Abstract
Normal vascular endothelium is essential for the synthesis and release of substances affecting vascular tone (e.g. nitric oxide; NO), cell adhesion (e.g. endothelins, interleukins), and the homeostasis of clotting and fibrinolysis (e.g. plasminogen inhibitors, von Willebrand factor). The degeneration of endothelial integrity promotes adverse events (AEs) leading to increased atherogenesis and to the development of vascular systemic and penile end-organ disease. Testosterone (T) is an important player in the regulation of vascular tone through non-genomic actions exerted via blockade of extracellular-calcium entry or activation of potassium channels; also, adequate T concentrations are paramount for the regulation of phosphodiesterase type-5 (PDE5) expression and finally, for the actions exerted by hydrogen sulphide, a gas involved in the alternative pathway controlling vasodilator responses in penile tissue. It is known that an age-related decline of serum T is reported in approximately 20 to 30% of men whereas T deficiency is reported in up to 50% of men with metabolic syndrome or diabetes. A number of laboratory and human studies have shown the combination of T and other treatments for erectile dysfunction (ED), such as PDE5 inhibitors, to be more beneficial in patients with ED and hypogonadism, who fail monotherapy for sexual disturbances.
The aim of this review is to show evidence on the role of T and PDE5 inhibitors, alone or in combination, as potential boosters of endothelial function in internal medicine diseases associated with reduced T or NO bioavailability, i.e. metabolic syndrome, obesity, diabetes, coronary artery disease, hyperhomocysteinemia, that share common risk factors with ED. Furthermore, the possibility of such a strategy to prevent endothelial dysfunction in men at increased cardiovascular risk is discussed.
Keywords: endothelial dysfunction, erectile dysfunction, phosphodiesterase type-5 inhibitors, testosterone, cardiovascular disease, hydrogen sulphide, adenosine diphosphate
Introduction
The endothelium is the single layer of cells that line the luminal surface of blood vessels. Over the past few years, it has become increasingly apparent that it is far more than just a structural lining and has a range of important physiological functions. The vascular endothelium is an active, dynamic tissue that controls many important functions on the vasculature, including regulation of vascular tone, local hemostasis, proliferative process, and maintenance of blood circulation, fluidity, coagulation, and inflammatory responses. Through these multiple functions, the endothelium is primarily responsible for enabling the arterial system to deliver sufficient tissue perfusion [Haller, 1997; Vane et al. 1990].
Endothelial dysfunction (EDys) has gained increasing notoriety as a key player in the pathogenesis of atherosclerosis [Ross, 1990]. As atherosclerosis is the most common cause of vasculogenic erectile dysfunction (ED) in older men, the recognition of ED as a warning sign of silent vascular disease has led to the concept that a man with ED and no cardiac symptoms is a cardiac (or vascular) patient until proven otherwise [Solomon et al. 2003]. Vasculogenic ED results from impairment of endothelial-dependent or -independent smooth muscle relaxation (functional vascular ED, initial stages), occlusion of the cavernosal arteries by atherosclerosis (structural vascular ED, late stages), or a combination of these. The association between ED and clinical atherosclerosis has been documented [Guay, 2007]. Furthermore, there is a high incidence of cardiovascular disease (CVD) in men with ED, and data suggest that ED may be an early manifestation of EDys in the presence or absence of cardiovascular risk factors (CRFs) [Gazzaruso et al. 2008]. The presence of traditional CRFs, such as aging, smoking, hypertension, dyslipidemia, diabetes and obesity, and some less-traditional risk factors, including inflammation, hypoxia, oxidative stress and homocysteinemia, are known to cause EDys [Brunner et al. 2005]. EDys frequently occurs in acute coronary syndromes [Gonzalez and Selwyn, 2003], heart failure, reperfusion injury, renal failure, systemic inflammatory disorders [Vlachopoulos et al. 2007] and ED [Bonetti et al. 2003]. EDys is the key event in the pathophysiology of ED and, importantly, men with penile vascular damage may have EDys in other vascular beds, as well [Kaiser et al. 2004]. Therefore, men with ED may be at increased risk for cardiovascular adverse events (AEs) and ED may be considered as a sentinel symptom in patients with occult CVD [Thompsom et al. 2005].
The age-related testosterone (T) decline may affect either arterial reactivity or sexual function [Allan and McLachlan, 2004]. Hypogonadism and ED in aging men are common disorders found in patients presenting to andrology clinics. Increasing evidence indicates that both disorders have important associations with the metabolic syndrome (MeS), diabetes and CVD, all conditions with an increased morbidity and mortality [Jones, 2007]. A low T-level is positively associated with the presence and severity of atherosclerosis and a reduction in plasma T might contribute to increased arterial stiffness, which in turn has been associated with increased cardiovascular risk [Hougaku et al. 2006]. The early recognition of these clinical conditions is important to allow treatment and hence reduce cardiovascular risk. The increased incidence of CVD in aging men compared with premenopausal women suggests an unfavourable effect of male sex hormone T on the cardiovascular system. However, numerous epidemiological and interventional studies reported a controversial relationship between T and CVD. T inversely correlates with the severity of atherosclerosis and has beneficial effects upon vascular reactivity, inflammatory cytokine, adhesion molecules, insulin resistance, serum lipids, and hemostatic factors [Fukui et al. 2007]. Interestingly, men with established coronary heart disease display reduced circulating T levels [Rosano et al. 2007] that are often associated with a certain degree of EDys independently of other vascular risk factors (VRFs), suggesting a protective role of endogenous T on the endothelium [Akishita et al. 2007]. Thus, a modern approach to ED should be geared not only towards ameliorating the symptom of erectile inadequacy, but also towards modifying the burden of any concomitant medical conditions in which EDys plays a pivotal role in worsening the course of disease and thus contributing to the severity of ED [Aversa et al. 2008a]. In this article, we shall discuss the role of T and PDE5-inhibitors to improve endothelial and erectile functions and their possible untoward effects on systemic endothelial function/dysfunction deriving from chronic exposure to these substances.
Endothelial function and dysfunction
In a normal physiologic state, healthy endothelium serves as an anticoagulant membrane, exerting predominantly fibrinolytic, anticoagulant and anti-aggregatory effects. These effects occur through the expression of anti-thrombin III (inhibiting fibrinogen to fibrin conversion), heparin-like molecules (which enhance anti-thrombin III activity), tissue factor pathway inhibitor (which inactivates the extrinsic pathway)[Kharbanda and Deanfield, 2001] and tissue-type plasminogen activator (tPA) from intact endothelial cells (ECs). Additionally, ECs bind thrombin, leading to protein-C activation and eventually inactivation of plasminogen activator inhibitor-1 (PAI-1) [Kharbanda and Deanfield, 2001]. In a haemostatic response, ECs produce key components of platelet activation and aggregation, including von Willebrand factor (vWf), fibronectin and thrombospondin, ultimately leading to the initiation of the coagulation cascade [Sagripanti and Carpi, 2000]. These dynamic thrombotic functions are important, as thrombus formation is a key element in atherosclerosis progression.
The endothelium regulates transmigration of circulating cells through a complex interplay of trans-signaling molecules. ECs recruit inflammatory cells via expression of cell adhesion molecules (CAMs) such as selectin and immunoglobulin superfamily adhesion molecules, and by responding to pro-adhesion signals from circulating cytokines [Panes et al. 1999]. Platelets also have a range of endothelial signalling abilities, including the release of vasodilating agents (such as adenosine diphosphate and serotonin), as well as vasoconstricting and procoagulant factors (such as endothelin-1 and vWf). The effects of these circulating cell signals can further be attenuated by some endothelially-derived substances such as nitric oxide (NO) and prostacyclin [Kharbanda and Deanfield, 2001]. The vascular endothelium also plays an obligatory role in vasodilation. These effects are mediated in large part by the action of endothelial derived nitric NO which has vasodilatory properties. NO is also known to inhibit platelet activation/aggregation, vascular smooth muscle proliferation, leukocyte adherence, and low density lipoprotein oxidation, all of which are known to contribute to CVD states. Endothelial nitric oxide synthase (eNOS), one of three nitric oxide synthase (NOS) isoforms, is responsible for the majority of endothelium derived production of NO from the substrate L-arginine [Vane et al. 1990]. Bioavailable NO can be increased by enhancing its production or reducing its inactivation. As a freely diffusible gas, NO acts not only within the lumen but also on the surrounding smooth muscle cells where it increases cyclic guanosine monophosphate (cGMP)-mediated vasodilation [Kharbanda and Deanfield, 2001]. In contrast, the endothelium also produces vasoconstricting hormones known as the endothelins [Maas et al. 2002]. A number of novel plasma markers have been associated with atherosclerosis and EDys, and the latter can be tested in vivo using several techniques that rely principally on measuring change in arterial diameter or flow in response to stimuli, and in vitro using circulating biomarkers, such as high-sensitivity C-reactive protein, P-selectin, CAMs and endothelial progenitor cells (EPCs) in clinical studies [Farouque and Meredith, 2001]. Longitudinal observations confirmed that EDys of the coronary and peripheral circulation is predictive of cardiovascular events, the sensitivity and specificity being greater for coronary artery EDys than for peripheral dysfunction [Vita and Keaney, 2002].
The mechanism underlying EDys induced by CRFs, such as diabetes, hypertension, smoking and dyslipidemia, involves two processes: the inhibition of dimethylarginine dimethylaminohydrolase, which catalyses the hydrolysis of asymmetric dimethyl arginine (ADMA), an inhibitor of eNOS [Boger, 2003]; and the uncoupling of eNOS activity [Watts et al. 2007]. Down-regulation of eNOS with these diseases results in reduced bioavailability of NO which determines EDys. The guanidino dimethyl arginine derivatives – symmetric (SDMA) and ADMA are derived from degradation of methylated proteins and are found in plasma [Krzyzanowska et al. 2008]. ADMA, in contrast to SDMA, has been shown to inhibit NOS, reduce NO levels and to be associated with cardiovascular events [Valkonen et al. 2001]. ADMA levels are ten times higher inside ECs [Elesber et al. 2006]. Both processes increase oxidative stress in the ECs [Cooke, 2005]. This increase in oxidative stress leads to further oxidative catabolism of NO, formation of peroxynitrite, and activation of the proinflammatory nuclear factor kappa B, which in turn induces cellular inflammation and adhesion molecule production [Cooke and Dzau, 1997]. One potent free radical, superoxide anion, inactivates NO resulting in the production of peroxynitrite – a potent oxidant that stimulates the production of vasoconstrictor prostanoids. NO production is also reduced in the presence of free radicals. Oxidative stress leads to the up-regulation of anti-oxidation enzymes, such as superoxide-dismutase, producing hydrogen peroxide [Browne et al. 2003]. These mechanisms, and the reduction in bone-marrow-derived endothelial progenitor cells (EPCs), could underpin a common pathogenesis for both ED and EDys [Baumhäkel et al. 2006]. In individuals with established ED, an elevated ADMA level has been shown to correlate with the severity of comorbidities, such as in patients with renal disease [Kielstein et al. 2001], insulin resistance and diabetes [Schiel et al. 2003], and CAD [Browne et al. 2003; Lu et al. 2003], indicating the impact that ADMA levels could have on endothelial function [Wierzbicki et al. 2006].
EDys is an important component of the metabolic or insulin resistance syndrome, as demonstrated by inadequate vasodilation and/or paradoxical vasoconstriction in coronary and peripheral arteries in response to stimuli that release NO [Cersosimo and DeFronzo, 2006]. Other distinct non-metabolic branches of insulin-signaling pathways regulate secretion of the vasoconstrictor endothelin-1 in endothelium. Metabolic insulin resistance is characterized by pathway-specific impairment in phosphatidylinositol 3-kinase-dependent signaling, which in endothelium may cause imbalance between production of NO and secretion of endothelin-1, leading to decreased blood flow, which worsens insulin resistance [Kim et al. 2006]. Deficiency of endothelial-derived NO is believed to be the primary defect that links insulin resistance and EDys. NO deficiency results from decreased synthesis and/or release, in combination with exaggerated consumption in tissues by high levels of reactive oxygen (ROS) and nitrogen (RNS) species, which are produced by cellular disturbances in glucose and lipid metabolism. EDys contributes to impaired insulin action, by altering the transcapillary passage of insulin to target tissues. Reduced expansion of the capillary network, with attenuation of microcirculatory blood flow to metabolically active tissues, contributes to the impairment of insulin-stimulated glucose and lipid metabolism. This establishes a reverberating negative feedback cycle in which progressive EDys and disturbances in glucose and lipid metabolism develop secondary to the insulin resistance [Kim et al. 2006].
The recent discovery that circulating endothelial microparticles (EMPs) are a hallmark of EDys [Brodsky et al. 2004] and that circulating EPCs of bone marrow origin contribute to the regeneration of damaged endothelium has opened up exciting avenues of research [Goldschmidt-Clermont et al. 2005]. EPCs play a key role in promoting endothelial repair processes after different injuries and their circulating levels are intimately correlated with the degree of vascular response to vasoconstrictor stimuli; in other words, the higher the EPCs, the better the vasodilator arterial response to shear stress. A low number of circulating EPCs is supposed to be an independent risk factor for coronary heart disease. Recent studies demonstrated a reduction of EPCs in patients with chronic heart failure [Valgimigli et al. 2004] or endothelial dysfunction [LinksHeiss et al. 2004]. In subjects with the MeS, circulating EPCs are synergistically decreased by clustering components of the syndrome [Fadini et al. 2006], and their levels negatively correlate with the homeostasis model assessment value, a measure of insulin resistance. Virtually all risk factors for atherosclerosis have been associated with decreased levels of circulating EPCs, while absent or insufficient EPCs in patients with endothelial-cell injury may affect the progression of cardiovascular disease, with EPCs as an independent predictor of cardiovascular outcomes [Werner et al. 2007]. The finding that men with ED of any origin have reduced number of EPCs and that men with type-2 diabetes [Esposito et al. 2007] or who are overweight [Esposito et al. 2009] have increased levels of EMPs which are independently involved in the pathogenesis of ED, opens a new scenario for the application of these potential novel markers in the early detection of ED. However, there is still a matter of debate as to whether any reliable surrogate marker of EDys in cardiovascular medicine may be applied [Braunwald, 2008]. We can conclude that endothelial integrity has a paramount role in preserving a man from cardiovascular and genital organ injuries.
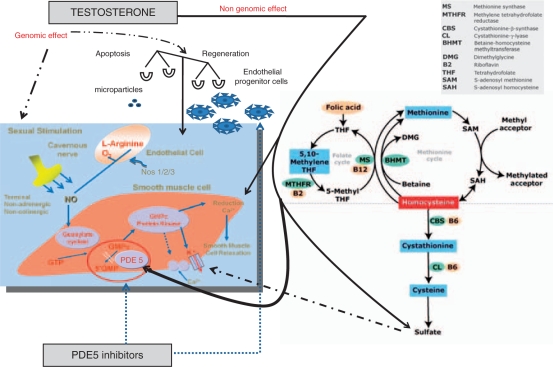
Hyperhomocysteinemia (HHcy) is considered one of the most important CRFs increasing considerably the risk of stroke and myocardial infarction. With respect to endothelial function, direct effects of HHcy on vascular endothelial cells have been demonstrated through the reduction of endothelial NO production. Also, the presence of mild elevations of plasma total homocysteine has been identified as an independent risk factor for early atherosclerotic vascular disease [Nygård et al. 1997]. Mild HHcy has a complex etiology, including insufficient intake of vitamins B6 and B12 and folate and genetic factors, of which an homozygote mutation of 5-methylenetetrahydrofolate reductase (MTHFR) thermolabile variant is probably the most important one (See Figure 1). The mechanism of premature CVD or ED in this context is not precisely known [Lombardo et al. 2004], but may be related to increased vulnerability to lipid toxicity, vascular smooth muscle-cell growth-factor properties of homocysteine, endothelial damage, or vasomotor dysfunction or to disorders of platelet aggregation and coagulation [Bellamy and McDowel, 1997].
Figure 1.
Schematic representation of different pathways related to penile erection in humans controlled by testosterone and phosphodiesterase type 5-inhibitors.
Testosterone and endothelium
The cerebral vasculature is a target tissue for sex steroid hormones. Estrogens, androgens, and progestins modulate the function and pathophysiology of cerebral circulation [Krause et al. 2006]. Hypogonadism related-ED represents the link between internal medicine and sexual medicine, so far. T circulating-levels are determinant for correct endothelial function, and tend to a stepward decrease with aging and with the presence of numerous comorbidities [Travison et al. 2007]. T plays a key role in coordinating and facilitating the delicate balance between the effect of endogenous vasoconstrictors and vasorelaxing agents of vascular tone [Aversa et al. 2005; Thompson and Khalil, 2003]. This process is supported by the fact that androgen receptors (ARs) have been localized within vascular endothelium and smooth muscle-cells. Thus, arterial functions may be directly subject to T influence and, most likely, two independent pathways of T-induced effects within the vessel wall can be assumed (i.e., genomic and nongenomic) [Orshal and Khalil, 2004; Wynne and Khalil, 2003]. The classic pathway of androgen action involves steroid binding to the AR, a ligand-activated transcription factor acting on the genome [Heinlein and Chang, 2002a; Kallio et al. 1996; Chang et al. 1995]. The genomic action of AR is modulated by a large variety of coregulators, which are proteins that fine-tune target gene expression by enhancing (coactivators) or restraining (corepressors) transcription [Lee and Chang, 2003; Heinlein and Chang, 2002a]. Although T circulates throughout the body, the factors responsible for variation in tissue androgen sensitivity remain to be further clarified. Intensity of expression of the single human AR [Kang et al. 2003; Lutz et al. 2003] partly defines androgen sensitivity, but AR is almost ubiquitously expressed to some degree in tissues. Further biologic determinants of tissue androgen sensitivity, including the functional AR polymorphisms as well as tissue distribution and regulation of AR coregulators, androgen metabolic enzymes, and nongenomic mechanisms, remain to be better defined so that their net integrated effects can be more fully understood.
Androgen sensitivity could be modulated by a functional polymorphism of the AR that influences the strength of the genomic signal transduced from its interaction with an androgen as a bound ligand. One such functional AR polymorphism is the exon 1 triplet CAG (polyglutamine), whereby the repeat length is directly correlated with T levels [Heinlein and Chang, 2002b]. There is now considerable evidence for rapid, non-genomic effects of steroid, including androgens [Cho et al. 2003]. Non-genomic steroid action is distinguished from classic genomic effects by rapid onset (seconds to minutes) that is faster than genomic mechanisms; insensitivity to inhibition of RNA and protein synthesis; effects produced by steroids unstable to access the nucleus (either covalently linked to membrane-impermeable macromolecules or in cells lacking a nucleus); and not usually blocked by classic antagonists resulting from different steroidal specificity from classic cognate nuclear receptors. As for other steroids, non-genomic androgen effects characteristically involve the rapid induction of conventional second-messenger signal transduction cascades, including increases in cytosolic calcium and activation of protein kinase A, protein kinase C, and MAP-kinase, leading to diverse cellular effects, including smooth muscle relaxation, neuromuscular and junctional signal transmission, and neuronal plasticity [Aversa et al. 2005]. Most non-genomic effects involve a membrane receptor, and putative-binding sites are described for all major classes of steroids, including androgens [Herve, 2002; Gerdes et al. 2000]. No membrane AR has been characterized, but preliminary evidence of a low-affinity microsomal membrane-binding site for alkylated androgens [Chirino et al. 1989] and an endothelial cell plasma membrane dehydroepiandrosterone (DHEA)-binding site [Williams et al. 2002] still require functional proof of specific receptor status. A plasma membrane sex harmone binding globuline (SHBG) receptor capable of modulating androgen action at the level of plasma membranes and initiating intracellular cyclic 3’-5’ adenosine monophosphate (cAMP) signaling has been described in humans. The SHBG receptor remains to be fully characterized, and it is not clear whether it has any physiological role in species like rodents that lack circulating SHBG [Rosner et al. 1999].
Recent animal and in vitro studies have further documented that T up-regulates the expression of arterial AR mRNA and is associated with an inhibitory effect on neo-intimal plaque formation [Hanke et al. 2001]. Additionally, positive acute hemodynamic effects of T on coronary vasomotion and stress-test induced ischemia were reported [Rosano et al. 1999]. Vascular ARs may mediate these effects of T on the arterial wall, and T has been shown to produce coronary, aortic, and brachial vasculature dilatation by activation of both endothelial-dependent and -independent mechanisms [Chou et al. 1996]. Endothelium-dependent effects of T are likely mediated, at least in part, through NO production, whereas mechanisms of endothelium-independent effects involve one or more types of smooth muscle ion conductance channels [Littleton-Kearney and Hurn, 2004]. The interaction of T with its specific nuclear receptors may trigger not only long-term genomic effects, but also acute non-genomic vasodilator responses [Wynne and Khalil, 2003]. T may activate the endothelium and stimulate the NO-cGMP and/or the hyperpolarization-mediated vascular relaxation pathway and may thus represent potential beneficial effects of T against coronary artery atherosclerosis. Additional endothelium-independent effects of T may involve inhibition of the signaling mechanism of vascular smooth muscle contraction such as intracellular concentration [Ca2 +] and protein kinase C, whereas a significant portion of the vasorelaxing effect of T appears to be endothelium independent because no significant difference is observed between the relaxing effect of the hormone in isolated vessels with or without endothelium [Crews and Khalil, 1999]. Also, inhibition of NO-synthase, prostaglandin synthase, and guanylate cyclase do not appear to affect the vasorelaxing effect of T, suggesting that the T-induced vascular relaxation may involve inhibition of the mechanism of vascular smooth muscle contraction [Murphy and Khalil, 1999; Yue et al. 1995]. Several studies have shown that acute administration of T induces a rapid relaxation in vascular tissues of different species including humans [Costarella et al. 1996; Perusquia et al. 1996], suggesting a non-genomic effect of this hormone on vascular reactivity [English et al. 2002]. Different mechanisms have been proposed to explain T-induced vasodilatation [Tep-Areenan et al. 2002] but it remains a matter of debate which is the effective mechanisms and which are the mediators involved of the T-induced vasorelaxation. T might induce relaxation in human isolated corpora cavernosa strips by activation of smooth muscle adenosine triphosphate-sensitive K(+) channels. This finding suggests that T, in addition to its known endothelial action, might regulate erectile function locally by its action on the smooth muscle of the human corpus cavernosum [Yildiz et al. 2009]. By contrast, contractile studies suggested that T may enhance thromboaxane A2 (TXA2)-induced coronary vasoconstriction in guinea pigs [Schrör et al. 1994]. Androgens may also regulate the expression and density of TXA2 receptors in cultured rat aortic and guinea pig coronary smooth-muscle cells [Higashiura et al. 1997]. TXA2 acts through membrane surface receptors to aggregate platelets by both constricting or modulating the proliferation vascular smooth-muscle cells [Aiayi et al. 1995]. Any systemic vascular effect of T, therefore, is likely to be a balance of vasodilatation by endothelial and non-endothelial effects and vasoconstriction resulting from TXA2 and possibly other mediators [Honda et al. 1999].
Recently, hydrogen sulphide (H2S) has been shown to act as gaseous modulator on rat vascular system both in vivo [Zhao et al. 2003] and in vitro [Hosoki et al. 1997]. H2S is an endogenous gas produced in mammals from L-cysteine by two different enzymes: cystathionine β-synthetase (CBS), predominantly present in the central nervous system, and cystathionine γ-lyase (CSE), predominantly localized in the cardiovascular network [Levonen et al. 2000; Yap et al. 2000; Van der Molen et al. 1978]. The finding that activation of K-channels is involved in T-induced vasodilatation [Deenadayalu et al. 2001; Ding and Stallone, 2001] led to the hypothesis of a possible involvement of H2S as a mediator involved, since drugs that block KATP channels, such as glibenclamide, have been shown to block the relaxant effect caused by exogenous H2S [Cheng et al. 2004; Zhao et al. 2001]. In a recent study, Bucci et al. elegantly suggested that the T non-genomic vascular effect might involve the L-cysteine/H2S pathway. In particular, the data presented demonstrated that, in rat aorta, the non-genomic effect of T is linked to a positive modulation of CSE/CBS activity. The H2S produced acts on KATP channels, contributing to the vasodilator effect of T. Thus, H2S involvement in the vascular activity of T may help to explain the beneficial effects of T on the cardiovascular system [Bucci et al. 2008]. On this basis, preliminary animal studies have suggested the involvement of H2S in facilitating erectile function [Srilatha et al. 2007, 2006]. However, the involvement of a functionally intact L-Cys/H2S pathway in human penile erection has not yet been demonstrated. In a recent work, by using human corpora obtained by a standardized surgical procedure, it has been demonstrated that human penile tissue expresses both CBS and CSE, and tissue homogenates efficiently convert L-Cys to H2S. Functional studies, performed in vitro, confirm that the L-Cys/H2S pathway plays a functional role in human tissue. Indeed, either sodium hydrogen sulfide (NaHS), an exogenous source of H2S, or L-Cys, the substrate for CBS/CSE, relaxed HCC strips in a concentration-related manner. Pharmacological modulation of CBS and CSE by using a CSE inhibitor and/or a CBS inhibitor confirmed the involvement of the L-Cys/H2S pathway both in vitro and in vivo in rats. Collectively, these observations indicate that a functional L-Cys/H2S pathway may be involved in mediating penile erection in humans and other mammals [D’Emmanuele di Villa Bianca et al. 2009]. (see Figure 1).
Moreover, the recent finding that hypotestosteronemia is associated with a low number of circulating EPCs in young hypogonadotropic hypogonadal patients [Foresta et al. 2006] and that T replacement is able to revert EPC reduced counts through a direct stimulatory effect on the bone marrow, [Foresta et al. 2008] clearly suggests a new potential field of application for T replacement therapy. Pilot studies suggest that T may have a role as an antiatherogenic therapy, by preserving endothelial and smooth-muscle cell integrity; also, recent evidence demonstrated that androgen deprivation for prostate cancer may reduce insulin sensitivity, thus suggesting a key role for T in the development of insulin resistance/MeS also [Smith et al. 2006].
PDE5-inhibitors and endothelium
The PDE5-inhibitors (PDE5-i) have revolutionized the management of ED since they appeared to offer advantages over other medical approaches in terms of ease of administration and costs, and oral drug treatment with PDE5-i is now widely advocated as first-line therapy. These agents act by potentiating the action of intracavernosal NO, thereby leading to a more sustained erection. Sildenafil was the first PDE5-i to be released and has been studied extensively. Subsequently two other agents – vardenafil and tadalafil – have been introduced. All of these drugs have been shown to be effective across a wide range of ED etiologies and have been shown to improve erectile function, penetration and maintenance of erection, resulting in more successful intercourse. Their effects are greater at higher doses [Lugg et al. 1995]. Sildenafil and vardenafil are short-acting agents, while tadalafil is a long-acting agent thus allowing the user more flexibility in planning sexual activity [Aversa et al. 2006]. Although the various classes of PDE5-i differ with respect to selectivity and pharmacokinetic profiles, their efficacy and safety are almost comparable in broad populations of men with ED. PDE5-i have no effect on the penis in the absence of sexual stimulation. The ability of a drug to select the target tissue (penis) while bypassing other tissues (vascular system) depends upon the fact that human corporeal smooth muscle is known to be rich in PDE5, which is abundant in the aorta and in some part of the peripheral vascular smooth muscle [Mercapide et al. 1999]. Experimental findings support the fact that chronic administration of PDE5-i may regulate the transduction pathway leading to the activation of endothelial-NOS but has no effect on NO bioavailability or on the cGMP pathway, thereby eliminating a possible concern for tachyphylaxis [Behr-Roussel et al. 2005].Since PDE5-i are widely used in treating male ED, it has been recently hypothesized that they may exert also important systemic effects, at the endothelial level, as well [Reffelmann and Kloner, 2006].
Recent research focused on PDE5-i has proven to be of great impact in the treatment of numerous human extra-sexual diseases [Aversa, 2008b]. In fact, specific inhibitors of members of the PDE super family are currently being investigated for the treatment of asthma, acute ischemic stroke, cancer, systemic inflammation, pulmonary hypertension, and many other conditions and they have been approved for clinical use in the treatment of claudicatio intermittens, congestive heart failure, chronic obstructive pulmonary disease [Lin, 2003]. The distribution of PDE activity has been determined with anti-PDE1 and anti-PDE5 antibodies in the human cardiac ventricle and saphenous vein, and in vitro studies were performed on the isolated human cardiac ventricle, corpora cavernosa, saphenous vein, and mesenteric artery as well as on rabbit aorta, dog coronary artery, dog trabecular tissue, and rabbit and human platelets. In fact, sildenafil selectively increases cGMP levels in coronary vascular smooth muscle tissue but produces no change in cyclic adenosine monophosphate (cAMP) levels, which is consistent with the drug’s selectivity for PDE5 [Wallis et al. 1999]. An interesting study by Turko et al. showed that sildenafil stimulates cGMP binding to the allosteric sites of PDE5 by interacting at the catalytic site of this enzyme, but the drug does not compete with cGMP for binding at the allosteric sites. In that study, it was concluded that the selectivity and potency of sildenafil was likely to be provided by a non-conserved residue or residues of specific aminoacids in the PDE5 catalytic domain [Turko et al. 1999].
As already outlined above, the event that triggers EDys is represented by the reduction of the overall antioxidant pool [Parodi et al. 2007] with the consequent reduced response to oxidative stress and the activation of several pro-atherogenic processes: reduction of NO bioavailability; increased levels of circulating free fatty acids, with subsequent sub-endothelial storage of lipid depots and increased smooth-muscle cell proliferation of the media layer of the vascular wall [Marks et al. 1995]. Pro-inflammatory and -infective processes may in turn contribute to activate and amplify the acute endothelial injury thus perpetuating such a vicious circle. In early atherogenic lesions, EDys causes adhesion and migration of monocytes and T-lymphocytes in the vascular inner layer in response to increased endothelial production of intercellular molecules, i.e. selectin, VCAM-1 and ICAM-1 that may quench NO [Basta et al. 2004]. This process may concur to determine impaired arterial inflow to the penis, thus contributing to persistent ED.
Growing evidence indicates that PDE5-i have a beneficial effect on inflammatory activation and surrogate markers of EDys. Although exact mechanisms are not fully known, the basis for these anti-inflammatory effects is the increased activity of the NO-cyclic guanosine monophosphate (NO-cGMP) axis [Vlachopoulos et al. 2005]. Through their up-regulation of nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase, cytokines not only increase formation of superoxide, but they also up-regulate the expression of PDE5. PDE5-i antagonize these inflammatory effects by enhancing the NO-cGMP pathway, which, apart from augmenting smooth-muscle-cell relaxation, inhibits NADPH-oxidase expression/activity [Hotston et al. 2007]. Beneficial acute and chronic effects of sildenafil on arterial function in men with ED are well known [Desouza et al. 2002]. Given the unfavourable effect of inflammation on arterial function, this effect could be partly attributed to a robust anti-inflammatory action of daily sildenafil [Aversa et al. 2008c]. In a pilot study, a 4-week treatment with tadalafil every other day produced a favourable effect on endothelium-dependent vasodilatation of cavernous arteries compared with an on-demand dosage in men with ED of any origin. In that study, positive effects on markers of endothelial function and inflammation, including VCAM and ICAM, ET-1, and high-sensitivity C-reactive protein, as well as on insulin were also found [Aversa et al. 2007a]. Daily tadalafil also significantly decreased hypoxia-induced up-regulation of tumor-necrosis factor alpha (TNF-α) and interleukin-1 beta (IL-1ß) expression in pulmonary arteries and improved erectile function in diabetic men, representing a preferred dosing option when compared with on-demand administration [Buvat et al. 2006]. Thus, an emerging role in the management of ED patients seems to be the possible use of daily dosing with a threefold purpose: First, recovery of non-responders to on-demand therapy; second, rehabilitation of erectile function which means endothelial rehabilitation; and third, modification of hormonal patterns, thus adding clinical evidence to the possibility of alternative regimens producing multiple systemic benefits [Bruzziches et al. 2008]. There is growing evidence that patients presenting with ED should be investigated for CVD, including diabetes, even if they have no symptoms. HHcy, known to be an important risk factor for EDys, seems to be an important determinant in ED especially in diabetic patients [Al-Hunayan et al. 2008]. The relationship between HHcy and low arterial blood supply to penile arteries in patients with ED [Demir et al. 2006] suggests that adopting strategies to reduce HHcy may offer the potential of improving the functioning of the entire vascular system thus turning out a men who is a PDE5-i non-responder into a responder [Lombardo et al. 2004]. On the basis of currently available evidence, it is possible to hypothesize that EDys and systemic cardiovascular pathology may be modulated by daily PDE5 inhibition, resulting in improved erectile function owing to effects upon both local and systemic targets. Unlike pulmonary hypertension, for which sildenafil may provide a net cost saving compared with inhaled or intravenous medications [Vida et al. 2007], daily PDE5-i use may be actually limited by high costs, given the average course frequency of five to six times per month. Should lower-dose daily administration confirm attractive efficacy and safety profiles compared with on-demand use, and equivalent net monthly cost approach, once-a-day PDE5-i use will become an important treatment option especially in complicated patients [Aversa et al. 2007b].
The endothelium links internal to sexual medicine
Since the metabolic aspect of ED is rather neglected or not sufficiently treated, it is our opinion that ED should mark the starting point for the evaluation and prevention of significant severe diseases (such as diabetes, dyslipidemia, atherosclerosis, hypertension, CAD, neuropathy, etc.) hitherto unknown by the patients [Foresta et al. 2009], all of which contribute to CVD and are associated with EDys. These abnormalities frequently cluster in individuals, and the term MeS is now widely used to define this cluster. On the other hand, many established therapies for CVD, such as β-blockers, angiotensin converting enzyme inhibitors (ACE-i), and statins, have been investigated for their impacts on the process of atherosclerosis and EDys [Shindel et al. 2008]. Recent studies have demonstrated that statin treatment increases the number of EPCs and improves EPC function. [Llevadot et al. 2001]. Moreover, statin therapy is able to promote vascular repair after balloon injury mediated by EPC [Walter et al. 2002]. By contrast, it has been reported that the statin dose during chronic and continuous treatment independently predicts reduced numbers of circulating as well as isolated EPCs in patients with CAD [Hristov et al. 2007]. A significant effect of statins in lowering total T and SHBG levels in a population of men with type-2 diabetes has been recently demonstrated [Stanworth et al, 2009]. These findings have important implications for the diagnosis of hypogonadism not only in diabetic men receiving statin treatment but also other drugs that are able to directly inhibit T synthesis, i.e. antiandrogens, antifungals. There is growing evidence that ED can be considered as a useful surrogate marker for CAD, with studies showing that a large proportion of men develop the problem before cardiac symptoms become evident [Aversa and Bruzziches, 2007]. One study showed that ED symptoms occurred before symptoms of CAD in 67% of men in a consecutive series presenting with chest pain and angiographically documented CAD. Of particular note, all patients with type-1 diabetes and ED developed sexual dysfunction before CAD onset [Montorsi et al. 2003a]. Gazzaruso et al. also evaluated the presence of ED in 133 uncomplicated type-2 diabetic men with angiographically verified silent CAD and in 127 type-2 diabetic men without myocardial infarction at exercise ECG, 48-h ambulatory ECG and stress echocardiography. The findings showed a strong, independent association between ED and silent CAD in apparently uncomplicated type-2 diabetic patients [Gazzaruso et al. 2004]. The obvious reason why ED may present before other signs of CVD is because penile artery diameter is smaller (1–2 mm) than the coronary artery (3–4 mm) or carotid artery (5–7 mm), so the symptoms associated with atherosclerosis occur sooner (the ‘artery size’ hypothesis). These studies have demonstrated that cardiovascular assessment of men with ED enables the detection of CVD and has been showed that ED presented well before angina symptoms of CAD in two-thirds of men with a mean time interval of almost 3 years [Kaiser et al. 2004; Montorsi et al. 2003b]. Another study revealed that 45% of men with ED had previously undiagnosed hyperlipidemia, 5% had undiagnosed diabetes and 7% had undiagnosed hypertension [Billups and Friedrich, 2000]. Kaiser et al. also looked at whether patients with vascular ED and no other clinical CVD had structural and functional abnormalities at other levels. The vascular parameters of 30 patients with ED (and 27 age-matched normal controls) were measured. Results showed that patients with ED have a peripheral vascular defect in endothelium-dependent and -independent vasodilation that occurs before the development of other overt functional or structural systemic vascular disease and is independent of other traditional risk factors [Kaiser et al. 2004]. Detecting atherosclerosis at this early stage could potentially prevent life-threatening events by facilitating appropriate intervention. This provides justification for investigating ED patients for other cardiovascular symptoms. Preliminary diagnostic testing for silent CAD by means of adenosine stress myocardial perfusion scintigraphy in diabetic patients with ED revealed that 45% of them had coronary alterations in the presence of concomitant penile artery damage as detected by penile ultrasound [Corona et al. 2008]. However, for the future, it will be important to perform studies in order to assess the sensitivity, specificity and cost-effectiveness of using ED as a marker for CVD [Kirby et al. 2005]. Recent data suggest tadalafil could exert prolonged beneficial effects on vascular endothelial function if taken regularly [Caretta et al. 2005]. In this view, once-a-day dosing with tadalafil should be an attractive alternative especially for those ED patients with overt EDys. We can speculate that precocious treatment of these men with daily long-acting PDE5-i, i.e. tadalafil, may represent a future strategy for preventing or reducing the extent of coronary dysfunction and/or myocardial infarction. A daily-based approach also may improve organic ED at the level of vascular endothelial function. Another possible explanation as to why chronic PDE5-i may induce endothelial rehabilitation comes from a recent study by Ayala et al. [Ayala et al. 2007] that demonstrated improvements in insulin action by chronic sildenafil in a murine model of diet-induced obesity and insulin resistance. In their experiment, this improvement occurred even in the absence of an exogenous NO donor, suggesting that the endogenous supply of NO in the high fat-fed state did not limit the effect of sildenafil on insulin action. Chronic PDE5 inhibition also resulted in increased energy expenditure, suggesting that improved energy balance and weight reduction might be partially responsible for the enhanced insulin action without any adverse effects on cardiac morphology or blood pressure measured in vivo, supporting human studies showing no association between long-term use of sildenafil and risk of ischemic events [Pegge et al. 2006]. These data have been confirmed with administration of chronic, short-acting [Aversa et al. 2008c] and long-acting PDE5-i tadalafil in men with CRFs even in the absence of ED [Rosano et al. 2005]. A pilot study by McMahon [McMahon, 2004] evaluated improvements in response rates once non-responders to on-demand tadalafil were shifted to daily dosing and concluded that up to 30% of non-responders may be ‘salvaged’ by daily dosing. Additional data on endothelial response to chronic tadalafil have been obtained in another pilot study involving men with ED and comorbidities. Results suggest a beneficial effect on endothelial function via decreasing plasma levels of surrogate markers (i.e., endothelin-1, C-reactive protein, CAMs). In this study, the authors added another possible mechanism underlying penile rehabilitation: improvement of morning erections, which may provide better end-organ oxygenation, especially in the diabetic population. This latter aspect was confirmed in another study [Proietti et al. 2007] carried out in male sclerodermic patients with ED. Besides improvement in penile vascular circulation, daily tadalafil markedly reduced endothelin-1 plasma levels and increased morning erections after 12 weeks of treatment. Moreover, in ED-patients with systemic sclerosis, it was hypothesized that the reduction of adrenomedullin levels obtained after daily tadalafil, was responsible for the reduction of Raynaud’s phenomenon number and severity of attacks over the time [Rosato et al. 2009]. Although this appears to be a fascinating hypothesis that opens new treatment perspectives, larger studies are needed in order to assess the possible clinical implications of chronic therapy with PDE5-i for cardioprotection and endothelial rehabilitation in patients with comorbidities. Most notably, these drugs are largely used for obstructive sleep apnea-induced ED. Because NO promotes upper airway congestion, muscle relaxation, and pulmonary vasodilation, it has been demonstrated that a single 50 mg dose of sildenafil at bedtime may worsen respiratory and desaturation events [Roizenblatt et al. 2006] so that caution is mandatory in this group of patients.
The relationship between diabetes, the MeS and T deficiency is complex. Male hypogonadism is generally characterized by abnormally low serum T-levels. Typical symptoms include change in mood and cognitive function disorders [Kenny et al. 2002], decreased bone mineral density [Snyder et al. 1999], increased visceral adiposity and body mass index (BMI) [Tan and Pu, 2002], decreased muscle mass and strength [Kenny et al. 2001], and sexual dysfunction [Morales and Heaton, 2001]. Free T-levels begin to decline at a rate of 1% per year after the age of 40 years. It is estimated to affect between 19 and 34% of men over the age of 60 [Kalyani and Dobs, 2007]. Cross-sectional studies have found that between 20 and 64% of men with diabetes have hypogonadism, with higher prevalence rates found in the elderly. Hypogonadism can be a risk factor for the development of diabetes and the MeS through various mechanisms including changes in body composition, androgen receptor polymorphisms, glucose transport, and reduced antioxidant effect. Conversely, diabetes and the MeS can be risk factors for hypogonadism through some similar but mostly distinct mechanisms, such as increased body weight and leptin levels, decreased sex hormone binding globulin levels, suppression of gonadotrophin release or Leydig cell T production, cytokine-mediated inhibition of testicular steroid production, and increased aromatase activity contributing to relative estrogen excess [Nieschlag et al. 2004]. Defects in the hypothalamic-pituitary-gonadal axis may also result from type-2 diabetes, visceral obesity (which is strongly associated with insulin resistance), CAD and MeS and from treatments with a wide range of medications [Kapoor et al. 2006]. Androgen deficiency can also occur at any time during a man’s life, but occurs more frequently with advancing age (LOH, late onset hypogonadism). Short-term interventional studies have also demonstrated that T replacement therapy produces an improvement in insulin sensitivity in men. Thus, hypotestosteronemia may have a role in the pathogenesis of insulin-resistant states and androgen replacement therapy could be a potential treatment that could be offered for improvements in glycemic control, insulin resistance, cholesterol and visceral adiposity and reduction in cardiovascular risk, particularly in diabetic men [Heinlein and Chang, 2002b]. Treatment of ED in men with diabetes has been changed by the introduction of PDE5-i. However, men with diabetes tend to respond less positively to these agents, at least as currently prescribed. This decreased responsiveness may be related to the severity of EDys that usually occurs in these patients. There has been much recent interest in the potential relationship between LOH and diabetes, but there is no evidence of a causal relationship and the evidence in favor of treating borderline hypogonadism in diabetes is limited.
Combination therapy in internal and sexual medicine
The role of T salvage in the setting of ‘sub-clinical’ hypogonadism of patients with ED has been evaluated in the presence and absence of PDE5-i effectiveness [Morelli et al. 2004]. Preclinical investigations reported by Traish et al. [Traish et al. 1999] provided convincing evidence that PDE5-i are ineffective in improving erectile function in androgen-deficient animals and that the re-administration of androgen facilitates PDE5-i action. As already outlined, T may directly control the expression and activity of PDE5 in human penile tissues [Traish et al. 2003]. Androgen deficiency or hypogonadism reduce the cavernosal expression of PDE5 mRNA, protein and enzyme activity, and T supplementation restores PDE5 expression and activity which represents the substrate for the inhibitory action of PDE5-i. The effects of androgens on penile tissues in experimental models demonstrated that androgen deprivation induces: smooth-muscle cell degeneration (apoptosis), adipose tissue deposition with associated fibrosis of corpus cavernosum [Traish and Guay, 2006]; reduction in the expression of eNOS and nNOS; decrease of arterial inflow and increase of venous out-flow in the corpus cavernosum; enhanced response to mediators of vasoconstriction and smooth muscle contraction such as α-adrenergic agents; decrease of NO-mediated smooth muscle relaxation during sexual stimuli; and down-regulation of expression of PDE5 enzyme. This latter aspect seems to be crucial in determining metabolic and structural imbalance in the corpus cavernosum, resulting in venous leakage and ED; this suggests a rationale for combination therapy with PDE5-i combined with T preparation for treating ED refractory to monotherapy [Shabsigh et al. 2006]. These clinical observations suggest a critical role for T in human erectile function. Aversa and co-workers were the very first to demonstrate that in the clinical setting, men with ED and low free-T may have an impaired relaxation of the penile smooth muscle, thus providing clinical evidence for the importance of androgen in regulating erectile function [Aversa et al. 2000]. More importantly, in all patients a strong direct correlation between resistive index values and free-T levels has been reported. Again, this relationship was maintained also when adjusted for age, SHBG, and estradiol. These results indicate that in men with ED, low free-T may correlate independently of age with the impaired relaxation of the cavernous smooth muscle cells. In a subsequent study, it has been shown that the treatment with transdermal T-patch and sildenafil on-demand, significantly increased scores in the erectile function domain of the International Index of Erectile Function (IIEF) [Aversa et al. 2003]. Other studies demonstrated that T therapy is able to improve erectile function and the response to PDE5-i in patients with ED and hypogonadism and also in men with LOH symptoms [Shabsigh et al. 2004; Kalinchenko et al. 2003]. Administration of intramuscular T and sildenafil was found to be efficacious in renal transplant patients and in patients on renal dialysis [Tas et al. 2006], and oral T has been reported to reverse ED associated with type-2 diabetes in patients failing on sildenafil therapy alone [Lin et al. 2001]. On the other hand, few studies suggested that in ED-associated hypogonadism, T alone may not be sufficient for early restoration of erectile potency [Shabsigh, 2003; Yassin et al. 2006] thus suggesting that the combination with a PDE5-i is initially recommended. In conclusion, T plus PDE5-i combination therapy improves the response to PDE5-i in patients previously not responding to PDE5-i therapy alone and in whom T levels at baseline are in the hypogonadal or normal-low adult range, i.e. late-onset hypogonadism, without any side effects on prostate size and PSA values in the long-term [El-Sakka et al. 2005]. Notewhorthy, T-induced remodelling of penile tissue structure is a process that may require a longer period of T administration, as long as 4 weeks for expected results on erection.
Regarding the hypothesis on steroid hormone changes induced by chronic treatments with PDE5-i for sexual disturbances, several studies indicate that resumption of sexual function with different oral therapies is able to increase T levels [Jannini et al. 1999]. It has been demonstrated as a beneficial effect of the administration of sildenafil or tadalafil on steroid hormones, i.e. total- and free-T that raised more significantly in those subjects using tadalafil versus sildenafil probably because of the more robust activation of the hypothalamic-pituitary axis due to higher number of sexual intercourses per month allowed by the long-acting drug [Carosa et al. 2004]. Successive studies have demonstrated that due to its indirect action, the raising effect on T is transient and is lost twelve months after continuative tadalafil assumption; T:estradiol ratio increased due to a significant reduction of estradiol levels which may account for by tadalafil persistent efficacy over the time [Greco et al. 2006a]. This study suggested for the first time a possible direct inhibitory effect of tadalafil on aromatase activity in humans independently from the body mass index and the quantity of adipose tissue. Preliminary studies from our laboratory aimed to investigate whether tadalafil could directly modulate aromatase expression in differentiated human adipocytes in culture demonstrated that it directly affect aromatase expression, hence it could positively modulate the T:estradiol circulating ratio in vivo [data not published]. Thus, the aromatase activity inhibition due to chronic exposure of tadalafil might be responsible for tadalafil sustained effectiveness in vivo during daily tadalafil low-dose treatment.
Conclusion
It is clear that the modification of reversible causes, i.e. inadequate lifestyle, cigarette smoking, alcohol or recreational drug abuse [Aversa et al. 2008b], hyperglycemia and hypertension, must represent the first approach to improve endothelial function and to promote general well-being and sexual health. Almost 20–30% of ED cases may be attributed to subnormal T levels [Morelli et al. 2005] and T deficiency is frequently associated with chronic diseases, that are in turn associated with increased deposition of visceral fat; this latter serves as an endocrine organ, producing inflammatory cytokines and thus promoting EDys and concurring to determine vascular disease. In fact, a considerable body of evidence exists suggesting a link among reduced T plasma levels and the presence of diabetes, MeS and systemic vascular diseases. Adequate T concentrations are also crucial for the regulation of a correct endothelial function, for the expression of penile PDE5 isoenzyme [Greco et al. 2006b; Morelli et al. 2004] as well as for the adequate production of H2S. Men with ED and low T levels are potential candidates to benefit from combination therapies if response to monotherapy is not sufficient [Rosano, 2000]. However, if we consider overall hormonal alterations of sex steroids in men complaining ED, it is noteworthy to remember that up to 41% of these men may present with alterations of T:estradiol ratios [Aversa et al. 2006a]. The beneficial effects of T supplementation along with chronic PDE5-i administration on endothelium in deficient men with or without ED appears to be a promising therapy to boost the effects on remodeling of vascular wall determined by single vasoactive agents used to treat internal medicine diseases [Aversa, 2008], and may represent a ‘salvage’ therapy especially in difficult-to-treat ED. Although the safety of this class of agents has been indisputably established for the treatment of ED on an on-demand basis, there remains a paucity of controlled data on the long-term endothelial effects and possible hormonal untoward effects deriving from chronic use.
Conflict of interest statement
None declared.
References
- Aiayi A.A., Mathur R., Halushka P.V. (1995) Testosterone increases human platelet thromboxane A2 receptor density and aggregation response? Circulation 91: 2742–2747 [DOI] [PubMed] [Google Scholar]
- Akishita M., Hashimoto M., Ohike Y., Ogawa S., Iijima K., Eto M., et al. (2007) Low testosterone level is an independent determinant of EDys in me? Hypertens Res 30: 1029–1034 [DOI] [PubMed] [Google Scholar]
- Al-Hunayan A., Thalib L., Kehinde E.O., Asfar S. (2008) Hyperhomocysteinemia is a risk factor for erectile dysfunction in men with adult-onset diabetes mellitu? Urology 71: 897–900 [DOI] [PubMed] [Google Scholar]
- Allan C.A., McLachlan R.I. (2004) Age-related changes in testosterone and the role of replacement therapy in older me? Clin Endocrinol (Oxf) 60: 653–670 [DOI] [PubMed] [Google Scholar]
- Aversa A., Isidori A.M., De Martino M.U., Caprio M., Fabbrini E., Rocchietti-March M., et al. (2000) Androgens and penile erection: evidence for a direct relationship between free testosterone and cavernous vasodilation in men with erectile dysfunctio? Clin Endocrinol 53: 517–522 [DOI] [PubMed] [Google Scholar]
- Aversa A., Isidori A.M., Spera G., Lenzi A., Fabbri A. (2003) Androgens improve cavernous vasodilation and response to sildenafil in patients with erectile dysfunctio? Clin Endocrinol 58: 632–638 [DOI] [PubMed] [Google Scholar]
- Aversa A., Bruzziches R., Spera G. (2005) A rationale for the use of testosterone salvage in treatment of men with erectile dysfunction failing phosphodiesterase inhibitor? The Endocrinologist 15: 99–105 [Google Scholar]
- Aversa A., Bruzziches R., Pili M., Spera G. (2006) Phosphodiesterase 5 inhibitors in the treatment of erectile dysfunctio? Curr Pharm Des 12: 3467–3484 [DOI] [PubMed] [Google Scholar]
- Aversa A., Bruzziches R., Greco E.A., Pili M., Spera G. (2006a) Possible involvement of gonadic steroids in determining erectile response to pharmacoerection test in men with erectile dysfunctio? It J Sex Reprod Med 13: 3–9 [Google Scholar]
- Aversa A., Greco E., Bruzziches R., Pili M., Rosano G., Spera G. (2007a) Relationship between chronic tadalafil administration and improvement of endothelial function in men with erectile dysfunction: a pilot stud? Int J Impot Res 19: 200–207 [DOI] [PubMed] [Google Scholar]
- Aversa A., Bruzziches R., Vitale C., Marazzi G., Francomano D., Barbaro G., et al. (2007b) Chronic sildenafil in men with diabetes and erectile dysfunctio? Expert Opin Drug Metab Toxicol 3: 451–464 [DOI] [PubMed] [Google Scholar]
- Aversa A., Bruzziches R. (2007) Phosphodiesterase type 5 inhibitors and endothelial functio? Curr Sex Health Rep 4: 157–162 [Google Scholar]
- Aversa A. (2008) Drugs targeted to improve endothelial function: clinical correlates between sexual and internal medicin? Curr Pharm Des 14: 3698–3699 [DOI] [PubMed] [Google Scholar]
- Aversa A., Caprio M., Rosano G.M., Spera G. (2008a) Endothelial effects of drugs designed to treat erectile dysfunctio? Curr Pharm Des 14: 3768–3778 [DOI] [PubMed] [Google Scholar]
- Aversa A., Rossi F., Francomano D., Bruzziches R., Bertone C., Santiemma V., et al. (2008b) Early endothelial dysfunction as a marker of vasculogenic erectile dysfunction in young habitual cannabis user? Int J Impot Res 20: 566–573 [DOI] [PubMed] [Google Scholar]
- Aversa A., Vitale C., Volterrani M., Fabbri A., Spera G., Fini M., et al. (2008c) Chronic administration of sildenafil improves markers of endothelial function in men with Type 2 diabete? Diabet Med 25: 37–44 [DOI] [PubMed] [Google Scholar]
- Ayala J.E., Bracy D.P., Julien B.M., Rottman J.N., Fueger P.T., Wasserman D.H. (2007) Chronic treatment with sildenafil improves energy balance and insulin action in high fat-fed conscious mic? Diabetes 56: 1025–1033 [DOI] [PubMed] [Google Scholar]
- Basta G., Schmidt A.M., De Caterina R. (2004) Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabete? Cardiovasc Res 63: 582–592 [DOI] [PubMed] [Google Scholar]
- Baumhäkel M., Werner N., Böhm M., Nickenig G. (2006) Circulating endothelial progenitor cells correlate with erectile function in patients with coronary heart diseas? Eur Heart J 27: 2184–2188 [DOI] [PubMed] [Google Scholar]
- Behr-Roussel D., Gorny D., Mevel K., Caisey S., Bernabe J., Burgess G. (2005) Chronic sildenafil improves erectile function and endothelium- dependent cavernosal relaxations in rats: lack of tachyphylaxi? Eur Urol 47: 87–91 [DOI] [PubMed] [Google Scholar]
- Bellamy M.F., McDowell I.F. (1997) Putative mechanisms for vascular damage by homocysteine? J Inherit Metab Dis 20: 307–315 [DOI] [PubMed] [Google Scholar]
- Billups K., Friedrich S. (2000) Assessment of fasting lipid profiles and Doppler ultrasound testing in men presenting with ED and no other medical problem? J Urol 163: 147–147 [Google Scholar]
- Bonetti P.O., Lerman L.O., Lerman A. (2003) Endothelial dysfunction: a marker of atherosclerotic ris? Arterioscler Thromb Vasc Biol 23: 168–175 [DOI] [PubMed] [Google Scholar]
- Boger R.H. (2003) The emerging role of asymmetric dimethylarginine as a novel cardiovascular risk facto? Cardiovasc Res 59: 824–833 [DOI] [PubMed] [Google Scholar]
- Braunwald E. (2008) Biomarkers in heart failur? N Engl J Med 358: 2148–2159 [DOI] [PubMed] [Google Scholar]
- Brodsky S.V., Zhang F., Nasjletti A., Goligorsky M.S. (2004) Endotheliumderived microparticles impair endothelial function in vitr? Am J Physiol Circ Heart Physiol 286: H1910–H1915 [DOI] [PubMed] [Google Scholar]
- Browne D., Meeking D., Shaw K., Cummings M. (2003) Endothelial dysfunction and presymptomatic atherosclerosis in Type 1 diabetes - pathogenesis and identificatio? Br J Diabetes Vasc Dis 3: 27–34 [Google Scholar]
- Brunner H., Cockcroft J.R., Deanfield J., Donald A., Ferrannini E., Halcox J., et al. (2005) Endothelial function and dysfunction. Part II: association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertensio? J Hypertens 23: 233–246 [DOI] [PubMed] [Google Scholar]
- Bruzziches R., Greco E.A., Pili M., Francomano D., Spera G., Aversa A. (2008) Redefining the role of long-acting phosphodiesterase inhibitor tadalafil in the treatment of diabetic erectile dysfunctio? Curr Diabetes Rev 4: 24–30 [DOI] [PubMed] [Google Scholar]
- Bucci M., Mirone V., Di Lorenzo A., Vellecco V., Roviezzo F., Brancaleone V., et al. (2009) Hydrogen sulphide is involved in testosterone vascular effec? Eur Urol In press. May 22. 56: 378–383 [DOI] [PubMed] [Google Scholar]
- Buvat J., Van Ahlen H., Schmitt H., Chan M., Kuepfer C., Varanese L. (2006) Efficacy and safety of two dosing regimens of tadalafil and patterns of sexual activity in men with diabetes mellitus and erectile dysfunction: scheduled use vs. on-demand regimen evaluation (SURE) study in 14 European countrie? J Sex Med 3: 512–520 [DOI] [PubMed] [Google Scholar]
- Caretta N., Palego P., Ferlin A., Garolla A., Bettella A., Selice R., et al. (2005) Resumption of spontaneous erections in selected patients affected by erectile dysfunction and various degrees of carotid wall alteration: Role of tadalafi? Eur Urol 48: 326–332 [DOI] [PubMed] [Google Scholar]
- Carosa E., Martini P., Brandetti F., Di Stasi S.M., Lombardo F., Lenzi A., et al. (2004) Type 5 phosphodiesterase inhibitor treatments for erectile dysfunction increase testosterone level? Clin Endocrinol 61: 382–386 [DOI] [PubMed] [Google Scholar]
- Cersosimo E., DeFronzo R.A. (2006) Insulin resistance and EDys: the road map to CVD? Diabetes Metab Res Rev 22: 423–436 [DOI] [PubMed] [Google Scholar]
- Chang C., Saltzman A., Yeh S., Young W., Keller E., Lee H.J., et al. (1995) Androgen receptor: an overvie? Crit Rev Eukaryot Gene Expr 5: 97–125 [DOI] [PubMed] [Google Scholar]
- Cheng Y., Ndisang J.F., Tang G., Cao K., Wang R. (2004) Hydrogen sulphide-induced relaxation of resistance mesenteric artery beds of rat? Am J Physiol Heart Circ Physiol 287: H2316–H2323 [DOI] [PubMed] [Google Scholar]
- Chirino R., López A., Navarro D., Cabrera J.J., Rivero J.F., Díaz-Chico B.N., et al. (1989) Steroid induction of low-affinity glucocorticoid binding sites in rat liver microsome? J Steroid Biochem 34: 97–105 [DOI] [PubMed] [Google Scholar]
- Cho J.J., Cadet P., Salamon E., Mantione K., Stefano G.B. (2003) The nongenomic protective effects of estrogen on the male cardiovascular system: clinical and therapeutic implications in aging me? Med Sci Monit 9: RA63–RA68 [PubMed] [Google Scholar]
- Chou T.M., Sudhir K., Hutchison S.J., Ko E., Amidon T.M., Collins P., et al. (1996) Testosterone induces dilatation in canine coronary conductance and resistance arteries in viv? Circulation 94: 2614–2619 [DOI] [PubMed] [Google Scholar]
- Cooke J.P., Dzau V.J. (1997) Nitric oxide synthase: role in the genesis of vascular diseas? Annu Rev Med 48: 489–509 [DOI] [PubMed] [Google Scholar]
- Cooke J.P. (2005) ADMA: its role in vascular diseas? Vasc Med 10: S11–S17 [DOI] [PubMed] [Google Scholar]
- Corona G., Fagioli G., Mannucci E., Romeo A., Rossi M., Lotti F., et al. (2008) Penile doppler ultrasound in patients with erectile dysfunction (ED): role of peak systolic velocity measured in the flaccid state in predicting arteriogenic ED and silent coronary artery diseas? J Sex Med 5: 2623–2634 [DOI] [PubMed] [Google Scholar]
- Costarella C.E., Stallone J.N., Rutecki G.W., Whittier F.C. (1996) Testosterone causes direct relaxation of rat thoracic aort? J Pharm Exp Ther 277: 349–349 [PubMed] [Google Scholar]
- Crews J.K., Khalil R.A. (1999) Antagonistic effect of 17 beta-oestradiol, progesterone and testosterone on Ca2+ entry mechanism of coronary vasoconstrictio? Arterioscler Thromb Vasc Biol 19: 1034–1040 [DOI] [PubMed] [Google Scholar]
- D’Emmanuele di Villa Bianca R., Sorrentino R., Maffia P., Mirone V., Imbimbo C., Fusco F., et al. (2009) Hydrogen sulfide as a mediator of human corpus cavernosum smooth-muscle relaxatio? Proceed Natl Acad Sci 106: 4513–4518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deenadayalu V.P., White R.E., Stallone J.N., Gao X., Garcia A.J. (2001) Testosterone relaxes coronary arteries by opening the large conductance, calcium-activated potassium channel? Am J Physiol Heart Circ Physiol 281: H1720–H1727 [DOI] [PubMed] [Google Scholar]
- Demir T., Comlekçi A., Demir O., Gülcü A., Calýpkan S., Argun L., et al. (2006) Hyperhomocysteinemia: a novel risk factor for erectile dysfunctio? Metabolism 55: 1564–1568 [DOI] [PubMed] [Google Scholar]
- Desouza C., Parulkar A., Lumpkin D., Akers D., Fonseca V.A. (2002) Acute and prolonged effects of sildenafil on brachial artery flow mediated dilatation in type 2 diabete? Diabetes Care 25: 1336–1339 [DOI] [PubMed] [Google Scholar]
- Ding A.Q., Stallone J.N. (2001) Testosterone-induced relaxation of rat aorta is androgen structure specific and involves K+ channel activatio? J Appl Physiol 91: 2742–2750 [DOI] [PubMed] [Google Scholar]
- El-Sakka A.I., Hassoba H.M., Elbakry A.M., Hassan H.A. (2005) Prostate Specific Antigen in patients with hypogonadism: effect of testosterone replacemen? J Sex Med 2: 235–240 [DOI] [PubMed] [Google Scholar]
- Elesber A.A., Solomon H., Lennon R.J., Mathew V., Prasad A., Pumper G., et al. (2006) Coronary EDys is associated with erectile dysfunction and elevated asymmetric dimethylarginine in patients with early atherosclerosi? Eur Heart J 27: 824–824 [DOI] [PubMed] [Google Scholar]
- English K.M., Jones R.D., Jones T.H., Morice A.H., Channer K.S. (2002) Testosterone acts as a coronary vasodilatator by calcium antagonist actio? J Endocrinol Invest 25: 455–458 [DOI] [PubMed] [Google Scholar]
- Esposito K., Ciotola M., Giugliano F., Schisano B., Improta L., Improta M.R., et al. (2007) Endothelial microparticles correlate with erectile dysfunction in diabetic me? Int J Impot Res 19: 161–166 [DOI] [PubMed] [Google Scholar]
- Esposito K., Ciotola M., Maiorino M.I., Giugliano F., Autorino R., De Sio M., et al. (2009) Circulating CD34+ KDR+ endothelial progenitor cells correlate with erectile function and endothelial function in overweight me? J Sex Med 6: 107–114 [DOI] [PubMed] [Google Scholar]
- Fadini G.P., de Kreutzenberg S.V., Coracina A., Baesso I., Agostini C., Tiengo A., et al. (2006) Circulating CD34+ cells, metabolic syndrome, and cardiovascular ris? Eur Heart J 27: 2247–2255 [DOI] [PubMed] [Google Scholar]
- Farouque H.M.O., Meredith I.T. (2001) The assessment of endothelial function in human? Coron Artery Dis 12: 445–454 [DOI] [PubMed] [Google Scholar]
- Foresta C., Caretta N., Lana A., De Toni L., Biagioli A., Ferlin A., et al. (2006) Reduced number of circulating endothelial progenitor cells in hypogonadal me? J Clin Endocrinol Metab 91: 4599–4602 [DOI] [PubMed] [Google Scholar]
- Foresta C., Zuccarello D., De Toni L., Garolla A., Caretta N., Ferlin A. (2008) Androgens stimulate endothelial progenitor cells through an androgen receptor-mediated pathwa? Clin Endocrinol 68: 284–289 [DOI] [PubMed] [Google Scholar]
- Foresta C., Caretta N., Corona G., Fabbri A., Francavilla S., Jannini E., et al. (2009) Clinical and metabolic evaluation of subjects with erectile dysfunction: a review with a proposal flowchar? Int J Androl 32: 198–211 [DOI] [PubMed] [Google Scholar]
- Fukui M., Kitagawa Y., Ose H., Hasegawa G., Yoshikawa T., Nakamura N. (2007) Role of endogenous androgen against insulin resistance and atherosclerosis in men with type 2 diabete? Curr Diabetes Rev 3: 25–31 [DOI] [PubMed] [Google Scholar]
- Gazzaruso C., Solerte S.B., Pujia A., Coppola A., Vezzosi M., Salvucci F., et al. (2008) Erectile dysfunction as a predictor of cardiovascular events and death in diabetic patients with angiographically proven asymptomatic coronary artery disease. A potential protective role for statins and 5-phosphodiesterase inhibitor? J Am Coll Cardiol 51: 2040–2044 [DOI] [PubMed] [Google Scholar]
- Gazzaruso C., Giordanetti S., De Amici E., Bertone G., Falcone C., Geroldi D., et al. (2004) Relationship between erectile dysfunction and silent myocardial ischemia in apparently uncomplicated type-2 diabetic patient? Circulation 110: 22–26 [DOI] [PubMed] [Google Scholar]
- Gerdes D., Christ M., Haseroth K., Notzon A., Falkenstein E., Wehling M. (2000) Nongenomic actions of steroids from the laboratory to clinical implication? J Pediatr Endocrinol Metab 13: 853–878 [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P.J., Creager M.A., Lorsordo D.W., Lam G.K.W., Wassef M., Dzau V.J. (2005) Atherosclerosis: recent discoveries and novel hypothese? Circulation 112: 3348–3353 [DOI] [PubMed] [Google Scholar]
- Gonzalez M.A., Selwyn A.P. (2003) Endothelial function, inflammation, and prognosis in CV? Am J Med 115: 99S–106S [DOI] [PubMed] [Google Scholar]
- Greco E.A., Pili M., Bruzziches R., Corona G., Spera G., Aversa A. (2006a) Testosterone: estradiol ratio changes associated with long-term tadalafil administration: a pilot stud? J Sex Med 3: 716–722 [DOI] [PubMed] [Google Scholar]
- Greco E.A., Spera G., Aversa A. (2006b) Combining testosterone and PDE5 inhibitors in erectile dysfunction: basic rationale and clinical evidence? Eur Urol 50: 940–947 [DOI] [PubMed] [Google Scholar]
- Guay A.T. (2007) ED2: Erectile dysfunction = Endothelial dysfunctio? Endocrinol Metab Clin N Am 36: 453–463 [DOI] [PubMed] [Google Scholar]
- Haller H. (1997) Endothelial functio? Drugs 53: 1–10 [DOI] [PubMed] [Google Scholar]
- Hanke H., Lenz C., Hess B., Spindler K.D., Weidemann W. (2001) Effect of testosterone on plaque development and androgen receptor expression in the arterial vessel wal? Circulation 103: 1382–1385 [DOI] [PubMed] [Google Scholar]
- Heinlein C.A., Chang C. (2002a) The roles of androgen receptors and androgenbinding proteins in nongenomic androgen action? Mol Endocrinol 16: 2181–2187 [DOI] [PubMed] [Google Scholar]
- Heinlein C.A., Chang C. (2002b) Androgen receptor (AR) coregulators: an overvie? Endocr Rev 23: 175–200 [DOI] [PubMed] [Google Scholar]
- Herve J.C. (2002) Non-genomic effects of steroid hormones on membrane channel? Mini Rev Med Chem 2: 411–417 [DOI] [PubMed] [Google Scholar]
- Higashiura K., Mathur R.S., Halushka P.V. (1997) Gender-related differences in androgen regulation of thromboxane A2 receptors in rat aortic smooth muscle cell? J Cardiovasc Pharmacol 29: 311–315 [DOI] [PubMed] [Google Scholar]
- Honda H., Unemoto T., Kogo H. (1999) Different mechanism for testosterone induced relaxation of aorta between normotensive and spontaneously hypertensive rat? Hypertension 34: 1232–1236 [DOI] [PubMed] [Google Scholar]
- Hosoki R., Matsiki N., Kimura H. (1997) The possible role of hydrogen sulphide as an endogenous smooth muscle relaxant in synergy with nitric oxid? Biochem Biophysic Res Comun 237: 527–531 [DOI] [PubMed] [Google Scholar]
- Hotston M.R., Jeremy J.Y., Bloor J., Koupparis A., Persad R., Shukla N. (2007) Sildenafil inhibits the up-regulation of phosphodiesterase type 5 elicited with nicotine and tumour necrosis factor-alpha in cavernosal vascular smooth muscle cells: mediation by superoxid? BJU Int 99: 612–618 [DOI] [PubMed] [Google Scholar]
- Hougaku H., Fleg J.L., Najjar S.S., Lakatta E.G., Harman S.M., Blackman M.R., et al. (2006) Relationship between androgenic hormones and arterial stiffness, based on longitudinal hormone measurement? Am J Physiol Endocrinol Metab 290: E234–E242 [DOI] [PubMed] [Google Scholar]
- Hristov M., Fach C., Becker C., Heussen N., Liehn E.A., Blindt R., Hanrath P., et al. (2007) Reduced numbers of circulating endothelial progenitor cells in patients with coronary artery disease associated with long-term statin treatmen? Atherosclerosis 192: 413–420 [DOI] [PubMed] [Google Scholar]
- Jannini E.A., Screponi E., Carosa E., Pepe M., Lo Giudice F., Trimarchi F., et al. (1999) Lack of sexual activity from erectile dysfunction is associated with a reversible reduction in serum testosteron? Int J Androl 22: 385–392 [DOI] [PubMed] [Google Scholar]
- Jones T.H. (2007) Testosterone associations with erectile dysfunction, diabetes, and the metabolic syndrom? Eur Urol 6: 847–857 [Google Scholar]
- Kaiser D.R., Billups K., Mason C., Wetterling R., Lundberg J.L., Bank A.J. (2004) Impaired brachial artery endothelium-dependent and -independent vasodilation in men with erectile dysfunction and no other clinical CV? J Am Coll Cardiol 43: 179–184 [DOI] [PubMed] [Google Scholar]
- Kalinchenko S.Y., Kozlov G.I., Gontcharov N.P., Katsiya G.V. (2003) Oral testosterone undecanoato reverses erectile dysfunction associated with diabetes mellitus in patients failing on sildenafil citrate therapy alon? Aging Male 6: 94–99 [PubMed] [Google Scholar]
- Kallio P.J., Pakvimo J.J., Janne O.A. (1996) Genetic regulation of androgen actio? Prostate Suppl 6: 45–51 [PubMed] [Google Scholar]
- Kalyani R.R., Dobs A.S. (2007) Androgen deficiency, diabetes, and the metabolic syndrome in me? Curr Opin Endocrinol Diabetes Obes 14: 226–234 [DOI] [PubMed] [Google Scholar]
- Kang H.Y., Tsai M.Y., Chang C., Huang K.E. (2003) Mechanisms and clinical relevance of androgens and androgen receptor action? Chang Gung Med J 26: 388–402 [PubMed] [Google Scholar]
- Kapoor D., Goodwin E., Channer K.S., Jones T.H. (2006) Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabete? Eur J Endocrinol 154: 899–906 [DOI] [PubMed] [Google Scholar]
- Kenny A.M., Prestwood K.M., Gruman C.A., Marcello K.M., Raisz L.G. (2001) Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone level? J Gerontol A Biol Sci Med Sci 56: M266–M272 [DOI] [PubMed] [Google Scholar]
- Kenny A.M., Bellantonio S., Gruman C.A., Acosta R.D., Prestwood K.M. (2002) Effect of transdermal testosterone on cognitive function and healt perception in older men with low bioavailable testosterone level? J Gerontol A Biol Sci Med Sci 57: M321–M325 [DOI] [PubMed] [Google Scholar]
- Kharbanda R.K., Deanfield J.E. (2001) Functions of the healthy endotheliu? Coron Artery Dis 12: 485–491 [DOI] [PubMed] [Google Scholar]
- Kielstein J.T., Frölich J.C., Haller H., Fliser D. (2001) ADMA (asymmetric dimethylarginine): an atherosclerotic disease mediating agent in patients with renal disease? Nephrol Dial Transplant 16(9): 1742–1745 [DOI] [PubMed] [Google Scholar]
- Kim J.A., Montagnani M., Koh K.K., Quon M.J. (2006) Reciprocal relationships between insulin resistance and EDys: molecular and pathophysiological mechanism? Circulation 113: 1888–1904 [DOI] [PubMed] [Google Scholar]
- Kirby M., Jackson G., Simonsen U. (2005) EDys links erectile dysfunction to heart diseas? Int J Clin Pract 59: 225–229 [DOI] [PubMed] [Google Scholar]
- Krause D.N., Duckles S.P., Pelligrino D.A. (2006) Influence of sex steroid hormones on cerebrovascular functio? J Appl Physiol 101: 1252–1261 [DOI] [PubMed] [Google Scholar]
- Krzyzanowska K., Mittermayer F., Wolzt M., Schernthaner G. (2008) ADMA, cardiovascular disease and diabete? Diabetes Res Clin Pract 82: S122–S126 [DOI] [PubMed] [Google Scholar]
- Lee H.J., Chang C. (2003) Recent advances in androgen receptor actio? Cell Mol Life Sci 60: 1613–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llevadot J., Murasawa S., Kureishi Y., Uchida S., Masuda H., Kawamoto A., et al. (2001) HMG-CoA reductase inhibitor mobilizes bone marrow — derived endothelial progenitor cell? J Clin Invest 108: 399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levonen A.L., Lapatto R., Saksela M., Raivio K.O. (2000) Human cystathionine gamma-lyase: developmental and in vitro expression of two isoform? Biochem J 347: 291–295 [PMC free article] [PubMed] [Google Scholar]
- Lin C.S., Chow S., Lau A., Tu R., Lue T.F. (2001) Identification and regulation of human PDE% A gene promote? Biochem Biophys Res Commun 280: 684–692 [DOI] [PubMed] [Google Scholar]
- Lin C.S. (2003) Phosphodiesterases as therapeutic target? Urology 61: 685–691 [DOI] [PubMed] [Google Scholar]
- LinksHeiss C., Keymel S., Niesler U., Ziemann J., Kelm M., Kalka C. (2005) Impaired progenitor cell activity in age-related endothelial dysfunctio? J Am Coll Cardiol 45: 1441–1448 [DOI] [PubMed] [Google Scholar]
- Littleton-Kearney M., Hurn P.D. (2004) Testosterone as a modulator of vascular behavio? Biol Res Nurs 5: 276–285 [DOI] [PubMed] [Google Scholar]
- Lombardo F., Sgrò P., Gandini L., Dondero F., Jannini E.A., Lenzi A. (2004) Might erectile dysfunction be due to the thermolabile variant of methylenetetrahydrofolate reductase? J Endocrinol Invest 27: 883–885 [DOI] [PubMed] [Google Scholar]
- Lu T.M., Ding Y.A., Lin S.J., Lee W.S., Tai H.C. (2003) Plasma levels of asymmetrical dimethylarginine and adverse cardiovascular events after percutaneous coronary interventio? Eur Heart J 24: 1912–1919 [DOI] [PubMed] [Google Scholar]
- Lugg J.A., Gonzalez-Cadaviz N.F., Rajfer J. (1995) The role of nitric oxide in erectile functio? J Androl 16: 2–4 [PubMed] [Google Scholar]
- Lutz L.B., Jamnongjit M., Yang W.H., Jahani D., Gill A., Hammes S.R. (2003) Selective modulation of genomic and nongenomic androgen responses by androgen receptor ligand? Mol Endocrinol 17: 1106–1116 [DOI] [PubMed] [Google Scholar]
- Maas R., Schwedhelm E., Albsmeier J., Böger R.H. (2002) The pathophysiology of erectile dysfunction related to EDys and mediators of vascular functio? Vasc Med 7: 213–225 [DOI] [PubMed] [Google Scholar]
- Marks D.S., Vita J.A., Folts J.D., Keaney Jr J.F., Welch G.N., Loscalzo J. (1995) Inhibition of neointimal proliferation in rabbits after vascular injury by a single treatment with a protein adduct of nitric oxid? J Clin Invest 96: 2630–2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon C. (2004) Efficacy and safety of daily tadalafil in men with erectile dysfunction previously unresponsive to on demand tadalafi? J Sex Med 1: 292–300 [DOI] [PubMed] [Google Scholar]
- Mercapide J., Santiago E., Alberdi E., Martinez-Irujo J.J. (1999) Contribution of phosphodiesterase isoenzymes and cyclic nucleotide efflux to the regulation of cyclic GMP levels in aortic smooth muscle cell? Biochem Pharmacol 58: 1675–1683 [DOI] [PubMed] [Google Scholar]
- Montorsi F., Briganti A., Salonia A., Rigatti P., Margonato A., Macchi A., et al. (2003a) Erectile dysfunction prevalence, time of onset and association with risk factors in 300 consecutive patients with acute chest pain and angiographically documented coronary artery diseas? Eur Urol 44: 360–365 [DOI] [PubMed] [Google Scholar]
- Montorsi P., Montorsi F., Schulman C.C. (2003) Is erectile dysfunction the ‘tip of the iceberg’ of a systematic vascular disorder? Eur Urol 44: 352–354 [DOI] [PubMed] [Google Scholar]
- Morales A., Heaton J.P. (2001) Hormonal erectile dysfunction. Evaluation and managemen? Urol Clin North Am 28: 279–288 [DOI] [PubMed] [Google Scholar]
- Morelli A., Filippi S., Mancina R., Luconi M., Vignozzi L., Marini M., et al. (2004) Androgens regulate phosphodiesterase type-5 expression and functional activity in corpora cavernos? Endocrinology 145: 2253–2263 [DOI] [PubMed] [Google Scholar]
- Morelli A., Filippi S., Zhang X.H., Luconi M., Vignozzi L., Mancina R., et al. (2005) Peripheral regulatory mechanisms in erectio? Int J Androl 28: 23–27 [DOI] [PubMed] [Google Scholar]
- Murphy J.G., Khalil R.A. (1999) Decreased [Ca2]i during inhibition of coronary smooth muscle contraction by 17β-estradiol, progesterone, and testosteron? J Pharmacol Exp Ther 291: 44–52 [PubMed] [Google Scholar]
- Nieschlag E., Behre E., Bouchard H.M., Corrales P., Jones J.J., Stalla T.H., et al. (2004) Testosterone replacement therapy: current trends and future direction? Hum Reprod Update 10: 409–419 [DOI] [PubMed] [Google Scholar]
- Nygård O., Nordrehaug J.E., Refsum H., Ueland P.M., Farstad M., Vollset S.E. (1997) Plasma homocysteine levels and mortality in patients with coronary heart diseas? N Engl J Med 337: 230–236 [DOI] [PubMed] [Google Scholar]
- Orshal J.M., Khalil R.A. (2004) Gender, sex hormones, and vascular ton? Am J Physiol Regul Integr Comp Physiol 286: R233–249 [DOI] [PubMed] [Google Scholar]
- Panes J., Perry M., Granger D.N. (1999) Leukocyte-endothelial cell adhesion: avenues for therapeutic interventio? Br J Pharmacol 126: 537–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi O., De Maria R., Roubina E. (2007) Redox state, oxidative stress and EDys in heart failure: the puzzle of nitrate-thiol interactio? J Cardiovasc Med (Hagerstown) 8: 765–774 [DOI] [PubMed] [Google Scholar]
- Pegge N.C., Twomey A.M., Vaughton K., Gravenor M.B., Ramsey M.W., Price D.E. (2006) The role of EDys in the pathophysiology of erectile dysfunction in diabetes and in determining response to treatmen? Diabet Med 23: 873–878 [DOI] [PubMed] [Google Scholar]
- Perusquia M., Hernandez R., Morales M.A., Campos M.G., Villalon M.G. (1996) Role of the endothelium in the vasodilating effect of progestins and androgens on the rat thoracic aort? Gen Pharmacol 27: 181–185 [DOI] [PubMed] [Google Scholar]
- Proietti M., Aversa A., Letizia C., Rossi C., Menghi G., Bruzziches R., et al. (2007) Erectile dysfunction in systemic sclerosis: effects of long-term inhibition of phosphodiesterase type-5 on erectile function and plasma endothelin-1 level? J Rheumatol 34: 1712–1717 [PubMed] [Google Scholar]
- Reffelmann T., Kloner R.A. (2006) Cardiovascular effects of phosphodiesterase 5 inhibitor? Curr Pharm Des 12: 3485–3494 [DOI] [PubMed] [Google Scholar]
- Roizenblatt S., Guilleminault C., Poyares D., Cintra F., Kauati A., Tufik S. (2006) A double-blind, placebo-controlled, crossover study of sildenafil in obstructive sleep apne? Arch Intern Med 166: 1763–1767 [DOI] [PubMed] [Google Scholar]
- Rosano G.M., Leonardo F., Pagnotta P., Pelliccia F., Panina G., Cerquetani E. (1999) Acute anti-ischemic effect of testosterone in men with coronary artery diseas? Circulation 99: 1666–1670 [DOI] [PubMed] [Google Scholar]
- Rosano G.M. (2000) Androgens and coronary artery disease. A sex-specific effect of sex hormones? Eur Heart J 21: 868–871 [DOI] [PubMed] [Google Scholar]
- Rosano G.M., Aversa A., Vitale C., Fabbri A., Fini M., Spera G. (2005) Chronic treatment with tadalafil improves endothelial function in men with increased cardiovascular ris? Eur Urol 47: 214–220 [DOI] [PubMed] [Google Scholar]
- Rosano G.M., Sheiban I., Massaro R., Pagnotta P., Marazzi G., Vitale C., et al. (2007) Low testosterone levels are associated with coronary artery disease in male patients with angin? Int J Impot Res 19: 176–182 [DOI] [PubMed] [Google Scholar]
- Rosato E., Letizia C., Proietti M., Aversa A., Menghi G., Rossi C., et al. (2009) Plasma adrenomedullin and endothelin-1 levels are reduced and Raynaud's phenomenon improved by daily Tadalafil administration in male patients with systemic sclerosi? J Biol Regul Homeost Agents 23: 23–29 [PubMed] [Google Scholar]
- Rosner W., Hryb D.J., Khan M.S., Nakhla A.M., Romas N.A. (1999) Sex hormone-binding globulin mediates steroid hormone signal transduction at the plasma membran? J Steroid Biochem Mol Biol 69: 481–485 [DOI] [PubMed] [Google Scholar]
- Ross R. (1993) The pathogenesis of atherosclerosis: a perspective for the 1990? Nature 362: 801–809 [DOI] [PubMed] [Google Scholar]
- Sagripanti A., Carpi A. (2000) Antithrombotic and prothrombotic activities of the vascular endotheliu? Biomed Pharmacother 54: 107–111 [DOI] [PubMed] [Google Scholar]
- Schiel R., Franke S., Busch M., Müller A., Fleck C., Müller U.A. (2003) Effect of smoking on risk factors for CVD in patients with diabetes mellitus and renal insufficienc? Eur J Med Res 8: 283–291 [PubMed] [Google Scholar]
- Schrör K., Morinelli T.A., Masuda A., Matsuda K., Mathur R.S., Halushka P.V. (1994) Testosterone treatment enhances thromboxane A2 mimetic induced coronary artery vasoconstriction in guinea pig? Eur J Clin Invest 24: 50–52 [DOI] [PubMed] [Google Scholar]
- Shabsigh R. (2003) Hypogonadism and erectile dysfunction: the role for testosterone therap? Int J Impot Res 15: S9–S13 [DOI] [PubMed] [Google Scholar]
- Shabsigh R., Kaufman J.M., Steidle C., Padma-Nathan H. (2004) Randomized study of testosterone gel as adjunctive therapy to sildenafil in hypogonadal men with erectile dysfunction who do not respond to sildenafil alon? J Urol 172: 658–663 [DOI] [PubMed] [Google Scholar]
- Shabsigh R., Rajfer J., Aversa A., Traish A.M., Yassin A., Kalinchenko S.Y., et al. (2006) The evolving role of testosterone in the treatment of erectile dysfunctio? Int J Clin Pract 60: 1087–1087 [DOI] [PubMed] [Google Scholar]
- Shindel A.W., Kishore S., Lue T.F. (2008) Drugs designed to improve endothelial function: effects on erectile dysfunctio? Curr Pharm Des 14: 3758–3767 [DOI] [PubMed] [Google Scholar]
- Snyder P.J., Peachey H., Hannoush P., Berlin J.A., Loh L., Holmes J.H., et al. (1999) Effect of testosterone treatment on bone mineral density in men over 65 years of ag? J Clin Endocrinol Metab 84: 1966–1972 [DOI] [PubMed] [Google Scholar]
- Smith M.R., Lee H., Nathan D.M. (2006) Insulin sensitivity during combined androgen blockade for prostate cance? J Clin Endocrinol Metab 91: 1305–1308 [DOI] [PubMed] [Google Scholar]
- Solomon H., Man J.W., Jackson G. (2003) Erectile dysfunction and the cardiovascular patient: endothelial dysfunction is the common denominato? Heart 89: 251–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srilatha B., Adaikan P.-G., Moore P.K. (2006) Possible role for the novel gasotransmitter hydrogen sulfide in erectile dysfunction: A pilot stud? Eur J Pharmacol 535: 280–282 [DOI] [PubMed] [Google Scholar]
- Srilatha B., Adaikan P.-G., Li L., Moore P.K. (2007) Hydrogen sulfide: A novel endogenous gasotransmitter facilitates erectile functio? J Sex Med 4: 1304–1311 [DOI] [PubMed] [Google Scholar]
- Stanworth R.D., Kapoor D., Channer K.S., Jones T.H. (2009) Statin therapy is associated with lower total but not bioavailable or free testosterone in men with type 2 diabete? Diabetes Care 32: 541–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan R.S., Pu S.J. (2002) Impact of obesity on hypogonadism in the andropaus? Int J Androl 25: 195–201 [DOI] [PubMed] [Google Scholar]
- Tas A., Ersoy A., Ersoy C., Gullulu M., Yurtkuran M. (2006) Efficacy of sildenafil in male dialysis patients with erectile dysfunction unresponsive to erythropoietin and/or testosterone treatment? Int J Impot Res 18: 61–68 [DOI] [PubMed] [Google Scholar]
- Tep-areenan P., Kendall D.A., Randall M.D. (2002) Testosteroneinduced vasorelaxation in the rat mesenteric arterial bed is mediated predominantly via potassium channel? Br J Pharmacol 135: 735–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Khalil R.A. (2003) Gender differences in the regulation of vascular ton? Clin Exp Pharmacol Physiol 30: 1–15 [DOI] [PubMed] [Google Scholar]
- Thompsom I.M., Tangen C.M., Goodman P.J., Probstfield J.L., Moinpour C.M., Coltman C.A. (2005) Erectile dysfunction and subsequent cardiovascular diseas? JAMA 294: 2996–3002 [DOI] [PubMed] [Google Scholar]
- Traish A.M., Park K., Dhir V., Kim N.N, Moreland R.B., Goldstein I. (1999) Effects of castration and androgen replacement on erectile function in a rabbit mode? Endocrinology 140: 1861–1868 [DOI] [PubMed] [Google Scholar]
- Traish A.M., Munarriz R., O’Connell L., Choi S., Kim S.W., Kim N.N., et al. (2003) Effects of medical or surgical castration on erectile function in an animal mode? J Androl 24: 381–387 [DOI] [PubMed] [Google Scholar]
- Traish A.M., Guay A.T. (2006) Are androgens critical for penile erections in humans? Examining the clinical and preclinical evidenc? J Sex Med 3: 382–407 [DOI] [PubMed] [Google Scholar]
- Travison T.G., Araujo A.B., Kupelian V., O’Donnell A.B., McKinlay J.B. (2007) The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in me? J Clin Endocrinol Metab 92: 549–555 [DOI] [PubMed] [Google Scholar]
- Turko I.V., Ballard S.A., Francis S.H., Corbin J.D. (1999) Inhibition of cyclic GMP-binding cyclic GMP-specific phosphodiesterase (Type 5) by sildenafil and related compound? Mol Pharmacol 56: 124–130 [DOI] [PubMed] [Google Scholar]
- Valgimigli M., Rigolin G.M., Fucili A., Porta M.D., Soukhomovskaia O., Malagutti P., et al. (2004) CD34+ and endothelial progenitor cells in patients with various degrees of congestive heart failur? Circulation 110: 1209–1212 [DOI] [PubMed] [Google Scholar]
- Valkonen V.P., Päivä H., Salonen J.T., Lakka T.A., Lehtimäki T., Laakso J., et al. (2001) Risk of acute coronary events and serum concentration of asymmetrical dimethylarginin? Lancet 358: 2127–2128 [DOI] [PubMed] [Google Scholar]
- Van der Molen E.F., Hiipakka M.J., van Lith-Zanders H., Boers G.H., van den Heuvel L.P., Monnens L.A., et al. (1978) Homocysteine metabolism in endothelial cells of a patient homozygous for cystathionine beta-synthase (CS) deficienc? Thromb Haemost 78: 827–833 [PubMed] [Google Scholar]
- Vane J.R., Anggard E.E., Botting R.M. (1990) Regulatory functions of the vascular endotheliu? N Engl J Med 323: 27–36 [DOI] [PubMed] [Google Scholar]
- Vida V.L., Gaitan G., Quezada E., Barnoya J., Castañeda A.R. (2007) Lowdose oral sildenafil for patients with pulmonary hypertension: a cost-effective solution in countries with limited resource? Cardiol Young 17: 72–77 [DOI] [PubMed] [Google Scholar]
- Vita J.A., Keaney Jr J.F. (2002) Endothelial function: a barometer for cardiovascular risk? Circulation 106: 640–642 [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C., Dima I., Aznaouridis K., Vasiliadou C., Ioakeimidis N., Aggeli C., et al. (2005) Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individual? Circulation 112: 2193–2200 [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C., Rokkas K., Ioakeimidis N., Stefanadis C. (2007) Inflammation, metabolic syndrome, erectile dysfunction, and coronary artery disease: common link? Eur Urol 52: 1590–1600 [DOI] [PubMed] [Google Scholar]
- Wallis R.M., Corbin J.D., Francis S.H., Ellis P. (1999) Tissue distribution of phosphodiesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitr? Am J Cardiol 83: 3–12C [DOI] [PubMed] [Google Scholar]
- Walter D.H., Rittig K., Bahlmann F.H., Kirchmair R., Silver M., Murayama T., et al. (2002) Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cell? Circulation 105: 3017–3024 [DOI] [PubMed] [Google Scholar]
- Watts G.F., Chew K.K., Stuckey B.G.A. (2007) The erectile-EDys nexus: new opportunities for cardiovascular risk preventio? Nature 4: 263–273 [DOI] [PubMed] [Google Scholar]
- Werner N., Wassmann S., Ahlers P., Schiegl T., Kosiol S., Link A., et al. (2007) Endothelial progenitor cells correlate with endothelial function in patients with coronary artery diseas? Basic Res Cardiol 102: 565–571 [DOI] [PubMed] [Google Scholar]
- Wierzbicki A., Solomon H., Lumb P., Lyttle K., Lambert-Hammill M., Jackson G. (2006) Asymmetrical dimethylarginine levels correlate with cardiovascular risk factors in patients with erectile dysfunctio? Atherosclerosis 185: 421–425 [DOI] [PubMed] [Google Scholar]
- Williams M.R., Ling S., Dawood T., Hashimura K., Dai A., Li, H, et al. (2002) Dehydroepiandrosterone inhibits human vascular smooth muscle cell proliferation independent of Ars and ER? J Clin Endocrinol Metab 87: 176–181 [DOI] [PubMed] [Google Scholar]
- Wynne F.L., Khalil R.A. (2003) Testosterone and coronary vascular tone: implications in coronary artery diseas? J Endocrinol Invest 26: 181–186 [DOI] [PubMed] [Google Scholar]
- Yap S., Naughten E.R., Wilcken B., Wilcken D.E., Boers G.H. (2000) Vascular complications of severe hyperhomocysteinemia in patients with homocystinuria due to cystathionine beta synthase deficiency: effects of homocysteine-lowering therap? Semin Thromb Hemost 26: 335–340 [DOI] [PubMed] [Google Scholar]
- Yassin A.A., Saad F., Diede H.E. (2006) Testosterone and erectile function in hypogonadal men unresponsive to tadalafil: results from an open-label uncontrolled stud? Andrologia 38: 61–68 [DOI] [PubMed] [Google Scholar]
- Yildiz O., Seyrek M., Irkilata H.C., Yildirim I., Tahmaz L., Dayanc M. (2009) Testosterone might cause relaxation of human corpus cavernosum by potassium channel opening actio? Urology 74: 229–232 [DOI] [PubMed] [Google Scholar]
- Yue P., Chatterjee K., Beale C., Poole-Wilson P.A., Collins P. (1995) Testosterone relaxes rabbit coronary arteries and aort? Circulation 91: 1154–1160 [DOI] [PubMed] [Google Scholar]
- Zhao W., Zhang J., Lu Y., Wang R. (2001) The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opene? EMBO J 20: 6008–6016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Ndisang J.F., Wang R. (2003) Modulation of endogenous production of H2S in rat tissue? Can J Physiol Pharmacol 81: 848–853 [DOI] [PubMed] [Google Scholar]