Abstract
Although the act of eating is voluntary, its initiation depends on several factors including its taste and the animal’s internal state as related to hunger or satiety. These factors together with the food’s hedonic value will determine whether food will be ingested. The taste of food will depend on the activation of receptors located on taste cells but also on the expectation of what it will taste like. For these reasons, it is important to investigate, in behaving animals, the neural correlates of feeding behavior in the taste-reward pathway. Here we review particular coding strategies, present experiments using freely licking rodents with chronically implanted arrays of electrodes throughout the taste-reward pathway to investigate the changes that occur when animals learn to discriminate among tastants and after they are ingested. In summary, we found that gustatory processing does not only depend on the input from the oral cavity but on expectation, learning, and post–ingestive effects.
INTRODUCTION
In a real world situation selecting food’s for ingestion involves extracting information from all sensory systems. Based on the food’s appearance, texture, smell, ones’ previous experience, and the extent of one’s hunger, the first decision that an animal has to make is whether to put it in its mouth. Once in the mouth, another decision is made regarding whether or not to ingest it. This second decision is dependent on the rapid evaluation of the multisensory aspects of the food. Once ingested the food is processed throughout the gastrointestinal (GI) system. This, in turn, signals either through the release of peptides and/or through vagal stimulation of chemical and stretch receptors the animals’ energy balance, the foods hedonic value and the extent that the animal is sated [1, 2]. Although the process of eating is inherently multisensory, and that many brain regions have neurons that respond to several sensory modalities [3–6], for reasons of brevity, here we will focus on the gustatory system.
The act of eating involves expectation, learning, attention, decision (to ingest) and post-ingestive effects. In this review we will touch on some of these contributions to gustatory processing. In addition, we will discuss one consequence of rhythmic orofacial movements such as licking, lapping or chewing that occur during eating. This active and rhythmic taste sensing will impose a temporal component to the flow of chemosensory information that is transmitted into the CNS, in quasi periodic chunks [7, 8]. We will show that this intrinsic rhythm affects the spike timing of neurons in a manner that increases tastant identification [9]. Furthermore, we will show that licking acts to synchronize different areas of the taste-reward pathway to enhance an animal’s ability to learn to discriminate among tastants.
CODING IN THE PERIPHERAL TASTE PATHWAY
The question of how chemosensory information is encoded in the periphery has been around for many years and generally falls into two broad categories. That is whether the taste information is transmitted via separate channels (labeled cells via dedicated lines) or via ensembles (across fiber patterns). There are several recent and excellent reviews on this topic [10–13] characterizing the various types of taste cells in taste buds [14] and is the topic of several papers in this Special issue of Flavors and Fragrance. For this reason we will not reiterate the pro and con arguments but rather point out some areas that perhaps need clarification and suggest possible arrangements that would be consistent with all these data.
It is generally agreed that many chemosensitive taste receptor cells (TRCs) are selective for a particular class of tastants. That is, ablating them selectively abolishes both the nerve and behavioral response to a particular classification of tastants (sweet, salty, acid, umami and bitter) [15–19]. For example, TRCs that contain T2R receptors respond only to bitter tasting stimuli. Moreover, depending on the concentration and internal state of the animal (thirst or hunger), the application of bitter tastants will cause the animal to reject the stimulus and produce stereotypic gaping mouth movements that does not depend on a functioning cortex or even for the animal to be conscious [20]. In contrast, TRCs that express T1R receptors respond to sweet and some aminoacids and thus for a hedonically positive stimulus, like sucrose, the animals will ingest it and produce mouth movements like licking that are associated with appetitive stimuli [21].
That is, information from these cells not only has to convey the tastant identity and quantity to the first taste relay in the rostral Nucleus of the Solitary Tract (rNST) but also must reliably signal the correct behavioral response to accept or reject the food [22]. The latter behavior is important for survival as the ingestion of several bitter tastants could be fatal.
We suggest that to produce the proper behavioral response (accept or reject) the tastant–specific taste cells with their tastant-selective dedicated fibers [10, 16, 17] synapse to rNTS neurons that project to motor pathways involved in rejection or ingestion [23]. Once into the NTS and into the cortex taste coding appears to utilize ensembles to encode the quality, quantity and hedonic value of the tastants [11, 24, 25].
ANATOMY OF THE GUSTATORY PATHWAY: TASTE IDENTIFICATION
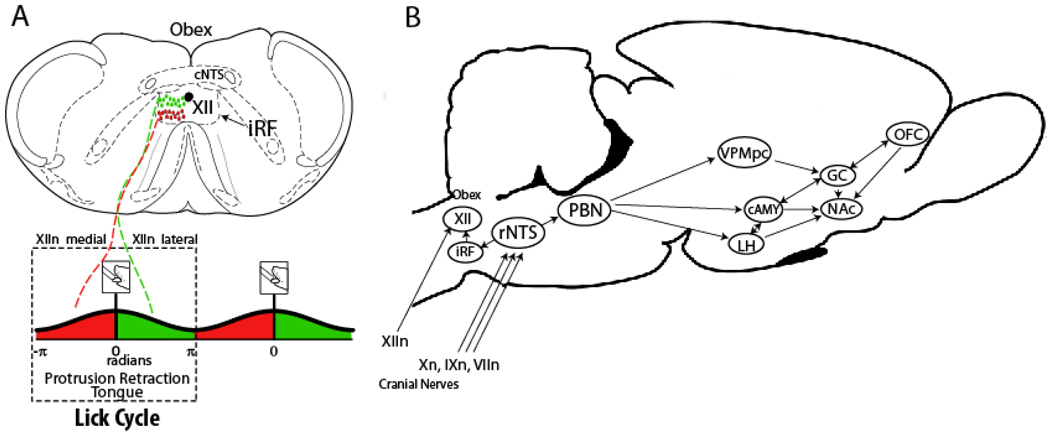
From taste cells that contain receptors and signal transduction pathways throughout the oral cavity, electrical signals from cranial nerves VIIn, IXn and Xn that contain information on the chemical properties of tastants (as well as somatosensory inputs, Vn) are conveyed to the rNTS division of the medulla. In addition to tastant-induced response, electrical stimulation of rNTS may also produce rhythmic licking behavior [22]. In fact, a subpopulation of NTS neurons project to pre-oromotor neurons located in the intermediate reticular formation [26] where they, in turn, project to motoneurons located in the hypoglossal nuclei (XII), where tongue movements, such as protusion and retraction are produced (Figure 1A). The ventral division of the NTS (vNTS) receives vagal input and projects to the Dorsal Motor Nucleus of X and the nucleus ambiguus where they regulate, among other things, the release of appetite regulating hormones[27, 28]. In rodents, rNTS efferents project to gustatory centers in pontine parabrachial nuclei (PBN) that synapse with neurons in the parvicellular part of the ventroposterior medial nucleus of the thalamus (VPMpc). A visceral PBN pathway has been shown to project to the central nucleus of the amygdala (cAMY) and lateral hypothalamus (LH). In primates, the rNTS projection fibers bypass the PBN to synapse directly into the VPMpc, whereas the vPBN conveys general visceral information to specialized thalamic nuclei. In either case, thalamic afferents project to the primary gustatory cortex (GC), which has both chemosensitive and visceral areas. In turn, the GC projects to the cAMY, from where gustatory information reaches the LH and the nucleus accumbens (NAc), the latter area releases dopamine in response to hedonically tasting stimuli such as glucose delivered either orally or postingestively [29, 30]. In fact, in sweet blind TRPM5 knockout mice, through metabolic post-ingestive responses (see below) dopamine is released in the nucleus accumbens in response to the intake of sucrose, but not artificial sweetners [31]. The GC also projects to the orbitofrontal cortex (OFC), which is sometimes referred to as the secondary taste cortex. With the noted exception of the nucleus accumbens, almost all the projections are reciprocal [32] (Figure 1B). For the purpose of this review the collection of several limbic regions involved in feeding, taste and reward, and that are intrinsically connected include the LH, cAMY, GC and OFC and the NAc, define the major components of the taste-reward network.
Figure 1. Schematic representation of the rodent taste – reward pathway and the central pattern generator for licking.
A. Schematic representation of preoromotor (iRF) and motor nuclei (XII) of the central pattern generator for rhythmic licking. The intrinsic and extrinsic tongue muscles (not shown) are innervated by the hypoglossal nerve (XIIn), which contains axons of motoneurons. Close to the muscles of the tongue, XIIn bifurcates to form medial and lateral branches [25]. Stimulation of the medial or lateral branch yields tongue protrusion (red) or retraction (green), respectively. The hypoglossal nuclei, XII, contains two types of motoneurons one that fires in phase with tongue protusion (−π to 0 radians) located more ventrally, and neurons that fires in phase with tongue retraction (0 to π radians) distributed more dorsally [71]. The intermediate reticular formation (iRF) contains a large portion of preoromotor neurons that sends axons to XII motoneurons to generate the characteristic 6.3 Hz rhythmic pattern of licking. B. Taste – reward pathway: Three cranial nerves VIIn, IXn, and Xn innervate different parts of the oral cavity and convey taste information to the rostral part of the nucleus of the solitary tract (rNTS). Input from the lingual branch of the trigeminal nerve (Vn) also contributes to gustatory processing (not shown). Taste information then projects to the pontine parabrachial nucleus (PBN) (for rodents), which, in turn, distributes gustatory information via a thalamo- cortical pathway or throughout a ventral forebrain pathway. The PBN projects to the parvicellular part of the ventroposterior medial nucleus of the thalamus (VPMpc). Then the VPMpc projects to the primary gustatory cortex (GC), which sends axon projections to the orbitofrontal cortex (OFC). Brain structures in the ventral forebrain that receive input from the PBN include the central nucleus of the amygdala (cAMY), and the lateral hypothalamus (LH). All four brain regions -LH, cAMY, GC and OFC- send unidirectional axons to medium spiny neurons of the nucleus accumbens (NAc), which defines the major components of the taste-reward network. For this reason, the nucleus accumbens is considered as a limbic – motor interface that transforms motivational information generated in limbic regions into movements to achieve a goal, such as eating [72].
GUT –BRAIN INTERACTIONS
Spatial maps reflect the current hedonic value of tastants
Having briefly described the peripheral gustatory system, we now turn to the cortex. The coding of tastants in cortical areas has been shown to be dynamic and require ensembles to best characterize the tastant (see [11] for review). Here we will characterize two other aspects of gustatory cortical responses, namely cortical plasticity and gut-brain interactions.
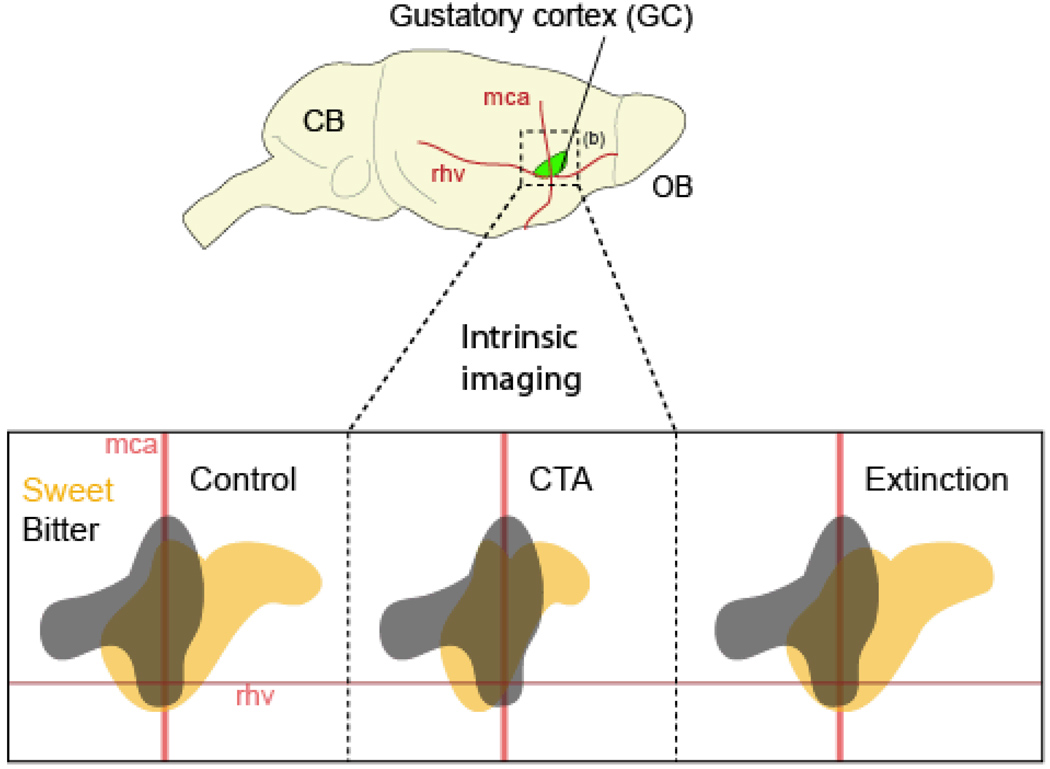
It is well known from acquired taste aversion studies that the hedonic value of a tastant can be changed as a function of the post-ingestive consequences experienced [33, 34]. To address the question of the cortical plasticity involved in the association between the response to a tastant and its post-ingestive consequences we refer to the study of Accolla and Carleton [35] in the rat GC. They induced a conditioned taste aversion (CTA) to a pleasant tastant (saccharin). Then using intrinsic imaging, they compared the cortical (GC) representation of saccharin to the response elicited by quinine, a reference aversive bitter tastant, before and after the CTA (Figure 2). Their results show that the taste maps for saccharin are plastic in the sense that they rearrange according to the shift of its hedonic value; both during the learning phases of CTA acquisition (where saccharin becomes aversive) and extinction (saccharin is attractive again). Because the taste modality remains unchanged (i.e., saccharin interacts with the same peripheral receptors), changes in correlation are directly related to shifts of the perceived hedonic value of saccharin [35]. These results, as well as those of others, provide strong evidence that spatial GC activation patterns carry information not only for the stimulus modality, but also on the palatability of the tastant [36–38].
Figure 2. Taste spatial representation in the gustatory cortex is dynamic.
Topographical representations in the rat gustatory cortex (green). Top, Approximate size and location of the GC with respect to anatomical landmarks (blood vessels: middle cerebral artery, mca; rhv, rhinal veins) and other sensory areas of the brain (olfactory bulb, OB). Below, schematic representation intrinsic imaging studies of the cortical territories activated following stimulation with bitter and sweet taste modalities. This panel also shows the relationship between behavioral state and cortical representation in the gustatory cortex. In a naive (i.e. control) rat, cortical representations of the hedonically positive (saccharin; orange) and negative (quinine; grey) tastants are quite different, although commonly-activated cortical territories exist. After conditioned taste aversion (CTA) training, in which the malaise-inducing agent lithium chloride (LiCl) is paired with the ingestion of saccharin, the normally positive stimulus of the latter becomes aversive and the pattern changes accordingly to become more similar (highly correlative) to the quinine response. After saccharin aversion extinction, the hedonic value of saccharin reverts to a positive response, and its cortical map is again less similar (low correlation) to the quinine pattern. Note that the new representation of saccharin after extinction might not be a simple return to the same one that existed prior to conditioning. From Carleton et, al. [35] with permission Trends in Neuroscience).
POST-INGESTIVE SENSORY CELLS IN THE GUT
Once the food is propelled beyond the oral cavity and enters the esophagus, stomach and intestine, chemical and electrical signals from specialized cells (e.g., enteroendocrine cells, see below) and associated nerves (vagal) become activated by chemical and mechanical stimuli (e.g., gastric distension). Although many of the physiological roles of these cells remain to be elucidated, we briefly outline some of their reported functions. They release a variety of appetite-regulating peptides and transmit electrical responses that inform the brain as to the identity of the chemical (e.g., glucose in β-cells), their hedonic and post-ingestive value via dopamine release in the NAc [30, 31, 38, 39]. Finally, these cells are also responsive to the organism’s energy needs by releasing signaling molecules such as ghrelin (stomach neuroendocrine cells) or leptin (from adipose cells or from the stomach fundus) that signal hunger or satiety, respectively [40, 41].
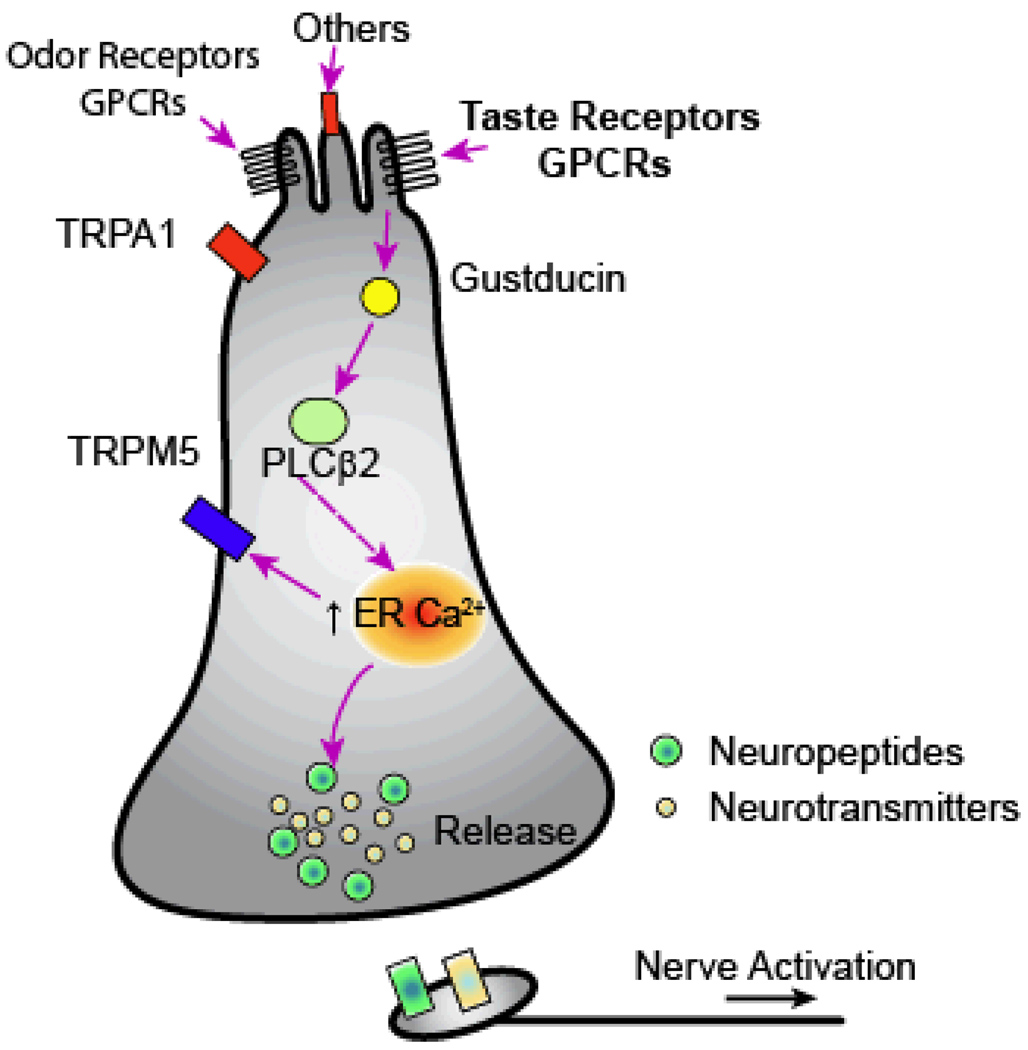
One major advance in elucidating gut-brain interactions that has taken place in recent years has been the discovery that in the gut peptide-secreting enteroendocrine (EE) or serotonin–secreting enterochromaffin (EC) cells use some of the same transduction machinery found in taste and olfaction [42–44]. As a group, these heterogeneous cell types have apical microvilli that are exposed to the ingested luminal contents and release peptides and/or transmitters directly onto sensory fibers (see Figure 3). In this regard, it is not surprising that they respond to chemical stimuli using some of the same peripheral receptors and signaling machinery that is found in taste cells.
Figure 3. Schematic of combination of enteroendocrine (EE) and enterochromaffin (EC) cells showing that these neuroepithelial cells, like taste cells, contain signaling pathways involving taste sensitive, GPCRs, α-gustducin, PLCβ2 and Ca2+ -activated TRPM5 channels.
The dotted arrow indicates the interaction between PLCβ2 and the endoplasmic reticulum (ER) is not direct but involves IP3 (not shown). EC cells, like some taste cells, contain synaptic vesicles with transmitters (yellow balls) and neuropeptides (green balls) that will bind to their receptors on nerve terminals (yellow and green rectangles, respectively) that in turn influence many physiological processes (see text). Also shown is the presence of TRPA1 ion channels that respond to a variety of spices and irritants. In addition, some of these cells also contain GPCR-odorant receptors and, other still to be identified receptors (“Others”).
For EE cells, like taste cells, these molecules include T1Rs (sweet tastants and amino acid receptors) and T2Rs (bitter tastant receptors) that couple through α-gustducin, PLCβ2 and TRPM5. Moreover, like taste cells, EE cells contain glucagon-like peptide (GLP-1)[45], or cholecystokinin (CCK- I Type EE cell) [46–48]. The levels of CCK in plasma peak maximally a few minutes after meal initiation and after meal termination return to baseline levels. In contrast, GLP-1 is released more slowly in response to food intake and levels remain elevated for hours after a meal [49]. Although with different temporal dynamics, both peptides are involved in satiety.
EC cells have been shown to contain several distinct odorant receptors that were responsive to physiologically relevant chemicals such as those found in bananas, strawberries, cloves, nutmeg, and oregano [43]. EC cells also contain thermally-sensitive TRPA1 channels [44] which are responsive to spices such as garlic and cinnamaldehyde as well as other irritants [50, 51]. EC cells may release transmitters (e.g., 5-HT) that regulate peristalsis and epithelial transport. One interesting difference between the EE-type gut cells and Type II taste cells with some of the same signaling machinery is that the latter do not seem to form synapses with primary taste fibers [52], whereas the EE cells apparently synapse with their appropriate neuron. In summary, chemical stimuli in the form of gaseous molecules (odorants) and dissolved solids interact with subpopulations of isolated cells lining the lumen of the gut and use some of the same signaling machinery that is found in Type II taste cells. The precise roles of these cells remain to be uncovered although one would expect differing responses in cells with receptors responsive to aversive stimuli (T2Rs, TRPA1) than cells responsive to nutritious stimuli (T1Rs).
GUT–BRAIN INTERACTIONS AND THE PREDICTION OF SATIETY
Having discussed peripheral transmission of nutrients and its post-ingestive effects, now we turn into the influence that satiety has in the motivation to eat. It is well known that the relative reward value of a palatable food is devalued by allowing hungry rats to feed to satiety. As food is consumed to satiety, its incentive value is gradually devalued through consummatory behavior. However, devaluation of the tastant is specific to the food consumed to satiety. This effect, called, sensory specific satiety, and it was clearly demonstrated in nonhuman primates [53] as well as in human fMRI studies [54]. Sensory-specific satiety occurs when a particular food eaten to satiety becomes less rewarding, without changing the taste of the food itself. In other words, the relative reward value of that particular food has been diminished while the reward value of other foods can remain unchanged. The neural correlates of sensory-specific satiety were first observed in neurons of the lateral hypothalamus [55] and later in the orbitofrontal cortex [53]. These neurons specifically decreased their firing rate as monkeys were fed to satiety with a particular juice flavor. The lower firing rate occurred when the monkey refused to drink more juice. However, the same neuron avidly fired to the presentation of a new tastant [53].
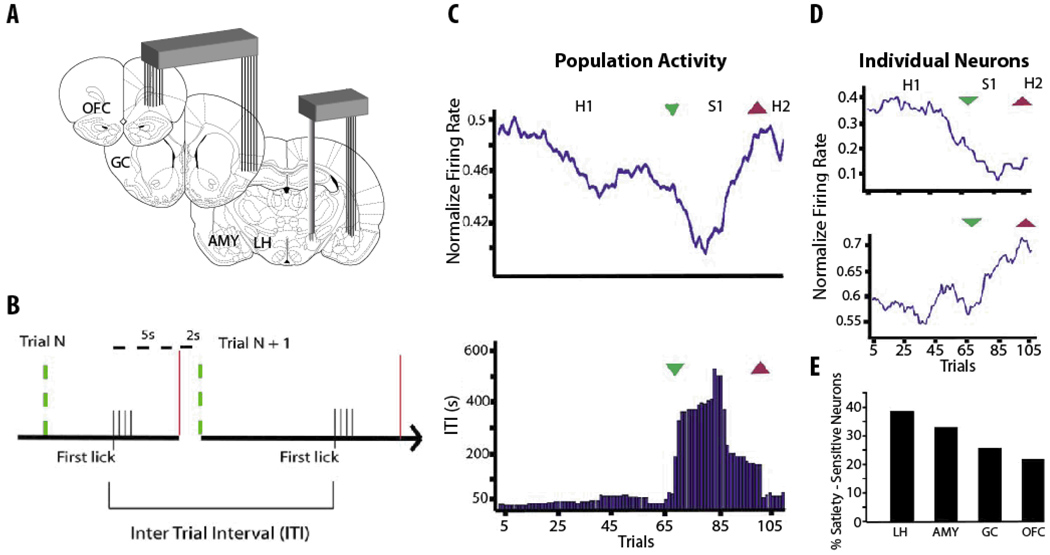
Simultaneous electrophysiological recordings from the LH, OFC, AMY, and GC in freely feeding rats [56] in states of satiety and hunger revealed lower firing rate levels during satiety state (Figure 4A). They found that whereas the large majority of satiety-modulated neurons preferentially responded to a unique phase of the feeding cycle (Figure 4D); the overall satiety state was only reflected by a global decrease in the population activity (Figure 4C). Thus, the sated state seems to employ a population code distributed across different brain regions that are sensitive to changes in metabolic status. Insofar as the activity in the particular areas change with satiety are in general agreement with functional neuroimaging studies [38, 54, 57, 58].
Figure 4. Ensemble coding of satiety states in the taste – feeding pathways of a behaving rat.
A. Schematic representation of four brain regions simultaneously recorded – The orbitofrontal cortex, OFC, gustatory cortex, GC, the amygdala, AMY, and lateral hypothalamus, LH. B. Scheme illustrating task structure. At a given trial n, sliding doors are open (green dashed lines), giving free access to sucrose solution from one sipping tube. Once the animal starts licking, lick timestamps are recorded and doors will be closed within 5 s after the first lick in the trial (red vertical lines). Two seconds later, doors reopen and trial n + 1 starts. The animal is allowed once again access to the drinking sipping tube. The time interval between the first lick in trial “n” and first lick in trial “n + 1” is defined as the Inter Trial Intervals (ITI). Larger ITIs indicate that the rat is sated. C. The population mean firing rate correlated significantly with behavioral ITIs (panel below). Mean population firing rate across trials throughout one experimental session. Green and red arrowheads indicate respectively start and end points for satiety phase (S1). The panel at bottom shows the corresponding ITIs for this session. Note a single significant satiety phase (S1, large ITI values), punctuated by two feeding cycles periods of hunger (Hunger 1 and 2, H1 and H2, respectively). D. Top. Shows the normalize activity of an individual neuron recorded in the same session. This neuron decreases its firing rate in trials where the rat gets sated, S1. Bottom. Shows the normalize activity of another individual neuron, but this neuron increases its firing rate after S1 and during phase H2. Unlike population activity, note that individual neurons do not encode the satiety state, instead they responded to a unique phase of the feeding cycle (H1-S1-H2). E. The histogram shows the percentage of cells modulated by satiety in each of the four recorded areas. Modified with permission from de Araujo et, al, 2006 [56].
TASTE DISCRIMINATION AND LEARNING
The study of taste discrimination is usually accomplished by employing a taste-guided task. In a discrimination task, the taste stimuli serve as cues for a reward or a punishment. This ensures that neural and behavioral responses are not driven by animal’s natural preference for a particular taste stimulus. Instead, taste guided tasks are used to study how an arbitrary taste-cue becomes predictive of a reward or a punishment. It also allows one to uncover the neuronal correlates of taste learning.
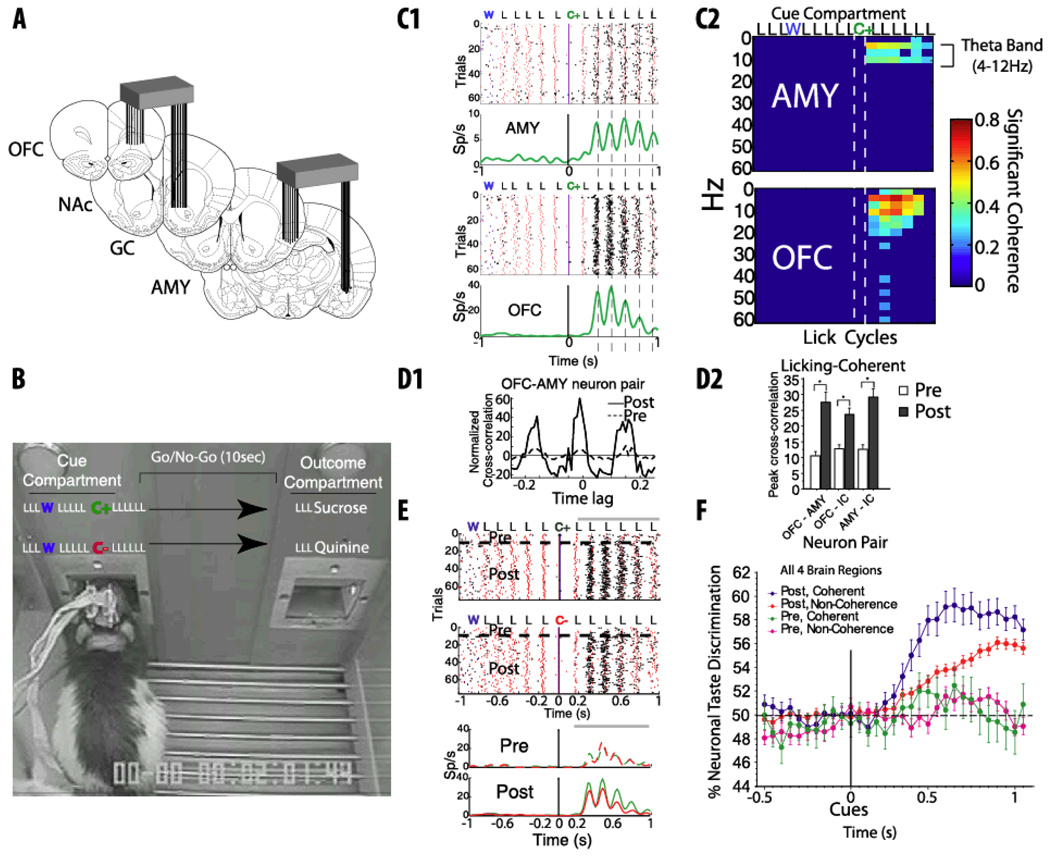
Freely moving rats drink tastant by rhythmically moving their tongues at 6–7 Hz (or ~160 ms) [59]. This rhythm generates a quasi-periodic signal that fragments taste information into discrete chunks [7, 60]. Recently, using a taste discrimination go/no-go task while simultaneously recording neuronal activity from the taste-reward circuit, we found how the neural responses of four brain regions (OFC, AMY, GC and NAc), process taste guided information while rats learn to discriminate between two taste cues (see Figure 5A) [9]. We found that rhythmic licking can entrain the spike timing of neurons located in disparate and distant brain regions (Figure 1A). For example, as seen in Figure 5C1 the activity of two simultaneously recorded neurons, one in amygdala and the other in the orbitofrontal cortex become synchronous after a taste cue comprised of a single lick had been delivered and during a period when the rat had to make a decision whether to “go” or “not go” to obtain a reward. In other words, both neurons fired in a manner that their spikes where both synchronous between areas (Figure 5D1) and coherent with licking (Figure 5C2).
Figure 5. Rhythmic licking serves as an internal “clock system” that dynamically coordinates spike-timing between brain regions and improves cue-taste discrimination as a function of learning.
A. Schematic representation of multielectrode recording sites in components of the rat taste-reward circuit. B. Picture of rat implanted with electrode bundles involved in taste discrimination go/no-go task. In each trial, rats approached a sipper in the Cue compartment, and then licked (L) an empty sipper three times, whereon on the fourth lick they received a water drop (W). They then licked the empty sipper another five times and on the 10th lick; they received a taste cue (either 0.1 M NaCl or 0.1 M MPG-monopotassium glutamate). In positive cue trials, C+, one of the arbitrary taste cues, signal the availability of sucrose, whereas in negative cue trials, C−, the other taste cue signaled the availability of quinine. After cue delivery, subjects had 10 s to leave the cue compartment and move to the outcome compartment where after three additional empty licks, they received either sucrose or quinine. C1. Rasters and Peri-Stimuli Time Histograms (PSTHs) showing the responses of two neurons simultaneously recorded from amygdala (AMY top) and orbitofrontal cortex (OFC bottom). Note that their action potentials were phase locked to the lick cycle and in synchrony only after cue delivery (vertical dashed lines). The red marks represent the tongue’s contact with the sipper tube. The black marks indicate the occurrence of action potentials. C2. This figure showing the coherence of the neurons in “C1” reveals that after cue delivery both AMY and OFC neurons fired at theta band (4–12Hz) in coherence with the lick cycle. D1. A cross-correlation between the same two neurons recorded in AMY-OFC (“C1”). It is seen that after learning (post) this neuron pair became more synchronous. D2. This histogram shows that synchronous spiking increased as a function of learning for pairs of OFC–AMY, OFC–IC (Insular Cortex), and AMY–IC neurons. A few nucleus accumbens (NAc) neurons fired in coherence with licking, thus they were not included in this analysis. E. It displays the rasters (top) and PSTHs (bottom) of the same OFC neuron plotted in “C1.” This neuron developed a stronger firing rate in C+ trials, after learning. The horizontal black lines separate trials in the Pre-learning and Post-learning of the go/no-go taste discrimination task. The red symbols represent the licks. F. The graph shows that, after learning (post learning), licking-coherent neurons (blue dots) were better at discriminating the taste-cues than non-coherent neurons (red dots). Modified with permission from Gutierrez et, al, 2010 [9].
In the vast majority of neurons in which spikes were phase-locked with licking do not arise from pure oromotor inputs such as those involved in jaw and tongue movements. This is because the neuronal activity was commonly found to be in synchrony with licking only during specific lick cycles in the trial (see Figure C1). Moreover, after learning the proportion of neurons that fire in coherence with licking dynamically increased. These results show that rhythmic licking has a more dynamic function than previously assigned to it. Under this scheme, we proposed that each lick can serve as a global internal “clock” signal against which neurons from distant brain regions can synchronize their spiking activity. Moreover, it was found that synchronous firing among pairs of neurons recorded simultaneously also increased after learning (Figure 2D1) and importantly, this effect was stronger in neurons that also fired in coherence with licking (Figure 2D2).
In order to solve this go/no-go task, rats needed to determine which taste cue predicted the reward (C+) and which predicted the aversive outcome (C−). Rats initially responded to each cue by making a go response and received the corresponding outcome until the cues acquired a predictive value. At this point, rats learned to avoid quinine, while continuing to respond after the positive cue to obtain sucrose. Thus, as rats learned to perform a go/no-go taste discrimination task, neuronal responses to initially non-predictive taste cues became more distinct and predictable in the four brain areas recorded, suggesting that learning induces a significant functional reorganization of neural activity throughout major components of the taste–reward circuit [9].
Finally, we identified two types of neurons that develop cue-selectivity with learning: one that fires in coherence with some phase of the licking cycle (Figures 1A, 5E) and a second that does not (data not shown). In general, cue-selective neurons that synchronize their activity with licking were significantly better at decoding the cue identity than non-coherent neurons (Figure 5F). A detailed analysis indicated that coherent cells fire with higher spike timing precision than non-licking coherent neurons, and that it was this finer degree of spike timing that conveyed the extra cue information of licking-coherent neurons. These results suggest that a major hallmark of taste learning is to increase spike timing precision, which in turn may allow licking-induced oscillations to enhance cue discrimination.
EXPECTATION AND TASTE PERCEPTION
Beyond post-ingestive effects, individual experience and history with tastants determines whether a tastant will be ingested or rejected. That is, taste perception is also modulated by expectations. In a study using functional magnetic resonance imaging in human GC, Nitschke et. al. [61] showed expectation can dampen neuronal taste responses. When humans were led to believe that a highly aversive bitter taste would be less distasteful than it actually was, they reported it to be less aversive than when they had accurate information about the taste and, the GC was less strongly activated. In another fMRI study the researchers showed pictures of appetizing foods to people (but not pictures of other objects) which selectively activated both the GC and OFC [62]. Indeed, even imagining consuming food has been shown to alter the amount of food ingested [63]. A final example of such behavior is referred as Anticipatory Reward Contrast Effect [64]. This behavioral effect occurs when a less preferred reward is devalued by the availability of one more preferred. One example of this effect was observed in rats that had daily access to a high concentration of sucrose (32%). In every experimental session these animals learn to expect to drink this high sucrose concentration. However, when this expectation is violated, for example by giving a lower (4%) sucrose concentration instead of the usual 32% solution, the rats rapidly refused to drink the otherwise palatable, but lower, sucrose solution. Interestingly, rats that had never been exposed to the high sucrose concentration avidly drink 4% sucrose solution [65]. Thus, it follows that exposure to a high concentration of sucrose devalued the reward value of lower concentrations of sucrose. This phenomenon indicates that exposure to sucrose can devaluate the reward value of a less palatable solution and less palatable tastants (e.g., salt, amino acids and acid).
SUMMARY OF MAIN RESULTS
In this review we covered many topics ranging from peripheral coding to gut-brain interactions to expectation. We first stated that to fully understand the processing of gustatory stimuli from the taste cells to the rNTS it is necessary to also identify the rNTS pathways involved in motor behavior regarding the food’s acceptance or rejection since these two processes are coupled. We next investigated Gut-Brain interactions in the rat GC by showing that the map produced by the positive hedonic value of an appetitive sweet tastant like saccharin can be made to appear like an hedonically negative aversive tastant like quinine by having the animal associate the sweet tastant with a malaise–evoking gut sensation, thus showing that for the same peripheral input a distinct change in the GC and associated behavioral output. Then we showed that when food is ingested it interacts with various classes of neuroepithelial enteroendocrine and enterochromaffin cells that synapse with primary vagal fibers where they regulate physiological responses involved with feeding. This occurs by regulating appetite and by releasing peptides. The third gut-brain topic involved an experiment in where recordings from ensembles of neurons were simultaneously obtained in the taste-reward pathway while hungry rats ate to satiety. These recordings showed that using a neural population code it is possible to accurately identify the rats’ internal state, namely when it was hungry and when it was sated. An experiment that involved recording ensembles throughout the taste-reward pathway while rats learned to discriminate among tastants revealed that when animals lick it sets up an internal “clock” (a common oscillatory drive) in which the evoked spike trains can covary with the lick cycle and synchronize the spike timing of multiple brain regions. The results indicated that neurons that covaried with licking were, after learning, better at identifying the tastant and accurately predicting the correct decision. We also touched on how responses in the GC are changed with the expectation of receiving food. Finally, we showed that receiving a less rewarding (but still rewarding) stimulus than was expected will influence the decision to ingest it.
IMPLICATIONS OF RESULTS
These results imply that to obtain a clearer understanding of gustatory processing it is necessary to investigate the taste-reward-motor pathways in awake, behaving animals that voluntarily ingest their food. Quasi-periodic dynamic inputs such as licking or chewing may act as an internal clock that may augment tastant discrimination and synchronize areas in the taste-reward pathway. Finally, these studies also indicate that gustatory processing does not only depend on the input from the oral cavity but on expectation, learning, and post–ingestive effects. The implications of these findings pertain to the world-wide epidemic of obesity and the associated metabolic consequences such as type II diabetes. Obesity must be approached systemically as it involves the interaction of taste, reward and post-ingestive physiological and pathological feedback in the taste-reward pathway [66–70].
ACKNOWLEDGEMENTS
We thank Professor Alan Carleton with help with the figures. This work was supported by NIH grant DC-01065 to SAS and CONACYT grant 78879 & ICYTDF PICDS08-59 to R.G.
REFERENCES
- 1.Oliveira-Maia AJ, Roberts CD, Simon SA, Nicolelis MA. In: Advances and Technical Standards in Neurosurgery. JD P, editor. Springer-Verlag/Wien; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rolls ET. Chem Senses. 2005;30 Suppl 1:i76. doi: 10.1093/chemse/bjh121. [DOI] [PubMed] [Google Scholar]
- 3.Small DA. Trends Neurosci. 2004;27:120. doi: 10.1016/j.tins.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Yaxley S, Rolls ET, Sienkiewicz ZJ. Physiol Behav. 1988;42:223. doi: 10.1016/0031-9384(88)90074-1. [DOI] [PubMed] [Google Scholar]
- 5.Kadohisa M, Rolls ET, Verhagen JV. Chem Senses. 2005;30:401. doi: 10.1093/chemse/bji036. [DOI] [PubMed] [Google Scholar]
- 6.Hanamori T, Kunitake T, Kato K, Kannan H. J Neurophysiol. 1998;79:2535. doi: 10.1152/jn.1998.79.5.2535. [DOI] [PubMed] [Google Scholar]
- 7.Halpern BP. In: Taste, Olfaction and the Central Nervous System. Pfaff DW, editor. New York: The Rockefeller Press; 1985. [Google Scholar]
- 8.Gutierrez R, Carmena JM, Nicolelis MA, Simon SA. J Neurophysiol. 2006;95:119. doi: 10.1152/jn.00467.2005. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez R, Simon SA, Nicolelis MA. J Neurosci. 2010;30:287. doi: 10.1523/JNEUROSCI.0855-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarmolinsky DA, Zuker CS, Ryba NJ. Cell. 2009;139:234. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carleton A, Accolla R, Simon SA. Trends Neurosci. 2010;33:326. doi: 10.1016/j.tins.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhari N, Roper SD. J Cell Biol. 2010;190:285. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spector AC, Glendinning JI. Curr Opin Neurobiol. 2009;19:370. doi: 10.1016/j.conb.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinnamon J, Yang R. In: The Senses: A Comprehensive Reference. S F, GK B, editors. San Diego: Academic Press; 2008. p. 135. [Google Scholar]
- 15.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. Cell. 2003;115:255. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Cell. 2003;112:293. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 17.Mueller KL, Hoon MA, Erlenbach I, Chandrashekar J, Zuker CS, Ryba NJ. Nature. 2005;434:225. doi: 10.1038/nature03352. [DOI] [PubMed] [Google Scholar]
- 18.Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. Cell. 2000;100:703. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 19.Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. Nature. 2010;464:297. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grill HJ, Norgren R. Science. 1978;201:267. doi: 10.1126/science.663655. [DOI] [PubMed] [Google Scholar]
- 21.Berridge KC. Inquiry (Oslo) 2009;52:378. doi: 10.1080/00201740903087359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinzeler NR, Travers SP. Am J Physiol Regul Integr Comp Physiol. 2008;295:R436. doi: 10.1152/ajpregu.00189.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaidi FN, Todd K, Enquist L, Whitehead MC. The Journal of Comparative Neurology. 2008;511:753. doi: 10.1002/cne.21869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fontanini A, Katz DB. J Neurophysiol. 2006;96:3183. doi: 10.1152/jn.00804.2006. [DOI] [PubMed] [Google Scholar]
- 25.Fontanini A, Grossman SE, Figueroa JA, Katz DB. J Neurosci. 2009;29:2486. doi: 10.1523/JNEUROSCI.3898-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobbins EG, Feldman JL. J Comp Neurol. 1995;357:376. doi: 10.1002/cne.903570305. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Fogel R, Renehan WE. The Journal of Comparative Neurology. 1992;323:432. doi: 10.1002/cne.903230310. [DOI] [PubMed] [Google Scholar]
- 28.Altschuler SM, Bao X, Miselis RR. The Journal of Comparative Neurology. 1991;309:402. doi: 10.1002/cne.903090309. [DOI] [PubMed] [Google Scholar]
- 29.Bernal SY, Dostova I, Kest A, Abayev Y, Kandova E, Touzani K, Sclafani A, Bodnar RJ. Behav Brain Res. 2008;190:59. doi: 10.1016/j.bbr.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren X, Ferreira JG, Zhou L, Shammah-Lagnado SJ, Yeckel CW, de Araujo IE. J Neurosci. 2010;30:8012. doi: 10.1523/JNEUROSCI.5749-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Neuron. 2008;57:930. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 32.de Araujo IE, Simon SA. Int J Obes. 2010;33:S34. doi: 10.1038/ijo.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia J, Lasiter PS, Bermudez-Rattoni F, Deems DA. Ann N Y Acad Sci. 1985;443:8. doi: 10.1111/j.1749-6632.1985.tb27060.x. [DOI] [PubMed] [Google Scholar]
- 34.Bermudez-Rattoni F. Nat Rev Neurosci. 2004;5:209. doi: 10.1038/nrn1344. [DOI] [PubMed] [Google Scholar]
- 35.Accolla R, Carleton A. Proc Natl Acad Sci U S A. 2008;105:4010. doi: 10.1073/pnas.0708927105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katz DB, Nicolelis MA, Simon SA. Curr Opin Neurobiol. 2002;12:448. doi: 10.1016/s0959-4388(02)00341-0. [DOI] [PubMed] [Google Scholar]
- 37.Fontanini A, Katz DB. J Neurophysiol. 2008;100:1160. doi: 10.1152/jn.90592.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Brain. 2001;124:1720. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 39.Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Nat Neurosci. 2008;11:1376. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berthoud HR, Morrison C. Annu Rev Psychol. 2008;59:55. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 41.Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y, Lewin MJ. Nature. 1998;394:790. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- 42.Bertrand PP. Front Neurosci. 2009;3:48. doi: 10.3389/neuro.21.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braun T, Voland P, Kunz L, Prinz C, Gratzl M. Gastroenterology. 2007;132:1890. doi: 10.1053/j.gastro.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 44.Nozawa K, Kawabata-Shoda E, Doihara H, Kojima R, Okada H, Mochizuki S, Sano Y, Inamura K, Matsushime H, Koizumi T, Yokoyama T, Ito H. Proc Natl Acad Sci U S A. 2009;106:3408. doi: 10.1073/pnas.0805323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kokrashvili Z, Mosinger B, Margolskee RF. Ann N Y Acad Sci. 2009;1170:91. doi: 10.1111/j.1749-6632.2009.04485.x. [DOI] [PubMed] [Google Scholar]
- 46.Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM. Proc Natl Acad Sci U S A. 2007;104:15069. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. Proc Natl Acad Sci U S A. 2007;104:15075. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emond M, Schwartz GJ, Ladenheim EE, Moran TH. Am J Physiol. 1999;276:R1545. doi: 10.1152/ajpregu.1999.276.5.R1545. [DOI] [PubMed] [Google Scholar]
- 49.Moran TH. Int J Obes (Lond) 2009;33 Suppl 1:S7. doi: 10.1038/ijo.2009.9. [DOI] [PubMed] [Google Scholar]
- 50.Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. J Clin Invest. 2008;118:1899. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simon SA, Liedtke W. J Clin Invest. 2008;118:2383. doi: 10.1172/JCI36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yee CL, Yang R, Bottger B, Finger TE, Kinnamon JC. J Comp Neurol. 2001;440:97. doi: 10.1002/cne.1372. [DOI] [PubMed] [Google Scholar]
- 53.Rolls ET, Sienkiewicz ZJ, Yaxley S. Eur J Neurosci. 1989;1:53. doi: 10.1111/j.1460-9568.1989.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 54.O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F, Kobal G, Renner B, Ahne G. Neuroreport. 2000;11:893. doi: 10.1097/00001756-200003200-00046. [DOI] [PubMed] [Google Scholar]
- 55.Rolls ET, Murzi E, Yaxley S, Thorpe SJ, Simpson SJ. Brain Res. 1986;368:79. doi: 10.1016/0006-8993(86)91044-9. [DOI] [PubMed] [Google Scholar]
- 56.de Araujo IE, Gutierrez R, Oliveira-Maia AJ, Pereira A, Jr, Nicolelis MA, Simon SA. Neuron. 2006;51:483. doi: 10.1016/j.neuron.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 57.Hinton EC, Parkinson JA, Holland AJ, Arana FS, Roberts AC, Owen AM. Eur J Neurosci. 2004;20:1411. doi: 10.1111/j.1460-9568.2004.03589.x. [DOI] [PubMed] [Google Scholar]
- 58.Fuhrer D, Zysset S, Stumvoll M. Obesity. 2008;16:945. doi: 10.1038/oby.2008.33. [DOI] [PubMed] [Google Scholar]
- 59.Weijnen JA. Neurosci Biobehav Rev. 1998;22:751. doi: 10.1016/s0149-7634(98)00003-7. [DOI] [PubMed] [Google Scholar]
- 60.Halpern BP, Tapper DN. Science. 1971;171:1256. doi: 10.1126/science.171.3977.1256. [DOI] [PubMed] [Google Scholar]
- 61.Nitschke JB, Dixon GE, Sarinopoulos I, Short SJ, Cohen JD, Smith EE, Kosslyn SM, Rose RM, Davidson RJ. Nat Neurosci. 2006;9:435. doi: 10.1038/nn1645. [DOI] [PubMed] [Google Scholar]
- 62.Simmons WK, Martin A, Barsalou LW. Cereb Cortex. 2005;15:1602. doi: 10.1093/cercor/bhi038. [DOI] [PubMed] [Google Scholar]
- 63.Morewedge CK, Huh YE, Vosgerau J. Science. 2010;330:1530. doi: 10.1126/science.1195701. [DOI] [PubMed] [Google Scholar]
- 64.Schroy PL, Wheeler RA, Davidson C, Scalera G, Twining RC, Grigson PS. Am J Physiol Regul Integr Comp Physiol. 2005;288:R966. doi: 10.1152/ajpregu.00292.2004. [DOI] [PubMed] [Google Scholar]
- 65.Leszczuk MH, Flaherty CF. Behavioural Brain Research. 2000;116:61. doi: 10.1016/s0166-4328(00)00265-5. [DOI] [PubMed] [Google Scholar]
- 66.Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. Brain Res. 2010;1350:43. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rolls ET. Int J Obes (Lond) 2010 August;3 [Google Scholar]
- 68.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. J Abnorm Psychol. 2008;117:924. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stice E, Spoor S, Bohon C, Small DM. Science. 2008;322:449. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Volkow ND, Wang GJ, Fowler JS, Telang F. Philos Trans R Soc Lond B Biol Sci. 2008;363:3191. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Travers JB, Jackson LM. J Neurophysiol. 1992;67:1171. doi: 10.1152/jn.1992.67.5.1171. [DOI] [PubMed] [Google Scholar]
- 72.Mogenson GJ, Yang CR. Adv Exp Med Biol. 1991;295:267. doi: 10.1007/978-1-4757-0145-6_14. [DOI] [PubMed] [Google Scholar]