Abstract
Natural clays have been used in ancient and modern medicine, but the mechanism(s) that make certain clays lethal against bacterial pathogens has not been identified. We have compared the depositional environments, mineralogies, and chemistries of clays that exhibit antibacterial effects on a broad spectrum of human pathogens including antibiotic resistant strains. Natural antibacterial clays contain nanoscale (<200 nm), illite-smectite and reduced iron phases. The role of clay minerals in the bactericidal process is to buffer the aqueous pH and oxidation state to conditions that promote Fe2+ solubility.
Chemical analyses of E. coli killed by aqueous leachates of an antibacterial clay show that intracellular concentrations of Fe and P are elevated relative to controls. Phosphorus uptake by the cells supports a regulatory role of polyphosphate or phospholipids in controlling Fe2+. Fenton reaction products can degrade critical cell components, but we deduce that extracellular processes do not cause cell death. Rather, Fe2+ overwhelms outer membrane regulatory proteins and is oxidized when it enters the cell, precipitating Fe3+ and producing lethal hydroxyl radicals.
INTRODUCTION
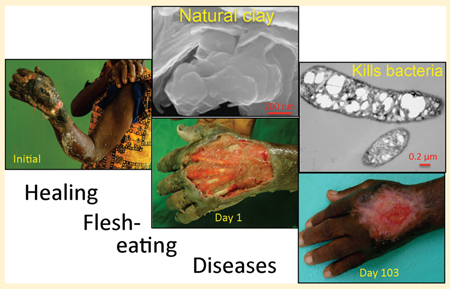
Overuse of antibiotics in healthcare is a major concern because of the consequential proliferation of antimicrobial resistance. Our studies of natural antibacterial minerals were initiated to investigate alternative antimicrobial mechanisms. Indigenous people worldwide have used clays for healing throughout history. French green clay poultices were documented for healing Buruli ulcer,1 a necrotizing fasciitis caused by Mycobacterium ulcerans. However, only one of the French clays used for healing proved to be antibacterial.2 Other sources of French green clay increased bacteria growth relative to controls.3 Continued testing of clays worldwide has revealed only a few deposits that are antibacterial. Each deposit is mineralogically different, but they are all from hydrothermally altered volcaniclastic environments; either altered pyroclastic material or bentonite (volcanic ash).
This paper reports the geochemical characteristics of the most effective antibacterial clay we have identified; supplied by Oregon Mineral Technologies (OMT) Grants Pass, Oregon. The clay source is an open pit mine in hydrothermally altered, pyroclastic material in the Cascade Mountains. It was shown to completely eliminate Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella typhimurium, and antibiotic resistant extended-spectrum beta lactamase (ESBL) E. coli and methicillin resistant S. aureus (MRSA) within 24 h.4
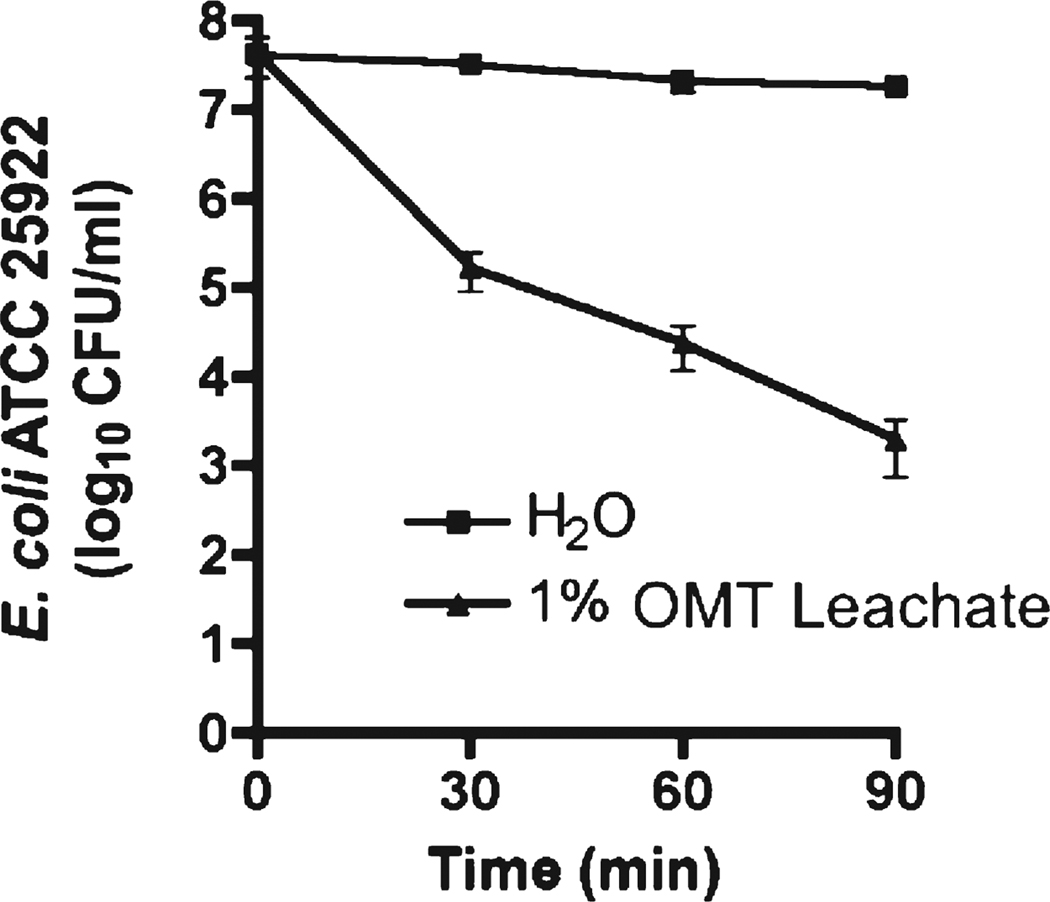
A variety of physical and/or chemical processes can make clays antibacterial. Physical bactericide can occur by surface attraction between clay minerals and bacteria, which can hamper passive and active uptake of essential nutrients, disrupt cell envelopes or impair efflux of metabolites.5 The natural antibacterial clays we have studied do not kill by physical associations between the clay and bacterial cells.6 The OMT clay shows no zone of inhibition when applied dry to bacterial colonies in vitro; however, the clays are antibacterial when hydrated. When an aqueous suspension of OMT clay (50 mg/mL water) was placed in dialysis tubing (25 000 MDCO) and submerged into a beaker of E. coli suspended in sterile Tryptic soy broth, the bacteria died over 24 h.7 Comparisons have been made between aqueous leachates of the OMT clay incubated with E. coli in nutrient broth and without nutrient broth2 to eliminate chemical speciation influenced by the broth chemistry. E. coli are completely killed by OMT leachates rapidly (Figure 1), compared to controls in distilled – deionized (DDI) water. Cell death occurs by exchange of soluble clay constituents toxic to the bacteria.
Figure 1.
Kill curve for a 10 mg/mL (1%) suspension of the OMT clay in DDI water. The top line shows that the control bacterial population was not significantly affected by the lack of nutrients over this time period.
Other clays, for example, allophane and imogolite, have been made antibacterial by chemical sorption of known bactericidal elements (Ag, Cu, Co, Zn) onto certain crystallographic sites of the mineral surfaces.8–10 Nanoparticulate metal oxides and ceramics can also be antibacterial;11 releasing soluble toxic compounds in proportion to their high specific surface area.12,13 Consequently, our objective is to document what soluble elements E. coli assimilate from the natural antibacterial OMT clay.
EXPERIMENTAL METHODS
Aqueous leachates were prepared using sterilized clay and water (50 mg/mL). The mixture was ultrasonified (Branson Sonifier 450) then shaken 24 h to equilibrate. The suspension of minerals in water was centrifuged (20 000 rpm; 67 000g) 1 h to remove solids14 and the solution was decanted.
E. coli (JM109) was grown to log phase and a cell density of ~109 cfu/mL in Luria Broth (LB). The cell population produced ~30 mg of cells (dry wt.) per experiment and each experiment was repeated in triplicate. E. coli was incubated in 1:1 volume ratio with clay leachate. All cultures were grown at 37 °C in a shaker-incubator for 24 h. Bacterial growth was evaluated after incubation, by standard plate counts. Bacteria (live or dead) from experiments were centrifuged at 7000 rpm for 5 min to pellet. Bacterial cells were then rinsed with DDI water (25 mL) in triplicate. Finally, one aliquot was rinsed in 25 mL of 50 mM EDTA:100 mM oxalic acid with 0.85% NaCl (pH adjusted to 6.95 with NaOH). This oxalate-EDTA solution removes metals from the exterior cell envelope15 without causing cell lysis.16 Test tubes containing bacteria were dried at room temperature, ground, and accurately weighed. Twelve milliliters of 10% nitric acid (Omnitrace Ultra, EMD chemicals) was used to digest cells, and then diluted to 40 mL.
Leachates, whole cells, and cells with exterior metals removed, were analyzed for elemental compositions with a Thermo Fisher Element 2 single-collector, double-focusing magnetic sector inductively coupled plasma mass spectrometer (ICP-MS) in low-, medium-, and high-resolution modes, depending on spectral interferences. The samples, blanks, and standards were acidified with nitric acid and spiked with 1 ppb indium solution to correct for instrumental response affected by solution matrix. Analyses of river water standard reference materials NIST 1640, NIST 1643e, and NRC SLRS4 determined analytical accuracy and precision. The measurement uncertainties were <5% (1σ). Major anion concentrations were measured by ion chromatography using a Dionex DX 600 Dual IC System with IonPac AS11-HC column.
RESULTS
Each antibacterial clay deposit is mineralogically different (Table 1) but they have in common the presence of expandable clay minerals (smectite) and Fe-rich phases (e.g., Fe-smectite, biotite, jarosite, pyrite, magnetite, hematite, goethite, amphibole). The presence of pyrite in some samples may be important for bactericidal action,18 but not all antibacterial clays contain pyrite. The average crystal diameter of the antibacterial clays (<200 nm) is an order of magnitude smaller than standard clay reference materials.19 The nanominerals in these deposits may enhance solubility of toxic elements.20 Additionally, the smectite may sequester toxic ions in the interlayer sites, which could be released upon rehydration in a clay poultice.
Table 1.
Mineralogy of Two Antibacterial Clays (ARG and OMT) Compared to Non-Antibacterial Clay (NAB), by Quantitative X-ray Diffraction17 Using CuKα Radiationa
| ARG |
OMT |
NAB |
|||
|---|---|---|---|---|---|
| mineral | wt% | mineral | wt% | mineral | wt% |
| Clay Minerals | Clay Minerals | Clay Minerals | |||
| 1Md Illite (+ smectite) | 30 | Illite-smectite (R1) | 49.6 | kaolins | 2.4 |
| Fe-smectite | 33 | chlorite | 3.1 | Ca smectite | 3.0 |
| 1 M Illite (R > 2) | 13 | Fe-smectite | 8.3 | ||
| mica (2M1) | 3.3 | biotite (1M) | 13.6 | ||
| mica (2M1) | 4.1 | ||||
| chlorite | 6.0 | ||||
| Non-Clays | Non-Clays | Non-Clays | |||
| quartz | 2.0 | quartz | 38.3 | quartz | 6.2 |
| calcite | 4.4 | amphibole | 0.5 | plagioclase | 43.9 |
| microcline | 3.4 | pyrite | 8.2 | Fe-amphibole | 3.8 |
| orthoclase | 2.9 | jarosite | 0.2 | pyrite | 0.7 |
| albite | 1.6 | gypsum | 0.9 | gypsum | 2.0 |
| apatite | 0.5 | ||||
| total nonclays | 14 | total nonclays | 48.1 | actinolite | 2.7 |
| calcite | 0.8 | ||||
Notation: R = Reichweite (ordering); M = monoclinic; Md = nonoclinic disordered.
Table 2 presents the chemistry of aqueous leachates from the OMT clay compared to the French antibacterial clay (ARG)3 and a nonantibacterial clay (NAB) 21 used medicinally. The elemental concentrations are all below minimum inhibitory concentrations (MIC) published for E. coli at circum-neutral pH.22–24 However, under the pH, oxidation state and aqueous speciation created within a clay poultice, the MIC may be quite different due to stabilization of different soluble species.
Table 2.
Chemical Analyses of Aqueous Clay Leachates, (A) ICP-MS Analyses of the Antibacterial ARG Clay3, the OMT (This Study) and NAB Clay Leachate21, (B) Ion Chromatography Analysis of Anions in the OMT Leachatea
| (A) |
ARG |
ARG |
OMT |
OMT |
NAB |
NAB |
|---|---|---|---|---|---|---|
| element | µg/L | µM | µg/L | µM | µg/L | µM |
| Mg | 620 | 26 | 20 900 | 860 | 93 000 | 3826 |
| Al | 5040 | 187 | 23 900 | 886 | 13 400 | 497 |
| Si | 7400 | 264 | 1430 | 51 | na | na |
| P | 446 | 14 | 96 | 3 | 7.3 | 0.24 |
| K | 7700 | 197 | 210 | 5 | na | na |
| Ca | 3500 | 87 | 2×105 | 4341 | 88 000 | 2196 |
| V | 21.5 | 0.42 | 3.3 | 0.06 | 1.1 | 0.02 |
| Cr | 1.3 | 0.03 | 16 | 0.30 | na | na |
| Mn | 55 | 1.0 | 5170 | 94 | 4300 | 78 |
| Fe | 280 | 5.0 | 51 600 | 924 | 10 500 | 188 |
| Co | 0.1 | 0.002 | 204 | 3 | 102 | 1.73 |
| Ni | 4.4 | 0.07 | 169 | 3 | 60 | 1.02 |
| Cu | 2.7 | 0.04 | 225 | 4 | 63 | 0.99 |
| Zn | 5.0 | 0.08 | 1090 | 17 | 66 | 1.01 |
| As | 260 | 3.47 | 1.1 | 0.01 | 0.4 | 0.01 |
| Se | 1.5 | 0.02 | 2.3 | 0.03 | 0.3 | 0.00 |
| Rb | 18 | 0.21 | 0.18 | 0.00 | 7.1 | 0.08 |
| Sr | 58 | 0.66 | 338 | 4 | 186 | 2.12 |
| Ba | 31 | 0.23 | 5.5 | 0.04 | 11 | 0.08 |
| Pb | 0.26 | 0.001 | 0.02 | 0.0001 | 0.08 | 0.0004 |
| pH | 10 | 4 | 8 |
| (B) |
OMT leachate anions |
|||||
|---|---|---|---|---|---|---|
| Cl− | NO2− | NO3− | Br− | SO42− | PO43− | |
| ppm | 0.88 | nd | nd | nd | 2065 | 6.6 |
| %rsd | 1.4 | 0.06 | 4.9 | |||
na = not analyzed; nd = none detected; %rsd = % relative std. dev. of three analyses.
E. coli (JM109) was used as a model species to evaluate mass transfer from the clay to bacteria. Incubation of the antibacterial OMT leachate 1:1 with a population of 109 cfu/mL E. coli grown in LB to log phase, showed no surviving bacteria in three independent experiments, compared to nonantibacterial (NAB) clay leachate and control (E. coli in LB without leachate). The control group produced on average 7.9 ± 0.4 × 109 cfu/mL, and the NAB clay leachate produced 9.0 ± 0.8 × 109 cfu/mL. Elements taken up by the OMT clay-amended E. coli suspensions were analyzed using direct injection ICP-MS25 (Table 3). Only elements showing significant differences in concentration from the controls are presented. Whole cells were rinsed in triplicate with filter-sterilized, distilled–deionized (DDI) water before elemental measurements. Intracellular elemental abundances were determined on oxalate-EDTA treated samples, which removes extracellular metals, particularly Fe.15
Table 3.
Metals Assimilated by Whole Bacterial Cells Compared to Intracellular Concentrationsa
| whole |
interior |
whole |
interior |
whole |
interior |
|
|---|---|---|---|---|---|---|
| element | control | control | NAB | NAB | OMT | OMT |
| Mg ppm | 23 600 | 7720 | 19 040 | 4960 | 6432 | 5434 |
| %rsd | 8 | 2 | 2 | 2 | 2 | 2 |
| Al ppm | 2360 | 560 | 1788 | 453 | 9936 | 703 |
| %rsd | 11 | 5 | 2 | 7 | 0.2 | 3 |
| Pppm | 6800 | 3580 | 20 000 | 5200 | 28 160 | 8552 |
| %rsd | 7 | 4 | 4 | 4 | 5 | 4 |
| Ca ppm | 17 200 | 3400 | 9600 | 2267 | 3360 | 2248 |
| %rsd | 7 | 4 | 13 | 6 | 10 | 5 |
| V ppm | 5 | 2 | 4 | 2 | 34 | 3 |
| %rsd | 8 | 41 | 52 | 25 | 8 | 13 |
| Fe ppm | 2160 | 354 | 1364 | 320 | 42 880 | 2662 |
| %rsd | 5 | 4 | 2 | 7 | 5 | 4 |
| Cu ppm | 40 | 10 | 60 | 20 | 125 | 51 |
| %rsd | 18 | 12 | 7 | 6 | 5 | 14 |
| Pb ppm | 4 | 2 | 3 | 2 | 50 | 3 |
| %rsd | 8 | 3 | 6 | 4 | 3 | 2 |
| cell | whole | interior | whole | interior | whole | interior |
| dry wt. | 10 mg | 20 mg | 25 mg | 29 mg | 25 mg | 29 mg |
ppm = µg/L × mL solution/mg dry cells; %rsd = percent relative standard deviaion.
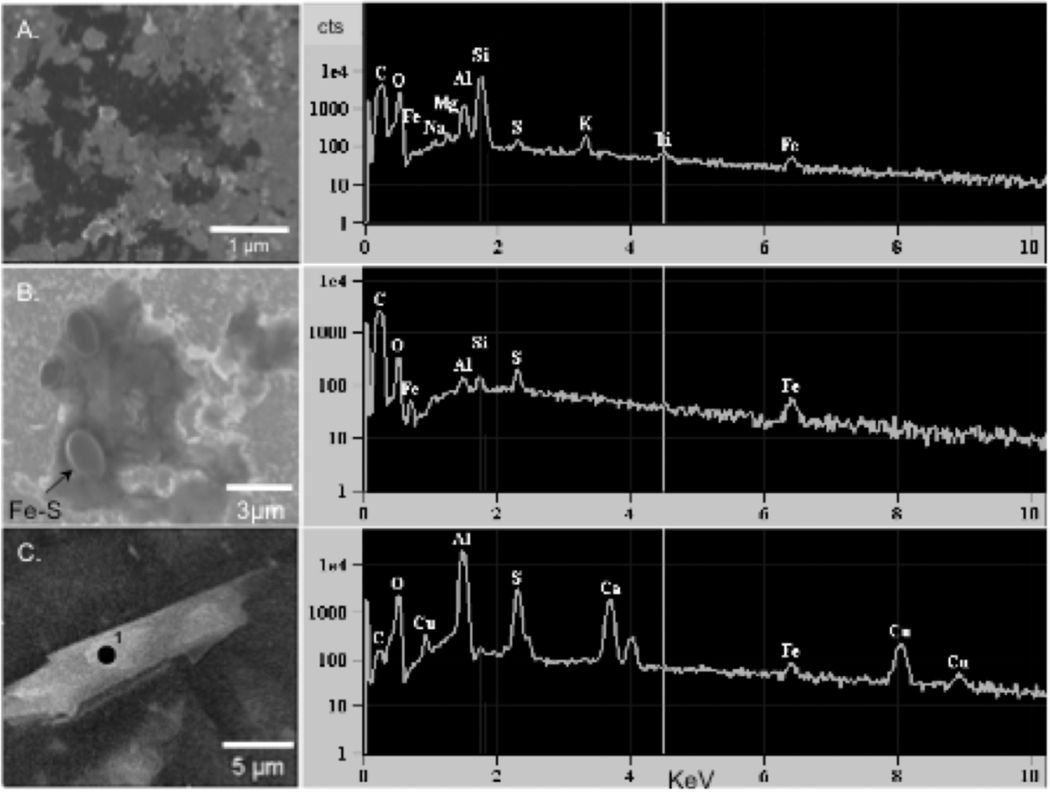
High-resolution scanning electron microscope (SEM) images of the OMT clay (Figure 2) show (a) the dominance of submicrometer crystals; (b) the clay matrix encompassing submicrometer spherical Fe–S particles; and (c) coexistence of a lath-shaped mineral rich in Ca, Al, S, and O (sulfate). Different batches of OMT contain up to 3wt.% gypsum and/or jarosite. Coexistence of gypsum and pyrite in the natural clay places the equilibrium chemical stability on the equal activity line for S–SO4.26
Figure 2.
SEM images using 30 kV, 2.1 nA current, (A) Clay particles on graphite (dark substrate). EDS (right) shows elements over a rastered area, (B) Close up of Fe–S spherules in aluminosilicate matrix. EDS spectrum is rastered over spherules, (C) Lath shaped particle with high Ca, S, and O (black dot) interpreted as gypsum or jarosite.
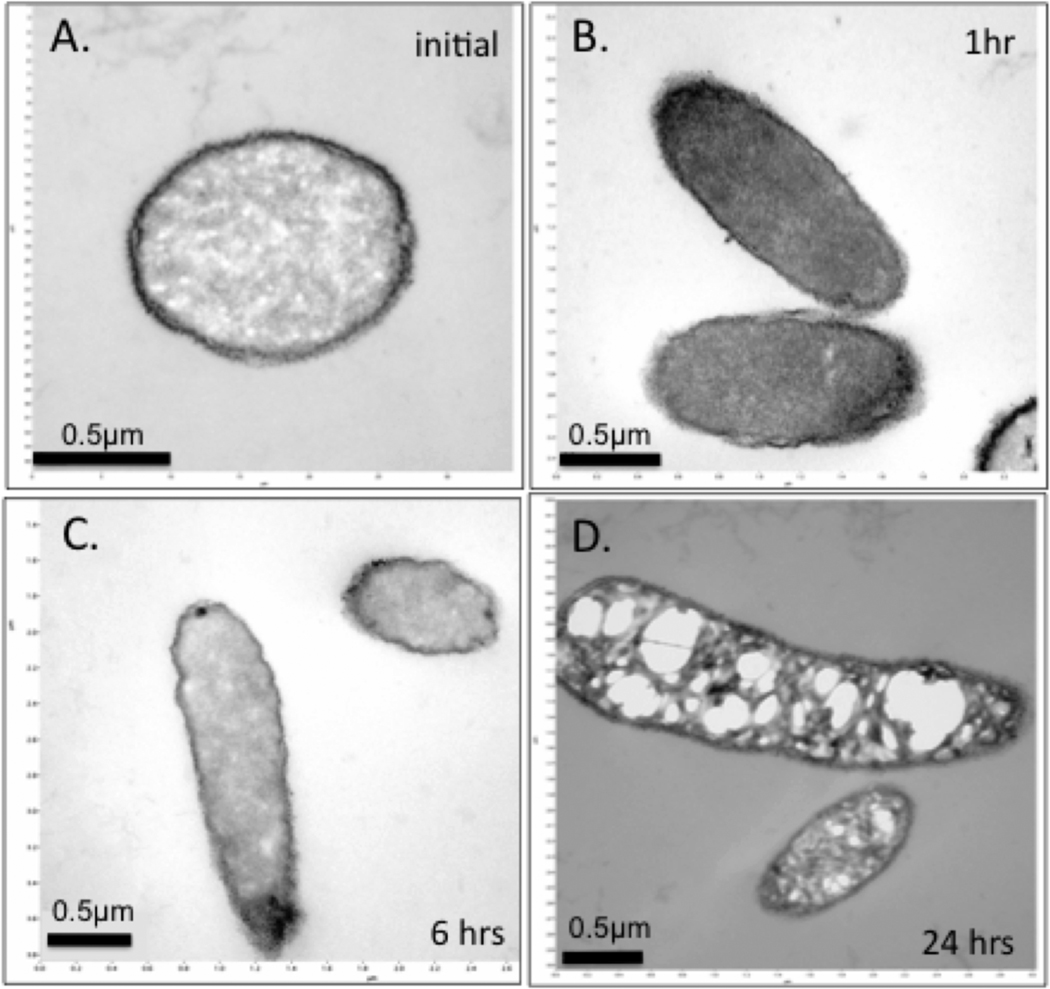
Important energy-requiring processes for nutrient uptake and metabolism, motility, and cell division occur at the cell membrane. Transmission electron microscope (TEM) imaging of E. coli treated with the OMT leachate (Figure 3) shows initial development of “hairy” vesicles on the cell membrane in response to acidic conditions.27 Black, electron-opaque, particles are initially evenly distributed on the cell walls. However, after 6 h of incubation, the black particles are concentrated at polar ends of the cells, indicating that the cells are metabolically active, as bacteria regulate uptake of metals through polar attractions.28 The greatest cell damage is observed after 24 h when the black particles appear on the interior of the E. coli cells, near large voids or vacuoles in the cytoplasm.
Figure 3.
Time series TEM images of pressure frozen, fixed (glutaraldehyde and Os vapor), resin embedded, sectioned E. coli after incubation with OMT leachate. (A) initial incubation shows uniform black precipitate of electron opaque metal, (B) after 1 h metal migrates toward cell poles, (C) metals are concentrated at cell poles after 6 h, (D) after 24 h metal has penetrated the cell interior and voids form accompanying cell death.
DISCUSSION
Significantly higher concentrations of Al, P, V, Fe Cu, and Pb are associated with the whole bacteria killed by the OMT leachate (Table 3), than in the live bacteria (controls). In contrast, the concentrations of Mg and Ca are much lower in the OMT leachate-treated whole bacteria. This is consistent with results of Borrok et al.29 who showed that protons irreversibly exchange with Mg and Ca on the surface of bacteria exposed to acidic solutions and increase adsorption of other metals. Nonetheless, the interior of the OMT treated bacteria shows Mg and Ca concentrations similar to the NAB sample that did not kill E. coli, indicating that limitation of these nutrients was not the cause for bacterial cell death.
On the cell interior of the OMT killed bacteria, Al, P, Fe, and Cu have elevated concentrations relative to controls. Whereas Cu can be toxic to bacteria, its abundance is not statistically different than the control or NAB samples. However, concentrations of P, Fe, and Al in the cell interior of OMT killed bacteria are significantly higher than controls.
The high concentration of Al in the OMT leachate is of interest because the antibacterial clay leachates have extreme pH (≤4 or ≥10; Table 2). Aluminum is soluble in extreme pH conditions, but precipitates in circum-neutral fluids.30 Notably, whole-cell concentrations of Al in OMT leachate-treated samples are four times higher than those in the controls, even though the cytoplasmic Al contents in treated and control samples were similar. Apparently, Al has limited transport through cell membranes, as tri- and tetra-valent metals are often precluded by cell pore diameters.31Aluminum precipitated on the cell envelope might inhibit influx of nutrients or efflux of waste. However, the elevated intracellular P and Fe suggest that Al did not block their influx channels.
The P content of the E. coli killed by OMT leachate is also four times greater than the control, and the interior cell concentration is twice as high. This indicates significant precipitation of P on the cell wall that does not preclude uptake into the cell. Phosphorus is an important part of ATP, DNA, polyphosphates, and phospholipids in cells, and phosphate anions have been found essential for the regulation of cation transport across the cell membrane.32 The negatively charged phosphate group in the lipid molecules of the cell membrane counterbalance positively charged arginine in a voltage-controlled gate across pore channels. The OMT leachate treated E. coli accumulated P, possibly in response to chemical stresses,33 whereas it was not required in excess for the normal cell function of the controls. The enhancement of P in the E. coli cell interior may represent an attempt by the bacteria to control the influx of Fe2+ across the cell membrane, or to remove the metal.
Whole cell Fe concentrations in the OMT leachate-treated E. coli are 20 times greater than in the controls. The intracellular Fe contents are eight times higher. Thus excess Fe was transported through the cell wall and is implicated as the primary reactant in the bactericidal process. The elevated concentrations of P and Fe in the E. coli interior imply that extracellular metals did not block transport channels through the cell envelope, at least not initially.
Images depicting the progression of dying E. coli incubated with OMT leachate (Figure 3) are similar to images of E. coli treated with FeSO4,20 where black particles were oxides of Fe3+. The vacuoles formed may result from (a) polyphosphate granules that regulate intracellular metals, (b) metabolic byproducts (e.g., NO), (c) destruction of DNA or other cell components, (d) leakage of cytoplasm after cell death. Bacteria require Fe for many metabolic processes, and use ferritin protein to regulate intracellular Fe levels and storage capacities. Ferritins can keep some Fe in solution, but excess Fe can form toxic precipitates.34 E. coli exposed to OMT leachate may have accumulated P as a stress response33 and may employ P to gate the cation flux across the cell wall,32 but eventually the bacteria were overwhelmed by the high Fe2+ concentration of the OMT leachate.
Evaluating the Antibacterial Process
The bioavailability of metals to bacteria depends on the aqueous metal speciation in the clay poultice. The process of transferring elements from a clay surface through water to a cell membrane involves numerous chemical reactions and the formation of rapidly reactive intermediates (radicals) that are affected by clay mineralogy and by surface complexation on the bacteria. The pH and oxidation state of the water added to clay to make a poultice is most influenced by the buffering capacity of the clay minerals with relative surface areas >100 m2/g.35
Clays that buffer water to circum-neutral pH values are not antibacterial,6 therefore, we tested the tolerance of E. coli to low pH fluids. When the OMT leachates are mixed with E. coli growing in LB, the pH increases to 5.3, but this increase does not change the solubility of Fe or Cu (Figure 4). Adjusting the pH of sodium phosphate buffer to acidic conditions similar to that of OMT leachate reduced the population of E. coli by 2 orders of magnitude (107.5 to 105.5 cfu/mL) over 2 h. However, the OMT leachate completely killed the bacteria in less than 1 h,4 indicating that it is not pH alone that killed the E. coli, and implicating soluble species derived from the clay.
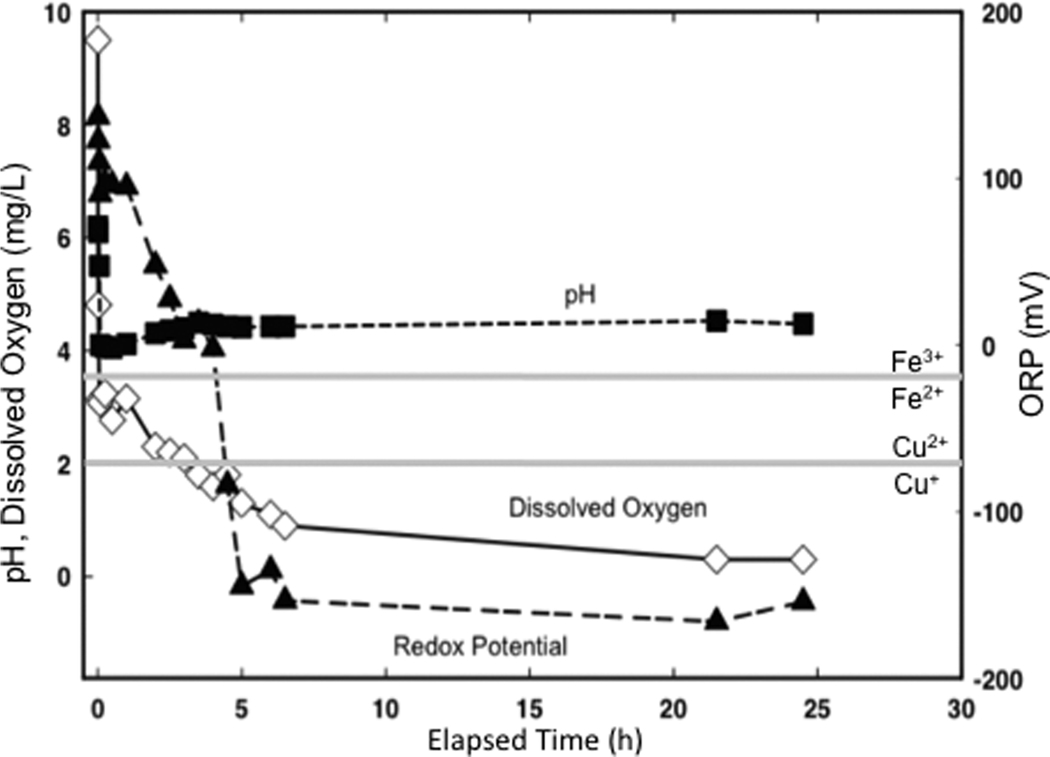
Figure 4.
Plot showing changes in pH, ORP and dissolved O2 content of DDI water over 24 h after addition of OMT clay (50 mg/mL). Redox couples for Fe3+/Fe2+ and Cu2+/Cu+ are shown (gray lines) for pH 4.
The oxidation state of the clay poultice is most critical to the antibacterial process. The highly reduced natural clay buffers the water chemistry of a poultice to low oxidation state where Fe2+ and Cu+ are stable (Figure 4). A rapid decrease of dissolved oxygen (D. O.), ORP and pH occurs when the OMT clay is added to DDI water. The pH stabilizes within the first hour, but the D.O. declines over 6 h, coincident with E. coli death. It is unlikely that decline in D.O. was responsible for the E. coli death, because this bacterium is fully capable of anaerobic respiration.36However, E. coli may take up Fe2+ and other metals under anaerobic conditions. When OMT leachates are oxidized, they lose antibacterial capacity coincident with precipitation of iron oxides. However, if small amounts (50 mg/mL) of clay are suspended in the aqueous solution, the leachate chemistry is stabilized and remains antibacterial. This demonstrates the buffering capacity of the clay.
The OMT clay contains 5–8% pyrite, which is known to be bactericidal. In the presence of water, pyrite produces reactive oxygen species (ROS) such as hydrogen peroxide and hydroxyl radicals that degrade nucleic acids in RNA and DNA via the Fenton reaction.37 Chelation of Fe2+ in solution by EDTA, or scavenging of hydroxyl radicals can prevent the destruction of DNA.38 Therefore, EDTA, thiourea, and bipyridal were used to chelate Fe and remove ROS from OMT aqueous leachates.4 The bactericidal effect on E. coli was reduced (compared to controls), but was not eliminated. Bacteria have developed mechanisms for tolerating external oxidative stress,39 therefore extracellular ROS are not implicated for bactericide.
Park and Imlay40 showed that significant damage to cellular DNA occurs when hydrogen peroxide reacts with Fe2+ to form hydroxyl radicals in vivo. The cell envelope accommodates exogenous hydrogen peroxide,41 but penetration of Fe2+ into the cell will catalyze the Fenton reaction. The rapid influx of reduced Fe2+ and possibly Cu+ produced by the OMT clay may overwhelm the metal resistance mechanisms of pathogenic bacteria. Oxidation of Fe2+ occurs within the bacteria and there produces destructive hydroxyl radical reactions precipitating Fe3+ near the intracellular voids (Figure 3d). Hydroxyl radical production may be further enhanced through cellular biochemical reduction of the Fe3+.40 Kohanski et al.42 showed that production of intracellular hydroxyl radicals is a common underlying mechanism for cellular death by synthetic antibiotics. Evidence suggests that some antibacterial clays promote similar bactericidal reactions.
Development of protective biochemical mechanisms by bacteria23 may not be possible on the time scale of a clay poultice application. Because of this, the external application of antibacterial clays may be more effective for wound care than systemic antibiotics. The growth of human tissue, coincident with the antibacterial action of clays1 remains unexplained. However, nanoparticles of Fe-oxide (magnetite) were recently found to be bactericidal against S. aureus, while increasing growth of human bone cells.43 The differential effect of Fe on eukaryotic versus prokaryotic cells is consistent with a role of host defense mechanisms that target bacterial Fe utilization,44 and may be key to understanding the observed clay healing process.1
ACKNOWLEDGMENT
We thank Oregon Mineral Technologies, Inc. for access to their mineral deposit. T. Cunningham and D. Lowry provided TEM images; S. Haydel assisted with microbiology pilot studies. NIH grant R21 AT003618 partially supported this research. We appreciate support from the ASU School of Life Sciences, Center for Solid State Science, and NASA Astrobiology Institute.
Footnotes
The author(s) declare that they have no competing interests.
REFERENCES
- 1.Williams LB, Holland M, Eberl DD, Brunet T, Brunet de Courssou L. Killer clays! Natural antibacterial clay minerals. Mineral. Soc. Bull., London. 2004;139:3–8. [Google Scholar]
- 2.Haydel SE, Remenih CM, Williams LB. Broad-spectrum in vitro antibacterial activities of clay minerals against antibiotic–susceptible and antibiotic-resistant bacterial pathogens. J. Antimicrob. Chemother. 2008;61:353–361. doi: 10.1093/jac/dkm468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams LB, Haydel SE, Geise RF, Eberl DD. Chemical and mineralogical characteristics of French green clays used for healing. Clays Clay Miner. 2008;56:437–452. doi: 10.1346/CCMN.2008.0560405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunningham TB, Koehl JL, Summers JS, Haydel SE. pH-dependent metal ion toxicity influences of the antibacterial activity of two natural mineral mixtures. PLoS-ONE. 2010;5:e9456. doi: 10.1371/journal.pone.0009456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferris FG, Fyfe WS, Beveridge TJ. Bacteria as nucleation sites for authigenic minerals in a metal-contaminated lake sediment. Chem. Geol. 1987;63:225–232. [Google Scholar]
- 6.Williams LB, Haydel SE. Evaluation of the medicinal use of clay minerals as antibacterial agents. Int. Geol. Rev. 2010;52:745–770. doi: 10.1080/00206811003679737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metge DW, Harvey RW, Eberl DD, Wasylenki LE, Williams LB. Evaluating the oxidation state of antibacterial minerals. Geochim. Cosmochim. Acta, Suppl. 2009;73:A875. [Google Scholar]
- 8.Clark CJ, McBride MB. Chemisorption of Cu2+ and Co2+ on allophane and imogolite. Clays Clay Miner. 1984;32:300–310. [Google Scholar]
- 9.Onodera Y, Sunayama S, Chatterjee A, Iwasaki T, Satoh T, Suzuki T, Mimura H. Bactericidal allophonic materials prepared from allophane soil II. Bactericidal activities of silver/phosphorus-silver loaded allophonic specimens. Appl. Clay Sci. 2001;18:135–144. [Google Scholar]
- 10.Magaña SM, Quintana P, Aguilar DH, Toledo JA, Angeles-Chavez C, Cortez MA, Leon L, Freile-Peleorio Y, Lopez T, Torrex-Sanches RM. Antibacterial activity of montmorillonites modified with silver. J. Mol. Catal. 2007;A281:192–199. [Google Scholar]
- 11.Sawai J, Yoshikawa T. Quantitative evaluation of antifungal activity of metallic oxide powder (MgO, CaO and ZnO) by an indirect conductimetric assay. J. Appl. Microbiol. 2004;96:803–809. doi: 10.1111/j.1365-2672.2004.02234.x. [DOI] [PubMed] [Google Scholar]
- 12.Waltimo T, Brunner TJ, Vollenweider M, Stark WJ, Zehnder M. Antibacterial effect of nanometric bioactive glass. J. Dent. Res. 2007;86:754–757. doi: 10.1177/154405910708600813. [DOI] [PubMed] [Google Scholar]
- 13.Yacoby I, Benhar I. Antibacterial Nanomedicine. Nanomedicine. 2008;3:329–341. doi: 10.2217/17435889.3.3.329. [DOI] [PubMed] [Google Scholar]
- 14.Jackson ML. Soil Chemical Analysis Advanced Course. 2nd ed. Madison, WI: 1979. [Google Scholar]
- 15.Tovar-Sanchez A, Sanudo-Wilhelmy SA, Garcia-Vargas M, Weaver RS, Popels LC, Hutchins DA. A trace metal clean reagent to remove surface-bound iron from marine phytoplankton. Mar. Chem. 2003;82:91–99. [Google Scholar]
- 16.Vogel C, Fisher NS. Metal accumulation by heterotrphic marine bacterioplankton. Limnol. Oceanogr. 2010;55:519–528. [Google Scholar]
- 17.Eberl DD. Users guide to RockJock: A Program for Determining Quantitative Mineralogy from Powder X-ray Diffraction Data, Open file Report 03-78; Denver, CO: U. S Geological Survey; 2003. [Google Scholar]
- 18.Cohn C, Laffers R, Simon SR, O’Riordan T, Schoonen MAA. Role of pyrite in formation of hydroxyl radicals in coal: possible implications for human health. Part. Fibre Toxicol. 2006;3 doi: 10.1186/1743-8977-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. [accessed February 27, 2011];Source Clays Repository, Clay Minerals Society Website. http://www.clays.org.
- 20.Lee C, Kim JY, Lee Y, Nelson KL, Yoon J, Sedlak DL. Bactericidal effect of zero-valent iron nanoparticles on Escherichia coli. Environ. Sci. Technol. 2008;42:4927–4933. doi: 10.1021/es800408u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hauser EA. Problems of Clay and Laterite Genesis. New York: American Institute of Mining and Metallurgical Engineering; 1952. Kisameet Bay clay deposit; pp. 178–190. [Google Scholar]
- 22.Dopson M, Baker-Austin C, Koppineedi PR, Bond PL. Growth in sulfidic mineral environments: metal resistance mechanisms in acidophilic microorganisms. Microbiology. 2003;1490:1959–1970. doi: 10.1099/mic.0.26296-0. [DOI] [PubMed] [Google Scholar]
- 23.Nies DH. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 1999;51:730–750. doi: 10.1007/s002530051457. [DOI] [PubMed] [Google Scholar]
- 24.Wackett LP, Dodge AG, Ellis LB. Microbial genomics and the periodic table. Appl. Environ. Microbiol. 2004;70:647–655. doi: 10.1128/AEM.70.2.647-655.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gikunju CM, Lev SM, Birenzvige A, Schaefer DM. Detection and identification of bacteria using direct injection inductively coupled plasma mass spectroscopy. Talanta. 2004;62:741–744. doi: 10.1016/j.talanta.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Helgeson HC. Thermodynamics of hydrothermal systems at elevated temperatures and pressures. Am. J. Sci. 1969;267:729–804. [Google Scholar]
- 27.Beveridge TJ. Structures of Gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 1999;181:4725–4733. doi: 10.1128/jb.181.16.4725-4733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shapiro L, McAdams H, Losick R. Generating and exploiting polarity in bacteria. Science. 2002;298:1942–1946. doi: 10.1126/science.1072163. [DOI] [PubMed] [Google Scholar]
- 29.Borrok D, Fein JB, Tischler M, O’Loughlin E, Meyer H, Liss M, Kemner KM. The effect of acidic solutions and growth conditions on the adsorptive properties of bacterial surfaces. Chem. Geol. 2004;209:107–119. [Google Scholar]
- 30.Wesolowski DJ. Aluminum speciation and equilibria in aqueous solution: I. The solubility of gibbsite in the system Na-K-Cl-OH-Al-A1(OH)4 from 0 to 100°C. Geochim. Cosmochim. Acta. 1992;56:1065–1091. [Google Scholar]
- 31.Williams RJP. What is wrong with aluminum?]?>. J. Inorg. Biochem. 1999;76:81–88. doi: 10.1016/s0162-0134(99)00118-x. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt D, Jiang Q, MacKinnon R. Phospholipids and the origin of cationic gating charges in voltage sensors. Nature. 2006;444:776–779. doi: 10.1038/nature05416. [DOI] [PubMed] [Google Scholar]
- 33.Rao NN, Liu S, Kornberg A. Inorganic polyphosphate in Escherichia coli: the phosphate regulon and the stringent response. J. Bacteriol. 1998;180:2186–2193. doi: 10.1128/jb.180.8.2186-2193.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arosio P, Levi S. Ferritin, iron homeostasis and oxidative damage. Free Radical Biol. Med. 2002;33:457–463. doi: 10.1016/s0891-5849(02)00842-0. [DOI] [PubMed] [Google Scholar]
- 35.Moore DM, Reynolds RC. X-ray Diffraction and the Identification and Analysis of Clay Minerals. New York: Oxford University Press; 1997. [Google Scholar]
- 36.Unden G, Achebach S, Holighaus G, Tran H, Wachwitz B, Zeuner Y. Control of FNR function of Escherichia coli by O2 and reducing conditions. J. Mol. Microbiol. Biotechnol. 2002;4:263–268. [PubMed] [Google Scholar]
- 37.Schoonen MAA, Harrington AD, Laffers R, Strongin DR. Role of hydrogen peroxide and hydroxyl radical in pyrite oxidation by molecula oxygen. Geochim. Cosmochim. Acta. 2010;74:4971–4987. [Google Scholar]
- 38.Cohn CA, Fisher SC, Brownawell BJ, Schoonen MAA. Adenine oxidation by pyrite generated hydroxyl radicals. Geochem. Trans. 2010;11(2):8. doi: 10.1186/1467-4866-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Storz G, Tartaglia LA, Farr SB, Ames BN. Bacterial defenses against oxidative stress. Trends Genet. 1990;6:363–368. doi: 10.1016/0168-9525(90)90278-e. [DOI] [PubMed] [Google Scholar]
- 40.Park S, Imlay JA. High levels of intracellular cysteine promote oxidative DNA damage by driving the Fenton reaction. J. Bacteriol. 2003;185:1942–1950. doi: 10.1128/JB.185.6.1942-1950.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imlay JA, Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988;240:640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- 42.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 43.Tran N, Mir A, Mallik D, Sinha A, Nayar S, Webster TJ. Bactericidal effect of iron oxide nanoparticles on Staphylococcus aureus. Int. J. Nanomed. 2010;5:277–283. doi: 10.2147/ijn.s9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Repine JE, Fox RB, Berger EM. Hydrogen peroxide kills Staphylococcus aureus by reacting with Staphylococcol iron to form hydroxyl radical. J. Biol. Chem. 1981;256:7094–7096. [PubMed] [Google Scholar]