Abstract
Improvements in prostate cancer diagnosis and treatment have resulted in a decreasing age-adjusted death rate. But improved diagnostic tools have not delivered a proportionate decrease in mortality, primarily because physicians now are diagnosing – and treating – more clinically insignificant tumors. Targeted focal therapy (TFT) uses three dimensional (3D) mapping biopsies to guide cryotherapy so that it targets lesions themselves while sparing surrounding healthy tissues, thereby avoiding side effects associated with more invasive treatments. As such, TFT can provide a bridge between active surveillance and more aggressive treatments for patients with low-risk tumors. It is appropriate for men who either do not want or are not good candidates for more aggressive therapies. Identifying patients who are appropriate candidates for TFT is challenging, but the mapping biopsy technique helps identify individuals who have localized disease and could benefit from this treatment. In recent years, improvements in cryotherapy have increased its efficacy while decreasing complications. At the present time using cryotherapy to target and destroy the cancer(s) is appealing. Other approaches to less aggressive therapies are discussed including gland hemi ablation, high intensity ultrasound (HIFU) and others. Going forward, patients will benefit further from development of better imaging technologies and completion of long-term survival studies.
Keywords: prostate cancer, cryotherapy, targeted focal therapy (TFT), PSA screening, digital rectal examination, transrectal ultrasound
Introduction
Prostate cancer is the second most common cause of cancer deaths in the United States [Jemal et al. 2007]. The incidence of prostate cancer is relatively constant at 165 cases per 100,000 men [Jemal et al. 2007]. Since 1990, the age-adjusted death rate has progressively decreased by 31%, which is most likely due to early detection and treatment [Jemal et al. 2007]. Even though there has been a decrease in mortality, it has not been as significant as expected based on the increased diagnosis of prostate cancer. The discrepancy between incidence and mortality is primarily due to increasing detection of clinically insignificant tumors.
Furthermore, prostate screening (e.g. digital rectal examination [DRE] and prostate specific antigen [PSA]) may identify many clinically insignificant cancers and result in overdiagnosis and overtreatment of prostate cancer. Our prostate cancer screening efforts may result in overtreatment of prostate cancer by at least 30% [Scattoni et al. 2007]. This is because DRE and PSA testing have shifted the diagnosis of prostate cancer to organ confined and lower grade disease [Crawford and Barqawi, 2007]. A recent review estimated that 10% of men with low grade prostate cancer were overtreated with radical surgery, and 45% were overtreated with radiation therapy [Etzioni et al. 2002].
The side effects of local treatments such as radical prostatectomy and radiation therapy include but are not limited to urinary incontinence and impotence. The incidence of these morbidities has decreased with improvement in treatment; however, these morbidities are significant for individuals who may have been overtreated for their prostate cancer [Crawford and Barqawi, 2007].
Once an individual is diagnosed with prostate cancer, a decision on the type of treatment required must be made. Treatment types include the following options: active surveillance, radical prostatectomy, cryotherapy, targeted focal therapy (TFT), and radiation therapy (either brachytherapy or external beam radiation) [Crawford and Barqawi, 2007]. Additionally, a number of new and innovative therapies such as high intensity ultrasound (HIFU) are being studied.
The type of treatment an individual chooses depends on clinical stage, Gleason Grade, and patient preference [Crawford and Barqawi, 2007]. If active surveillance and radical prostatectomy are acceptable treatment options for an individual man with prostate cancer, then there is a place for the middle ground of cryotherapy or TFT.
Cryotherapy was approved as treatment for primary prostate cancer by the Centers for Medicare and Medicaid Services approximately 5 years ago [Onik, 2008]. Cryotherapy was initially developed in the 1960s by Cooper and Lee. They developed the first cryotherapy probe system involving circulation of liquid nitrogen. These probes froze tissue to −200°C. These experiments resulted in incontinence, rectourethral fistulas, urethral sloughing and urethral strictures. The 1990s witnessed the development of multiple improvements in the technology of cryosurgery. They include the urethral warmer, the use of trans-rectal ultrasound (TRUS) to aid in probe placement and real time visualization of the ice ball formation, the replacement of liquid nitrogen by argon for cooling and helium for warming, as well as the advent of thinner cryoneedles (17-guage, 1.5 mm diameter), placement of cryoprobes through a brachytherapy-like template grid, thermocouples, and computer software developed to generate preoperative isotherm maps based on theoretical cryoprobe placements [Babaian et al. 2008]. These refinements and technological developments have reduced the side effects of cryosurgery while enhancing its effectiveness [Babaian et al. 2008].
One of the challenges of prostate cancer and the use of cryotherapy as TFT is that prostate cancer tends to be multifocal [Crawford and Barqawi, 2007]. Two-thirds of patients with prostate cancer have multifocal disease. However, approximately 33% will have unifocal disease, and this population can be isolated from the multifocal disease population with a sensitivity of 90% [Djavan et al. 1999]. Also, approximately 40–80% of the individual components of multifocal tumors measure less than 0.5 ml in volume, which is the definition of clinically insignificant prostate cancer [Noguchi et al. 2003; Rukstalis et al. 2002; Villers et al. 1992].
Recent response to our overdiagnosis of prostate cancer has led to the need to consider other forms of therapy. Consider the transition which occurred in breast cancer treatment from radical mastectomy to lumpectomy in certain individuals. Currently, there is an ongoing clinical trial evaluating TFT as a treatment for prostate cancer.
We define TFT as complete ablation of all clinically significant cancer foci within the prostate using a minimally invasive technique with preservation of the sphincter, normal gland tissue, and the neurovascular bundles [Crawford and Barqawi, 2007].
In this manuscript we will review the current literature on this experimental therapy regarding diagnosis, patient selection, mapping biopsy technique, cryotherapy technique, current efficacy studies on focal therapy, follow-up care after TFT, cost, and future developments.
Diagnosis
Screening for prostate cancer consists of a combination of PSA and DRE. Prostate biopsy is the standard of care for diagnosis of prostate cancer in men with an elevated PSA, a PSA velocity of >0.75 ng/ml, or abnormal DRE findings [Crawford and Barqawi, 2007]. Although PSA screening and DREs are used to screen for prostate cancer, these tools are not accurate in clinically staging the extent of disease [Crawford and Barqawi, 2007]. Currently, at least 12 core biopsies are recommended as the standard biopsy scheme [Presti et al. 2003]. Clinically significant disease is defined as a combination of the largest tumor volume (>5 mm3), tumor grade (≥7), life expectancy, and cancer doubling time [Dugan et al. 1996; Stamey et al. 1993].
Accurate staging at diagnosis would aid in better categorizing patients into correct treatment types for their tumor and help in making more informed decisions about their treatment [Crawford and Barqawi, 2007]. However, clinical overstaging of prostate biopsies occurs about 23% of the time [Hsu et al. 2007], and about 30% of prostate biopsies will detect clinically insignificant prostate cancer [Scattoni et al. 2007].
Mapping biopsy technique
Prostate needle biopsies are used to diagnose prostate cancer. However, they cannot be used to exclude contralateral disease or identify all cancer foci. Since the PSA screening era, we are diagnosing earlier stage disease, and Yoon et al. found a mean of 2.9 cancer foci in men with low-risk prostate cancer [Yoon et al. 2008]. This is significantly less than the mean of 7.3 cancer foci found in men before the PSA screening era [Bastacky et al. 1995; Villers et al. 1992]. Mapping biopsies are very helpful in detailing the foci and precise location of the prostate cancer. The mapping biopsy information will allow the patient and physician to make better informed decisions regarding treatment, including surveillance, TFT, surgery, or radiation therapy. There are two techniques for mapping biopsies: transrectal and transperineal [Crawford and Barqawi, 2007]. A transrectal approach limits access to the anterior and apical regions of the prostate and may increase the risk of rectal bleeding and sepsis [Barzell et al. 2003]. Due to the limitations in transrectal biopsies, the transperineal technique will be discussed further in this paper.
Indications for transperineal mapping biopsies include multiple negative TRUS biopsies with persistent high-risk parameters; e.g., high PSA, rapid PSA doubling time, abnormal DRE [Crawford and Barqawi, 2007; Barzell et al. 2003], or the need to further define the foci of prostate cancer in patients who desire TFT [Barzell et al. 2003].
The transperitoneal procedure for saturation mapping biopsies was initially pioneered by Barzell and Whitmore [Barzell et al. 2003], (see Figure 1). Pre-procedure patient preparation includes a sodium bisphosphate and sodium phosphate enema (Fleet) the morning of and one hour before the procedure, as well as prophylactic antibiotics [Barzell et al. 2003]. The patient must be under light general or IV sedation anesthesia for the procedure [Crawford et al. 2005; Barzell et al. 2003]. The patient is then placed in the dorsal lithotomy position with the scrotum elevated toward the abdomen [Barzell et al. 2003].
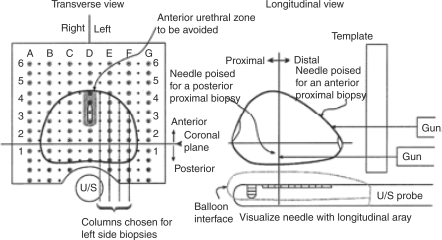
Figure 1.
Systematic Mapping Biopsy of the Prostate–Grid placement and needle projectory into the prostate. U/S = ultrasound. Adapted, with permission, from Brazell and Whitmore. [Barzell et al. 2003].
These investigators divide the prostate into eight regions, and cores are sent from each region for pathological examination [Barzell et al. 2003]. To make the eight regions the following three planes are used: a transverse plane divides the prostate into proximal (base) and distal (apex) regions; a sagittal plane divides the prostate into right and left lobes; and a coronal plane divides the prostate into anterior and posterior regions [Barzell et al. 2003].
The number of cores taken from each region of the prostate depends on the size of the prostate. To begin taking cores, in the transverse view, on the largest cross-sectional area of the gland, center the prostate on the D column on the grid. This divides the prostate into right and left. To choose the midway point in the prostate, one must use the stepper on the ultrasound to estimate the length of the prostate and identify the midpoint, then take one or two steps closer to the base than the apex [Barzell et al. 2003]. The coronal reference plane is arbitrarily chosen but may be the plane between the transition and peripheral zones. Biopsies are taken from the outer aspect of the prostate, proceeding medially in the same row toward the midline at 5 mm intervals. Then one must move down a row and repeat until the prostate has been completely biopsied in all eight regions. Ultrasound guidance is used throughout the procedure to identify the prostate and surrounding anatomy, as well as the location of the biopsies [Crawford and Barqawi, 2007; Crawford et al. 2005; Barzell et al. 2003].
Some descriptions of transperineal biopsies describe the use of a Foley catheter (with aerated gel) in the urethra during the procedure, which can help identity the urethra [Crawford et al. 2005]. Three days of oral antibiotic and alpha-blockers are given after the procedure [Barzell et al. 2003]. Unilateral saturation biopsies can be performed on individuals planning on hemi-gland abalation to prove the absence of cancer on the uninvolved side [Barzell et al. 2003]. Once this step is completed, the entire cancer-containing half of the prostate is ablated with cryotherapy.
Our approach to mapping biopsies is a variation of the description above, in that we use the perineal brachytherapy template and take biopsies at 5 mm intervals on the grid. We have found that 5 mm grid spacing is the most effective in detecting prostate cancer. Crawford et al. evaluated the use of the template-guided transperineal saturation biopsy method in 86 autopsy specimens with no known diagnosis of prostate cancer but were found to have prostate cancer during an autopsy. They also evaluated 20 prostate specimens after radical prostatectomy that were cT1c at diagnosis and did not have prior hormone therapy. The specimens were evaluated and graded by a pathologist. Then the sections were transformed into a 3D computer model of the prostate simulating the transperineal saturation biopsy schemes at both 5 mm and 10 mm grid points. The study showed that compared to 10 mm grid point spacing, 5 mm spacing achieved a higher cancer detection rate and a higher detection rate of clinically nonthreatening tumors, and it adequately identified Gleason Grade 4 and 5 tumors [Crawford et al. 2005]. This study showed that 5 mm grid spacing was most effective in detecting prostate cancer and should be used when performing transperineal mapping biopsies to further define the foci of prostate cancer before treating a patient with minimally invasive TFT and hemiablative techniques [Crawford et al. 2005]. The use of the 5 mm grid allows physicians to accurately place needles, localize areas of disease, and target areas above the urethra [Crawford et al. 2005].
Transperineal template-guided saturation biopsies use the stabilized bi-plane transrectal ultrasound to visualize and perform the systematic biopsies [Barzell et al. 2003]. The grid and ultrasound make the biopsy samples uniform throughout the prostate and the coordinates of the sample are reproducible [Barzell et al. 2003]. Weaknesses of the transperineal approach include the need for general anesthesia and slight difficulty biopsying the peripheral zone adjacent to the rectal wall [Barzell et al. 2003]. The peripheral zone is the area where prostate cancer is most likely to be located and transrectal mapping biopsies may better sample this area. However, the transrectal biopsies have disadvantages that include a theoretical increase risk of infection, limitations to the apex and anterior portion of the prostate and the inaccuracies of the location of the biopsies when using unstabilized manual placement of needles into the prostate. The grid used in transperitoneal mapping biopsies makes each biopsy location reproducible so the area can be treated in the future by TFT. The peripheral zone is easily sampled during transrectal biopsies and some groups recommend both transperitoneal mapping biopsies and transrectal biopsies [Barzell et al. 2003].
Large prostates (>60 cc) may have inadequate sampling because the pubic arch can limit access to the anterior prostate, which results in inadequate biopsies when biopsying through the perineum [Crawford and Barqawi, 2007]. A combination approach with transrectal and transperineal biopsies can allow for a complete mapping of these large prostates [Crawford and Barqawi, 2007; Barzell et al. 2003]. Additionally, short-term (2–6 months) of therapy with a 5-alpha-reductase inhibitor, or hormonal manipulation, may help reduce the size of the prostate and allow for better access to the prostate [Crawford and Barqawi, 2007].
There also appears to be more complications (hematuria, UTI, and urinary retention) following mapping biopsies in larger prostates. We have found that treatment with a 5-alpha-reductase inhibitor or hormonal manipulation decreases the rate of complications [Crawford and Barqawi, 2007]. Barzell et al. found that complications including hematuria, blood in the ejaculate, UTI, and sepsis do not appear to have higher incidence when compared to transrectal biopsies [Barzell et al. 2003].
Patient selection for TFT
We consider TFT as a treatment option in a variety of men diagnosed with prostate cancer. In a way, it provides a bridge between surveillance and more definitive therapies. It appeals to patients (and physicians) who are not comfortable with surveillance, who want to avoid more radical treatments, and who have cancers that are amenable to this therapy. The average man with prostate cancer, that we are seeing in our multidisciplinary clinic, is 59 years old, has a PSA of 5, and one or two cores with a Gleason 6 cancer.
Cryosurgery is appropriate for men who either do not want or who are not good candidates for more invasive therapies due to comorbidities [Babaian et al. 2008]. Primary TFT is an option for men with organ confined disease (up to cT2b), any Gleason Grade, with a negative metastatic evaluation [Crawford and Barqawi, 2007; Babaian et al. 2008]. Other characteristics that need to be considered are tumor burden, which can be accurately determined from the mapping biopsies and Gleason Grade [Crawford and Barqawi, 2007]. The Best Practice Statement on Cryosurgery released in 2008 indicated that subtotal cryosurgery or hemiablation needs further evaluation in order to make a recommendation about its usefulness as a treatment type for prostate cancer [Babaian et al. 2008]. Also, strict patient selection criteria have not been determined for the hemiablation technique; however, it may be used for patients who are active surveillance candidates with organ-confined unilateral disease but would prefer some form of therapy [Babaian et al. 2008].
The best results for cryoablation have occurred in men with PSA <10 ng/ml [Babaian et al. 2008]. However, cryosurgery is effective in patients with intermediate disease (Gleason Grade of ≤7, a PSA between 10 and 20, or clinical T2b) [Babaian et al. 2008]. Other possible indications for cryotherapy include patients who need salvage therapy following radiation therapy or failed cryotherapy [Barzell et al. 2003]. Additionally, select individuals who have a narrow pelvis and cannot tolerate electron beam radiation therapy, persons who have had previous pelvic radiation, persons with irritable bowel disease, or those with rectal disorders may be good candidates for cryotherapy [Babaian et al. 2008].
Contraindications to cryosurgery include the presence of tumor foci near the urethra or neurovascular bundles (unless there is no concern over sacrificing the nerve with the treatment), severe lower urinary tract symptoms due to benign prostatic hyperplasia, multiple foci of cancer, and large prostates [Crawford and Barqawi, 2007]. Much like complications from mapping biopsies, treatment of larger prostates with cryotherapy has been associated with urinary obstruction. Treatment with 5-alpha-reductase inhibitors may help decrease the volume of the gland before therapy [Crawford and Barqawi, 2007]. Larger prostates can be difficult to treat using cryoablation, secondary to non-uniformity of the temperature [Babaian et al. 2008]. Neoadjuvant hormonal therapy (with an LHRH agonist and antiandrogen) can be used to decrease the size of the prostate before cryosurgery; however, no studies have shown beneficial outcomes of hormone therapy in this setting [Babaian et al. 2008].
Overview of cryotherapy technique
Cryotherapy has had a long history in the treatment of prostate cancer. Contemporary cryotechnology includes better monitoring and urethral warmers [Crawford and Barqawi, 2007]. Improvements in cryotechnology have led to improvement in efficacy and decreased complications [Crawford and Barqawi, 2007].
The cryotherapy technique begins with enemas the night before and the morning of the procedure. The patient is placed in exaggerated lithotomy position. The transperineal brachytherapy template guide is placed in front of the peritoneum. The biplanar transrectal ultrasound is used to visualize the prostate and perform the procedure. Temperature monitors or thermocouples may be placed at the mid-glands, at the apex or the external sphincter, at bilateral neurovascular bundles, and at Denonvillier’s fascia. Lastly, the urethral warmer is placed under cystoscopic guidance. Tumor freezing temperatures are carried out to −40°C for two cycles. The thermocouple's temperature is continuously monitored [Han and Belldegrun, 2004].
The transperineal brachytherapy template guide is placed on the peritoneum and it is used with the biplanar transrectal ultrasound to visualize the prostate. The cryoneedles are inserted with TRUS guidance into the grid and spaced approximately 1 cm apart to cover the area of concern. In targeted focal therapy the needles can be placed at an exact location identified by pre-operative mapping biopsies. The needles are placed within 5 mm of the capsule and urethra in order to reduce the injury to the urethra and neurovascular bundles. Figure 2 is an example of the cryotherapy unit and the extent of freezing of different cryoprobes.
Figure 2.
The top portion is an example of a cryotherapy unit. The lower portion is an example of different cryoprobes. Picture courtesy of Endocare.
Mechanism of action of cryotherapy
There are several mechanisms of action by which cryotherapy works to destroy prostate cancer cells. The first mechanism is the cells’ response to freezing that induces cell death. ‘Freeze rupture’ is the term used to describe the method by which the extracellular fluid freezes and forms ice crystals. The extracellular fluid then becomes hyperosmotic, which causes the cells to lose water, followed by cell shrinkage and damage to the intracellular proteins. The extracellular osmolality can get as high as 8,000 mOsm by −15°C. Another mechanism is that at temperatures less than −15°C, intracellular ice begins to form, and cell metabolism fails, causing cell destruction. After such a thermal injury the cells begin to die by programmed cell death, or apoptosis. Also, once ice crystals form in the cell, they disrupt the cell membrane. As temperatures rise, vasodilatation occurs, causing increased cellular permeability and edema. This leads to endothelial damage and platelet aggregation. Cell fragments resulting from freeze rupture are responsible for initiating the inflammation cascade. Another mechanism of cell death is through vascular cells, which are injured by the freeze cycle and cause vascular stasis. Vascular stasis causes local hypoxia and secondary necrosis of the tissue [Babaian et al. 2008; Han and Belldegrun, 2004].
The freezing and thaw rates have a significant impact on destruction of prostate cancer cells. The minimum freezing temperature is −40°C, and cells must remain at that temperature for at least three minutes [Babaian et al. 2008; Han and Belldegrun, 2004]. Prostate cancer cells have a lethal temperature of −20°C [Babaian et al. 2008]. A slow thaw rate, or passive thaw rate, has been shown to improve prostate cancer ablation. The active thaw step of cryotherapy affects only the tissue next to the cryoprobe. The tissue at the edge of the ice ball around the cryoprobe will still thaw passively [Babaian et al. 2008]. Lastly, two cycles of freezing and thawing have shown increased effectiveness in destroying tissue compared to a single cycle [Babaian et al. 2008; Han and Belldegrun, 2004].
Current studies on efficacy of focal ablation, hemigland ablation, and TFT
Cryotherapy can be used to treat a small foci of cancer involving only a portion of the gland. Mapping biopsies are used to identify the location of the foci of cancer which then can be treated during a second procedure using TFT (see Figure 3). Targeted cryotherapy allows us to treat small volume disease either unilaterally or bilaterally. Other institutions perform similar cryotherapy procedures with hemi-gland ablation for unilateral disease. Hemi-ablation includes destruction of the neurovascular bundles on the involved side. Crawford et al. found that 60% of patients at autopsy without known prostate cancer prior to death, were candidates for subtotal techniques [Crawford et al. 2005]. Mouraviev et al. found that 19.2% of patients who had a radical prostatectomy had unilateral cancers and may have benefited from hemi-gland ablation [Mouraviev et al. 2007b].
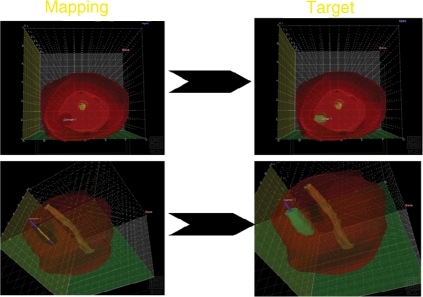
Figure 3.
3D reconstruction of mapping biopsies which identify foci of prostate cancer. The recreation of the foci can be used to treat with TFT.
In 2002, Onik et al. reported on nine patients who had hemi-cryoablation for unilaterally confined prostate cancer [Onik et al. 2002], and the authors updated their data in 2007 and 2008 to include 21 and 48 patients, respectively [Onik, 2008; Onik et al. 2008]. In the 2008 study, mean follow-up was 4.5 years, with a range from 2 to 10 years [Onik et al. 2008]. Patients were selected if they had unilateral disease on either extended biopsy schemes (14–16 cores) or mapping biopsies. Patients were also required to be potent by report. Cryoablation was performed on the side with the tumor foci, and the neurovascular bundle on this side was destroyed during the procedure. Ninety-four percent of the patients had stable PSAs that met the ASTRO criteria. Twenty-four patients were re-biopsied after cryotherapy, and all were negative for recurrent cancer. Potency rates of 90% were reported. No other complications were noted, and no urinary incontinence occurred in any of the 48 patients [Onik et al. 2008].
Likewise, Bahn et al. performed hemiablation on 31 men with unilateral prostate cancer and reported a 92.8% biochemical DFS at a mean of 70 months follow-up [Bahn et al. 2006]. These patients’ potency rate was 88%. Lambert et al. also reported on 25 patients who had hemiablation for unilateral foci of prostate cancer. Patients in this study demonstrated 84% DFS at a median follow-up of 28 months. The definition of biochemical failure was a PSA nadir greater than 50% of the pretreatment PSA level. Repeat prostate biopsies were positive in 8% of the contralateral lobes and 4% of the treated lobes. The potency rate was 71%, and no urinary incontinence was reported [Lambert et al. 2007].
The Best Practice Statement on Cryosurgery released in 2008 indicated that subtotal cryosurgery or hemiablation needs further evaluation in order to make a recommendation about its usefulness as a treatment type for prostate cancer [Babaian et al. 2008]. Hemiablation of the prostate will allow for treatment of unilateral disease while sparing at least one neurovascular bundle, therefore sparing potency while treating prostate cancer.
At our insititution we perform TFT, utilizing cryotherapy as the form of ablation. The foci of prostate cancer are localized using mapping biopsies. And instead of hemiablation of the prostate gland, we perform focal ablation of the area of prostate cancer [Crawford and Barqawi, 2007]. Patients qualify for our study if they have Gleason grade ≤7, stage T1c, and serum PSA <10 ng/dL. First we perform a mapping biopsy on each participant to accurately establish the extent of tumor burden and to obtain the coordinates of the foci of prostate cancer. In our series the average number of positive cores is two, and the median prostate size is 34 cc. Results from the study are pending.
Follow up after TFT
Follow-up after focal cryoablation is with serial PSA levels; however, there is no accepted biochemical definition of PSA failure [Babaian et al. 2008]. An undetectable PSA level is highly unlikely in any form of cryotherapy because some PSA-producing tissue will remain, especially around the urethra [Babaian et al. 2008]. However, the lower the PSA nadir, the more likely it is that future PSA levels will be stable, and that follow-up prostate biopsies will be negative [Babaian et al. 2008]. Serial PSA studies are the most reliable measures for assessing biochemical failure [Crawford and Barqawi, 2007]. Onik et al. follow their patients after TFT with PSA every 3 months for 2 years, then every 6 months indefinitely. This study also recommends biopsies at one year after TFT [Onik et al. 2008]. We utilize the same follow-up.
Other post-cryotherapy follow-up challenges include the fact that there are multiple definitions of ‘disease free,’ and of progression after subtotal therapy. They include include PSA <0.4 ng/ml, <0.5 mg/ml, and <1.0 ng/ml; 3 consecutive PSA increases; or nadir plus 2.0 ng/ml [Babaian et al. 2008]. The multiple definitions of ‘disease free’ make patient follow-up and comparison of different treatment types very difficult. The initial ASTRO criterion for PSA failure after cryotherapy is three consecutive rises in PSA after the post-treatment nadir has been reached [Babaian et al. 2008].
Furthermore, the comparable procedure is a prostatectomy, in which all the prostate tissue is removed, making the follow-up PSA very accurate in identifying recurrent disease. However, post-cryotherapy treatment PSA levels are not as accurate [Babaian et al. 2008].
Some centers have advocated for prostate biopsies after cryotherapy without elevated PSA at 6- and 12-month intervals. The incidence of negative biopsy after one or more whole gland cryotherapy treatments ranges from 87 to 98% if the biopsy is done at 6- to 12-month intervals after treatment [Babaian et al. 2008]. In fact, most cryotherapy practitioners have stopped routine post-treatment biopsies because of the high negative biopsy rate. However, if PSA is increasing, experts recommend that a prostate biopsy be performed, and that patients with positive biopsies be retreated [Crawford and Barqawi, 2007]. PSA failures after TFT have the option of additional treatment with cryotherapy, prostatectomy, or radiation therapy [Crawford and Barqawi, 2007].
Complications
In hemiablation or TFT there may be no injury to the neurovascular bundle or injury to one bundle as seen in hemiablation cryosurgery techniques. Therefore, impotence rates for TFT should be less than other treatment modalities. Prostatectomy patients have incontinence rates of 6% and impotence of 30% and can take up to two years for return of potency after surgery [Onik et al. 2008]. Impotence rates after hemi-gland cryoablation range from 10–29% and may take up to one year after treatment for return of potency [Onik et al. 2008; Lambert et al. 2007 Onik et al. 2002]. Individuals on hormones may experience a longer period before the return of potency [Onik, 2008]. Fistula formation was one of the main concerns when cryotherapy was reintroduced in the 1990s and was one of the most common complications in the 1960s. The incidence of fistula formation in the current literature ranges between 0 and 0.5% which is similar to rectal injury after RRP [Babaian et al. 2008].
Cost of TFT
A single-institution study that compared the cost of radical and perineal prostatectomy to the cost of robotic prostatectomy and cryosurgery found that the direct surgical costs were lower for radical ($2,471) and perineal prostatectomy ($2,788) than for laparoscopic robotic prostatectomy ($3,441) and cryosurgery ($5,702). However, total hospital costs were lowest for cryosurgery ($9,195), compared to RRP ($10,704), RPP ($10,536), and laproscopic robotic prostatectomy ($10,047). Reasons for the lower overall cost for cryotherapy are that the hospital stay is much shorter, patients require no pathology evaluation, and there is little or no need for blood transfusions [Mouraviev et al. 2007a].
Future direction of research
Imaging of the prostate and identification of the amount and location of prostate cancer will play pivotal roles in the future success of TFT [Etzioni et al. 2002]. Future imaging techniques need the following characteristics: high sensitivity for identifying prostate cancer foci; the ability to localize foci of cancer in a reproducible fashion (e.g. being able to regenerate the coordinates of tumor foci by generating a 4-dimensional image); minimal risk to the patient; and cost effectiveness when implemented on a large scale [Etzioni et al. 2002]. Other imaging techniques such as color Doppler ultrasounds and ultrasonic elastography have not produced a significant increase in sensitivity for detecting foci of prostate cancer [Etzioni et al. 2002].
Since the 1980s, magnetic resonance imaging (MRI) has been an area of research in the identification of prostate cancer. A new technique in the science of MRIs is being studied for the localization and detection of prostate cancer. The new technique is magnetic resonance spectroscopic imaging (MRSI). MRSI can detect differences in the biochemical make up of tumor tissue. The MRSI uses an endorectal coil which has improved the ability of the MRSI to evaluate the anatomy of the prostate and the tissues of the prostate. The MRSI detects elevations and depressions in certain metabolites that are present in tissues with prostate cancer but not in normal tissues. Evaluation of the data produced by the MRSI needs to be processed by special software which makes access to this modality limited [Puech et al. 2009; Kurhanewicz et al. 2008].
A combination of the normal MRI and the MRSI have shown a specificity of 91% and a sensitivity of 95%. The specificity of localization of prostate cancer with the combined exams increases to 98% when a sextant biopsy is positive [Kurhanewicz et al. 2008]. The sensitivity and specificity of the MRI techniques increases with higher volume and grade prostate cancer [Puech et al. 2009].
Another variation in the different MRI techniques is dynamic contrast enhanced (DCE) MRI. This type of MRI is performed by injecting a gadolinium based contrast agent into the patient and measuring the signal intensity on T1 weighted images. Areas of increased angiogenesis, such as prostate cancer, will have increased enhancement on T1 weighted images [Puech et al. 2009; Kurhanewicz et al. 2008]. A recent study showed that DCE MRI has a 89% sensitivity and 90% specificity for localizing prostate cancer [Crawford and Barqawi, 2007]. Other studies have found DCE MRI has a sensitivity of 77% and a specificity of 91% for foci of cancer greater than 0.2 cc, and 90% and 88% for foci greater than 0.5 cc, respectively. This technique may help physicians identify individuals with unilateral prostate cancer [Villers et al. 2006].
Benign pathologies of the prostate tissue (e.g. inflammation and BPH) can cause decreased specificity of the MRI techniques [Puech et al. 2009; Zelhof et al,. 2009; Kurhanewicz et al. 2008]. A minimum of six weeks is required between the prostate biopsy and the MRI. Prostatic hemorrhage after biopsy can cause artifact that will overestimate the amount of cancer on the MRI [Puech et al. 2009; Zelhof et al. 2009; Kurhanewicz et al. 2008].
Additional areas of investigation should include treatments for any remaining small foci of prostate cancer not involved in the TFT treatment, such as neoadjavant radiation therapy [Etzioni et al. 2002]. Additionally, investigation into the molecular basis of prostate cancer will allow for evaluation of genetic markers for aggressive prostate cancer and evaluation of smaller foci of cancer. We also must investigate the natural history of each foci of cancer in the gland, as well as whether certain foci are indolent and never require treatment, whether we can identify the foci of cancer that will progress, and whether it's possible to treat just those areas while decreasing morbidity of the cancer.
Conclusion
Prostate screening for cancer has increased the identification of many clinically insignificant cancers and resulted in overdiagnosis and overtreatment of prostate cancer. Current treatments for prostate cancer have significant morbidities, which is especially troubling in light of the number of individuals who may be overtreated for their prostate cancer. For these individuals TFT or other methods of partial gland ablation may be a bridge between active surveillance and more aggressive therapies. The mapping biopsy technique has been developed to isolate individuals who have localized disease and are candidates for TFT. Improvements in cryotherapy technology have led to better efficacy and decreased complications. We still need to develop better imaging to identify the amount and location of prostate cancer. This is a pivotal element in future success of TFT, as are long-term survival studies.
Conflicts of interest statement
This is a complete disclosure of all significant financial interests related to this project for Dr. E. David Crawford. He is an advisor or speaker for the following: Sanofi-Aventis, Poinard, Astra Zeneca, Glaxo Smith Kline, Ferring, Indevus, Soar BioDynamics. He performs research and has grants with the following: Endocare, Oncura/Galil/Eigen, Bostwick, Genprobe, Aeterna Zentaris, EDAP - HIFU, Ferring, NIH/NCI, Cancer Center. He performs volunteer work with Prostate Conditions Education Council as the Chairman and US Too.
References
- Babaian R.J., Donnelly B., Bahn D., Baust J.G., Dineen M., Ellis D., et al. (2008) Best Practice Statement on Cryosurgery for the Treatment of Localized Prostate Cancer. J Urol 180: 1993–2004 [DOI] [PubMed] [Google Scholar]
- Bahn D.K., Silverman P., Lee F., Sr,, Badalament R., Bahn E.D., Rewcastle J.C. (2006) Focal prostate cryoablation: Initial results show cancer control and potency preservation. J Endourol 20(9): 688–692 [DOI] [PubMed] [Google Scholar]
- Barzell W.E., Whitmore III W.F., Andriole G.L. (2003) How to perform transperineal saturation prostate biopsy: technique addresses diagnostic, therapeutic dilemmas that arise following TRUS biopsies. Urology Times 31: 41–41 [Google Scholar]
- Bastacky S.I., Wonjno K.U., Walsh P.C., Carmichael M.J., Epstein J.I. (1995) Pathological features of hereditary prostate cancer. J Urol 153: 987–992 [PubMed] [Google Scholar]
- Crawford E.D., Barqawi A. (2007) Targeted Focal Therapy: A minimally invasive Ablation Technique for Early Prostate Cancer. Oncology 21(1): 27–32 [PubMed] [Google Scholar]
- Crawford E.D., Wilson S.S., Torkko K.C., Hirano D., Stewart J.S., Brammell C., et al. (2005) Clinical staging of prostate cancer: A computer-simulated study of transperineal prostate biopsy. BJU Int 96(7): 999–1004 [DOI] [PubMed] [Google Scholar]
- Djavan B., Susani M., Bursa B., Basharkhah A., Simak R., Marberger M. (1999) Predictability and significance of multifocal prostate cancer in the radical prostatectomy specimen. Tech Urol 5(3): 139–142 [PubMed] [Google Scholar]
- Dugan J.A., Bostwick D.G., Myers R.P., Qian J., Bergstralh E.J., Oesterling J.E. (1996) The definition and preoperative prediction of clinically insignificant prostate cancer. JAMA 276: 288–294 [PubMed] [Google Scholar]
- Etzioni R., Penson D.F., Legler J.M., di Tommaso D., Boer R., Gann P.H., et al. (2002) Overdiagnosis due to prostate-specific antigen screening: Lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst 94: 981–990 [DOI] [PubMed] [Google Scholar]
- Han K.R., Belldegrun A.S. (2004) Third-generation cryosurgery for primary and recurrent prostate cancer. BJU Int 93: 14–18 [DOI] [PubMed] [Google Scholar]
- Hsu C.Y., Joniau S., Oyen R., Roskams T., Van Poppel H. (2007) Outcome of surgery for clinical unilateral T3a prostate cancer: A single-institution experience. Eur Urol 51(1): 121–128 [DOI] [PubMed] [Google Scholar]
- Jemal A., Siegel R., Ward E., Murray T., Xu J., Thun M. (2007) Cancer statistics, 2007. CA Cancer J Clin 57: 43–66 [DOI] [PubMed] [Google Scholar]
- Kurhanewicz J., Vigneron D., Carroll P., Coakley F. (2008) Multiparametric magnetic resonance imaging in prostate cancer: present and future. Curr Opin Urol 18(1): 71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert E.H., Bolte K., Masson P., Katz A.E. (2007) Focal cryosurgery: encouraging health outcomes for unifocal prostate cancer. Urology 69(6): 1117–1120 [DOI] [PubMed] [Google Scholar]
- Mouraviev V., Nosnik I., Sun L., Robertson C.N., Walther P., Albala D., et al. (2007a) Financial comparative analysis of minimally invasive surgery to open surgery for localized prostate cancer: A single-institution experience. Urology 69(2): 311–314 [DOI] [PubMed] [Google Scholar]
- Mouraviev V., Mayes J.M., Sun L., Madden J.F., Moul J.W., Polascik T.J. (2007b) Prostate cancer laterality as a rationale for focal ablative therapy for the treatment of clinically localized prostate cancer. Cancer 110(4): 906–910 [DOI] [PubMed] [Google Scholar]
- Noguchi M., Stamey T.A., McNeal J.E., Nolley R. (2003) Prognostic factors for multifocal prostate cancer in radical prostatectomy specimens: Lack of significance of secondary cancers. J Urol 170: 459–463 [DOI] [PubMed] [Google Scholar]
- Onik G., Narayan P., Vaughan D., Dineen M., Brunelle R. (2002) Focal "nerve-sparing" cryosurgery for treatment of primary prostate cancer: A new approach to preserving potency. Urology 60(1): 109–114 [DOI] [PubMed] [Google Scholar]
- Onik G. (2008) Rationale for a “Male Lumpectomy” a prostate cancer targeted approach using cryoablation: results in 21 patients with at least 2 years of follow-up. Cardiovasc Intervent Radiol 31(1): 98–106 [DOI] [PubMed] [Google Scholar]
- Onik G., Vaughan D., Lotenfoe R., Dineen M., Brady J. (2008) The “male lumpectomy”: Focal therapy for prostate cancer using cryoablation results in 48 patients with at least 2-year follow-up. Urol Oncol 26(5): 500–505 [DOI] [PubMed] [Google Scholar]
- Presti J.C., O’Dowd G.J., Miller C., Mattu R., Veltri R. (2003) Extended peripheral zone biopsy schemes increase cancer detection rates and minimize variance in prostate specific antigen and age related cancer rates: results of a community multi-practice study. J Urol 169(1): 125–129 [DOI] [PubMed] [Google Scholar]
- Puech P., Huglo D., Petyt G., Lemaitre L., Villers A. (2009) Imaging of organ-confined prostate cancer: functional ultrasound, MRI and PET/computed tomography. Curr Opin Urol 19(2): 168–176 [DOI] [PubMed] [Google Scholar]
- Rukstalis D.B., Goldknopf J.L., Crowley E.M., Garcia F.U. (2002) Prostate cryoablation: A scientific rationale for future modifications. Urology 60: 19–25 [DOI] [PubMed] [Google Scholar]
- Scattoni V., Zlotta A., Montironi R., Schulman C., Rigatti P., Montorsi F. (2007) Extended and saturation prostatic biopsy in the Diagnosis and Characterization of Prostate Cancer: A Critical Analysis of the Liturature. Eur Urol 52: 1309–1322 [DOI] [PubMed] [Google Scholar]
- Stamey T.A., Freiha F.S., McNeal J.E., Redwine E.A., Whittemore A.S., Schmid H.P. (1993) Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer 71: 933–938 [DOI] [PubMed] [Google Scholar]
- Villers A., McNeal J.E., Freiha F.S., Stamey T.A. (1992) Multiple cancers in the prostate. Morphologic features of clinically recognized versus incidental tumors. Cancer 70(9): 2313–2318 [DOI] [PubMed] [Google Scholar]
- Villers A., Puech P., Mouton D., Leroy X., Ballereau C., Lemaitre L. (2006) Dynamic contrast enhanced, pelvic phased array magnetic resonance imaging of localized prostate cancer for predicting tumor volume: correlation with radical prostatectomy findings. J Urol 176: 2432–2437 [DOI] [PubMed] [Google Scholar]
- Yoon G.S., Wang W., Osunkoya A.O., Lane Z., Partin A.W., Epstein J.I. (2008) Residual tumor potentially left behind after local ablation therapy in prostate adenocarcinoma. J Urol 179(6): 2203–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelhof B., Lowry M., Rodrigues G., Kraus S., Turnbull L. (2009) Description of magnetic resonance imaging derived enhancement variables in pathologically confirmed prostate cancer and normal peripheral zone regions. BJU Int 103(7): 883–888 [DOI] [PubMed] [Google Scholar]