Abstract
Owing to an increased use of diagnostic imaging for evaluating patients with other abdominal conditions, incidentally discovered kidney masses now account for a majority of renal tumors. Renal ablative therapy is assuming a more important role in patients with borderline renal impairment. Renal ablation uses heat or cold to bring about cell death. Radiofrequency ablation and cryoablation are two such procedures, and 5-year results are now emerging from both modalities. Renal biopsy at the time of ablation is extremely important in order to establish tissue diagnosis. Real-time temperature monitoring at the time of radiofrequency ablation is very useful to ensure adequacy of ablation.
Keywords: renal ablation, radiofrequency ablation, cryoablation, small renal masses, renal cancer treatment
Introduction
Renal cancer accounts for approximately 3.5% of all malignancies [Jemal et al. 2007] with renal cell carcinomas (RCCs) accounting for approximately 85% of kidney cancers [Lipworth et al. 2006], and it is estimated that 57,760 individuals (35,430 men and 22,330 women) will be diagnosed with and 12,980 men and women will die of cancer of the kidney and renal pelvis in 2009 [National Cancer Institute, 2009].
Since sectional imaging is now routinely employed to evaluate patients with other abdominal conditions, incidentally discovered kidney tumors now account for 48–66% of RCCs diagnosed [Volpe et al. 2004; Jayson and Sanders, 1998]. This inadvertent ‘screening effect’ has resulted in an average increase in incidence of 2–3% per year [Mathew et al. 2002], an associated stage migration, and an increase in the rates of surgical intervention [Hollingsworth et al. 2006].
Until recently, surgical excision (radical or partial nephrectomy) for pT1a tumors (4 cm or less) with its excellent 5-year cause-specific survival (CSS) rates in excess of 95%, or laparoscopic excision with a similarly favorable early results [Moinzadeh et al. 2006], were considered standard therapy for these patients. However, the thrust to use more conservative, ablative techniques for T1a or T1b renal tumors is increasing partly because more tumors are diagnosed in the elderly patients and only 26% are aggressive grade 3 (considered potentially hazardous) [Remzi et al. 2006], many tumors are found to be benign, and some patients may also have renal insufficiency at presentation requiring a more nephron-conserving approach. In this review on the role of ablative techniques in renal tumors, we discuss the principles of therapy, mechanism of action of both ‘heating’ and ‘freezing’ of tissues, methods of delivery, appropriate patient selection for treatment and controversies in this newly developed field.
Principles of ablative therapy for small renal masses
Overview
Renal ablative treatment uses the cell destroying properties of temperature (hot or cold) to bring about apoptosis in cancer cells. An ideal ablative treatment should be able to destroy all cancer cells, without affecting normal tissue and the zone of treatment should be under the control of the physician. Principles of ablation have been studied previously [Leveillee and Hoey, 2003]. Ablation, unlike random tissue destruction by diathermy, depends on the development of a controlled tissue-based thermodynamic equilibrium, with controlled delivery of temperatures to a predetermined ablation zone, which brings about apoptosis within that zone. This is dependent on the rate of delivery of thermal energy, tissue thermal conductivity and the rate of dissipation via the heat sink mechanism. Studies in the author’s (RJL) Joint Biomedical Engineering lab at the University of Miami School of Medicine, Florida, USA, have previously supported the concept, that when the heat delivery exceeds dissipation by the heat sink, charring and carbonization will occur, producing a suboptimal ablation zone. This has the same effect as inadequate heat energy delivery with a proportionally higher dissipation by the heat sink.
Heat-sink mechanisms
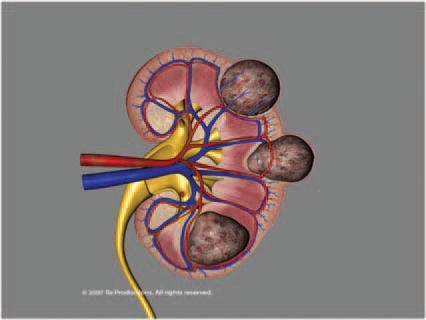
Heat-sink mechanisms affect both radiofrequency (RF) and cryoablation zones. One of the main reasons for heat dissipation is tissue vascularity within and surrounding the renal tumor, and this has an impact on the volume of the ablated area [Sterrett et al. 2008]. Depending on the adjacent blood flow, heat may dissipate (the ‘heat-sink’ phenomenon), making it more challenging for the urologist to achieve target temperatures for the requisite duration, in highly vascularized lesions or lesions adjacent to large blood vessels such as the renal hilum (Figure 1). Larger tumors may sometimes require adjunctive selective embolization to decrease the heat-sink effect [Yamakado et al. 2006]. Clamping the renal artery alone, in order to avoid the heat-sink effect of renal blood flow has not been shown to increase the size of the cryolesion significantly [Campbell et al. 1998], but clamping both the renal artery and vein may do so [Collyer et al. 2004].
Figure 1.
Superficial exophytic, and deeper endophytic types of renal masses. The deeper the location (more endophytic) the closer the tumor is to larger blood vessels and the greater the ‘heat-sink’ effect.
Principles of therapy
Radiofrequency ablation
Radiofrequency ablation (RFA) induces thermal damage through frictional heating due to ionic oscillation by a monopolar or bipolar high-frequency alternating current (375–500 kHz). It can induce temperatures between 50 and 120°C throughout the tumor [Gervais et al. 2005a; Goldberg et al. 2000]. The diameter of the zone of ablation can vary due to the length, diameter, surface area, and temperature of the electrode. Not all RFA devices perform similarly, therefore, the preliminary results of effectiveness need to be interpreted appropriately.
Thermal damage by RFA ultimately leads to coagulative necrosis. Damage is believed to manifest through protein denaturation, DNA/RNA chain disruption, and vascular congestion.
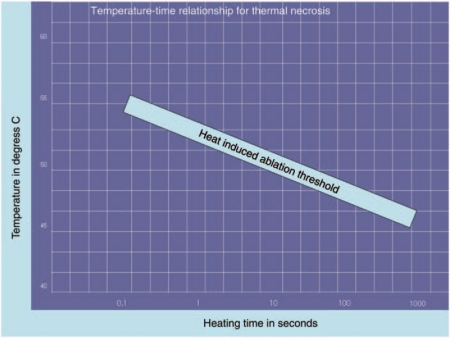
Effective ablation and cell damage is a time-temperature dependent process (Figure 2). A wide range of temperature–time combinations (heat) will damage tissue and result in cell death. More time is required at lower temperatures while temperatures above the boiling point of water (>100°C) result in immediate structural damage to the cells with desiccation, vaporization, and carbonization [Sterrett et al. 2008]. In experimental models, tissue ablation occurs when the temperature is elevated for a ‘sufficient period’ of time which can be specific for a given tissue (tissue-specific time–temperature curve for necrosis) [Lele, 1977]. Reliable tissue destruction has been demonstrated at a temperature of 55°C when maintained for 15 seconds [Bhowmick et al. 2001; Djavan et al. 1997; McGahan et al. 1992]. At temperatures between 60 and 100°C, irreversible damage is instantaneous.
Figure 2.
Temperature–time relationship for thermal ablation.
Lower temperatures (45–55°C) are associated with less grossly apparent changes but biochemical changes involving cellular enzymes or damaged membrane channels manifest after several hours in the form of cellular edema, organelle swelling, and bleb formation [Sterrett et al. 2008]. Elevated temperatures cause the intracellular buffering capacity and transport mechanisms to fail causing an overload of intracellular calcium and cell death. Disruption of the delicate intracellular balance causes localized inflammatory changes to appear followed by an ischemic response leading to acidosis and eventual coagulative necrosis [Sterrett et al. 2008].
Following thermal injury the basic structure of the cell is preserved. Three to seven days after RF treatment the damaged tissue begins to show signs of coagulative necrosis with interspersed inflammatory cells. The necrotic debris is then removed by fragmentation or phagocytosis. Concomitant apoptosis can increase the total area of cell kill through the promotion of nuclear pyknosis in cells adjacent to directly injured cells [Leveillee and Hoey, 2003]. All evidence of organized renal cellular architecture disappears by 30 days. The necrotic tissue absorbed with fragmentation and phagocytosis transforms into avascular scar tissue that may become smaller in size and does not enhance on contrasted imaging [Crowley et al. 2001; Hsu et al. 2000].
Energy delivery
Three different monopolar RFA devices are currently available for use in the USA, and vary in the way energy delivery is controlled (temperature versus electrical impedance), and whether they are internally cooled or not. The three instruments are Cool Tip device (Covidien, Mansfield, MA, USA), the LeVeen needle electrode (Boston Scientific, Natick, MA, USA), and the RITA (Angiodynamics, Queensbury, NY, USA).
Cryoablation
Cryoablation causes apoptosis due to mechanical cell damage in the acute setting, and vascular damage in the subacute setting because of crystallization–recrystallization caused by tissue freezing [Gage and Baust, 1998]. This multistep process [Sterrett et al. 2008] starts with initial extracellular ice formation, increased osmolarity in the extracellular space and efflux of intracellular fluid into the extracellular compartment [Berger et al. 2008], along with changes in pH and mechanical disruption of the cell membranes due to intracellular ice formation seen with lower temperatures [Sterrett et al. 2008]. Lastly, ischemic cell death due to microcirculatory failure correlating with the thaw phase of the freeze–thaw cycle occurs due to endothelial damage and microvascular thrombosis [Gill and Novick, 1999; Mazur, 1970]. Later, fibrosis, and collagen deposition occurs which lasts over weeks to a month [Chosy et al. 1998].
Because the thaw cycle plays such an important role, multiple freeze–thaw cycles exacerbate tissue damage and increase liquefaction necrosis [Auge et al. 2006]. Argon gas is used for freezing, and helium gas for thawing of the tumor according to the Joule–Thomson effect. A slow, passive thaw may be more effective than a rapid and active thaw [Berger et al. 2008].
Temperatures of –19.4°C or less cause cell death in the animal models [Chosy et al. 1998], but exposure of RCC lines to –10°C for 60 minutes resulted in cell death in only 5% of cells while exposure to –20°C, resulted in 85% cell death [Stephenson et al. 1996]. Similarly to RFA, cryoablation also follows a temperature–time necrosis pattern. Optimal freeze times have been studied in the pig model and a 10-minute freeze appears to produce necrosis with the fewest complications [Collyer et al. 2004]. Five-minute freeze times produced inadequate effect but were associated with excessive bleeding, whereas 15-minute freeze times produced consistent necrosis but were associated with renal fracture [Collyer et al. 2004].
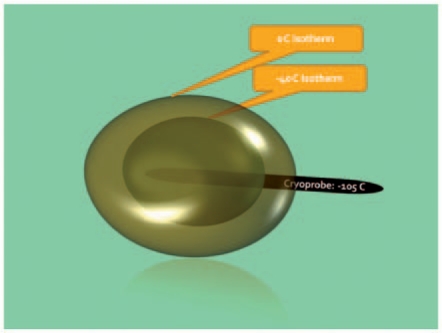
Since there seems to be a rapid warming of the tissue toward the periphery of the ice ball (Figure 3), the edge of the ice ball in a canine model needs to extend at least 3.1 mm beyond the edge of the target lesion for adequate cell death [Campbell et al. 1998].
Figure 3.
Temperature decay at the edge of the ice ball formation in a cryoablated zone. Various mechanisms play a role, including to some extent the ‘heat-sink’ effect and the insulating properties of the ice ball itself.
The target temperature in clinical protocols is therefore approximately –40°C with extent of the ice ball at least 0.5 cm beyond the target lesion [Berger et al. 2008]. The duration of freezing is an important factor. After achieving target temperatures, further cell destruction can be achieved by prolonged freezing at this temperature [Hoffmann and Bischof, 2002].
Delivery systems
There are currently four cryoablation manufacturers [Matin and Ahrar, 2008]: Endocare (Irvine, CA, USA), Galil Medical (Yokneam, Israel), Oncura (Arlington Heights, IL, USA), and Cryomedical Sciences (Rockville, MD, USA). The first three use argon gas to cause rapid freezing at the probe tip, based on the Joule–Thomson effect. The Cryomedical Sciences unit is a nitrogen-based system and is currently only utilized for prostate and liver applications [Matin and Ahrar, 2008].
Microwave
Microwave energy with frequencies from 900 to 2450 MHz can cause ionic oscillation at high rates. Water molecules have a net positive charge on the hydrogen side and a negative charge on the oxygen side [Simon et al. 2005] and this charge changes signs nearly 2 billion times a second (9.2 × 108 Hz) [Simon et al. 2005] causing resistive heating. This causes a rise in temperature, proportional to the vigorous movement of water molecules, thus inducing cellular death via coagulation necrosis.
High-intensity focused ultrasound
High-intensity focused ultrasound (HIFU) uses focused ultrasound waves to generate heat. As the ultrasound wave propagates through biological tissues, it is progressively absorbed dissipating its mechanical energy into heat. If the wave is brought to a tight focus at a point within tissues (HIFU), the high-energy density results in heat generation sufficient to cause coagulative necrosis [Klingler et al. 2008; Madersbacher et al. 1995]. The energy density decreases rapidly outside the focal zone, so that surrounding tissues remain unharmed. As heat generation is extremely rapid, tumor cell dissemination or metastases are less commonly seen [Murat et al. 2007; Oosterhof et al. 1997], and potential heat sinks such as large blood vessels have a lesser impact than with other, slower techniques of thermal ablation [Klingler et al. 2008; Madersbacher et al. 1995].
HIFU ablation is more efficient if homogeneous tissue structures without significant acoustic interphases along the path of ultrasound delivery are present, along with a stationary target for precise energy deposition [Klingler et al. 2008]. Clinically this can be achieved when the kidney is surgically exposed [Susani et al. 1993]. HIFU of renal tumors by an extracorporeal approach has proven unreliable both in animal experiments and clinical pilot studies [Illing et al. 2005; Marberger et al. 2005], and this can be a major drawback of this technology.
Patient selection
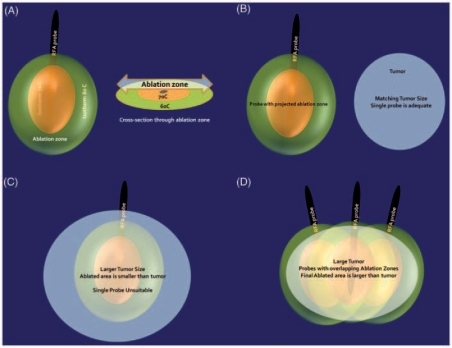
Both RFA and cryoablation are good tools for renal tumor ablation and success may be a function of patient selection and the technique used, rather than the ablation technology [Cadeddu and Raman, 2008]. Proper patient selection needs to be based on an understanding of the mechanisms by which they work and following the principles of delivery. Cryoablation failures may be related to improperly performed freeze–thaw cycles. RFA failures maybe related to improper selection of patients with larger tumor sizes, or tumors nearer large blood vessels, rapid heating causing tissue charring and therefore more thermal insulation, and nonuniform heating of the tumor. It is essential to make an appropriate probe selection. For example, a 2.5-cm tumor could be treated with one probe, but a 4.5-cm tumor would require two or even three probes (Figure 4). Appropriate real-time thermal monitoring of tissues is extremely important while performing a RFA in order to ensure uniform heating. Lastly, we feel that the use of general anesthesia with controlled respiration helps to improve results.
Figure 4.
The zone of ablation and need for multiple probes for larger tumors. (A) Isotherms around the radiofrequency ablation probe with corresponding zone of ablation. (B) Close match between the ablation zone and the size of a smaller T1a tumor. (C) Gross mismatch between the ablation zone and larger tumor. (D) Need to use multiple probes (or alternatively multiple cycles) to achieve proper match between zone of necrosis and larger tumor.
Controversies
Renal ablative therapy, like any newly developed field, has many controversial issues. We now look at some of the commonly addressed areas.
Renal biopsy
Renal biopsies for renal masses have had a high incidence of false-negative results [Dechet et al. 2003; Brierly et al. 2000; Campbell et al. 1997; Herts and Baker, 1995] and were selectively used in patients suspected of having a lymphoma or metastases to the kidneys [Herts and Baker, 1995]. A better understanding of the tumor biology has required a revision of policies concerning renal biopsy. Approximately 15–20% of clinical stage T1 renal masses may be benign and could be considered for less aggressive management [Schmidbauer et al. 2008; Lane et al. 2007; Remzi et al. 2006]; but only 17% of lesions were correctly defined as benign on computed tomography (CT) before surgery. On the other hand, around 26% of tumors are aggressive G3 [Remzi et al. 2006] and may require more definitive surgical treatment. It would therefore be very useful to have a definitive preoperative diagnosis by a needle biopsy.
The false-negative rate with renal mass biopsy is now only 1%, with another 10–15% of biopsies turning out to be indeterminate (not as concerning as a false negative) [Novick et al. 2009] and the incidence of symptomatic complications requiring any form of intervention is also relatively low (<2%) [Remzi and Marberger, 2008a; Schmidbauer et al. 2008; Lebret et al. 2007; Volpe et al. 2007]. Renal biopsies are therefore becoming more popular. We routinely perform renal biopsies at the time of the RFA. The biopsy is just an extension of the procedure and in our opinion cannot be considered any more invasive than the process of ablation. It gives valuable information about the pathology and grade of tumor. When used with special stains, as is routinely done in our institution, its yield is very high and there are significant implications for follow up of the patient. For example, a patient with a fat poor angiomyolipoma that was treated with RFA need not undergo CT scanning with the same frequency as a patient with clear cell RCC.
Hot versus cold: which one to use
RFA and cryoablation are two popular modalities in use today.
RFA is technically easy to setup, uses a small portable generator and has excellent results when appropriate cases are selected. Long-term follow up of up to 5 years has shown that 94% of patients with biopsy-proven RCC treated with curative intent had no evidence of local or distant recurrence at 5 years [McDougal et al. 2005], with five patients dying of other causes. Outcomes depend on tumor grade, with excellent overall recurrence-free rate of 96.8% and 94.8% cancer-specific survival in a series of 94 tumors with a predominance of Fuhrman grade 1 [Park et al. 2006]. The cumulative local control rate could be different depending on duration of follow up and was found to be 96% at 2 years and 86.4% at 3 years [Varkarakis et al. 2005]. Percutaneous RFA has been tried under conscious sedation [Arzola et al. 2006], with a cancer-free survival rate of 90% (18/20) at a mean follow up of 24 months [Arzola et al. 2006], RFA can be particularly useful in patients with solitary kidneys. In 10 patients with a history of prior contralateral nephrectomies, CT-guided RFA was performed with (tumor >2.5 cm) or without (tumor <2.5 cm) selective adjunctive arterial embolization. No tumor recurrence or major complication was found in the follow up of 3–24 months and none of the patients developed renal failure [Hoffmann et al. 2010].
Repeat interventions may, however, be required. In one series only 69% had successful ablation with a single treatment [Arzola et al. 2006] and in another study 10.7% had incomplete therapy and underwent repeat intervention [Varkarakis et al. 2005]. Repeat intervention improved the radiological success rate from 86% to 89% in another study [Veltri et al. 2004]. In our series (unpublished data) repeat interventions are infrequent since we use multiple probes or a single probe with multiple cycles (in different places of the tumor) to offset mismatches in tumor volume and predicted ablation zone.
Distant progression can occur and has been described in 2/44 patients in one series [Veltri et al. 2004], and in 4 patients in another [Mahnken et al. 2005] at 19 and 13.9 months, respectively, in spite of imaging showing successful ablation of the treated area and stable renal function, emphasizing the need for appropriate surveillance.
One of the concerns with RFA has been ‘skip areas’ of ablation. Skipping can occur due to the ‘heat-sink’ effect (see previous discussion) due to proximity to rapidly flowing blood, and this renders RFA unreliable in highly vascularized tumors larger than 3 cm, with 23% vital tumors to be found at histological work up [Fernandez Rosado et al. 2006]. Techniques to optimize the uniformity of ablation in these situations include clamping the renal artery prior to cryotherapy [Orihuela et al. 1999], or performing a superselective embolization to eliminate the heat-sink effect [Yamakado et al. 2006]. The embolization before RFA can be done even in the setting of renal artery stenosis or abdominal aortic aneurysm [Mondshine et al. 2008], and can be done under local anesthesia with intravenous sedation [Arima et al. 2007]. RFA compares well with partial nephrectomy for clinical T1a tumors, and overall actuarial disease-free probabilities for the partial-nephrectomy and RFA groups, respectively, were 95.8% and 93.4% (p = 0.67) [Stern et al. 2007].
Cryoablation on the other hand, has been associated with a significantly lower rate of incomplete ablation (4.8%) than RFA (14.2%) [Novick et al. 2009], although this comparison could be biased due to use of dissimilar groups. Cancer-specific survival of 98% at 3 years and complete resolution of the lesion in 38% on MRI have been described [Gill et al. 2005]. However, conversion rates for cryoablation (3.5%) are almost twice as high as RFA rates (1.6%) [Novick et al. 2009].
Laparoscopic cryoablation is a good choice for elderly patients with comorbidities [O’Malley et al. 2007]. Laparoscopic cryoablation is a feasible option even in angiomyolipoma [Byrd et al. 2006], and in such vascularized tumors, it may be advantageous to use cryotherapy [Klingler et al. 2007]. The mean thermal treatment time for laparoscopic cryoablations was 19.3 min compared with 32.2 min for percutaneous RFA [Hegarty et al. 2006], and radiologic evidence of tumor recurrence or persistence of disease was noted in three patients (1.8%) with cryoablation and in nine (11.1%) with RFA [Hegarty et al. 2006]. The patients in this study were, however, not evenly matched with a higher percentage of patients having solitary kidneys as well as hilar/central tumors treated with CT RFA as compared with more peripheral, exophytic tumors treated with laparoscopic cryoablation (the cryoablation group had a greater number of anteriorly located tumors, 39% versus 10%, as well as fewer central tumors, 6% versus 37%, and fewer solitary kidneys, 24% versus 49% [Hegarty et al. 2006]). The mean thermal time may depend on many variables. The technique used could have an impact: in our center, we wait for all temperature probes to register temperatures above 60°C, and this could take longer, depending upon the endpoint of treatment. Since most centers do not use independent temperature sensors, the endpoint of ablation treatment is determined by the manufacturer using a preset algorithm. The number of probes used could also have a direct impact on the time and expense. Three probes could be used synchronously for a total of 14–20 minutes; or one probe could be deployed three times in three different places for 14–20 minutes each. A head-to-head comparison may only be possible if the methodology is standardized.
Current relapse-free survival (RFS) for laparoscopic cryoablation has been reported to be as high as 94% [Lawatsch et al. 2006]. Repeat intervention may be required. Tumor location and size were the major determinants for achieving tumor eradication, and 82.6% had a single treatment [Permpongkosol et al. 2006]. In another series the RFS was 84.3% after one cryoablation, and 96.8% after repeat intervention [Davol et al. 2006].
Percutaneous cryoablation of small RCCs under horizontal open MRI guidance appears to be safe, but a few patients experience incomplete ablation as confirmed in patients undergoing secondary nephrectomy [Miki et al. 2006]. Percutaneous cryoablation is also effective in patients with recurrent tumors [Nosnik et al. 2006], in patients with bilateral RCC and even in a patient with an incidental 3.2-cm mass in a renal allograft [Hruby et al. 2006].
In a 5-year follow up after cryosurgical ablation of renal tumors ranging from 1.1 to 4.6 cm (median lesion size 2.6 cm), 12.5% of patients were diagnosed with persistent disease during follow up [Davol et al. 2006].
In a meta-analysis [Kunkle and Uzzo, 2008] involving 47 studies representing 1375 kidney lesions comparing cryoablation with RFA, analysis showed that the patients were well matched for age, tumor size, or duration of follow up. In this study, RFA had a higher rate of repeat ablation (8.5% versus 1.3%; p < 0.0001), local tumor progression (12.9% versus 5.2%; p < 0.0001) and metastasis (2.5% versus 1.0%; p < 0.06). The higher incidence of local tumor progression was found to be correlated significantly with treatment by RFA on univariate analysis (p < 0.001) and on multivariate regression analysis (p < 0.003). Cryoablation was usually performed laparoscopically (65%), whereas 94% of RFAs were performed percutaneously [Kunkle and Uzzo, 2008].
Complications
Head-to-head comparison has been difficult since no randomized, prospective trials are available. However, both procedures are popular, can be performed percutaneously as well as laparoscopically, should ideally have intraoperative biopsies, need sectional imaging in order to optimize results, and need similar follow-up protocols. They also have similar complications seen with any laparoscopic or percutaneous procedure on the kidney.
Complications described with RFA include hemorrhage requiring blood transfusion (2.3%) [Gervais et al. 2005b], abscess formation mimicking tumor recurrence [Roarke et al. 2006], ureteral strictures, ureteropelvic junction obstruction and delayed hemorrhage [Carey and Leveillee, 2007], renocolic fistula [Uribe et al. 2006], a renoduodenal fistula [de Arruda et al. 2006] and neuromuscular complications [Lee et al. 2006] with paresthesia in the distribution of the genitor–femoral nerve while ablating lesions near the psoas muscle. The likelihood of tumor seeding along the probe track after biopsy or ablation is minimized by ablating the probe track during removal [Stone et al. 2007]. Treatment of central tumors is most likely to cause hematuria which is usually self-limiting [Ahrar et al. 2005]. Many of the above complications are related to proximity of the tumor to neighboring organs [Park and Kim, 2009]. In order to avoid many of these complications, the distance between the tumor and neighboring organs may be increased artificially by using methods such as changing the patient’s position, using the RF electrode as a lever and hydrodissection [Park and Kim, 2009].
Cryoablation has a complications rate of 1.8%, and as with RFA, percutaneous techniques are less effective as compared with laparoscopy, with recurrence rates ranging between 13% and 21% [Klingler, 2007a]. Hemorrhage due to renal fracture is a complication unique to cryoablation, and is seen with breakage at the junction of the ice ball formation. This may require transfusion [Davol et al. 2006; Schwartz et al. 2006] or conversion to open surgery [Lee et al. 2003]. Other complications include pancreatic injury [Lee et al. 2003], ureteral obstruction by clots causing anuria in a solitary kidney [Schwartz et al. 2006; Lane et al. 2005; Lee et al. 2003] have been described. The major urological complication rate for cryoablation was 4.9%; this rate is similar to rates for RFA but renal loss has been reported in the presence of complications [Novick et al. 2009].
What is an ideal tumor size
Tumor size is an important determinant of local ablation success with smaller tumors having a better chance of success. Good candidates for ablation include those with small, contrast enhancing, solid renal masses less than 4 cm (T1a) in a noncentral location: they should not be located too close to important structures such as the ureteropelvic junction, or renal hilar vessels, and preferably should not abut the pyelocalyceal system [Park and Cadeddu, 2007]. The thermal damage through frictional heating seen in RFA results in an ablation diameter of around 1.6 cm around a 17 gauge needle electrode [Gervais et al. 2005a; Goldberg et al. 2000]. Technical innovations that can control the size and shape of the ablation zone include pulsing the current and using cooling of the electrode to prevent carbonization [Tacke et al. 2004], using multiple electrodes, or multitined expandable electrodes [Gervais et al. 2005a], or increasing the effective electrode size by injection of an ionic solution such as interstitial saline.
For cryoprobes, the rate of temperature change and thermal conductivity of the target tissue affects the volume of tissue that is ablated. A 3.4-mm probe cooling down at 50°C/min to a low of –175°C will create a cryolesion 4 cm in diameter in 20 minutes, but an 8-mm probe cooling at the rate of 100°C/min to a nadir tip temperature of –190°C will result in a cryolesion 7 cm in diameter in 20 minutes [Gage and Baust, 1998]. In general, complete ablation is achieved in all tumors less than 3 cm in size, in 92% of tumors between 3 and 5 cm, but in only 25% of tumors larger than 5 cm [Gervais et al. 2005b] and for each 1-cm increase in tumor diameter over 3.6 cm, the likelihood of RFS decreased by a factor of 2.19 [Zagoria et al. 2007]. A retrospective study looking at percutaneous cryoablation performed on 40 patients with renal tumors 3 cm in diameter or larger found that extension of the ice ball beyond the tumor margin was seen on all patients on the CT, and technical success defined as postablation imaging findings of no contrast enhancement in the area encompassing the original tumor, was achieved in 38 (95%) of 40 cryoablation procedures. The authors reported one National Cancer Institute Common Terminology Criteria for Adverse Events grade 3 adverse event (3%). No local tumor recurrence or tumor progression was found in 26 (65%) of the 40 patients that were available for follow up [Atwell et al. 2007]. Cryoablation may be a better treatment alternative for larger tumors and the capacity to form a very large ice ball would be advantageous in this situation. A caveat was raised by Lehman et al. [2008], who retrospectively evaluated patients treated with laparoscopic cryoablation who were divided into two groups. Group 1 (30 tumors <3.0 cm) had no complications. Group 2 (21 tumors >3.0 cm) demonstrated 62% complication rates, including a 38% transfusion rate and two mortalities. These authors concluded that cryoablation should be reserved for tumors <3.0 cm [Lehman et al. 2008].
Until further improvements in technology are available and long-term results show technical feasibility, tumor sizes of 4 cm or less are considered good candidates. Masses over 4 cm can be treated with multiple probes in order to optimally overlap zones of ablation to involve the whole tumor (Figure 4).
This would minimize the risk of residual tumor and the need to perform repeated ablations. Masses over 7 cm are rarely selected for ablative therapy unless there is a compelling need to do so.
Tumor location
Noncentral tumors are easier to access and treat with a low complication rate. Posterior and posterolateral tumors can be treated percutaneously, while anterior and medially placed tumors may require a laparoscopic intervention (Figure 5).
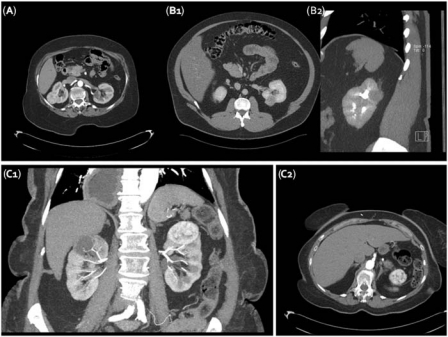
Figure 5.
Tumor location and route of access. (A) Patient with posterolateral and another patient (B1 and B2) with posteromedial tumors could be considered good candidates for a percutaneous RFA. A third patient (C1 and C2) with upper pole tumor sitting very close to the bowel and (C1 and C2) and the spleen (C1) would be better served by a laparoscopic approach.
Urologist versus interventional radiologist
Endourologists with skills in percutaneous and laparoscopic procedures are comfortable performing both routes. However the laparoscopic method for renal cryoablation has been the exclusive domain of the urologist. The transperitoneal approach, in particular, is comfortingly familiar, affords excellent access to the kidney, allows use of intraoperative ultrasound, visualization of probe placement and, in the case of cryoablation, monitoring of ice ball formation [Sterrett et al. 2008].
The percutaneous approach can be performed either by the urologist or the interventional radiologist. In our institution it is performed by a team in the radiology suite (Figure 6). Percutaneous RFA using multitined probes under moderate sedation with adjunctive procedures such as water injection for pancreatic or colonic displacement has been described [Clark et al. 2006]. A mean involution of 15% per year was noted by these authors after the use of multitined probes [Clark et al. 2006].
Figure 6.
Percutaneous CT-guided radiofrequency ablation: the urologist and interventional radiologist need to work as a team. Note the extensive use of sectional imaging.
Percutaneous techniques are more cost effective, most often do not require a hospital stay and patients return to work earlier. Percutaneous RFA has been compared with nephron-sparing surgery (NSS) in patients with T1a tumors [Pandharipande et al. 2008] using a decision-analytic Markov model developed to estimate life expectancy and lifetime costs for 65-year-old patients with a small RCC treated with RFA versus NSS. NSS yielded a minimally greater average quality-adjusted life expectancy than did RF ablation (2.5 days) but was more expensive. NSS had an incremental cost-effectiveness ratio greatly exceeding US$75,000 per quality-adjusted life year (QALY), and RFA was considered preferred if the annual probability of post-RFA local recurrence was up to 48% higher relative to that post-NSS. NSS preference required an estimated NSS cost reduction of US$7500 or RF ablation cost increase of US$6229 [Pandharipande et al. 2008]. Percutaneous cryoablation has also been found to be 2.2–2.7 times less costly when compared with open or laparoscopic partial nephrectomy or when compared with laparoscopic cryoablation [Link et al. 2006], with cryoprobe consumables accounting for more than 70% of the total cost of the procedure [Link et al. 2006]. The lack of visual cues afforded by the laparoscopic technique is offset by the availability of excellent new-generation sectional imaging devices. With the team approach, either interventional radiologist or the urologist should be available to deal with any complications that may arise.
Is there an age limit
Patients with a solitary kidney, multiple synchronous RCC, von Hippel Lindau disease, familial RCC, or those with limited renal function are appropriate candidates for ablative treatment irrespective of age [Sterrett et al. 2008]. However, controversy exists in younger patients without significant comorbid conditions, or those with normal contralateral renal function and minimal future risk of renal function loss. In such patients the results of definitive treatment such as radical nephrectomy (open or laparoscopic) or partial nephrectomy far outweigh the available results, and limited follow up from relatively small series of renal ablation. As experience with ablative therapy accumulates and long-term oncologic results become available, favorable outcomes may justify the treatment of smaller, potentially less-aggressive tumors in younger, healthier patients.
Definition of success
Undertreatment in RFA could be indicated by a rapid decline in temperature during cooling (indicating persistent cooling perfusion at the tip), a suboptimal rise in impedance or a delayed decline, or a lack of pulsing of the generator [Stone et al. 2007]. However, long-term success is measured by surrogate markers such as radiographic demonstration of loss of contrast enhancement (Figure 7).
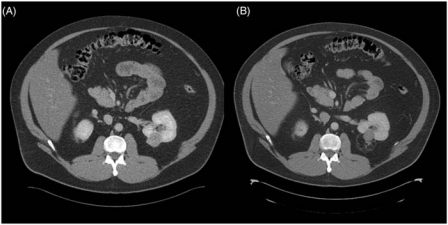
Figure 7.
Patient with a preablation and postablation CT. (A) Preablation CT showing good enhancement of tumor. Biopsy showed this to be a clear cell renal cell carcinoma, Fuhrman Grade 2. (B) Eighteen-month postablation CT scan shows no enhancement and postoperative changes are seen surrounding the lesion. Note that even after 18 months the lesion has not shrunk completely.
Lack of enhancement (a rise of Hounsfield units [HU] of less than 10 HU [Farrell et al. 2003] or less than 20 HU [Sterrett et al. 2008]), and no evidence of growth are reasonably reliable indicators of the presence of nonviable tissue in the place where the tumor had been present [Sterrett et al. 2008]. However, this has come into question, due to the demonstration of viable tissue in non-enhancing lesions on pathological examination [Weight et al. 2008].
The presence of incompletely ablated original tumor is now considered a recurrence, and not a persistence, in accordance with the recommendations of the Working Group on Image-Guided Tumor Ablation [Goldberg et al. 2005].
The percutaneous approach for ablation, although less invasive, may have a higher incomplete ablation rate compared with the laparoscopic approach with recurrence rates for RFA ranging between 14% and 18% [Novick et al. 2009], although more randomized studies are required to investigate this issue. Patient selection and ‘intent to treat’ decisions may influence the aggressiveness of the surgeon or radiologist performing the treatment. Percutaneous ablations were compared with surgical ablations [Hui et al. 2008]. Primary effectiveness was defined by the proportion of tumors without residual enhancement after one treatment session and secondary effectiveness as repeated treatments required. A meta-analysis of 46 series (28 percutaneous, 18 surgical) showed a significantly lower primary effectiveness rate for the percutaneous group (87% versus 94%; p < 0.05) compared with the surgical group, but no significant difference for the retreatment rates (92% versus 95%; p > 0.05). However the major complication rate in the percutaneous treatment group (3%) was significantly lower than that in the surgical treatment group (7%; p < 0.05) [Hui et al. 2008].
Unlike cryoablated lesions, RFA lesions tend not to shrink in size (Figure 7). Matsumoto et al. described the typical characteristics of the RF ablated mass. These characteristics include a nonenhancing wedge-shaped lesion frequently with a thin rim of fat between the lesion and the normal parenchyma. Exophytic tumors tend to retain their preablation shape and size [Matsumoto et al. 2004]. Imaging characteristics can change at any time and strict adherence to the follow-up protocol is encouraged in all patients since late recurrences (up to 31 months) have been documented [Sterrett et al. 2008].
Technical aspects of renal ablation
Image-guided (ultrasound, MRI, CT) renal tumor ablations can be performed through open surgical, laparoscopic or percutaneous routes. The device can be inserted under ultrasound, CT, or MRI guidance. These patients are usually discharged home the same day as the procedure. Patients not suitable for a percutaneous approach are treated laparoscopically. The optimal trajectory for the device for the percutaneous approach should be via a posterior or lateral approach with the patient in a semiprone or lateral decubitus position with coordinated breathing to help position the target lesion without injuring surrounding structures [Stone et al. 2007]. We favor general anesthesia with suspended respiration to improve targeting and outcomes [Gupta et al. 2009]. The use of real-time virtual ultrasonography as a navigational tool for percutaneous RFA has been described [Ukimura et al. 2008].
Cortical renal tumors and exophytic lesions are often more readily ablated due to the presence of surrounding fat, which provides an insulating ‘oven effect’ during ablation, and the absence of a heat-sink effect seen with medullary lesions (Figure 1).
Ablation of cystic tumors can be challenging. The probe might need to be moved to different locations within the cystic tumor to ablate the solid components [Stone et al. 2007].
The ureters, surrounding bowel, and several nerves (genitofemoral and ilioinguinal nerves coursing along the psoas muscle) and muscles are at risk for thermal injury and the operator needs to be cognizant of the presence of these structures. The proximity of the adrenal gland to the kidney must also be taken into consideration, as damage to the adrenal gland during renal ablation can lead to an acute hypertensive crisis or delayed adrenal insufficiency in patients with a prior contralateral adrenalectomy [Stone et al. 2007].
Intraoperative monitoring is recommended. Laparoscopic ultrasonography may facilitate identification of tumor and renal anatomy and help in excluding satellite lesions. It also makes assessment of ice ball formation during laparoscopic renal cryotherapy [Fazio et al. 2006]. Our group [Carey and Leveillee, 2007] has previously described the use of nonconducting temperature probes independent of the RFA electrode in order to achieve real-time temperature monitoring of the ablation zone. The ablation can be continued until all of the peripheral temperature monitors registered 60°C for at least 15 s [Carey and Leveillee, 2007]. Ultrasound or CT placement of these ‘peripheral sensors’ is essential to ensure adequate positioning (Figure 8).
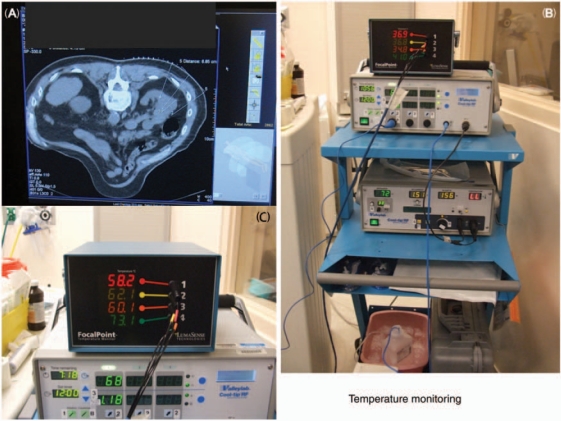
Figure 8.
Importance of real-time temperature monitoring. (A) Probe placement under sectional image guidance. (B) Temperature measurement cart. (C) Temperatures at the end of procedure show consistent temperatures near and above 60°C.
Hydrodissection with a nonelectrolyte solution such as dextrose 5% in water (D5W) [Laeseke et al. 2005] has been described to try to protect adjacent structures and can create an insulating envelope around the structures, minimizing the risk for complications such as bowel perforation [Chen et al. 2006]. Pneumodissection using CO2 has also been used successfully, and separation of the target tumors from adjacent structures by injecting CO2 around the tumor avoided thermal injury [Kariya et al. 2005].
However, an aspect of hydrodissection or pneumodissection that has not been fully assessed is the effect of dissipation. Both CO2 and D5W can dissipate or undergo resorption, and therefore repeated injections in the proper plane of dissection may be required to maintain adjacent organ displacement, away from the probe. Repeated injections have their own risks, and injection in the wrong planes can have serious adverse effects. A thorough understanding of the surgical planes is instrumental in deciding which patients are optimally treated with CT guidance versus laparoscopic exposure.
Additional maneuvers to achieve extra bowel displacement could include additional manual torquing of the RFA probe, and the use of angioplasty balloon interposition [Ginat et al. 2009]. When the electrode is used as a lever, the distance between the tumor and bowel during percutaneous CT-guided RFA increased by over 5 mm [Park and Kim, 2008] and no thermal injury to the bowel was noted [Park and Kim, 2008].
We favor laparoscopic exposure for those situations where bowel or ureter injury is particularly concerning and do not advocate hydro/pneumodissection as a routine (Figure 5). The ureter is particularly at risk for thermal damage with centrally located neoplasms or transitional cell tumors, and can be thermoprotected by using infusions of chilled solution via a ureteral catheter or nephrostomy [Schultze et al. 2003]. The technique of using cooled D5W solution retrograde pyeloperfusion is particularly recommended for patients undergoing ablation of tumors within 1.5 cm of the ureter [Cantwell et al. 2008]. In 17 patients (mean distance from ureter, 7 mm) good technical success was reported in all patients, no patient developed a ureteral stricture or hydronephrosis during a mean of 14 months of follow up and 3 patients had residual tumor on the first follow-up imaging study, but all three tumors were completely ablated after a second RF ablation session [Cantwell et al. 2008]. This relatively high (17.6%) retreatment rate may be attributed to a cautious approach to treating in such proximity to the ureter or to a detrimental effect of the ‘cooling/protection’ employed. Alternative strategies for the surgeon would be to utilize a laparoscopic mobilization of the ureter away from the field to ensure optimal distances.
For cryoablations, two freezing and two active and passive thawing cycles are often performed [Klingler, 2007b]. Percutaneous renal cryoablation is currently performed with the use of CT scan guidance, open gantry MRI or ultrasound [Permpongkosol et al. 2008; Stein and Kaouk, 2007].
With microwave ablation, after image-guided tumor localization the microwave antenna is then placed directly into the tumor. When the antenna is attached to the microwave generator with a coaxial cable, an electromagnetic microwave is emitted from the exposed, noninsulated portion of the antenna [Simon et al. 2005]. Owing to the inherent properties of the electromagnetic wave, the device does not need to be grounded.
For laparoscopic HIFU after core tumor biopsies are obtained from the tumor, one 18-mm port (Ethicon, Somerville, NJ, USA) is required to allow access for the HIFU probe [Klingler et al. 2008]. If, because of tumor size, location or access port placement issues, the tumor area cannot be completely covered by a single treatment, second overlapping treatment can be used to completely ablate the entire tumor volume. The transducer’s focal length limits the maximum HIFU penetration depth to approximately 35 mm. The maximum linear scanning extent of the transducer is 50 mm and the maximum angular scanning extent is 90° [Klingler et al. 2008].
Conclusions
RFA and cryoablation are popular techniques for the treatment of small renal masses. Many series with over 5 years of follow up are emerging to reassure the urologist of their safety and efficacy. RFA uses heat to kill cells whereas cryoablation uses freezing. Both produce consistently good results which could be a function of patient selection and technique, rather than the ablation technology [Cadeddu and Raman, 2008]. Either procedure could be used to treat small T1a lesions in the kidney. Temperature monitoring is an essential part of the treatment and should be done to ensure adequacy of treatment. Biopsy of the renal lesion should be done prior to any form of ablation. It would be reasonable to expect that renal ablation could be incorporated as a standard treatment option for small renal masses, especially, for the patient with renal impairment requiring a nephron-sparing option.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest statement
The authors have declared that there is no conflict of interest.
References
- Ahrar K., Matin S., Wood C.G., Wallace M.J., Gupta S., Madoff D.C., et al. (2005) Percutaneous radiofrequency ablation of renal tumors: technique, complications, and outcomes. J Vasc Interv Radiol 16: 679–688 [DOI] [PubMed] [Google Scholar]
- Arima K., Yamakado K., Kinbara H., Nakatsuka A., Takeda K., Sugimura Y. (2007) Percutaneous radiofrequency ablation with transarterial embolization is useful for treatment of stage 1 renal cell carcinoma with surgical risk: results at 2-year mean follow up. Int J Urol 14: 585–590, discussion 590 [DOI] [PubMed] [Google Scholar]
- Arzola J., Baughman S.M., Hernandez J., Bishoff J.T. (2006) Computed tomography-guided, resistance-based, percutaneous radiofrequency ablation of renal malignancies under conscious sedation at two years of follow-up. Urology 68: 983–987 [DOI] [PubMed] [Google Scholar]
- Atwell T.D., Farrell M.A., Callstrom M.R., Charboneau J.W., Leibovich B.C., Frank I., et al. (2007) Percutaneous cryoablation of large renal masses: technical feasibility and short-term outcome. AJR Am J Roentgenol 188: 1195–1200 [DOI] [PubMed] [Google Scholar]
- Auge B.K., Santa-Cruz R.W., Polascik T.J. (2006) Effect of freeze time during renal cryoablation: a swine model. J Endourol 20: 1101–1105 [DOI] [PubMed] [Google Scholar]
- Berger A., Crouzet S., Canes D., Haber G.P., Gill I.S. (2008) Minimally invasive nephron-sparing surgery. Curr Opin Urol 18: 462–466 [DOI] [PubMed] [Google Scholar]
- Bhowmick S., Swanlund D.J., Coad J.E., Lulloff L., Hoey M.F., Bischof J.C. (2001) Evaluation of thermal therapy in a prostate cancer model using a wet electrode radiofrequency probe. J Endourol 15: 629–640 [DOI] [PubMed] [Google Scholar]
- Brierly R.D., Thomas P.J., Harrison N.W., Fletcher M.S., Nawrocki J.D., Ashton-Key M. (2000) Evaluation of fine-needle aspiration cytology for renal masses. BJU Int 85: 14–18 [DOI] [PubMed] [Google Scholar]
- Byrd G.F., Lawatsch E.J., Mesrobian H.G., Begun F., Langenstroer P. (2006) Laparoscopic cryoablation of renal angiomyolipoma. J Urol 176: 1512–1516, discussion 1516 [DOI] [PubMed] [Google Scholar]
- Cadeddu J.A., Raman J.D. (2008) Renal tumor ablation is a function of patient selection and technique—not the ablation technology. Cancer 113: 2623–2626 [DOI] [PubMed] [Google Scholar]
- Campbell S.C., Krishnamurthi V., Chow G., Hale J., Myles J., Novick A.C. (1998) Renal cryosurgery: experimental evaluation of treatment parameters. Urology 52(1): 29–34 [DOI] [PubMed] [Google Scholar]
- Campbell S.C., Novick A.C., Herts B., Fischler D.F., Meyer J., Levin H.S., et al. (1997) Prospective evaluation of fine needle aspiration of small, solid renal masses: accuracy and morbidity. Urology 50: 25–29 [DOI] [PubMed] [Google Scholar]
- Cantwell C.P., Wah T.M., Gervais D.A., Eisner B.H., Arellano R., Uppot R.N., et al. (2008) Protecting the ureter during radiofrequency ablation of renal cell cancer: a pilot study of retrograde pyeloperfusion with cooled dextrose 5% in water. J Vasc Interv Radiol 19: 1034–1040 [DOI] [PubMed] [Google Scholar]
- Carey R.I., Leveillee R.J. (2007) First prize: direct real-time temperature monitoring for laparoscopic and CT-guided radiofrequency ablation of renal tumors between 3 and 5 cm. J Endourol 21: 807–813 [DOI] [PubMed] [Google Scholar]
- Chen E.A., Neeman Z., Lee F.T., Kam A., Wood B. (2006) Thermal protection with 5% dextrose solution blanket during radiofrequency ablation. Cardiovasc Intervent Radiol 29: 1093–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chosy S.G., Nakada S.Y., Lee F.T., Jr, Warner T.F. (1998) Monitoring renal cryosurgery: predictors of tissue necrosis in swine. J Urol 159: 1370–1374 [DOI] [PubMed] [Google Scholar]
- Clark T.W., Malkowicz B., Stavropoulos S.W., Sanchez R., Soulen M.C., Itkin M., et al. (2006) Radiofrequency ablation of small renal cell carcinomas using multitined expandable electrodes: preliminary experience. J Vasc Interv Radiol 17: 513–519 [DOI] [PubMed] [Google Scholar]
- Collyer W., Venkatesh R., Vanlangendonck R., Morissey K., Humphrey P., Yan Y., et al. (2004) Enhanced renal cryoablation with hilar clamping and intrarenal cooling in a porcine model. Urology 63(6): 1209–1212 [DOI] [PubMed] [Google Scholar]
- Crowley J.D., Shelton J., Iverson A.J., Burton M.P., Dalrymple N.C., Bishoff J.T. (2001) Laparoscopic and computed tomography-guided percutaneous radiofrequency ablation of renal tissue: acute and chronic effects in an animal model. Urology 57: 976–980 [DOI] [PubMed] [Google Scholar]
- Davol P.E., Fulmer B.R., Rukstalis D.B. (2006) Long-term results of cryoablation for renal cancer and complex renal masses. Urology 68(1 Suppl): 2–6 [DOI] [PubMed] [Google Scholar]
- de Arruda H.O., Goldman S., Andreoni C., Maia R.S., Szejnfeld J., Ortiz V. (2006) Renoduodenal fistula after renal tumor ablation with radiofrequency. Surg Laparosc Endosc Percutan Tech 16: 342–343 [DOI] [PubMed] [Google Scholar]
- Dechet C.B., Zincke H., Sebo T.J., King B.F., LeRoy A.J., Farrow G.M., et al. (2003) Prospective analysis of computerized tomography and needle biopsy with permanent sectioning to determine the nature of solid renal masses in adults. J Urol 169: 71–74 [DOI] [PubMed] [Google Scholar]
- Djavan B., Zlotta A.R., Susani M., Heinz G., Shariat S., Silverman D.E., et al. (1997) Transperineal radiofrequency interstitial tumor ablation of the prostate: correlation of magnetic resonance imaging with histopathologic examination. Urology 50: 986–992, discussion 992–983 [DOI] [PubMed] [Google Scholar]
- Farrell M.A., Charboneau W.J., DiMarco D.S., Chow G.K., Zincke H., Callstrom M.R., et al. (2003) Imaging-guided radiofrequency ablation of solid renal tumors. AJR Am J Roentgenol 180: 1509–1513 [DOI] [PubMed] [Google Scholar]
- Fazio L.M., Downey D., Nguan C.Y., Karnik V., Al-Omar M., Kwan K., et al. (2006) Intraoperative laparoscopic renal ultrasonography: use in advanced laparoscopic renal surgery. Urology 68: 723–727 [DOI] [PubMed] [Google Scholar]
- Fernandez Rosado E., Sanchez Rodriguez-Losada J., Chantada Abal V., Rois Soto J.M. (2006) [Nephron sparing renal surgery as salvage treatment after failure of radiofrequency thermal ablation in a patient with a single kidney and renal adenocarcinoma]. Arch Esp Urol 59: 577–582 [DOI] [PubMed] [Google Scholar]
- Gage A.A., Baust J. (1998) Mechanisms of tissue injury in cryosurgery. Cryobiology 37: 171–186 [DOI] [PubMed] [Google Scholar]
- Gervais D.A., Arellano R.S., Mueller P.R. (2005a) Percutaneous radiofrequency ablation of renal cell carcinoma. Eur Radiol 15: 960–967 [DOI] [PubMed] [Google Scholar]
- Gervais D.A., McGovern F.J., Arellano R.S., McDougal W.S., Mueller P.R. (2005b) Radiofrequency ablation of renal cell carcinoma: part 1, Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol 185: 64–71 [DOI] [PubMed] [Google Scholar]
- Gill I.S., Novick A.C. (1999) Renal cryosurgery. Urology 54: 215–219 [DOI] [PubMed] [Google Scholar]
- Gill I.S., Remer E.M., Hasan W.A., Strzempkowski B., Spaliviero M., Steinberg A.P., et al. (2005) Renal cryoablation: outcome at 3 years. J Urol 173: 1903–1907 [DOI] [PubMed] [Google Scholar]
- Ginat D.T., Saad W., Davies M., Walman D., Erturk E. (2009) Bowel displacement for CT-guided tumor radiofrequency ablation: techniques and anatomic considerations. J Endourol 23: 1259–1264 [DOI] [PubMed] [Google Scholar]
- Goldberg S.N., Gazelle G.S., Mueller P.R. (2000) Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol 174: 323–331 [DOI] [PubMed] [Google Scholar]
- Goldberg S.N., Grassi C.J., Cardella J.F., Charboneau J.W., Dodd G.D., III, Dupuy D.E., et al. (2005) Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology 235: 728–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Raman J.D., Leveillee R.J., Wingo M.S., Zeltser I.S., Lotan Y., et al. (2009) General anesthesia and contrast-enhanced computed tomography to optimize renal percutaneous radiofrequency ablation: multi-institutional intermediate-term results. J Endourol 23: 1099–1105 [DOI] [PubMed] [Google Scholar]
- Hegarty N.J., Gill I.S., Desai M.M., Remer E.M., O’Malley C.M., Kaouk J.H. (2006) Probe-ablative nephron-sparing surgery: cryoablation versus radiofrequency ablation. Urology 68(1 Suppl): 7–13 [DOI] [PubMed] [Google Scholar]
- Herts B.R., Baker M.E. (1995) The current role of percutaneous biopsy in the evaluation of renal masses. Semin Urol Oncol 13: 254–261 [PubMed] [Google Scholar]
- Hoffmann N.E., Bischof J.C. (2002) The cryobiology of cryosurgical injury. Urology 60(2 Suppl 1): 40–49 [DOI] [PubMed] [Google Scholar]
- Hoffmann R.T., Jakobs T.F., Kubisch C.H., Trumm C., Weber C., Siebels M., et al. (2010) Renal cell carcinoma in patients with a solitary kidney after nephrectomy treated with radiofrequency ablation: Mid term results. Eur J Radiol 73(3): 652–656, [Epub 31 Jan 2009] [DOI] [PubMed] [Google Scholar]
- Hollingsworth J.M., Miller D.C., Daignault S., Hollenbeck B.K. (2006) Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst 98: 1331–1334 [DOI] [PubMed] [Google Scholar]
- Hruby G.W., Fine J.K., Landman J. (2006) Ultrasound-guided percutaneous ablation of a renal mass in a renal allograft. Urology 68: 891, 895–896 [DOI] [PubMed] [Google Scholar]
- Hsu T.H., Fidler M.E., Gill I.S. (2000) Radiofrequency ablation of the kidney: acute and chronic histology in porcine model. Urology 56: 872–875 [DOI] [PubMed] [Google Scholar]
- Hui G.C., Tuncali K., Tatli S., Morrison P.R., Silverman S.G. (2008) Comparison of percutaneous and surgical approaches to renal tumor ablation: metaanalysis of effectiveness and complication rates. J Vasc Interv Radiol 19: 1311–1320 [DOI] [PubMed] [Google Scholar]
- Illing R.O., Kennedy J.E., Wu F., ter Haar G.R., Protheroe A.S., Friend P.J., et al. (2005) The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer 93: 890–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayson M., Sanders H. (1998) Increased incidence of serendipitously discovered renal cell carcinoma. Urology 51: 203–205 [DOI] [PubMed] [Google Scholar]
- Jemal A., Siegel R., Ward E., Murray T., Xu J., Thun M.J. (2007) Cancer statistics, 2007. CA Cancer J Clin 57: 43–66 [DOI] [PubMed] [Google Scholar]
- Kariya S., Tanigawa N., Kojima H., Komemushi A., Shomura Y., Ueno Y., et al. (2005) Radiofrequency ablation combined with CO2 injection for treatment of retroperitoneal tumor: protecting surrounding organs against thermal injury. AJR Am J Roentgenol 185: 890–893 [DOI] [PubMed] [Google Scholar]
- Klingler H.C. (2007a) [Energy ablative therapy of renal tumours]. Urologe A 46: 485–486, 488–490, 492–495 [DOI] [PubMed] [Google Scholar]
- Klingler H.C. (2007b) Kidney cancer: energy ablation. Curr Opin Urol 17: 322–326 [DOI] [PubMed] [Google Scholar]
- Klingler H.C., Marberger M., Mauermann J., Remzi M., Susani M. (2007) ‘Skipping’ is still a problem with radiofrequency ablation of small renal tumours. BJU Int 99: 998–1001 [DOI] [PubMed] [Google Scholar]
- Klingler H.C., Susani M., Seip R., Mauermann J., Sanghvi N., Marberger M.J. (2008) A novel approach to energy ablative therapy of small renal tumours: laparoscopic high-intensity focused ultrasound. Eur Urol 53: 810–816, discussion 817–818 [DOI] [PubMed] [Google Scholar]
- Kunkle D.A., Uzzo R.G. (2008) Cryoablation or radiofrequency ablation of the small renal mass: a meta-analysis. Cancer 113: 2671–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeseke P.F., Sampson L.A., Winter T.C., III, Lee F.T., Jr (2005) Use of dextrose 5% in water instead of saline to protect against inadvertent radiofrequency injuries. AJR Am J Roentgenol 184: 1026–1027 [DOI] [PubMed] [Google Scholar]
- Lane B.R., Babineau D., Kattan M.W., Novick A.C., Gill I.S., Zhou M., et al. (2007) A preoperative prognostic nomogram for solid enhancing renal tumors 7 cm or less amenable to partial nephrectomy. J Urol 178: 429–434 [DOI] [PubMed] [Google Scholar]
- Lane B.R., Moinzadeh A., Kaouk J.H. (2005) Acute obstructive renal failure after laparoscopic cryoablation of multiple renal tumors in a solitary kidney. Urology 65: 593–593 [DOI] [PubMed] [Google Scholar]
- Lawatsch E.J., Langenstroer P., Byrd G.F., See W.A., Quiroz F.A., Begun F.P. (2006) Intermediate results of laparoscopic cryoablation in 59 patients at the Medical College of Wisconsin. J Urol 175: 1225–1229, discussion 1229 [DOI] [PubMed] [Google Scholar]
- Lebret T., Poulain J.E., Molinie V., Herve J.M., Denoux Y., Guth A., et al. (2007) Percutaneous core biopsy for renal masses: indications, accuracy and results. J Urol 178: 1184–1188, discussion 1188 [DOI] [PubMed] [Google Scholar]
- Lee D.I., McGinnis D.E., Feld R., Strup S.E. (2003) Retroperitoneal laparoscopic cryoablation of small renal tumors: intermediate results. Urology 61: 83–88 [DOI] [PubMed] [Google Scholar]
- Lee S.J., Choyke L.T., Locklin J.K., Wood B.J. (2006) Use of hydrodissection to prevent nerve and muscular damage during radiofrequency ablation of kidney tumors. J Vasc Interv Radiol 17: 1967–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman D.S., Hruby G.W., Phillips C.K., McKiernan J.M., Benson M.C., Landman J. (2008) First Prize (tie): Laparoscopic renal cryoablation: efficacy and complications for larger renal masses. J Endourol 22: 1123–1127 [DOI] [PubMed] [Google Scholar]
- Lele, P. (1977) Thresholds and mechanisms of ultrasonic damage to organized animal tissues. In: Proceedings of the Symposium on Biological Effects and Characterizations of Ultrasound Sources, Rockville, MD, Vol. 78-8048.
- Leveillee R.J., Hoey M.F. (2003) Radiofrequency interstitial tissue ablation: wet electrode. J Endourol 17: 563–577 [DOI] [PubMed] [Google Scholar]
- Link R.E., Permpongkosol S., Gupta A., Jarrett T.W., Solomon S.B., Kavoussi L.R. (2006) Cost analysis of open, laparoscopic, and percutaneous treatment options for nephron-sparing surgery. J Endourol 20: 782–789 [DOI] [PubMed] [Google Scholar]
- Lipworth L., Tarone R.E., McLaughlin J.K. (2006) The epidemiology of renal cell carcinoma. J Urol 176: 2353–2358 [DOI] [PubMed] [Google Scholar]
- Madersbacher S., Pedevilla M., Vingers L., Susani M., Marberger M. (1995) Effect of high-intensity focused ultrasound on human prostate cancer in vivo. Cancer Res 55: 3346–3351 [PubMed] [Google Scholar]
- Mahnken A.H., Rohde D., Brkovic D., Gunther R.W., Tacke J.A. (2005) Percutaneous radiofrequency ablation of renal cell carcinoma: preliminary results. Acta Radiol 46: 208–214 [DOI] [PubMed] [Google Scholar]
- Marberger M., Schatzl G., Cranston D., Kennedy J.E. (2005) Extracorporeal ablation of renal tumours with high-intensity focused ultrasound. BJU Int 95(Suppl 2): 52–55 [DOI] [PubMed] [Google Scholar]
- Mathew A., Devesa S.S., Fraumeni J.F., Jr, Chow W.H. (2002) Global increases in kidney cancer incidence, 1973–1992. Eur J Cancer Prev 11: 171–178 [DOI] [PubMed] [Google Scholar]
- Matin S.F., Ahrar K. (2008) Nephron-sparing probe ablative therapy: long-term outcomes. Curr Opin Urol 18: 150–156 [DOI] [PubMed] [Google Scholar]
- Matsumoto E.D., Watumull L., Johnson D.B., Ogan K., Taylor G.D., Josephs S., et al. (2004) The radiographic evolution of radio frequency ablated renal tumors. J Urol 172: 45–48 [DOI] [PubMed] [Google Scholar]
- Mazur P. (1970) Cryobiology: the freezing of biological systems. Science 168: 939–949 [DOI] [PubMed] [Google Scholar]
- McDougal W.S., Gervais D.A., McGovern F.J., Mueller P.R. (2005) Long-term followup of patients with renal cell carcinoma treated with radio frequency ablation with curative intent. J Urol 174: 61–63 [DOI] [PubMed] [Google Scholar]
- McGahan J.P., Brock J.M., Tesluk H., Gu W.Z., Schneider P., Browning P.D. (1992) Hepatic ablation with use of radio-frequency electrocautery in the animal model. J Vasc Interv Radiol 3: 291–297 [DOI] [PubMed] [Google Scholar]
- Miki K., Shimomura T., Yamada H., Kishimoto K., Ohishi Y., Harada J., et al. (2006) Percutaneous cryoablation of renal cell carcinoma guided by horizontal open magnetic resonance imaging. Int J Urol 13: 880–884 [DOI] [PubMed] [Google Scholar]
- Moinzadeh A., Gill I.S., Finelli A., Kaouk J., Desai M. (2006) Laparoscopic partial nephrectomy: 3-year followup. J Urol 175: 459–462 [DOI] [PubMed] [Google Scholar]
- Mondshine R.T., Owens S., Mondschein J.I., Cizman B., Stavropoulos S.W., Clark T.W. (2008) Combination embolization and radiofrequency ablation therapy for renal cell carcinoma in the setting of coexisting arterial disease. J Vasc Interv Radiol 19: 616–620 [DOI] [PubMed] [Google Scholar]
- Murat F.J., Poissonnier L., Pasticier G., Gelet A. (2007) High-intensity focused ultrasound (HIFU) for prostate cancer. Cancer Control 14: 244–249 [DOI] [PubMed] [Google Scholar]
- National Cancer Institute (2009) Surveillance, Epidemiology and End Results (SEER) Cancer Facts & Figures – 2009, American Cancer Society (ACS), Atlanta, Georgia, 2009: http://seer.cancer.gov/csr/1975_2006/results_single/sect_01_table.01.pdf.
- Nosnik I.P., Mouraviev V., Nelson R., Polascik T.J. (2006) Multiple nephron-sparing procedures in solitary kidney with recurrent, metachronous, nonfamilial renal cell carcinoma. Urology 68: 1341–1343 [DOI] [PubMed] [Google Scholar]
- Novick, A.C., Campbell, S.C., Belldegrun, A., Blute, M.L., Chow, G.K., Derweesh, I.H. et al. (2009) Guideline for management of the clinical stage 1 renal mass: http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines/main-reports/renalmass09.pdf. [DOI] [PubMed]
- O’Malley R.L., Berger A.D., Kanofsky J.A., Phillips C.K., Stifelman M., Taneja S.S. (2007) A matched-cohort comparison of laparoscopic cryoablation and laparoscopic partial nephrectomy for treating renal masses. BJU Int 99: 395–398 [DOI] [PubMed] [Google Scholar]
- Oosterhof G.O., Cornel E.B., Smits G.A., Debruyne F.M., Schalken J.A. (1997) Influence of high-intensity focused ultrasound on the development of metastases. Eur Urol 32: 91–95 [PubMed] [Google Scholar]
- Pandharipande P.V., Gervais D.A., Mueller P.R., Hur C., Gazelle G.S. (2008) Radiofrequency ablation versus nephron-sparing surgery for small unilateral renal cell carcinoma: cost-effectiveness analysis. Radiology 248: 169–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B.K., Kim C.K. (2008) Using an electrode as a lever to increase the distance between renal cell carcinoma and bowel during CT-guided radiofrequency ablation. Eur Radiol 18: 743–746 [DOI] [PubMed] [Google Scholar]
- Park B.K., Kim C.K. (2009) Complications of image-guided radiofrequency ablation of renal cell carcinoma: causes, imaging features and prevention methods. Eur Radiol 19: 2180–2190 [DOI] [PubMed] [Google Scholar]
- Park S., Anderson J.K., Matsumoto E.D., Lotan Y., Josephs S., Cadeddu J.A. (2006) Radiofrequency ablation of renal tumors: intermediate-term results. J Endourol 20: 569–573 [DOI] [PubMed] [Google Scholar]
- Park S., Cadeddu J.A. (2007) Outcomes of radiofrequency ablation for kidney cancer. Cancer Control 14: 205–210 [DOI] [PubMed] [Google Scholar]
- Permpongkosol S., Link R.E., Kavoussi L.R., Solomon S.B. (2006) Percutaneous computerized tomography guided cryoablation for localized renal cell carcinoma: factors influencing success. J Urol 176: 1963–1968, discussion 1968 [DOI] [PubMed] [Google Scholar]
- Permpongkosol S., Link R.E., Kavoussi L.R., Solomon S.B. (2008) Temperature measurements of the low-attenuation radiographic ice ball during CT-guided renal cryoablation. Cardiovasc Intervent Radiol 31: 116–121 [DOI] [PubMed] [Google Scholar]
- doi: 10.1016/j.eururo.2008.09.053. Remzi, M. and Marberger, M. (2009) Renal tumor biopsies for evaluation of small renal tumors: why, in whom, and how? Eur Urol 55(2): 359–367. [DOI] [PubMed] [Google Scholar]
- Remzi M., Ozsoy M., Klingler H.C., Susani M., Waldert M., Seitz C., et al. (2006) Are small renal tumors harmless? Analysis of histopathological features according to tumors 4 cm or less in diameter. J Urol 176: 896–899 [DOI] [PubMed] [Google Scholar]
- Roarke M.C., Collins J.M., Nguyen B.D. (2006) Indolent enterococcal abscess mimicking recurrent renal cell carcinoma on MR imaging and PET/CT after radiofrequency ablation. J Vasc Interv Radiol 17: 1851–1854 [DOI] [PubMed] [Google Scholar]
- Schmidbauer J., Remzi M., Memarsadeghi M., Haitel A., Klingler H.C., Katzenbeisser D., et al. (2008) Diagnostic accuracy of computed tomography-guided percutaneous biopsy of renal masses. Eur Urol 53: 1003–1011 [DOI] [PubMed] [Google Scholar]
- Schultze D., Morris C.S., Bhave A.D., Worgan B.A., Najarian K.E. (2003) Radiofrequency ablation of renal transitional cell carcinoma with protective cold saline infusion. J Vasc Interv Radiol 14: 489–492 [DOI] [PubMed] [Google Scholar]
- Schwartz B.F., Rewcastle J.C., Powell T., Whelan C., Manny T., Jr, Vestal J.C. (2006) Cryoablation of small peripheral renal masses: a retrospective analysis. Urology 68(1 Suppl): 14–18 [DOI] [PubMed] [Google Scholar]
- Simon C.J., Dupuy D.E., Mayo-Smith W.W. (2005) Microwave ablation: principles and applications. Radiographics 25(Suppl 1): S69–S83 [DOI] [PubMed] [Google Scholar]
- Stein R.J., Kaouk J.H. (2007) Renal cryotherapy: a detailed review including a 5-year follow-up. BJU Int 99: 1265–1270 [DOI] [PubMed] [Google Scholar]
- Stephenson R.A., King D.K., Rohr L.R. (1996) Renal cryoablation in a canine model. Urology 47: 772–776 [DOI] [PubMed] [Google Scholar]
- Stern J.M., Svatek R., Park S., Hermann M., Lotan Y., Sagalowsky A.I., et al. (2007) Intermediate comparison of partial nephrectomy and radiofrequency ablation for clinical T1a renal tumours. BJU Int 100: 287–290 [DOI] [PubMed] [Google Scholar]
- Sterrett S.P., Nakada S.Y., Wingo M.S., Williams S.K., Leveillee R.J. (2008) Renal thermal ablative therapy. Urol Clin North Am 35: 397–414, viii [DOI] [PubMed] [Google Scholar]
- Stone M.J., Venkatesan A.M., Locklin J., Pinto P., Linehan M., Wood B.J. (2007) Radiofrequency ablation of renal tumors. Tech Vasc Interv Radiol 10: 132–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susani M., Madersbacher S., Kratzik C., Vingers L., Marberger M. (1993) Morphology of tissue destruction induced by focused ultrasound. Eur Urol 23(Suppl 1): 34–38 [DOI] [PubMed] [Google Scholar]
- Tacke J., Mahnken A., Roggan A., Gunther R.W. (2004) Multipolar radiofrequency ablation: first clinical results. Rofo 176: 324–329 [DOI] [PubMed] [Google Scholar]
- Ukimura O., Mitterberger M., Okihara K., Miki T., Pinggera G.M., Neururer R., et al. (2008) Real-time virtual ultrasonographic radiofrequency ablation of renal cell carcinoma. BJU Int 101: 707–711 [DOI] [PubMed] [Google Scholar]
- Uribe P.S., Costabile R.A., Peterson A.C. (2006) Progression of renal tumors after laparoscopic radiofrequency ablation. Urology 68: 968–971 [DOI] [PubMed] [Google Scholar]
- Varkarakis I.M., Allaf M.E., Inagaki T., Bhayani S.B., Chan D.Y., Su L.M., et al. (2005) Percutaneous radio frequency ablation of renal masses: results at a 2-year mean followup. J Urol 174: 456–460, discussion 460 [DOI] [PubMed] [Google Scholar]
- Veltri A., De Fazio G., Malfitana V., Isolato G., Fontana D., Tizzani A., et al. (2004) Percutaneous US-guided RF thermal ablation for malignant renal tumors: preliminary results in 13 patients. Eur Radiol 14: 2303–2310 [DOI] [PubMed] [Google Scholar]
- Volpe A., Kachura J.R., Geddie W.R., Evans A.J., Gharajeh A., Saravanan A., et al. (2007) Techniques, safety and accuracy of sampling of renal tumors by fine needle aspiration and core biopsy. J Urol 178: 379–386 [DOI] [PubMed] [Google Scholar]
- Volpe A., Panzarella T., Rendon R.A., Haider M.A., Kondylis F.I., Jewett M.A. (2004) The natural history of incidentally detected small renal masses. Cancer 100: 738–745 [DOI] [PubMed] [Google Scholar]
- Weight C.J., Kaouk J.H., Hegarty N.J., Remer E.M., O’Malley C.M., Lane B.R., et al. (2008) Correlation of radiographic imaging and histopathology following cryoablation and radio frequency ablation for renal tumors. J Urol 179: 1277–1281, discussion 1281–1273 [DOI] [PubMed] [Google Scholar]
- Yamakado K., Nakatsuka A., Kobayashi S., Akeboshi M., Takaki H., Kariya Z., et al. (2006) Radiofrequency ablation combined with renal arterial embolization for the treatment of unresectable renal cell carcinoma larger than 3.5 cm: initial experience. Cardiovasc Intervent Radiol 29: 389–394 [DOI] [PubMed] [Google Scholar]
- Zagoria R.J., Traver M.A., Werle D.M., Perini M., Hayasaka S., Clark P.E. (2007) Oncologic efficacy of CT-guided percutaneous radiofrequency ablation of renal cell carcinomas. AJR Am J Roentgenol 189: 429–436 [DOI] [PubMed] [Google Scholar]