Abstract
Purpose
Since 1992, the Centers for Disease Control and Prevention recommends that women of childbearing age consume 400 µg of folic acid per day to reduce the risk of neural tube defects (NTD). It has been speculated that both NTD and nervous system tumors (NST) may share common mechanisms of altered development. It examines the association between folic acid supplementation and the risk for childhood NST.
Methods
Incident cases of children with cancer in Spain registered between 2004 and 2006 were identified through the MACAPE Network Group. Tumors were classified as tumors derived from the neuroectoderm (cases) and those with a mesoderm origin (controls). In a second analysis, NST were further divided into central nervous system tumors (CNST) and sympathetic nervous system tumors (SNST). We compared folic acid supplementation between the groups.
Results
Overall, folic acid supplementation any time during pregnancy was similar between cases and controls (odds ratio (OR)=1.05; 95% confidence interval (CI) 0.92–1.20). However, supplementation before the 21st and 36th days of gestation resulted in significantly lower NST than in children with mesoderm tumors (OR=0.34; 95% CI 0.17–0.69 and OR=0.58; 95% CI 0.37–0.91, respectively). Preconceptional intakes of folic acid were also lower in NST although marginally nonsignificant (OR=0.44; 95% CI 0.10–1.02). When NST were divided into CNST and SNST, significant differences between tumors of mesoderm origin were only found for CNST.
Conclusions
Our results support the hypothesis that folate supplementation reduces the risk of childhood NST, especially CNST. The specific mechanism and cellular role that folate may play in the development of CNST have yet to be elucidated.
Keywords: Folic acid, Folate supplementation, Pediatric cancer, Nervous system tumors
Introduction
In September of 1992, the US Centers for Disease Control and Prevention (CDC) recommended that women of childbearing age consume 400 µg of folic acid per day prior to and during pregnancy to reduce the risk of neural tube defects (NTD) [1]. Dietary folate is naturally obtained from fruits, vegetables, and legumes. In Spain, folate is not available in fortified grains.
The importance of folate intake during early embryogenesis is of scientific interest and comprises a prolific research area in the field of pediatric cancers, where nervous system tumors (NST) are the second most common type of malignant tumors. It is thought that folate, through a common mechanism of altered development, may be involved in both NTD and NST. A relationship between folate intake and several types of cancers, including colorectal, breast, cervical, pancreatic, brain, and lung cancers, has been observed in several population-based studies [2–4].
Folate is known to protect against NTD, and is suspected to be particularly involved in primitive neuroectoderm tumors (PNET) [5]. A case-control study that looked at PNET risks associated with specific vitamin intake found a significant reduction in risk with folate supplementation (odds ratio (OR)=0.38, 95% confidence interval (CI)=0.21–0.73) [6]. In molecular analyses of pediatric central nervous system (CNS) malignancies, especially ependymomas, the folate receptor was found to be overexpressed [7]. Polymorphisms in the metabolic pathway of folate may alter the risk of NTD, and the same may apply to central nervous system tumors (CNST) [5, 8]. The specific cellular role that folate plays in CNST development is unknown, but some studies have hypothesized that folate is a protective factor.
Most studies published in the scientific literature have looked at the multivitamin supplementation of mothers during pregnancy, making it difficult to investigate the potentially independent effects of the micronutrients of interest. These have shown that multivitamin and folate supplement intake during preconceptional and some postconception periods reduce the risk of some sympathetic nervous system tumors (SNST) and leukemias during infancy [9–11].
Medio Ambiente y Cáncer Pediátrico (Environment and Paediatric Cancer Group) is a project for the compilation of pediatric environmental history (PEH) in children with cancer in the USA, Argentina, and Spain [12]. As part of a larger and ongoing study about the determinants of pediatric cancer (PC) that uses the PEH, we present data about folic acid supplement intake during periods critical to fetal development in mothers of children with cancer in Spain. This study analyzes the association between maternal supplementation with folic acid and NST in children.
Methods
Incident cases of children with cancer were diagnosed between 1 January 2004 and 1 January 2006 at six collaborating hospitals. Families were identified from the hospital cancer registries and invited to participate in the study. Centralized care reference units of PC in Spain facilitated access to medical records in the hospitals of the network. Furthermore, in each reference area, the hospital registry included over 98% of the children younger than 15 years of age who were diagnosed with a malignant neoplasm. The study was approved by the hospital network ethics committees and the institutional review boards.
Families were contacted by telephone to schedule interviews. Completion of the PEH questionnaire lasted 2–3 h. The interview was conducted in person, with one or both parents present. Informed consents were obtained from all parents. Children over 12 years of age were besides offered assent forms. The same pediatrician conducted the interviews at the collaborating hospitals and at the sites of local parent associations of children with cancer. The pediatrician has expertise in environmental health and oncology, and experience in interacting with PC patients and their families.
Information about folic acid supplement intake was obtained during the in-person interview with the mother or both parents and from the maternal ambulatory history, detailing last menstrual period and the date when folic acid supplements were initiated. For each mother, we estimated the date of the last period, the most probable day of conception, and noted the date when folic acid vitamin supplementation was initiated (≥400 µg/day). These dates (date supplementation was initiated (≥400 µg/day) and the probable conception date) were used for the preconceptional classification of each child: before the 21st day of gestation, before the 36th day of gestation, or anytime during pregnancy. Multivitamin supplementation intake was also noted. Three categorical variables were created, one for each trimester of pregnancy, to reflect whether multivitamins had been taken or not during that specific trimester.
Tumors were divided into two groups: neuroectoderm derivatives (including central and sympathetic NST—classified following the standards from the International Classification of Diseases for Oncology, 3rd edition) and mesoderm derivatives (leukemias, lymphomas, kidney, liver, bone, and soft tissue tumors). Seven tumors derived from multiple germ layers, like teratomas, were excluded from analysis. As the CDC only published the recommendations for folic acid supplementation in 1992, we excluded children born earlier than 1993.
Cases were children with NST, while the controls for this study were children with tumors of mesodermal origin. Of the 222 children included in the study 67 were NST and 155 children with tumors of mesodermal origin. The 67 NST cases were categorized in a secondary analysis into CNST and SNST. ORs were estimated from contingency tables comparing the differences in the periconceptional folic acid supplementation in cases (NST, and in secondary analyses CNST and SNST) and controls. An OR >1 would be interpreted as that folic acid supplementation would be greater in NST than in controls, while an OR <1 would suggest that supplementation was more frequent in controls. Analyses were conducted using SPSS 15. Logistic multivariable regression models were used to control for possible confounding factors (such as age, sex, socioeconomic status, associated familial syndrome, cancer in first degree relative, mother's and father's smoking habits during pregnancy, smokers fetus (overall exposure to tobacco during intrauterine period), exposure to traffic contaminants, and multivitamin intake). In the stepwise logistic regression analysis for NST and controls, all variables that showed statistically significant associations were included in the model. We investigated the existence of interaction and/or confusion between variables by constructing and comparing logistic regression models.
Results
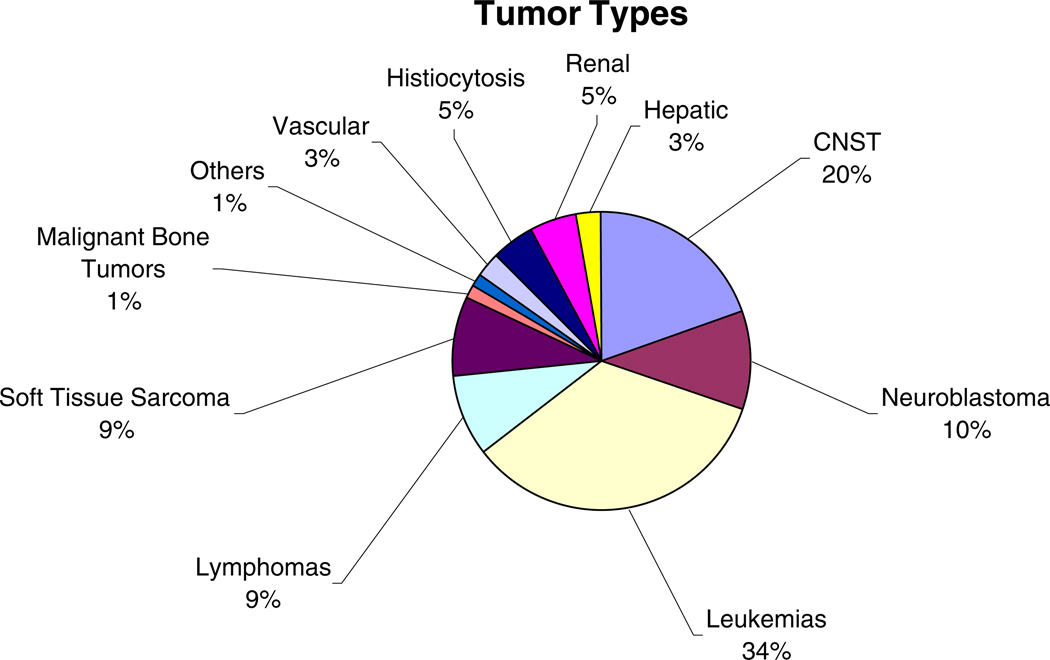
Participation reached 100% of the 325 cases originally identified in the study. Seven tumors derived from multiple germ layers, like teratomas, were excluded from analysis. Seventy-seven children who were born before 1993 were excluded from the study since the CDC only published the recommendations for folic acid supplementation in 1992. Of the remaining 241 children, information regarding folic acid supplementation was incomplete in 19 (8%), leaving, therefore, 222 children available for the study. Figure 1 shows the distribution of tumor types in the 222 children. Acute leukemia represented 34% of all tumors and CNST 20%. Table 1 lists the main characteristics and differences between cases and controls. As may be observed, there were significant differences between the two groups in maternal tobacco smoke exposure. More mothers of children with NST smoke than mothers of control children. However, when NST were further categorized into CNST and SNST, significant differences were only observed in smoking status between mothers of children with SNST and mesoderm tumors (OR=1.53; 95% CI 1.08–2.18) but not for CNST and children with mesoderm tumors.
Fig. 1.
Distribution of tumor types. Together, leukemias and CNST comprised 54% of the tumors found in the 222 children in our study. The three tumors forming the 1% labeled Others are a pulmonary blastoma, a thyroid carcinoma, and a melanoma
Table 1.
NST and mesoderm group characteristics
| NST cases; n=67 | Mesoderm controls; n=155 | OR | |
|---|---|---|---|
| Sex | |||
| Male | 42 (62.7%) | 95 (61.3%) | NS |
| Female | 25 (37.3%) | 60 (38.7%) | NS |
| Age at diagnosis (years) | |||
| Mean | 3.4 | 3.8 | NS |
| Net income/month (€) | |||
| <800 | 3 (4.5%) | 11 (7.1%) | NS |
| 800–1,500 | 15 (22.4%) | 22 (14.2%) | NS |
| 1,500–2,000 | 6 (9.0%) | 23 (14.8%) | NS |
| 2,000–2,500 | 7 (10.4%) | 17 (11.0%) | NS |
| 2,500–3,600 | 4 (6.0%) | 18 (11.6%) | NS |
| >3,500 | 4 (6.0%) | 11 (7.1%) | NS |
| Not answered | 28 (41.8%) | 53 (34.2%) | |
| Associated familial syndrome | |||
| Yes | 3 (4.5%) | 2 (1.3%) | NS |
| No | 64 (95.5%) | 153 (98.7%) | NS |
| Cancer in first-degree relative | |||
| Yes | 4 (6.0%) | 5 (3.2%) | NS |
| No | 63 (94.0%) | 150 (96.8%) | NS |
| Mother smoked during pregnancy | |||
| Yes | 38 (57.6%) | 65 (42.5%) | 1.4 (1.0–1.8)a |
| No | 28 (42.4%) | 88 (96.8%) | 0.7 (0.5–1.0)a |
| Fetal exposure (second-hand smoke) | |||
| Yes | 50 (75.8%) | 116 (76.3%) | NS |
| No | 16 (24.2%) | 36 (23.7%) | NS |
| Father smoked during pregnancy | |||
| Yes | 41 (62.1%) | 88 (58.3%) | NS |
| No | 25 (37.9%) | 63 (41.7%) | NS |
| Distance major road (≤100 m) | |||
| Yes | 15 (23.8%) | 40 (30.8%) | NS |
| No | 48 (76.2%) | 90 (69.2%) | NS |
There were significant differences. While the percentage of associated familial syndromes and cancer in first-degree relatives was greater in the NST group, our study population is too small to make inferences regarding these
Table 2 shows the distribution of folic acid supplementation in the study population. Though only 16.7% of all mothers took supplements before conception, up to 80.6% took supplements at any time during the pregnancy. Overall, folic acid supplementation any time during pregnancy was similar between cases and controls (OR=1.05; 95% CI 0.92–1.20). The odds of folic acid supplementation before the 21st and 36th days of gestational age were significantly lower in NST than in children with mesoderm tumors (OR=0.34; 95% CI 0.17–0.69 and OR=0.58; 95%CI 0.37–0.91, respectively). Preconceptional intakes of folic acid were also lower in NST although marginally nonsignificant (OR=0.44; 95% CI 0.10–1.02). When NST were divided into CNST and SNST, stronger significant differences with tumors of mesoderm origin were found for CNST but not for SNST. The largest differences were found in the frequency of folic acid supplementation before the 21st day of gestation for CNST compared with controls (OR=0.19; 95% CI=0.06–0.60). Table 3 shows the association between multivitamin intake and NST versus mesoderm tumors.
Table 2.
Distribution of folic acid (≥400 µg/day) supplement intake
| Period of ≥400µg/ day folic acid supplement intake |
All tumors (NST and mesoderm tumors) |
Neuroectoderm-derived tumors (NST) |
Mesoderm tumors |
Odds ratios (NST versus mesoderm tumors) |
Odds ratios (CNST versus mesoderm tumors) |
Odds ratios (SNST versus mesoderm tumors) |
||
|---|---|---|---|---|---|---|---|---|
| All NST |
Only CNST |
Only SNST |
||||||
| Preconceptional | ||||||||
| Yes | 37 | 6 | 3 | 3 | 31 | 0.44 (0.19–1.02) | 0.34 (0.10–1.06) | 0.65 (0.25–1.96) |
| No | 185 | 61 | 41 | 20 | 124 | |||
| Total | 222 | 67 | 44 | 23 | 155 | |||
| Before 21 days gestation | ||||||||
| Yes | 61 | 8 | 3 | 5 | 53 | 0.34 (0.17–0.69) | 0.19 (0.06–0.60) | 0.63 (0.28–1.42) |
| No | 151 | 59 | 41 | 18 | 102 | |||
| Total | 222 | 67 | 44 | 23 | 155 | |||
| Before 36 days gestation | ||||||||
| Yes | 84 | 17 | 11 | 6 | 67 | 0.58 (0.37–0.91) | 0.57 (0.33–0.99) | 0.60 (0.29–1.22) |
| No | 138 | 50 | 33 | 17 | 88 | |||
| Total | 222 | 67 | 44 | 23 | 155 | |||
| During any period | ||||||||
| Yes | 179 | 56 | 33 | 23 | 123 | 1.05 (0.92–1.20) | 0.94 (0.78–1.14) | |
| No | 43 | 11 | 11 | 0 | 32 | |||
| Total | 222 | 67 | 44 | 23 | 155 | |||
Distribution of folic acid intake by period is shown above. OR of folic acid supplement intake were significantly lower in children with NST than controls, but when NST were broken down by CNST and SNST, OR only remained significant for CNST in which folic acid intake occurred before the 21st (0.19, 0.06–0.60) or 36th day (0.57, 0.33–0.99) postconception
Table 3.
Distribution of multivitamin supplementation intake
| All | CNST | SNST | Mesoderm | Odds ratios (NST versus mesoderm tumors) |
Odds ratios (CNST versus mesoderm tumors) |
Odds ratios (SNST versus mesoderm tumors) |
|
|---|---|---|---|---|---|---|---|
| None | 55 | 41 | 14 | 108 | |||
| 1st trimester | 11 | 3 | 8 | 32 | 0.72 (0.39–1.35) | 0.29 (0.09–0.92) | 1.59 (0.84–2.99) |
| 2nd trimester | 2 | 1 | 2 | 16 | 0.27 (0.06–1.14) | 0.18 (0.02–1.35) | 0.96 (0.24–3.84) |
| 3rd trimester | 1 | 0 | 1 | 12 | 0.18 (0.02–1.35) | – | 0.67 (0.09–4.81) |
| Any intake | 12 | 3 | 9 | 47 | 0.59 (0.33–1.04) | 0.22 (0.07–0.68) | 1.29 (0.73–2.26) |
After logistic regression, the factor remaining significant for NST was folate supplementation before the 21st day gestation (OR=0.26; 95% CI 0.11–0.58). Although the overall number of cases is small, we carried out an exploratory study of the CNST and SNST. In the stepwise logistic regression analysis for CNST, significant folate supplementation before the 21st day (OR=0.16; 95% CI 0.04–0.57) and any multivitamin intake during pregnancy (OR=0.20; 95% CI 0.06–0.71) remained. For SNST, logistic regression showed that maternal smoking during pregnancy remained in the model, with an OR 2.53 (95% CI 1.01–6.3). No interaction and confusion were detected.
Discussion
Our results indicate that folate supplementation reduces the risk of childhood NST, especially CNST. The specific cellular mechanism that folate may play in the development of CNST has yet to be elucidated.
In support of the hypothesis of a true association, we argue that the association between malformations and tumors during infancy is well known. Thus, the possibility of shared metabolic pathways during neuro-oncogenic and neuromorphogenic periods could explain the role of folic acid in CNST [13, 14]. The different polymorphisms could explain the particular susceptibility for developing NST [5].
Folic acid deficiency may contribute to the synthesis of aberrant DNA and initiate oncogenesis by reducing the bioavailability of methionine and interfering with normal DNA methylation [15]. In fact, several epidemiologic studies show that vitamin supplementation with folic acid reduces the risk of colon cancer by up to 75% and breast cancer by 25%, and is even more effective in alcohol consumers [2–4]. A case-control study found a reduction in risk of meduloblastoma/PNET with periconceptional folic acid supplement intake in the quartile of women with the highest dietary intake [16]. Another study, which looked at the role of multivitamin intake and tried to elucidate the role of some micronutrients, including folate, found a reduced risk in CNST of 0.5 (0.3–0.8) with intake ≥400 µg/day, but it did not differentiate by the intake period [17]. The maternal ingestion of prenatal multivitamins is associated with a decreased risk for pediatric brain tumors and neuroblastoma. Presently, it is not known which constituent(s) among the multivitamins confers this protective effect [18]. Although many multivitamins contain folic acid (<200 ug), no interaction and confusion were detected in our study. The composition was different, as did the timing and duration of exposure. It is therefore difficult to interpret the protective effect at this point, and certainly deserves further analysis.
Currently, the known risk factors for CNST in children include [19]: sex, therapeutic doses of ionizing radiation to the head, genetic conditions such as neurofibromatosis, tuberous sclerosis, Turcot syndrome, Li–Fraumeni syndrome, and nevoid basal cell syndrome. Factors for which evidence is suggestive but not conclusive are frequent cured meat consumption, having a first-degree relative with a brain tumor, and family history of bone cancer, leukemia, or lymphoma. Factors for which evidence is inconsistent or limited are electromagnetic fields, products containing N-nitroso compounds, father's occupation, pesticide exposure, history of head injury, and family history of epilepsy, seizures, or mental retardation.
The finding of a higher rate of maternal smoking in the groups of SNST (smoking during pregnancy in SNST (65.5%)) was significantly higher than other different tumor types (p<0.05). Our study, similar to other studies, suggests a relationship between maternal smoking during pregnancy and SNST risk [9, 20]. The National Cancer Institute claim the evidence for an association between maternal smoking during pregnancy and the risk for neuroblastoma is inconsistent or limited [21].
In our study, we cannot specify whether doses <400 µg/day would be sufficient to prevent all CNST or if higher doses would have a greater protective effect. We explored the three cases of CNST whose mothers use folic acid supplementation. Out of those three, one had an inherited type I neurofibromatosis and another nonaffiliated familial syndrome (father and uncle had cleft lip and palate). Both of these constitutional characteristics could counterbalance the protective effect of folates.
Finally, it is possible that folic acid supplementation could protect against the development of CNST or could be confounded by factors associated with that intake and therefore be a marker of other true risk factors.
We could not analyze the dietary intake of folic acid, but unless systematic differences existed in the diet of the NST and the control group, the observed effect could be attributable to supplementation alone. In Spain, folate is not available in fortified grains.
Recall bias with regard to folic acid supplement intake could also be a confounding factor especially in mothers whose children were diagnosed at a later age. However, since the possible association of cancer is not regarded by the general public or the medical community as possibly related to folate or vitamin intakes in general, it is unlikely that a bias might have been introduced. And even so, recall bias would most likely be nondifferential, as controls were also tumor patients. Nonetheless, we repeated the analyses using only children who were diagnosed before the age of 6, and the results were consistent with the findings presented here.
A final consideration is whether any possible association might exist between the exposure of interest (folate supplementation) and controls. No association has been described between folate supplementation and a higher incidence of tumors of mesoderm origin in children, nor any other type of tumors. It is therefore unlikely that the association found might be explained by a higher intake in our control group than in the general population. To what extent the use of supplementation in our control group is comparable to that of the general population is unknown as there was no comparable data available on the use of folic acid supplementation in the general population at the time of the study. If the general use of folate supplementation were to be higher in the general population than in our control groups, then the odds ratios we found may underestimate the true magnitude of the protective effect of folate supplementation on NST.
Finally, several strengths of our study should be mentioned. One is that the study is based on a population-based registry on pediatric tumors and therefore uses incident cases. Details on the registry have been described elsewhere [12, 20, 22]. Another strength of our study is that, unlike most studies, our patient's information was collected through personal interviews conducted by specially trained personnel. Therefore, the possibilities for error, recall bias, or missing information were minimized and comparable in the two groups. A third strength is that our cases and controls were derived from the same population, and controls would most likely have been included as cases in our study if they had developed a NST instead of a tumor of mesoderm origin.
Our results suggest a possible protective effect of folic acid supplement intake in NST, especially CNST. Until now, recommendations for folic acid supplement intake have pertained to NTD. In the future, we hope to extend these recommendations to folic acid's protection against CNST. Under current public health authority recommendations of folic acid supplement intake to protect against NTD, we found that only 16.7% of mothers of children with cancer in our study population took folic acid supplements. With folate's possible protective effect against NST, the recommendations and public awareness of the importance of folic acid supplementation must rise.
Acknowledgments
The authors express their gratitude for the support and funding granted by the Scientific Foundation of the AECC (Asociación Española Contra el Cáncer). Additionally, we thank Isaedmarie Fevo and Rayden Llano from the Mount Sinai International Exchange Program for Minority Students. Their work is supported by grant MD001452 from the National Center on Minority Health and Health Disparities of the National Institutes of Health.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Juan Antonio Ortega-García, Paediatric Environmental Health Speciality Unit, Translational Cancer Research Center, University Hospital Virgen of Arrixaca, 30120 Murcia, Spain, ortega@pehsu.org, URL: www.pehsu.org.
Josep Ferrís-Tortajada, Paediatric Environmental Health Speciality Unit, Children’s University Hospital La Fe, Valencia, Spain.
Luz Claudio, Department of Community and Preventive Medicine, Mount Sinai School of Medicine, New York, NY, USA.
Offie Porat Soldin, Departments of Oncology, Medicine, Physiology and Biophysics, Georgetown University Medical Center, Washington, DC, USA.
Miguel Felipe Sanchez-Sauco, Paediatric Environmental Health Speciality Unit, Translational Cancer Research Center, University Hospital Virgen of Arrixaca, 30120 Murcia, Spain.
Jose Luís Fuster-Soler, Paediatric Oncology Section, University Hospital Virgen of Arrixaca, Murcia, Spain.
Juan Francisco Martínez-Lage, Regional Service of Neurosurgery, University Hospital Virgen of Arrixaca, Murcia, Spain.
References
- 1.Center Disease Control (CDC) Recommendations for the use of folic acid to reduce the number of cases of spine bifida and other neural tube defects. MMWR. 1992;41(No. RR-14):1–7. [PubMed] [Google Scholar]
- 2.Glynn SA, Albanes D. Folate and cancer: a review of the literature. Nutr Cancer. 1994;22:101–119. doi: 10.1080/01635589409514336. [DOI] [PubMed] [Google Scholar]
- 3.Giovarmucci E, Stampfer MJ, Coldlitz GA, Hunter DJ, Fuchs C, Rosner BA, Speizer FE, Willett WC. Multivitamin use, folate, and colon cancer in women in the Nurse’s Health Study. Ann Intern Med. 1998;129:517–534. doi: 10.7326/0003-4819-129-7-199810010-00002. [DOI] [PubMed] [Google Scholar]
- 4.Zhang S, Hunter DJ, Hankinson SE, Giovannucci EL, Rosner BA, Colditz GA, Speizer FE, Willett WC. A prospective study of folate intake and the risk of breast cancer. JAMA. 1999;281:1632–1637. doi: 10.1001/jama.281.17.1632. [DOI] [PubMed] [Google Scholar]
- 5.Gurney JG, Smith MA, Olshan AF, Hecht SS, Kasum CM. Clues to the etiology of childhood brain cancer: N-nitroso compounds, polyomaviruses, and other factors of interest. Cancer Invest. 2001;19:630–640. doi: 10.1081/cnv-100104291. [DOI] [PubMed] [Google Scholar]
- 6.Bunin GR, Kuijten RR, Buckley JD, Rorke LB, Meadows AT. Relation between maternal diet and subsequent primitive neuroectodermal brain tumors in young children. N Engl J Med. 1993;329:536–541. doi: 10.1056/NEJM199308193290804. [DOI] [PubMed] [Google Scholar]
- 7.Weitman SD, Frazier KM, Kamen BA. The folate receptor in central nervous system malignancies of childhood. J Neurooncol. 1994;21:107–1012. doi: 10.1007/BF01052894. [DOI] [PubMed] [Google Scholar]
- 8.Sirachainan N, Wongruangsri S, Kajanachumpol S, Pakakasama S, Visudtibhan A, Nuchprayoon I, Lusawat A, Phudhicharoenrat S, Shuangshoti S, Hongeng S. Folate pathway genetic polymorphisms and susceptibility of central nervous system tumors in Thai children. Cancer Detect Prev. 2008;32:72–78. doi: 10.1016/j.cdp.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Ferris i Tortajada J, Ortega Garcia JA, Garcia i Castell J, Lopez Andreu JA, Berbel Tornero O, Crehua Gaudiza E. Risk factors for neuroblastoma. An Pediatr (Barc) 2005;63:50–60. doi: 10.1157/13076768. [DOI] [PubMed] [Google Scholar]
- 10.French AE, Grant R, Weitzman S, Ray JG, Vermeulen MJ, Sung L, Greenberg M, Koren G. Folic acid food fortification is associated with a decline in neuroblastoma. Clin Pharmacol Ther. 2003;74:288–294. doi: 10.1016/S0009-9236(03)00200-5. [DOI] [PubMed] [Google Scholar]
- 11.Thompson JR, Gerald PF, Willoughby ML, Armstrong BK. Maternal folate supplementation in pregnancy and protection against acute lymphoblastic leukaemia in childhood: a case-control study. Lancet. 2001;358:1935–1940. doi: 10.1016/S0140-6736(01)06959-8. [DOI] [PubMed] [Google Scholar]
- 12.Ferrís Tortajada J, Ortega García JA, Marco Macián A, García Castell J. Environment and pediatric cancer. An Pediatr (Barc) 2004;61:42–50. doi: 10.1016/s1695-4033(04)78352-6. [DOI] [PubMed] [Google Scholar]
- 13.Berbel-Tornero O, Ortega-Garcia JA, Ferris-Tortajada J. Congenital abnormalities and childhood cancer: a cohort record-linkage study. Cancer. 2006;106:1418–1419. doi: 10.1002/cncr.21685. [DOI] [PubMed] [Google Scholar]
- 14.Berbel Tornero O, Ortega García JA, Ferrís I Tortajada J, García Castell J, Donat I Colomer J, Soldin OP, Fuster Soler JL. Neonatal tumours and congenital malformations. An Pediatr (Barc) 2008;68:589–595. doi: 10.1157/13123291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blount BC, Ames BN. DNA damage in folate deficiency. Baillières Clin Haematol. 1995;8:461–478. doi: 10.1016/s0950-3536(05)80216-1. [DOI] [PubMed] [Google Scholar]
- 16.Bunin GR, Gallagher PR, Rorke-Adams LB, Robison LL, Cnaan A. Maternal supplement, micronutrient, and cured meat intake during pregnancy and risk of medulloblastoma during childhood: a children’s oncology group study. Cancer Epidemiol Biomark Prev. 2006;15:1660–1667. doi: 10.1158/1055-9965.EPI-06-0254. [DOI] [PubMed] [Google Scholar]
- 17.Preston-Martin S, Pogoda JM, Mueller BA, Lubin F, Modan B, Holly EA, Filippini G, Cordier S, Peris-Bonet R, Choi W, Little J, Arslan A. Results from an international case-control study of childhood brain tumors: the role of prenatal vitamin supplementation. Environ Health Perspect. 1998;106:887–892. doi: 10.1289/ehp.98106887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goh YI, Bollano E, Einarson TR, Koren G. Prenatal multivitamin supplementation and rates of pediatric cancers: a meta-analysis. Clin Pharmacol Ther. 2007;81:685–691. doi: 10.1038/sj.clpt.6100100. [DOI] [PubMed] [Google Scholar]
- 19.Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, et al. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. Betsheda: National Cancer Institute; 1999. SEER Program NIH Pub No. 99–4649. [Google Scholar]
- 20.Ortega García JA, Martin M, López Fernández MT, Fuster Soler JL, Donat-Colomer J, López-Ibor B, Claudio L, Ferrís-Tortajada J. Transgenerational tobacco smoke exposure and childhood cancer: an observational study. 2010 doi: 10.1111/j.1440-1754.2010.01710.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ries LAG, Smith MA, Gurney JG, et al., editors. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. Bethesda: National Cancer Institute; 1999. pp. 99–4649. SEER Program NIG Pub No. [Google Scholar]
- 22.Ortega-García JA, Ferrís-Tortajada J, Torres-Cantero AM, Soldin OP, Torres EP, Fuster-Soler JL, Lopez-Ibor B, Madero-López L. Full breastfeeding and paediatric cancer. J Paediatr Child Health. 2008;44:10–13. doi: 10.1111/j.1440-1754.2007.01252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]