Abstract
Male hypogonadism, or testosterone deficiency syndrome (TDS), results from a failure of the testes to produce adequate androgen. Patients have low circulating testosterone in combination with clinical symptoms such as fatigue, erectile dysfunction, and body composition changes. The cause may be primary (genetic anomaly, Klinefelter’s syndrome) or secondary (defect in hypothalamus or pituitary), but often presents with the same symptomatology. In the older patient, androgen deficiency of the aging male (ADAM) is an important cause of secondary hypogonadism because testosterone levels decline progressively after age 40. Hypogonadal patients have alterations not only in sexual function and body composition, but also in cognition and metabolism. Regardless of etiology, hypogonadal patients who are both symptomatic and who have clinically significant alterations in laboratory values are candidates for treatment. The goal of hormone replacement therapy in these men is to restore hormone levels to the normal range and to alleviate symptoms suggestive of hormone deficiency. This can be accomplished in a variety of ways, although most commonly testosterone replacement therapy (TRT) is employed.
Keywords: androgen deficiency, hypogonadism, testosterone deficiency syndrome, testosterone replacement
Hypogonadism and ADAM
Male hypogonadism, also known as testosterone deficiency syndrome (TDS), results from a failure of the testes to produce adequate androgen. This syndrome is characterized by low circulating levels of androgens, most often decreased bioavailable testosterone. The diagnosis of hypogonadism relies on the combination of laboratory measurements of serum testosterone and clinical signs and symptoms of androgen deficiency. Decreased libido is the symptom most often associated with hypogonadism, but patients may also exhibit fatigue, erectile dysfunction (ED), and body composition changes [Bassil et al. 2009]. A patient may have normal libido, sexual function, and energy levels despite having low testosterone levels. Such asymptomatic men can be a diagnostic challenge to the clinician. Discrepancies among hypogonadal men arise not only from different individual thresholds to the actions of testosterone, but also from the varying etiologies that underlie hypogonadism.
Primary hypogonadism, or hypergonadotropic hypogonadism, is characterized by low levels of circulating androgens despite high levels of pituitary gonadotropins, and is often referred to as primary testicular failure. Klinefelter’s syndrome (47,XXY or mosaic 46,XY/47,XXY) is the classic example of primary hypogonadism. Interestingly, some patients may have normal testosterone levels but have low bioavailable testosterone levels due to elevated estrogen and sex-hormone binding globulin (SHBG) levels. However, luteinizing hormone (LH) is usually elevated and follicle-stimulating hormone (FSH) is markedly elevated in these patients. Other causes of primary testicular failure range from infection and trauma to drug use and exposure to chemotherapeutic agents or radiation. Chemotherapy is a well-known cause of azoospermia, although many cases are temporary and reproductive function is usually recovered over time.
Secondary hypogonadism, or hypogonadotropic hypogonadism, generally refers to conditions resulting from decreased release of LH and/or FSH with the defect being in the hypothalamus or the pituitary. Kallman’s syndrome is a congenital hypogonadal syndrome with delayed puberty, anosmia, and midline facial defects resulting from impaired migration of gonadotropin-releasing hormone (GnRH)-releasing neurons to the hypothalamus during the embryonic period. Idiopathic hypogonadotropic hypogonadism results in a similar clinical picture. Any damage to the hypothalamus or pituitary before puberty can result in low gonadotropin levels. Infection, tumors, exposure to radiation, surgery, and infarction of the hypothalamus or pituitary gland can lead to low levels of gonadotropins and resultant hypogonadism. For example, elevated prolactin secretion from pituitary prolactinomas causes a decrease in gonadotropin secretion and subsequent hypogonadism.
Androgen deficiency of the aging male (ADAM) is a cause of secondary hypogonadism that often goes unrecognized. This phenomenon of hypogonadism due to aging has also been described as testosterone deficiency syndrome, late-onset hypogonadism, and andropause. Symptoms of this condition resemble those of ‘normal’ aging and include changing body composition (osteopenia, increased adiposity, decreased muscle mass), decline in energy and stamina, decreased cognitive function, decreased libido, and erectile dysfunction [American Society for Reproductive Medicine Practice Committee (ASRMPC), 2008]. Testosterone levels in men begin to decline in the late third or early fourth decade and diminish at a constant rate thereafter [Allan and McLachlan, 2004]. Longitudinal studies, such as the Massachusetts Male Aging Study, suggest that total testosterone decreases at a rate of about 1.6% annually, with a concomitant 1.3% annual increase in SHBG after age 40 [Feldman et al. 2002]. The fraction of circulating testosterone that is bound to SHBG is inactive, so its increase results in even lower levels of bioavailable testosterone. An estimated 30% of men aged 70–79 have low serum total testosterone and approximately 70% have low bioavailable testosterone levels [Harman et al. 2001].
Validated questionnaires have been developed to assess symptoms associated with androgen deficiency, such as the ADAM questionnaire and the Aging Male Survey [Moore et al. 2004; Morley et al. 2000]. Exhibiting high statistical sensitivity and low specificity, the ADAM questionnaire will rarely miss the diagnosis in hypogonadal individuals, but will also incorrectly identify many nonhypogonadal men [Bassil et al. 2009; Harman et al. 2001] (see Table 1). The lack of specificity is not only due to the fact that many positive responses in the questionnaire may be indicative of other conditions such as depression, but also because scores derived from these questionnaires do not predict or correlate well with measured free and total testosterone [ASRMPC, 2008; Harman et al. 2001]. It is therefore up to the physician to use one’s own clinical judgment when combining the results of these questionnaires and laboratory measurements of androgen levels to formulate a diagnosis of hypogonadism.
Table 1.
Androgen Deficiency of the Aging Male (ADAM) Questionnaire. The ADAM questionnaire is considered positive if the patient answers ‘yes’ to questions 1 and 7, as well as two to four of the other items [Morley et al. 2000].
| 1. Do you have a decrease in libido or sex drive? |
| 2. Do you have a lack of energy? |
| 3. Do you have a decrease in strength and/or endurance? |
| 4. Have you lost weight? |
| 5. Have you noticed a decresed ‘enjoyment of life’? |
| 6. Are you sad and/or grumpy? |
| 7. Are your erections less strong? |
| 8. Have you noticed a recent deterioration in your ability to play sports? |
| 9. Are you falling asleep after dinner? |
| 10. Has there been a recent deterioration in your work performance? |
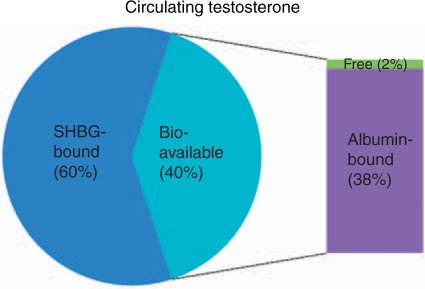
The laboratory diagnosis of testosterone levels, however, also has its own drawbacks. Testosterone circulates in three fractions, and the 60% bound to SHBG is inactive. Only 1–2% of circulating testosterone is free and available for cellular activity, but the remaining one-third of circulating testosterone that is bound to albumin is easily released and also considered to be bioavailable testosterone (see Figure 1). Although bioavailable testosterone is the component that best correlates with bone mineral density, sexual function, and cognition, it is not established that it is the clinically relevant fraction for every target organ of androgen action [ASRMPC, 2008]. Since circulating levels of SHBG can change, measurements of total testosterone can vary greatly. Recent trends have led towards obtaining SHBG levels in addition to total testosterone to calculate the free testosterone index (FTI) by dividing SHBG into total testosterone (a calculation tool is available online at http://www.issam.ch). A FTI of less than 0.153 supports the diagnosis of hypogonadism [ASRMPC, 2008]. While reference values for total testosterone vary significantly, consensus recommendations issued by the Endocrine Society have proposed that total testosterone less than 200 ng/dl identify hypogonadism requiring treatment, whereas those with total testosterone in the range of 200–400 ng/dl may benefit from treatment [ASRMPC, 2008]. Given the variability in testing for total and bioavailable testosterone, an ADAM patient in the ‘low–normal’ total testosterone range of 200–400 ng/dl with clinical symptoms of hypogonadism is certainly a candidate for a trial of testosterone replacement.
Figure 1.
Testosterone circulates in three fractions: SHBG-bound (60%), albumin-bound (38%), and free (2%). Bioavailable testosterone is composed of free and albumin-bound testosterone [ASRMPC, 2008].
Effects of hypogonadism
Hypogonadal men often experience a decline in libido, and occasionally problems with erectile function. Androgens are known to play a significant role in sexual function, although erectile function may not be directly androgen dependent. Testosterone supplementation in hypogonadal men appears to have a lasting improvement in libido, but improvement in erectile function based on a validated questionnaire (such as the International Index of Erectile Function [IIEF]) is short lived [Mulhall et al. 2004]. Androgens also have a direct impact on bone mineral density since testosterone and estrogens both play a vital role in bone health. Estrogens, which in men are dependent on aromatization of androgens, activate osteoblasts and inhibit osteoclastic bone resorption [Michael et al. 2005]. Therefore, low testosterone levels can actually cause an increase in osteoclast-induced bone resorption. Body mass and composition are also affected by androgens. Studies of hypogonadal men show that there are increases in weight and overall body fat mass as well as decreases in lean body mass with declining androgen levels [Smith et al. 2002]. Androgens also appear to play an important role in adipose tissue distribution, as testosterone replacement therapy (TRT) in hypogonadal men decreases the amount of adiposity and alter its distribution to increase lean body mass [Wang et al. 2000]. Page and colleagues studied 70 hypogonadal men treated with TRT and found increases in lean body mass and decreases in total body fat [Page et al. 2005].
On a metabolic level, men with lower androgen levels have demonstrated higher glucose and insulin levels, higher rates of obesity, and an increased incidence of type 2 diabetes [Grossmann et al. 2008; Ding et al. 2006]. Several studies have shown a significant improvement in insulin sensitivity in diabetic men treated with supplemental testosterone [Kapoor et al. 2006; Boyanov et al. 2003]. Studies have also suggested a link between hypogonadism and cardiovascular disease, which is not surprising given the relationship with hypogonadism and the metabolic syndrome [Basaria, 2008; Makinen et al. 2008]. The possibility of a causal relationship between hypogonadism and cardiovascular disease has been demonstrated by finding a direct correlation between low testosterone levels and increased risk of aortic atherosclerosis independent of age, body mass index (BMI), total cholesterol, smoking status, or diabetes [Hak et al. 2002]. Page and colleagues’ study mentioned above also found decreases in low-density lipoprotein (LDL) and triglyceride levels, although interestingly no improvement in high-density lipoprotein (HDL) levels [Page et al. 2005].
In addition to the physical effects on the body, there is evidence suggesting a role of androgens in cognition, especially in elderly men. One study examining cognition in elderly men found a relationship between low testosterone levels and significant declines in memory and visuospatial performance [Moffat et al. 2004, 2002]. Recent prospective studies have identified a link between low androgen levels in aging men and an increased risk of Alzheimer’s disease [Moffat et al. 2004; Rosario et al. 2004]. In vitro animal studies suggest that low levels of testosterone may increase β-amyloid deposition and decrease neuronal survival in response to toxic insult [Ramsden et al. 2003]. In animal studies, testosterone has been shown to upregulate acetylcholine release and the expression of nerve growth factors and nicotinic receptors in areas of the brain involved in spatial learning, such as the hippocampus [Janowsky, 2006].
Hormone replacement
The goal of hormone replacement therapy in hypogonadal men is to restore hormone levels to the normal range of young adult males and to alleviate symptoms suggestive of hormone deficiency [Bassil et al. 2009; ASRMPC, 2008]. Restoration of normal testosterone levels with replacement therapy, as mentioned previously, can improve muscle mass, prevent osteoporosis, maintain mental acuity, and restore libido, especially in elderly males. While these benefits of TRT in hypogonadal men are well described, not all hypogonadal patients receive only testosterone as a means of hormone replacement. In general, treatment is either in the form of direct androgen replacement with testosterone therapy, or in the form of replacement of pituitary gonadotropins to stimulate endogenous androgen production. Treatment of hypogonadism needs to be tailored to the underlying cause. Since most men over the age of 50 have declining levels of testosterone (a so-called ‘relative hypogonadism’), such men should only be considered candidates for hormone therapy if they have clinical manifestations of ADAM in addition to low testosterone or decreased FTI [Bassil et al. 2009]. The form of treatment for men with hypogonadotropic hypogonadism depends on the desire for future fertility.
TRT is the easiest and most cost-effective form of treatment, but results in suppression of the endogenous hypothalamic–pituitary–gonadal axis leading to infertility. If fertility is desired, treatment can be given by supplementation with GnRH, human chorionic gonadotropin (hCG, an analog of LH), or human menopausal gonadotropin (hMG, mimics both LH and FSH). Supplementation of LH and FSH, as well as pulsatile supplementation of GnRH with an infusion pump, can result in sperm production in hypogonadotropic hypogonadal men. Use of this treatment protocol requires the patient to have an intact and functional pituitary gland, so patients with previous pituitary surgery, trauma, or radiation may not be candidates for GnRH therapy. Administration of hCG by subcutaneous injection two to three times weekly (2000–2500 IU) is sufficient to initiate sperm production, but FSH supplementation is also necessary for sperm maturation. Therefore, FSH can be supplemented either by administering recombinant FSH two to three times weekly (75 IU) or by administering hMG which contains both FSH and LH [Schiff et al. 2007]. Testosterone levels are usually normalized with supplementation of gonadotropins. More recently, the use of clomiphene citrate has gained popularity in both the treatment of male factor infertility and male hypogonadism. Clomiphene citrate is a selective estrogen receptor modulator that acts at the level of the hypothalamus to block feedback inhibition of estradiol, thereby increasing release of LH and FSH from the pituitary. The LH and FSH then act on the Leydig and Sertoli cells of the testes, respectively, to increase serum testosterone and levels and spermatogenesis [Taylor and Levine, 2009]. In just 4–6 weeks of a low dose of clomiphene citrate treatment (25 mg per day), hypogonadal men exhibit significant increases in serum testosterone and testosterone/estradiol ratios [Shabsigh et al. 2005]. By stimulating endogenous androgen production, clomiphene citrate has proven to be a viable alternative to TRT.
If fertility is not an issue, then supplementation with testosterone is provided (see Table 2). In general, testosterone can be supplemented using intramuscular injections of testosterone preparations (testosterone enanthate, testosterone cypionate, testosterone undecanoate), testosterone gels (Androgel®, Solvay, Marietta, GA; and Testim®, Auxilium, Malvern, PA), patches (Androderm®, Watson, Corona, CA), or long-acting subcutaneous pellets of testosterone (Testopel®, Slate, Durham, NC). The testosterone esters in the injectable preparations are put in depot injections that act over a period of weeks, with an average dose of 100 mg per week, with injection intervals of every 2–3 weeks for enanthate and cypionate, and every 2–3 months for undecanoate [Bassil et al. 2009]. In subcutaneous pellet form, a typical dosing regimen is 450–900 mg testosterone (implantation of 6–12 pellets, 75 mg each, in the posterior gluteal or abdominal region) every 3–6 months. In the United States, buccal testosterone tablets and other currently available oral androgen preparations have fallen out of favor due to their extensive hepatic first-pass metabolism. This first-pass effect in the liver not only leads to a very small amount of hormone entering the circulation, but also to an increased risk of toxicity when compared with other preparations [Page et al. 2008].
Table 2.
Methods of testosterone replacement therapy (TRT) and common dosing regimens [Bassil et al. 2009].
| TRT modality | Dosing regimen |
|---|---|
| Intramuscular injection | Testosterone enanthate/cypionate: 200 mg Q2 weeks or 300 mg Q3 weeks |
| Testosterone undecanoate: injection Q2–3 months | |
| Transdermal gels/patches | Androderm: 5 mg/day (2.5 mg patch BID or 5 mg patch; nonscrotal) |
| Testoderm: 40 cm2 scrotal patch (1 patch/day) | |
| Gels (Testim/Androgel): 5–10 mg/day | |
| Subcutaneous pellets | Testopel: 75 mg pellets; 6–12 pellets (450–900 mg) Q3–6 months |
| Oral | Oral preparations available but not commonly used in the USA |
Each method of TRT carries different risks and benefits. Selection of treatment modality is a matter of patient preference once they have been well informed of the risks and benefits by their physician. Injectable forms are the most cost effective, but typically cause significant variations in testosterone levels. Transdermal forms of testosterone replacement, while more costly, offer more physiologic levels of testosterone. Since they are administered daily in low doses, the risk of supraphysiological or subtherapeutic levels is minimized. These topical preparations are also noteworthy for their minimal risk of adverse events. Although there is a slight risk of person-to-person androgen transfer, especially with the use of a testosterone gel, most series examining the toxicity of topical agents find that adverse events are almost nonexistent [Ohl and Quallich, 2006]. Implant therapy with subcutaneous pellets of testosterone also has minimal adverse events. Issues with implant theory therapy include pellet extrusion and bleeding and infection from the implantation procedure. Recent retrospective analysis of Testopel® patients found substantially lower rates of pellet extrusion and infection as compared with their European predecessor [Cavender and Fairwall, 2009]. In general, all physicians providing testosterone replacement should monitor their patients for fluid retention, weight gain, benign prostate hyperplasia (BPH), gynecomastia, problems sleeping, and polycythemia [ASRMPC, 2008; Ohl and Quallich, 2006]. Blood counts should be monitored periodically, especially with injectable forms of testosterone. With any form of TRT, routine digital rectal exam and prostate-specific androgen (PSA) monitoring is necessary. PSA production is androgen dependent, so a rapid rise in PSA in the first few months of treatment may suggest the presence of an unrecognized prostate cancer [ASRMPC, 2008].
Treatment of hypogonadal men with a history of prostate cancer is more controversial, but there is emerging evidence suggesting that the practice is safe [Khera and Lipshultz, 2007; Morgentaler, 2007]. However, metastatic prostate cancer remains an absolute contraindication to treatment, as does history of or current breast cancer, and a hematocrit greater than 55%. Relative contraindications include hematocrits from 52% to 55%, severe obstructive sleep apnea, severe BPH-related lower urinary tract symptoms, and medical conditions that cause fluid retention such as congestive heart failure [Bassil et al. 2009; ASRMPC, 2008]. After beginning therapy, clinical evaluations need to be conducted initially at 3–6 months, and then annually [ASRMPC, 2008]. Since therapy is empiric, and time to reach optimal testosterone levels depends on mode of replacement, clinicians need to use standardized laboratory values in conjunction with symptom questionnaires to monitor progress of therapy.
Conflict of interest statement
The authors declare that there is no conflict of interest.
References
- Allan C.A., McLachlan R.I. (2004) Age-related changes in testosterone and the role of replacement therapy in older men. Clin Endocrinol 60: 653–670 [DOI] [PubMed] [Google Scholar]
- American Society for Reproductive Medicine Practice Committee (ASRMPC) (2008) Androgen deficiency in the aging male. Fertil Steril 90: S83–S87 [DOI] [PubMed] [Google Scholar]
- Basaria S. (2008) Androgen deprivation therapy, insulin resistance, and cardiovascular mortality: an inconvenient truth. J Androl 29: 534–539 [DOI] [PubMed] [Google Scholar]
- Bassil N., Alkaade S., Morley J.E. (2009) The benefits and risks of testosterone replacement therapy: a review. Therapeut Clin Risk Manage 5: 427–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyanov M.A., Boneva Z., Christov V.G. (2003) Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male 6: 1–7 [PubMed] [Google Scholar]
- Cavender R.K., Fairwall M. (2009) Subcutaneous testosterone pellet implant (Testopel®) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analsysis. J Sex Med 6: 3177–3192 [DOI] [PubMed] [Google Scholar]
- Ding E.L., Song Y., Malik V.S., Liu S. (2006) Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 295: 1288–1299 [DOI] [PubMed] [Google Scholar]
- Feldman H.A., Longcope C., Derby C.A., Johannes C.B., Araujo A.B., Coviello A., et al. (2002) Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 87: 589–598 [DOI] [PubMed] [Google Scholar]
- Grossmann M., Thomas M.C., Panagiotopoulos S., Macisaac R., Clarke S.J., Zajac G., et al. (2008) Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 93: 1834–1840 [DOI] [PubMed] [Google Scholar]
- Hak A.E., Witteman J.C., de Jong F.H., Geerlings M.I., Hofman A., Pols H.A. (2002) Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab 87: 3632–3639 [DOI] [PubMed] [Google Scholar]
- Harman S.M., Metter E.J., Tobin J.D., Pearson J., Blackman M.R. (2001) Longitudinal effects of aging on serum total and free testosterone levels in healthy men: Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 86: 724–731 [DOI] [PubMed] [Google Scholar]
- Janowsky J.S. (2006) The role of androgens in cognition and brain aging in men. Neuroscience 138: 1015–1020 [DOI] [PubMed] [Google Scholar]
- Kapoor D., Goodwin E., Channer K.S., Jones T.H. (2006) Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 154: 899–906 [DOI] [PubMed] [Google Scholar]
- Khera M., Lipshultz L.I. (2007) The role of testosterone replacement therapy following radical prostatectomy. Urol Clin North Am 34: 549–553, vi [DOI] [PubMed] [Google Scholar]
- Makinen J.I., Perheentupa A., Irjala K., Pollanen P., Makinen J., Huhtaniemi I., et al. (2008) Endogenous testosterone and serum lipids in middle-aged men. Atherosclerosis 197: 688–693 [DOI] [PubMed] [Google Scholar]
- Michael H., Harkonen P.L., Vaananen H.K., Hentunen T.A. (2005) Estrogen and testosterone use different cellular pathways to inhibit osteoclastogenesis and bone resorption. J Bone Miner Res 20: 2224–2232 [DOI] [PubMed] [Google Scholar]
- Moffat S.D., Zonderman A.B., Metter E.J., Blackman M.R., Harman S.M., Resnick S.M. (2002) Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J Clin Endocrinol Metab 87: 5001–5007 [DOI] [PubMed] [Google Scholar]
- Moffat S.D., Zonderman A.B., Metter E.J., Kawas C., Blackman M.R., Harman S.M., et al. (2004) Free testosterone and risk for Alzheimer disease in older men. Neurology 62: 188–193 [DOI] [PubMed] [Google Scholar]
- Moore C., Huebler D., Zimmermann T., Heinemann L.A., Saad F., Thai D.M. (2004) The Aging Males' Symptoms scale (AMS) as outcome measure for treatment of androgen deficiency. Eur Urol 46: 80–87 [DOI] [PubMed] [Google Scholar]
- Morgentaler A. (2007) Testosterone replacement therapy and prostate cancer. Urol Clin North Am 34: 555–563 [DOI] [PubMed] [Google Scholar]
- Morley J.E., Charlton E., Patrick P., Kaiser F.E., Cadeau P., McCready D., et al. (2000) Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 49: 1239–1242 [DOI] [PubMed] [Google Scholar]
- Mulhall J.P., Valenzuela R., Aviv N., Parker M. (2004) Effect of testosterone supplementation on sexual function in hypogonadal men with erectile dysfunction. Urology 63: 348–352 discussion 352–343 [DOI] [PubMed] [Google Scholar]
- Ohl D., Quallich S. (2006) Clinical hypogonadism and androgen replacement therapy: an overview. Urol Nurs 26: 253–259 [PubMed] [Google Scholar]
- Page S.T., Amory J.K., Bowman F.D., Anawalt B.D., Matsumoto A.M., Bremner W.J., et al. (2005) Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab 90: 1502–1510 [DOI] [PubMed] [Google Scholar]
- Page S.T., Bremner W.J., Clark R.V., Bush M.A., Zhi H., Caricofe R.B., et al. (2008) Nanomilled oral testosterone plus dutasteride effectively normalizes serum testosterone in normal men with induced hypogonadism. J Androl 29: 222–227 [DOI] [PubMed] [Google Scholar]
- Rosario E.R., Chang L., Stanczyk F.Z., Pike C.J. (2004) Age-related testosterone depletion and the development of Alzheimer disease. JAMA 292: 1431–1432 [DOI] [PubMed] [Google Scholar]
- Ramsden M., Nyborg A.C., Murphy M.P., Chang L., Stanczyk F.Z., Golde T.E., et al. (2003) Androgens modulate beta-amyloid levels in male rat brain. J Neurochem 87: 1052–1055 [DOI] [PubMed] [Google Scholar]
- Schiff J.D., Ramirez M.L., Bar-Chama N. (2007) Medical and surgical management male infertility. Endocrinol Metab Clin North Am 36: 313–331 [DOI] [PubMed] [Google Scholar]
- Shabsigh A., Kang Y., Shabsigh R., Gonzalez M., Liberson G., Fisch H., et al. (2005) Clomiphene citrate effects on testosterone/estrogen ratio in male hypogonadism. J Sex Med 2: 716–721 [DOI] [PubMed] [Google Scholar]
- Smith M.R., Finkelstein J.S., McGovern F.J., Zietman A.L., Fallon M.A., Schoenfeld D.A., et al. (2002) Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab 87: 599–603 [DOI] [PubMed] [Google Scholar]
- Taylor F., Levine L. (2009) Clomiphene citrate and testosterone gel replacement therapy for male hypogonadism: efficacy and treatment cost. J Sex Med 7: 269–276 [DOI] [PubMed] [Google Scholar]
- Wang C., Swerdloff R.S., Iranmanesh A., Dobs A., Snyder P.J., Cunningham G., et al. (2000) Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 85: 2839–2853 [DOI] [PubMed] [Google Scholar]