Abstract
Concerning the ureterovesical junction – the region most important for the anti-reflux mechanism – there is still a lot of misunderstanding and misinterpretation with regard to normal fetal development. Data are scarce on possible causes of primary vesicoureteral reflux and on involved mechanisms of the so-called maturation process of refluxing ureteral endings. The ratio of the intravesical ureteral length to the ureteral diameter is obviously lower than assumed so far, as clearly revealed by some studies. Therefore it can be doubted that the length and course of the intravesical ureter is of sole importance in the prevention of reflux. Additionally refluxing intravesical ureteral endings present with dysplasia, atrophy, and architectural derangement of smooth muscle fibers. Besides, a pathologically increased matrix remodeling combined with deprivation of the intramural nerve supply has been confirmed. Consequently, symmetrical narrowing of the very distal ureteral smooth muscle coat creating the active valve mechanism to defend reflux is not achievable. It is apparent that primary congenital vesicoureteral reflux seems to be the result of an abnormality within the ureterovesical junction with an insufficient muscular wrap. Nature is believed to establish much more sophisticated mechanisms than the so-called passive anti-reflux mechanism. Remodeling processes within the ureterovesical junction of refluxing ureteral endings support that maturation itself is nothing else than wound or defect healing and not a restitution of a morphological normal ureterovesical junction. Lacking the nerve supply a restoration of any muscular structure can not be achieved.
Keywords: human fetal development, ureterovesical junction, vesicoureteral reflux, children, extracellular matrix, nerve supply
Introduction
It is established that the ureter fuses with the urogenital sinus by day 37 of fetal development [Keith, 1948]. The ureteric bud evaginates out of the metanephric (Wolffian) duct. On the other hand there is very little data and understanding about the subsequent caudal growth although this region is most important and crucial for the anti-reflux mechanism. Many existing morphological concepts of the configuration of the ureterovesical junction explaining the mechanisms preventing reflux into the ureter and kidney are disappointing. The ureterovesical junction represents the bounding part between the low pressure in the upper urinary tract and the highly variable demands of the lower urinary tract. The mechanism of ureteric motility as well as comprehensive physiology of the normal valve mechanism of the ureterovesical junction has been examined in some detail. Despite this the clinical and anatomical literature does not offer a compromise on the topography and the growth of the intravesical ureter in fetuses.
Mackie and Stephens [Mackie and Stephens, 1975] claimed that in congenital anomalies of the kidney and the urinary tract the original ureteric budding arises at an irregular position of the metanephric duct. The ending of the ureter fuses with the urogenital sinus by day 37, the subsequent caudal growth remains vague, mainly the distention and intravesical submucosal enlargement which is supposed most responsible for the anti-reflux mechanism. Relocation of the ureteral orifice in the bladder is described in a cranial and lateral course with the concluding position of the orifice at danger for reflux. Determining aspects in passive valve function are the diagonal course and the length of the submucosal section of the ureter; this intravesical route of the ureter is considered to be of functional significance in preserving the ureterovesical closure preventing reflux [King et al. 1974].
The active valve function is thought to consist of an ‘ureterovesical sphincter’ which contracts in response to vesical contraction and settles down following contraction of the external urethral sphincter [Shafik, 1997]. Since a histological definable sphincter could not be identified, a ‘physiologic sphincter,’ like that described in the rectosigmoid junction, is postulated [Shafik, 1996].
While King and Stephens [King and Stephens, 1977] considered the ureterovesical valve to be activated by the intrinsic muscle of the ureter, Tanagho [Tanagho et al. 1965] attributed this task to contraction of the muscle of the superficial trigone of the bladder. This hypothesis of trigonal attachment of the ureteric function was weakened by Hannan and Stephens [Hannan and Stephens, 1973], who excised the canine trigone except for the mucosal layer and found reflux in only one specimen of their series.
Regardless of which of these models of valve function is truthful, the effectiveness of both depends on the functioning ureteric muscle. Accordingly, it seems clear that the requirement of a patent ureterovesical junction is a timely and full development of the smooth muscle coat of the intravesical ureter. Minor notice has been paid on a potential congenital muscular structural insufficiency of the very distal refluxing ureter with thorough consideration to general morphology, smooth muscle architecture, chronic inflammatory markers and the distribution of the collagen composition, respectively. As well only limited data on extracellular matrix (ECM) changes, the remodeling process and altered innervation of the intravesical part of the ureter are available. The ECM is a biologically active and dynamic composition of structural, adhesive and counteradhesive fibrous proteins embedded in a hydrated ground substance of glycosaminoglycans and proteoglycans. Smooth muscle cells participate in ECM transformation through localized production of various proteinases and their inhibitors. One can hypothesize that these ureteral smooth muscle cells participate in repair and resynthesis of structural matrix proteins influencing the proteolytic activity of other cell types. They might therefore have an impact on the progression of potential maturation processes in refluxing ureters [Ferri et al. 2003].
Based on morphological data confirming muscle deficiency in the ureterovesical junction (UVJ) [Oswald et al. 2003], alterations of innervation in association with matrix remodeling have been revealed. Mutilation of smooth muscle function and changes of the ECM microenvironment can compromise the nerve supply in this region.
The key purposes of these studies are to portray the chronology of development of these tissues, in particular to explore the growth curves of the ureterovesical junction and of the mesenchymal and muscular distal and intravesical wall, the meticulous description of the morphology of refluxing ureteral endings, and in conclusion the analysis of extracellular matrix remodeling processes in combination with the nerve supply in these specimens to gain insight into potential maturation procedures.
Developmental studies
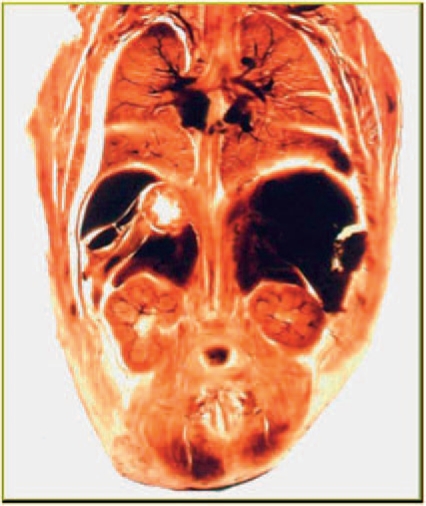
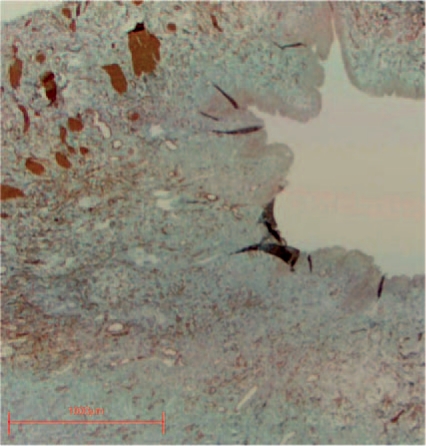
Fetal development studies based on plastinated sections of whole pelvises, allow the study of the sectional anatomy of all pelvic structures in a condition closely resembling the in vivo status. Architecture and cellular integrity are preserved, cytomorphologic alterations with artificial tissue shrinkage are avoided by using plastination histology and a special fixation protocol [Oswald et al. 2003]. Because plastinated sections are transparent, it is possible to recognize the exact width and borders of the mesenchymal and muscle structure of the ureter wall allowing precise sequential measurements (Figure 1).
Figure 1.
An example of a plastination specimen clearly revealing the advantages of architectural integrity.
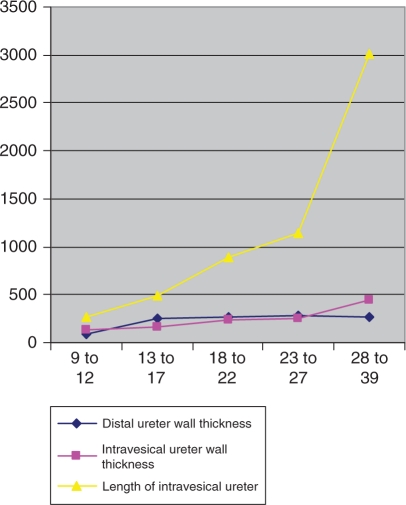
Baskin et al. [Baskin et al. 2001] confirmed that mesenchymal-epithelial interactions are essential for the growth of bladder smooth muscle. These changes in the pattern of the ureter point to a progressive change of the mesenchyme around the epithelial tube to smooth muscle. Other interpretations [Oswald et al. 2003] demonstrated a continuous linear increase of the mesenchymal ureter wall to smooth muscle in human fetal ureters. Statistical analysis showed an almost linear growth profile. The distal and intravesical ureter thickness increased with 8.98 μm and 11.25 μm per week, respectively and the intravesical tunnel grows with 97.4 μm per week [Oswald et al. 2003] (Figure 2).
Figure 2.
Dimensions of distal ureter wall thickness, intravesical ureter wall thickness and length of intravesical ureter according to age groups.
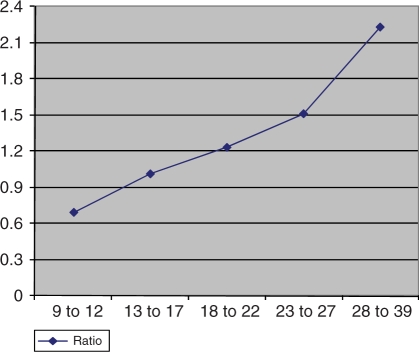
The timing of smooth muscle differentiation in the distal ureter is not known. During a first or mesenchymal period (between the eighth and twelfth gestational weeks), the wall of the pre- and intravesical metanephric duct consists of undifferentiated mesenchyme. Subsequently a second period (between the thirteenth and twentieth gestational weeks) can be differentiated, in which condensed mesenchyme predominates. Paquin provided standard values for the length of the submucosal segment of the ureter (orifice to hiatus into the bladder) and in infants aged 1–3 years he found a 7 mm long intravesical ureter [Paquin, 1959]. Hutch estimated intravesical ureter lengths of 5 mm in neonates [Hutch, 1961]. The macroscopic method using blunt-nosed cylindrical probes or ureteral catheters measuring the ureterovesical junction is a disadvantage of previous measurements. The length of the intravesical ureter in gestational weeks 20–30 as described by Cussen [Cussen, 1967], is 2–5 mm (mean 3 mm), with a 1–3 mm submucosal and a 1–2 mm intramuscular segment. During gestational weeks 30–40, the intravesical ureter has a mean length of 4 mm. A shorter intravesical human fetal ureter with a length of 271.7 μm (±191.01 μm) at the ninth to twelfth weeks and 3017.2 μm (±388.9 μm) at birth was ascertained by another group [Oswald et al. 2003] (Figure 2). The tunnel length relative to its diameter is thought to be important in the prevention of reflux by closing the junction’s valvular mechanism. Children aged 1–3 years showed an intravesical ureteral length of 7000 μm by an intravesical ureteral diameter of 1400 μm. The ratio of tunnel length to ureteral diameter at the ureterovesical junction was found to average 5 : 1 in Paquin’s study [Paquin, 1959]. In children with reflux a pathological length to diameter ratio of 1.4 : 1 was reported [Tanagho et al. 1969]. The mean values of the intravesical length in another series of nonrefluxing ureters revealed somewhat lower values (3017.2 μm and ±388.9 μm) that implies a proportion of the intravesical ureteral length to intravesical ureteral diameter of 2.23 : 1 [Oswald et al. 2003] (Figure 3). In contrast to the newborn, in 11- and 20-week old fetuses the ratio of the intravesical ureteral length to the ureteral diameter decreased to 0.69 : 1 and 1.23 : 1, respectively. This might be one reason for the higher prevalence of vesicoureteral reflux in newborns and fetuses. Even so, the standard ratio of 5 : 1 for reimplantation surgery seems sufficient albeit this relation does not mimic nature regarding the theory of the passive antireflux mechanism. One can speculate that a lower ratio may be sufficient to obviate reflux [Shokeir, 2001]. Other factors of the anti-reflux mechanism such as the dynamic valve mechanism, the intravesical pressure or the connection with the trigone and posterior urethra must be taken into account in re-evaluating the function of the ureterovesical junction. This might elucidate the fact that neonatal reflux is a special entity with a much higher predisposition to vanish spontaneously than the reflux found in older children [Tamminen-Mobius et al. 1992; Anderson and Rickwood, 1991; Edwards et al. 1977].
Figure 3.
Ratio of tunnel length to ureteral diameter according to gestational age.
Refluxing uretal endings and their morphological changes
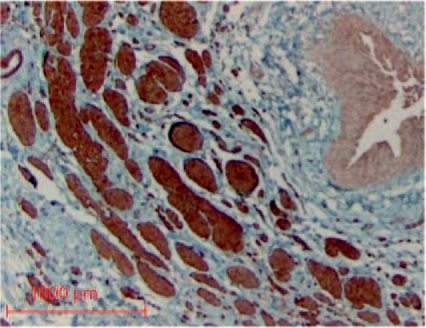
Spontaneous reflux resolution in children is explained with the growth of the bladder for the elongation of the submucosal tunnel will take place [Stephens and Lenaghan, 1962]. On the other hand, Cussen [Cussen, 1967] has shown that growth of the intravesical ureter takes place concurrently in relation to the surrounding tissue components. Correlating length, muscle mass and muscle population of the intravesical ureter with height, weight, body surface area and age he found a definitive development in all of these elements with time. The morphological and functional integrity of the ureterotrigonal unit providing the active muscular control of the ostial lock mechanism seems as important as the so-called passive anti-reflux mechanism most notably since we demonstrated that the ratio of the intravesical ureteral length to the ureteral diameter is obviously lower than assumed so far [Oswald et al. 2003]. Refluxing ureteral endings clearly demonstrated a reduction of smooth muscle alpha-actin, myosin and desmin expression [Oswald et al. 2003]. This very distal part demonstrated a high level of muscle atrophy and degeneration as well as a disordered, disrupted and scattered arrangement of fibers associated with changes in ECM collagen composition (). The proportion of muscle to collagen decreased consequently from normal 1 : 0.3 to 1 : 3. Also in lower reflux grades disintegrated or lacking smooth muscle filaments either at the periphery or at the interior part of the muscular wall were noted. The findings of an enhancement in type I collagen and a lower amount of type III collagen could point to a higher collagen synthesis by interstitial fibroblasts typically for later stages of fibrotic lesions [Bornstein and Sage, 1980].
Figure 4.
Alpha-actin staining of a normal ostial ureter with predominantly inner longitudinal and slight outer circular layers of smooth muscle.
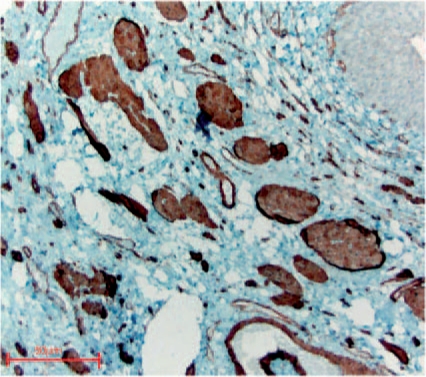
Figure 5.
Massive derangement of refluxive intravesical ureter showing severe absent smooth muscular wall (alpha-actin SM staining).
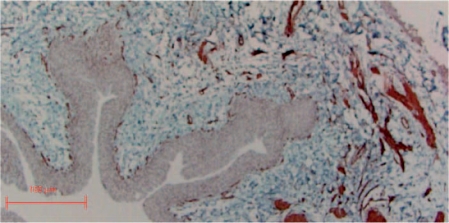
Figure 6.
Smooth muscle fascicle showing replacement of detoriated smooth muscle by connective tissue in perimysial and endomysial regions (myosin SM staining).
Figure 7.
Immunohistochemical staining for desmin demonstrating massive derangement of smooth muscle wall with disintegration and dissolving muscles fascicles.
Interestingly, no significant association between the degree of ureteral muscle wall damage and the clinical reflux grade was observed, whereas some degree of damage can be found in all cases of reflux [Oswald et al. 2003]. Extra research is needed to resolve whether the degree of muscle wall impairment can alter clinical outcomes concerning the so-called spontaneous maturation of the ureterovesical junction in childhood.
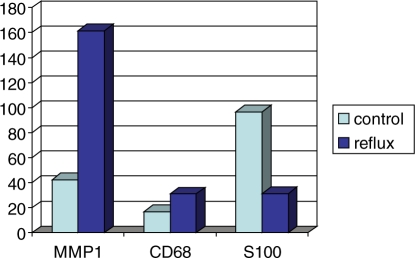
Studies also revealed that MMP1 production was approximately four fold and the CD-68+ macrophages twice as much increased in the very distal part of refluxing ureteral specimens [Oswald et al. 2004], both in the ECM and in the fascicles of the longitudinal muscle’s coat, compared to controls (Figure 8). Cells undergoing programmed cell death are cleared rapidly in vivo by phagocytes without inducing inflammation [Henson et al. 2001]. Due to the lack of any inflammatory cells, which are present in necrosis, apoptotic cell removal is an acceptable hypothesis. The increased activity of CD68+ cells accounts for the efficient deletion of damaged cells, often considered a counterpoint to apoptosis. As the patients in that study presented without any signs of urinary tract infections at the time of surgery, an inherent anatomical or developmental difference from children without reflux must be discussed. On the other hand there are animal models available using congenital non-infected refluxing ureterovesical junctions [Mendelsohn, 2009]. Using these strategies might offer the possibility to help to clearly distinguish whether or not some of the findings might be influenced by urinary tract infections [Murawski et al. 2007]. On the other hand these studies have the disadvantages of all animal models when you want to transfer and interpret the results for human beings. Therefore in this paper we only focus on available data in studies human patients. It is evident that in cases of muscle injury or muscle dysplasia CD68+ cells infiltrate the damaged tissue and scavenge cellular remnants as well as replace muscle bundles with connective tissue [Best and Hunter, 2000]. The capability of these macrophages to detect tiny differences in the specific combination, concentration and distribution of matrix components suggests that perturbations of the matrix homeostasis may guide remodeling of the ureteral ending. In this ECM environment adhesion of macrophages, modification or denaturation of the major extracellular matrix component (type I and III collagen) occurs [Gowen et al. 2000]. Once in the underlying tissues, monocytes differentiate into macrophages, which are essential for proper immune function and ECM transmutation and production of collagens [Duerksen-Hughes and Gooding, 1993]. Above all in collagen rich regions an increased secretion of matrix metalloproteinase 1 (MMP1) cleaving collagen types I and III is noted. This simultaneous synthesis and secretion of matrix proteins and a degrading enzyme by the same cell indicates a switch from a collagen-producing to a collagen-degrading phenotype [Weitkamp et al. 1999]. Matrix metalloproteinases have a number of physiologically important functions in wound healing processes and angiogenesis. The increased synthesis of MMP1 is stimulated by various cytokines such as transforming growth factor-β, released from macrophages [Schuppan et al. 1994]. Increased ECM turnover has an influence on the neuronal network within the ureter wall. It has been shown that some MMPs are neurotoxic by degrading extracellular matrix proteins like collagen type I. They are normally able to protect cultured neurons from cytotoxic cell death. Cytotoxicity by MMP1 to the ureteral neurons at the UVJ is probable; discrimination to a congenital absence of neuronal nerves is not conceivable at present [Vos et al. 2000]. A significant diminution of S-100 positive neuronal cells as a result of potential neuronal harm was observed suggesting that defective innervation may have an additional impact on the pathogenesis of the impaired active anti-reflux mechanism [Oswald et al. 2004] (Figure 8). Based upon this significant reduction in S-100 positive myelinated nerves, an intact innervation may be one of the prerequisites for normal morphogenesis of the intravesical smooth muscle wrap [Oswald et al. 2004]. No correlation between the degree of MMP1 expression, accumulation of CD68+ macrophages as well as degradation of the neuronal supply and the clinical reflux grade was observed. However at the moment it is not clear whether a congenital absence of an intact innervated muscle wall or an additional deterioration in the framework of tissue remodeling as a result of restoring damaged tissue combined with increased MMP activity is the main cause of the significant reduction within the neuronal network at the UVJ. In the maturation process of refluxing ureters it is postulated that somatic growth of the child causes a morphological development of the pathologic UVJ resulting in a restoration of the anti-reflux valve mechanism [Weiss et al. 1992]. While the ratio of the intravesical ureteral length-to-ureteral diameter is obviously lower than assumed previously [Oswald et al. 2003] the morphological and functional integrity of the intravesical longitudinal muscle coat, which provides the active muscular control of the ostial lock mechanism, seems to be a crucial factor to prevent reflux [Oswald et al. 2003]. The mechanisms in charge of this rearrangement of the UVJ have never been elucidated. One can assume that the increased concentrations of MMP1+ cells as well as CD68+ macrophages in the ECM of refluxing ureteral endings represent a causal correlation of these MMPs to tissue degradation and defect healing. Replacing the smooth muscle fibers by collagen types I and III on the basis of an increased ECM turnover can lead to scarring and fibrosis [Zhu et al. 2001]. The structural changes observed in refluxing ureteral endings indicate a tissue response that culminates in cell and matrix turnover as well as in matrix remodeling and maturation, which in conclusion may cause tissue shrinkage equivalent to wound contraction. One can consider this ureter wall shrinkage results in an adequate ureteral diameter to ureteral length relation causing a sufficient passive anti-reflux mechanism.
Figure 8.
Illustration of MMP1, CD68 and S100 production in refluxing and normal ureteral endings including statistical significance at p < 0.05 (Mann-Whitney U test).
Conclusion
Concerning the normal fetal development of the UVJ, there is still a lot of misunderstanding and misinterpretation; the region most important for the anti-reflux mechanism. No more than a little information exists on possible causes of primary vesicoureteral reflux and implicated mechanisms of the so-called maturation course of refluxing ureteral endings. Using age-continuous series, significant positive linear relationships between gestation week, distal and intravesical ureter wall thickness of the mesenchym- and smooth muscle growth and the length of the intravesical ureter in 9–39-week old fetuses and newborns is obvious. The intravesical tunnel does not grow before the late fetal phase and the relative amount of the intravesical ureteral length to the ureteral diameter in newborns is apparently lower than assumed so far. It gives the impression that in nature the passive anti-reflux mechanism may not be as essential as previously thought.
Primary vesicoureteral reflux is the outcome of a congenital abnormality of the ureterovesical junction. A paucity of the longitudinal muscle coat leading to dysfunction of the ostial valve mechanism may participate in the active anti-reflux mechanism. The muscular wall of the very distal ureter is diminished. Incompetent muscle fiber architecture pilots to a dysfunction of the dynamic valve mechanism. The atrophic and dysplastic smooth muscle cells packed in a bulky basal lamina are increasingly separated by expanded extracellular matrix. These morphological transformations in the ureteral wall often results in deformation of the smooth muscle wall arrangement. Refluxing ureters are characterized by reduced and degraded muscle fascicles of the distal ureteral wall leading to an inadequate active valve mechanism, consequently ample contraction of the muscular conduit in order to secure the ostium and preventing vesicoureteral reflux is doubtful.
Furthermore MMP1 expression as well as CD68+ macrophages are increased notably in refluxing ureters compared to healthy controls. In contrast, S-100-positive nerve fibers are significantly reduced illustrating deficiency of the intramural nerve supply. The enhanced activity of CD68+ cells together with an increased synthesis of MMP1 is indicative of pathologically increased ECM remodeling. The increased collagenolytic activity results in the manifestation of collagen cleavages, which may be implied in the triggering of mesenchymal proliferation. One can theorize that the spontaneous maturation of the primary refluxing ureteral orifice appears to be associated with the adjustments of the ECM. Further research has to focus on smooth muscle cell-matrix interactions, matrix degradation and turnover mediated by cell proteases such as the MMP family in order to elucidate the mechanism of reflux maturation [Radmayr et al. 2005].
Conflict of interest statement
None declared.
References
- Anderson P., Rickwood A. (1991) Features of primary vesicoureteric reflux detected by prenatal sonography. Br J Urol 67: 267–271 [DOI] [PubMed] [Google Scholar]
- Baskin L., DiSandro M., Li Y., Li W., Hayward S., Cunha G. (2001) Mesenchymal-epithelial interactions in bladder smooth muscle development: effects of the local tissue environment. J Urol 165: 1283–1288 [PubMed] [Google Scholar]
- Best T.M., Hunter K.D. (2000) Muscle injury and repair. Phys Med Rehabil Clin Am 11: 251–266 [PubMed] [Google Scholar]
- Bornstein P., Sage H. (1980) Structurally distinct collagen types. Annu Rev Biochem 49: 957–1003 [DOI] [PubMed] [Google Scholar]
- Cussen L.J. (1967) Dimensions of the normal ureter in infancy and childhood. Invest Urol 5: 164–178 [PubMed] [Google Scholar]
- Cussen L.J. (1967) The structure of the normal human ureter in infancy and childhood. A quantitative study of the muscular and elastic tissue. Invest Urol 5: 179–194 [PubMed] [Google Scholar]
- Duerksen-Hughes P.J., Gooding L.R. Macrophage-mediated cytotoxicity.. In Sitkovsky M.V., Henkart P.H. (eds) ((1993)) Cytotoxic Cells: Recognition, Effector Function and Methods Birkhauser: Boston: 439–454 [Google Scholar]
- Edwards D., Normand I.C., Prescod N. (1977) Disappearance of vesicoureteric reflux during long term prophylaxis of urinary tract infection in children. BMJ 2: 285–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri N., Garton K.J., Raines E.W. (2003) An NF-kappa B-dependent transcriptional program is required for collagen remodeling by human smooth muscle cells. J Biol Chem 30: 19757–19764 [DOI] [PubMed] [Google Scholar]
- Gowen B., Borg T.K., Ghaffar A., Mayer E.P. (2000) Selective adhesion of macrophages to denatured forms of type I collagen is mediated by scavenger receptors. Matrix Biol 19: 61–71 [DOI] [PubMed] [Google Scholar]
- Hannan Q.H.A., Stephens F.D. (1973) The influence of trigonectomy on vesicoureteral reflux in dogs. Invest Urol 10: 469–472 [PubMed] [Google Scholar]
- Henson P.M., Bratton D.L., Fadok V.A. (2001) Apoptotic cell removal. Current Biology 11: 795–805 [DOI] [PubMed] [Google Scholar]
- Hutch J.A. (1961) Theory of maturation of the intravesical ureter. J Urol 86: 534–538 [DOI] [PubMed] [Google Scholar]
- Keith A. Human Embryology and Morphology (1948) 6th edn, Edward Arnold & Co: London [Google Scholar]
- King L.R., Kazmi S.O., Belman A.B. (1974) Natural history of vesicoureteral reflux. Outcome of a trial of nonoperative therapy. Urol Clin North Am 1: 441–455 [PubMed] [Google Scholar]
- King P.A., Stephens F.D. (1977) Ureteral muscle tone in prevention of vesicoureteral reflux. Invest Urol 14: 488–491 [PubMed] [Google Scholar]
- Mackie G.G., Stephens F.D. (1975) Duplex kidneys: a correlation of renal dysplasia with position of the ureteral orifice. J Urol 114: 274–280 [DOI] [PubMed] [Google Scholar]
- Mendelsohn C. (2009) Using mouse models to understand normal and abnormal urogenital tract development. Organogenesis 5: 306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski I., Myburgh D., Favor J., Gupta I. (2007) Vesico-ureteric reflux and urinary tract development in the Pax2 1Neu+/− mouse. Am J Physiol Renal Physiol 293: 1736–1745 [DOI] [PubMed] [Google Scholar]
- Oswald J., Brenner E., Schwentner C., Deibl M., Bartsch G., Fritsch H., Radmayr C. (2003) The intravesical ureter in children with vesicoureteral reflux – a morphological and immunohistochemical characterisation. J Urol 170: 2423–2427 [DOI] [PubMed] [Google Scholar]
- Oswald J., Brenner E., Deibl M., Fritsch H., Bartsch G., Radmayr C. (2003) Longitudinal and thickness measurement of the normal distal and intravesical ureter in human fetuses. J Urol 169: 1501–1504 [DOI] [PubMed] [Google Scholar]
- Oswald J., Schwentner C., Brenner E., Deibl M., Fritsch H., Bartsch G., et al. (2004) Extracellular matrix degradation and reduced nerve supply in refluxing ureteral endings. J Urol 172: 1099–1102 [DOI] [PubMed] [Google Scholar]
- Paquin A.J. (1959) Ureterovesical anastomosis: The description and evaluation of a technique. J Urol 82: 573–583 [DOI] [PubMed] [Google Scholar]
- Radmayr C., Fritsch H., Schwentner C., Lunacek A., Deibl M., Bartsch G., et al. (2005) Fetal development of the ureterovesical junction and immunohistochemistry of the ends of refluxing ureters. J Ped Urol 1: 53–59 [DOI] [PubMed] [Google Scholar]
- Schuppan D., Somasundaram R., Dieterich W., Ehnis T., Bauer M. (1994) The extracellular matrix in cellular proliferation and differentiation. Ann N Y Acad Sci 733: 87–102 [DOI] [PubMed] [Google Scholar]
- Shafik A. (1997) Study of the effect of external urethral sphincter contraction on the mechanical activity of the ureterovesical junction and urinary bladder: recognition of the sphinctero-ureterovesical reflex. Urology 6: 949–952 [DOI] [PubMed] [Google Scholar]
- Shafik A. (1996) Sigmoido-rectal junction reflex: role in the defecation mechanism. Clin Anat 9: 391–394 [DOI] [PubMed] [Google Scholar]
- Shokeir A.A. (2001) A novel technique of ureteroneocystostomy (extravesical seromuscular tunnel): a preliminary clinical study. Urology 57: 1055–1058 [DOI] [PubMed] [Google Scholar]
- Stephens F.D., Lenaghan D. (1962) The anatomical basis and dynamics of vesicoureteral reflux. J Urol 87: 669–680 [DOI] [PubMed] [Google Scholar]
- Tamminen-Mobius T., Brunier E., Ebel K.D. (1992) Cessation of vesicoureteral reflux for 5 years in infants and children allocated to medical treatment. The International Reflux Study in Children. J Urol 148: 1662–1666 [DOI] [PubMed] [Google Scholar]
- Tanagho E.A., Hutch J.A., Meyers F.H., Rambo Jr O.N. (1965) Primary vesicoureteral reflux: experimental studies of its etiology. J Urol 93: 165–176 [DOI] [PubMed] [Google Scholar]
- Tanagho E.A., Guthrie T.H., Lyon R.P. (1969) The intravesical ureter in primary reflux. J Urol 101: 824–832 [DOI] [PubMed] [Google Scholar]
- Vos C.M., Sjulson L., McArthur J.C., Pardo C.A., Rothstein J., Conant K. (2000) Cytotoxicity by matrix metalloproteinase-1 in organotypic spinal cord and dissociated neuronal cultures. Exp Neurol 163: 324–330 [DOI] [PubMed] [Google Scholar]
- Weiss R., Duckett J., Spiter A. (1992) On behalf of the International Reflux Study in Children: Results of randomized clinical trial of medical versus surgical management of infants and children with grades III and IV primary vesicoureteral reflux (United States). J Urol 148: 1667–1673 [DOI] [PubMed] [Google Scholar]
- Weitkamp B., Cullen P., Plenz G., Robenek H., Rauterberg J. (1999) Human macrophages synthesize type VIII collagen in vitro and in the atherosclerotic plaque. FASEB J 13: 1445–1457 [DOI] [PubMed] [Google Scholar]
- Zhu Y.K., Liu X., Wang H., Kohyama T., Wen F.Q., Skold C.M., Rennard S.I. (2001) Interactions between monocytes and smooth-muscle cells can lead to extracellular matrix degradation. J Allergy Clin Immunol 108: 989–996 [DOI] [PubMed] [Google Scholar]