltrating lymphocytes extracted from the primary renal cancer at time of nephrectomy [Figlin et al. 1999].
A meta-analysis and systemic review of immunotherapy in RCC was undertaken for the Cochrane database in 2000 and updated in 2005 [Coppin et al. 2005]. This review concluded that immunotherapies resulted in a partial or complete response rate of 12.9% compared to 2.5% in nonimmunotherapy controls and 4.3% in placebo controls. The median OS benefit attributed to immunotherapy was 3.8 months compared to controls. Moreover, there was limited correlation of response with survival. These conclusions emphasized the limited effect of interferon-α over best supportive care, (incorporating MPA) seen in the MRC trial where the difference in median OS was 2.8 months [Collaborators, 1999]. This review also noted that nephrectomy in metastatic RCC patients given interferon-α resulted in a median 4.8 month improvement in OS [Flanigan et al. 2001; Mickisch et al. 2001].
Subsequently, two important randomized trials of immunotherapy were undertaken. A comparison of high-dose interleukin-2 and low-dose subcutaneous interferon-α and interleukin-2 in combination demonstrated superior response rates for high-dose interleukin-2 and poorer quality-of-life for patients given the low-dose combination regimen [Mcdermott et al. 2005]. Most recently with improved clinical categorization of patients into risk groups, the Groupe Francais undertook the PERCY (Programme Etude Rein Cytokines) Quattro trial comparing MPA, interferon-α alone, interleukin-2 alone or a combination of interleukin-2 and interferon-α in patients with intermediate-risk clear cell carcinoma [Negrier et al. 2007]. The OS outcomes for all arms were not different to supportive care offered as the control arm with MPA. In addition, in quality-of-life evaluation, treatment with either cytokine resulted in further decrement compared to MPA and the combination arm was even worse. This suggests that when low-dose cytokines are given to this selected population of RCC patients with intermediate-risk disease (which comprises 60–70% of patients seen) there is no effect on survival, despite significant morbidity. On this basis low-dose subcutaneous regimens are now limited to combination with targeted agents (see below) or use solely in patients with good-risk disease who are not suitable for high-dose interleukin-2, who have declined or are unsuitable for targeted therapy.
Sorafenib
Sorafenib (BAY 43-9006, Nexavar®), a dual-specificity multikinase inhibitor, inhibits both tyrosine and serine/threonine-specific kinases, and has been implicated as an inhibitor of kinase targets involved at different levels of the cascade [Wilhelm et al. 2004]. Biochemical assays have demonstrated that sorafenib inhibits the autophosphorylation of VEGFR-2 and -3 and PDGFRβ, c-KIT and FMS-like tyrosine kinase-3 (FLT-3) and, further, can inhibit the kinase activity of the serine/threonine kinases, c-Raf, BRaf, and p38MAPK (see Figure 1)[Wilhelm et al. 2004]. This repertoire suggests sorafenib is a potential inhibitor of tumorigenic signaling in both endothelial cells and tumor cells.
Two phase I studies defined sorafenib 400 mg, taken orally twice daily on a continuous basis, as a tolerable dose with a high rate of disease stability [Awada et al. 2005; Strumberg et al. 2005], while a third trial suggested that higher doses of 600 mg or even 800 mg twice daily were tolerable in many patients [Clark et al. 2005]. Initially felt to act predominantly through the raf kinase pathway, it soon became evident that the major effect for sorafenib was through VEGF receptor inhibition. A phase II, placebo-controlled trial of sorafenib, 400 mg twice daily, using a randomized discontinuation of treatment (RDT) design was undertaken [Ratain et al. 2004; Rosner et al. 2002]. A total of 202 metastatic RCC patients were treated for 12 weeks with sorafenib and 71% demonstrated a response or had stabilized disease. Patients with a 25% reduction in measurable disease were deemed responders and continued on sorafenib while those whose disease had progressed with a 25% or greater increase in disease ceased treatment. Patients with stable disease were offered randomization and 65 consented to this. Patients continuing treatment with sorafenib had four times longer PFS compared to patients that were randomized to stop the drug (24 versus 6 weeks, p = 0.0087)[Ratain et al. 2006].
After the results of the RDT trial became available, the decision was made to design TARGET (Therapeutic Approaches in Renal Cancer Global Evaluation Trial), a large randomized trial of sorafenib given at a dose of 400 mg twice daily orally on a continuous basis compared to placebo [Escudier et al. 2007b]. The study population was selected to have clear cell histology, failed at least one therapy within 8 months of enrolment, good performance status and measurable disease. The study enrolled 905 patients with low- or intermediate-MSKCC risk advanced disease who had received prior systemic therapy; patients with brain metastases or poor risk stratification were excluded. The major endpoints were OS and PFS. Although only one patient had a complete response and 10% qualified as partial response according to investigator reported RECIST criteria (Response Evaluation Criteria in Solid Tumors), about 75% had some shrinkage in their tumors compared to 25% of those in the placebo group. This relatively subtle change in tumor growth kinetics yielded a dramatic difference in PFS, which was doubled from 12 weeks in the placebo group to 24 weeks in the sorafenib group (Hazard ratio (HR) 0.44, 95% confidence interval (CI) 0.35–0.55, p < 0.00001, see Figure 1, Table 2).
Table 2.
Randomized phase III trial of sorafenib compared to placebo for renal cell cancer patients progressing on prior, predominantly cytokine, therapy [Escudier et al. 2009a, 2007b].
| Treatment | PFS | p value | OS | p value |
|---|---|---|---|---|
| Sorafenib | 5.5 | <0.0001 | 17.8 | 0.029 |
| Placebo | 2.8 | 14.3 |
OS, overall survival; PFS, progression-free survival.
Given the clear advantage of sorafenib at this first planned PFS analysis, it was decided on ethical grounds to allow patients in the placebo arm to crossover to active treatment. This crossover impacted 215 patients in the placebo arm and necessitated a change in the trial design to evaluate OS: crossover patients were censored as ‘not relapsed’ at the point of crossover but with no further follow up taken into account. In this analysis, OS in the placebo group was 14.3 months, while median survival in the sorafenib group was 17.8 months (HR 0.78, 95% CIs 0.62–0.97, p = 0.0287) [Escudier et al. 2009a; Bukowski et al. 2007a]. The median OS benefit in favour of sorafenib was approximately 3.5 months; an important finding for this drug with implications for the potential benefits of other angiogenesis inhibiting agents in RCC.
Subsequently, sorafenib was evaluated in untreated patients with RCC in a two-arm first-line randomized phase II trial with interferon-α as the comparator arm [Escudier et al. 2009]. While conclusions from such studies can be difficult to interpret, there was no difference in median PFS between the arms, a result that stands in contrast to the phase III trial comparing sunitinib with interferon-α in the same setting [Motzer et al. 2007]. Sorafenib did have a superior response rate and quality-of-life profile compared to interferon-α [Escudier et al. 2009b].
Overall, sorafenib has a favourable safety profile. In the TARGETs trial the incidence of any grade 3–4 adverse event was 30% in sorafenib-treated patients and 22% in the placebo group [Escudier et al. 2007b]. Common adverse events recorded for the sorafenib group were hypertension, a known effect of inhibition of VEGFR signalling, hand-foot syndrome, fatigue, and diarrhea. Despite these side effects, sorafenib was associated with an improved quality of life compared to placebo in this trial [Bukowski et al. 2007c].
The range of sorafenib side-effects in clinical practice outside of a trial setting is similar to that seen in the clinical trials, although the incidence may vary. For instance, more patients may develop hypertension after nephrectomy, due to limited residual renal function. The mechanism of blood pressure elevation is not clear, but one study demonstrated an increase in systolic blood pressure of 10 mm Hg in 75% of cases, with 60% of patients experiencing an increase >20 mm Hg [Veronese et al. 2006]. Nevertheless, this can be managed with careful home blood pressure monitoring and the adjustment of ongoing antihypertensive agents or the addition of new ones; to date there is no preferred class of antihypertensive drug for this indication. The most common toxicity with sorafenib is hand-foot skin syndrome and rash, which can occur with varying severity in 60–70% of patients. Even in severe cases, however, most patients can continue treatment after temporary cessation of the drug has facilitated resolution of the symptoms. Lanolin-based emollient creams applied twice daily can help control skin reactions; urease-containing emollients can also be helpful. Diarrhea is a third common adverse event. There are two broad types: one that is an increased frequency, which can be managed with anti-diarrheal agents, and the other which is more sporadic, unpredictable and explosive, which patients need to be warned about. Interestingly, the presence of skin toxicity or diarrhea correlates with increased time-to-progression compared to patients that do not experience these side effects [Strumberg et al. 2006].
Sorafenib has also recently been approved for hepatocellular carcinoma after the completion of an international phase III trial demonstrating superior OS compared with placebo [Llovet et al. 2008].
Sunitinib
Sunitinib (SU11248, Sutent®) is a multiple kinase inhibitor with activity against VEGFR-1, VEGFR-2, VEGFR-3, PDGFR-α, PDGFR-ß, c-kit, and Flt-3 [Rini and Small, 2005]. In vitro data indicated that sunitinib could block VEGF-induced endothelial proliferation as well as PDGF-induced proliferation of murine fibroblasts [Mendel et al. 2003]. Mice injected with human melanoma and glioma cell lines demonstrated in vivo that sunitinib treatment could block VEGFR- and PDGFR-mediated signaling cascades [Sun et al. 2003]. More importantly, treatment with sunitinib inhibited growth of xenografts in mice, and eradicated established tumors. After treatment, immunohistochemical staining for CD31 in xenograft sections revealed that microvessel density was reduced [Sun et al. 2003]. Sunitinib’s activity in suppressing angiogenesis and cell proliferation in the pre-clinical setting was significant enough to warrant further investigation [Osusky et al. 2004; Abrams et al. 2003].
A phase I study established a tolerable dose for sunitinib of 50 mg daily orally for 28 days followed by a 14-day break, where attempts at continuous dosing proved too toxic [Faivre et al. 2006]. Analysis of two single arm phase II trials of sunitinib as second-line therapy in patients who had prior cytokine failure revealed that it had substantial anti-tumor activity in this setting [Motzer et al. 2006a, 2006b]. A total of 169 patients were treated with a regimen of 50 mg of daily oral therapy given for 4 weeks, repeated in 6-week cycles. A combined objective response rate of approximately 40% and disease stabilization in 25% was reported, with an associated median PFS of 8.8 months and median OS of 24 months [Motzer et al. 2007a, 2006a, 2006b].
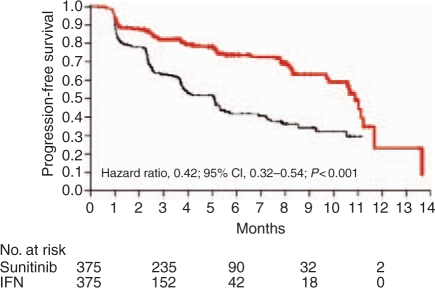
To evaluate the efficacy of sunitinib in treatment naïve patients with metastatic RCC, an international phase III trial was conducted with thrice weekly interferon-α as the comparator arm [Motzer et al. 2007b]. Seven hundred and fifty patients with metastatic clear cell RCC were randomized in a 1:1 ratio to interferon-α or sunitinib 50 mg daily, 4 weeks on then 2 weeks off. While no patients in the trial had a complete response, more patients receiving sunitinib achieved partial response (31%) than those in the interferon-α arm (6%). The rates of stable disease in the treatment groups were similar; 48 and 49% for sunitinib and interferon-α respectively, but fewer patients receiving targeted therapy had progressive disease. Similar to the results seen with sorafenib in the second-line setting, there was a doubling of PFS in the sunitinib group (11 months) compared to the control group (5.1 months) (HR 0.538, 95% CIs 0.439–0.658,p < 0.00001) (Figure 2, Table 3). OS data show a median survival of 26 months in the sunitinib arm compared to 21 in the interferon-α arm [Motzer et al. 2009]. The difference has marginal statistical significance (p = 0.051, HR = 0.821; 95% CI, 0.673–1.001); however, given the large number of patients who receive active drugs such as sorafenib after failing interferon-α, one can hypothesize that the survival difference in the trial was diluted by this effect. Post-hoc analyses excluding patients who received any targeted therapy after study therapy with either sunitinib or interferon-α, did demonstrate substantive differences in OS that many feel reflect a true advantage for sunitinib.
Figure 2.
Sunitinib (red line) versus interferon-α (IFN, black line) study progression-free survival curves for first-line renal cell cancer [Motzer et al. 2007]. With permission, New England Medical Society. CI, confidence interval.
Table 3.
Randomized phase III trial of sunitinib compared to interferon-α in advanced renal cell cancer patients not having prior systemic therapy [Motzer et al. 2009, 2007b].
| Treatment | PFS | p value | OS | p value |
|---|---|---|---|---|
| Sunitinib | 11 | <0.001 | 26.4 | 0.051 |
| Interferon-α | 5 | 21.8 |
OS, overall survival; PFS, progression-free survival.
Treatment with sunitinib was well tolerated over a long period of time in some patients, with fewer than 10% of patients experiencing grade 3–4 toxicity, though 50% required a dose reduction. Grade 2–3 treatment-related adverse events noted during sunitinib trials included fatigue, diarrhea, stomatitis, nausea and hypertension. Hand-foot syndrome was far less commonly seen than with sorafenib [Robert et al. 2005]. Laboratory abnormalities described for sunitinib treated patients were neutropenia, anemia, and thrombocytopenia. There was a 13% incidence of left ventricular dysfunction in the sunitinib arm compared to 3% with interferon-α [Motzer et al. 2007b] and patients treated with sunitinib should be monitoring with echocardiography or nuclear-labeled LVEF assessment at baseline and periodically during treatment [Khakoo et al. 2008; Telli et al. 2008; Chu et al. 2007]. Thyroid function anomalies are also common with sunitinib therapy, with hypothyroidism being relatively common and transient hyperthyroidism reported [Rini et al. 2007]. Routine monitoring of serum thyroid stimulating hormone levels is now undertaken in most centers. Another idiosyncratic side effect of sunitinib is hair depigmentation, the nature of which varies with patient race [Faivre et al. 2006].
Sunitinib has also recently been approved for the treatment of gastrointestinal stromal tumors that are refractory to first line therapy with imatinib [Demetri et al. 2006].
Bevacizumab
Bevacizumab (Avastin®), a recombinant human monoclonal antibody against VEGF isomer A (see Figure 1), was the first targeted agent to show efficacy in RCC. In a phase II study, high-dose bevacizumab (10 mg/kg IV every 2 weeks) doubled the time-to-progression compared with placebo (4.8 months versus 2.5 months, p < 0.001) in patients with metastatic RCC who had progressed on high-dose interleukin-2 [Yang et al. 2003]. These data were promising, but to prove the benefit a very large phase III study was required, which was not initially feasible. However, subsequent trials incorporating bevacizumab in combination with interferon-α have now been completed [Rini et al. 2008; Escudier et al. 2007c]. These trials, the AVOREN (Roche B017705) trial and the CALGB-90206 trial, were launched in the absence of formal phase I testing but incorporated safety evaluations to identify potential significant toxicity of the combinations.
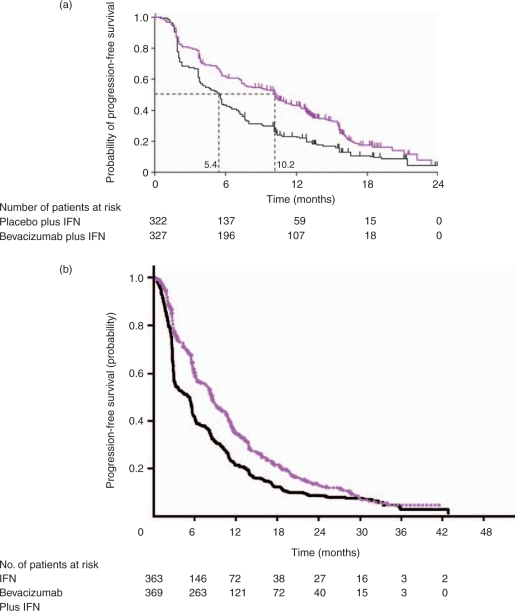
The AVOREN trial selected treatment-naïve patients with RCC to be randomized to interferon-α with either intravenous (IV) bevacizumab, 10 mg/kg, or placebo every 2 weeks. Interferon-α was given at a dose of 9 million units (MU) subcutaneously, thrice weekly [Escudier et al. 2007c]. The combination arm showed a markedly better response rate of 31% compared to 13% in the interferon-α plus placebo arm (p < 0.0001). Median PFS was 10.2 months for interferon-α with bevacizumab and 5.4 months for interferon-α with placebo (p < 0.0001; Figure 3a, Table 4). Toxicities and adverse events were similar between the two arms, although patients getting bevacizumab were more likely to experience hypertension and/or proteinuria.
Figure 3.
(a) Interferon-α (IFN) with placebo (black line) versus IFN with bevacizumab (purple line) in untreated patients with recurrent or advanced renal cell carcinoma – AVOREN Trial – progression-free survival [Escudier et al. 2007c]. With permission, The Lancet, (b) Interferon-α (IFN) alone (black line) and with bevacizumab (purple line), in untreated patients with recurrent or advanced renal cell carcinoma – CALGB Trial 90206 – progression-free survival [Rini et al. 2008]. With permission, American Society for Clinical Oncology.
Table 4.
Randomized phase III trials of interferon-α with or without bevacizumab in advanced renal cell cancer patients not having prior systemic therapy [Rini et al. 2008c; Escudier et al. 2007c].
| Treatment | PFS | p value | OS | p value |
|---|---|---|---|---|
| AVOREN Study | ||||
| Interferon-α + bevacizumab | 10.2 | 0.0001 | NR | NA |
| Interferon-α | 5.4 | 19.8 | ||
| CALGB 90206 | ||||
| Interferon-α + bevacizumab | 8.5 | 0.0001 | NR | NA |
| Interferon-α | 5.2 | NR | ||
NR, not reached; OS, overall survival; PFS, progression-free survival.
In the CALGB-90206 trial, the design and patient selection were similar to AVOREN except that patients were not given placebo infusions in the control arm and therefore patients and clinicians were aware of which patients were receiving bevacizumab [Rini et al. 2008]. The combination arm showed better response rate of 26% compared to 13% in the interferon-α alone arm (p < 0.0001; Figure 3b, Table 4). Median PFS was 8.5 months for interferon-α with bevacizumab and 5.2 months for interferon-α alone (p < 0.0001). Toxicities and adverse events were similar between the two arms, although patients who received bevacizumab were more likely to experience hypertension, anorexia, fatigue and/or proteinuria. Full analysis for OS effect, which was the primary endpoint in both trials, awaits further follow up. These response and PFS data attest to the additive effect for bevacizumab in addition to interferon-α. Prior phase II studies have reported similar data for single-agent bevacizumab [Bukowski et al. 2007b] A retrospective analysis of the AVOREN trial found that patients who had dose reductions of interferon-α for toxicity had no detriment in PFS [Melichar et al. 2008]. This is hardly definitive, but suggests that lower dose interferon-α with bevacizumab may be an option. The question of whether single-agent bevacizumab given in the absence of interferon-α could produce similar outcomes to the combination remains unresolved and will require additional trials.
Bevacizumab is approved for the treatment of advanced colorectal cancer and advanced non-small cell non-squamous histology lung cancer in combination with chemotherapy [Sandler et al. 2006; Hurwitz et al. 2004].
Temsirolimus
Temsirolimus (CCI-779, Torisel®) is an ester of rapamycin, which inhibits mTOR (mammalian Target Of Rapamycin), regulates cellular glucose homeostasis and inhibits production of VEGF through direct and indirect effects on hypoxia-inducible factor. In addition, the drug mediates proliferation through cyclin and c-myc, at least partially through regulation of S-6 kinase activity [Guba et al. 2002; Dudkin et al. 2001]. A randomized phase II trial investigated the efficacy of temsirolimus over a range of doses administered on a weekly schedule (25, 75, or 250 mg) in 111 patients with refractory, advanced RCC [Atkins et al. 2004]. An overall response rate of 7% and a minor response rate of 26% were observed. The median survival was 15 months, and the median observed time-to-progression was 5.8 months. Importantly, the investigators noted that patients who entered this trial who were in the MSKCC poor-risk category had a better than expected OS of around 8 months compared to a predicted median of 5 months. Phase I data suggested that temsirolimus and interferon-α could be given together but required reduced doses due to stomatitis, fatigue and nausea/vomiting [Motzer et al. 2007c].
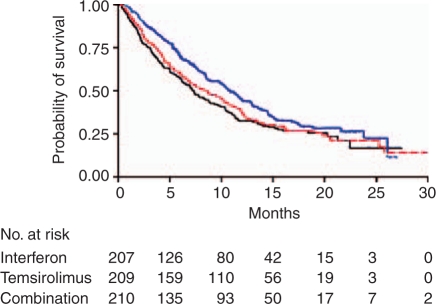
Subsequently, a phase III trial was undertaken, which randomized 626 patients with largely poor-risk, but also some intermediate-risk, metastatic RCC between three arms: interferon-α (up to 18 MU subcutaneously three times/week), temsirolimus (25 mg IV weekly) or temsirolimus (15 mg IV weekly) plus interferon-α (6 MU subcutaneously three times/week). Distinct from the sorafenib and sunitinib trials, non-clear cell histological subtypes were not excluded [Hudes et al. 2007]. This trial demonstrated that temsirolimus alone conferred a survival benefit (Figure 4, Table 5) compared to temsirolimus plus interferon-α or interferon-α alone [Hudes et al. 2007]. However, both the temsirolimus plus interferon-α-group and the temsirolimus-alone group performed similarly in terms of PFS, suggesting that temsirolimus elongates the time to progression and OS, while the addition of interferon-α does not provide translation of the improved PFS (compared to interferon-α alone) into better OS. Similar objective responses were observed in 7, 9, and 11% of patients in the interferon-α, temsirolimus, and combination arms, respectively. This trial has been the subject of criticism. The use of interferon-α as the standard therapy arm is considered inappropriate by some, in the context that cytokine therapy does not benefit patients with poor-risk features. In addition, accrual was relatively slow to the trial given the rarity and fragility of poor-risk patients being recruited. On this basis, eligibility criteria were broadened to allow patients into the trial who had multiple sites of metastatic disease. The result was improved accrual with addition of patients who fell strictly into the MSKCC intermediate-risk category. A yet-to-be-published post-hoc analysis of this trial demonstrated that the benefit of temsirolimus over interferon-α only occurred in patients with poor-risk MSKCC disease, with no benefit in intermediate-risk disease. This same post-hoc analysis demonstrated that patients with non-clear cell RCC benefited from temsirolimus while those with clear cell histology did not and that patients less than 65 years old at study entry benefited from temsirolimus while old patients did not. The implications of these findings for clinical practice are unclear but provocative enough to rate consideration in particular patients.
Figure 4.
Temsirolimus (blue line) versus temsirolimus and interferon-α (IFN, red line) versus IFN alone (black line) in first-line poor-risk renal cell cancer patients – overall survival [Hudes et al. 2007]. With permission, New England Medical Society.
Table 5.
Randomized phase III trial of temsirolimus, interferon-α or both in poor-risk metastatic renal cell cancer patients [Hudes et al. 2007].
| Treatment | PFS | p value | OS | p value |
|---|---|---|---|---|
| Temsirolimus | 3.7 | 0.0001 | 10.9 | 0.0069 |
| Interferon-α | 1.9 | 0.0019 | 7.3 | 0.6912 |
| Interferon-α + temsirolimus | 3.7 | 8.4 |
OS, overall survival; PFS, progression-free survival.
Significant grade 3 toxicities reported for patients treated with temsirolimus given as 25 mg IV each week were mucositis, thrombocytopenia, hypophosphatemia, leucopenia, anemia, asymptomatic hyperlipidemia, hyperglycemia and skin and nail toxicities. Notably, asthenia was more common in patients receiving interferon-α, while rash was more common the temsirolimus arm.
Temsirolimus is now considered a standard for patients with poor risk metastatic RCC. The role of this drug and other mTOR inhibitors in other RCC settings and in other tumor types is under investigation.
Everolimus
Everolimus, an esteric derivative of rapamycin with some structural homology with temsirolimus, binds to an intracellular protein, FKBP-12, forming a complex that inhibits the mTOR serine-threonine kinase. Its effects are mediated through hypoxia-inducible factors to angiogenesis and S-6 kinase for cell cycle regulation.
Pharmacodynamic-pharmacokinetic modeling of dose and dose scheduling based on plasma sirolimus levels and inhibition of patients peripheral blood lymphocytes of S6 kinase, suggested that the optimal tumor kill would occur with daily dosing of the everolimus [O’Donnell et al. 2008; Tanaka et al. 2008]. In one phase I study, initial weekly dosing schedules were explored but pharmacodymanic data led to the trial being amended to incorporate daily dosing. This eventually led to optimal dosing at 10 mg/day orally for moving forward into later phase trials. The study recorded four partial responses and 12 patients with protracted stable disease. Of ten RCC patients treated, five had protracted response or stable disease. The daily dosing schedule of everolimus was further explored in a phase I/II study in patients with hematological disorders [Yee et al. 2006].
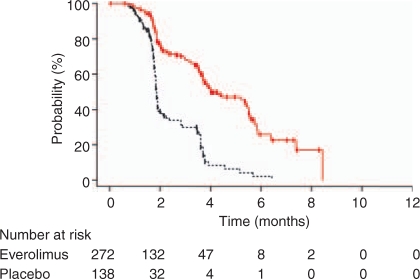
Subsequently, the RECORD-1 trial was designed to evaluate oral daily everolimus compared to placebo in patients who had failed one or both of the approved VEGF TKIs (TKIs), sorafenib and/or sunitinib [Motzer et al. 2008b]. Patients were permitted to have had other therapies apart from a VEGF TKI, such as cytokines, chemotherapy or bevacizumab. The trial accrued 410 patients randomized in a 2:1 ratio to everolimus and placebo. At interim analysis the trial was halted because the median PFS in everolimus was 4.1 months compared to 1.9 months in the placebo group, (HR 0.30, 95% CI 0.22–0.40, p < 0.0001). Only one person in the everolimus groups achieved a partial response but 65% of patients on placebo progressed compared to 37% on everolimus (Table 6, Figure 5).
Table 6.
Randomized phase III trial of everolimus with best supportive care or placebo with best supportive care in metastatic renal cell cancer patients who have failed at least one vascular endothelial growth factor tyrosine kinase inhibitor [Motzer et al. 2008b].
| Treatment | PFS | p value | OS | p value |
|---|---|---|---|---|
| Everolimus | 4.0 | 0.0001 | NR | NA |
| Placebo | 1.9 | 8.8 |
NR, not reached; OS, overall survival; PFS, progression-free survival.
Figure 5.
Everolimus (red line) compared to placebo (black line) on patients who have progressed on at least one vascular endothelial growth factor tyrosine kinase inhibitor – RECORD-1 Trial [Motzer et al. 2008b]. With permission, The Lancet.
Overall everolimus therapy was well tolerated. A small number of patients developed pneumonitis on everolimus, a side effect seen with other agents in this class, which responded to a break in therapy and institution of corticosteroids in symptomatic cases. Stomatitis, rash and fatigue were more common in the everolimus group compared with the placebo group. Everolimus is the first drug to demonstrate a PFS benefit for patients who are no longer responding to VEGF TKIs. Patients will be followed up in the coming months to determine whether there is a survival advantage.
The potential roles of everolimus prior to VEGF TKI treatment, in the adjuvant setting and in non-clear cell histologies are the subject of several accruing or planned clinical trials.
Sequencing of targeted drugs in renal cell cancer
Given the activity of newer agents targeting either VEGF or mTOR in advanced RCC, the potential benefit or toxicity of sequential or combination therapy raise obvious questions [Hutson and Figlin, 2007].
The selection of first-line therapy has become extremely complex. Pre-therapy predictors of outcome are lacking and leave much to the clinician’s judgement and the therapeutic aspirations of an individual patient. Options are summarized in Table 7 related to levels of evidence for different MSK categories in first-line and for the prior therapy failed subsequently. Generally, first-line patients should be offered sunitinib, which has activity across all three MSK categories, although there is strong data for the use of temsirolimus in poor-risk patients and potential for cure in a small fraction of patients with good-risk disease given high-dose interleukin-2 [Hutson and Quinn, 2005]. Patients might also be offered bevacizumab and interferon-α or potentially sorafenib if they have evidence of cardiac failure or other vascular risk factors [Telli et al. 2008; Chu et al. 2007]. A clinical trial always remains a good option for every patient.
Table 7.
Summary of evidence-based approach to therapy for advanced or recurrent renal clear cell carcinoma in 2009 (Adapted from Rini B, American Society for Clinical Oncology Annual Meeting 2008 [Rini, 2008b]).
| Setting | Patients | Therapy (level 1) | Other options (≥ level 2) |
|---|---|---|---|
| Untreated | Good or intermediate MSK risk | Sunitinib [Motzer et al. 2007b] Bevacizumab + IFN [Rini et al. 2008; Escudier et al. 2007c] Clinical trial | HD IL-2 [Fisher et al. 2000] Sorafenib [Ratain et al. 2006] Clinical trial observation |
| Untreated | Poor MSK risk | Temsirolimus [Hudes et al. 2007a] Clinical trial | Sunitinib [Motzer et al. 2007a] Clinical trial |
| Refractory | Cytokine | Sorafenib [Escudier et al. 2007a] Pazopanib [Hutson et al. 2007b] Clinical trial | Sunitinib [Motzer et al. 2006], Bevacizumab [Bukowski et al. 2007b] |
| Refractory | VEGF; mTOR | Everolimus[Motzer et al. 2008b] Clinical trial | Other VEGF TKIs Axitinib [Dutcher et al. 2008; Rixe et al. 2007] |
IFN, interferon; MSK, Memorial Sloan Kettering; mTOR, mammalian target of rapamycin; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor.
For patients failing first-line therapy, phase III data supports the use of sorafenib for patients failing cytokine therapy and everolimus for patients failing VEGF TKIs [Motzer et al. 2008b; Escudier et al. 2007a]. There is also phase II data demonstrating activity for sunitinib in this setting. A potentially important issue relates to how an individual fails therapy.
Patients developing progressive disease on full doses of a VEGF TKI may be more likely to have VEGF-pathway resistance and less likely to respond to another VEGF-pathway therapy. Logic might suggest that they would be better served by being treated with an agent that has a different mechanism of action such as an mTOR modulator.
Patients who stop therapy with a VEGF TKI because of toxicity, or reduce dose of the drug because of side effects, and then progress are less likely to have VEGF-pathway resistance. They may be candidates for further VEGF-pathway targeted therapy with agents that have a different profile of side effects and toxicities.
To address these issues, accruing studies will compare mTOR modulation to further VEGF TKI therapy (STAR Trial) and different VEGF TKIs for patients failing first-line therapy (AXIS Trial) [Rini, 2008a; Hutson, 2007]. In addition, another study will examine the effect of commencing therapy with either sunitinib (considered a first-line standard in many clinician’s eyes) or sorafenib and switching to the other at progression or intolerance with total time-to-progression on the second VEGF TKI as a major endpoint.
Combination therapy with targeted drugs
Efforts at combining targeted agents with one another and with cytokine therapy have met with limited success, mainly because of toxicity. The addition of bevacizumab to VEGF TKIs has been difficult due to vascular toxicity, most commonly hypertension, but on occasion renal failure and central nervous system vaso-occlusive phenomena, and hand-foot syndrome [Feldman et al. 2009]. Early phase trials combining mTOR inhibitors with VEGF pathway directed drugs look to provide a better toxicity profile. Combinations of sorafenib, bevacizumab and temsirolimus will be evaluated by the North American Cooperative Groups in the BEST trial [Flaherty et al. 2007]. Combination of VEGF-directed agents with cytokines therapies also look promising as evidenced by the combination of bevacizumab and interferon-α in the AVOREN and CALGB trials. Combination of interferon-α with VEGF TKIs does appear to increase response but at the cost of increased toxicity which is considerable in some cases [Bracarda et al. 2007; Gollob et al. 2007; Jonasch et al. 2007; Ryan et al. 2007].
Adjuvant therapy after nephrectomy for high-risk renal cell cancer
As many as 50% of patients treated with curative intent for localized RCC will relapse. Risk of relapse is predicted by cancer nuclear grade, disease stage and performance status in the University California Los Angeles integrated staging system (UISS) [Karakiewicz et al. 2007; Zisman et al. 2001]. Symptomatic presentation as opposed to incidental diagnosis also has an adverse impact on outcome [Patard et al. 2004]. Given the high rate of relapse for certain identifiable groups, adjuvant therapy appears a logical approach. Unfortunately, no treatment represents a standard intervention in the adjuvant setting in RCC. Previously the Eastern Cooperative Oncology Group (ECOG) led a trial of the use of interferon-α for a year after nephrectomy compared to observation. This trial found that patients given interferon-α had a trend toward a worse survival (p = 0.09) and a poor quality-of-life during treatment [Messing et al. 2003]. High-dose interleukin-2 therapy has also failed to demonstrate benefit in this setting, although data are limited. The Cytokine Working Group evaluated the potential of one course of high-dose interleukin-2 therapy compared to observation in the adjuvant setting; this underpowered study failed to suggest an advantage over observation [Clark et al. 2003].
Current trials are examining the potential of newer TKIs such as sorafenib and sunitinib in the adjuvant setting for patients at high risk of relapse [Yap and Eisen, 2006]. ECOG Trial E2805, also known as ASSURE (Adjuvant Sorafenib or Sunitinib for Unfavorable Renal Carcinoma) will accrue 1,736 patients with locally advanced clear cell RCC treated with nephrectomy to one year of therapy with sorafenib or sunitinib or placebo, with a primary endpoint of OS. This trial is accessible through ECOG, SWOG, CALGB and NCI Canada [Balzer-Hass et al. 2007]. In Europe, the SORCE trial will examine the use of one of year of sorafenib, three years of sorafenib or placebo in a similarly selected group of patients [Eisen, 2007]. It will answer a further question about the duration of adjuvant therapy for these patients. Other adjuvant trials are planned, including one comparing the mTOR inhibitor, everolimus, given for one year versus placebo.
Given that our experience with sorafenib and sunitinib is still relatively limited, we need to be vigilant for chronic side-effects in patients on long-term treatment. This is especially applicable to patients getting adjuvant treatment after surgery on a range of ongoing trials, where dose maintenance and compliance have been more problematic than for patients with advanced disease on the same regimen.
New targeted agents for renal cell cancer
The plethora of targeted agents coming to trials and being licensed in the last 3 years represents only the first wave of agents for these groups. It is likely that as many as a dozen VEGF pathway and six mTOR/akt modulators could be marketed for RCC in the next several years. A detailed overview of these agents is outside the scope of this current article but has recently been skillfully reviewed by Sonpavde and Hutson [Sonpavde and Hutson, 2008]. Two agents are, however, worthy of note. Pazopanib, a VEGF TKI [Hurwitz et al. 2009], has been evaluated in a large phase II trial in RCC patients with encouraging tumor response rate and a median OS of around 11 months for patients not previously treated for advanced disease [Hutson et al. 2007]. This compares favourably with other agents licensed for use in RCC. A recently presented phase III trial demonstrated a significant PFS advantage compared to placebo for first-line patients and also for those who had previously been treated with cytokines [Sternberg et al. 2009]. Axitinib, another VEGF TKI, has shown significant activity in a phase II trial of cytokine refractory patients [Rixe et al. 2007] and also appears to have activity in patients progressing on other targeted agents [Dutcher et al. 2008]. It is currently being compared to sorafenib in a phase III trial for patients who have progressed on or failed to tolerate a range of first-line treatments including sunitinib, temsirolimus and cytokine-based immunotherapy.
Conclusion
Clinicians and patients now have multiple drugs available to them which are capable of doubling time to disease progression in RCC, and which also extend OS. The sequential, combination and dose-adjusted use of these drugs provide challenges to be tackled in future trials. New drugs targeting the VEGF ligand, tyrosine kinases and mTOR, as well as a myriad of other interesting targets are in trial and hold much promise. In the meantime, we must perfect the art of individual application of these new agents to patients in our clinics, revisiting our internal medicine roots and mastering supportive care, to maximize tolerability and benefit.
Conflict of interest statement
TBD, AG – none. DIQ has received honoraria from speakers’ bureau and consulting as well as clinical trials support from Sanofi-Aventis, Millennium, Bayer Schering Healthcare, Onyx Pharmaceuticals, Pfizer, Genentech, Roche and Wyeth.
References
- Aaltomaa S., Lipponen P., Ala-Opas M., Eskelinen M., Syrjanen K., Kosma V.M. (1999) Expression of cyclins A and D and p21(waf1/cip1) proteins in renal cell cancer and their relation to clinicopathological variables and patient survival. Br J Cancer 80: 2001–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams T.J., Lee L.B., Murray L.J., Pryer N.K., Cherrington J.M. (2003) Su11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther 2: 471–478 [PubMed] [Google Scholar]
- Amin M.B., Tamboli P., Javidan J., Stricker H., Venturina M.D., Deshpande A., et al. (2002) Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am J Surg Pathol 26: 281–291 [DOI] [PubMed] [Google Scholar]
- Atkins M., Regan M., Mcdermott D., Mier J., Stanbridge E., Youmans A., et al. (2005) Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin Cancer Res 11: 3714–3721 [DOI] [PubMed] [Google Scholar]
- Atkins M.B., Hidalgo M., Stadler W.M., Logan T.F., Dutcher J.P., Hudes G.R., et al. (2004) Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol 22: 909–918 [DOI] [PubMed] [Google Scholar]
- Atkins M.B., Sparano J., Fisher R.I., Weiss G.R., Margolin K.A., Fink K.I., et al. (1993) Randomized phase II trial of high-dose interleukin-2 either alone or in combination with interferon alfa-2b in advanced renal cell carcinoma. J Clin Oncol 11: 661–670 [DOI] [PubMed] [Google Scholar]
- Awada A., Hendlisz A., Gil T., Bartholomeus S., Mano M., De Valeriola D., et al. (2005) Phase I safety and pharmacokinetics of bay 43-9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br J Cancer 92: 1855–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzer-Hass, N.S., Flaherty, K.T., Uzzo, R., Kane, C.J., Wood, C.G. and Jewett, M.A.S. (2007) Phase III randomized study of adjuvant sunitinib malate versus sorafenib in patients with resected renal cell carcinoma; Nct00326898: ECOG/Intergroup E2805.
- Bensalah K., Pantuck A.J., Crepel M., Verhoest G., Mejean A., Valeri A., et al. (2008) Prognostic variables to predict cancer-related death in incidental renal tumours. BJU Int 102: 1376–1380 [DOI] [PubMed] [Google Scholar]
- Blancher C., Moore J.W., Robertson N., Harris A.L. (2001) Effects of RAS and Von Hippel-Lindau (VHL) gene mutations on hypoxia-inducible factor (HIF)-1alpha, HIF-2alpha, and vascular endothelial growth factor expression and their regulation by the phosphatidylinositol 3’-kinase/Akt signaling pathway. Cancer Res 61: 7349–7355 [PubMed] [Google Scholar]
- Bracarda S., Porta C., Boni C., Santoro A., Artioli F., Di Bartolomeo C., et al. (2007) Randomized prospective phase II trial of two schedules of sorafenib daily and interferon-Α2a (IFN) in metastatic renal cell carcinoma (rapsody): GOIRC (Italian Oncology Group for Clinical Research) Study 0681. J Clin Oncol, ASCO Annual Meeting Proceedings, Vol. 25: Chicago, IL–Chicago, IL [Google Scholar]
- Brauch H., Weirich G., Brieger J., Glavac D., Rodl H., Eichinger M., et al. (2000) VHL alterations in human clear cell renal cell carcinoma: association with advanced tumor stage and a novel hot spot mutation. Cancer Res 60: 1942–1948 [PubMed] [Google Scholar]
- Brown L.F., Berse B., Jackman R.W., Tognazzi K., Manseau E.J., Dvorak H.F., et al. (1993) Increased expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in kidney and bladder carcinomas. Am J Pathol 143: 1255–1262 [PMC free article] [PubMed] [Google Scholar]
- Brugarolas J.B., Vazquez F., Reddy A., Sellers W.R., Kaelin Jr W.G. (2003) TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell 4: 147–158 [DOI] [PubMed] [Google Scholar]
- Bukowski R., Cella D., Gondek K., Escudier B. (2007c) Effects of sorafenib on symptoms and quality of life: results from a large randomized placebo-controlled study in renal cancer. Am J Clin Oncol 30: 220–227 [DOI] [PubMed] [Google Scholar]
- Bukowski R.M., Eisen T., Szczylik C., Stadler W.M., Simantov R., Shan M., et al. (2007a) Final results of the randomized phase III trial of sorafenib in advanced renal cell carcinoma: survival and biomarker analysis. J Clin Oncol, ASCO Annual Meeting Proceedings 25: 5023–5023 [Google Scholar]
- Bukowski R.M., Kabbinavar F.F., Figlin R.A., Flaherty K., Srinivas S., Vaishampayan U., et al. (2007b) Randomized phase II study of erlotinib combined with bevacizumab compared with bevacizumab alone in metastatic renal cell cancer. J Clin Oncol 25: 4536–4541 [DOI] [PubMed] [Google Scholar]
- Carmeliet P., Ferreira V., Breier G., Pollefeyt S., Kieckens L., Gertsenstein M., et al. (1996) Abnormal blood vessel development and lethality in embryos lacking a single VEGF Allele. Nature 380: 435–439 [DOI] [PubMed] [Google Scholar]
- Chan A.S., Leung S.Y., Wong M.P., Yuen S.T., Cheung N., Fan Y.W., et al. (1998) Expression of vascular endothelial growth factor and its receptors in the anaplastic progression of astrocytoma, oligodendroglioma, and ependymoma. Am J Surg Pathol 22: 816–826 [DOI] [PubMed] [Google Scholar]
- Cheng S.Y., Huang H.J., Nagane M., Ji X.D., Wang D., Shih C.C., et al. (1996) Suppression of glioblastoma angiogenicity and tumorigenicity by inhibition of endogenous expression of vascular endothelial growth factor. Proceedings of the National Academy of Sciences of the United States of America 93: 8502–8507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choueiri T.K., Garcia J.A., Elson P., Khasawneh M., Usman S., Golshayan A.R., et al. (2007) Clinical factors associated with outcome in patients with metastatic clear-cell renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. Cancer 110: 543–550 [DOI] [PubMed] [Google Scholar]
- Choueiri T.K., Vaziri S.A., Jaeger E., Elson P., Wood L., Bhalla I.P., et al. (2008) Von Hippel-Lindau gene status and response to vascular endothelial growth factor targeted therapy for metastatic clear cell renal cell carcinoma. J Urol 180: 860–865 [DOI] [PubMed] [Google Scholar]
- Chow W.H., Devesa S.S., Warren J.L., Fraumeni Jr J.F. (1999) Rising incidence of renal cell cancer in the United States. J Am Med Assoc 281: 1628–1631 [DOI] [PubMed] [Google Scholar]
- Chu T.F., Rupnick M.A., Kerkela R., Dallabrida S.M., Zurakowski D., Nguyen L., et al. (2007) Cardiotoxicity associated with TKI sunitinib. Lancet 370: 2011–2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J.I., Atkins M.B., Urba W.J., Creech S., Figlin R.A., Dutcher J.P., et al. (2003) Adjuvant high-dose bolus interleukin-2 for patients with high-risk renal cell carcinoma: a cytokine working group randomized trial. J Clin Oncol 21: 3133–3140 [DOI] [PubMed] [Google Scholar]
- Clark J.W., Eder J.P., Ryan D., Lathia C., Lenz H.J. (2005) Safety and pharmacokinetics of the dual action RAF kinase and vascular endothelial growth factor receptor inhibitor, bay 43-9006, in patients with advanced, refractory solid tumors. Clin Cancer Res 11: 5472–5480 [DOI] [PubMed] [Google Scholar]
- Clifford S.C., Astuti D., Hooper L., Maxwell P.H., Ratcliffe P.J., Maher E.R. (2001) The pVHL-associated SCF ubiquitin ligase complex: Molecular genetic analysis of elongin B and C, RBXL and HIF-1alpha in renal cell carcinoma. Oncogene 20: 5067–5074 [DOI] [PubMed] [Google Scholar]
- Clifford S.C., Maher E.R. (2001) Von Hippel-Lindau disease: Clinical and molecular perspectives. Adv Cancer Res 82: 85–105 [DOI] [PubMed] [Google Scholar]
- Collaborators M.R.C.R.C. (1999) Interferon-alpha and survival in metastatic renal carcinoma: Early results of a randomised controlled trial. Lancet 353: 14–17 [PubMed] [Google Scholar]
- Coppin C., Porzsolt F., Awa A., Kumpf J., Coldman A., Wilt T. (2005) Immunotherapy for advanced renal cell cancer. Cochrane Database Syst Rev CD001425–CD001425 [DOI] [PubMed] [Google Scholar]
- David K.A., Milowsky M.I., Nanus D.M. (2006) Chemotherapy for non-clear-cell renal cell carcinoma. Clin Genitourin Cancer 4: 263–268 [DOI] [PubMed] [Google Scholar]
- Decastro G.J., Mckiernan J.M. (2008) Epidemiology, clinical staging, and presentation of renal cell carcinoma. Urol Clin North Am 35: 581–592 [DOI] [PubMed] [Google Scholar]
- Del Bufalo D., Ciuffreda L., Trisciuoglio D., Desideri M., Cognetti F., Zupi G., et al. (2006) Antiangiogenic potential of the mammalian target of rapamycin inhibitor temsirolimus. Cancer Res 66: 5549–5554 [DOI] [PubMed] [Google Scholar]
- Demetri G.D., Van Oosterom A.T., Garrett C.R., Blackstein M.E., Shah M.H., Verweij J., et al. (2006) Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 368: 1329–1338 [DOI] [PubMed] [Google Scholar]
- Dosquet C., Coudert M.C., Lepage E., Cabane J., Richard F. (1997) Are angiogenic factors, cytokines, and soluble adhesion molecules prognostic factors in patients with renal cell carcinoma? Clin Cancer Res 3: 2451–2458 [PubMed] [Google Scholar]
- Duan D.R., Pause A., Burgess W.H., Aso T., Chen D.Y., Garrett K.P., et al. (1995) Inhibition of transcription elongation by the VHL tumor suppressor protein. Science 269: 1402–1406 [DOI] [PubMed] [Google Scholar]
- Dudkin L., Dilling M.B., Cheshire P.J., Harwood F.C., Hollingshead M., Arbuck S.G., et al. (2001) Biochemical correlates of mTOR inhibition by the rapamycin ester CCI-779 and tumor growth inhibition. Clin Cancer Res 7: 1758–1764 [PubMed] [Google Scholar]
- Dulaimi E., DeCaceres I.I., Uzzo, R.G, Al-Saleem T., Greenberg T., Polascik R.E., et al. (2004) Promoter hypermethylation profile of kidney cancer. Clin Cancer Res 10: 3972–3979 [DOI] [PubMed] [Google Scholar]
- Dutcher J., Atkins M.B., Margolin K., Weiss G., Clark J., Sosman J., et al. (2001) Kidney cancer: the cytokine working group experience (1986–2001): Part II. management of II-2 toxicity and studies with other cytokines. Med Oncol 18: 209–219 [DOI] [PubMed] [Google Scholar]
- Dutcher J.P., Logan T., Gordon M., Sosman J., Weiss G., Margolin K., et al. (2000) Phase II trial of interleukin 2, interferon alpha, and 5-fluorouracil in metastatic renal cell cancer: A cytokine working group study. Clin Cancer Res 6: 3442–3450 [PubMed] [Google Scholar]
- Dutcher J.P., Wilding G., Hudes G.R., Stadler W.M., Kim S., Tarazi J.C., et al. (2008) Sequential axitinib (Ag-013736) therapy of patients (Pts) with metastatic clear cell renal cell cancer (RCC) refractory to sunitinib and sorafenib, cytokines and sorafenib, or sorafenib alone. J Clin Oncol, ASCO Annual Meeting Proceedings, Vol. 26: : Chicago, IL. [Google Scholar]
- Dvorak H.F., Brown L.F., Detmar M., Dvorak A.M. (1995) Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. American Journal of Pathology 146: 1029–1039 [PMC free article] [PubMed] [Google Scholar]
- Eisen, T. (2007) Sorafenib in treating patients at risk of relapse after undergoing surgery to remove kidney; Nct00492258; Mrc-Re05-Sorce; Eudract Id 2006-006079-19; Eu-20734.
- Escudier B., Choueiri T.K., Oudard S., Szczylik C., Negrier S., Ravaud A., et al. (2007a) Prognostic factors of metastatic renal cell carcinoma after failure of immunotherapy: New paradigm from a large phase III trial with shark cartilage extract Ae 941. J Urol 178: 1901–1905 [DOI] [PubMed] [Google Scholar]
- Escudier B., Eisen T., Stadler W.M., Szczylik C., Oudard S., Siebels M., et al. (2007b) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356: 125–134 [DOI] [PubMed] [Google Scholar]
- Escudier B., Eisen T., Stadler W.M., Szczylik C., Oudard S., Staehler M., et al. (2009a) Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol 27: 3312–3318 [DOI] [PubMed] [Google Scholar]
- Escudier B., Pluzanska A., Koralewski P., Ravaud A., Bracarda S., Szczylik C., et al. (2007c) Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet 370: 2103–2111 [DOI] [PubMed] [Google Scholar]
- Escudier B., Szczylik C., Hutson T.E., Demkow T., Staehler M., Rolland F., et al. (2009b) Randomized phase II trial of first-line treatment with sorafenib versus interferon alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol 27: 1280–1289 [DOI] [PubMed] [Google Scholar]
- Faivre S., Delbaldo C., Vera K., Robert C., Lozahic S., Lassau N., et al. (2006) Safety, pharmacokinetic, and antitumor activity of Su11248, a novel oral multitarget TKI, in patients with cancer. J Clin Oncol 24: 25–35 [DOI] [PubMed] [Google Scholar]
- Feldman D.R., Baum M.S., Ginsberg M.S., Hassoun H., Flombaum C.D., Velasco S., et al. (2009) Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol 27: 1432–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N., Carver-Moore K., Chen H., Dowd M., Lu L., O’Shea K.S., et al. (1996) Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380: 439–442 [DOI] [PubMed] [Google Scholar]
- Fidler I.J., Ellis L.M. (1994) The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell 79: 185–188 [DOI] [PubMed] [Google Scholar]
- Figlin R.A., Belldegrun A., Moldawer N., Zeffren J., Dekernion J. (1992) Concomitant administration of recombinant human interleukin-2 and recombinant interferon alfa-2a: an active outpatient regimen in metastatic renal cell carcinoma. J Clin Oncol 10: 414–421 [DOI] [PubMed] [Google Scholar]
- Figlin R.A., Thompson J.A., Bukowski R.M., Vogelzang N.J., Novick A.C., Lange P., et al. (1999) Multicenter, randomized, phase III trial of Cd8(+) tumor-infiltrating lymphocytes in combination with recombinant interleukin-2 in metastatic renal cell carcinoma. J Clin Oncol 17: 2521–2529 [DOI] [PubMed] [Google Scholar]
- Finke J.H., Rayman P., George R., Tannenbaum C.S., Kolenko V., Uzzo R., et al. (2001) Tumor-induced sensitivity to apoptosis in T cells from patients with renal cell carcinoma: role of nuclear factor-kappab suppression. Clin Cancer Res 7: 940s–946s [PubMed] [Google Scholar]
- Fisher R.I., Coltman Jr C.A., Doroshow J.H., Rayner A.A., Hawkins M.J., Mier J.W., et al. (1988) Metastatic renal cancer treated with interleukin-2 and lymphokine-activated killer cells. A phase II clinical trial. Ann Intern Med 108: 518–523 [DOI] [PubMed] [Google Scholar]
- Fisher R.I., Rosenberg S.A., Fyfe G. (2000) Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am 6 Suppl 1: S55–57 [PubMed] [Google Scholar]
- Flaherty, K.T. and Mcdermott, D.F. (2007) The BeST trial: A randomized phase II study of VEGF, RAF kinase, and mTOR combination targeted therapy (CTT) with bevacizumab, sorafenib and temsirolimus in advanced renal cell carcinoma. Nct00378703; ECOG-E2804. [DOI] [PMC free article] [PubMed]
- Flanigan R.C., Salmon S.E., Blumenstein B.A., Bearman S.I., Roy V., Mcgrath P.C., et al. (2001) Nephrectomy followed by interferon alpha-2b compared with interferon alpha-2b alone for metastatic renal-cell cancer. N Engl J Med 345: 1655–1659 [DOI] [PubMed] [Google Scholar]
- Fong G.H., Rossant J., Gertsenstein M., Breitman M.L. (1995) Role of the FLT-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 376: 66–70 [DOI] [PubMed] [Google Scholar]
- Furge K.A., Tan M.H., Dykema K., Kort E., Stadler W., Yao X., et al. (2007) Identification of deregulated oncogenic pathways in renal cell carcinoma: an integrated oncogenomic approach based on gene expression profiling. Oncogene 26: 1346–1350 [DOI] [PubMed] [Google Scholar]
- Gabrilovich D., Ishida T., Oyama T., Ran S., Kravtsov V., Nadaf S., et al. (1998) Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 92: 4150–4166 [PubMed] [Google Scholar]
- Gabrilovich D.I., Ishida T., Nadaf S., Ohm J.E., Carbone D.P. (1999) Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res 5: 2963–2970 [PubMed] [Google Scholar]
- George D.J., Kaelin W.G., Jr (2003) The Von Hippel-Lindau protein, vascular endothelial growth factor, and kidney cancer. N Engl J Med 349: 419–421 [DOI] [PubMed] [Google Scholar]
- Gitlitz B.J., Hoffman D.M., Moldawer N., Belldegrun A., Figlin R.A. (2001) Treatment of metastatic renal cell carcinoma with high-dose bolus interleukin-2 in a non-intensive care unit: an analysis of 124 consecutively treated patients. Cancer J 7: 112–120 [PubMed] [Google Scholar]
- Gnarra J.R., Duan D.R., Weng Y., Humphrey J.S., Chen D.Y., Lee S., et al. (1996a) Molecular cloning of the Von Hippel-Lindau tumor suppressor gene and its role in renal carcinoma. Biochim Biophys Acta 1242: 201–210 [DOI] [PubMed] [Google Scholar]
- Gnarra J.R., Zhou S., Merrill M.J., Wagner J.R., Krumm A., Papavassiliou E., et al. (1996b) Post-transcriptional regulation of vascular endothelial growth factor MRNA by the product of the VHL tumor suppressor gene. Proc Natl Acad Sci U S A 93: 10589–10594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobé G., Rubin M., Williams G., Sawczuk I., Buttyan R. (2002) Apoptosis and expression of Bcl-2, Bcl-XL, and Bax in renal cell carcinomas. Cancer Invest 20: 324–332 [DOI] [PubMed] [Google Scholar]
- Gollob J.A., Rathmell W.K., Richmond T.M., Marino C.B., Miller E.K., Grigson G., et al. (2007) Phase II trial of sorafenib plus interferon alfa-2b as first- or second-line therapy in patients with metastatic renal cell cancer. J Clin Oncol 25: 3288–3295 [DOI] [PubMed] [Google Scholar]
- Gomella L.G., Anglard P., Sargent E.R., Robertson C.N., Kasid A., Linehan W.M. (1990) Epidermal growth factor receptor gene analysis in renal cell carcinoma. J Urol 143: 191–193 [DOI] [PubMed] [Google Scholar]
- Grumont R.J., Rourke I.J., O’Reilly L.A., Strasser A., Miyake K., Sha W., et al. (1998) B lymphocytes differentially use the Rel and nuclear factor kappaB1 (Nf-kappaB1) transcription factors to regulate cell cycle progression and apoptosis in quiescent and mitogen-activated cells. J Exp Med 187: 663–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guba M., Von Breitenbuch P., Steinbauer M., Koehl G., Flegel S., Hornung M., et al. (2002) Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: Involvement of vascular endothelial growth factor. Nat Med 8: 128–135 [DOI] [PubMed] [Google Scholar]
- Gunaratnam L., Morley M., Franovic A., De Paulsen N., Mekhail K., Parolin D.A., et al. (2003) HIF activates the TGF-alpha/EGF-R growth stimulatory pathway in VHL/renal cell carcinoma cells. J Biol Chem 27: 27–27 [DOI] [PubMed] [Google Scholar]
- Gunningham S.P., Currie M.J., Han C., Turner K., Scott P.A., Robinson B.A., et al. (2001) Vascular endothelial growth factor-B and vascular endothelial growth factor-C expression in renal cell carcinomas: regulation by the Von Hippel-Lindau gene and hypoxia. Cancer Res 61: 3206–3211 [PubMed] [Google Scholar]
- Haitel A., Wiener H.G., Neudert B., Marberger M., Susani M. (2001) Expression of the cell cycle proteins P21, P27, and PRB in clear cell renal cell carcinoma and their prognostic significance. Urology 58: 477–481 [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. (2000) The hallmarks of cancer. Cell 100: 57–70 [DOI] [PubMed] [Google Scholar]
- Hansen W.J., Ohh M., Moslehi J., Kondo K., Kaelin W.G., Welch W.J. (2002) Diverse effects of mutations in exon II of the Von Hippel-Lindau (VHL) tumor suppressor gene on the interaction of pVHL with the cytosolic chaperonin and pVHL-dependent ubiquitin ligase activity. Mol Cell Biol 22: 1947–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara S., Oya M., Mizuno R., Horiguchi A., Marumo K., Murai M. (2005) Akt activation in renal cell carcinoma: contribution of a decreased PTEN expression and the induction of apoptosis by an Akt inhibitor. Ann Oncol 16: 928–933 [DOI] [PubMed] [Google Scholar]
- Hedberg Y., Davoodi E., Roos G., Ljungberg B., Landberg G. (1999) Cyclin-D1 expression in human renal-cell carcinoma. Int J Cancer 84: 268–272 [DOI] [PubMed] [Google Scholar]
- Hofmockel G., Riess S., Bassukas I.D., Dammrich J. (1997) Epidermal growth factor family and renal cell carcinoma: expression and prognostic impact. Eur Urol 31: 478–484 [DOI] [PubMed] [Google Scholar]
- Hon W.C., Wilson M.I., Harlos K., Claridge T.D., Schofield C.J., Pugh C.W., et al. (2002) Structural basis for the recognition of hydroxyproline in HIF-1 alpha by pVHL. Nature 417: 975–978 [DOI] [PubMed] [Google Scholar]
- Horiguchi A., Oya M., Uchida A., Marumo K., Murai M. (2003) Elevated Akt activation and its impact on clinicopathological features of renal cell carcinoma. J Urol 169: 710–713 [DOI] [PubMed] [Google Scholar]
- Huang A., Fone P.D., Gandour-Edwards R., White R.W., Low R.K. (1999) Immunohistochemical analysis of bcl-2 protein expression in renal cell carcinoma. J Urol 162: 610–613 [PubMed] [Google Scholar]
- Hudes G., Carducci M., Tomczak P., Dutcher J., Figlin R., Kapoor A., et al. (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356: 2271–2281 [DOI] [PubMed] [Google Scholar]
- Hurwitz H., Fehrenbacher L., Novotny W., Cartwright T., Hainsworth J., Heim W., et al. (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335–2342 [DOI] [PubMed] [Google Scholar]
- Hurwitz H.I., Dowlati A., Saini S., Savage S., Suttle A.B., Gibson D.M., et al. (2009) Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res 15: 4220–4227 [DOI] [PubMed] [Google Scholar]
- Hutson, T.E. (2007) Temsirolimus and sorafenib as second-line therapy in patients with advanced RCC who failed first-line sunitinib: Nct00474786: Wyeth 3066k1–404.
- Hutson T.E., Davis I.D., Machiels J.P., De Souza P.L., Hong B.F., Rottey S., et al. (2007) Pazopanib (Gw786034) is active in metastatic renal cell carcinoma (RCC): interim results of a phase II randomized discontinuation trial (RDT). J Clin Oncol (Meeting Abstracts) 25: 5031–5031 [Google Scholar]
- Hutson T.E., Figlin R.A. (2007) Evolving role of novel targeted agents in renal cell carcinoma. Oncology (Williston Park) 21: 1175–1180 [PubMed] [Google Scholar]
- Hutson T.E., Quinn D.I. (2005) Cytokine therapy: a standard of care for metastatic renal cell carcinoma? Clin Genitourin Cancer 4: 181–186 [DOI] [PubMed] [Google Scholar]
- Iliopoulos O., Levy A.P., Jiang C., Kaelin Jr W.G., Goldberg M.A. (1996) Negative regulation of hypoxia-inducible genes by the Von Hippel-Lindau protein. Proc Natl Acad Sci U S A 93: 10595–10599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai K., Yamanaka K., Kamura T., Minato N., Conaway R.C., Conaway J.W., et al. (1999) Identification of the Von Hippel-Lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc Natl Acad Sci U S A 96: 12436–12441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J., Rasmuson T., Grankvist K., Ljungberg B. (2000) Vascular endothelial growth factor as prognostic factor in renal cell carcinoma. J Urol 163: 343–347 [PubMed] [Google Scholar]
- Jonasch E., Corn P., Ashe R.G., Do K., Tannir N.M. (2007) Randomized phase II study of sorafenib with or without low-dose IFN in patients with metastatic tenal cell carcinoma. J Clin Oncol, ASCO Annual Meeting Proceedings, Vol. 25: : Chiacgo, IL. [Google Scholar]
- Kaelin W.G., Jr (2009) Treatment of kidney cancer: insights provided by the VHL tumor-suppressor protein. Cancer 115: 2262–2272 [DOI] [PubMed] [Google Scholar]
- Kallakury B.V., Karikehalli S., Haholu A., Sheehan C.E., Azumi N., Ross J.S. (2001) Increased expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma. Clin Cancer Res 7: 3113–3119 [PubMed] [Google Scholar]
- Karakiewicz P.I., Briganti A., Chun F.K., Trinh Q.D., Perrotte P., Ficarra V., et al. (2007) Multi-institutional validation of a new renal cancer-specific survival nomogram. J Clin Oncol 25: 1316–1322 [DOI] [PubMed] [Google Scholar]
- Kenerson H.L., Aicher L.D., True L.D., Yeung R.S. (2002) Activated mammalian target of rapamycin pathway in the pathogenesis of tuberous sclerosis complex renal tumors. Cancer Res 62: 5645–5650 [PubMed] [Google Scholar]
- Khakoo A.Y., Kassiotis C.M., Tannir N., Plana J.C., Halushka M., Bickford C., et al. (2008) Heart failure associated with sunitinib malate: a multitargeted receptor TKI. Cancer 112: 2500–2508 [DOI] [PubMed] [Google Scholar]
- Kim H.J., Park J.K., Kim Y.G. (1999) Suppression of NF-Kappab activation in normal T cells by supernatant fluid from human renal cell carcinomas. J Korean Med Sci 14: 299–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.J., Li B., Winer J., Armanini M., Gillett N., Phillips H.S., et al. (1993) Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362: 841–844 [DOI] [PubMed] [Google Scholar]
- Krieg M., Haas R., Brauch H., Acker T., Flamme I., Plate K.H. (2000) Up-regulation of hypoxia-inducible factors HIF-1alpha and HIF-2alpha under normoxic conditions in renal carcinoma cells by Von Hippel-Lindau tumor suppressor gene loss of function. Oncogene 19: 5435–5443 [DOI] [PubMed] [Google Scholar]
- Krishnan B., Truong L.D. (2002) Renal epithelial neoplasms: the diagnostic implications of electron microscopic study in 55 cases. Hum Pathol 33: 68–79 [DOI] [PubMed] [Google Scholar]
- Leung S.Y., Chan A.S., Wong M.P., Yuen S.T., Cheung N., Chung L.P. (1997) Expression of vascular endothelial growth factor and its receptors in pilocytic astrocytoma. Am J Surg Pathol 21: 941–950 [DOI] [PubMed] [Google Scholar]
- Li Q., Verma I.M. (2002) NF-Kappab regulation in the immune system. Nat Rev Immunol 2: 725–734 [DOI] [PubMed] [Google Scholar]
- Li X., Liu J., Park J.K., Hamilton T.A., Rayman P., Klein E., et al. (1994) T cells from renal cell carcinoma patients exhibit an abnormal pattern of kappa B-specific DNA-binding activity: a preliminary report. Cancer Res 54: 5424–5429 [PubMed] [Google Scholar]
- Li Y., Wang M.N., Li H., King K.D., Bassi R., Sun H., et al. (2002) Active immunization against the vascular endothelial growth factor receptor FLK1 inhibits tumor angiogenesis and metastasis. J Exp Med 195: 1575–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F., et al. (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359: 378–390 [DOI] [PubMed] [Google Scholar]
- Mack F.A., Rathmell W.K., Arsham A.M., Gnarra J., Keith B., Simon M.C. (2003) Loss of pVHL is sufficient to cause HIF dysregulation in primary cells but does not promote tumor growth. Cancer Cell 3: 75–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher E.R., Kaelin W.G., Jr (1997) Von Hippel-Lindau disease. Medicine (Baltimore) 76: 381–391 [DOI] [PubMed] [Google Scholar]
- Majumder P.K., Febbo P.G., Bikoff R., Berger R., Xue Q., McMahon L.M., et al. (2004) mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med 10: 594–601 [DOI] [PubMed] [Google Scholar]
- Mathew A., Devesa S.S., Fraumeni Jr J.F., Chow W.H. (2002) Global increases in kidney cancer incidence, 1973–1992. Eur J Cancer Prev 11: 171–178 [DOI] [PubMed] [Google Scholar]
- Maxwell P.H., Wiesener M.S., Chang G.W., Clifford S.C., Vaux E.C., Cockman M.E., et al. (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399: 271–275 [DOI] [PubMed] [Google Scholar]
- Clement J.M., McDermott D.F. (2009) The high-dose aldesleukin (IL-2) ‘select’ trial: a trial designed to prospectively validate predictive models of response to high dose IL-2 treatment in patients with metastatic renal cell carcinoma. Clin Genitourin Cancer 7: E7–E9 [DOI] [PubMed] [Google Scholar]
- McDermott D.F., Regan M.M., Clark J.I., Flaherty L.E., Weiss G.R., Logan T.F., et al. (2005) Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol 23: 133–141 [DOI] [PubMed] [Google Scholar]
- McDonald P.P., Bald A., Cassatella M.A. (1997) Activation of the NF-Kappab pathway by inflammatory stimuli in human neutrophils. Blood 89: 3421–3433 [PubMed] [Google Scholar]
- Mekhail T.M., Abou-Jawde R.M., Boumerhi G., Malhi S., Wood L., Elson P., et al. (2005) Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol 23: 832–841 [DOI] [PubMed] [Google Scholar]
- Melichar B., Koralewski P., Ravaud A., Pluzanska A., Bracarda S., Szczylik C., et al. (2008) First-line bevacizumab combined with reduced dose interferon-alpha2a is active in patients with metastatic renal cell carcinoma. Ann Oncol 19: 1470–1476 [DOI] [PubMed] [Google Scholar]
- Mendel D.B., Laird A.D., Xin X., Louie S.G., Christensen J.G., Li G., et al. (2003) In vivo antitumor activity of Su11248, a novel TKI targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 9: 327–337 [PubMed] [Google Scholar]
- Messing E.M., Manola J., Wilding G., Propert K., Fleischmann J., Crawford E.D., et al. (2003) Phase III study of interferon alfa-a as adjuvant treatment for resectable renal cell carcinoma: an Eastern Cooperative Oncology Group/Intergroup trial. J Clin Oncol 21: 1214–1222 [DOI] [PubMed] [Google Scholar]
- Mickisch G.H., Garin A., Van Poppel H., De Prijck L., Sylvester R. (2001) Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet 358: 966–970 [DOI] [PubMed] [Google Scholar]
- Migita T., Oda Y., Naito S., Tsuneyoshi M. (2002) Low expression of P27(KIP1) is associated with tumor size and poor prognosis in patients with renal cell carcinoma. Cancer 94: 973–979 [PubMed] [Google Scholar]
- Motzer R.J., Bacik J., Mariani T., Russo P., Mazumdar M., Reuter V. (2002) Treatment outcome and survival associated with metastatic renal cell carcinoma of non-clear-cell histology. J Clin Oncol 20: 2376–2381 [DOI] [PubMed] [Google Scholar]
- Motzer R.J., Bacik J., Schwartz L.H., Reuter V., Russo P., Marion S., et al. (2004) Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol 22: 454–463 [DOI] [PubMed] [Google Scholar]
- Motzer R.J., Bukowski R.M., Figlin R.A., Hutson T.E., Michaelson M.D., Kim S.T., et al. (2008a) Prognostic nomogram for sunitinib in patients with metastatic renal cell carcinoma. Cancer 113: 1552–1558 [DOI] [PubMed] [Google Scholar]
- Motzer R.J., Escudier B., Oudard S., Hutson T.E., Porta C., Bracarda S., et al. (2008b) Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372: 449–456 [DOI] [PubMed] [Google Scholar]
- Motzer R.J., Hudes G.R., Curti B.D., Mcdermott D.F., Escudier B.J., Negrier S., et al. (2007c) Phase I/II trial of temsirolimus combined with interferon alfa for advanced renal cell carcinoma. J Clin Oncol 25: 3958–3964 [DOI] [PubMed] [Google Scholar]
- Motzer R.J., Hutson T.E., Tomczak P., Michaelson M.D., Bukowski R.M., Oudard S., et al. (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27: 3584–3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer R.J., Hutson T.E., Tomczak P., Michaelson M.D., Bukowski R.M., Rixe O., et al. (2007b) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356: 115–124 [DOI] [PubMed] [Google Scholar]
- Motzer R.J., Mazumdar M., Bacik J., Berg W., Amsterdam A., Ferrara J. (1999) Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 17: 2530–2540 [DOI] [PubMed] [Google Scholar]
- Motzer R.J., Mazumdar M., Bacik J., Russo P., Berg W.J., Metz E.M. (2000) Effect of cytokine therapy on survival for patients with advanced renal cell carcinoma. J Clin Oncol 18: 1928–1935 [DOI] [PubMed] [Google Scholar]
- Motzer R.J., Michaelson M.D., Redman B.G., Hudes G.R., Wilding G., Figlin R.A., et al. (2006a) Activity of Su11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 24: 16–24 [DOI] [PubMed] [Google Scholar]
- Motzer R.J., Michaelson M.D., Rosenberg J., Bukowski R.M., Curti B.D., George D.J., et al. (2007a) Sunitinib efficacy against advanced renal cell carcinoma. J Urol 178: 1883–1887 [DOI] [PubMed] [Google Scholar]
- Motzer R.J., Rini B.I., Bukowski R.M., Curti B.D., George D.J., Hudes G.R., et al. (2006b) Sunitinib in patients with metastatic renal cell carcinoma. J Am Med Assoc 295: 2516–2524 [DOI] [PubMed] [Google Scholar]
- Na X., Wu G., Ryan C.K., Schoen S.R., Di’santagnese P.A., Messing E.M. (2003) Overproduction of vascular endothelial growth factor related to Von Hippel-Lindau tumor suppressor gene mutations and hypoxia-inducible factor-1 alpha expression in renal cell carcinomas. J Urol 170: 588–592 [DOI] [PubMed] [Google Scholar]
- Negrier S., Caty A., Lesimple T., Douillard J.Y., Escudier B., Rossi J.F., et al. (2000a) Treatment of patients with metastatic renal carcinoma with a combination of subcutaneous interleukin-2 and interferon alpha with or without fluorouracil. Groupe Francais D'immunotherapie, Federation Nationale Des Centres De Lutte Contre Le Cancer. J Clin Oncol 18: 4009–4015 [DOI] [PubMed] [Google Scholar]
- Negrier S., Escudier B., Gomez F., Douillard J.Y., Ravaud A., Chevreau C., et al. (2002) Prognostic factors of survival and rapid progression in 782 patients with metastatic renal carcinomas treated by cytokines: a report from the Groupe Francais D'immunotherapie. Ann Oncol 13: 1460–1468 [DOI] [PubMed] [Google Scholar]
- Negrier S., Escudier B., Lasset C., Douillard J.Y., Savary J., Chevreau C., et al. (1998) Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Francais D'immunotherapie. N Engl J Med 338: 1272–1278 [DOI] [PubMed] [Google Scholar]
- Negrier S., Maral J., Drevon M., Vinke J., Escudier B., Philip T. (2000b) Long-term follow-up of patients with metastatic renal cell carcinoma treated with intravenous recombinant interleukin-2 in Europe. Cancer J Sci Am 6(Suppl 1): S93–98 [PubMed] [Google Scholar]
- Negrier S., Perol D., Ravaud A., Chevreau C., Bay J.O., Delva R., et al. (2007) Medroxyprogesterone, interferon alfa-2a, interleukin 2, or combination of both cytokines in patients with metastatic renal carcinoma of intermediate prognosis: results of a randomized controlled trial. Cancer 110: 2366–2369 [DOI] [PubMed] [Google Scholar]
- Niethammer A.G., Xiang R., Becker J.C., Wodrich H., Pertl U., Karsten G., et al. (2002) A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nat Med 8: 1369–1375 [DOI] [PubMed] [Google Scholar]
- O’Donnell A., Faivre S., Burris H.A., Rea D., Papadimitrakopoulou V., Shand N., et al. (2008) Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol 26: 1588–1595 [DOI] [PubMed] [Google Scholar]
- Oda H., Nakatsuru Y., Ishikawa T. (1995) Mutations of the P53 gene and P53 protein overexpression are associated with sarcomatoid transformation in renal cell carcinomas. Cancer Res 55: 658–662 [PubMed] [Google Scholar]
- Ohm J.E., Gabrilovich D.I., Sempowski G.D., Kisseleva E., Parman K.S., Nadaf S., et al. (2003) VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood 101: 4878–4886 [DOI] [PubMed] [Google Scholar]
- Osusky K.L., Hallahan D.E., Fu A., Ye F., Shyr Y., Geng L. (2004) The receptor TKI Su11248 impedes endothelial cell migration, tubule formation, and blood vessel formation in vivo, but has little effect on existing tumor vessels. Angiogenesis 7: 225–233 [DOI] [PubMed] [Google Scholar]
- Oyama T., Ran S., Ishida T., Nadaf S., Kerr L., Carbone D.P., et al. (1998) Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J Immunol 160: 1224–1232 [PubMed] [Google Scholar]
- Pantuck A.J., Seligson D.B., Klatte T., Yu H., Leppert J.T., Moore L., et al. (2007) Prognostic relevance of the mTOR pathway in renal cell carcinoma: implications for molecular patient selection for targeted therapy. Cancer 109: 2257–2267 [DOI] [PubMed] [Google Scholar]
- Patard J.J., Dorey F.J., Cindolo L., Ficarra A.D.L.T., Tostain J., et al. (2004a) Symptoms as well as tumor size provide prognostic information on patients with localized renal tumors. J Urol 172: 2167–2171 [DOI] [PubMed] [Google Scholar]
- Patard J.J., Kim H.L., Lam J.S., Dorey F.J., Pantuck A.J., Zisman A., et al. (2004b) Use of the University of California Los Angeles integrated staging system to predict survival in renal cell carcinoma: an international multicenter study. J Clin Oncol 22: 3316–3322 [DOI] [PubMed] [Google Scholar]
- Patel P.H., Chadalavada R.S., Ishill N.M., Patil S., Reuter V.E., Motzer R.J., et al. (2008) Hypoxia-inducible factor (HIF) 1{Alpha} and 2{Alpha} levels in cell lines and human tumor predicts response to sunitinib in renal cell carcinoma (RCC). J Clin Oncol (Meeting Abstracts) 26: 5008–5008 [Google Scholar]
- Prowse A.H., Webster A.R., Richards F.M., Richard S., Olschwang S., Resche F., et al. (1997) Somatic inactivation of the VHL gene in Von Hippel-Lindau disease tumors. Am J Hum Genet 60: 765–771 [PMC free article] [PubMed] [Google Scholar]
- Ratain M.J., Eisen T., Stadler W.M., Flaherty K.T., Kaye S.B., Rosner G.L., et al. (2006) Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 24: 2505–2512 [DOI] [PubMed] [Google Scholar]
- Ratain M.J., Flaherty K.T., Stadler W.M., O’Dwyer P., Kaye S., Xiong H., et al. (2004) Preliminary antitumor activity of bay 43-9006 in metastatic renal cell carcinoma and other advanced refractory solid tumors in a phase II randomized discontinuation trial, Proc Am Soc Clin Oncol, Vol. 23: New Orleans, LA–New Orleans, LA [Google Scholar]
- Reiter R.E., Anglard P., Liu S., Gnarra J.R., Linehan W.M. (1993) Chromosome 17p deletions and P53 mutations in renal cell carcinoma. Cancer Res 53: 3092–3097 [PubMed] [Google Scholar]
- Rini, B.I. (2008a) Axitinib (Ag 013736) as second line therapy for metastatic renal cell cancer, a phase III trial with sorafenib; Nct00678392, A4061032, Axis Trial.
- Rini B.I. (2008b) Renal cancer – commentary, J Clin Oncol, ASCO Annual Meeting Proceedings, Vol. 26: Chicago, IL–Chicago, IL [Google Scholar]
- Rini B.I., Halabi S., Rosenberg J.E., Stadler W.M., Vaena D.A., Ou S.S., et al. (2008) Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: Calgb 90206. J Clin Oncol 26: 5422–5428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rini B.I., Small E.J. (2005) Biology and clinical development of vascular endothelial growth factor-targeted therapy in renal cell carcinoma. J Clin Oncol 23: 1028–1043 [DOI] [PubMed] [Google Scholar]
- Rini B.I., Tamaskar I., Shaheen P., Salas R., Garcia J., Wood L., et al. (2007) Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst 99: 81–83 [DOI] [PubMed] [Google Scholar]
- Rixe O., Bukowski R.M., Michaelson M.D., Wilding G., Hudes G.R., Bolte O., et al. (2007) Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study. Lancet Oncol 8: 975–984 [DOI] [PubMed] [Google Scholar]
- Robert C., Soria J.C., Spatz A., Le Cesne A., Malka D., Pautier P., et al. (2005) Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol 6: 491–500 [DOI] [PubMed] [Google Scholar]
- Ronnen E.A., Kondagunta G.V., Ishill N., Spodek L., Russo P., Reuter V., et al. (2006) Treatment outcome for metastatic papillary renal cell carcinoma patients. Cancer 107: 2617–2621 [DOI] [PubMed] [Google Scholar]
- Rosenberg S.A., Lotze M.T., Yang J.C., Linehan W.M., Seipp C., Calabro S., et al. (1989) Combination therapy with interleukin-2 and alpha-interferon for the treatment of patients with advanced cancer. J Clin Oncol 7: 1863–1874 [DOI] [PubMed] [Google Scholar]
- Rosenberg S.A., Yang J.C., Topalian S.L., Schwartzentruber D.J., Weber J.S., Parkinson D.R., et al. (1994) Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. J Am Med Assoc 271: 907–913 [PubMed] [Google Scholar]
- Rosner G.L., Stadler W., Ratain M.J. (2002) Randomized discontinuation design: application to cytostatic antineoplastic agents. J Clin Oncol 20: 4478–4484 [DOI] [PubMed] [Google Scholar]
- Ryan C.W., Goldman B.H., Lara Jr P.N., Mack P.C., Beer T.M., Tangen M., et al. (2007) Sorafenib with interferon alfa-2b as first-line treatment of advanced renal carcinoma: a phase II study of the Southwest Oncology Group. J Clin Oncol 25: 3296–3301 [DOI] [PubMed] [Google Scholar]
- Ryan C.W., Vogelzang N.J., Stadler W.M. (2002) A phase II trial of intravenous gemcitabine and 5-fluorouracil with subcutaneous interleukin-2 and interferon-alpha in patients with metastatic renal cell carcinoma. Cancer 94: 2602–2609 [DOI] [PubMed] [Google Scholar]
- Sandler A., Gray R., Perry M.C., Brahmer J., Schiller J.H., Dowlati A., et al. (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355: 2542–2550 [DOI] [PubMed] [Google Scholar]
- Sargent E.R., Gomella L.G., Belldegrun A., Linehan W.M., Kasid A. (1989) Epidermal growth factor receptor gene expression in normal human kidney and renal cell carcinoma. J Urol 142: 1364–1368 [DOI] [PubMed] [Google Scholar]
- Senger D.R., Van De Water L., Brown L.F., Nagy J.A., Yeo K.T., Yeo T.K., et al. (1993) Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer & Metastasis Reviews 12: 303–324 [DOI] [PubMed] [Google Scholar]
- Shalaby F., Rossant J., Yamaguchi T.P., Gertsenstein M., Wu X.F., Breitman M.L., et al. (1995) Failure of blood-island formation and vasculogenesis in FLK-1-deficient mice. Nature 376: 62–66 [DOI] [PubMed] [Google Scholar]
- Simons J.P., Aaronson N.K., Vansteenkiste J.F., Ten Velde G.P., Muller M.J., Drenth B.M., et al. (1996) Effects of medroxyprogesterone acetate on appetite, weight, and quality of life in advanced-stage non-hormone-sensitive cancer: a placebo-controlled multicenter study. J Clin Oncol 14: 1077–1084 [DOI] [PubMed] [Google Scholar]
- Sionov R.V., Haupt Y. (1999) The cellular response to P53: the decision between life and death. Oncogene 18: 6145–6157 [DOI] [PubMed] [Google Scholar]
- Slaton J.W., Inoue K., Perrotte P., El-Naggar A.K., Swanson D.A., Fidler I.J., et al. (2001) Expression levels of genes that regulate metastasis and angiogenesis correlate with advanced pathological stage of renal cell carcinoma. Am J Pathol 158: 735–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonpavde G., Hutson T.E. (2008) New treatments for metastatic renal cell carcinoma. Clin Genitourin Cancer 6: S29–36 [DOI] [PubMed] [Google Scholar]
- Sternberg C.N., Szczylik C., Lee E., Salman P.V., Mardiak J., Davis I.D., et al. (2009) A randomized, double-blind phase III study of pazopanib in treatment-naive and cytokine-pretreated patients with advanced renal cell carcinoma (RCC). J Clin Oncol (Meeting Abstracts) 27: 5021–5021 [Google Scholar]
- Strumberg D., Awada A., Hirte H., Clark J.W., Seeber S., Piccart P., et al. (2006) Pooled safety analysis of bay 43-9006 (sorafenib) monotherapy in patients with advanced solid tumours: is rash associated with treatment outcome? Eur J Cancer 42: 548–556 [DOI] [PubMed] [Google Scholar]
- Strumberg D., Richly H., Hilger R.A., Schleucher N., Korfee S., Tewes M., et al. (2005) Phase I clinical and pharmacokinetic study of the novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol 23: 965–972 [DOI] [PubMed] [Google Scholar]
- Stumm G., Eberwein S., Rostock-Wolf S., Stein H., Pomer S., Schlegel J., et al. (1996) Concomitant overexpression of the EGFR and ERBB-2 genes in renal cell carcinoma (RCC) is correlated with dedifferentiation and metastasis. Int J Cancer 69: 17–22 [DOI] [PubMed] [Google Scholar]
- Sun L., Liang C., Shirazian S., Zhou Y., Miller T., Cui J., et al. (2003) Discovery of 5-[5-fluoro-2-oxo-1,2- dihydroindol-(3z)-ylidenemethyl]-2,4- dimethyl-1h-pyrrole-3-carboxylic acid (2-diethylaminoethyl)amide, a novel TKI targeting vascular endothelial and platelet-derived growth factor receptor tyrosine kinase. J Med Chem 46: 1116–1119 [DOI] [PubMed] [Google Scholar]
- Takahashi A., Sasaki H., Kim S.J., Tobisu K., Kakizoe T., Tsukamoto T., et al. (1994) Markedly increased amounts of messenger RNAs for vascular endothelial growth factor and placenta growth factor in renal cell carcinoma associated with angiogenesis. Cancer Res 54: 4233–4237 [PubMed] [Google Scholar]
- Takahashi M., Yang X.J., Sugimura J., Backdahl J., Tretiakova M., Qian C.N., et al. (2003) Molecular subclassification of kidney tumors and the discovery of new diagnostic markers. Oncogene 22: 6810–6818 [DOI] [PubMed] [Google Scholar]
- Tamaskar I., Choueiri T.K., Sercia L., Rini B., Bukowski R., Zhou M. (2007) Differential expression of caveolin-1 in renal neoplasms. Cancer 110: 776–782 [DOI] [PubMed] [Google Scholar]
- Tanaka C., O’Reilly T., Kovarik J.M., Shand N., Hazell K., Judson I., et al. (2008) Identifying optimal biologic doses of everolimus (Rad001) in patients with cancer based on the modeling of preclinical and clinical pharmacokinetic and pharmacodynamic data. J Clin Oncol 26: 1596–1602 [DOI] [PubMed] [Google Scholar]
- Telli M.L., Witteles R.M., Fisher G.A., Srinivas S. (2008) Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann Oncol 19: 1613–1618 [DOI] [PubMed] [Google Scholar]
- Textor S.C., Margolin K., Blayney D., Carlson J., Doroshow J. (1987) Renal, volume, and hormonal changes during therapeutic administration of recombinant interleukin-2 in man. Am J Med 83: 1055–1061 [DOI] [PubMed] [Google Scholar]
- Thrash-Bingham C.A., Tartof K.D. (1999) HIF-alpha: a natural antisense transcript overexpressed in human renal cancer and during hypoxia. J Natl Cancer Inst 91: 143–151 [DOI] [PubMed] [Google Scholar]
- Turner J.S., Cheung E.M., George J., Quinn D.I. (2007) Pain management, supportive and palliative care in patients with renal cell carcinoma. BJU Int 99: 1305–1312 [DOI] [PubMed] [Google Scholar]
- Uchida T., Gao J.P., Wang C., Jiang S.X., Muramoto M., Satoh T., et al. (2002) Clinical significance of P53, Mdm2, and Bcl-2 proteins in renal cell carcinoma. Urology 59: 615–620 [DOI] [PubMed] [Google Scholar]
- Uhlman D.L., Nguyen P., Manivel J.C., Zhang G., Hagen K., Fraley E., et al. (1995) Epidermal growth factor receptor and transforming growth factor alpha expression in papillary and nonpapillary renal cell carcinoma: correlation with metastatic behavior and prognosis. Clin Cancer Res 1: 913–920 [PubMed] [Google Scholar]
- Uhlman D.L., Nguyen P.L., Manivel J.C., Aeppli D., Resnick J.M., Fraley E.E., et al. (1994) Association of immunohistochemical staining for P53 with metastatic progression and poor survival in patients with renal cell carcinoma. J Natl Cancer Inst 86: 1470–1475 [DOI] [PubMed] [Google Scholar]
- Upton M.P., Parker R.A., Youmans A., Mcdermott D.F., Atkins M.B. (2005) Histologic predictors of renal cell carcinoma response to interleukin-2-based therapy. J Immunother 28: 488–495 [DOI] [PubMed] [Google Scholar]
- Uzzo R.G., Clark P.E., Rayman P., Bloom T., Rybicki L., Novick A.C., et al. (1999) Alterations in NF kappab activation in T lymphocytes of patients with renal cell carcinoma. J Natl Cancer Inst 91: 718–721 [DOI] [PubMed] [Google Scholar]
- Uzzo R.G., Rayman P., Kolenko V., Clark P.E., Cathcart M.K., Bloom T., et al. (1999) Renal cell carcinoma-derived gangliosides suppress nuclear factor-kappab activation in T cells. J Clin Invest 104: 769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese M.L., Mosenkis A., Flaherty K.T., Gallagher M., Stevenson J.P., Townsend R.R., et al. (2006) Mechanisms of hypertension associated with Bay 43-9006. J Clin Oncol 24: 1363–1369 [DOI] [PubMed] [Google Scholar]
- Vogelzang N.J., Stadler W.M. (1998) Kidney cancer. Lancet 352: 1691–1696 [DOI] [PubMed] [Google Scholar]
- Walpole E.T., Dutcher J.P., Sparano J., Gucalp R., Einzig A., Paietta E., et al. (1993) Survival after phase II treatment of advanced renal cell carcinoma with taxol or high-dose interleukin-2. J Immunother 13: 275–281 [DOI] [PubMed] [Google Scholar]
- Wilhelm S.M., Carter C., Tang L., Wilkie D., Mcnabola A., Rong H., et al. (2004) Bay 43-9006 exhibits broad spectrum oral antitumor activity and targets the Raf/Mek/Erk pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64: 7099–7109 [DOI] [PubMed] [Google Scholar]
- Wykoff C.C., Sotiriou C., Cockman M.E., Ratcliffe P.J., Maxwell P., Liu E., et al. (2004) Gene array of VHL mutation and hypoxia shows novel hypoxia-induced genes and that cyclin D1 is a VHL target gene. Br J Cancer 90: 1235–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagoda A., Abi-Rached B., Petrylak D. (1995) Chemotherapy for advanced renal-cell carcinoma: 1983–1993. Semin Oncol 22: 42–60 [PubMed] [Google Scholar]
- Yang J.C., Haworth L., Sherry R.M., Hwu P., Schwartzentruber D.J., Topalian S.L., et al. (2003) A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349: 427–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap T.A., Eisen T.G. (2006) Adjuvant therapy of renal cell carcinoma. Clin Genitourin Cancer 5: 120–130 [DOI] [PubMed] [Google Scholar]
- Yee K.W., Zeng Z., Konopleva M., Verstovsek S., Ravandi F., Ferrajoli A., et al. (2006) Phase I/II study of the mammalian target of rapamycin inhibitor everolimus (Rad001) in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res 12: 5165–5173 [DOI] [PubMed] [Google Scholar]
- Zisman A., Pantuck A.J., Dorey F., Said J.W., Shvarts O., Quintana D., et al. (2001) Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol 19: 1649–1657 [DOI] [PubMed] [Google Scholar]