Abstract
Rationale
Investigations of the neural consequences of the effects of cocaine on cognition have centered on specific brain circuits including prefrontal cortex, medial temporal lobe and striatum and their roles in controlling drug dependent behavior and addiction. These regions are critical to many aspects of drug abuse; however recent investigations in addicted individuals have reported possible cognitive deficits that impact recovery and other therapeutic interventions.
Objectives
Therefore a direct assessment of the effects of cocaine as a reward for cognitive function provides a means of determining how brain systems involved such as prefrontal cortex are affected under normal vs. conditions of acute drug exposure as a precursor to the final impaired function in the addicted state.
Methods
Nonhuman primates (NHPs) were tested in a delayed-match-to-sample decision making task to determine effects of high vs. low cognitive load trials on single neuron activity and fluorodeoxyglucose-positron emission tomography (FDG-PET) determined metabolic activation of prefrontal cortex when juice vs. intravenous cocaine were employed as rewards for successful performance.
Results
Cognitive processing in prefrontal cortex was altered primarily on high load trials in which cocaine was randomly presented as the signaled and delivered reward on particular trials. The detrimental actions of cocaine rewards were also shown to persist and impair task performance on subsequent juice rewarded trials.
Conclusions
The findings indicate that one of the ways in which cocaine use may disrupt performance of a cognitive task is to alter neural processing in prefrontal cortex when involved in discriminating circumstances on the basis of low vs. high cognitive demand.
Keywords: Prefrontal Cortex, Neural recording, PET Imaging, Cocaine rewards, Decision processes
Introduction
A major factor underlying substance abuse as a societal problem is that people under the influence of addictive drugs cannot always make correct decisions (Bechara et al. 2001; Bechara and Martin 2004). The transformation of decision making from rational to irrational within the same social, professional, and behavioral contexts with respect to drug seeking is obvious in most cases, yet many decisions are made that maintain a modicum of the addict’s former lifestyle to allow continued drug use (Rogers and Robbins 2001). Therefore, a major problem with respect to countering drug use in society is the lack information related to the underlying cognitive processes that are impaired in chronic drug users (Goldstein et al. 2004; Sun and Rebec 2006). Given recent advances in describing the neural basis of information processing using current imaging and recording methods (Berns 1999; Browndyke et al. 2004; Tsujimoto et al. 2004; Stout et al. 2004), it is now possible to determine which brain areas are critical for certain types of mental activity, including different types of memories, planning via executive function and intentional motor activity (Ashby and Spiering 2004; Buckner et al. 2000; Fogassi et al. 2005; Keri 2003; Miller and Cohen 2001; Murray et al. 2000). Similar studies have also shown how mental processes are affected by recreational and chronic drug use, including gambling strategies, short-term memory, and motor control (Adinoff et al. 2003; Hester and Garavan 2004). Historically much of the emphasis in understanding drug actions in animals and humans has been directed at investigating the “rewarding” properties and what cellular and brain processes related to motivational factors such as response inhibition dysfunction, have been altered by continued abuse of drugs such as cocaine (Staley and Mash 1996; Goldstein and Volkow 2002; Bolla et al. 2003). While it is important to eventually understand the cellular, gene and neural substrates of drug reward as a basis for addiction (Cornish and Kalivas 2000; Toda et al. 2006; Volkow et al. 2006; Opris et al. 2009), assessment of how abused drugs such as cocaine modulate other cognitive processes involved in executive function, such as memory impairment, decision making and performance maintenance, can also reveal important factors related to the major problem of response inhibition dysfunction in humans (Stout et al. 2004; Karila et al. 2007).
Employment of nonhuman primates (NHPs) to assess cocaine actions has been one means of addressing both of these issues (Grant et al. 2000; Porrino and Lyons 2000; Bradberry et al. 2000; Porrino et al. 2001; Nader et al. 2002; Jentsch et al. 2002; Bradberry 2007). In the current study, we examine the neuronal and behavioral effects of cocaine in NHPs performing a delayed short-term memory task that provokes cognitive demand from low to high extremes with respect to types of trials presented. Differences in performance level is examined with respect to the type of reward delivered on each trial, either (1) the customary natural fruit juice, or (2) intravenous (IV) doses of cocaine infused at the end of successful trials. The neural basis of differences in juice vs. cocaine rewards are assessed in the same animals using either functional PET imaging and/or electrophysiological readout from regions of prefrontal cortex (PFC) involved in making the necessary cognitive decisions within the task. The results show that cocaine, when delivered as a reward for correct performance, decreases performance of the task in a dose-dependent manner accompanied by disruption of associated neuronal firing in dorsal (PFC) on trials with low vs. high cognitive load.
Methods
Animals
All procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Wake Forest University, and performed in accordance with established practices as described in the National Institutes of Health and Public Health Service policy on humane care and use of laboratory animals. Six adult male rhesus (Macaca mulatta) monkeys (6–15 kg) housed in stainless steel cages in temperature and humidity controlled colony rooms with lighting maintained on a 0600:1800 hours day/night cycle and visual contact with conspecifics at all times except during experimental sessions. Animals were fed a daily diet of NHP chow supplemented by fresh fruit and chewable multiple vitamin tablets. All six animals were tested using fluorodeoxyglucose-positron emission tomography (FDG PET) procedures and four of these animals were utilized for electrophysiological recording.
Behavioral apparatus and training
NHPs were trained to sit quietly in a custom designed primate chair (Crist Instruments, Hagerstown, MD) configured for free arm movement across a horizontal surface in front of the chair positioned 1.5 m from a 1.0 × 1.0 m LCD-front-projection screen. The chairs were housed in a sound attenuated cubicle in a larger animal testing room with low light CCD cameras to allow constant observation. The primate chair contained two reward delivery systems: (1) a juice delivery system consisting of a 500-ml reservoir for juice mounted on the wall, an electrically operated juice valve and plastic tube to gravity feed juice to a sipper tube mounted in front of the animal’s lips, and (2) a drug delivery system consisting of a 250-ml sterile saline reservoir containing mixed cocaine solution connected to the animal via "IV drip" tubing and a peristaltic pump with computer and timer operated remote control. Limb position was tracked via a UV-fluorescent adhesive reflector affixed to the back of the hand by a small LCD camera positioned 30 cm above. Horizontal position of the illuminated target was computed using a Plexon Video Tracker (Plexon, Inc., Dallas TX) that digitized and displayed hand position as a bright yellow cursor on the projection screen. Animals were trained to move a cursor on the projection screen by positioning the hand within a two-dimensional coordinate system on the chair counter into 25 cm clip-art images downloaded from a website and stored for daily usage.
Multi-item visual delayed-match-to-sample task
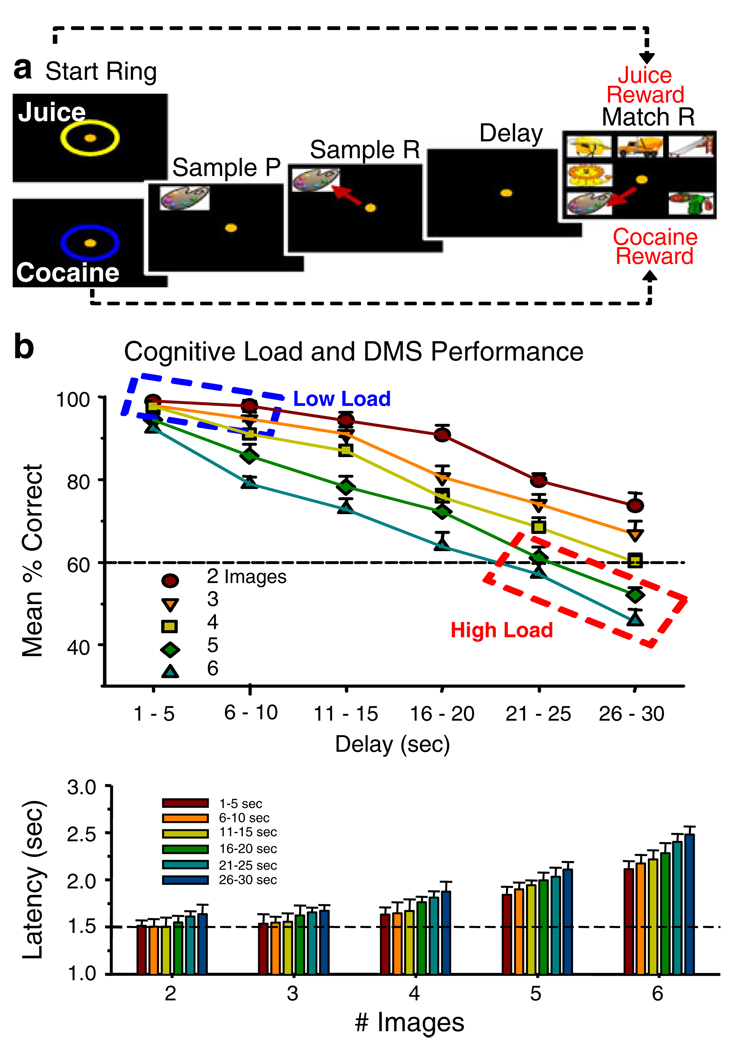
A previously utilized delayed-match-to-sample (DMS) short-term memory task (Porrino et al. 2005; Deadwyler et al. 2007; Hampson et al. 2009, 2010) with different degrees of “cognitive load” or task difficulty for NHPs was modified to test the effects of the two different rewards. Specifically the task examined the relationship between performance on trials with orally administered fruit juice as the reward and trials in which direct 10.0 s IV infusion of cocaine (0.03–0.09 mg/kg/infusion) was the reward (Bowman et al. 1996; Opris et al. 2009). Figure 1a describes the features of the DMS task and shows the sequence of trial events dependent on the type of reward delivered for correct responses in the Match phase. The only difference from previous versions of the DMS task was that trials initiated by the animal placing the cursor into a Start Ring, coded, and signaled the type of reward delivered. As shown in Fig. 1a, if the Start Ring was yellow it signaled a juice rewarded trial (Start Ring: Juice); or if the Start Ring was blue it signaled delivery of IV cocaine (Start Ring: Cocaine) for successful performance of the trial. Correct juice-signaled reward trials were completed with 0.5 ml of dilute fruit juice delivered to a sipper tube placed in front of the animal’s mouth following the Match response (Fig. 1a). Correct cocaine-signaled reward trials consisted of operation of the peristaltic pump to infuse (IV) drug for 10 s after the appropriate Match response.
Fig. 1.
Delayed match to sample (DMS) task: a Schematic of Juice-and Cocaine-rewarded DMS trials. Appearance of Start Ring signals condition for initiation of trial which is started when the cursor (yellow dot) is placed within either ring for 1.0 s. The color of Start Ring (blue for cocaine and yellow for juice) was the signal for the type of reward to be delivered for successful performance at the end of the trial. The remaining series of screen display events after the Start Ring were the same for each trial regardless of type of reward. Placement of the cursor into the Start Ring produced a randomly selected “Sample” clip-art image (Sample P) displayed for 2 s in one of nine different screen positions. Placement of the cursor in the Sample image (Sample R) blanked the screen and initiated the Delay Phase with 1–30 s duration selected randomly on each trial. Time-out of the Delay initiated the Match Phase of the task in which two to eight images appeared on the screen randomly at nine different possible locations, one of which was the previous Sample image. Placement of the cursor into the Sample image for 1.0 s constituted a correct Match response (Match R) followed immediately by delivery of either juice or cocaine reward dependent on the type of trial signaled by the color of the Start Ring. Placement of the cursor into other images for >0.5 s constituted an error causing the screen to blank with no reward delivery. The intertrial interval (ITI) was 10 or 30 s depending on the type of reward (juice or cocaine) delivered. The number of non-match “distracter” images presented with the Sample image in the Match phase varied randomly from one to seven on each trial. All clip-art images in a given session were unique, no Sample or distracter clip-art image was utilized more than once within the same session (100–150 trials/session) and all images were new in each session (Hampson et al. 2004). b Illustration of differences in performance as a function of cognitive load in the DMS task. Upper: percent correct performance in DMS task as a function of duration of delay interval (Delay sec) and number of images presented in the Match phase signified by the five different delay curves plotted for each number of distracter images in the Match phase of the task. The dotted boxes enclose the parameters at the extremes of the continuum of cognitive workload from low (blue) vs. high (red) difficulty (Hampson et al. 2009). Lower: decrease in performance accuracy (% correct) as a function of cognitive load is also shown by the systematic change in response latency in the Match phase of the task plotted for the number of images as a function of increased duration of delay (separate bars)
Figure 1b illustrates the different parameters of trial conditions of the DMS task and the average performance for juice-rewarded trials summed over nine NHPs (four of which were subjects in the current study). The upper graph shows different curves plotted which show the decline in performance as a function of increased duration of Delay. Each curve represents performance with a different number of images in the Match phase of the task. The dashed boxes indicate the extremes of difficulty or cognitive load in the DMS task for Low Load (blue dashed box) vs. High Load (red dashed box) trials. Below is plotted latency to respond (seconds) in the Match phase as a function of number of images (two to six) with each bar reflecting the trials with different delay durations in the task. All combinations of delay and # images were presented randomly in normal (juice only) “Mixed” (cocaine+juice) testing sessions with a minimum of 2.0 min (interspersed with juice trials) between presentations of cocaine reward trials. Cocaine rewards were delivered on 40% of trials in Mixed daily sessions consisting of 100–150 total trials. For cognitive load comparisons during FDG PET imaging DMS sessions consisted “Exclusively” of only Low load (short delays, two to three images), or High load (long delays, six to eight images) trials (Hampson et al. 2009) as shown in Fig. 1b.
Drug administration
Each animal was previously prepared with a vascular access port implanted into a major vein (internal or external jugular, femoral, or brachial). Prior to each session, the animal was scrubbed with betadine disinfectant and a 20-gauge Huber Point needle inserted into the port, connecting the infusion pump to the catheter. Correct responses on cocaine-reward trials were followed by delivery of 0.03, 0.06, or 0.09 mg/kg/infusion (inf) injections of a cocaine hydrochloride/saline solution (1.5 ml over 10 s) via computer controlled peristaltic pump (Opris et al. 2009). The intertrial interval (ITI) of 30 s following drug signaled trials was long enough to accommodate the duration of infusion. All cocaine trials were followed by juice trials at 10 s ITIs for 2.0 min to space out drug delivery and maintain a constant systemic concentration. The dose levels of cocaine over the entire session (0.1–0.6 mg/kg) were previously shown to correlate with differences in NHPs self-administering cocaine (Beveridge et al. 2006) and to alter striatal neural activity in NHPs performing a different task to receive similar cocaine rewards (Opris et al. 2009). Following each session, the injection port was filled with heparinized saline (100 units/ml) the animal returned to its home cage.
Surgery
Each animal was anesthetized with ketamine (10 mg/kg) then intubated and maintained with isoflurane (1–2% in oxygen 6 l/min) during surgical placement of recording cylinders (Crist Instruments) on the skull over 11 mm diameter craniotomies (Opris et al. 2009; Hampson et al. 2010) to access targeted areas of PFC (Fig. 2a). Cylinder placement was determined by magnetic resonance imaging (MRI) to overlie frontal cortex at stereotaxic coordinates 25 mm anterior to interaural line and 12 mm lateral to the midline (Paxinos et al. 2003). This location allowed access to the caudal region of principal sulcus, the dorsal limb of arcuate sulcus in area 8 and in the dorsal premotor area 6 (Fig. 2). Two titanium posts were secured to the skull for head restraint with titanium screws embedded in bone cement. Following surgery, animals were given 0.025 mg/kg buprenorphine for analgesia and penicillin injections to prevent infection. All cylinders were cleaned with betadine after surgery and routinely during daily recording sessions. Animals were allowed to recover for at least 1 month prior to commencement of recording within behavioral sessions.
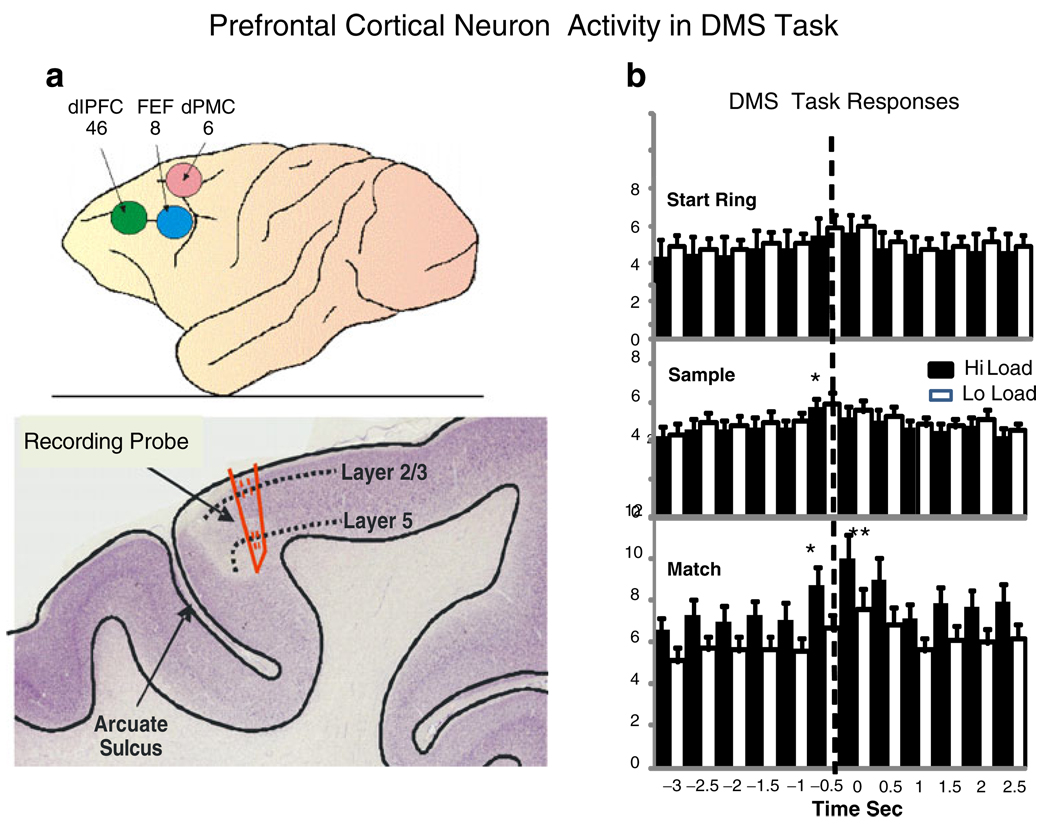
Fig. 2.
Prefrontal cortex (PFC) recording areas and neuron activity in DMS task. a Upper: areas 6, 8, and 46 of NHP prefrontal cortex which bracketed recording tracks for PFC neurons. Lower: cross section of NHP location of recording probe track near the arcuate sulcus in the premotor area. dlPFC dorsolateral prefrontal cortex, FEF frontal eye fields, dPMC dorsal premotor cortex. b Firing of PFC cells in each phase of the DMS task as a function of low vs. high cognitive load for juice reward trials. The PEHs show mean firing rate across all PFC cells (n=96) for the Start Ring Response, Sample Response, and Match Response at t=0.0 s (dotted line), on low (white bars) vs. high (black bars) cognitive load trials. Asterisks indicate significant differences in mean firing rate stated in text
Recording procedures
Recording of PFC neurons utilized data acquisition software for 64 channel simultaneous recordings employing the MAP Spike Sorter by Plexon, Inc. (Dallas, TX). Regular spiking pyramidal neurons (Rao et al. 1997; Elstron et al. 2006) were recorded in layer 2/3 and layer 5 and from the dorsal bank of the arcuate sulcus in the dorsolateral premotor area of PFC. Tungsten microelectrodes were employed for recording single and extracellular neuron action potentials (spikes) identified and isolated online by separation of extracellular action potential waveform duration and amplitude, and confirmed offline using principal components analysis and autocorrelation (Hampson et al. 2010). Neural spike trains were analyzed with respect to firing rate during specific events within DMS trials (Hampson et al. 2004) and segregated as a function of the type of signaled reward (juice or cocaine; Opris et al. 2009) and cognitive load (low or high). Figure 2a shows the areas in PFC in which single neurons were recorded during performance of the DMS task.
Positron emission tomography
Procedures employed for the measurement of rates of cerebral glucose utilization in NPHs were the same as described previously (Porrino et al. 2005; Deadwyler et al. 2007). Awake, conscious NHPs were placed in the primate chair to perform the DMS task and after 10 min injected IV with [18F]fluorodeoxyglucose (18FDG) 5–6 mCi and continued performance of the DMS task to allow the tracer to be incorporated in brain. Blood samples were obtained from a different vein prior to, then 8 and 45 min after FDG injection. All animals were thoroughly habituated to these procedures. After 40 min of task performance following 18FDG injections, animals were anesthetized with ketamine (15 mg/kg) and transported to a Siemens ECAT 951/31 scanner with in-plane (transaxial) resolution of approximately 6.0–6.5 mm in stationary mode and 5.2 mm in wobbled mode. PET assessment consisted of a 5-min scan to set parameters, followed by a 15-min emission scan to detect FDG concentrations in brain. Glucose concentrations were computed from baseline 8- and 45-min blood samples, calibrated from a standard arterial/venous blood glucose, time × concentration, curve. A 30-min transmission scan was obtained once per animal for accurate data reconstruction. PET image data were normalized for glucose metabolic constants, then subjected to 3D region-of-interest (ROI) and voxel-based multivariate statistical analyses using standard SPM99 programs for human brain image analyses adapted to rhesus macca brain coordinates (Porrino et al. 2005). Each animal also received an MRI scan for PET reconstruction and determination of stereotaxic coordinates of brain areas. Determination of regions activated or modified specifically during performance of DMS task was via subtraction of images in the same animal obtained under separate conditions and the resulting alterations displayed as difference images superimposed on MRIs of the frontal cortex (Porrino et al. 2005). The “difference maps” show the regions activated by the task in terms of statistically (SPM99) assessed plots of voxel-by-voxel differences in the PET scans taken under separate conditions. The effects of the different types of rewards were assessed in terms of modifications of task-related brain activity, via PET (18FDG) imaged regions, during sessions with only juice-rewarded trials vs. images from sessions in which cocaine rewards were delivered on 40% and juice rewards on the remaining proportion of trials.
Results
The effect of cocaine-rewarded trials was to suppress DMS task performance in a dose-dependent manner. However, cocaine rewards affected performance in terms of (1) the degree of difficulty of the task as determined on trials of low vs. high cognitive load, (2) the dose of cocaine administered as a reward for successful performance, and (3) performance on juice-rewarded trials that immediately followed cocaine rewarded trials during the session. These changes were accompanied by a significant alteration in 18FDG activation patterns in sessions with cocaine rewarded trials as well as changes in the robustness and latency of firing of FCx neurons correlated with behavioral response latencies on low vs. high cognitive load trials. The results described show that cocaine rewards selectively disrupted DMS performance on trials with greater difficulty (high cognitive load) that required differential metabolic activation and neural firing of FCx neurons, in comparison to low cognitive load trials which were not affected to the same degree.
Effect of cognitive load on frontal cortical activity during performance for juice rewards
Figure 2a shows the areas in PFC in which single neurons (n=96) were recorded that exhibited significant changes in firing rate during performance of the DMS task (see Methods). These include 47 neurons in or near dorsolateral prefrontal cortex (dlPFC), 30 neurons in or near frontal eye fields (FEF), and 19 adjacent to FEF in the dPMC. The number of neurons recorded differed across animals but was ≥4 for each animal in each brain region. Some of the areas (dlPFC, dPMC) are included in those areas in which changes in metabolic rates were observed for low vs. high load trials (Fig. 3a). Figure 2b shows the corresponding mean firing rates of all PFC cells in each of the three phases of the task (Fig. 1a) synchronized to the animal’s behavioral response of placing the cursor into the image for the required 1.0 s (dotted line) to receive a reward. Mean firing rates on low vs. high cognitive load (juice rewarded) trials revealed that significant differences in PFC mean firing rate relative to pre-event (−3.0 to −1.0 s) baseline (F(1,3011)>6.71, p<0.01, F(1,3011)>10.35, p<0.001) only occurred in the Match phase at the time of the Match R (dotted line in bottom perievent histograms [PEHs]) for both low and high cognitive load (Fig. 1b) juice rewarded trials.
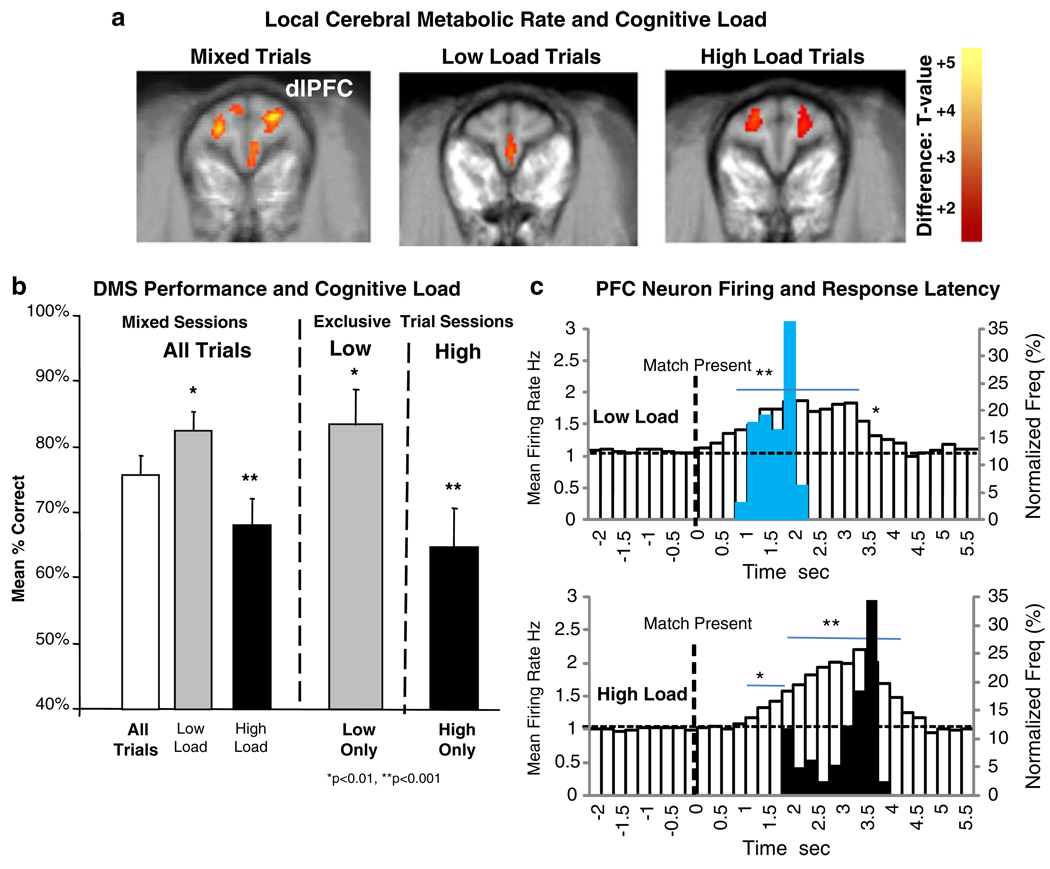
Fig. 3.
Effect of cognitive load on prefrontal local cerebral metabolic rate (LCMR) during DMS performance on juice reward trials. a [18]FDG PET scan images of LCMR in dorsolateral prefrontal cortex (dlPFC) for either Mixed sessions: all types of trials presented over the full range of parameters (Fig. 1b), or Exclusive sessions: only Low or High Load trials presented during the entire session. Difference images obtained from sessions (≥100 trials) with only Low Load or High load trials compared to Mixed sessions with All types of trials. Level of [18]FDG uptake indicated as voxel × voxel change in color from red to yellow as increasing levels of significance (scale shown at right t-test value) for differences in intensities of label (Porrino et al. 2005). b Differences in mean percent correct performance in DMS sessions corresponding to those in which [18]FDG PET scans were obtained in a. Levels of performance in sessions with only low or high load trials (Exclusive) shown with performance in Mixed sessions in which low and high load trials were randomly distributed among the full range of trials presented (Fig. 1b). Asterisks indicate significant differences (*p<0.01, **p<0.001) in mean performance compared to Mixed Sessions (All Trials). c Mean PEHs of PFC neuron firing rate on low vs. high cognitive load juice reward trials associated with corresponding differences in Match R (Fig. 1a) latency on the same trials. Upper and lower white bar PEHs show changes in mean firing rate of PFC neurons following presentation of Match phase images (0.0 s, dotted line) for respective low vs. high load trials. Blue and black bars superimposed on the PEHs indicate distribution of Match R latencies for the same low and high load trials. Asterisks and horizontal bars indicate mean rates in 0.5 s time bins that differ significantly (*p< 0.01, **p<0.001) from mean pre-Match phase (−2.0 to 0.0 s) baseline rate
Past studies employing PET scans of the tracer [18F] fluoro-2-deoxyglucose (18FDG) have provided accurate estimates of localized cerebral metabolic rates (LCMR) of glucose utilization as a sensitive indicator of functional brain areas in NHPs during performance of this DMS task (Porrino et al. 2005; Deadwyler et al. 2007; Hampson et al. 2009). The 18FDG isotope is incorporated during behavioral performance of the DMS task and assessments made later when the animal is anesthetized (Eberling et al. 1995; Martinez et al. 1997; Howell et al. 2001). Prior PET analysis of more than 24 different NHPs in this DMS task have shown the brain areas most activated to be dlPFC, medial temporal lobe (MTL), and dorsal striatum (DStr) during normal performance of the task for juice rewards vs. similar non-task conditions in which the animals watched videos (Porrino et al. 2005; Deadwyler et al. 2007; Hampson et al. 2009).
Figure 3a shows the effects of cognitive load on metabolic activity in the PFC (dlPFC) under normal juice reward conditions (DMS Performance), and when low or high load trials (High Only and Low Only) were exclusively presented (?) (Hampson et al. 2009). This was done in order to obtain activation patterns in sessions in which isotope uptake only occurred when animals were performing either low or high load trials thereby providing DLPFC metabolic activation patterns that were specific for these two trial types. The first difference image in Fig. 3a shows the regions activated during performance of the task under normal testing conditions in which all types of trials (Fig. 1b) were presented randomly during the (Mixed trial) session. The middle and right composite scans in Fig. 3a show dlPFC metabolic activity in the same animals exposed to sessions composed exclusively of low cognitive load or high cognitive load trials (Fig. 1b). Mean % correct DMS performance within the same sessions is shown in Fig. 3b (Mixed and Exclusive sessions). It is important to note that performance in sessions with exclusively low or high load trials was similar to the mean level of the same types of trials sorted from Mixed sessions (Low Load, High Load) when all types of trials (All trials) were presented. Trial specific accuracy in Excusive sessions was different than the overall mean performance level (Mixed All: 75.7± 5.1%) in both cases showing a significantly higher mean level on low load trials (≥83.4±5.5%, F(1,61)>7.48, p< 0.01) and significantly less accuracy on high load trials (≤67.1±6.3%, F(1,61)>10.64, p<0.001) compared to the overall mean of Mixed Sessions (All Trials, Fig. 3b).
Further verification of PFC neuron involvement in the decision making process in the Match phase is shown in Fig. 3c in which the same PFC neuron mean firing rates are plotted in PEHs from 2.0 s prior to the time of Match image presentation (Match Present, 0.0 s, dotted line) to 6.0 s afterward which encompassed the distributions of Match R latencies on low and high load trials. The normalized frequency distributions of Match R latencies (blue and black bars) are superimposed on the PFC neuron PEHs to illustrate the correspondence between the behavioral and neural indicators of the effects of cognitive load. The distributions in Fig. 3c show marked distinctions in both latency to respond and the patterns of PFC neural firing associated with low vs. high cognitive load trials (blue and black bars in Fig. 3c). The asterisks and horizontal bars in Fig. 3c indicate firing rates in 0.5 s time bins that differ significantly (*F(1,3011)>6.95, p<0.01, **F(1,3011)>10.17, p<0.001) from pre-Match phase baseline (−2.0 to 0.0 s).
Effects of cocaine reward on frontal cortical correlates of DMS performance
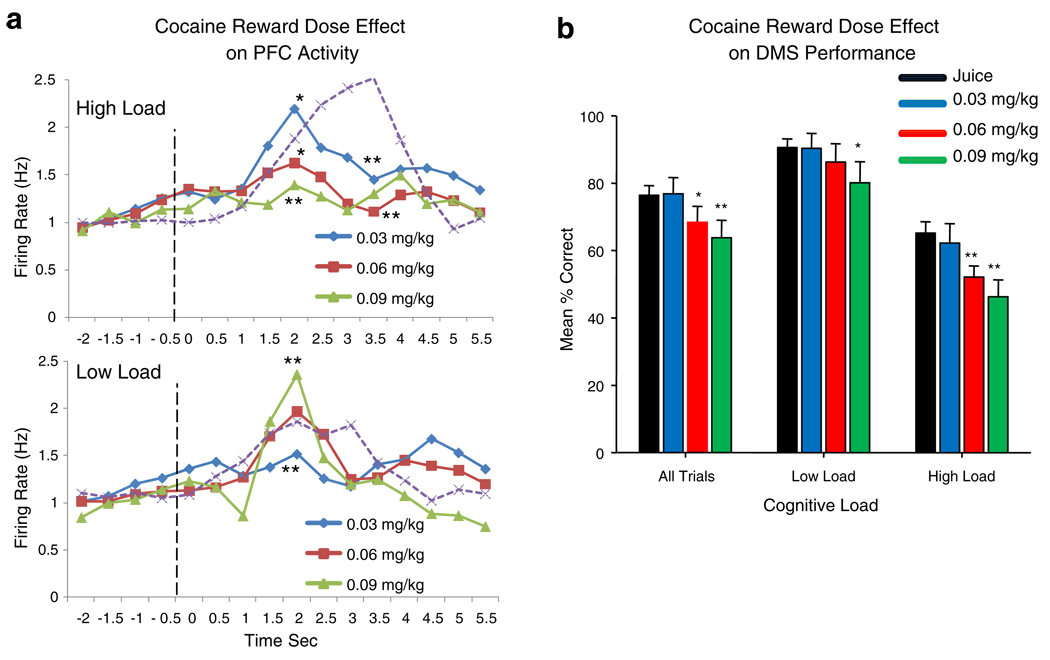
Figure 4a shows mean DMS performance as a function of the number of images presented in the Match phase of the task (Fig. 1a) in Mixed sessions with 40% of trials rewarded by IV delivery of cocaine at doses of 0.03, 0.06, and 0.09 mg/kg/inf in different sessions, compared to sessions (Control) with only juice rewarded trials. Performance was significantly decreased in sessions with doses of cocaine ≥0.06 mg/kg/inf on trials with ≥4 images (high load) in the Match phase, compared to performance on trials of the same type in sessions with only juice rewards (Control). The effect was to significantly suppress mean performance in sessions with cocaine rewards relative to juice only sessions (F(1,247)>7.65, p<0.01; F(1,247)>12.69, p<0.001) on trials with four or more distracter images in the Match phase in a dose-dependent manner for doses >0.03 mg/kg/inf. The dose effect of cocaine rewards on DMS performance was also exhibited by significantly decreased mean % correct trials (F(1,247)>7.65, p<0.01) as a function of increased duration of delay on high cognitive load trials (Fig. 1b, delays >20.0 s), but to a lesser degree than the effect of number of Match phase images as shown in Fig. 4a.
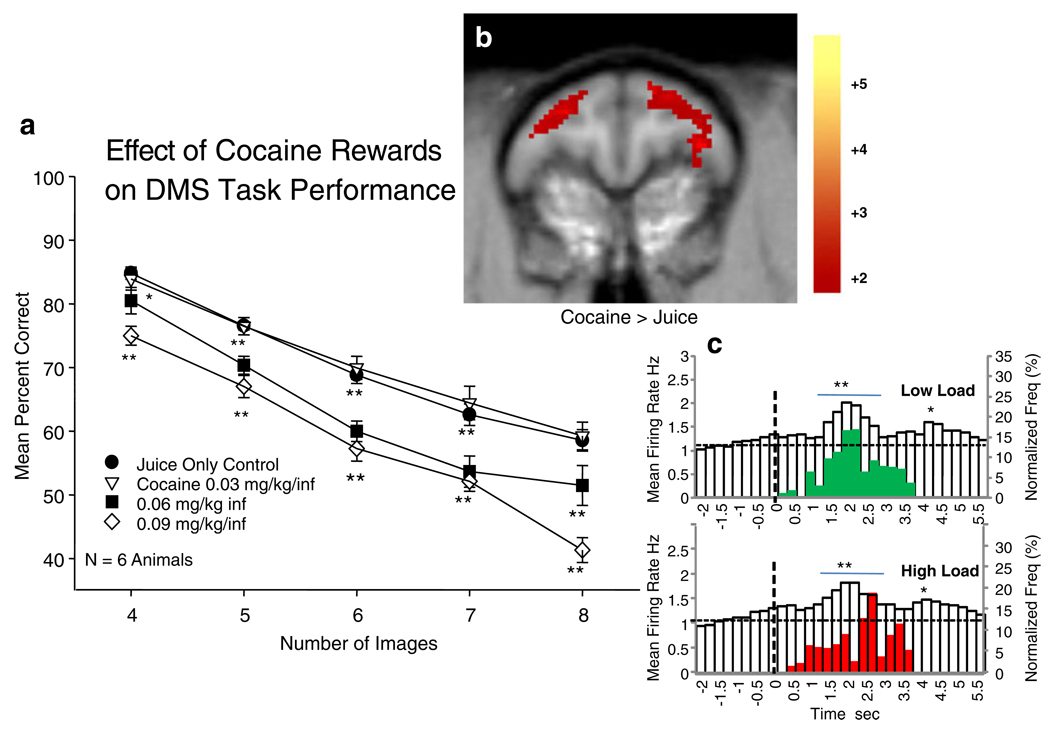
Fig. 4.
Effects of cocaine reward trials on DMS task performance and PFC activity as a function of cognitive load. a Dose effect of cocaine reward infusion (0.03–0.09 mg/kg/inf) on mean DMS performance as a function of increased number of Match phase images in sessions with both cocaine (40%) and juice (60%) reward trials. Asterisks indicate significant differences (*p<0.01, **p<0.001) from Control (juice reward) sessions. b [18]FDG PET scan difference image between juice only and Mixed trial cocaine+juice reward sessions showing increased activation of dlPFC during cocaine + juice reward sessions (Cocaine > Juice) compared to sessions with only juice reward trials. Color bar at right indicates degree of significance with respect to t values as in Fig. 1a. c Mean PEHs of PFC neuron firing rates in Match phase on low vs. high cognitive load cocaine reward trials from the same sessions as in a (summed across all dose levels) plotted with superimposed Match R latencies (red and green bars) on the same trials (see Fig. 3c). Presentation of Match phase images at t=0.0 s (dotted line). Superimposed bars (green, low load; red, high load) indicate normalized frequency distributions of Match R latencies on the same cocaine rewarded trials. Asterisks and horizontal bars indicate time bins rates that differ significantly (*p<0.01, **p<0.001) from pre-Match phase baseline (−2.0 to 0.0 s)
Performance of the DMS task was assessed during juice-only and cocaine+juice reward sessions and [18]FDG related rates of DLPFC cerebral metabolism compared between the two different sessions as shown in Fig. 3a, b. While the changes in DLPFC were similar to those shown in Fig. 3a (increased activation for higher cognitive load trials), metabolic activity was significantly increased in sessions with cocaine (0.06 mg/kg/inf) rewarded trials compared to sessions containing only juice rewarded (Control) trials (t= 18.5, z=4.01, p<0.01).
The PEHs in Fig. 4c show the mean firing rates of PFC neurons in the Match phase on low and high cognitive load trials during cocaine reward sessions in relation to the latency to respond on the same trials. PFC cell firing was significantly increased relative to pre-Match phase baseline levels (−2.0 to 0.0 s) for both low and high load trials (F(1,3011)>6.95, p<0.01; F(1,3011)>10.17, p<0.001), however, in contrast to Fig. 3c peak firing (as shown below) and Match R latencies (F(1,198)=1.25, n.s.) were not significantly different on low vs. high load trials which was consistent with the nearly complete overlap in distribution of response latencies for those same trials (red and green bars in Fig. 4c).
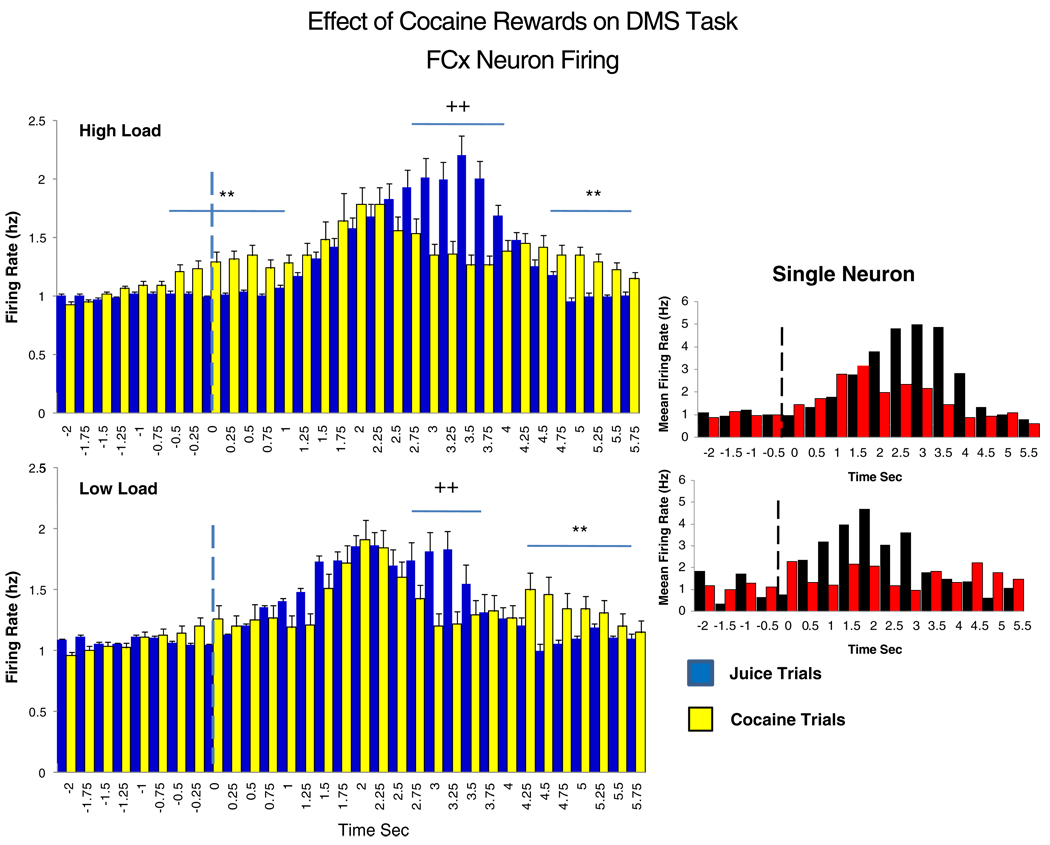
Figure 5 shows the same mean PFC neuron firing rates for low and high cognitive load trials as in Figs. 3c and 4c but at higher temporal resolution (250 ms), during the Match phase for cocaine (yellow bars) vs. juice (blue bars) rewarded trials. The respective Match R latencies are the same as shown in Figs. 3c and 4c. Significant differences in mean firing rate for cocaine vs. juice reward trials are indicated by horizontal bars with asterisks (**) representing higher rates (F(1,3011)>12.51, p<0.001) on cocaine rewarded trials and plusses (++) indicating higher rates (F(1,3011) >15.06, p<0.001) on juice rewarded trials. The major differences between types of rewarded trials were the temporal features of PFC neuron firing on low vs. high load trials. These were reflected as: (1) increased discharges on juice trials persisting longer than on cocaine trials (++> 3.0 s, Fig. 5, upper), (2) additional firing occurring prior to the main peak (−0.75 to +1.25 s) on high load cocaine rewarded trials, and (3) increased firing after peak discharges and Match R completion on high and low load cocaine trials (≥4.0 s), the latter corresponding to the time of drug infusion (see Methods). The insets at the right illustrate similar differences in mean firing rate of a single PFC neuron recorded in Area 46 under the same juice (black bars) vs. cocaine (red bars) high vs. low load trial conditions.
Fig. 5.
Detailed comparison of PFC neuron firing changes on cocaine vs. juice rewarded trials as a function of low vs. high cognitive load. PEHs show mean (±SEM) firing rate of PFC neurons at 250 ms resolution following Match phase onset vertical (dotted line) for cocaine (yellow bars) vs. juice (blue bars) reward on high and low cognitive load trials within the same sessions. Inset: Example of similar firing changes of a single PFC neuron during a single cocaine/juice session for cocaine (red bars) vs. juice (black bars) reward trials. Asterisks (**cocaine > juice) and plusses (++juice > cocaine) indicate significant differences (p< 0.001) over respective 250 ms time bins (horizontal bars)
Dose–effect relation of cocaine reward disruption on DMS performance
Figure 6 shows the effect of different doses of cocaine rewards for successful DMS performance together with altered PFC neuronal firing in relation to low vs. high cognitive load trials. In Fig. 6a, Match phase firing is shown for three different doses of cocaine rewards on high and low cognitive trials in comparison to firing on juice rewarded trials (dotted curves). There was a clear inverse relationship between the effects of the lowest (0.03 mg/kg) vs. highest (0.09 mg/kg) dose of cocaine as a function of type of trial. Sessions with the lowest dose of cocaine showed the largest increase in mean firing on high cognitive load trials, while sessions with the high cocaine dose exhibited the highest mean rates on low load trials. Asterisks indicate significant differences in mean firing rates (F(1,3011)>6.70, p<0.01, F(1,3011)>10.75, p<0.001) for cocaine vs. juice reward trials for all time bins equal to or exceeding the labeled mean values. Fig. 6b shows the corresponding significant dose-dependent decrease in mean % correct performance for cocaine vs. juice rewarded trials (F(1,247)>7.14, p<0.01, F(1,247)>11.04, p<0.001) with low and high cognitive load trials for the same sessions shown in Fig. 6a. There was a clear linear decrease in performance as a function of increased dose of cocaine rewards >0.03 mg/kg, with the demarcation between low and high load trials maintained at all dose levels and the largest performance decrements exhibited on high load trials.
Fig. 6.
Dose effect of cocaine signaled rewards on PFC neuron activity and DMS performance as a function of cognitive load. a Effects of different doses (0.03–0.09 mg/kg/inf) of cocaine reward on Match phase mean PFC neuron firing rate on high and low cognitive load trials. Dotted curves show mean PFC neuron firing rates for the same cells on high and low load juice reward trials. Asterisks indicate significant differences (*p<0.01, **p<0.001) for cocaine vs. juice (dotted curves) reward trials at the indicated time bins for different dose levels. b Performance differences (mean % correct) related to cocaine reward trials for different cocaine reward dose levels on low vs. high cognitive load trials compared to performance across all trials (All Trials) within the same sessions. Asterisks indicate significant differences (*p<0.01, **p<0.001) in mean % correct for cocaine vs. juice reward trials within the same sessions
Residual effects of cocaine delivery on subsequent juice reward trials
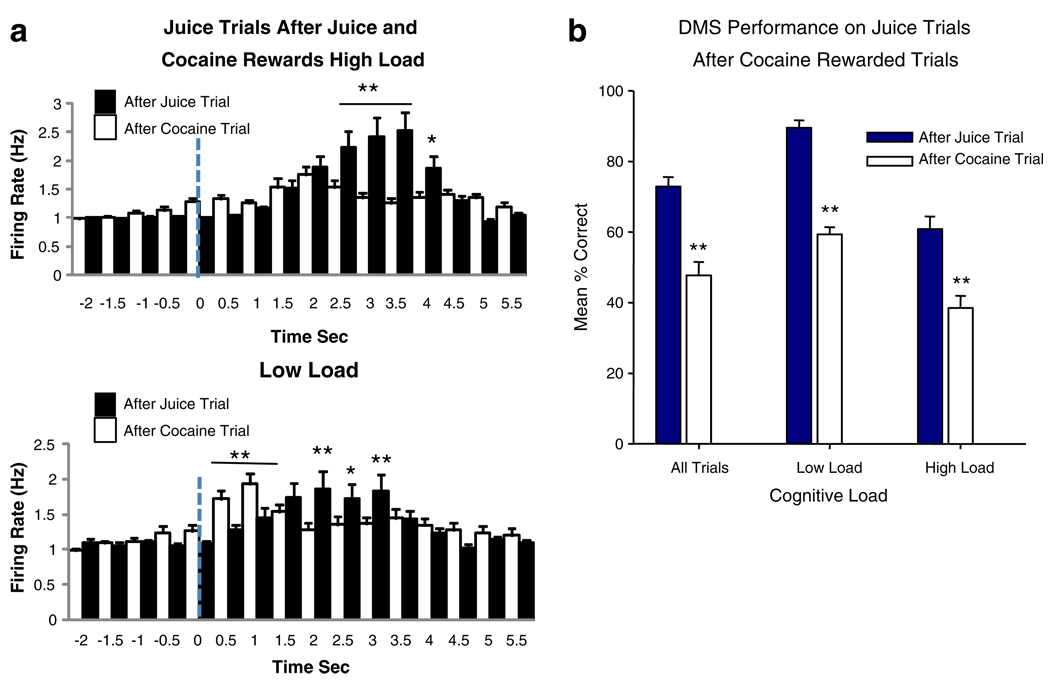
The above-described changes in PFC metabolic activity and PFC neuron firing were observed in sessions in which cocaine was the “signaled” reward (Fig. 1a) and was not delivered until after successful performance of the task in the Match phase (Figs. 4, 5 and 6). As indicated in Figs. 4a and 6b, performance was also dose-dependently reduced in sessions where cocaine signaled reward trials were interspersed with juice rewarded trials. Such analyses therefore determined the cumulative effects of cocaine delivery over the session, but do not take into account the immediate effect of cocaine infusion on performance. Therefore, the direct effects of the dose of cocaine were determined by assessing performance on juice signaled reward trials that, because of the means of drug administration, always followed cocaine rewarded trials (see Methods). Figures 7a, b show the respective changes in PFC cell firing and DMS performance on juice reward trials that were immediately preceded by IV delivery of cocaine (0.06 and 0.09 mg/kg) rewards compared to juice trials preceded by juice rewards. Figure 7a shows the same type of distortion of PFC neuron firing on succeeding juice signaled reward trials as in Figs. 4, 5 and 6 for cocaine signaled reward trials, including the early discharges immediately following Match phase onset (Fig. 5). Significant differences in mean firing rates between juice trials after cocaine rewarded trials vs. juice trials after juice rewarded trials (F(1,3011)>7.05, p<0.01, F(1,3011)>11.91, p<0.001) are indicated for time bins following onset of the Match phase in Fig. 7a. Figure 7b shows that cocaine reward delivery had a marked influence on mean % correct performance on juice trials that followed cocaine reward trials vs. juice trials that followed juice reward trials (F(1,61) >13.44, p<0.001), irrespective of cognitive load. The distribution of Match R latencies for juice trials following cocaine rewarded trials also exhibited a significant change (χ(20)2=49.9, p<0.001) from juice trials that followed juice rewarded trials (not shown), and did not differ significantly (χ(20)2=22.4, n.s.) from the distribution of latencies on cocaine rewarded trials (Fig. 4c).
Fig. 7.
Effects of cocaine and juice reward delivery on the next (immediately following) juice reward trial. a PFC neuron firing in Match phase on high and low load juice rewarded trials that were immediately preceded by either cocaine (white bars), or juice (black bars) rewarded trials. Asterisks and horizontal bars indicate significant differences in firing rate (*p<0.01, **p<0.001) between juice trials preceded by cocaine vs. juice rewards. b Performance (mean % correct) on juice trials that followed either cocaine (white bars) or a juice (black bars) rewarded trial. Differences are shown for all trials presented (All trials) and for only high vs. low load trials in the same sessions. Asterisks indicate significant differences (**p<0.001) in mean % correct for juice trials that followed cocaine vs. juice rewarded trials
Discussion
Neural basis of cognitive factors controlling DMS performance
In agreement with the current results, it has been shown previously that neurons in the PFC (ventral and dorsal) are selectively responsive to particular stimulus features or response components of tasks that employ those elements to satisfy experimental contingencies (Wallis and Miller 2003; Hoshi and Tanji 2004; Opris et al. 2010). In the current view of prefrontal function, relations between stimuli as determined via exposure to stimulus–response criteria, become incorporated into rules or strategies as performance increases through learning (Lundstrom et al. 2003; Genovesio et al. 2006; Muhammad et al. 2006). The results reported here provide a clear correlation between activation of PFC neurons and performance of the DMS task under high vs. low cognitive load conditions (Figs. 2, 3, 4, 5 and 6). Prior studies using electrophysiological recording methods (Hampson et al. 2004; Lindner et al. 2008; Opris et al. 2009; Deadwyler 2010; Hampson et al. 2010) and functional imaging techniques (Porrino et al. 2005; Deadwyler et al. 2007; Hampson et al. 2009) in NHPs have identified hippocampus, PFC, and dorsal striatum as areas differentially activated by the DMS task. The roles of the above three structures in human cognition have been demonstrated in similar testing conditions (Boettiger and D'Esposito 2005) while deficits in cognitive function that accrue as the result of trauma and/or elimination of these areas in human brain are also similar to deficits produced in experimental animals sustaining similar manipulations (Cho et al. 1993; Buckner et al. 2000; Zola and Squire 2001; Izquierdo et al. 2005).
Figure 3c shows that both low and high load trials produced increased firing prior to occurrence of the Match R on juice rewarded trials and, in the case of low load trials, firing persisted beyond the distribution of Match R latencies. Therefore, it is unlikely that PFC cell discharges were merely the reflections of limb movements, rather it is more likely that the distinct temporal differences in the PFC neuron firing profiles on low and high load trials reflected the difference in processing time required to (1) search the screen for the Sample image and (2) make the correct response within the required 5.0 s timeout period (Hampson et al. 2010). The fact that the Match R latency distributions showed little or no overlap for low vs. high load trials reflects the likelihood that image choice was controlled by task complexity or cognitive load as defined in Fig. 1b. Since PET scans were obtained under the same conditions and in the same animals from which recordings were obtained, it is possible that the differences in PFC metabolic activity on high vs. low cognitive load trials (Fig. 3a) reflected the segregated PFC neural firing patterns associated with Match R latencies shown in Fig. 3c. Because there were no significant differences in performance on the same trial types when presented in either Mixed or Exclusive sessions (Fig. 3b), the scans in Fig. 3a likely reflect the range of differential activation of DLPFC during routine performance of the DMS task (Simmons and Richmond 2008) when trials of variable cognitive load are presented randomly during the session.
Effects of cocaine rewards on DMS performance
The increase in DLPFC metabolic activity during cocaine + juice reward sessions compared to sessions with only juice rewarded trials (Fig. 4b), was similar to that shown in Fig. 3a for high vs. low load juice trials as reported previously (Hampson et al. 2009). However in contrast, Fig. 4a and c shows that cocaine signaled reward trials during the session impaired task performance and disrupted PFC neural firing in the Match phase of the task compared to juice rewarded trials (Figs. 3 and 5). In agreement with other studies (Robbins and Everitt 2002), these detrimental effects were related to the dose of cocaine reward as shown in Fig. 6a, where PFC firing was distorted in a complex manner at all cocaine dose levels relative to firing on juice rewarded trials (dotted curves) especially on high load trials. This suggests that the increased DLPFC metabolic activity during cocaine reward sessions was related to the lack of differentiation of PFC firing and latency to respond on low vs. high cognitive load trials (Figs. 3c and 4c). Under no circumstances was there a clear differentiation of mean peak discharge of PFC neurons or Match R latencies on low vs. high load trials on cocaine signaled reward trials (Figs. 4, 5 and 6). In most cases the patterns of PFC neuron activation resembled firing on low load trials and never transitioned to the high cognitive load pattern on high load trials when cocaine was the signaled reward (Fig. 5). The same neurons showed clear differentiation within the same sessions on high cognitive load trials that were juice rewarded (Figs. 3, 4, 5 and 6) and with the exception of trials that followed cocaine rewards, response latencies were also differentiated with respect to cognitive load (F(1,198)=11.51, p<0.001). Since the effect of the prior cocaine reward was to significantly suppress PFC neuron firing and performance only on immediately succeeding juice trials (Fig. 7), it was likely that the residual pharmacological actions of cocaine reward delivery did not extend beyond 2 min (Wakazono and Kiyatkin 2008) and therefore did not affect responding on other juice trials in the same session.
Studies of chronic cocaine use in humans show decreased function of the PFC, and it has been well documented that brain glucose utilization rates measured with PET scans in chronic cocaine users are lower than in non-user control populations (Volkow et al. 1992, 1993, 1999; Adinoff et al. 2003; Goldstein et al. 2004; Kalivas and Volkow 2005; Volkow et al. 2005). Other reports have also shown decreased performance of chronic cocaine users correlated with decreased activation of the medial PFC and anterior cingulate gyrus (Bolla et al. 2003; Kaufman et al. 2003; Hester and Garavan 2004; Stout et al. 2004; Bechara et al. 2001; Bechara and Martin 2004; Kalivas 2004). The results reported here show that initial “acute” exposure to cocaine as a reward for successful cognitive performance is associated with enhanced DLPFC metabolic activity in contrast to the above reported reduced activation seen in chronically exposed subjects. However, the increase in metabolic activity under acute cocaine reward delivery conditions was accompanied by “distorted” task-specific firing of PFC neurons in relation to trials with low vs. high cognitive load (Figs. 3c, 4 and 5). The systematic decrease in performance accuracy as a function of number of images (four to eight) demonstrates that cognitive processing within cocaine reward sessions was not completely eliminated but was decreased in a dose-dependent manner (doses of 0.06 and 0.09 mg/kg/inf). Thus, the dose-dependent reduction in cognitive performance associated with the disrupted PFC firing on cocaine rewarded trials (Figs. 4, 5 and 6), as well as immediately following cocaine delivery (Fig. 7), may serve as the precursor condition for the reduced DLPFC metabolic activity and other reduced brain functions reported in chronic cocaine abusers.
Acknowledgements
We appreciate the technical assistance from the following individuals in the above studies: Joshua Long, Michael Todd, Mack Miller, Joseph Noto, and Brian Parrish. This work was supported by NIH grants DA023573, DA06634, and DA026487 to SAD.
References
- Adinoff B, Devous MD, Sr, Cooper DB, Best SE, Chandler P, Harris T, Cervin CA, Cullum CM. Resting regional cerebral blood flow and gambling task performance in cocaine-dependent subjects and healthy comparison subjects. Am J Psychiatry. 2003;160:1892–1894. doi: 10.1176/appi.ajp.160.10.1892. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Spiering BJ. The neurobiology of category learning. Behav Cogn Neurosci Rev. 2004;3:101–113. doi: 10.1177/1534582304270782. [DOI] [PubMed] [Google Scholar]
- Bechara A, Martin EM. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology. 2004;18:152–162. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Berns GS. Functional neural imaging. Life Sci. 1999;65:2531–2540. doi: 10.1016/s0024-3205(99)00297-0. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Daunais JB, Nader MA, Porrino LJ. Chronic cocaine self-administration is associated with altered functional activity in the temporal lobes of non human primates. Eur J Neurosci. 2006;23(11):3109–3118. doi: 10.1111/j.1460-9568.2006.04788.x. [DOI] [PubMed] [Google Scholar]
- Boettiger CA, D'Esposito M. Frontal networks for learning and executing arbitrary stimulus-response associations. J Neurosci. 2005;25:2723–2732. doi: 10.1523/JNEUROSCI.3697-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman EM, Aigner TG, Richmond BJ. Neural signals in the monkey ventral striatum related to motivation for juice and cocaine. J Neurophysiol. 1996;75:1061–1073. doi: 10.1152/jn.1996.75.3.1061. [DOI] [PubMed] [Google Scholar]
- Bradberry CW. Cocaine sensitization and dopamine mediation of cue effects in rodents, monkeys, and humans: areas of agreement, disagreement, and implications for addiction. Psychopharmacology. 2007;191:705–717. doi: 10.1007/s00213-006-0561-6. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Barrett B, Larimore RL, Jatlow P, Rubino SR. Impact of self-administered cocaine and cocaine cues on extracellular dopamine in mesolimbic and sensorimotor striatum in rhesus monkeys. J Neurosci. 2000;20:3874–3883. doi: 10.1523/JNEUROSCI.20-10-03874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browndyke JN, Tucker KA, Woods SP, Beauvals J, Cohen RA, Gottschalk PC, Kosten TR. Examining the effect of cerebral perfusion abnormality magnitude on cognitive performance in recently abstinent chronic cocaine abusers. J Neuroimaging. 2004;14:162–169. [PubMed] [Google Scholar]
- Buckner RL, Logan J, Donaldson DI, Wheeler ME. Cognitive neuroscience of episodic memory encoding. Acta Psychol. 2000;105:127–139. doi: 10.1016/s0001-6918(00)00057-3. [DOI] [PubMed] [Google Scholar]
- Cho YH, Beracochea D, Jaffard R. Extended temporal gradient for the retrograde and anterogradeamnesia produced by ibotenate entorhinal cortex lesions in mice. J Neurosci. 1993;13:1759–1766. doi: 10.1523/JNEUROSCI.13-04-01759.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deadwyler SA. Electrophysiological correlates of abused drugs: relation to natural rewards. Ann NY Acad Sci Addict Rev. 2010;2(1187):140–147. doi: 10.1111/j.1749-6632.2009.05155.x. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Porrino LP, Siegel JE, Hampson RE. Systemic and nasal delivery of orexin-A (hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. J Neurosci. 2007;27:14239–14247. doi: 10.1523/JNEUROSCI.3878-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberling JL, Roberts JA, De Manincor DJ, Brennan KM, Hanrahan SM, Vanbrocklin HF, Roos MS, Jagust WJ. PET studies of cerebral glucose metabolism in conscious rhesus macaques. Neurobiol Aging. 1995;16:825–832. doi: 10.1016/0197-4580(95)00085-s. [DOI] [PubMed] [Google Scholar]
- Elston GN, Benavides-Piccione R, Elston A, Zietsch B, Defelipe J, Manger P, Casagrande V, Kaas JH. Specializations of the Granular Prefrontal Cortex of Primates: implications for Cognitive Processing. The Anatomical Record Part A. 2006;288A:26–35. doi: 10.1002/ar.a.20278. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308:662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Genovesio A, Brasted PJ, Wise SP. Representation of future and previous spatial goals by separate neural populations in prefrontal cortex. J Neurosci. 2006;26:7305–7316. doi: 10.1523/JNEUROSCI.0699-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, Wang GJ, Fowler JS, Volkow ND. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuro-psychologia. 2004;42:1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38:1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Pons TP, Stanford TR, Deadwyler SA. Categorization in the monkey hippocampus: a possible mechanism for encoding information into memory. Proc Natl Acad Sci USA. 2004;101:3184–3189. doi: 10.1073/pnas.0400162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, España RA, Rogers GA, Porrino LJ, Deadwyler SA. Mechanisms underlying cognitive enhancement and reversal of cognitive deficits in nonhuman primates by the ampakine CX717. Psychopharmacology. 2009;202:355–369. doi: 10.1007/s00213-008-1360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Opris I, Deadwyler SA. Neural correlates of fast pupil dilation in nonhuman primates: Relation to behavioral performance and cognitive workload. Behav Brain Res. 2010;212:1–11. doi: 10.1016/j.bbr.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cere-bellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Area-selective neuronal activity in the dorsolateral prefrontal cortex for information retrieval and action planning. J Neurophysiol. 2004;91:2707–2722. doi: 10.1152/jn.00904.2003. [DOI] [PubMed] [Google Scholar]
- Howell LL, Hoffman JM, Votaw JR, Landrum AM, Jordan JF. An apparatus and behavioral training protocol to conduct positron emission tomography (PET) neuroimaging in conscious rhesus monkeys. J Neurosci Methods. 2001;106:161–169. doi: 10.1016/s0165-0270(01)00345-4. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. J Neurosci. 2005;25:8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–29. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Karila L, Gorelick D, Weinstein A, Noble F, Benyamina A, Coscas S, Blecha L, Lowenstein W, Martinot JL, Reynaud M, Lepine JP. New treatments for cocaine dependence: a focused review. Int J Neuropsychopharmacol. 2007:1–14. doi: 10.1017/S1461145707008097. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keri S. The cognitive neuroscience of category learning. Brain Res Brain Res Rev. 2003;43:85–109. doi: 10.1016/s0165-0173(03)00204-2. [DOI] [PubMed] [Google Scholar]
- Lindner MD, McArthur RA, Deadwyler S, Hampson R, Tariot PN. Development, optimization and use of preclinical behavioral models to maximize the productivity of drug discovery for Alzheimer’s disease. In: McArthur RA, Borsini F, editors. Animal and translational models of behavioural disorders. Vol. 2. NY: Elsevier; 2008. [Google Scholar]
- Lundstrom BN, Peterson KM, Anderson J, Johnson M, Franson P, Ingvar M. Isolating the retreival of imagined pictures during episodic memory: activation of the left precuneus and the left inferior frontal cortex. Neuroimage. 2003;27:1934–1943. doi: 10.1016/j.neuroimage.2003.07.017. [DOI] [PubMed] [Google Scholar]
- Martinez ZA, Colgan M, Baxter LR, Jr, Quintana J, Siegel S, Chatziioannou A, Cherry SR, Mazziotta JC, Phelps ME. Oral 18F-fluoro-2-deoxyglucose for primate PET studies without behavioral restraint: demonstration of principle. Am J Primatol. 1997;42:215–224. doi: 10.1002/(SICI)1098-2345(1997)42:3<215::AID-AJP4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Muhammad R, Wallis JD, Miller EK. A comparison of abstract rules in the prefrontal cortex, premotor cortex, the inferior temporal cortex and the striatum. J Cogn Neurosci. 2006;18:974–989. doi: 10.1162/jocn.2006.18.6.974. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Wise SP. Role of prefrontal cortex in a network for arbitrary visuomotor mapping. Exp Brain Res. 2000;133:114–129. doi: 10.1007/s002210000406. [DOI] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore M, Nader S, Moore R, Smith HR, Friedman DP, Porrino LJ. Effects of long-term cocaine self-administration on mesolimbic and nigrostriatal dopamine systems in rhesus monkeys. Neuropsychopharmacology. 2002;27:35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Opris I, Hampson RE, Deadwyler SA. Comparison of neuronal correlates of cocaine and appetitive rewards in nonhuman primates. Neuroscience. 2009;163:40–54. doi: 10.1016/j.neuroscience.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opris I, Hampson RE, Stanford TR, Gerhardt GA, Deadwyler SA. Neural activity in frontal cortical cell layers: evidence for columnar sensorimotor processing. J Cogn Neurosci. 2010 doi: 10.1162/jocn.2010.21534. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Huang XF, Toga AW. The rhesus monkey brain in stereotaxic coordinates. San Diego: Academic; 2003. [Google Scholar]
- Porrino LJ, Lyons D. Orbital and medial prefrontal cortex and psychostimulant abuse: studies in animal models. Cereb Cortex. 2000;10:326–333. doi: 10.1093/cercor/10.3.326. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Nader ME, Miller MD, Friedman DP. Metabolic mapping of the effects of cocaine self-administration in the nonhuman primate Neuropsychopharmacology, rhesus monkey. Brain Res. 2001;578:235–243. [Google Scholar]
- Porrino LJ, Daunais JB, Rogers GA, Hampson RE, Deadwyler SA. Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates. PLoS Biol. 2005;3:e299. doi: 10.1371/journal.pbio.0030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SC, Rainer G, Miller EK. Integration of what and where in the primate prefrontal cortex. Science. 1997;276:821–824. doi: 10.1126/science.276.5313.821. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Curr Opin Neurobiol. 2001;11:250–257. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Simmons JM, Richmond BJ. Dynamic changes in representations of preceding and upcoming reward in monkey orbitofrontal cortex. Cereb Cortex. 2008;18:93–103. doi: 10.1093/cercor/bhm034. [DOI] [PubMed] [Google Scholar]
- Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout JC, Busemeyer JR, Lin A, Grant SJ, Bonson KR. Cognitive modeling analysis of decision-making processes in cocaine abusers. Psychon Bull Rev. 2004;11:742–747. doi: 10.3758/bf03196629. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J Neurosci. 2006;26:8004–8008. doi: 10.1523/JNEUROSCI.1413-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda S, Shen H, Peters J, Cagle S, Kalivas P. Cocaine Increases Actin Cycling: effects in the Reinstatement Model of Drug Seeking. J Neurosci. 2006;26:1579–1587. doi: 10.1523/JNEUROSCI.4132-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto T, Ogawa M, Tsukada H, Takeharu K, Sasaki K. Activation of the ventral and mesial frontal cortex of the monkey by self-initiated movement tasks as revealed by positron emission tomography. Neurosci Lett. 2004;258:117–120. doi: 10.1016/s0304-3940(98)00868-4. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Wong C, Hitemann R, Pappas NR. Reinforcing effects of psychos-timulants in humans are associated with increases in brain dopamine and occupancy of D2 receptors. J Pharmacol Exp Ther. 1999;291:409–415. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Telang F, Fowler JS, Logan J, Childress A, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakazono Y, Kiyatkin EA. Electrophysiological evaluation of the time-course of dopamine uptake inhibition induced by intravenous cocaine at a reinforcing dose. Neuroscience. 2008;6:824–835. doi: 10.1016/j.neuroscience.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. From rule to response: neuronal processes in the premotor and prefrontal cortex. J Neurophysiol. 2003;90:1790–1806. doi: 10.1152/jn.00086.2003. [DOI] [PubMed] [Google Scholar]
- Zola SM, Squire LR. Relationship between magnitude of damage to the hippocampus and impaired recognition memory in monkeys. Hippocampus. 2001;11:92–98. doi: 10.1002/hipo.1027. [DOI] [PubMed] [Google Scholar]