Abstract
Angiogenesis has been identified as a relevant target for melanoma experimental therapeutics, based on preclinical and clinical studies. A variety of angiogenesis inhibitors are currently being tested in both metastatic and adjuvant melanoma clinical trials. To date, the most promising evidence of benefit is based on a statistically nonsignificant trend in survival gain reported in a randomized phase II trial combining bevacizumab, a monoclonal antibody targeting vascular endothelial growth factor, with cytotoxic chemotherapy. Larger phase III studies are required to determine the true extent of clinical benefit with this class of agents. Key to these clinical trials is the need to include translational endpoints, since correlation of biological and clinical data will provide the opportunity to identify biomarkers predictive of treatment response. These biological studies will also aid our, as yet, poor understanding of the mechanism of action of angiogenesis inhibitors, as well as drug-related side effects. Finally, if these trials show meaningful clinical benefit, then careful consideration will need to be given when designing second-generation trials, in the light of novel gene-directed therapies currently showing promise in melanoma.
Keywords: angiogenesis, angiogenesis inhibitors, melanoma, treatment, vascular endothelial growth factor
Introduction
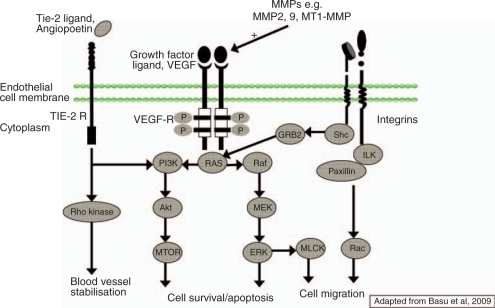
Evidence suggests that most tumours cannot grow beyond 1–2 mm without the need to establish an independent blood supply, which is generated by the process known as angiogenesis. In 1971, Judah Folkman described angiogenesis as being essential for tumour growth and proposed that its inhibition might be an effective therapeutic approach against cancer [Folkman, 1971]. Subsequently, a series of putative angiogenic factors were described. Vascular endothelial growth factor (VEGF) was cloned in 1989 and appears to be the most relevant promoter of angiogenesis in both normal conditions and malignant disease (Figure 1 and Table 1) [Ellis and Hicklin, 2008; Ferrara et al. 2003; Hanahan and Weinberg, 2000; Hanahan and Folkman, 1996]. It is now known that VEGF comprises a family of five glycoproteins (VEGF-A, -B, -C, -D and placental growth factor). The primary regulator of VEGF secretion is the hypoxic microenvironment, which is mediated by the transcription factor hypoxia-inducible factor 1-α (HIF-1α). VEGF-A (commonly referred to as VEGF) is overexpressed in a variety of human tumours. The VEGF ligands bind with differing affinities to the extracellular domains of three structurally similar receptor tyrosine kinases: VEGFR-1, -2 and -3. VEGFR-1 and -2 are expressed on the surface of most endothelial cells and bind VEGF-A. VEGFR-3 is expressed on lymphatic endothelial cells and is primarily involved in lymphangiogenesis. It does not bind VEGF-A, but does bind other VEGF isoforms. VEGFR-2 binding with VEGFs appears to be the major mediator of proliferation, chemotaxis, prosurvival and permeability enhancing effects in cells.
Figure 1.
Pathways implicated in angiogenesis.
Table 1.
Role of vascular endothelial growth factor (VEGF) in promoting angiogenesis.
| • Regulates new blood vessel growth by controlling endothelial cell activation, survival, migration, invasion, proliferation, chemotaxis of bone-marrow-derived progenitor cells. |
| • Promotes survival of immature vasculature. |
| • Promotes vascular function: flow (vasodilatation) and permeability. |
| • Stimulates lymphangiogenesis |
| - growth of new lymphatic vessels often accompanies angiogenesis |
| - VEGF overexpression leads to functionally abnormal lymphatic vessels in experimental models. |
| • Affects the immune response |
| - ↓ dendritic cell maturation |
| - ↑ survival and migration of immune cells. |
The first report of angiogenesis inhibition provided meaningful clinical benefit was published in 2004 when the combination of the monoclonal antibody, bevacizumab (Avastin), which targets VEGF, with conventional chemotherapy was shown to significantly improve survival in patients with metastatic colorectal cancer compared with chemotherapy alone [Hurwitz et al. 2004]. Bevacizumab has now been licensed for use in a variety of tumour types, supporting accumulating preclinical evidence that angiogenesis is a process which is common to all cancers. There are currently over 20 VEGF-targeted agents in clinical trials and many more novel agents for which angiogenesis inhibition is thought to contribute to their mechanism of action. Melanoma is a highly vascular tumour and theoretically should be amenable to treatment with angiogenesis inhibitors. There is a good scientific rationale to support this argument; however, the clinical evidence of benefit from employing this strategy has yet to be convincingly established. This may reflect a lack of full understanding of the mechanisms responsible for the antitumour activity of angiogenesis inhibitors, as well as a need to better understand how the role of angiogenesis and the microenvironment in the different stages of melanoma progression. Thus, alongside ongoing clinical trials, biological studies are crucial to informing whether angiogenesis inhibition is likely to offer clinical benefit to melanoma patients in the coming years. While angiogenesis inhibitors are being established in clinical practice for a number of tumour types, identification of biomarkers predicting for response has proved elusive. Affordability and toxicity pose problems to both healthcare providers and patients alike. Finally, if angiogenesis inhibition is shown to be effective, their place needs to be viewed within the growing portfolio of genetically driven therapies currently in development for the treatment of melanoma.
Angiogenesis is a valid target in melanoma therapeutics
The evidence base for angiogenesis being relevant to melanoma progression and metastasis has recently been summarised in two reviews [Basu et al. 2009; Streit and Detmar, 2003]. The purpose of this review is therefore not to reiterate this evidence, but the most compelling data will be summarized.
The overproduction of VEGF and its association with VEGFR expression promotes melanoma cell growth and survival through MAP kinase and phosphatidyl inositol-3-kinase (PI3K) signalling pathways [Graells et al. 2004]. This suggests, at least in vitro, that melanoma proliferation may involve a VEGF-dependent autocrine loop. Immunohistochemical studies suggest that VEGF is expressed by 20–77% of human primary melanomas [Potti et al. 2003; Simonetti et al. 2002]. Elevated VEGF expression and other soluble pro-angiogenic factors have been noted at both the mRNA and protein level, and have been demonstrated to correlate strongly with poor clinical outcome in melanoma patients [Goydos and Gorski, 2003; Poon et al. 2001; Ugurel et al. 2001]. Using immunoenzymatic techniques, raised serum VEGF levels have been observed in melanoma patients with advanced disease and a higher rate of relapse was noted in patients with resected primary melanoma whose serum VEGF increased during follow up [Osella-Abate et al. 2002]. In the largest tissue microarray study performed to interrogate the VEGF pathway in melanoma, over 1000 pathological specimens of melanoma and benign naevi were assessed quantitatively for expression of VEGF, VEGFR-1 and VEGFR-2. Expression of all three proteins was found to be higher in malignant melanocytes when compared with their benign counterparts samples and VEGF and VEGFR-2 were expressed in higher amounts in metastatic compared with primary melanoma [Mehnert et al. 2007]. Serum VEGF, VEGF-C and VEGFR-3 have also been noted to be significantly higher in metastatic melanoma patients compared with healthy controls, with higher serum VEGFR-3 levels in patients with high tumour burden and in nonresponding patients compared with responding patients, suggesting that VEGF isoforms and receptors important in lymphangiogenesis may play an important role in outcome of melanoma patients [Mouawad et al. 2009]. Inhibition of tumour growth has been achieved in different melanoma xenograft models by various anti-VEGF strategies [Li et al. 2002; Wedge et al. 2002; Oku et al. 1998].
Taken as a whole, these data justify exploratory studies targeting the VEGF pathway as a means of more effectively treating melanoma.
Angiogenesis is implicated in the mechanism of action of historical melanoma therapies
Prior to the development of specific inhibitors of angiogenesis, several drugs historically associated with melanoma treatment are now thought to exert at least some of their antitumour effect by inhibiting angiogenesis. These include thalidomide, interferon-γ and interferon-α. While thalidomide and interferon-γ have been rejected as being ineffective treatment for melanoma, interferon-α is licensed for use as adjuvant therapy and remains a standard of care at least in North America. Clinical trials consistently show that interferon-α delays time to relapse, while evidence supporting a survival benefit has only been hinted at by meta-analysis [Wheatley et al. 2007]. Interferon-α is a cytokine with pleiotropic cellular functions, including immunomodulatory, antiviral, antiproliferative, and anti-angiogenic effects. Clinical studies demonstrate that interferon-α treatment can induce impressive responses in angioproliferative diseases such as Kaposi’s sarcoma and hemangiomas. Preclinical studies suggest that the anti-angiogenic properties may be associated with regulation of endothelial cell motility and survival, as well as inhibition of other molecules such as basic fibroblast growth factor, interleukin 8 and matrix metalloproteinases (MMPs), all of which appear to be involved in the angiogenic response [Indraccolo, 2010]. The contribution angiogenesis inhibition plays in determining how interferon-α therapy delays time to relapse in melanoma patients is not known.
VEGF-targeted agents tested in melanoma
VEGF-targeted therapies were initially developed with the notion that they would inhibit new blood vessel growth and thus starve tumours of necessary oxygen and nutrients. It has become increasingly apparent that the therapeutic benefit associated with VEGF-targeted therapy is more complex than this, involving multiple mechanisms [Ellis and Hicklin, 2008]. VEGF-targeted therapy has an impact on numerous cell types within the tumour microenvironment, including endothelial cells, haematopoietic progenitor cells, dendritic cells and tumour cells. It is not yet clear whether the various mechanisms of action are dependent on tumour type. Currently, VEGF-targeted monotherapy has only been shown to be effective in renal cell and hepatocellular carcinoma, whereas it only appears to confer a benefit in epithelial cancers when combined with cytotoxic chemotherapy.
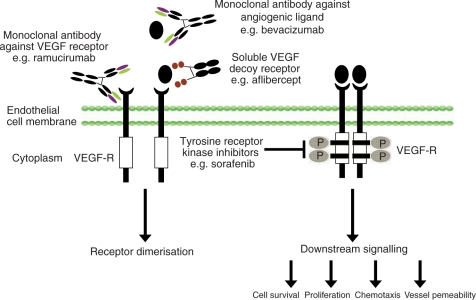
Angiogenesis inhibitors currently in clinical practice primarily target the VEGF ligand/receptor pathway (Figure 2). They largely fall into two camps: tyrosine kinase inhibitors (recognized by their generic name ending in ‘-ib’) and monocolonal antibodies (generic names ending in ‘-ab’). The two sets of agents contrast one another in that tyrosine kinase inhibitors are oral small molecules which act intracellularly and are selective rather than specific in their ability to inhibit certain tyrosine kinase receptors. Their ability to block enzyme phosphorylation of more than one target polypeptide may be advantageous in that multiple signalling pathways might be affected; however, this might also be relevant to generating drug-related side effects, which can be problematic.
Figure 2.
Vascular endothelial growth factor (VEGF) signalling inhibition.
Monocolonal antibodies, on the other hand, are delivered by intermittent intravenous infusion and have a specific defined inhibitory role outside of the cell or at the cell surface. The most promising agents to date intercept VEGF ligand from binding to its receptor at the cell surface. The resulting effects on tumour interstitial pressure and blood vessel permeability, a process described as ‘vessel normalization’, suggests that these antibodies might enhance the delivery of chemotherapy to tumour cells [Jain, 2001] in addition to possessing de novo antitumour activity. These antibodies are also not without side effects, the most common being hypertension, proteinuria, as well as increased incidence of thrombo-embolic events and bleeding episodes, reflecting the significant role played by VEGF signalling in regulating normal vasculature.
Both classes of drugs are being tested in melanoma.
VEGFR tyrosine kinase inhibitors
Most reports of VEGF receptor tyrosine kinase inhibitors tested in metastatic melanoma patients are based on phase II clinical trials (Table 2). The first published study of 20 patients receiving the selective VEGFR-2 inhibitor, semaxinib (SU5416, Sugen), reported no objective responses [Kuenen et al. 2003]. Following the groundbreaking news that a substantial proportion of melanomas carried mutations in the BRAF gene [Davies et al. 2002], clinical trials of sorafenib were promptly initiated. Although originally developed as a BRAF inhibitor, sorafenib also selectively inhibits VEGFR-2 and -3, as well as having some effect on platelet-derived growth factor receptor (PDGFR). Either as single agent [Eisen et al. 2006], or combined with conventional dacarbazine [McDermott et al. 2008], temozolamide [Amaravadi et al. 2009], or carboplatin/paclitaxel chemotherapy as first- or second-line treatment [Hauschild et al. 2009], sorafenib has not been shown to improve the standard of care. Even so, these studies were important in expanding our knowledge of drug-related toxicities, which are now well recognized with this class of agent: namely hand–foot skin reactions, hypertension, fatigue and gastrointestinal toxicities. Studies combining sorafenib with other cytotoxic drugs, hormonal therapies, immunotherapy and targeted agents such as bevacizumab are still ongoing.
Table 2.
Results of phase II and III* trials testing selective vascular endothelial growth factor receptor tyrosine kinase inhibitors in metastatic melanoma.
| Author | Description | Regimen | Number of patients | Response rate (PRs only) (%) | Stable disease (%) | Median PFS | Median OS |
|---|---|---|---|---|---|---|---|
| Kuenen et al. [2008] | First line | Semaxanib | 20 | 0 | |||
| Fruehauf et al. [2008] | First line and previously treated | Axitinib | 32 | 16 | 2.3 months | 6.8 months | |
| Cook et al. [2010] | First line and previously treated | Vatalanib | 34 | 3 | 32 | 1.8 months | 6.5 months |
| Decoster et al. [2009] | Previously treated | Sunitinib | 18 | 11 | 28 | ||
| Eisen et al. [2006] | Pre-treated | Sorafenib | 37 | 19 | 11 weeks | ||
| Kim et al. [2009] | Pre-treated | Sorafenib and temsirolimus | 21 | 42.8 | |||
| Hauschild et al. [2009] | Previously treated | Sorafenib and paclitaxel and carboplatin | 135 | 12 | 17.9 weeks | ||
| Placebo and paclitaxel and carboplatin | 135 | 11 | 17.4 weeks | ||||
| Amaravadi et al. [2007] | First line (no brain metastases) | Sorafenib and temozolomide (standard dose) | 38 | 24 | 39 | ||
| (known brain metastases) | Sorafenib and temozolomide (extended dose) | 40 | 15 | 55 | |||
| Previously treated(no brain metastases) | Sorafenib and temozolomide (standard dose) | 35 | 17 | 49 | |||
| Sorafenib and temozolomide (extended dose) | 34 | 0 | 27 | ||||
| Eisen et al. [2007] | First line | Sorafenib and dacarbazine | 83 | 10 | 41 | 14 weeks | 41 weeks |
| McDermott et al. [2008] | First line | Sorafenib and dacarbazine | 51 | 24 | 21.1 weeks | ||
| Placebo and dacarbazine | 50 | 12 | 11.7 weeks |
IFN, interferon; OS, overall survival; PFS, progression-free survival; PRs, partial responses.
Axitinib (AGO13736, Pfizer), a multikinase pan-VEGFR inhibitor (inhibiting VEGFR- 1, -2 and -3), as well as PDGFR and c-kit, was tested in a phase II trial in metastatic melanoma, but the overall response rate of 15.6% and median overall survival (OS) of 6.8 months were unimpressive [Fruehauf et al. 2008]. Again, common treatment-related adverse effects included fatigue, hypertension and diarrhoea. In this study, an unplanned retrospective subgroup analysis of patients treated with axitinib in this and other studies involving other tumour sites identified longer OS in patients who developed hypertension (diastolic pressure >90 mmHg). The investigators proposed that hypertension might be a marker of treatment response [Rini et al. 2008]. This hypothesis has not been substantiated in other trials testing similar agents. Even so, Pfizer has not progressed further trials of axitinib in melanoma.
Other negative trials testing various VEGFR selective tyrosine kinase inhibitors, including sunitinib [Decoster et al. 2009], dovitinib [Kim et al. 2008] and vatalanib [Cook et al. 2010], have followed. Clearly, these results are disappointing. However, they were probably destined to fail from the outset for the following reasons. First, the negative outcomes may simply reflect the chemoresistant nature of metastatic melanoma, which has hitherto plagued our ability to identify any effective systemic therapy for these patients. Second, it is now apparent that tyrosine kinase inhibitors are not likely to be cytotoxic, but cytostatic in nature, thus applying conventional trial design using an objective response rate as the primary endpoint is not ideal. Third, attempting to block angiogenesis in already established metastatic tumours is probably the equivalent of trying to lock the gate once the horse has bolted.
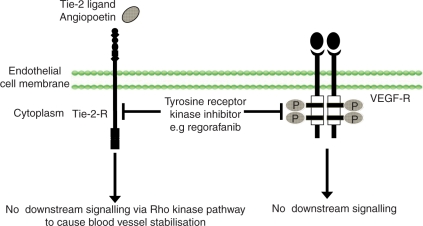
New agents with novel and distinct antiangiogenic profiles are being identified in drug development programmes which inhibit kinases across multiple different signalling pathways and show ‘tandem’ inhibition of kinases within the same signalling path. The attraction of this method of inhibition is that a single molecule may bypass the activity of downstream effectors that have undergone feedback upregulation. An example of this concept is regorafenib (Bayer), which inhibits both VEGFR-2 and the Tie-2 receptor tyrosine kinase. Tie-2 receptor activation by its ligand, angiopoietin, appears to be involved in blood vessel stabilization and VEGF is also able to activate Tie-dependent signalling pathways (Figure 3). The dual receptor inhibitory effect of regorafenib potentially offers advantages over inhibition of the VEGF axis alone, which have yet to be evaluated in clinical trials.
Figure 3.
Tandem kinase inhibition.
When testing these agents, the trial endpoints used to evaluate efficacy need to be carefully considered. If, as many believe, for ethical reasons these agents need to be tested in advanced disease in the first instance, the trials should incorporate endpoints other than clinical objective response. Disease control rate (the proportion of all complete responses, partial responses and stable disease) or progression-free survival (PFS) may be more relevant endpoints to evaluate agents predicted to generate cytostasis. Inclusion of pharmacodynamic endpoints is critical to provide some early biological, pharmacological or functional signal of proof of principle, in order to avoid discarding drugs inappropriately from further testing in melanoma (Table 3). For inhibitors of angiogenesis, novel imaging tools are available which can reliably assess tumour vascularity and permeability. Thus, dynamic contrast enhanced (DCE) MRI and DCE ultrasound have been incorporated into early phase studies of dovitinib [Kim et al. 2008], vatalanib [Cook et al. 2010] and sorafenib combined with temozolamide [Robert et al. 2009] give some early indication that tumour devascularization may be associated with a subset of melanoma patients who might respond to treatment. Key to the new era of personalized medicine is the ability to identify individuals most likely to respond to a specific treatment. In the context of VEGF-targeted therapy, identification of biomarkers predictive for response has proved elusive to date [Murukesh et al. 2010]. Until they are discovered and validated, drug costs may remain prohibitive to clinical use in most cases, and certainly within the UK National Health Service.
Table 3.
Potential biomarkers for evaluating angiogenesis inhibitors.
| Noninvasive | Minimally invasive | Invasive |
|---|---|---|
| Computed tomography imaging | Blood circulating endothelial cells | Tissue biopsy |
| Positron emission tomography imaging | Blood circulating endothelial progenitor cells | Interstitial fluid pressure measurement |
| Dynamic contrast enhanced MRI or ultrasound | Protein levels in plasma (ie. VEGF, bFGF | Measurement of tissue oxygenation |
| Urine protein (MMP, VEGF) | Protein levels in ascites/pleural effusion | Skin wound healing |
| BP monitoring | HU177 levels (cryptic collagen epitope) |
bFGF, basic fibroblast growth factor; BP, blood pressure; MMP, matrix metalloproteinase; VEGF, vascular endothelial growth factor.
Monoclonal antibodies to VEGF
While there is now a plethora of small molecule tyrosine kinases targeting angiogenesis, the number of monoclonal antibodies remains remarkably small. Bevacizumab, a humanized monoclonal IgG antibody directed against VEGF ligand, remains the only agent in this class licensed for use in several cancer types. A growing number of small studies conducted in metastatic melanoma have been undertaken combining bevacizumab with interferon or cytotoxic chemotherapy and have shown modest activity (Table 4). Recently, the results of a larger randomized phase II trial tested carboplatin and paclitaxel chemotherapy with or without bevacizumab as first-line therapy for metastatic melanoma (the BEAM trial) were reported [O’Day et al. 2009]. The addition of antibody to chemotherapy improved PFS by 22% (95% confidence interval [CI]: 0.55–1.13; p = 0.19), and OS by 21% (hazard ratio 0.78) compared with chemotherapy alone. The primary endpoint of the study was improvement in PFS, which was not met, but the OS gain was intriguing. A subsequent definitive trial is planned.
Table 4.
Results of phase II clinical trials with bevacizumab in metastatic melanoma.
| Author | Description | Regimen | Number of patients | Response rate (%) | Partial response (%) | Stable disease (%) | Median PFS (months) | Median OS (months) |
|---|---|---|---|---|---|---|---|---|
| Varker et al. [2007] | Previously treated | Bevacizumab | 16 | 3 | 8.5 | |||
| Bevacizumab and IFN | 16 | 3 | 10 | |||||
| González-Cao et al. [2008] | Previously treated | Bevacizumab and weekly paclitaxel | 12 | 17 | 17 | 58 | 3.7 | 7.8 |
| Perez et al. [2009] | Previously treated | Bevacizumab and weekly paclitaxel and carboplatin | 53 | 17 | 17 | 57 | 6 | 12 |
| Peyton et al. [2009] | Previously treated | Bevacizumab and everolimus | 28 | 4 | 4 | 68 | 3.5 | |
| Si et al. [2009] | Previously treated acral | Bevacizumab and temozolomide and sorafenib | 11 | 18 | 18 | 54.5 | ||
| Wyman et al. [2007] | First line and Previously treated | Bevacizumab and erlotinib | 29 | 9 | 9 | 22 | 3.2 | |
| Munzone et al. [2007] | First line | Bevacizumab and dacarbazine | 8 | 20 | 20 | 40 | ||
| Boasberg et al. [2009] | First line | Bevacizumab and nab-paclitaxel | 41 | 6.25 | 91% (6 months) | |||
| 83% (12 months) | ||||||||
| O’Day et al. [2009] | First line | Bevacizumab and paclitaxel and carboplatin | 143 | 25.5 | 23.4 | 50.4 | 5.6 | 12.3 |
| Paclitaxel and carboplatin | 71 | 16.4 | 14.9 | 37.4 | 4.2 | 8.6 |
IFN, interferon; OS, overall survival; PFS, progression-free survival.
Bevacizumab combined with a cremaphor-free nanoparticle albumin-bound paclitaxel formulation (Nab paclitaxel, Abraxane) designed to improve paclitaxel tumour cell penetration [Boasberg et al. 2009] has been investigated as first-line therapy in patients with metastatic melanoma. Over half of the patients enrolled had extremely poor prognosis disease, yet the 12-month survival rate of 83% was extremely encouraging.
The results from clinical studies of bevacizumab in metastatic melanoma are not yet robust enough to change practice and larger phase III randomized trials are still required. Even so, with the knowledge that angiogenesis is an essential prerequisite for the establishment of systemic metastases, a natural question to investigate is whether anti-VEGF treatment should be administered prior to development of metastatic disease, in the preventative setting. Large numbers of patients undergo surgical resection of primary melanoma, which for many will be curative. However, a significant proportion of patients remain at high risk of recurrence due to invasion of melanoma early into the dermal tissues. Despite huge international efforts to identify effective treatment to improve outcomes after surgery (‘adjuvant therapy’) in melanoma patients at high risk of recurrence, no systemic therapy has yet been shown convincingly to improve survival. Most studies have focused on immunotherapy approaches. Alternatively, inhibition of VEGF might suppress the angiogenic switch to malignancy and prevent micrometastases from establishing themselves. This hypothesis is being tested in the currently recruiting United Kingdom Adjuvant Trial of AVASTin in high-risk Melanoma (AVAST-M), where bevacizumab is being given for a period of 1 year to melanoma patients in the adjuvant setting and survival will be compared with a randomized control group who undergo routine follow up after their surgery [Corrie et al. 2009].
This trial is important for a number of reasons. First, with 1320 patients entered, it will be the largest UK adjuvant melanoma trial conducted to date, and one of the largest worldwide. Second, it will be the largest existing dataset of patients treated with bevacizumab monotherapy, thus affording the opportunity to closely monitor and study drug-related side effects. Third, archived tumour tissue, paired normal tissue and blood are being collected on all recruited patients prior to entry, with additional samples being collected at relapse. This unique tissue bank will provide the opportunity to study the relevance of angiogenesis in melanoma progression as well as identification of novel markers of both prognosis and treatment response. The trial is recruiting on time, is expected to complete accrual in late 2012, with a first interim analysis planned by the end of 2013.
VEGF-trap (soluble VEGF receptor)
Other routes of intercepting the VEGF ligand/VEGFR axis are actively being investigated in melanoma. Aflibercept is a fusion protein that incorporates portions of human VEGFR-1 and VEGFR-2, fused to the constant region of human IgG1. This acts as a soluble decoy VEGF receptor which, in preclinical studies, showed a favourable profile over other VEGF inhibitors, with increased binding affinity (dissociation constant 0.5 picomolar) for VEGF-A, as well as binding of placental growth factor [Holash et al. 2002]. Its efficacy has been tested in a phase II study of treatment-naive patients with metastatic melanoma and early results of the interim analysis of the first 21 patients (which included patients with ocular melanoma) were reported recently, in which one patient achieved a complete response [Tarhini et al. 2009].
VEGFR antibodies
Anti-angiogenic antibodies have also been directed against the extracellular domain of VEGFRs thereby preventing the binding interaction with VEGF ligand. A fully humanized anti-VEGFR-2 IgG1 monoclonal antibody, ramucirumab (IMC-1121B, Imclone), raised interest as a potential therapeutic option in melanoma when a phase I dose-escalation study of IMC-1121B in 37 patients with advanced cancer showed promising results, with one partial response lasting 31 weeks seen in a melanoma patient [Spratlin et al. 2010]. On the basis of this, a multicentre, randomized, open-label phase II study of ramucirumab with or without dacarbazine has enrolled treatment-naive metastatic melanoma patients and results are awaited with interest.
Other novel strategies targeting angiogenesis
MMPs
Remodelling of the extracellular matrix during angiogenesis is accomplished largely through the activity of MMPs. Angiogenic mitogens such as basic fibroblast growth factor (bFGF) and VEGF can stimulate the production of MMPs by capillary endothelial cells. MMP-2 and MMP-9 levels have frequently been reported to be elevated in human malignancies and MMP inhibition using BB-94 (batimastat), has been shown to reduce vascularity and growth of liver metastases in a B16F1 mouse melanoma model [Chirivi et al. 1994]. These findings suggest that MMP activity is critical to both the initiation of angiogenesis and to the maintenance of the growing vascular bed required to support tumour growth and metastasis. However, MMP activity has been shown to generate endogenous inhibitors of angiogenesis, including angiostatin and endostatin, so MMPs may act as both positive and negative regulators of angiogenesis in cancer. This may explain why MMP inhibitors have proved so unsuccessful in clinical practice to date. Marimastat was one of the first agents to enter the clinic, was tested in a variety of cancer types including melanoma [Quirt et al. 2002], all of which singularly failed, largely due to a lack of specificity. Peptidomimetic MMP inhibitors mimic the structure of collagen at the MMP cleavage site and musculoskeletal damage to normal tissues has limited patient tolerance.
More recent evidence suggests that some MMPs may possess antitumour properties of their own, for instance MMP8 has been identified as a tumour suppressor in melanoma, so broad spectrum blockade of multiple MMPs would not be expected to be beneficial [Palavalli et al. 2009]. Trials testing newer generations of more specific inhibitors of the MMPs that are unambiguously implicated in the progression of melanoma and angiogenesis may prove more fruitful [Pavlaki and Zucker, 2003].
As argued with other inhibitors of angiogenesis, it may be that MMP inhibitors would actually be more effective in earlier stages of cancer progression, which might also allow smaller, better tolerated doses to be delivered to patients. This hypothesis was borne out in the RIP-Tag2 model of pancreatic carcinogenesis, when the broad spectrum MMPI, batimastat, was effective in the early stages of progression. While research is ongoing to better understand the functions of MMPs and to identify effective MMP-targeted therapy, a variety of drugs with MMP inhibitory action are in clinical trial. Of interest to melanoma is a current study of genestein, the soy isoflavone with MMP-2 and -9 inhibitory activity, currently being tested in a phase II prevention study involving melanoma patients among other tumour types [Roy et al. 2009].
Anti-integrin strategies
Integrins are members of the immunoglobulin superfamily, comprising noncovalently linked heterodimers of α- and β-subunits. They act as cell surface receptors for extracellular membrane proteins and, via these interactions, they mediate cell attachment and migration. Integrins on endothelial cells have been implicated in the control of cell growth, migration and survival during angiogenesis. The αvβ3 integrin has been shown to be upregulated in endothelial cells by angiogenic factors in response to inflammation, wound healing and tumourigenesis [Mahabeshwar and Byzova, 2007; Brooks et al. 1994]. Since then, numerous members of the integrin family have been implicated in angiogenesis.
Antagonism of integrins using function blocking antibodies, peptides and small molecule inhibitors has been tested in a several melanoma clinical trials. The best-characterized inhibitors are the function-blocking anti-integrin antibodies such as etaracizumab (MEDI-522, Abegrin), a humanized antibody against αvβ3. Its early prototype, Vitaxin was well tolerated in a phase I trial [Gutheil et al. 2000]. Etaracizumab has since been evaluated in a randomized phase II trial as monotherapy versus a combination of etaracizumab plus dacarbazine in 112 metastatic melanoma patients. Although no objective responses were demonstrated in the etaracizumab-only arm compared with 13% in the combination arm, surprisingly, patients who received etaracizumab alone had a median OS greater than 12 months, compared with 9.4 months for the combination arm. The 1-year survival rate was 53% for patients receiving etaracizumab alone and 42% for those on combination treatment, while both groups were noted to have better survival than is usually reported or most first-line regimens in metastatic melanoma. Unfortunately, these early promising results were not borne out with longer patient follow up and this antibody is no longer being investigated in metastatic melanoma [Schadendorf et al. 2009].
The fully human monoclonal antibody, intetumumab (CNTO 95, Centocor), blocks both αvβ3 and αvβ5 integrins, and inhibits angiogenesis and tumour growth by 80% in human melanoma xenografts in vivo [Trikha et al. 2004]. A four-arm randomized phase II study compared two doses of intetumumab given alone with the higher dose of intetumumab combined with DTIC (dacarbazine) versus DTIC alone in metastatic melanoma patients. At 2 years of follow up, a trend towards improved PFS and OS with the highest dose of antibody with or without dacarbazine has been reported [Loquai et al. 2009]. Further evaluation of this agent is justified.
The αv integrins have also been targeted using a cyclic peptide inhibitor of integrins αvβ3 and αvβ5, cilengitide, which in preclinical studies was observed to increase the antitumour activity of temozolomide against melanoma [Tentori et al. 2008]. Cilengitide was well tolerated in phase I trials and mature data are awaited from a phase II trial that has been completed in melanoma patients [Kim et al. 2007]. A chimeric mouse–human anti-α5β1 antibody M200 (volociximab), was developed, since β1 integrin on endothelial cells appears to be required for ligation of fibronectin during angiogenesis. It has been tested in melanoma patients in combination with dacarbazine with evidence of response in 62% of patients [Kuwada, 2007]. While it is too early to judge how useful integrin inhibitors will be in clinical practice, it is of note that the side-effect profile of these agents is generally mild. This favourable attribute would suggest their use in combination with other drugs such as anti-VEGF agents would be justified, to provide a broader coverage of angiogenesis inhibition.
Future challenges for angiogenesis inhibition
The list of angiogenesis inhibitors discussed here is by no means exhaustive. As the mechanisms governing angiogenesis are unravelled, new opportunities for therapeutic intervention are being generated. Other classes of agents currently in clinical trial include those mimicking endogenous angiogenesis inhibitors (e.g. endostatin) [Cui et al. 2009; Ling et al. 2007], inhibitors of placental growth factor [Fischer et al. 2007], and agents blocking HIFα, the key molecule in hypoxia signalling [Patnaik et al. 2009; Semenza, 2007; Hewitson and Schofield, 2004]. Many of these classes of agents are still at the early stages of their development and over the next decade, the clinical impact of targeting angiogenesis in melanoma will become apparent.
As already alluded to, key to their evaluation will be the need to design clinical trials appropriately, testing in the most appropriate patient population and ensuring pharmacodynamic endpoints are given adequate consideration when judging signals for further development, since the likelihood of generating objective responses over and above the level seen consistently with conventional therapy for metastatic melanoma is extremely remote. Pharmacodynamics are also crucial for identifying biomarkers of response.
A precedent has already been set for evaluating angiogenesis inhibitors at an early stage of melanoma progression. Furthermore, there is increasing evidence that the angiogenic process begins in the premalignant stages of most cancers. In premalignant melanoma, microvessel density has been reported to be significantly increased in dysplastic nodules compared with benign naevi. Thus, angiopreventive strategies warrant exploration, if appropriate agents could be identified [Menakuru et al. 2008].
Melanoma is entering a new era of genetically driven treatment, affording the potential for ‘stratified’ therapy in future years. The first trials of treatments being tested specifically in BRAF mutant and c-kit mutant patients are now underway. Even so, the majority of melanoma patients will not carry these mutations, and the benefit of treatment in those who do may be short lasting, so the need to find more effective treatments and combination regimens for this devastating disease remains absolutely paramount.
While focusing on this first generation of angiogenesis inhibitor trials for signals of activity, melanoma investigators must maintain an awareness of findings with similar agents tested in other cancer types. Drug-related toxicities including hypertension, skin toxicity and fatigue limit patient tolerance of VEGF-targeted therapy. These need to be better understood and managed effectively. Questions about optimal duration of therapy [Wolmark et al. 2009] and the practicality of delivering what might be long-term treatment over many years have been raised from the first trials of adjuvant therapy with bevacizumab undertaken in colon cancer. Finally, emerging resistance to long-term treatment [Hanahan and Bergers, 2008] will inevitably pose further challenges to clinical application.
Summary
Treatment of metastatic melanoma remains unsatisfactory, since, to date, no systemic therapy has been shown to improve patient survival. There is therefore a clear case for testing new therapies in the first-line setting. VEGF is the principal ligand thought to regulate angiogenesis in human tumours. In melanoma, altered expression of VEGF and other proangiogenic ligands, in addition to their receptors, has been found to correlate with both disease stage and progression. However, strategies to block VEGF ligands and receptors have not yet translated into meaningful clinical benefit to patients. The most promising evidence of activity in melanoma is based on a single randomized phase II study with the VEGF-directed monoclonal antibody, bevacizumab, combined with cytotoxic chemotherapy. The VEGF ligand/receptor pathway is by no means the only mechanism for inhibiting angiogenesis and many new agents with diverse mechanisms of action are at different stages of preclinical and clinical development. Careful attention needs to be paid to the design of key clinical trials to ensure both clinical and pharmacodynamic endpoints are weighted appropriately when making go/no-go decisions to develop new treatments. Whether or not these trials generate evidence of benefit to patients, they will surely contribute significantly to the understanding of angiogenesis-directed therapy in melanoma specifically, with wider implications for its use in all cancers where there is an established role.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
P. Corrie is chief investigator of the AVAST-M clinical trial and is in receipt of an unrestricted educational grant from L Hoffman La Roche in support of this trial.
References
- Amaravadi R.K., Schuchter L.M., McDermott D.F., Kramer A., Giles L., Gramlich K., et al. (2009) Phase II trial of temozolomide and sorafenib in advanced melanoma patients with or without brain metastases. Clin Cancer Res 15: 7711–7718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu B., Biswas S., Wrigley J., Sirohi B., Corrie P. (2009) Angiogenesis in cutaneous malignant melanoma and potential therapeutic strategies. Expert Rev Anticancer Ther 9: 1583–1598 [DOI] [PubMed] [Google Scholar]
- Boasberg P., Cruickshank S., Hamid O., O'Day S., Weber R., Spitler L. (2009) Nab-paclitaxel and bevacizumab as first-line therapy in patients with unresectable stage III and IV melanoma. J Clin Oncol 27: Abstract 9061 [DOI] [PubMed] [Google Scholar]
- Brooks P.C., Montgomery A.M., Rosenfeld M., Reisfeld R.A., Hu T., Klier G., et al. (1994) Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 79: 1157–1164 [DOI] [PubMed] [Google Scholar]
- Chirivi R.G., Garofalo A., Crimmin M.J., Bawden L.J., Stoppacciaro A., Brown P.D., et al. (1994) Inhibition of the metastatic spread and growth of B16-BL6 murine melanoma by a synthetic matrix metalloproteinase inhibitor. Int J Cancer 58: 460–464 [DOI] [PubMed] [Google Scholar]
- Cook, N., Basu, B., Biswas, S., Kareclas, P., Mann, C., Palmer, C. et al. (2010) A phase 2 study of vatalanib in metastatic melanoma patients. Eur J Cancer (in press) [DOI] [PubMed]
- Corrie, P., Marshall, A., East, C., Dunn, J., Ahern, A., Lorigan, P. et al. (2009) Safety of adjuvant bevacizumab as treatment for melanoma patients at high risk of recurrence. In 7th World Congress on Melanoma, Abstract p237.
- Cui C., Chi Z., Yuan X., Si L., Sheng X., Shen L., et al. (2009) Endostatin combined with chemotherapy as first-line therapy for stage IV melanoma patients: A phase II clinical study. J Clin Oncol 27: Abstract e20003 [Google Scholar]
- Davies H., Bignell G.R., Cox C., Stephens P., Edkins S., Clegg S., et al. (2002) Mutations of the BRAF gene in human cancer. Nature 417: 949–954 [DOI] [PubMed] [Google Scholar]
- Decoster L., Broek I.V., Declerq D., et al. (2009) Activity of sunitinib in advanced malignant melanoma and its correlation with potential predictive biomarkers. Eur J Cancer Suppl 7: 577–578 [Google Scholar]
- Eisen T., Ahmad T., Flaherty K.T., Gore M., Kaye S., Marais R., et al. (2006) Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer 95: 581–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen, T., Marais, R., Affolter, A., Lorigan, P.,Ottensmeier, C., Robert, C., et al. (2007) An open-label phase II study of sorafenib and dacarbazine as first-line therapy in patients with advanced melanoma. J Clin Oncol 25: Abstract 8529.
- Ellis L.M., Hicklin D.J. (2008) VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer 8: 579–591 [DOI] [PubMed] [Google Scholar]
- Ferrara N., Gerber H.P., LeCouter J. (2003) The biology of VEGF and its receptors. Nat Med 9: 669–676 [DOI] [PubMed] [Google Scholar]
- Fischer C., Jonckx B., Mazzone M., Zacchigna S., Loges S., Pattarini L., et al. (2007) Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 131: 463–475 [DOI] [PubMed] [Google Scholar]
- Folkman J. (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285: 1182–1186 [DOI] [PubMed] [Google Scholar]
- Fruehauf J., Lutzky J., McDermott D., Brown C., Pithavala Y., Bycott P., et al. (2008) Axitinib (AG-013736) in patients with metastatic melanoma: A phase II study. J Clin Oncol 26: Abstract 9006 [Google Scholar]
- González-Cao M., Viteri S., Díaz-Lagares A., et al. (2008) Preliminary results of the combination of bevacizumab and weekly paclitaxel in advanced melanoma. Oncology 74: 12–16 [DOI] [PubMed] [Google Scholar]
- Goydos J.S., Gorski D.H. (2003) Vascular endothelial growth factor C mRNA expression correlates with stage of progression in patients with melanoma. Clin Cancer Res 9: 5962–5967 [PubMed] [Google Scholar]
- Graells J., Vinyals A., Figueras A., Llorens A., Moreno A., Marcoval J., et al. (2004) Overproduction of VEGF concomitantly expressed with its receptors promotes growth and survival of melanoma cells through MAPK and PI3K signaling. J Invest Dermatol 123: 1151–1161 [DOI] [PubMed] [Google Scholar]
- Gutheil J.C., Campbell T.N., Pierce P.R., Watkins J.D., Huse W.D., Bodkin D.J., et al. (2000) Targeted antiangiogenic therapy for cancer using Vitaxin: a humanized monoclonal antibody to the integrin alphavbeta3. Clin Cancer Res 6: 3056–3061 [PubMed] [Google Scholar]
- Hanahan D., Bergers G. (2008) Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 8: 592–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Folkman J. (1996) Patterns and emerging mechanisms of the angiogeneic switch during tumorigenesis. Cell 86: 353–364 [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. (2000) The hallmarks of cancer. Cell 100: 57–70 [DOI] [PubMed] [Google Scholar]
- Hauschild A., Agarwala S.S., Trefzer U., Hogg D., Robert C., Hersey P., et al. (2009) Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol 27: 2823–2830 [DOI] [PubMed] [Google Scholar]
- Hewitson K.S., Schofield C.J. (2004) The HIF pathway as a therapeutic target. Drug Discov Today 9: 704–711 [DOI] [PubMed] [Google Scholar]
- Holash J., Davis S., Papadopoulos N., Croll S.D., Ho L., Russell M., et al. (2002) VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA 99: 11393–11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz H., Fehrenbacher L., Novotny W., Cartwright T., Hainsworth J., Heim W., et al. (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335–2342 [DOI] [PubMed] [Google Scholar]
- Indraccolo S. (2010) Interferon-alpha as angiogenesis inhibitor: learning from tumour models. Autoimmunity 43: 244–247 [DOI] [PubMed] [Google Scholar]
- Jain R.K. (2001) Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 7: 987–989 [DOI] [PubMed] [Google Scholar]
- Kim K., Diwan A., Papadopoulos N., Bedikian A., Camacho L., Hwu P., et al. (2007) A randomized phase II study of EMD 121974 in patients (pts) with metastatic melanoma (MM). J Clin Oncol 25: 8548–8548 [Google Scholar]
- Kim K., Saro J., Moschos S., Hwu P., Tarhini A., Hwu W., et al. (2008) A phase I dose finding and biomarker study of TKI258 (dovitinib lactate) in patients with advanced melanoma. J Clin Oncol 26: Abstract v9026 [Google Scholar]
- Kim K.B., Davies M.A., Papadopoulos N.E., Bedidian A.Y., Hwu W., Woodward K., et al. (2009) Phase I/II study of the combination of sorafenib and temsirolimus in patients with metastatic melanoma. J Clin Oncol 27: Abstract 9026 [Google Scholar]
- Kuenen B.C., Tabernero J., Baselga J., Cavalli F., Pfanner E., Conte P.F., et al. (2003) Efficacy and toxicity of the angiogenesis inhibitor SU5416 as a single agent in patients with advanced renal cell carcinoma, melanoma, and soft tissue sarcoma. Clin Cancer Res 9: 1648–1655 [PubMed] [Google Scholar]
- Kuwada S.K. (2007) Drug evaluation: Volociximab, an angiogenesis-inhibiting chimeric monoclonal antibody. Curr Opin Mol Ther 9: 92–98 [PubMed] [Google Scholar]
- Li Y., Wang M.N., Li H., King K.D., Bassi R., Sun H., et al. (2002) Active immunization against the vascular endothelial growth factor receptor flk1 inhibits tumor angiogenesis and metastasis. J Exp Med 195: 1575–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y., Yang Y., Lu N., You Q.D., Wang S., Gao Y., et al. (2007) Endostar, a novel recombinant human endostatin, exerts antiangiogenic effect via blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of endothelial cells. Biochem Biophys Res Commun 361: 79–84 [DOI] [PubMed] [Google Scholar]
- Loquai, C., Pavlick, A., Lawson, D., Gutzmer, R., Richards, J., Gore, M.E. et al. (2009) Randomized phase II study of the safety and efficacy of a human anti-αv integrin monoclonal antibody (CNTO 95) alone and in combination with dacarbazine in patients with stage IV metastatic melanoma: 12-month results. J Clin Oncol 27: Abstract 9029.
- Mahabeshwar G.H., Byzova T.V. (2007) Angiogenesis in melanoma. Semin Oncol 34: 555–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott D.F., Sosman J.A., Gonzalez R., Hodi F.S., Linette G.P., Richards J., et al. (2008) Double-blind randomized phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: a report from the 11715 Study Group. J Clin Oncol 26: 2178–2185 [DOI] [PubMed] [Google Scholar]
- Mehnert J., McCarthy M., Aziz S., Sznol M., Flaherty K., Camp R., et al. (2007) VEGF, VEGFR1 and VEGFR2 expression in melanoma. J Clin Oncol 25: Abstract 8520–Abstract 8520 [Google Scholar]
- Menakuru S.R., Brown N.J., Staton C.A., Reed M.W.R. (2008) Angiogenesis in pre-malignant conditions. Br J Cancer 99: 1961–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouawad R., Spano J.P., Comperat E., Capron F., Khayat D. (2009) Tumoural expression and circulating level of VEGFR-3 (Flt-4) in metastatic melanoma patients: correlation with clinical parameters and outcome. Eur J Cancer 45: 1407–1414 [DOI] [PubMed] [Google Scholar]
- Munzone E., Testori A., Minchella I., Mosconi C., Passoni E., Verri M., et al. (2007) A phase II trial of dacarbazine (DTIC) and bevacizumab in patients with metastatic melanoma. J Clin Oncol 25: Abstract 8579 [Google Scholar]
- Murukesh N., Dive C., Jayson G.C. (2010) Biomarkers of angiogenesis and their role in the development of VEGF inhibitors. Br J Cancer 102: 8–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Day S., Kim K.J., Sosman A., Peterson A., Feng S., Minor D., et al. (2009) A randomized phase II study evaluating the activity of Bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated Advanced Melanoma. Eur J Cancer Suppl 7: Abstract 23LBA–Abstract 23LBA [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku T., Tjuvajev J.G., Miyagawa T., Sasajima T., Joshi A., Joshi R., et al. (1998) Tumor growth modulation by sense and antisense vascular endothelial growth factor gene expression: effects on angiogenesis, vascular permeability, blood volume, blood flow, fluorodeoxyglucose uptake, and proliferation of human melanoma intracerebral xenografts. Cancer Res 58: 4185–4192 [PubMed] [Google Scholar]
- Osella-Abate S., Quaglino P., Savoia P., Leporati C., Comessatti A., Bernengo M.G. (2002) VEGF-165 serum levels and tyrosinase expression in melanoma patients: correlation with the clinical course. Melanoma Res 12: 325–334 [DOI] [PubMed] [Google Scholar]
- Palavalli, L.H., Prickett, T.D., Wunderlich, J.R., Wei, X., Burrell, A.S., Porter-Gill, P. et al. (2009) Analysis of the matrix metalloproteinase family reveals that MMP8 is often mutated in melanoma. Nat Genet 41: 518–520. [DOI] [PMC free article] [PubMed]
- Patnaik A., Chiorean E., Tolcher A., Papadopoulos K., Beeram M., Kee D., et al. (2009) EZN-2968, a novel hypoxia-inducible factor-1α (HIF-1α) messenger ribonucleic acid (mRNA) antagonist: Results of a phase I, pharmacokinetic (PK), dose-escalation study of daily administration in patients (pts) with advanced malignancies. J Clin Oncol 27: Abstract 2564 [Google Scholar]
- Pavlaki M., Zucker S. (2003) Matrix metalloproteinase inhibitors (MMPIs): the beginning of phase I or the termination of phase III clinical trials. Cancer Metastasis Rev 22: 177–203 [DOI] [PubMed] [Google Scholar]
- Perez D.G., Suman V.J., Fitch T.R., Amatruda T., Morton R.F., Jilani S.Z., et al. (2009) Phase 2 trial of carboplatin, weekly paclitaxel, and biweekly bevacizumab in patients with unresectable stage IV melanoma: a North Central Cancer Treatment Group study, N047A. Cancer 115: 119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyton J.D., Spigel D.R., Burris H.A., et al. (2009) Phase II trial of bevacizumab and everolimus in the treatment of patients with metastatic melanoma: preliminary results. J Clin Oncol 27: Abstract 9027 [Google Scholar]
- Poon R.T., Fan S., Wong J. (2001) Clinical implications of circulating angiogeneic factors in cancer patients. J Clin Oncol 19: 1207–1225 [DOI] [PubMed] [Google Scholar]
- Potti A., Moazzam N., Tendulkar K., Javed N.A., Koch M., Kargas S. (2003) Immunohistochemical determination of vascular endothelial growth factor (VEGF) overexpression in malignant melanoma. Anticancer Res 23: 4023–4026 [PubMed] [Google Scholar]
- Quirt I., Bodurth A., Lohmann R., Rusthoven J., Belanger K., Young V., et al. (2002) Phase II study of marimastat (BB-2516) in malignant melanoma: a clinical and tumor biopsy study of the National Cancer Institute of Canada Clinical Trials Group. Invest New Drugs 20: 431–437 [DOI] [PubMed] [Google Scholar]
- Rini B., Scjiller J., Fruehauf J., Cohen E., Tarazi J., Rosbrook B., et al. (2008) Association of diastolic blood pressure (dBP) ≥ 90 mmHg with overall survival (OS) in patients treated with axitinib (AG- 013736). J Clin Oncol 26: Abstract 3543 [Google Scholar]
- Robert C., Lazar V., Lacroix L., Farace F., Lassau N., Dromain C., et al. (2009) Phase I/II study of the association of sorafenib and temozolimde (extended schedule) in patients with metastatic melanoma: A new clinical response profile with massive tumor necroses. J Clin Oncol 27: Abstract 9062 [Google Scholar]
- Roy R., Yang J., Moses M.A. (2009) Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol 27: 5287–5296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadendorf D., Algarra S.M., Bastholt L., Cinat G., Dreno B., Eggermont A.M., et al. (2009) Immunotherapy of distant metastatic disease. Ann Oncol 20(Suppl 6): vi41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G.L. (2007) Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discov Today 12: 853–859 [DOI] [PubMed] [Google Scholar]
- Si L., Chi X., Yuan C., Cui X., Sheng Y., Zhu Y., et al. (2009) Durable response of the triple combination of temozolomide, sorafenib and bevacizumab as second-line therapy for patients with stage IV acral melanoma. J Clin Oncol 27: Abstract e20010 [Google Scholar]
- Simonetti O., Lucarini G., Brancorsini D., Nita P., Bernardini M.L., Biagini G., et al. (2002) Immunohistochemical expression of vascular endothelial growth factor, matrix metalloproteinase 2, and matrix metalloproteinase 9 in cutaneous melanocytic lesions. Cancer 95: 1963–1970 [DOI] [PubMed] [Google Scholar]
- Spratlin J.L., Cohen R., Eadens M., Gore L., Camidge R., Diab S., et al. (2010) Phase 1 pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monocolonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol 28: 780–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit M., Detmar M. (2003) Angiogenesis, lymphangiogenesis, and melanoma metastasis. Oncogene 22: 3172–3179 [DOI] [PubMed] [Google Scholar]
- Tarhini A., Christensen S., Frankel P., Margolin K., Ruel C., Shipe-Spotloe J., et al. (2009) Phase II study of aflibercept (VEGF trap) in recurrent inoperable stage III or stage IV melanoma of cutaneous or ocular origin. J Clin Oncol 27: Abstract 9028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tentori L., Dorio A.S., Muzi A., Lacal P.M., Ruffini F., Navarra P., et al. (2008) The integrin antagonist cilengitide increases the antitumor activity of temozolomide against malignant melanoma. Oncol Rep 19: 1039–1043 [PubMed] [Google Scholar]
- Trikha M., Zhou Z., Nemeth J.A., Chen Q., Sharp C., Emmell E., et al. (2004) CNTO 95, a fully human monoclonal antibody that inhibits alphav integrins, has antitumor and antiangiogenic activity in vivo. Int J Cancer 110: 326–335 [DOI] [PubMed] [Google Scholar]
- Ugurel S., Rappl G., Tilgen W., Reinhold U. (2001) Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol 19: 577–583 [DOI] [PubMed] [Google Scholar]
- Varker K.A., Biber J.E., Kefauver C., Jensen R., Lehman A., Young D., et al. (2007) A randomized phase 2 trial of bevacizumab with or without daily low-dose interferon alfa-2b in metastatic malignant melanoma. Ann Surg Oncol 14: 2367–2376 [DOI] [PubMed] [Google Scholar]
- Wedge S.R., Ogilvie D.J., Dukes M., Kendrew J., Chester R., Jackson J.A., et al. (2002) ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res 62: 4645–4655 [PubMed] [Google Scholar]
- Wheatley K., Ives N., Eggermont J., Kirkwood N., Cascinelli S., Markovic S.N., et al. (2007) Interferon-alfa as adjuvant therapy for melanoma: an individual patient data meta-analysis of randomised trials. J Clin Oncol 25, Abstract 8526 [Google Scholar]
- Wolmark N., Yothers G., O’Connell M.J., Sharif S., Atkins J.N., Seay T.E., et al. (2009) A phase III trial comparing mFOLFOX6 to mFOLFOX6 plus bevacizumab in stage II or III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol 27: Abstract LBA4–Abstract LBA4 [Google Scholar]
- Wyman K., Spigel D., Puzanov I., Hainswroth J., Kelley P., Krozely D., et al. (2007) A multicentre phase II study of erlotinib and bevacizumab in patients with metastatic melanoma. J Clin Oncol 25: Abstract 8539 [Google Scholar]