Abstract
The androgen receptor (AR) is a key transcriptional regulator and therapeutic target in prostate cancer. During androgen deprivation therapy to treat metastatic prostate cancer, surviving cells acquire increased AR signaling through a variety of mechanisms, one of which is enhanced interactions with AR coactivators. One recently identified AR-specific coregulator expressed only in human and nonhuman primates is the melanoma antigen gene protein-A11 (MAGE-11). MAGE-11 increases AR transcriptional activity through direct interactions with AR and other coactivators, and its levels increase during prostate cancer progression to castration-recurrent growth. The MAGE-11 gene is located at Xq28 on the human X chromosome as part of an X-linked MAGE gene family of cancer–testis antigens. MAGE-11 stabilizes AR when androgen levels are low, and functions in a transcriptional hub to promote AR-mediated gene activation. The evolutionary development and organization of the MAGE-11 gene within the cancer–testis antigen family suggests that MAGE-11 provides a gain-of-function to AR among primates in both normal physiology and cancer, and may serve as a therapeutic target in the treatment of advanced prostate cancer.
Keywords: androgen receptor, cancer–testis antigens, MAGE-11, MAGE-A11, N/C interaction, X chromosome, X-linked genes
Introduction
Prostate cancer develops initially as an androgen-dependent disease that relies on the androgen receptor (AR) for growth and progression. The androgen dependence of prostate cancer reflects the properties of the normal prostate gland and explains the initial effectiveness of androgen deprivation therapy as a first-line therapy. However, resistance to androgen deprivation develops with time and prostate cancer cells undergo recurrent growth despite low levels of circulating androgen. Relapsed prostate cancer, known as castration-recurrent or castration-resistant prostate cancer, is associated with high mortality due to the lack of effective long-term treatment strategies. Castration-recurrent prostate cancer is a complex disease that develops during the course of androgen deprivation therapy and maintains a dependence on AR for its metastatic potential.
One major goal of prostate cancer research is to understand the mechanisms by which AR signaling promotes castration-recurrent prostate cancer growth so that new treatments can be developed to prevent or treat relapse of the disease. Although research has progressed rapidly in the field, there has been a lack of therapies that provide a significant survival benefit. Many aspects of prostate cancer research have been summarized in recent reviews that address a range of topics related to AR signaling and clinical management of the disease. These include the development of inhibitors that target steroid metabolic pathways [Lassie and Dawson, 2010; Reid et al. 2010; Attard et al. 2009] based on evidence that castration-recurrent prostate cancer cells acquire the ability to synthesize androgen [Locke et al. 2008; Montgomery et al. 2008; Stanbrough et al. 2006; Titus et al. 2005b; Mohler et al. 2004], therapeutic and vaccine approaches [Cha and Fong, 2010; Stavridi et al. 2010; Chi et al. 2009; Vis and Schröder, 2009], molecular mechanisms that underlie prostate cancer progression [Dutt and Gao, 2009], and the role of AR mutations [Brooke and Bevan, 2009], epigenetic mechanisms [Schulz and Hoffmann, 2009] and AR coregulators in prostate cancer progression [Golias et al. 2009; Heemers and Tindall, 2007]. The present review focuses on molecular aspects of AR function in relation to the AR selective coregulator, melanoma antigen gene protein-A11 (MAGE-11).
AR signaling in prostate cancer
AR is a member of the steroid/nuclear receptor superfamily of ligand-dependent transcription factors and acts in the nucleus to regulate androgen-dependent gene expression in response to the two active androgens, testosterone or dihydrotestosterone (DHT). Androgen binding induces a conformational change in AR that is associated with the androgen-dependent AR NH2- and carboxyl-terminal (N/C) interaction, the recruitment of coactivators, and stabilization of a multiprotein transcription complex involved in chromatin remodeling. AR binds to specific DNA androgen response elements associated with androgen-regulated genes. Loss-of-function AR gene mutations that cause the androgen insensitivity syndrome demonstrate that AR is required to mediate the effects of androgen. AR DNA binding domain mutations that cause androgen insensitivity demonstrate that AR must bind DNA in the nucleus.
Under normal physiological conditions, AR function depends on high affinity binding of testis-derived androgen. However, in castration-recurrent prostate cancer cells, AR is no longer dependent on circulating androgen and may be activated by androgen synthesized locally. Evidence suggests that AR transcriptional activity is a major factor in the growth of androgen-dependent and castration-recurrent prostate cancer. This is evident from studies in which prostate cancer cell growth is arrested by lowering AR levels using small inhibitory RNAs [Ponguta et al. 2008; Li et al. 2007; Guo et al. 2006; Yuan et al. 2006; Furutani et al. 2005; Zegarra-Moro et al. 2002]. The proposed mechanisms for increased AR signaling in castration-recurrent prostate cancer include local prostate cancer tissue androgen production [Titus et al. 2005b; Mohler et al. 2004], AR gene amplification [Linja et al. 2001; Visakorpi et al. 1995], increased mitogen signaling [Culig et al. 2005; Gregory et al. 2004], increased levels of AR coactivators [O’Malley, 2009; Gregory et al. 2001], increased sensitivity to low androgen levels [Waltering et al. 2009; Gregory et al. 2001; Klein et al. 1997] and the expression of AR somatic mutants. The identification of sufficient levels of androgen in castration-recurrent prostate cancer cells to activate AR has challenged the notion that AR can be activated independent of androgen binding. Some studies suggest intratumor testosterone or DHT produced through the metabolic conversion of adrenal androgens, which do not activate wild-type AR, or the de novo synthesis of active androgens from cholesterol, can activate AR when circulating testicular testosterone is undetectable. On the other hand, there is in vitro evidence that AR can be transcriptionally activated in the absence of androgen through mechanisms that include enhanced mitogen signaling and interactions with coactivators.
Somatic AR mutations are uncommon in prostate cancer, but occur with greater frequency in more advanced stages of the disease. The gain-in-function associated with these mutations exemplifies the ability of prostate cancer cells to adapt to androgen deprivation or antiandrogen therapy and maintain AR function. Unlike naturally occurring loss-of-function AR germline mutations that cause incomplete masculine development in 46XY genetic males with the androgen insensitivity syndrome [Quigley et al. 1995], somatic AR mutations that develop in prostate cancer more often increase AR responsiveness to adrenal androgens, other steroids and AR antagonists. Depending on the location and nature of the amino acid mutation, AR signaling can be increased by binding other steroids [Askew et al. 2007; He et al. 2006; Chang et al. 2001; Tan et al. 1997; Harris et al. 1991; Veldscholte et al. 1990]. The gain-of-function AR somatic mutations that occur under the selective pressure of androgen deprivation within the genetically unstable environment of cancer may be further facilitated by the single allele status of the AR gene on the human male X chromosome.
Primate-specific cancer–testis antigens in the MAGE gene family
The single allele human male X chromosome has been subject to selective pressure during the evolution of primates that has resulted in the accumulation of male-advantage genes involved in sex development and reproduction [Delbridge and Graves, 2007; Saifi and Chandra, 1999]. In addition to the AR gene at Xq11-12 [Brown et al. 1989], there are X-linked germ cell specific genes required for male reproductive function [Zheng et al. 2010], and a group of X-linked cancer–testis antigen genes whose function has been associated with spermatogenesis. Of the 153 cancer–testis antigen genes [Almeida et al. 2009], 83 cancer–testis antigens occur within multigene families and represent ∼10% of the genes on the human X chromosome [Ross et al. 2005; Simpson et al. 2005]. One class of cancer–testis antigen is the melanoma antigen gene (MAGE) family that has 52 members. MAGE genes were named based on their initial identification in melanoma and have been divided into eight subclasses, MAGE-A through H, whose functions are mostly unknown. Members of the MAGE family share a conserved ∼200 amino acid MAGE homology domain in the carboxyl-terminal region [Barker and Salehi, 2002; Chomez et al. 2001]. The AR specific coregulator MAGE-A11 (MAGE-11) is one of 12 members of the MAGE-A subfamily of cancer–testis antigen genes located in the Xq28 region of the human X chromosome [Rogner et al. 1995; De Plaen et al. 1994]. The MAGE homology domain in human MAGE-11 includes amino acid residues 222 to 421 in the 429 amino acid full-length protein [Bai and Wilson, 2008, 2008].
Similar to other cancer–testis antigen genes, MAGE genes have undergone species-specific expansion through gene duplication by retrotransposition from the MAGE-D subfamily that rapidly diverged among mammals [Chomez et al. 2001]. Some MAGE genes are conserved between mouse and man, such as the gene that codes for Necdin, a cell cycle regulatory protein. Deletion of the Necdin gene results in the neurogenetic disorder known as Prader-Willi syndrome [Lee et al. 2005]. However, many MAGE genes including MAGE-11 are poorly conserved through evolution, suggesting they evolved more recently through retrotransposition. The MAGE-11 gene arose by gene amplification within the primate lineage [Delbridge and Graves, 2007] and is expressed only in human and nonhuman primates. Thus, while some human MAGE genes have homologues in mice [De Plaen et al. 1999], the species-specific expansion of the MAGE gene family resulted in MAGE-11 being unique to primates. It is noteworthy that primates have a large number of retroposed insertions that have contributed to the evolution of new functions [Baertsch et al. 2008]. The more recent evolution of genes involved in reproduction and fertilization is associated with speciation [Turner and Hoekstra, 2008], and primate-specific genes are preferentially expressed in tissues of the reproductive tract [Tay et al. 2009].
Genes in the MAGE-A subfamily were first thought to be expressed mainly in cancer and code for ∼300 amino acid residue proteins from a single exon that included the MAGE homology domain [Artamonova and Gelfand, 2004; Barker and Salehi, 2002; Chomez et al. 2001; Jurk et al. 1998; Rogner et al. 1995]. However, the MAGE-11 gene has four coding exons in the human Xq28 chromosomal region. The first of the three short coding exons contains a nuclear targeting signal consistent with the predominantly nuclear localization of MAGE-11 [Bai and Wilson, 2008, 2008; Irvine and Coetzee, 1999]. Based on the prevalence of retroposed elements in the primate lineage, the three 5’ upstream coding exons of the MAGE-11 gene most likely derived from the splicing of mRNA-derived retrocopies that amended the function of the MAGE-A subfamily and enabled MAGE-11 to be an AR coregulator. This is supported by evidence that deletion of the MAGE-11 NH2-terminal region causes loss of its AR coactivator function. The MAGE-11 protein is 429 amino acid residues in length and migrates as a 60–65 kDa protein on denaturing gels. Unlike some MAGE genes that are expressed primarily in cancer with the exception of male germ cells, MAGE-11 is expressed in both normal reproductive tissues and cancer [Irvine and Coetzee, 1999; Rogner et al. 1995].
The physiological function of most cancer–testis antigens remains unknown [Simpson et al. 2005; Scanlan et al. 2004]. However, several MAGE genes have been shown to be involved in cell cycle progression, neural development and apoptosis. For example, p53 function is modulated by hNRAGE (MAGE-D1), Necdin (MAGE-L2) and MAGE-A2, all members of the MAGE gene family [Monte et al. 2006; Wen et al. 2004; Taniura et al. 1999]. MAGE-D1 interacts with proteins involved in cell cycle control and apoptosis [Barker and Salehi, 2002], and regulates the cell cycle by enhancing p75 neurotrophin-mediated apoptosis [Salehi et al. 2000]. Necdin is a neuron-specific growth suppressor that interacts with cell growth promoting proteins that include viral oncogenes [Ohman Forslund and Nordqvist, 2001; Taniura et al. 1999]. Necdin facilitates cell cycle exit and cell survival [Matsumoto et al. 2001], and its loss results in the rare neurogenetic disorder known as Prader–Willi syndrome [Lee et al. 2005; Boccaccio et al. 1999]. The entire Necdin MAGE homology domain was required for the interaction with p53 [Taniura et al. 2005]. MAGE-A2 acts as a p53-histone deacetylase 3 assembly protein that provides a survival advantage to cancer cells [Monte et al. 2006]. Suppression of class I MAGE-A, B and C members coded on the human-X-chromosome-induced apoptosis and the acetylation of p53 in melanoma cell lines, suggesting that MAGE proteins promote tumor survival [Yang et al. 2007]. MAGE-11 shares sequence similarity with the adenovirus early region 1A viral oncoprotein E1A that binds p300, a potent acetyltransferase and cell cycle regulator. This suggests that MAGE-11 in association with AR functions in cell cycle regulation and provides a growth advantage to prostate cancer cells.
MAGE-11 as AR coregulator
More than 50 coactivator proteins are reported to interact with AR and the levels of some of these proteins increase during prostate cancer progression [Heemers and Tindall, 2007]. AR transcriptional activity depends on interactions with coregulator proteins mediated primarily through two activation domains. Activation function 1 (AF1) is in the AR NH2-terminal region, and activation function 2 (AF2) is a hydrophobic surface of the highly structured carboxyl-terminal ligand-binding domain. The p160 coactivators, named for their molecular weight of ∼160 kDa and referred to as SRC1, SRC2/TIF2/GRIP1 or SRC3/AIB1/TRAM1/ACTR, interact principally with AF2 in the AR ligand-binding domain through the coactivator LXXLL motifs. Other AR coactivators that interact with the AR NH2-terminal region are less well characterized. Increased levels of AR coactivators is one mechanism by which castration-recurrent prostate cancer may acquire enhanced AR signaling to promote tumor growth during androgen deprivation therapy.
The AR NH2-terminal region contains the AR FXXLF motif sequence 23FQNLF27 that interacts with AF2 as an amphipathic alpha helix to mediate the androgen-dependent AR N/C interaction [He et al. 2004, 2000]. The AR N/C interaction is required for the activation of many androgen-regulated genes [He et al. 2002, 2000]. Androgen-dependent binding of the AR FXXLF motif to AF2 can competitively inhibit binding of the p160 coactivator LXXLL motifs, which bind with lower affinity to AF2 than the AR FXXLF motif [Askew et al. 2007; He et al. 2001].
MAGE-11 was identified as an AR coactivator in a yeast two hybrid screen of a human testis library using an AR NH2-terminal FXXLF motif fragment as bait [Bai et al. 2005]. The screen was based on the ability of mutations introduced into the AR FXXLF motif to expose AF2 and increase p160 coactivator LXXLL motif binding. It was postulated that a protein that binds the AR FXXLF motif might similarly relieve inhibition of AF2 caused by the AR N/C interaction. As predicted, through its interaction with the AR NH2-terminal FXXLF motif, one mechanism by which MAGE-11 increases AR transcriptional activity is to expose AF2 for increased p160 coactivator recruitment [He et al. 2002, 2001].
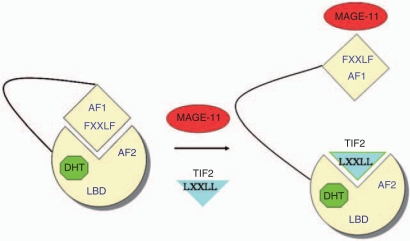
The AR NH2-terminal FXXLF motif therefore serves at least two functions. In addition to binding the AR AF2 site to mediate the androgen-dependent AR N/C interaction, the AR FXXLF motif binds MAGE-11. The competitive relationship between the AR N/C interaction and MAGE-11 binding modulates AR transcriptional activity. In the interaction with MAGE-11, the AR FXXLF motif binds a MAGE-11 F-box region that resembles the F-box in cyclin F for which it was originally named [Askew et al. 2009]. The F-box is a ∼40 amino acid sequence that has a conserved spacing of hydrophobic residues. The presence of an F-box in MAGE-11 suggests that it functions as part of a ubiquitin ligase complex. The MAGE-11 F-box contains threonine 360 (Thr-360), which is phosphorylated by cell cycle checkpoint kinase Chk1 in response to epidermal growth factor signaling [Bai and Wilson, 2008, 2008]. Phosphorylation at Thr-360 signals the monoubiquitinylation of MAGE-11 at two lysine residues outside the F-box. Both phosphorylation and monoubiquitinylation of MAGE-11 are required to bind the AR FXXLF motif (Figure 1).
Figure 1.
MAGE-11 binds the AR NH2-terminal FXXLF motif and competes for the AR N/C interaction to increase AF2 binding of p160 coactivators and AR transcriptional activity. AR (yellow) bound to DHT (green) undergoes the N/C interaction between the AR NH2-terminal FXXLF and AF2 in the ligand-binding domain. The AR FXXLF motif also binds MAGE-11 (red), which exposes AF2 for p160 coactivator TIF2 (blue) recruitment by LXXLL motifs and increases AR transcriptional activity. MAGE-11 also increases AR transactivation by bridging to other coregulatory proteins. AF1, activation function 1; AF2, activation function 2; AR, androgen receptor; DHT, dihydrotestosterone; LBD, ligand-binding domain; MAGE-11, melanoma antigen gene protein-A11; TIF2, transcriptional mediator protein 2.
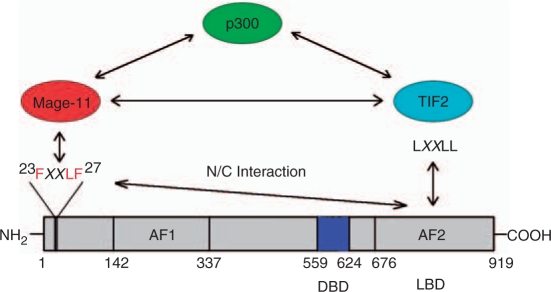
AR signaling is also influenced by the ability of MAGE-11 to stabilize AR when androgens levels are low. This may be an important mechanism to maintain AR levels in castration-recurrent prostate cancer. MAGE-11 also increases AR transcriptional activity through direct interactions with p160 coactivators and p300 (Figure 2) [Askew et al. 2010, 2009]. MAGE-11 functions synergistically with transcriptional mediator protein 2 (TIF2), a p160 coactivator, and with p300, a potent and ubiquitous transcriptional regulator with histone acetyltransferase activity.
Figure 2.
MAGE-11 increases androgen receptor (AR) transcriptional activity by interacting with TIF2 and p300. Binding of MAGE-11 to the AR NH2-terminal FXXLF motif opens AF2 in the ligand-binding domain for p160 coactivator TIF2 binding. Direct interactions between MAGE-11, TIF2 and p300 provide additional mechanisms for increased AR signaling in prostate cancer. AF1, activation function 1; AF2, activation function 2; DBD, DNA-binding domain; LBD, ligand-binding domain; MAGE-11, melanoma antigen gene protein-A11; TIF2, transcriptional mediator protein 2.
In addition to being an AR-specific coregulator, MAGE-11 has been shown to interact with the hypoxia-inducible factor prolyl hydroxylase (PHD), a cytoplasmic enzyme that hydroxylates and thereby controls the stability of hypoxia-inducible factor-α (HIF-1α) [Aprelikova et al. 2009; Berra et al. 2003]. The inhibitory effect of MAGE-11 on PHD hydroxylase activity results in the stabilization of HIF-1α, a protein that allows tumors to adapt to the hypoxic conditions associated with low oxygen [Wang et al. 1995].
Regulated expression of MAGE-11 in normal tissues and prostate cancer
MAGE-11 is expressed predominantly in tissues of the human male and female reproductive tracts. Cancer–testis antigen gene expression was initially thought to be limited to human germ cells of the adult testis with limited expression in placenta and ovary, and overexpression in different types of cancer [Kalejs and Erenpreisa, 2005; Simpson et al. 2005; Scanlan et al. 2002]. However, MAGE-11 is expressed in normal human testis, ovary, endometrium, adrenal gland and placenta [Bai et al. 2008, 2005; De Plaen et al. 1994] and in prostate cancer.
The MAGE-11 gene is regulated through at least two mechanisms. MAGE-11 expression is upregulated by cyclic AMP in human endometrial and prostate cancer cells [Karpf et al. 2009; Bai et al. 2008]. MAGE-11 levels increase in human endometrial epithelial cells after the LH surge during the early to mid secretory phase of the menstrual cycle with highest levels during the window of implantation [Bai et al. 2008]. This suggests that LH signaling through cyclic AMP is responsible for the increase in MAGE-11, and that MAGE-11 has a role in human embryo implantation. MAGE-11 mRNA levels are low in most normal tissues, but increase by ∼50-fold in the endometrium epithelium during the window for implantation, and up to 1000 fold during prostate cancer progression to castration-recurrent growth [Karpf et al. 2009]. Thus, one mechanism for increased MAGE-11 expression and possibly other cancer–testis antigens in advanced forms of cancer may be increased cyclic AMP signaling. However, in DU145 prostate cancer cells that do not express MAGE-11, the MAGE-11 gene is fully methylated near the transcription start site and levels of MAGE-11 mRNA do not increase in response to cyclic AMP. This suggests that promoter DNA methylation can render the MAGE-11 gene refractory to stimulation by cyclic AMP.
A second predominant mechanism for increased cancer–testis antigen expression widely demonstrated in cancer is DNA hypomethylation. This is supported by evidence that the demethylating agent 5-aza-2-deoxycytidine increases cancer–testis antigen expression [Shichijo et al. 1996]. MAGE genes are normally repressed by DNA methylation and histone deacetylation [Wischnewski et al. 2006; De Smet et al. 1999] that may involve methyl-CpG binding proteins that recruit histone deacetylases [Wischnewski et al. 2007]. In agreement with these findings, the MAGE-11 promoter has a high CpG content that is subject to DNA methylation. The MAGE-11 gene promoter is progressively hypomethylated at the transcription start site during prostate cancer progression, and is partially to fully hypomethylated in castration-recurrent prostate cancer to an extent that correlates with MAGE-11 mRNA levels [Karpf et al. 2009]. In agreement with the higher expression of cancer–testis antigens in higher-grade cancers with poor prognosis [Andrade et al. 2008; Napoletano et al. 2008; Velazquez et al. 2007; Gure et al. 2005], MAGE-11 levels were higher in castration-recurrent prostate cancer. During the transition to castration-recurrent growth, MAGE-11 mRNA levels increase to a greater extent than AR mRNA [Karpf et al. 2009], suggesting that stabilization of AR by MAGE-11 is one mechanism to promote prostate cancer progression.
The increased expression of MAGE-11 by DNA hypomethylation in castration-recurrent prostate cancer may reflect genomewide DNA hypomethylation, an epigenetic event associated with cancer [Ehrlich, 2002; De Smet et al. 1996]. Although global hypomethylation was reported for colorectal cancer without increased cancer–testis antigen expression [Park and Lee, 2002], global hypomethylation in other types of cancer included the tumor-associated MAGE-A genes [Wischnewski et al. 2007]. Specific hypomethylation of the MAGE-A1 promoter in tumors was associated with inhibition of DNA methylation by transcription factor binding required to maintain an active promoter [De Smet et al. 2004]. Epigenetic activation of the MAGE gene family could be associated with activity similar to BORIS, an epigenetic reprogramming factor in the male germ line shown to activate MAGE-A subfamily gene expression in the testis [Hong et al. 2005; Loukinov et al. 2002]. The studies suggest that MAGE-11 and possibly other cancer–testis antigen genes are hormone regulated in normal reproductive tissues, and are subject to deregulated DNA hypomethylation that increases expression in advanced forms of cancer. Expression levels may be further enhanced through the activation of constitutive second messenger pathways since MAGE-11 levels increased in prostate cancer cells in response to cyclic AMP [Karpf et al. 2009].
The Xq28 chromosomal locus that contains the MAGE-11 gene harbors genes involved in over 40 diseases, with 17 diseases mapped to the region for which a gene has yet to be identified [Kolb-Kokocinski et al. 2006]. A hereditary form of prostate cancer has been linked to the Xq27-28 locus by gene linkage analysis [Brown et al. 2004; Xu et al. 1998], which raises the possibility that changes in MAGE-11 expression may be associated with hereditary prostate cancer [Stephan et al. 2002]. Cancer–testis antigens have also been identified in estrogen receptor-negative forms of breast cancer that have increased expression of cancer–testis antigens that include the MAGE-A subfamily [Grigoriadis et al. 2009].
AR as a therapeutic target in castration-recurrent prostate cancer
AR has been the traditional prostate cancer drug target of the pharmaceutical industry and more recently of academic laboratories supported by the National Institutes of Health. Small molecule inhibitors can be effective agents because of their ability to diffuse freely into cells. The best-known AR small-molecule inhibitors are the antiandrogens used in the treatment of androgen-dependent prostate cancer as part of combined therapy with inhibition of gonadotrophin-releasing hormone. Antiandrogens competitively inhibit AR binding of androgen and AR transcriptional activity. Used in combined therapy, antiandrogens can prolong the period of remission [Akaza et al. 2009; Schmitt et al. 2001], but have not been effective in preventing castration-recurrent growth. The presence of testosterone and DHT at levels sufficient to activate AR [Titus et al. 2005b; Mohler et al. 2004] has escalated research on the intracrine synthesis of androgens in castration-recurrent prostate cancer cells [Knudsen and Penning, 2010; Locke et al. 2008; Montgomery et al. 2008; Stanbrough et al. 2006; Titus et al. 2005a, 2005b; Mohler et al. 2004] and renewed efforts to inhibit AR through inhibition of androgen biosynthesis [Reid et al. 2010; Attard et al. 2009].
The inability of antiandrogens to inhibit the growth of castration-recurrent prostate cancer even though AR is an essential transcription factor that drives tumor recurrence may have several causes. AR activated by local tissue androgen may not be effectively competed by antagonists that bind AR with lower affinity. AR binds testosterone and DHT with 100–500-fold higher affinity than it binds antiandrogens such as hydroxyflutamide, the active form of flutamide [Kemppainen et al. 1999]. If local tissue androgen production accounts for AR signaling and castration-recurrent growth, these lower affinity AR antagonists may not compete sufficiently with the more readily available higher affinity androgens. However, ligand-binding affinity alone does not differentiate agonists from antagonists. Some anabolic steroids bind AR with only moderate affinity but are potent androgens in vivo, in part because of their ability to induce the AR N/C interaction, whereas traditional AR antagonists inhibit the AR N/C interaction. Thus, to compete for local androgen production, higher-affinity AR antagonists are needed that inhibit the AR N/C interaction and AR signaling to block castration-recurrent prostate cancer growth.
On the other hand, numerous studies suggest that AR activation can be enhanced through alternative pathways independent of androgen binding [Ponguta et al. 2008; Culig and Bartsch, 2006; Culig et al. 2005] in which case antiandrogen or androgen deprivation therapy may be ineffective. Alternative AR transactivation pathways could provide potential new targets for small molecule inhibitors. Those currently being targeted include inhibition of AR binding to DNA and inhibition of AR interaction with coregulatory proteins.
The approach of small-molecule inhibitors that block ERα binding to DNA was recently developed as a potential therapy for breast cancer. First- and second-generation small-molecule inhibitors such as theophylline-8-[(benzylthio)methyl]-(7CI,8CI) act outside the ERα ligand-binding pocket and inhibit ERα DNA binding and transcription and the estrogen-dependent growth of tamoxifen-resistant ERα positive MCF-7 breast cancer cells, with no effect on estrogen-independent cell growth [Mao et al. 2008]. A potential limitation of this approach is that transcriptional effects may occur independent of direct receptor binding to DNA [Carroll et al. 2006]. On the other hand, the functional effects of AR require binding to DNA based on complete androgen resistance that results from naturally occurring AR DNA binding mutations that cause the androgen insensitivity syndrome [Brown, 1995; Quigley et al. 1995]. Thus, a small-molecule inhibitor that targets AR binding to DNA may be a promising approach for new drug development.
MAGE-11 as a therapeutic target in castration-recurrent prostate cancer
Nuclear receptor interactions with coregulators is another treatment target in cancer [O’Malley, 2009]. Targeting protein–protein interactions with small-molecule inhibitors is a challenging but promising approach supported by recent reports. The proteasome inhibitor bortezomid (Velcade) was developed for the treatment of multiple myeloma [Arkin and Wells, 2004]. A high throughput screen using fluorescence polarization that measured binding of c-Jun N-terminal kinase 1 (JNK1) interaction to its binding partner, JNK interacting protein 1 (JIP1), identified two classes of small-molecule inhibitors that allosterically block the JNK/JIP interaction site [Chen et al. 2009]. A small-molecule inhibitor class known as Nutlins inhibit mouse double minute 2 (mdm2) degradation of p53 [Graat et al. 2007]. Other less-specific targets for small-molecule inhibitors of AR action in prostate cancer include the inhibition of interactions with heat shock proteins [Hieronymus et al. 2006; Solit et al. 2003], histone deacetylases [Dobosy et al. 2007] and HER2-neu kinase [Mellinghoff et al. 2004].
Small-molecule inhibitors that target AR interactions with p160 coactivators or MAGE-11 may be a strategy to inhibit castration-recurrent prostate cancer growth [Karpf et al. 2009; Gregory et al. 2001]. Because MAGE-11 levels are low in most normal cells and increase during prostate cancer progression to recurrent growth, inhibition of the AR–MAGE-11 interaction may have minimal side effects. The potential effectiveness of small chemical inhibitors is enhanced by a well-defined interaction surface. The interaction between the carboxyl-terminal MAGE-11 F-box and AR NH2-terminal FXXLF motif, where both are predicted to form an α-helical structure, may be amenable to inhibition by drug-like compounds [Askew et al. 2009; Bai et al. 2005; Arkin and Wells, 2004].
An alternative approach to inhibit AR signaling in castration-recurrent prostate cancer is to target MAGE-11 using immunotherapy. Cancer–testis antigens are immunogenic in cancer cells, and tumor-specific antigens can bring about antibody-directed tumor rejection. This and the largely restricted expression of cancer–testis antigens in cancer has led to the development of cancer vaccines [Scanlan et al. 2004, 2002]. Cancer–testis antigens are cleaved in cancer cells and presented on the cell surface in association with the human leukocyte antigen (HLA) class I (or major histocompatibility) complex where they elicit a cytotoxic T-cell and humoral response as shown in melanoma [Kalejs and Erenpreisa, 2005; Gaugler et al. 1994]. MAGE-A1 was the first tumor-specific antigen shown to be recognized by specific cytotoxic T cells [Hérin et al. 1987]. The vaccination of patients with MAGE-A peptides resulted in tumor regression [Mischo et al. 2006; Marchand et al. 2003; Sadanaga et al. 2001; Thurner et al. 1999]. NY-ESO-1 of the MAGE gene family is considered one of the most immunogenic cancer–testis antigens and has been the focus of humoral immune approaches in clinical trials for different types of cancer expressing MAGE-A and NY-ESO-1 [Atanackovic et al. 2008; Bender et al. 2007; Odunsi et al. 2007; Scanlan et al. 2004, 2002]. A similar immune therapy approach targeting MAGE-11 may provide an effective prostate cancer vaccine. However, it remains to be established whether MAGE-11 peptides are presented on the prostate cancer cell surface in association with the HLA class I complex and induce a cytotoxic T-cell immune response. It may be that cancer–testis antigen-directed tumor vaccines combined with epigenetic modulatory drugs that inhibit DNA methyltransferases and histone deacetylases [Karpf, 2006] will provide new approaches for the treatment of advanced prostate cancer.
Acknowledgements
Support was from the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development US Public Health Service Grant HD16910 and National Cancer Institute Grant P01-CA77739.
Conflict of interest statement
The author declares that there is no conflict of interest.
References
- Akaza H., Hinotsu S., Usami M., Arai Y., Kanetake H., Naito S., et al. (2009) Combined androgen blockade with bicalutamide for advanced prostate cancer: long-term follow-up of a phase 3, double-blind, randomized study for survival. Cancer 115: 3437–3445 [DOI] [PubMed] [Google Scholar]
- Almeida L.G., Sakabe N.J., deOliveira A.R., Silva M.C., Mundstein A.S., Cohen T., et al. (2009) CTdatabase: a knowledge-base of high-throughput and curated data on cancer–testis antigens. Nucleic Acids Res 37: D816–D819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade V.C., Vettore A.L., Felix R.S., Almeida M.S., Carvalho F., Oliveira J.S., et al. (2008) Prognostic impact of cancer/testis antigen expression in advanced stage multiple myeloma patients. Cancer Immun 8: 2–2 [PMC free article] [PubMed] [Google Scholar]
- Aprelikova O., Pandolfi S., Tackett S., Ferreira M., Salnikow K., Ward Y., et al. (2009) Melanoma antigen-11 inhibits the hypoxia-inducible factor prolyl hydroxylase 2 and activates hypoxic response. Cancer Res 69: 616–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkin M.R., Wells J.A. (2004) Small-molecule inhibitors of protein–protein interactions: progressing towards the dream. Nat Rev Drug Discov 3: 301–317 [DOI] [PubMed] [Google Scholar]
- Artamonova I.I., Gelfand M.S. (2004) Evolution of the exon-intron structure and alternative splicing of the MAGE-A family of cancer/testis antigens. J Mol Evol 59: 620–631 [DOI] [PubMed] [Google Scholar]
- Askew E.B., Bai S., Blackwelder A.J., Wilson E.M. (2010) Transcriptional synergy between melanoma antigen gene protein-A11 (MAGE-11) and p300 in androgen receptor signaling. J Biol Chem [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew E.B., Bai S., Hnat A.T., Minges J.T., Wilson E.M. (2009) Melanoma antigen gene protein-A11 (MAGE-11) F-box links the androgen receptor NH2-terminal transactivation domain to p160 coactivators. J Biol Chem 284: 34793–34808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew E.B., Gampe R.T., Stanley T.B., Faggart J.L., Wilson E.M. (2007) Modulation of androgen receptor activation function 2 by testosterone and dihydrotestosterone. J Biol Chem 282: 25801–25816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanackovic D., Altorki N.K., Cao Y., Ritter E., Ferrara C.A., Ritter G., et al. (2008) Booster vaccination of cancer patients with MAGE-A3 protein reveals long-term immunological memory or tolerance depending on priming. Proc Natl Acad Sci U S A 105: 1650–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attard G., Reid A.H., Olmos D., de Bono J.S. (2009) Antitumor activity with CYP17 blockade indicates that castration-resistant prostate cancer frequently remains hormone driven. Cancer Res 69: 4937–4940 [DOI] [PubMed] [Google Scholar]
- Baertsch R., Diekhans M., Kent W.J., Haussler D., Brosius J. (2008) Retrocopy contributions to the evolution of the human genome. BMC Genomics 9: 466–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai S., Grossman G., Yuan L., Lessey B.A., French F.S., Young S.L., et al. (2008) Hormone control and expression of androgen receptor coregulator MAGE-11 in human endometrium during the window of receptivity to embryo implantation. Mol Hum Reproduction 14: 107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai S., He B., Wilson E.M. (2005) Melanoma antigen gene protein MAGE-11 regulates androgen receptor function by modulating the interdomain interaction. Mol Cell Biol 25: 1238–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai S., Wilson E.M. (2008) Epidermal growth factor-dependent phosphorylation and ubiquitinylation of MAGE-11 regulates its interaction with the androgen receptor. Mol Cell Biol 28: 1947–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker P.A., Salehi A. (2002) The MAGE proteins: emerging roles in cell cycle progression, apoptosis, and neurogenetic disease. J Neurosci Res 67: 705–712 [DOI] [PubMed] [Google Scholar]
- Bender A., Karbach J., Neumann A., Jäger D., Al-Batran S.E., Atmaca A., et al. (2007) LUD 00-009: phase 1 study of intensive course immunization with NY-ESO-1 peptides in HLA-A2 positive patients with NY-ESO-1-expressing cancer. Cancer Immun 7: 16–16 [PMC free article] [PubMed] [Google Scholar]
- Berra E., Benizri E., Ginouvès A., Volmat V., Roux D., Pouysségur J. (2003) HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J 22: 4082–4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccaccio I., Glatt-Deeley H., Watrin F., Roëckel N., Lalande M., Muscatelli F. (1999) The human MAGEL2 gene and its mouse homologue are paternally expressed and mapped to the Prader-Willi region. Hum Mol Genet 8: 2497–2505 [DOI] [PubMed] [Google Scholar]
- Brooke G.N., Bevan C.L. (2009) The role of androgen receptor mutations in prostate cancer progression. Curr Genomics 10: 18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C.J., Goss S.J., Lubahn D.B., Joseph D.R., Wilson E.M., French F.S., et al. (1989) Androgen receptor locus on the human X chromosome: regional localization to Xq11–12 and description of a DNA polymorphism. Am J Hum Genet 44: 264–269 [PMC free article] [PubMed] [Google Scholar]
- Brown T.R. (1995) Human androgen insensitivity syndrome. J Androl 16: 299–303 [PubMed] [Google Scholar]
- Brown W.M., Lange E.M., Chen H., Zheng S.L., Chang B., Wiley K.E., et al. (2004) Hereditary prostate cancer in African American families: linkage analysis using markers that map to five candidate susceptibility loci. Br J Cancer 90: 510–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J.S., Meyer C.A., Song J., Li W., Geistlinger T.R., Eeckhoute J., et al. (2006) Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38: 1289–1297 [DOI] [PubMed] [Google Scholar]
- Cha E., Fong L. (2010) Therapeutic vaccines for prostate cancer. Curr Opin Mol Ther 12: 77–85 [PubMed] [Google Scholar]
- Chang C.Y., Walther P.J., McDonnell D.P. (2001) Glucocorticoids manifest androgenic activity in a cell line derived from a metastatic prostate cancer. Cancer Res 61: 8712–8717 [PubMed] [Google Scholar]
- Chen T., Kablaoui N., Little J., Timofeevski S., Tschantz W.R., Chen P., et al. (2009) Identification of small molecule inhibitors of the JIP and JNK interaction. Biochem J 420: 283–294 [DOI] [PubMed] [Google Scholar]
- Chi K.N., Bjartell A., Dearnaley D., Saad F., Schröder F.H., Sternberg C., et al. (2009) Castration-resistant prostate cancer: from new pathophysiology to new treatment targets. Eur Urol 56: 594–605 [DOI] [PubMed] [Google Scholar]
- Chomez P., De Backer O., Bertrand M., De Plaen E., Boon T., Lucas S. (2001) An overview of the MAGE gene family with the identification of all human members of the family. Cancer Res 61: 5544–5551 [PubMed] [Google Scholar]
- Culig Z., Bartsch G. (2006) Androgen axis in prostate cancer. J Cell Biochem 99: 373–381 [DOI] [PubMed] [Google Scholar]
- Culig Z., Steiner H., Bartsch G., Hobisch A. (2005) Interleukin-6 regulation of prostate cancer cell growth. J Cell Biochem 95: 497–505 [DOI] [PubMed] [Google Scholar]
- Delbridge M.L., Graves J.A. (2007) Origin and evolution of spermatogenesis genes on the human sex chromosomes. Soc Reprod Fertil Suppl 65: 1–17 [PubMed] [Google Scholar]
- De Plaen E., Arden K., Traversari C., Gaforio J.J., Szikora J.P., De Smet C., et al. (1994) Structure, chromosomal localization, and expression of 12 genes of the MAGE family. Immunogenetics 40: 360–369 [DOI] [PubMed] [Google Scholar]
- De Plaen E., De Backer O., Arnaud D., Bonjean B., Chomez P., Martelange V., et al. (1999) A new family of mouse genes homologous to the human MAGE genes. Genomics 55: 176–184 [DOI] [PubMed] [Google Scholar]
- De Smet C., De Backer O., Faraoni I., Lurquin C., Brasseur F., Boon T. (1996) The activation of human gene MAGE-1 in tumor cells is correlated with genome-wide demethylation. Proc Natl Acad Sci U S A 93: 7149–7153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet C., Loriot A., Boon T. (2004) Promoter-dependent mechanism leading to selective hypomethylation within the 5′ region of gene MAGE-A1 in tumor cells. Mol Cell Biol 24: 4781–4790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet C., Lurquin C., Lethé B., Martelange V., Boon T. (1999) DNA methylation is the primary silencing mechanism for a set of germ line- and tumor-specific genes with a CpG-rich promoter. Mol Cell Biol 19: 7327–7335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobosy J.R., Roberts J.L., Fu V.X., Jarrard D.F. (2007) The expanding role of epigenetics in the development, diagnosis and treatment of prostate cancer and benign prostatic hyperplasia. J Urol 177: 822–831 [DOI] [PubMed] [Google Scholar]
- Dutt S.S., Gao A.C. (2009) Molecular mechanisms of castration-resistant prostate cancer progression. Future Oncol 5: 1403–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M. (2002) DNA methylation in cancer: too much, but also too little. Oncogene 21: 5400–5413 [DOI] [PubMed] [Google Scholar]
- Furutani T., Takeyama K., Koutoku H., Ito S., Taniguchi N., Suzuki E., et al. (2005) A role of androgen receptor protein in cell growth of an androgen-independent prostate cancer cell line. Biosci Biotechnol Biochem 69: 2236–2239 [DOI] [PubMed] [Google Scholar]
- Gaugler B., Van den Eynde B., van der Bruggen P., Romero P., Gaforio J.J., De Plaen E., et al. (1994) Human gene MAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. J Exp Med 179: 921–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golias C., Iliadis I., Peschos D., Charalabopoulos K. (2009) Amplification and co-regulators of androgen receptor gene in prostate cancer. Exp Oncol 31: 3–8 [PubMed] [Google Scholar]
- Graat H.C., Carette J.E., Schagen F.H., Vassilev L.T., Gerritsen W.R., Kaspers G.J., et al. (2007) Enhanced tumor cell kill by combined treatment with a small-molecule antagonist of mouse double minute 2 and adenoviruses encoding p53. Mol Cancer Ther 6: 1552–1561 [DOI] [PubMed] [Google Scholar]
- Gregory C.W., Fei X., Ponguta L.A., He B., Bill H.M., French F.S., Wilson E.M. (2004) Epidermal growth factor increases coactivation of the androgen receptor in recurrent prostate cancer. J Biol Chem 279: 7119–7130 [DOI] [PubMed] [Google Scholar]
- Gregory C.W., He B., Johnson R.T., Ford O.H., Mohler J.L., French F.S., Wilson E.M. (2001) A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res 61: 4315–4319 [PubMed] [Google Scholar]
- Gregory C.W., Johnson R.T., Mohler J.L., French F.S., Wilson E.M. (2001) Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res 61: 2892–2898 [PubMed] [Google Scholar]
- Grigoriadis A., Caballero O.L., Hoek K.S., da Silva L., Chen Y.T., Shin S.J., et al. (2009) CT-X antigen expression in human breast cancer. Proc Natl Acad Sci U S A 106: 13493–13498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Dai B., Jiang T., Xu K., Xie Y., Kim O., et al. (2006) Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell 10: 309–319 [DOI] [PubMed] [Google Scholar]
- Gure A.O., Chua R., Williamson B., Gonen M., Ferrera C.A., Gnjatic S., et al. (2005) Cancer–testis genes are coordinately expressed and are markers of poor outcome in non-small cell lung cancer. Clin Cancer Res 11: 8055–8062 [DOI] [PubMed] [Google Scholar]
- Harris S.E., Harris M.A., Rong Z., Hall J., Judge S., French F.S., et al. Karr J.P., Coffey D.S., Smith R.G., Tindall D.J. (1991) Androgen regulation of HBGF-1 (alpha FGF) mRNA and characterization of the androgen receptor mRNA in the human prostate carcinoma cell line LNCaP/A-Dep. Molecular and Cellular Biology of Prostate Cancer Plenum Press: New York, 315–330 [Google Scholar]
- He B., Bowen N.T., Minges J.T., Wilson E.M. (2001) Androgen-induced NH2- and carboxyl-terminal interaction inhibits p160 coactivator recruitment by activation function 2. J Biol Chem 276: 42293–42301 [DOI] [PubMed] [Google Scholar]
- He B., Gampe R.T., Hnat A.T., Faggart J.L., Minges J.T., French F.S., Wilson E.M. (2006) Probing the functional link between androgen receptor coactivator and ligand binding sites in prostate cancer and androgen insensitivity. J Biol Chem 281: 6648–6663 [DOI] [PubMed] [Google Scholar]
- He B., Gampe R.T., Kole A.J., Hnat A.T., Stanley T.B., An G., et al. (2004) Structural basis for androgen receptor interdomain and coactivator interactions suggests a transition in nuclear receptor activation function dominance. Mol Cell 16: 425–438 [DOI] [PubMed] [Google Scholar]
- He B., Kemppainen J.A., Wilson E.M. (2000) FXXLF and WXXLF sequences mediate the NH2-terminal interaction with the ligand binding domain of the androgen receptor. J Biol Chem 275: 22986–22994 [DOI] [PubMed] [Google Scholar]
- He B., Lee L.W., Minges J.T., Wilson E.M. (2002) Dependence of selective gene activation on the androgen receptor NH2- and carboxyl-terminal interaction. J Biol Chem 277: 25631–25639 [DOI] [PubMed] [Google Scholar]
- Heemers H.V., Tindall D.J. (2007) Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev 28: 778–808 [DOI] [PubMed] [Google Scholar]
- Hérin M., Lemoine C., Weynants P., Vessière F., Van Pel A., Knuth A., et al. (1987) Production of stable cytolytic T-cell clones directed against autologous human melanoma. Int J Cancer 39: 390–396 [DOI] [PubMed] [Google Scholar]
- Hieronymus H., Lamb J., Ross K.N., Peng X.P., Clement C., Rodina A., et al. (2006) Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer Cell 10: 321–330 [DOI] [PubMed] [Google Scholar]
- Hong J.A., Kang Y., Abdullaev Z., Flanagan P.T., Pack S.D., Fischette M.R., et al. (2005) Reciprocal binding of CTCF and BORIS to the NY-ESO-1 promoter coincides with derepression of this cancer–testis gene in lung cancer cells. Cancer Res 65: 7763–7774 [DOI] [PubMed] [Google Scholar]
- Irvine R.A., Coetzee G.A. (1999) Additional upstream coding sequences of MAGE-11. Immunogenetics 49: 585–585 [DOI] [PubMed] [Google Scholar]
- Jurk M., Kremmer E., Schwarz U., Förster R., Winnacker E.L. (1998) MAGE-11 protein is highly conserved in higher organisms and located predominantly in the nucleus. Int J Cancer 75: 762–766 [DOI] [PubMed] [Google Scholar]
- Kalejs M., Erenpreisa J. (2005) Cancer/testis antigens and gametogenesis: a review and “brain-storming” session. Cancer Cell Int 5: 4–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpf A.R. (2006) A potential role for epigenetic modulatory drugs in the enhancement of cancer/ germ-line antigen vaccine efficacy. Epigenetics 1: 116–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpf A.R., Bai S., James S.R., Mohler J.L., Wilson E.M. (2009) Increased expression of androgen receptor coregulator MAGE-11 in prostate cancer by DNA hypomethylation and cyclic AMP. Mol Cancer Res 7: 523–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen J.A., Langley E., Wong C.I., Bobseine K., Kelce W.R., Wilson E.M. (1999) Distinguishing androgen receptor agonists and antagonists: distinct mechanisms of activation by medroxyprogesterone acetate and dihydrotestosterone. Mol Endocrinol 13: 440–454 [DOI] [PubMed] [Google Scholar]
- Klein K.A., Reiter R.E., Redula J., Moradi H., Zhu X.L., Brothman A.R., et al. (1997) Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nat Med 3: 402–408 [DOI] [PubMed] [Google Scholar]
- Knudsen K.E., Penning T.M. (2010) Partners in crime: deregulation of AR activity and androgen synthesis in prostate cancer. Trends Endocrinol Metab 21: 315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb-Kokocinski A., Mehrle A., Bechtel S., Simpson J.C., Kioschis P., Wiemann S., et al. (2006) The systematic functional characterisation of Xq28 genes prioritises candidate disease genes. BMC Genomics 7: 29–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassie K., Dawson N.A. (2010) Update on castrate-resistant prostate cancer: 2010. Curr Opin Oncol 22: 263–267 [DOI] [PubMed] [Google Scholar]
- Lee S., Walker C.L., Karten B., Kuny S.L., Tennese A.A., O'Neill M.A., Wevrick R. (2005) Essential role for the Prader-Willi syndrome protein necdin in axonal outgrowth. Hum Mol Genet 14: 627–637 [DOI] [PubMed] [Google Scholar]
- Li T.H., Zhao H., Peng Y., Beliakoff J., Brooks J.D., Sun Z.A. (2007) A promoting role of androgen receptor in androgen-sensitive and -insensitive prostate cancer cells. Nucleic Acids Res 35: 2767–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linja M.J., Savinainen K.J., Saramäki O.R., Tammela T.L., Vessella R.L., Visakorpi T. (2001) Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res 61: 3550–3555 [PubMed] [Google Scholar]
- Locke J.A., Guns E.S., Lubik A.A., Adomat H.H., Hendy S.C., Wood C.A., et al. (2008) Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res 68: 6407–6415 [DOI] [PubMed] [Google Scholar]
- Loukinov D.I., Pugacheva E., Vatolin S., Pack S.D., Moon H., Chernukhin I., et al. (2002) BORIS, a novel male germ-line-specific protein associated with epigenetic reprogramming events, shares the same 11-zinc-finger domain with CTCF, the insulator protein involved in reading imprinting marks in the soma. Proc Natl Acad Sci U S A 99: 6806–6811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C., Patterson N.M., Cherian M.T., Aninye I.O., Montoya J.B., Zhang C., et al. (2008) A new small molecule inhibitor of estrogen receptor alpha binding to estrogen response elements blocks estrogen-dependent growth of cancer cells. J Biol Chem 283: 12819–12830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand M., Punt C.J., Aamdal S., Escudier B., Kruit W.H., Keilholz U., et al. (2003) Immunization of metastatic cancer patients with MAGE-3 protein combined with adjuvant SBAS-2: a clinical report. Eur J Cancer 39: 70–77 [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Taniura H., Uetsuki T., Yoshikawa K. (2001) Necdin acts as a transcriptional repressor that interacts with multiple guanosine clusters. Gene 272: 173–179 [DOI] [PubMed] [Google Scholar]
- Mellinghoff I.K., Vivanco I., Kwon A., Tran C., Wongvipat J., Sawyers C.L. (2004) HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell 6: 517–527 [DOI] [PubMed] [Google Scholar]
- Mischo A., Kubuschok B., Ertan K., Preuss K.D., Romeike B., Regitz E., et al. (2006) Prospective study on the expression of cancer testis genes and antibody responses in 100 consecutive patients with primary breast cancer. Int J Cancer 118: 696–703 [DOI] [PubMed] [Google Scholar]
- Mohler J.L., Gregory C.W., Ford O.H., Kim D., Weaver C.M., Petrusz P., et al. (2004) The androgen axis in recurrent prostate cancer. Clin Can Res 10: 440–448 [DOI] [PubMed] [Google Scholar]
- Monte M., Simonatto M., Peche L.Y., Bublik D.R., Gobessi S., Pierotti M.A., et al. (2006) MAGE-A tumor antigens target p53 transactivation function through histone deacetylase recruitment and confer resistance to chemotherapeutic agents. Proc Natl Acad Sci U S A 103: 11160–11165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery R.B., Mostaghel E.A., Vessella R., Hess D.L., Kalhorn T.F., Higano C.S., et al. (2008) Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res 68: 4447–4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoletano C., Bellati F., Tarquini E., Tomao F., Taurino F., Spagnoli G., et al. (2008) MAGE-A and NY-ESO-1 expression in cervical cancer: prognostic factors and effects of chemotherapy. Am J Obstet Gynecol 198: 99.e1–7 [DOI] [PubMed] [Google Scholar]
- Odunsi K., Qian F., Matsuzaki J., Mhawech-Fauceglia P., Andrews C., Hoffman E.W., et al. (2007) Vaccination with an NY-ESO-1 peptide of HLA class I/II specificities induces integrated humoral and T cell responses in ovarian cancer. Proc Natl Acad Sci U S A 104: 12837–12842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman Forslund K., Nordqvist K. (2001) The melanoma antigen genes—any clues to their functions in normal tissues? Exp Cell Res 265: 185–194 [DOI] [PubMed] [Google Scholar]
- O’Malley B.W. (2009) The year in basic science: nuclear receptors and coregulators. Mol Endocrinol 22: 2751–2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.H., Lee S.W. (2002) Hypertonicity induction of melanoma antigen, a tumor-associated antigen. Mol Cells 13: 288–295 [PubMed] [Google Scholar]
- Ponguta L.A., Gregory C.W., French F.S., Wilson E.M. (2008) Site-specific androgen receptor serine phosphorylation linked to epidermal growth factor-dependent growth of castration-recurrent prostate cancer. J Biol Chem 283: 20989–21001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley C.A., De Bellis A., Marschke K.B., El-Awady M.K., Wilson E.M., French F.S. (1995) Androgen receptor defects: historical, clinical and molecular perspectives. Endocrine Rev 16: 271–321 [DOI] [PubMed] [Google Scholar]
- Reid A.H., Attard G., Danila D.C., Oommen N.B., Olmos D., Fong P.C., et al. (2010) Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the cyp17 inhibitor abiraterone acetate. J Clin Oncol 28: 1489–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogner U.C., Wilke K., Steck E., Korn B., Poustka A. (1995) The melanoma antigen gene (MAGE) family is clustered in the chromosomal band Xq28. Genomics 29: 725–731 [DOI] [PubMed] [Google Scholar]
- Ross M.T., Grafham D.V., Coffey A.J., Scherer S., McLay K., Muzny D., et al. (2005) The DNA sequence of the human X chromosome. Nature 434: 325–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadanaga N., Nagashima H., Mashino K., Tahara K., Yamaguchi H., Ohta M., et al. (2001) Dendritic cell vaccination with MAGE peptide is a novel therapeutic approach for gastrointestinal carcinomas. Clin Cancer Res 7: 2277–2284 [PubMed] [Google Scholar]
- Saifi G.M., Chandra H.S. (1999) An apparent excess of sex- and reproduction-related genes on the human X chromosome. Proc Biol Sci 266: 203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi A.H., Roux P.P., Kubu C.J., Zeindler C., Bhakar A., Tannis L.L., et al. (2000) NRAGE, a novel MAGE protein, interacts with the p75 neurotrophin receptor and facilitates nerve growth factor-dependent apoptosis. Neuron 27: 279–288 [DOI] [PubMed] [Google Scholar]
- Scanlan M.J., Gure A.O., Jungbluth A.A., Old L.J., Chen Y.T. (2002) Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev 188: 22–32 [DOI] [PubMed] [Google Scholar]
- Scanlan M.J., Simpson A.J., Old L.J. (2004) The cancer/testis genes: review, standardization, and commentary. Cancer Immun 4: 1–1 [PubMed] [Google Scholar]
- Schmitt B., Wilt T.J., Schellhammer P.F., DeMasi V., Sartor O., Crawford E.D., Bennett C.L. (2001) Combined androgen blockade with nonsteroidal antiandrogens for advanced prostate cancer: a systematic review. Urology 57: 727–732 [DOI] [PubMed] [Google Scholar]
- Schulz W.A., Hoffmann M.J. (2009) Epigenetic mechanisms in the biology of prostate cancer. Semin Cancer Biol 19: 172–180 [DOI] [PubMed] [Google Scholar]
- Shichijo S., Yamada A., Sagawa K., Iwamoto O., Sakata M., Nagai K., Itoh K. (1996) Induction of MAGE genes in lymphoid cells by the demethylating agent 5-aza-2′-deoxycytidine. Jpn J Cancer Res 87: 751–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson A.J., Caballero O.L., Jungbluth A., Chen Y.T., Old L.J. (2005) Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer 5: 615–625 [DOI] [PubMed] [Google Scholar]
- Solit D.B., Scher H.I., Rosen N. (2003) Hsp90 as a therapeutic target in prostate cancer. Semin Oncol 30: 709–716 [DOI] [PubMed] [Google Scholar]
- Stanbrough M., Bubley G.J., Ross K., Golub T.R., Rubin M.A., Penning T.M., et al. (2006) Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res 66: 2815–2825 [DOI] [PubMed] [Google Scholar]
- Stavridi F., Karapanagiotou E.M., Syrigos K.N. (2010) Targeted therapeutic approaches for hormone-refractory prostate cancer. Cancer Treat Rev 36: 122–130 [DOI] [PubMed] [Google Scholar]
- Stephan D.A., Howell G.R., Teslovich T.M., Coffey A.J., Smith L., Bailey-Wilson J.E., et al. (2002) Physical and transcript map of the hereditary prostate cancer region at xq27. Genomics 79: 41–50 [DOI] [PubMed] [Google Scholar]
- Tan J., Sharief Y., Hamil K.G., Gregory C.W., Zang D.Y., Sar M., et al. (1997) Dehydroepiandrosterone activates mutant androgen receptors expressed in the androgen-dependent human prostate cancer xenograft CWR22 and LNCaP cells. Mol Endocrinol 11: 450–459 [DOI] [PubMed] [Google Scholar]
- Taniura H., Kobayashi M., Yoshikawa K. (2005) Functional domains of necdin for protein-protein interaction, nuclear matrix targeting, and cell growth suppression. J Cell Biochem 94: 804–815 [DOI] [PubMed] [Google Scholar]
- Taniura H., Matsumoto K., Yoshikawa K. (1999) Physical and functional interactions of neuronal growth suppressor necdin with p53. J Biol Chem 274: 16242–16248 [DOI] [PubMed] [Google Scholar]
- Tay S.K., Blythe J., Lipovich L. (2009) Global discovery of primate-specific genes in the human genome. Proc Natl Acad Sci U S A 106: 12019–12024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurner B., Haendle I., Röder C., Dieckmann D., Keikavoussi P., Jonuleit H., et al. (1999) Vaccination with MAGE-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med 190: 1669–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus M.A., Gregory C.W., Ford O.H., Schell M.J., Maygarden S.J., Mohler J.L. (2005a) Steroid 5alpha-reductase isozymes I and II in recurrent prostate cancer. Clin Cancer Res 11: 4365–4371 [DOI] [PubMed] [Google Scholar]
- Titus M.A., Schell M.J., Lih F.B., Tomer K.B., Mohler J.L. (2005b) Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res 11: 4653–4657 [DOI] [PubMed] [Google Scholar]
- Turner L.M., Hoekstra H.E. (2008) Causes and consequences of the evolution of reproductive proteins. Int J Dev Biol 52: 769–780 [DOI] [PubMed] [Google Scholar]
- Velazquez E.F., Jungbluth A.A., Yancovitz M., Gnjatic S., Adams S., O'Neill D., et al. (2007) Expression of the cancer/testis antigen NY-ESO-1 in primary and metastatic malignant melanoma (MM)—correlation with prognostic factors. Cancer Immun 7: 11–11 [PMC free article] [PubMed] [Google Scholar]
- Veldscholte J., Ris-Stalpers C., Kuiper G.G., Jenster G., Berrevoets C., Claassen E., et al. (1990) A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun 173: 534–540 [DOI] [PubMed] [Google Scholar]
- Vis A.N., Schröder F.H. (2009) Key targets of hormonal treatment of prostate cancer. Part 1: the androgen receptor and steroidogenic pathways. BJU Int 104: 438–448 [DOI] [PubMed] [Google Scholar]
- Visakorpi T., Hyytinen E., Koivisto P., Tanner M., Keinänen R., Palmberg C., et al. (1995) In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet 9: 401–406 [DOI] [PubMed] [Google Scholar]
- Waltering K.K., Helenius M.A., Sahu B., Manni V., Linja M.J., Jänne O.A., Visakorpi T. (2009) Increased expression of androgen receptor sensitizes prostate cancer cells to low levels of androgens. Cancer Res 69: 8141–8149 [DOI] [PubMed] [Google Scholar]
- Wang G.L., Jiang B.H., Rue E.A., Semenza G.L. (1995) Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A 92: 5510–5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C.J., Xue B., Qin W.X., Yu M., Zhang M.Y., Zhao D.H., et al. (2004) hNRAGE, a human neurotrophin receptor interacting MAGE homologue, regulates p53 transcriptional activity and inhibits cell proliferation. FEBS Lett 564: 171–176 [DOI] [PubMed] [Google Scholar]
- Wischnewski F., Friese O., Pantel K., Schwarzenbach H. (2007) Methyl-CpG binding domain proteins and their involvement in the regulation of the MAGE-A1, MAGE-A2, MAGE-A3, and MAGE-A12 gene promoters. Mol Cancer Res 5: 749–759 [DOI] [PubMed] [Google Scholar]
- Wischnewski F., Pantel K., Schwarzenbach H. (2006) Promoter demethylation and histone acetylation mediate gene expression of MAGE-A1, -A2, -A3, and -A12 in human cancer cells. Mol Cancer Res 4: 339–349 [DOI] [PubMed] [Google Scholar]
- Xu J., Meyers D., Freije D., Isaacs S., Wiley K., Nusskern D., et al. (1998) Evidence for a prostate cancer susceptibility locus on the X chromosome. Nat Genet 20: 175–179 [DOI] [PubMed] [Google Scholar]
- Yang B., O'Herrin S.M., Wu J., Reagan-Shaw S., Ma Y., Bhat K.M., et al. (2007) MAGE-A, mMage-b, and MAGE-C proteins form complexes with KAP1 and suppress p53-dependent apoptosis in MAGE-positive cell lines. Cancer Res 67: 9954–9962 [DOI] [PubMed] [Google Scholar]
- Yuan X., Li T., Wang H., Zhang T., Barua M., Borgesi R.A., et al. (2006) Androgen receptor remains critical for cell-cycle progression in androgen-independent CWR22 prostate cancer cells. Am J Pathol 169: 682–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegarra-Moro O.L., Schmidt L.J., Huang H., Tindall D.J. (2002) Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res 62: 1008–1013 [PubMed] [Google Scholar]
- Zheng K., Yang F., Wang P.J. (2010) Regulation of male fertility by X-linked genes. J Androl 31: 79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]