Abstract
Clear cell renal cell carcinoma (ccRCC) presents problems for urologists in diagnosis, treatment selection, intraoperative surgical margin analysis, and long term monitoring. In this paper we describe the development of a radiolabeled antibody specific to ccRCC (124I-cG250) and its potential to help urologists manage each of these problems. We believe 124I-cG250, in conjunction with perioperative Positron emission tomography/computed tomography imaging and intraoperative handheld gamma probe use, has the potential to diagnose ccRCC, aid in determining a proper course of treatment (operative or otherwise), confirm complete resection of malignant tissue in real time, and monitor patients post-operatively.
Keywords: advanced kidney cancer, kidney cancer basic research, kidney cancer evaluation/staging, kidney cancer localized, kidney cancer
In the 1980s, researchers characterized G250, a monoclonal antibody (MAb) that binds to carbonic anhydrase IX, a transmembrane protein that is activated in hypoxic conditions to sustain tumor progression. Since that time, G250 has been shown to localize in primary (98%) and metastatic (88%) clear cell renal cell carcinoma (ccRCC) lesions found on human histologic slides under light microscopy [Oosterwijk et al. 1986a; b]. Although MAb G250 occasionally stains positive in several other tumors, it was shown to be a very strong biomarker for ccRCC due to its absence in normal kidney tissue. Thus, the potential use of this MAb for radioimmunoscintigraphy was immediately recognized [Oosterwijk et al. 1986a; b]. However, we are only now seeing that dream coming to fruition.
It was not until 1997, that a chimeric form of G250 (cG250) was developed to reduce human anti-mouse antibody responses (HAMA), and it took an additional 10 years to achieve successful radiolabeling with a suitable radionuclide [Divgi et al. 2007; Steffens et al. 1997]. For example, cG250 labeled with Iodine-131 localized just 30% of metastatic ccRCC lesions using radioimmunoscintigraphy [Brouwers et al. 2002]. Today, however, human clinical trials using Iodine-124 as a radiolabel have demonstrated excellent sensitivity, specificity, positive predictive values, and negative predictive values (94, 100, 100, and 90%, respectively) [Divgi et al. 2007]. As such, positron emission tomography/computed tomography (PET/CT) imaging of ccRCC with 124I-cG250 may change the workup of a patient with an incidental kidney mass, the treatment of ccRCC, and the way patients with a history of ccRCC are monitored.
As diagnostic imaging advanced, the presentation of ccRCC changed from a large palpable symptomatic mass, to an ‘incidentaloma’ found on CT. Given that up to 30% of these lesions, when less than 4 cm in size, are benign, urologists are faced with a dilemma: perform a nephrectomy on a potentially benign mass, or watch a potentially aggressive tumor progress. It would be an advantage to be able to obtain a histologic diagnosis based on preoperative imaging to avoid invasive biopsies and, worse yet, unnecessary surgeries.
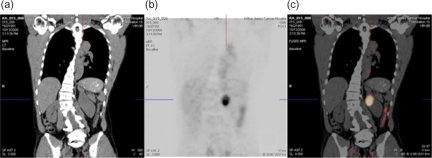
PET/CT imaging with 124I-cG250 may eliminate this predicament, as it is both highly sensitive and specific for ccRCC. Thus, those ‘incidentalomas’ that are, in fact, ccRCC appear on PET/CT imaging (Figure 1). If there is a signal on PET imaging, the patient likely has ccRCC.
Figure 1.
Preoperative CT (a), PET (b), and fused PET/CT (c) images obtained 4 days post-injection of 5.2 mCi (192.4 MBq) 124-I cG250 show a well-defined tumor mass in the left kidney.
The utilization of PET/CT imaging with chimeric MAbs has been given the name: ‘imaging histology’ amongst our imaging and radio-guided surgery group, and describes the non-invasive, in vivo, three-dimensional ‘mapping’ of pathology on a histologic level. However, the potential utility of PET/CT imaging of ccRCC with 124I-cG250 extends beyond mere diagnosis. Imaging histology allows a surgeon to more precisely plan their approach and, perhaps, predict and plan surgical margins before the patient even enters the operating suite [Hall et al. 2008]. Once in the operating suite, a hand-held gamma probe may be used in real-time to verify that all of the radioactivity, and thus, the tumor, has been excised. This intraoperative information may then be further verified by immediately acquiring an ex vivo PET/CT scan of the whole specimen and/or repeating the PET/CT scan of the patient after surgery (Figure 2) [Povoski et al. 2009; Cohn et al. 2008, Hall et al. 2008, Povoski et al. 2008; Hall et al. 2007; Sarikaya et al. 2007]. The combined use of perioperative 124I-cG250 PET/CT imaging and the intraoperative handheld gamma probe may provide more accurate information than does frozen section with the additional advantage of providing real-time information to the urologist.
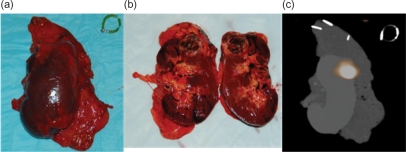
Figure 2.
Digital photographs of a whole (a) and bisected (b) ex vivo left kidney specimen show extent of ccRCC. A fused PET/CT image (c) of the kidney obtained prior to bisection of the tissue indicates a region of intense tracer uptake that correlates well with the gross tumor location.
After a diagnosis of a solid renal neoplasm is made, urologists face a dilemma whether to perform a partial or total nephrectomy. Recent evidence has shown that partial nephrectomy has similar oncologic outcomes with the added advantage of sparing useful nephrons [Fergany et al. 2006; 2000]. Yet a study based on NCI data from 1995–2002 showed that only 18% of patients with tumors measuring 4 cm or smaller were treated with partial nephrectomy [Huang et al. 2009]. The percentage of patients treated with partial nephrectomy increased each year (up to 26% in 2002), but the treatment is slow to become the standard of care. Whether this is due to a lack of training, relative comfort with traditional radical excision, concern over leaving tumor behind, or any number of other factors is unclear. Whatever the reason, we believe that perioperative PET/CT utilizing 124I-cG250 could influence more widespread acceptance of partial nephrectomy with its advantages of preoperative planning and ex vivo margin analysis.
Additionally, the utility of 124I-cG250 PET/CT imaging for verification of complete tumor resection could extend to other treatment modalities such as radiofrequency ablation (RFA) and cryotherapy (cryo). Since neither RFA nor cryo involve resection of malignant tissue, the urologist would have to allow for the radioactive iodine molecules to completely decay, a delay of approximately 40 days from the time of injection, before assessment of the procedure is possible. As a result, real-time RFA and cryo treatment assessment will be unavailable, but a follow-up PET/CT image utilizing 124I-cG250 may assess the effectiveness of these therapies; no signal, no ccRCC.
Finally, 124I-cG250 imaging histology may be a powerful tool for the evaluation of recurrent and metastatic disease. Current PET/CT imaging to evaluate ccRCC patients for both recurrent and/or distant metastatic disease is problematic, largely due to the non-specific nature of 18F-fluorodeoxyglucose (18F-FDG) as a radiotracer. The theory behind 18F-FDG PET imaging is that tumors will utilize a higher concentration of radiolabeled glucose than will normal cells. However, cells (both tumor and normal cells) uptake glucose at varying rates, and the difference between glucose concentration in normal cells as compared with tumor cells is not always large enough to be appreciated by PET/CT imaging. While 18F-FDG has shown promising results in several other tumor types, the excretion of 18F-FDG via the urinary system, its low sensitivity, and high rates of both false positivity and false negativity in primary and metastatic RCC patients limits its use for urologists [Kang et al. 2004; Majhail et al. 2003; Miyakita et al. 2002]. The high sensitivity and positive predictive value of 124I-cG250 PET/CT would make it a very effective alternative to 18F-FDG PET/CT for monitoring ccRCC patients for metastasis and recurrence.
It is important to realize that 124I-cG250 is specific only to ccRCC, and therefore provides no further useful information concerning other tumor types. This limits the utility of 124I-cG250 PET/CT as a potential diagnostic evaluation for all renal masses. However, it is still a strong biomarker for the most frequent renal tumor type. Moreover, the data and methodologies presented here illustrate the potential for using a specific, radiolabeled MAb for detection, diagnosis, confirmation of resection, and postoperative surveillance for cRCC. However, this paradigm could be extended to a wide range of cancer types with the development of cancer specific antibodies linked with radionuclides.
At this time, modern imaging modalities have proved inadequate for the detection and monitoring of ccRCC patients. Specifically, PET/CT imaging with 18F-FDG has been shown to have poor sensitivity and specificity in the urinary system. However, new imaging methodologies utilizing radiolabeled chimeric antibodies, such as 124I-cG250 for ccRCC, can potentially extend far beyond the traditional diagnostic role of PET/CT imaging. The urologist may now have a reliable tool to identify patients with ccRCC. Images will aid the determination of tumor margins preoperatively, in the decision to perform a partial or total nephrectomy, and help ensure clear surgical margins intraoperatively with the utilization of a handheld gamma probe. It can also provide an accurate method for monitoring all three non-pharmacologic therapies (surgery, cryoablation, and radiofrequency ablation) for ccRCC. Long term data are not available at this time, but there is promise that PET/CT imaging with chimeric antibodies may lead to improved patient outcomes.
Conflicts of interest statement
None declared.
References
- Brouwers A.H., Dorr U., Lang O., Boerman O.C., Oyen W.J., Steffens M.G., et al. (2002) 131 I-Cg250 Monoclonal Antibody Immunoscintigraphy Versus [18 F]Fdg-Pet Imaging in Patients with Metastatic Renal Cell Carcinoma: A Comparative Study. Nucl Med Commun 23: 229–236 [DOI] [PubMed] [Google Scholar]
- Cohn D.E., Hall N.C., Povoski S.P., Seamon L.G., Farrar W.B., Martin Jr E.W. (2008) Novel Perioperative Imaging with 18f-Fdg Pet/Ct and Intraoperative 18f-Fdg Detection Using a Handheld Gamma Probe in Recurrent Ovarian Cancer. Gynecol Oncol 110: 152–157 [DOI] [PubMed] [Google Scholar]
- Divgi C.R., Pandit-Taskar N., Jungbluth A.A., Reuter V.E., Gonen M., Ruan S., et al. (2007) Preoperative Characterisation of Clear–Cell Renal Carcinoma Using Iodine-124-Labelled Antibody Chimeric G250 (124i-Cg250) and Pet in Patients with Renal Masses: A Phase I Trial. Lancet Oncol 8: 304–310 [DOI] [PubMed] [Google Scholar]
- Fergany A.F., Hafez K.S., Novick A.C. (2000) Long-Term Results of Nephron Sparing Surgery for Localized Renal Cell Carcinoma: 10-Year Followup. J Urol 163: 442–445 [PubMed] [Google Scholar]
- Fergany A.F., Saad I.R., Woo L., Novick A.C. (2006) Open Partial Nephrectomy for Tumor in a Solitary Kidney: Experience with 400 Cases. J Urol 175: 1630–1633; discussion 1633 [DOI] [PubMed] [Google Scholar]
- Hall N.C., Povoski S.P., Murrey D.A., Knopp M.V., Martin E.W. (2008) Bringing Advanced Medical Imaging into the Operative Arena Could Revolutionize the Surgical Care of Cancer Patients. Expert Rev Med Devices 5: 663–667 [DOI] [PubMed] [Google Scholar]
- Hall N.C., Povoski S.P., Murrey D.A., Knopp M.V., Martin Jr E.W. (2007) Combined Approach of Perioperative 18f-Fdg Pet/Ct Imaging and Intraoperative 18f-Fdg Handheld Gamma Probe Detection for Tumor Localization and Verification of Complete Tumor Resection in Breast Cancer. World J Surg Oncol 5: 143–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W.C., Elkin E.B., Levey A.S., Jang T.L., Russo P. (2009) Partial Nephrectomy Versus Radical Nephrectomy in Patients with Small Renal Tumors––Is There a Difference in Mortality and Cardiovascular Outcomes? J Urol 181: 55–61; discussion 61–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D.E., White Jr R.L., Zuger J.H., Sasser H.C., Teigland C.M. (2004) Clinical Use of Fluorodeoxyglucose F 18 Positron Emission Tomography for Detection of Renal Cell Carcinoma. J Urol 171: 1806–1809 [DOI] [PubMed] [Google Scholar]
- Majhail N.S., Urbain J.L., Albani J.M., Kanvinde M.H., Rice T.W., Novick A.C., et al. (2003) F-18 Fluorodeoxyglucose Positron Emission Tomography in the Evaluation of Distant Metastases from Renal Cell Carcinoma. J Clin Oncol 21: 3995–4000 [DOI] [PubMed] [Google Scholar]
- Miyakita H., Tokunaga M., Onda H., Usui Y., Kinoshita H., Kawamura N., et al. (2002) Significance of 18f-Fluorodeoxyglucose Positron Emission Tomography (Fdg-Pet) for Detection of Renal Cell Carcinoma and Immunohistochemical Glucose Transporter 1 (Glut-1) Expression in the Cancer. Int J Urol 9: 15–18 [DOI] [PubMed] [Google Scholar]
- Oosterwijk E., Ruiter D.J., Hoedemaeker P.J., Pauwels E.K., Jonas U., Zwartendijk J., et al. (1986a) Monoclonal Antibody G 250 Recognizes a Determinant Present in Renal-Cell Carcinoma and Absent from Normal Kidney. Int J Cancer 38: 489–494 [DOI] [PubMed] [Google Scholar]
- Oosterwijk E., Ruiter D.J., Wakka J.C., Huiskens-Van Der Meij J.W., Jonas U., Fleuren G.J., et al. (1986b) Immunohistochemical Analysis of Monoclonal Antibodies to Renal Antigens. Application in the Diagnosis of Renal Cell Carcinoma. Am J Pathol 123: 301–309 [PMC free article] [PubMed] [Google Scholar]
- Povoski S.P., Hall N.C., Martin Jr E.W., Walker M.J. (2008) Multimodality Approach of Perioperative 18f-Fdg Pet/Ct Imaging, Intraoperative 18f-Fdg Handheld Gamma Probe Detection, and Intraoperative Ultrasound for Tumor Localization and Verification of Resection of All Sites of Hypermetabolic Activity in a Case of Occult Recurrent Metastatic Melanoma. World J Surg Oncol 6: 1–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povoski S.P., Neff R.L., Mojzisik C.M., O'malley D.M., Hinkle G.H., Hall N.C., et al. (2009) A Comprehensive Overview of Radioguided Surgery Using Gamma Detection Probe Technology. World J Surg Oncol 7: 11–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarikaya I., Povoski S.P., Al-Saif O.H., Kocak E., Bloomston M., Marsh S., et al. (2007) Combined Use of Preoperative 18f Fdg-Pet Imaging and Intraoperative Gamma Probe Detection for Accurate Assessment of Tumor Recurrence in Patients with Colorectal Cancer. World J Surg Oncol 5: 80–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens M.G., Boerman O.C., Oosterwijk-Wakka J.C., Oosterhof G.O., Witjes J.A., Koenders E.B., et al. (1997) Targeting of Renal Cell Carcinoma with Iodine-131-Labeled Chimeric Monoclonal Antibody G250. J Clin Oncol 15: 1529–1537 [DOI] [PubMed] [Google Scholar]