Abstract
Sipuleucel-T represents a novel immunotherapeutic compound designed to stimulate an immune response against castration-resistant prostate cancer (CRPC). Sipuleucel-T is an autologous active cellular immunotherapy product, which includes autologous dendritic cells pulsed ex vivo with PAP2024, a recombinant fusion protein made of prostatic acid phosphatase and granulocyte-macrophage colony-stimulating factor. Despite the lack of prostate-specific antigen and objective response, a recent phase III randomized trial demonstrated a significant improvement in overall survival in asymptomatic and minimally symptomatic CRPC patients. This review summarizes the clinical development of Sipuleucel-T in CRPC that led to the regulatory approval of this compound in the USA.
Keywords: castrate-resistant prostate cancer, immunotherapy, Sipuleucel-T, vaccine
Introduction
Prostate cancer is the second leading cause of death in men in the USA and more than 217,730 new cases are expected to be diagnosed in 2010 [Jemal et al. 2010]. Although the majority of patients with advanced prostate cancer have an initial response to androgen deprivation, most patients will eventually progress to a castration-resistant state, manifested by rising levels of prostate-specific antigen (PSA), progressive disease (PD) on imaging studies, and/or worsening of symptoms all in the setting of an anorchid testosterone level [Oh et al. 1998]. Treatment options for patients with metastatic castration-resistant prostate cancer (CRPC), once thought to be a futile endeavor, have changed significantly with the understanding that new interventions, including secondary hormonal manipulations, chemotherapy, and a variety of new investigational approaches have clear anticancer effects [Chi et al. 2009].
Recently, immunotherapy has been recognized as a potential therapeutic strategy in prostate cancer. Several features of this epithelial malignancy including its long natural history [Coffey and Isaacs, 1981], its ability to induce auto-antibodies [Wang et al. 2005], and the availability of several tumor-specific antigens, such as prostatic acid phosphatase (PAP) [Taylor et al. 2006; Rhodes et al. 2002], have allowed the development of several immune-based strategies in the treatment of this disease [Antonarakis and Drake, 2010]. In this review, we discuss the clinical data of Sipuleucel-T, a novel immunotherapeutic agent recently approved by the US Food and Drug Administration for the management of patients with asymptomatic or minimally symptomatic metastatic CRPC.
Sipuleucel-T: an autologous dendritic cell product
Sipuleucel-T is an autologous active cellular immunotherapy product designed to stimulate an immune response against prostate cancer. Sipuleucel-T consists of autologous peripheral blood mononuclear cells (PBMCs), including antigen-presenting cells (APCs) pulsed ex vivo and activated in vitro with a recombinant fusion protein (PA2024). PA2024 is composed of a full-length PAP linked via its COOH terminus to the NH2 terminus of full-length granulocyte-macrophage colony-stimulating factor (GM-CSF). PAP is an antigen expressed in the majority of prostate cancers [Goldstein, 2002; Haines et al. 1989]. GM-CSF is a small secreted polypeptide that binds to specific cell surface receptors, functioning as a potent pleiotropic cytokine capable of enhancing hematopoietic differentiation and activation from several lineage precursors, including phagocytic macrophages and dendritic cells (DCs) [Steinman et al. 1991]. When administered in vivo, GM-CSF promotes the growth and antigen-presenting capabilities of DCs, leading to T-cell cross-priming [Lieschke and Burgess, 1992].
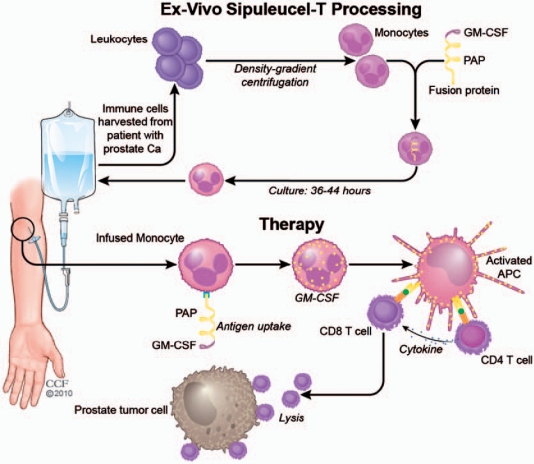
As seen in Figure 1, the preparation and processing of Sipuleucel-T involves a standard leukapheresis of approximately 1.5–2.0 blood volumes with isolation of PBMCs through density-gradient centrifugation to allow for the removal of platelets and monocytes. The cell pellet containing DC precursors is then washed and incubated in the absence of exogenous cytokines or serum with the recombinant fusion protein PAP2024 containing PAP and GM-CSF. After incubation, the cells were washed and formulated at the desired clinical dose in 250 ml of lactated Ringer’s solution. The final Sipuleucel-T product is then transported to the outpatient infusion center at the respective facility and infused into the patients within 8 h of formulation. Once infused back to the patient, this autologous product is thought to activate PAP-specific CD4+ and CD8+ T cells responsible for mediating the antitumor response in prostate cancer patients. Multiple phase I/II sequential trials evaluated the clinical and immune effects of Sipuleucel-T in patients with CRPC. In a trial that included 31 metastatic and nonmetastatic CRPC patients treatment with Sipuleucel-T was administered on weeks 0, 4, and 8, with a fourth infusion on week 24 only to those patients whose disease was stable or better after the initial three infusions. All patients receiving Sipuleucel-T developed a T-cell response defined by the immune response to the recall antigen influenza and to the naïve antigen KLH. These responses to the fusion protein were maximal after either two or three infusions of Sipuleucel-T, although no obvious correlation between the dose infused and the magnitude of T-cell proliferation was observed. Antibodies to PAP and GM-CSF were also evaluated by specific enzyme-linked immunosorbent assay (ELISA) on serum samples obtained at baseline and then every 4 weeks. None of the patients had pre-existing antibodies to PAP (isolated from human seminal fluid), whereas after treatment, 16 (52%) out of 31 patients had antibodies. The median antibody titer was 1/240 (range 1/40–1/5120). Similar to the T-cell experience, 10 patients (33%) had pre-existing antibodies to GM-CSF, and after treatment, 25 out of 31 patients (80.6%) had antibodies [Small et al. 2000].
Figure 1.
Processing of Sipuleucel-T. Sipuleucel-T immunotherapy is similar to a DC vaccine and is based on cells from a patient-derived leukapheresis product. APC, antigen-presenting cells; DC, dendritic cell; GM-CSF, granulocyte-macrophage colony-stimulating factor; PAP, prostatic acid phophatase.
Sipuleucel-T in prostate cancer: clinical data
Three small prospective phase II studies that included patients with castrate resistant and androgen-dependent, biochemically relapsed disease initially demonstrated the safety and modest clinical activity of this agent in prostate cancer. The first trial, which included 19 men with CRPC, demonstrated a modest PSA response in 2 out of 19 patients (PSA decline >50% compared with baseline), and no objective disease response in those with response evaluation criteria in solid tumors (RECIST)-defined measurable disease. In terms of immune parameters, 15 out of the 19 patients were evaluated every 4 weeks from week 0 to week 16 and then every 8 weeks until PD. All patients elicited T-cell responses to PAP2024 that lasted for the entire duration of the clinical trial [Burch et al. 2004]. Similarly, two small single institutional studies demonstrated that treatment with Sipuleucel-T alone [Beinart et al. 2005], or in combination with bevacizumab, a monoclonal humanized antibody against vascular endothelial growth factor [Rini et al. 2006], leads to an increase in PSA doubling time (PSADT) in patients with androgen-dependent, biochemically relapsed prostate cancer patients. While the single Sipuleucel-T study demonstrated a median increase in PSADT of 62% (4.9 months before treatment vs. 7.9 months after treatment; p = 0.09; signed-rank test), the median increase in PSADT when bevacizumab is added to Sipuleucel-T appears to be greater than 150% (6.9 months vs. 12.7 months; p = 0.01). Although no immune parameters were reported in the monotherapy trial, all patients in the combination study with bevacizumab demonstrated induction of an immune response against Sipuleucel-T [Rini et al. 2006].
The initial phase III clinical program development of Sipuleucel-T in CRPC disease included two parallel identical randomized, double-blind, placebo-controlled studies, D9901 and D9902A [Higano et al. 2009; Small et al. 2006]. D9901 was a multi-institutional double-blind, placebo-controlled, randomized phase III trial designed to test the effect of Sipuleucel-T on time to progression (TTP) and overall survival (OS) in 127 men with asymptomatic metastatic CRPC. Patients in this trial were randomly assigned in a 2 : 1 ratio to receive three infusions of Sipuleucel-T (n = 82) or placebo (n = 45) every 2 weeks. On disease progression, placebo patients received a similar product made with frozen leukapheresis cells. D9902A was an identical study to D9901 but was prematurely stopped after the initial TTP results from D9901 were reported. Thus, the study was underpowered and ultimately enrolled 98 patients. Among these patients, 65 were randomized to Sipuleucel-T.
The primary endpoint of both studies was TTP defined as objective or clinical progression. Imaging studies were completed every 8 weeks until week 32 then every 12 weeks thereafter, and progression was confirmed by independent review. PSA was not used to determine disease progression or to trigger radiographic evaluations. Sample size calculations were based on attaining a power of 80% with a two-sided 0.05 significance level and the assumption that treatment with Sipuleucel-T would result in an increase in median TTP from 4 months to 7.7 months. The median TTP for patients receiving Sipuleucel-T in study D9901 was 11.7 weeks compared with 10.0 weeks for placebo (p = 0.052, log-rank; hazard ratio [HR] 1.45; 95% confidence interval [CI] 0.99–2.11). Similarly, the TTP in D9902 was 10.9 and 9.9 weeks, respectively (p = 0.719, log-rank; HR 1.09; 95% CI 0.69–1.70). When looking at the impact of treatment in serum PSA values, of the 147 patients receiving Sipuleucel-T in both trials, only five patients had a PSA reduction of ≥50% for an overall PSA response rate of 4.8%. Similarly, no objective responses were reported in those patients with measurable disease who received treatment with Sipuleucel-T.
While neither study was powered to determine the impact of Sipuleucel-T on OS, a preplanned 3-year OS assessment was carried out. The median OS in the D9901 study was 25.9 months for Sipuleucel-T-treated patients and 21.4 months for placebo (p = 0.01, log-rank; HR 1.70; 95% CI 1.13–2.56). Although no survival benefit was observed in the D9902A trial (19 vs. 15.7 months; p = 0.331) [Higano et al. 2009], when both trials are integrated together, the median OS was 23.2 months for Sipuleucel-T and 18.9 months for placebo (p = 0.011). Similarly, the percentage of patients alive at 36 months was 33% and 15%, respectively [Higano et al. 2009]. These trials also evaluated the correlation between CD54 upregulation and OS. CD54, also known as intracellular adhesion molecule-1, is expressed on DCs and plays an important role in the synapse between DCs and T cells. There was a strong correlation between CD54 upregulation and survival (p = 0.009). The OS benefits found in the trial were maintained after adjusting for multiple predefined CRPC prognostic factors [Kantoff et al. 2010; Smaletz et al. 2002], including the subsequent use of docetaxel-based chemotherapy (p = 0.022) [Higano et al. 2009].
To confirm the survival impact of Sipuleucel-T in metastatic CRPC patients, a double-blind, placebo-controlled, multicenter trial, called the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) study was conducted [Kantoff et al. 2010]. This study enrolled 512 men with metastatic CRPC and randomly assigned them in a 2 : 1 fashion to either Sipuleucel-T or placebo every 2 weeks for a total of three infusions. Eligibility on this trial was similar to that in previous CRPC studies [Higano et al. 2009; Small et al. 2006]. All patients were stratified according to Gleason grade, number of bone metastases, and bisphosphonate use. PD was independently monitored using PSA and imaging studies at weeks 6, 14, 26, and 34 and every 12 weeks. During the trial, placebo patients developing PD could enroll in an open-label salvage protocol and receive APC8015F, a product manufactured according to the same specifications as Sipuleucel-T from cells cryopreserved at the time the placebo was prepared.
Contrary to previous studies, OS was the primary endpoint of the trial. Survival was analyzed on the basis of a stratified Cox regression model with adjustment for the natural log of the baseline levels of PSA and lactate dehydrogenase and stratified according to randomization factors [Halabi et al. 2003; Smaletz et al. 2002] Antibody titers were also assessed by means of an ELISA assay with threshold levels for response defined as 2 SD above the median of the baseline values for all assessed patients. T-cell proliferation was assessed with the use of a stimulation index, calculated as the median value for 3H-thymidine incorporation from triplicate wells cultured with antigen, divided by the median incorporation value in the absence of antigen.
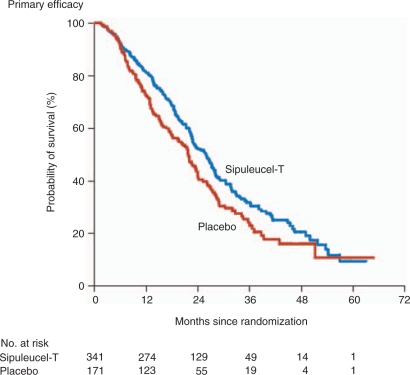
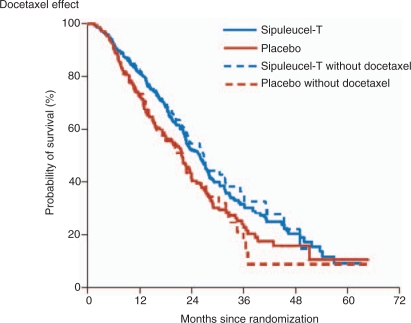
The median OS was 25.8 months for Sipuleucel-T-treated patients compared with 21.7 months for patients receiving placebo with an adjusted HR for death of 0.78 (95% CI, 0.61–0.98), representing a relative reduction in the risk of death of 22% (p = 0.03) (Figure 2). Similar results were obtained with the use of the unadjusted, stratified model and the log-rank test (HR 0.77; 95% CI 0.61–0.97; p = 0.02). The reduction in the risk of death from prostate cancer in the Sipuleucel-T group (HR 0.77; 95% CI 0.61–0.98; p = 0.04) was similar to the reduction in the risk of death from any cause. There was no difference in the median time to objective PD (3.7 months in the Sipuleucel-T group vs. 3.6 months in the placebo group: HR 0.95; 95% CI 0.77–1.17; p = 0.63). Similar results were observed for the time to clinical PD (HR 0.92; 95% CI 0.75–1.12; p = 0.40). Only one patient on Sipuleucel-T achieved a partial remission (PR) while 2.6% had a PSA decline of ≤50%. When evaluating the impact of subsequent therapy with outcome, the estimated effect of Sipuleucel-T treatment for those patients receiving subsequent docetaxel-based chemotherapy was consistent with the result of the primary efficacy analysis (HR for death 0.78; 95% CI 0.62–0.98; p = 0.03) (Figure 3).
Figure 2.
Kaplan–Meier estimates of overall survival. The figure shows the results of the primary efficacy analysis of treatment with Sipuleucel-T as compared with placebo (hazard ratio for death in the Sipuleucel-T group 0.78; 95% confidence interval 0.61–0.98; p = 0.03). (Figure reproduced with permission from Kantoff et al. [2010]).
Figure 3.
Kaplan–Meier estimates of overall survival with subsequent treatment. This figure shows the results of the analysis with and without censoring at the time of the initiation of docetaxel therapy after study treatment. After censoring at the time of docetaxel initiation, a consistent treatment effect with Sipuleucel-T was observed (hazard ratio 0.65; 95% confidence interval 0.47–0.90; p = 0.009). (Figure reproduced with permission from Kantoff et al. [2010]).
When evaluating immune changes on study, titers of antibodies against the immunizing antigen PA2024 at any time after baseline were observed in 100 out of 151 patients (66.2%) in the Sipuleucel-T group and 2 out of 70 patients (2.9%) in the placebo group. Similarly, titers of antibodies against PAP were observed in 43 out of 151 patients (28.5%) in the Sipuleucel-T group and 1 out of 70 patients (1.4%) in the placebo group. At week 6 of treatment, T-cell proliferation responses (stimulation index >5) to PA2024 were observed in 73% of patients treated with Sipuleucel-T compared with 12% in the placebo group. No changes in the immune correlative studies conducted in this trial correlated with outcome.
Toxicity and safety profile of Sipuleucel-T
Overall, therapy with Sipuleucel-T is well tolerated (Table 1). Almost all patients in the IMPACT trial received all three planned infusions and no patient discontinued study secondary to toxicity. The most common toxicities were grade (G) 1 or 2 and included chills/rigors, fever (pyrexia), headache, influenza-like illness, myalgia, hypertension, hyperhidrosis, and groin pain. Except for groin pain, most of these events were temporary, occurred within 1 day after infusion and resolved within 1 to 2 days. Adverse events reported in the IMPACT trial were mostly G1 and G2 and included chills, fever (pyrexia), headache, influenza-like illness, myalgia, hypertension, hyperhidrosis, and groin pain. Most of these adverse events (AEs) occurred within 1 day after infusion and resolved within 1 to 2 days. Among patients in the Sipuleucel-T group, G3 events that were reported for at least one patient within 1 day after infusion were chills (in four patients), fatigue (three patients), and back pain, hypertension, hypokalemia, and muscular weakness (in two patients each); one G4 event was reported (intravenous catheter-associated bacteremia). Although not statistically significant when compared with placebo, less than 3% (8/338) of patients receiving Sipuleucel-T experienced a cerebrovascular event. The true relationship between Sipuleucel-T and this AE remains unclear.
Table 1.
Selected adverse event reported with Sipuleucel-T.
| Event | All grades n (%) | Grade 3–5 n (%) |
|---|---|---|
| Any | 334 (98.8) | 107 (31.7) |
| Chills | 183 (54.1) | 4 (1.2) |
| Pyrexia | 99 (29.3) | 1 (0.3) |
| Fatigue | 132 (39.1) | 4 (1.2) |
| Back pain | 116 (34.3) | 12 (3.6) |
| Headache | 54 (16%) | 1 (0.3) |
| Myalgia | 33 (9.8) | 2 (0.6) |
| Hyperhidrosis | 18 (5.3) | 0 |
| Groin pain | 17 (5.0) | 0 |
Discussion
Although new treatments for the management of men with CRPC are always welcome, the regulatory approval of Sipuleucel-T has generated more questions than answers. Albeit treatment with Sipuleucel-T led to a significant improvement in OS in men with symptomatic or minimally symptomatic metastatic CRPC, this improvement came without evidence of a measurable antitumor effect, such as TTP, objective response rate and serologic response (PSA reduction of at least 50%). The Prostate Cancer Clinical Trials Working Group 2 (PCWG2) consensus guidelines currently emphasize the need to reconsider clinical trial design and endpoints when developing novel agents in CRPC [Scher et al. 2008]. Although both biochemical response and biochemical progression-free survival are predictive of survival in CRPC patients treated with cytotoxic agents [Halabi et al. 2009; Armstrong et al. 2007], reductions in PSA levels as the principal surrogate marker for therapeutic efficacy of immunotherapy studies might not be optimal. Similarly, it is somewhat perplexing that despite the lack of tumor burden reduction when measurable disease was present, treatment with Sipuleucel-T could impact the natural history of this disease in a positive manner. Although sensitivity analyses in the IMPACT trial demonstrated that subsequent treatment (mostly docetaxel-based chemotherapy) did not account for the survival difference observed, the study was not powered to detect such a difference and, as the investigators have disclosed, statistical methods commonly used to address postbaseline confounding factors in any randomized clinical study are limited and subject to potential biases.
One of the most intriguing and yet unanswered questions is the true mechanism of action of Sipuleucel-T. The presumption that this immune product works by activating APCs, which in turn, will prime and activate T-cell function ultimately leading to antitumor activity, requires further validation. As has been reported, treatment with Sipuleucel-T was capable of producing antigen-specific T-cell responses and those with elevated antibody titers after treatment had a better survival than those without titer elevations. Of interest however, was the fact that T-cell proliferation responses to PA2024 or PAP did not impact a patient’s outcome. A better understanding of the immune effects of this product is required to characterize further the appropriate population likely to obtain the greatest benefit from treatment with Sipuleucel-T. Another major challenge facing this agent is its cost in the USA. This is a very expensive treatment for a modest gain for many US providers, especially when it is not known which patients will truly benefit from this immune approach. Currently there are no studies assessing the cost effectiveness of this agent against a standard of care for this patient population. Such a study would be somewhat difficult to conduct due to the heterogeneity in the patient population as not every patient with asymptomatic metastatic disease will go on to receive docetaxel-based chemotherapy. Likewise, the number of cycles of docetaxel required to derive the OS benefit observed in SWOG 9916 and TAX327 has been established.
Despite all the caveats with this novel compound, immunotherapy with Sipuleucel-T for CRPC is here to stay. Therefore, patient selection and education about the limitations of this agent are crucial in the management of CRPC. Although the economics and technical logistics linked to this compound are likely to impact its wide use in the USA, it is unclear what role if any Sipuleucel-T will have should other promising agents be approved, as the CYP-17 inhibitor, abiraterone, has recently demonstrated survival benefit over placebo in men with metastatic docetaxel-refractory CRPC.
Notwithstanding the challenges involved in the development of immunotherapy for prostate cancer, the regulatory approval of Sipuleucel-T has opened the door for multiple opportunities for immune drug development. Future studies will require thoughtful clinical trial designs, including appropriate patient selection and translational and clinical endpoints.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
The authors declare no conflict of interest in preparing this article.
References
- Antonarakis E.S., Drake C.G. (2010) Current status of immunological therapies for prostate cancer. Curr Opin Urol 20: 241–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong A.J., Garrett-Mayer E., OuYang Y.C., Carducci M.A., Tannock I., deWit R., et al. (2007) Prostate specific antigen and pain surrogacy analysis in metastatic hormone-refractory prostate cancer. J Clin Oncol 25: 3965–3970 [DOI] [PubMed] [Google Scholar]
- Beinart G., Rini B.I., Weinberg V., Small E.J. (2005) Antigen-presenting cells 8015 (Provenge) in patients with androgen-dependent, biochemically relapsed prostate cancer. Clin Prostate Cancer 4: 55–60 [DOI] [PubMed] [Google Scholar]
- Burch P.A., Croghan G.A., Gastineau D.A., Jones L.A., Kaur J.S., Kylstra J.W., et al. (2004) Immunotherapy (APC8015, Provenge) targeting prostatic acid phosphatase can induce durable remission of metastatic androgen-independent prostate cancer: A Phase 2 trial. Prostate 60: 197–204 [DOI] [PubMed] [Google Scholar]
- Chi K.N., Bjartell A., Dearnaley D., Saad F., Schröder F.H., Sternberg C., et al. (2009) Castration-resistant prostate cancer: From new pathophysiology to new treatment targets. Eur Urol 56: 594–605 [DOI] [PubMed] [Google Scholar]
- Coffey D.S., Isaacs J.T. (1981) Control of prostate growth. Urology 17: 17–24 [PubMed] [Google Scholar]
- Goldstein N.S. (2002) Immunophenotypic characterization of 225 prostate adenocarcinomas with intermediate or high Gleason scores. Am J Clin Pathol 117: 471–477 [DOI] [PubMed] [Google Scholar]
- Haines A.M., Larkin S.E., Richardson A.P., Stirling R.W., Heyderman E. (1989) A novel hybridoma antibody (PASE/4LJ) to human prostatic acid phosphatase suitable for immunohistochemistry. Br J Cancer 60: 887–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halabi S., Small E.J., Kantoff P.W., Kattan M.W., Kaplan E.B., Dawson N.A., et al. (2003) Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol 21: 1232–1237 [DOI] [PubMed] [Google Scholar]
- Halabi S., Vogelzang N.J., Ou S.S., Owzar K., Archer L., Small E.J. (2009) Progression-free survival as a predictor of overall survival in men with castrate-resistant prostate cancer. J Clin Oncol 27: 2766–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higano C.S., Schellhammer P.F., Small E.J., Burch P.A., Nemunaitis J., Yuh L., et al. (2009) Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with Sipuleucel-T in advanced prostate cancer. Cancer 115: 3670–3679 [DOI] [PubMed] [Google Scholar]
- Jemal A., Siegel R., Xu J., Ward E. (2010) Cancer statistics, 2010. CA Cancer J Clin 60(5): 277–300 [DOI] [PubMed] [Google Scholar]
- Kantoff P.W., Higano C.S., Shore N.D., Berger E.R., Small E.J., Penson D.F., et al. (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363: 411–422 [DOI] [PubMed] [Google Scholar]
- Lieschke G.J., Burgess A.W. (1992) Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor. N Engl J Med 372: 28–35 [DOI] [PubMed] [Google Scholar]
- Oh W.K., Kantoff P.W. (1998) Management of hormone refractory prostate cancer: Current standards and future prospects. J Urol 1160: 1220–1229 [PubMed] [Google Scholar]
- Rhodes D.R., Barrette T.R., Rubin M.A., Ghosh D., Chinnaiyan A.M. (2002) Meta-analysis of microarrays: Interstudy validation of gene expression profiles reveals pathway dysregulation in prostate cancer. Cancer Res 62: 4427–4433 [PubMed] [Google Scholar]
- Rini B.I., Weinberg V., Fong L., Conry S., Hershberg R.M., Small E.J. (2006) Combination immunotherapy with prostatic acid phosphatase pulsed antigen-presenting cells (provenge) plus bevacizumab in patients with serologic progression of prostate cancer after definitive local therapy. Cancer 107: 67–74 [DOI] [PubMed] [Google Scholar]
- Scher H.I., Halabi S., Tannock I., Morris M., Sternberg C.N., Carducci M.A., et al. (2008) Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 26: 1148–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaletz O., Scher H.I., Small E.J., Verbel D.A., McMillan A., Regan K., et al. (2002) Nomogram for overall survival of patients with progressive metastatic prostate cancer after castration. J Clin Oncol 20: 3972–3982 [DOI] [PubMed] [Google Scholar]
- Small E.J., Fratesi P., Reese D.M., Strang G., Laus R., Peshwa M.V., et al. (2000) Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. Clin Oncol 18: 3894–3903 [DOI] [PubMed] [Google Scholar]
- Small E.J., Schellhammer P.F., Higano C.S., Redfern C.H., Nemunaitis J.J., Valone F.H., et al. (2006) Placebo-controlled phase III trial of immunologic therapy with Sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 24: 3089–3094 [DOI] [PubMed] [Google Scholar]
- Steinman R.M. (1991) The dendritic cell system and its role in immunogenicity. Annu Rev Immunol 9: 271–296 [DOI] [PubMed] [Google Scholar]
- Taylor B.S., Varambally S., Chinnaiyan A.M. (2006) Differential proteomic alterations between localized and metastatic prostate cancer. Br J Cancer 95: 425–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Yu J., Sreekumar A., Varambally S., Shen R., Giacherio D., et al. (2005) Autoantibody signatures in prostate cancer. N Engl J Med 353: 1224–1235 [DOI] [PubMed] [Google Scholar]