Abstract
The mammalian target of rapamycin (mTOR) kinase occurs in mTOR complex 1 (mTORC1) and complex 2 (mTORC2), primarily differing by the substrate specificity factors raptor (in mTORC1) and rictor (in mTORC2). Both complexes are activated during human cytomegalovirus (HCMV) infection. mTORC1 phosphorylates eukaryotic initiation factor 4E (eIF4E)-binding protein (4E-BP1) and p70S6 kinase (S6K) in uninfected cells, and this activity is lost upon raptor depletion. In infected cells, 4E-BP1 and S6K phosphorylation is maintained when raptor or rictor is depleted, suggesting that either mTOR complex can phosphorylate 4E-BP1 and S6K. Studies using the mTOR inhibitor Torin1 show that phosphorylation of 4E-BP1 and S6K in infected cells depends on mTOR kinase. The total levels of 4E-BP1 and viral proteins representative of all temporal classes were lowered by Torin1 treatment and by raptor, but not rictor, depletion, suggesting that mTORC1 is involved in the production of all classes of HCMV proteins. We also show that Torin1 inhibition of mTOR kinase is rapid and most deleterious at early times of infection. While Torin1 treatment from the beginning of infection significantly inhibited translation of viral proteins, its addition at later time points had far less effect. Thus, with respect to mTOR's role in translational control, HCMV depends on it early in infection but can bypass it at later times of infection. Depletion of 4E-BP1 by use of short hairpin RNAs (shRNAs) did not rescue HCMV growth in Torin1-treated human fibroblasts as it has been shown to in murine cytomegalovirus (MCMV)-infected 4E-BP1−/− mouse embryo fibroblasts (MEFs), suggesting that during HCMV infection mTOR kinase has additional roles other than phosphorylating and inactivating 4E-BP1. Overall, our data suggest a dynamic relationship between HCMV and mTOR kinase which changes during the course of infection.
INTRODUCTION
The mammalian target of rapamycin (mTOR) kinase exists in two complexes, mTOR complex 1 (mTORC1) and complex 2 (mTORC2). The major difference between the two is the substrate specificity factors, raptor in mTORC1 and rictor in mTORC2 (17, 29). Under normal conditions, the two complexes differ in their sensitivity to the drug rapamycin: mTORC1 is sensitive, and mTORC2 is insensitive (29). However, increasing evidence suggests that some mTORC1 functions are rapamycin resistant (33).
Functionally, the two complexes have very different substrates in uninfected cells. The substrates and functions of mTORC2 are less well characterized than those of mTORC1; however, data suggest that mTORC2 functions in (i) organizing the actin cytoskeleton (16, 29), (ii) regulating cell growth and proliferation, (iii) promoting the activation of the serum glucocorticoid-induced protein kinase (SGK), and (iv) promoting the phosphorylation of proline-directed serine or threonine sites in the turn motif of Akt and protein kinase C isoforms (reviewed in reference 1). A primary function of mTORC1 is to control cap-dependent translation (21, 28, 30). When mTORC1 is active, it phosphorylates p70S6 kinase (S6K) and the eukaryotic initiation factor 4E (eIF4E)-binding protein (4E-BP1). Phosphorylation of S6K activates it, promoting the formation of translation initiation complexes (21); this includes the phosphorylation of ribosomal protein S6. The phosphorylation of 4E-BP1 is a major point of control in cap-dependent translation and regulates the function of the eIF4F translation-initiation complex. eIF4F binds to the 5′ cap of an mRNA, which is the first step in the initiation of cap-dependent translation. The eIF4F complex contains eIF4E, which is the subunit that actually binds to the 5′ cap. To form the eIF4F complex, eIF4E must bind to eIF4G, the scaffolding protein of the eIF4F complex. However, eIF4G binding, and cap-dependent translation, can be inhibited by binding of 4E-BP1 to eIF4E, which displaces eIF4G and inhibits eIF4F complex formation. mTORC1 regulates the binding of 4E-BP1 to eIF4E by controlling 4E-BP1 phosphorylation. Under positive growth conditions, mTORC1 is active and maintains 4E-BP1 in its hyperphosphorylated state, where it is incapable of binding eIF4E. This allows eIF4E to remain active in the eIF4F complex and promote cap-dependent translation. However, under negative growth conditions, for example during stress or inhibition of mTOR kinase activity, mTORC1 is inactive; 4E-BP1 becomes hypophosphorylated, binds efficiently to eIF4E, displaces eIF4G, and inhibits cap-dependent translation (reviewed in reference 8).
Human cytomegalovirus (HCMV), a betaherpesvirus, is the largest human herpesvirus, with a 230-kb double-stranded DNA genome and the potential to encode over 200 proteins (25, 26). HCMV shows slow growth in culture and, therefore, must maintain favorable cellular conditions for a long period. However, during this time, the increased transcription, translation, and metabolism that accompany infection will cause cellular stress. This, in turn, results in the induction of a repertoire of cellular stress responses which provide means for the cell to survive and control the stress. Many of these stress responses target mTORC1 for inactivation to reduce cellular translation and save energy and resources during times of stress. While this would be good for the stressed cell, inhibition of translation would be deleterious to an HCMV infection. We have previously shown that both mTORC1 and mTORC2 are activated during HCMV infection (18, 20). In addition, we and others have shown that HCMV has multiple mechanisms for dealing with the deleterious aspects of cellular stress responses while maintaining beneficial ones (2, 6, 8, 9, 12, 14, 15, 18–20, 22, 23, 35, 36). From these studies, it has become clear that HCMV strives to maintain mTOR kinase activity, as measured by 4E-BP1, S6K, and Akt phosphorylation, even under conditions that normally induce strong inhibitory stress responses (2, 18–20).
Our previous studies have shown that the phosphorylation of 4E-BP1 becomes resistant to rapamycin in HCMV-infected cells and that rapamycin has only a transient effect on HCMV growth in human fibroblasts (18, 20). These studies suggested that, during HCMV infection, mTORC1 and mTORC2 are modified such that their substrate specificities and rapamycin sensitivities are altered. This was shown by using short hairpin RNAs (shRNAs) to deplete rictor or raptor; it was found that phosphorylation of 4E-BP1 and S6K was maintained when either raptor or rictor was depleted in infected cells. These results suggested that, in infected cells, the substrate specificity of mTORC2 was broadened to include 4E-BP1 and S6K.
Recently, a more effective inhibitor of mTOR kinase, Torin1, was developed. Torin1 specifically targets the catalytic site of mTOR kinase and inhibits all known phosphorylation by either mTORC1 or mTORC2 (33). Recent HCMV studies (24) using Torin1 suggested that HCMV replication requires both rapamycin-sensitive and rapamycin-resistant mTOR activity and confirmed our previous observations (18, 20) that phosphorylation of 4E-BP1 and the formation of the eIF4F complex become resistant to rapamycin in HCMV-infected cells. In contrast to rapamycin, mTOR phosphorylation of 4E-BP1 in infected cells is sensitive to Torin1 (24). These studies also showed that similar mTOR signaling and sensitivity to Torin1 occurred in murine cytomegalovirus (MCMV)-infected mouse embryo fibroblasts (MEFs). In the MCMV system, the inhibition of MCMV replication by Torin1 was rescued in MEFs lacking 4E-BP1, suggesting that the primary reason for preserving mTOR kinase activity in MCMV-infected MEFs was to maintain the ability to phosphorylate and inactivate 4E-BP1.
In the following experiments, we further examined the function of mTORC1 and mTORC2 in HCMV-infected cells by use of shRNAs to deplete rictor or raptor or Torin1 treatment to inhibit mTOR kinase. For the Torin1 experiments, we treated with Torin1 not only from the beginning of the infection but also for 24-h periods prior to harvest. We did this because Torin1 addition from the beginning of infection attenuates and significantly delays the establishment of an infection. The addition of Torin1 at various time points after infection allowed us to determine the effect of mTOR kinase inhibition at different points in an established infection.
We find that the phosphorylation of 4E-BP1 on amino acid residues T37, T46, and S65 and the phosphorylation of S6K on amino acid residue T389 are all maintained under conditions of either rictor or raptor depletion. This agrees with and expands upon our previous data (20), which suggested that 4E-BP1 and S6K become substrates of either mTOR complex in infected cells. However, we find that the depletion of rictor or raptor had significantly different effects on the total level of 4E-BP1; while hyperphosphorylation is maintained, the total level of 4E-BP decreased in raptor-depleted, but not rictor-depleted, cells. Substrate specificity for the phosphorylation of Akt S473 was not altered in infected cells; it remained mTORC2 specific and was inhibited only by rictor depletion. That mTOR kinase remained the kinase that phosphorylated 4E-BP1, S6K, and Akt in infected cells was demonstrated by the complete loss of 4E-BP1, S6K, and Akt phosphorylation by the addition of Torin1 to infected cells. The depletion of rictor or raptor also showed differing effects on the accumulation of viral proteins. Representatives of immediate-early, early, and late proteins were all lowered by raptor depletion but not by rictor depletion. Similar effects on the accumulation of these proteins were noted after Torin1 treatment. Thus, the loss of raptor and the inhibition of mTOR kinase present similar phenotypes with respect to viral protein production. This suggests that mTORC1, where mTOR kinase and raptor function together, plays a significant role in the production of all classes of HCMV proteins.
We also show that Torin1 inhibition of mTOR kinase is rapid, being complete by 120 min after addition, and is most deleterious at early times of infection. While Torin1 treatment from the beginning of infection significantly inhibited translation of viral proteins, its addition at later time points had far less effect. Thus, with respect to mTOR's role in translational control of viral proteins, HCMV depends on it early in infection but becomes increasingly less dependent as the viral time course proceeds. Finally, the depletion of 4E-BP1 by use of shRNAs did not rescue HCMV growth in Torin1-treated human fibroblasts as it has been shown to in MCMV-infected, 4E-BP1−/− MEFs. This suggests that during HCMV infection mTOR kinase has additional roles other than phosphorylating and inactivating 4E-BP1, potentially roles that are not related to translation. Overall, our data suggest a dynamic relationship between HCMV and mTOR kinase which changes during the course of the infection.
MATERIALS AND METHODS
Cell culture.
Life-extended human foreskin fibroblasts (HFs) (5) were cultured at 37°C in 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM Gluta-Max (Gibco). Serum starvation experiments were done with the same medium lacking FCS. HFs were used between passage 2 and passage 10 after thawing.
Reagents.
Torin1 was kindly provided by David Sabatini (Whitehead Institute) and was used at a concentration of 250 nM. The primary antibodies used in this study were total and phospho-4E-BP1 (T37/46 and S65), phospho-S6K (T389), and phospho-Akt (Ser 473) (Cell Signaling), Rictor (Bethyl), Raptor (Millipore), UL44 and pp28 (Santa Cruz), gB (East Coast Bio), and β-actin (Chemicon). The antibody that recognizes the common exon 2 and 3 sequences of the HCMV major immediate-early proteins (MIEPs) was prepared by this laboratory and has previously been described (13).
Virus preparation, titration, and infection.
HCMV (Towne strain) stocks were prepared and purified as previously described (18). Titers were determined using the 50% tissue culture infective dose (TCID50) method. All experiments were performed using a multiplicity of infection (MOI) of 3.
Plasmids encoding lentiviruses that express shRNAs against rictor (1857) and raptor (1853) were purchased from Addgene. Plasmids encoding lentiviruses that express shRNAs against 4E-BP1 (TRCN0000040206) were purchased from Open Biosystems. The lentivirus containing the luciferase shRNA, used as a control, was constructed in this laboratory. Lentiviruses were prepared in 293T cells as previously described (37). Where applicable, lentiviruses were incubated with cells for 7 h in the presence of 10 μg/ml polybrene. Cells were then maintained in serum-containing medium for 4 days before serum starvation prior to mock or HCMV infection.
HCMV growth in the presence of Torin1.
HFs grown in six-well plates were serum starved for 24 h, followed by mock or HCMV infection at a MOI of 3. After a 2-h incubation, the cells were washed three times with 1× phosphate-buffered saline (PBS), and 2 ml of serum-free medium, with or without 250 nM Torin1, was added to each well. Drug treatments began at 2 h postinfection (hpi) or 24 h prior to cell harvest. At designated time points, 1 ml of medium was removed from the well and transferred to a 15-ml conical tube. The cells in the well were scraped into the remaining 1 ml of medium, sonicated 10 times with 1-s pulses, and combined with the previously collected medium. The medium was then centrifuged at 4°C for 10 min at 1,800 rpm. The supernatant was transferred to a fresh tube, flash frozen in liquid nitrogen, and stored at −80°C. Viral titers were calculated using the TCID50 method.
Time course viral infections.
HFs grown in six-well plates were serum starved for 24 h, followed by mock or HCMV infection at a MOI of 3. After a 2-h incubation, cells were washed three times with 1× PBS and fresh serum-free DMEM was added. Where applicable, Torin1 was added as described above. At various time points after infection, cells were incubated for 5 min at 4°C in cold radioimmunoprecipitation assay (RIPA) lysis buffer (1% NP-40, 1% sodium deoxycholate, 0.1% SDS, 0.15 M NaCl, 0.01 M sodium phosphate, pH 7.2, 2 mM EDTA, 50 mM sodium fluoride, 200 μM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 1.5 μg aprotinin/ml, 1 μg leupeptin/ml). Cells were then scraped into Eppendorf tubes, and lysates were centrifuged at 4°C for 10 min at maximum speed in a Beckman microcentrifuge. The supernatant was transferred to a fresh tube and stored at −20°C until ready for Western blot analysis.
Western analyses.
Loading dye (3×; 187.5 mM Tris-HCl [pH 6.8], 6% sodium dodecyl sulfate, 30% glycerol, 0.3% bromophenol blue, 467 mM β-mercaptoethanol) was added to lysates, and the samples were boiled for 5 min. Proteins were separated by 8% or 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. The membranes were blocked for 1 h in 5% nonfat dry milk in Tris-buffered saline with 0.1% Tween 20 (TBST). Membranes were incubated in primary antibody overnight at 4°C with the appropriate antibody diluted in 2% bovine serum albumin in TBST. Membranes were then incubated for 1 h with horseradish peroxidase-conjugated secondary antibodies (Thermo) diluted in 5% milk and visualized with enhanced chemiluminescence (ECL) reagents (Roche).
Metabolic labeling.
Mock- or HCMV-infected HFs (MOI = 3) were treated with 250 nM Torin1 starting at 2 hpi, 24 hpi, or 48 hpi or similarly treated with dimethyl sulfoxide (DMSO), the solvent control. Cells were harvested at 72 hpi. Metabolic labeling was performed as previously described (15). Briefly, prior to harvest at 72 hpi, the cells were washed and incubated in prewarmed DMEM lacking methionine and cysteine (Gibco) for 15 min. Torin1 was included in the medium during the wash step, where applicable. Cells were then labeled for 30 min with prewarmed methionine/cysteine-free DMEM containing 125 μCi/ml [35S]methionine and [35S]cysteine. Torin1 was included in the medium during labeling, where applicable. Cells were subsequently washed three times in 1× PBS and lysed in RIPA buffer. 35S labeling of HCMV-encoded proteins was determined by immunoprecipitation of the major immediate-early proteins (MIEPs), pp28 and gB. Immune complexes were purified by binding to Dynabeads protein G (Invitrogen), followed by SDS-PAGE. The gel was fixed in 46% methanol, 7% acetic acid and dried for 2 h before being exposed to film.
Cap-binding protein affinity purification.
HFs were infected with lentiviral vectors encoding shRNAs to 4E-BP or luciferase. After 4 days, the cells were serum starved for 24 h, followed by mock or HCMV infection (MOI = 3). Torin1 (250 nM) was added from 2 to 72 hpi or from 48 to 72 hpi or the cells were treated with the solvent control, DMSO. At 72 hpi, the cells were extracted using affinity purification (AP) buffer (0.5% Triton X-100, 50 mM Tris-Cl, pH 7.5, 150 mM NaCl, 1.5 mM EDTA, 20 mM β-glycerophosphate, 10 mM sodium fluoride, 200 μM sodium orthovanadate, 2 mM phenylmethylsulfonyl fluoride, 1.5 μg/ml aprotinin, and 1.0 μg/ml leupeptin) as previously described (38). Two hundred micrograms of extracted protein was incubated with 10 μl of 7-methyl GTP Sepharose beads (GE Healthcare) for 2 h at 4°C. The beads were then washed twice in cold AP buffer and prepared for Western analysis as described above.
RESULTS
The phosphorylation of 4E-BP1 and S6K is retained in HCMV-infected cells depleted of either raptor or rictor.
Previous studies from our laboratory (18, 20) examined 4E-BP1 and S6K phosphorylation at 10 and 24 h postinfection (hpi); at these early time points following HCMV infection, hyperphosphorylation of 4E-BP1 and S6K was retained when either rictor or raptor was depleted using shRNAs. This suggested that the substrate specificity of the rictor-containing complex (mTORC2) was extended by HCMV infection to include 4E-BP1 and S6K. In Fig. 1, we expanded upon these results to examine the effects of rictor or raptor depletion in addition to the effects of Torin1 inhibition on the phosphorylation of mTOR kinase substrates and the expression of viral genes at various time points during HCMV infection.
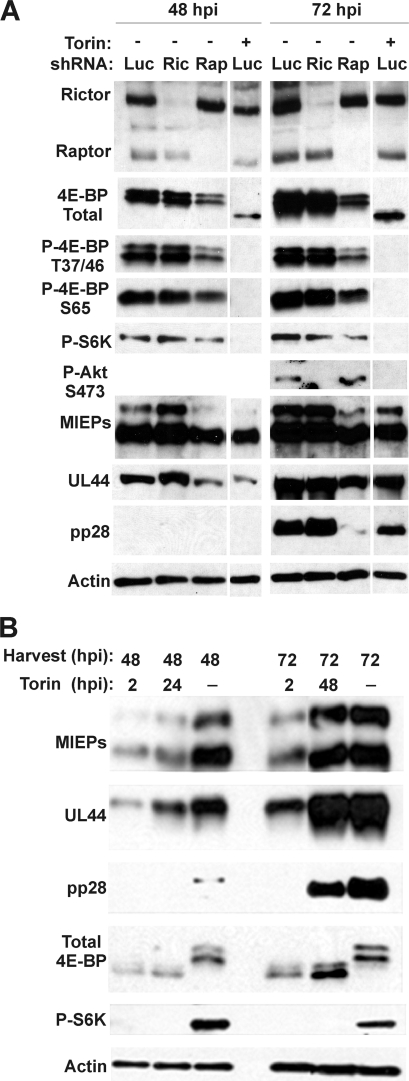
Fig. 1.
Effects of rictor or raptor depletion or Torin1 treatment on 4E-BP1, S6K, and Akt phosphorylation and expression of representative HCMV proteins. (A) Western analysis of HCMV-infected HFs following shRNA-mediated depletion using lentiviruses expressing control (Luc) or rictor (Ric)- or raptor (Rap)-specific shRNA or HFs treated with 250 nM Torin1 for 24 h prior to cell harvest. Cells were collected at 48 and 72 hpi and analyzed for expression of rictor, raptor, 4E-BP1, S6K, Akt, and various viral proteins representing the different temporal classes of viral genes. Actin expression was monitored as a loading control. All the lanes shown were from the same experiment and the same gel; some intervening lanes were removed for clarity of presentation. (B) Western analysis of HCMV-infected HFs following Torin1 treatment beginning at 2 hpi or duing the last 24 h before harvest.
HF cells were infected with lentivirus vectors that expressed either a control luciferase-specific shRNA (Luc) or shRNAs specific for either rictor or raptor. After 4 days, the cells were serum starved for 24 h, then infected with HCMV (MOI = 3), and harvested at 48 and 72 hpi. The Western blot in Fig. 1A shows that rictor and raptor depletions were effective at both 48 and 72 hpi. Consistent with previous reports, hyperphosphorylation of 4E-BP1 was retained under either rictor or raptor depletion conditions, as indicated by analysis using an antibody that detects total 4E-BP1 (similar results were seen for 4E-BP2; not shown). In addition, we show that phosphorylations of 4E-BP1 on amino acid residues T37, T46, and S65 are all maintained following either rictor or raptor depletion, as indicated by phosphospecific antibodies. However, it is clear that the total level of 4E-BP1 is decreased in raptor-depleted cells; this is also reflected by the decreased detection of the specifically phosphorylated forms of 4E-BP1. Phosphorylation of S6K is also not significantly altered after depletion of either rictor or raptor. The phosphorylation of Akt on amino acid residue S473 is rictor dependent, suggesting that Akt is solely a substrate of mTORC2 in infected cells, as it is in uninfected cells (18, 31). Thus, the substrate specificity for Akt by the mTOR complexes is not altered by HCMV infection, whereas the substrate specificity for 4E-BP1 and S6K appears to be altered such that either the rictor- or the raptor-containing complexes can phosphorylate them.
One possibility for this expanded substrate specificity is that mTOR kinase is not the kinase that phosphorylates 4E-BP1 and S6K in HCMV-infected cells. We show that this is not the case when the mTOR kinase-specific inhibitor, Torin1, is used (Fig. 1A). Phosphorylation of 4E-BP1, S6K, and Akt is completely lost by the addition of Torin1 (250 nM) for 24 h prior to harvest (i.e., between 24 and 48 hpi and between 48 and 72 hpi). Note the faster migration of the hypophosphorylated form of 4E-BP1 than of the hyperphosphorylated forms seen in the rictor- and raptor-depleted, HCMV-infected cells; significantly, there is no detectable hypophosphorylated 4E-BP1 under rictor or raptor depletion conditions. In these experiments, we chose to use 24 h of Torin1 treatment because adding Torin1 from the beginning of the infection (at 2 hpi) does not allow an infection to establish. For example, under our normal infection conditions, a titer of 5 ×104 to 1 × 105 infectious virions can be obtained by 72 hpi; however, when Torin1 is added from 2 to 72 hpi between 0 and 102 infectious virions are detected (not shown). This is also demonstrated in Fig. 1B; Torin1 treatment from 2 to 48 hpi or from 2 to 72 hpi inhibited the accumulation of the major immediate-early proteins (MIEPs; which include IEP86, also called IE2, and IEP72, also called IE1), the early protein UL44, and the late protein pp28.
Depletion of raptor and mTOR inhibition by Torin1 have similar effects on immediate-early, early, and late viral gene expression.
The data in Fig. 1A also show the effects of rictor or raptor depletion, and Torin1 treatment, on the expression of proteins representing the immediate-early, early, and late classes of viral genes. The depletion of rictor had no noticeable effect on the expression of any of these genes. However, the depletion of raptor resulted in several notable effects: (i) a specific loss of the expression of IEP86, the 86-kDa major immediate-early protein; (ii) a decrease in the early protein UL44; and (iii) a considerable loss of expression of the late protein pp28 seen in the 72-hpi sample. When compared with the effects of Torin1 treatment for 24 h prior to harvest (Fig. 1A), similar effects were observed: IEP86, UL44, and pp28 expression levels were all diminished. The diminution of pp28 after the 48- to 72-hpi Torin1 treatment was less than that seen under conditions of raptor depletion, and this can be explained by the accumulation of pp28 prior to the addition of Torin1. Overall, these data show that the depletion of raptor and the inhibition of mTOR kinase by Torin1 have similar effects on viral protein expression. This suggests that mTORC1, where mTOR kinase and raptor function together, plays a significant role in the production of all classes of HCMV proteins. Our findings differ from those of the recent HCMV study using Torin1 (24), which suggested that Torin1 caused only modest reductions in immediate-early- and early-viral-protein expression levels; in contrast, we see significant losses of IEP86 and UL44. However, the studies agree that there is a decrease in late protein accumulation.
Inhibition of mTOR kinase is most deleterious at early times in infection and does not affect virion assembly.
To more specifically examine the effects of Torin1 on HCMV infection, we performed an infection time course experiment examining both protein expression and infectious virus production (Fig. 2). As in the previous experiment, 250 nM Torin1 was added for 24 h prior to harvest; for example, Torin1 was present from 24 to 48 hpi for the 48-hpi time point and from 48 to 72 hpi for the 72-hpi time point. The only exception is the 24-hpi time point, where Torin1 was added 2 h after infection (2 to 24 hpi). The addition of Torin1 at various time points after infection allows us to determine when during infection the inhibition of mTOR kinase is most deleterious.
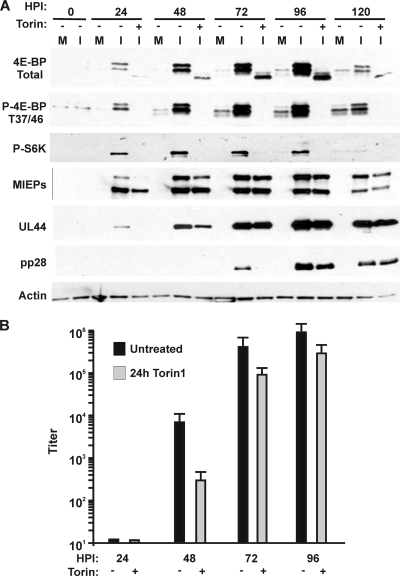
Fig. 2.
Effects of Torin1-mediated inhibition of mTOR kinase on 4E-BP1 and S6K phosphorylation, representative viral protein synthesis, and viral growth. An HCMV infection time course experiment (0 to 120 hpi) was performed with HFs where the cells either were left untreated or were treated with 250 nM Torin1 for 24 h prior to the harvest time point. (A) Western analysis of 4E-BP1 or S6K phosphorylation or of various viral proteins in the presence or absence of Torin1 treatment (M, mock-infected; I, HCMV-infected). (B) Viral titer was determined at 24, 48, 72, and 96 hpi from untreated cultures (black bars) or cultures treated with Torin1 for 24 h prior to harvest (gray bars).
Figure 2A shows that the level of total 4E-BP1 significantly increases during infection up to 96 hpi and that it is hyperphosphorylated; this can be seen in the analysis using the total 4E-BP1 antibody as well as the antibody specific to 4E-BP1 phosphorylated on threonine residues 37 and 46. At each time point, 24 h of Torin1 treatment eliminated 4E-BP1 hyperphosphorylation. Phosphorylation of S6K shows a similar increase during the course of infection and is also inhibited by Torin1 treatment. Furthermore, the data show that the effect of Torin1 on IEP86 accumulation is most significant when present from 2 to 24 hpi and from 24 to 48 hpi. Addition from 48 to 72 hpi had less effect, suggesting that the steady-state level of IEP86 accumulated at 48 hpi changed little between 48 and 72 hpi. Similarly, UL44 accumulation was most significantly affected by the 2- to 24-hpi and the 24- to 48-hpi Torin1 treatments, after which Torin1 treatment had little effect on UL44 levels. Despite the accumulation of immediate-early and early proteins during the 48- to 72-hpi Torin1 treatment, accumulation of pp28 was delayed.
Figure 2B shows the effect of 24 h of Torin1 treatment on infectious virion formation. The effect is greatest at earlier times of infection; the 24- to 48-hpi treatment lowered the titer by over 1.5 log, whereas the 48- to 72-hpi and 72- to 96-hpi treatments lowered the titer by only 0.5 to 0.6 log. At time points after 48 hpi, sufficient levels of viral proteins have accumulated, which may maintain the formation of virions despite Torin1 inhibition of mTOR kinase. However, it is also possible that between 48 and 72 hpi, the loss of phosphorylated 4E-BP1 due to Torin1 addition is slow, and the consequences of 4E-BP1 dephosphorylation take effect only toward the end of the 48- to 72-hpi treatment period. To show that this is not the case, cells were treated at 48 hpi with 250 nM Torin for 30, 60, and 120 min to determine how quickly Torin1 affected 4E-BP1 phosphorylation. Control mock-infected cells were serum stimulated to activate mTOR kinase and then subjected to the same Torin1 treatment. Figure 3 shows that after just 30 min of Torin1 treatment, 4E-BP1 hyperphosphorylation in infected and serum-stimulated uninfected cells is being lost, and by 120 min, only the hypophosphorylated form remains. Thus, inhibition of mTOR kinase, as measured by reduced levels of 4E-BP1 phosphorylation, occurs rapidly after Torin1 addition in both serum-stimulated and infected cells.
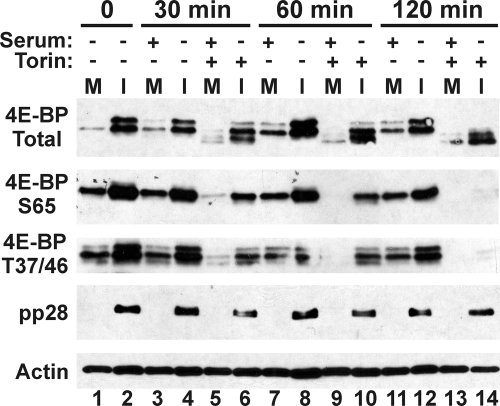
Fig. 3.
Inhibition of mTOR kinase by Torin1 is rapid. At 48 hpi, HFs were treated with 250 nM Torin1 and harvested at 30, 60, and 120 min posttreatment. Control mock-infected cells were serum stimulated to activate mTOR kinase and then subjected to the same Torin1 treatment. Western analysis was conducted to examine levels of total and phospho-4E-BP1, pp28, and actin (M, mock-infected; I, HCMV-infected).
Pulse-labeling studies show that Torin1 treatment has less effect on protein synthesis late in infection.
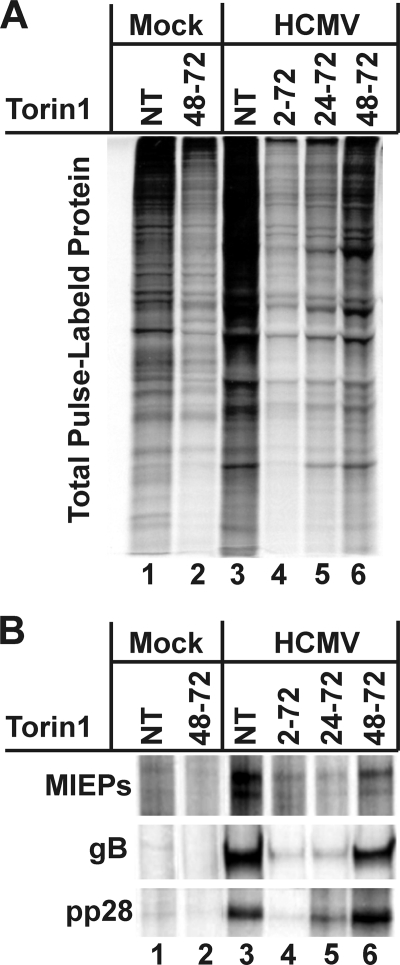
Another possible cause of the less significant effect of Torin1 treatment late in infection is that the virus may mediate translational modifications that minimize the effects of hypophosphorylated 4E-BP1 or other effects mediated by mTOR kinase inhibition. Thus, cells were pulse-labeled with [35S]methionine and [35S]cysteine to examine protein synthesis under normal and Torin1-treated conditions. As described above, mock- and HCMV-infected cells were Torin1 treated at 2 hpi, 24 hpi, or 48 hpi or similarly treated with DMSO, the solvent control. All of the samples were metabolically labeled and then harvested at 72 hpi. Specifically, at 72 hpi, the cells were washed and incubated in prewarmed DMEM lacking methionine and cysteine for 15 min. Torin1 was included in the medium, where applicable. Cells were then labeled for 30 min with prewarmed methionine/cysteine-free DMEM containing 125 μCi/ml [35S]methionine and [35S]cysteine. Torin1 was included in the medium during labeling, where applicable. Cells were then extracted, and total protein or immunoprecipitated proteins were analyzed by SDS-PAGE and autoradiography (Fig. 4A and B). The data show that the overall level of protein synthesis, as indicated by 35S labeling, decreased following Torin1 treatment in both the mock- and the HCMV-infected cells (Fig. 4A). In infected cells, this decrease is more apparent when Torin1 is added for longer periods of time; for example, there is a more significant decrease in total protein pulse-labeling when Torin1 is present from 2 to 72 hpi than when it is added from 48 to 72 hpi, and the 24- to 72-hpi treatment is intermediate. A similar trend is observed following immunoprecipitation of the viral major immediate-early proteins (MIEPs), gB and pp28 (Fig. 4B); the expression of each protein, as measured by pulse-labeling, increases inversely with the length of Torin1 treatment. Indeed, the labeling of the immunoprecipitated viral proteins occurring in the samples treated with Torin1 from 48 to 72 hpi is very comparable to the labeling in the untreated sample (compare lanes 3 and 6 in Fig. 4B). This suggests that by 48 hpi, translation of the viral proteins is essentially unaffected by the inhibition of mTOR kinase but that the background labeling of cellular proteins has not recovered as significantly (Fig. 4A). Again, an argument can be made that Torin1 may not be as effective when added later in infection; however, the data in Fig. 1, 2, and 3 show very convincingly that a 24-h Torin1 treatment between 48 and 72 hpi is very effective in inhibiting 4E-BP and S6K phosphorylation. Thus, the data suggest that, in infected cells, inhibition of mTOR kinase is deleterious to viral replication when it occurs early, before an infection can be established. However, as the infection proceeds and is allowed to establish, mTOR kinase inhibition has progressively less effect on the synthesis of viral proteins. Hence, it appears that during the course of infection, translational mechanisms that are resistant to inhibition by hypophosphorylated 4E-BP1 and resistant to the loss of S6K activity are activated.
Fig. 4.
Protein synthesis is less affected by Torin1 at late times in infection than at early times in infection. HFs were mock or HCMV infected in the presence or absence of 250 nM Torin1. Where applicable, Torin1 or DMSO (solvent control) was added at 2 hpi, 24 hpi, or 48 hpi and cells were pulse-labeled with [35S]methionine/[35S]cysteine for 30 min just prior to harvest at 72 hpi. Total protein (A) or immunoprecipitated proteins (B) were analyzed by SDS-PAGE and autoradiography. NT, not treated.
The depletion of 4E-BP1 does not rescue infectious virion formation in HCMV-infected human fibroblasts.
As discussed in the introduction, recent evidence obtained using the MCMV system in MEFs showed that the inhibition of MCMV growth caused by Torin1 could be rescued in MEFs lacking 4E-BP1 (24). In these studies, there was a significant decrease in viral growth following Torin1 treatment in wild-type MEFs; however, MCMV replication was not inhibited when Torin1 was added to 4E-BP1−/− MEFs. These studies concluded that Torin1 is solely inhibiting MCMV growth through a 4E-BP1-dependent mechanism in MEFs. In other words, the primary function of mTOR kinase in MCMV-infected MEFs is to maintain the ability to phosphorylate and inactive 4E-BP1.
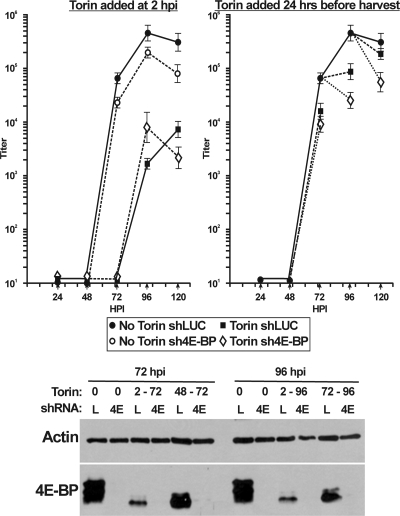
To determine whether this is also true in HCMV-infected HFs, we infected HFs with lentiviral vectors encoding shRNAs specific for 4E-BP1 (sh4E-BP) or a luciferase control (shLuc). Four days later, cells were serum starved for 24 h, followed by mock or HCMV infection. Infected cells were harvested at 24, 48, 72, 96, and 120 hpi for Western analysis or for determination of viral titer by use of the TCID50 method. Either Torin1 treatment was begun at 2 hpi and maintained throughout the time course or Torin1 was added for 24 h before harvest (i.e., 48 to 72 hpi, 72 to 96 hpi, and 96 to 120 hpi). The Western analysis in Fig. 5 shows the status of 4E-BP1 phosphorylation and depletion at the 72- and 96-h time points. In the shLuc (Fig. 5, lanes L) control samples, the addition of Torin1 from 2 hpi (2 to 72 and 2 to 96 hpi) or for 24 h before harvest (48 to 72 or 72 to 96 hpi) resulted in the hypophosphorylation of 4E-BP1. 4E-BP1 depletion was very effective in the sh4E-BP1 samples (Fig. 5, lanes 4E).
Fig. 5.
4E-BP1 depletion does not rescue infectious virion formation in Torin1-treated, HCMV-infected HFs. HFs were infected with lentiviral vectors encoding shRNAs specific for 4E-BP1 (sh4E-BP; 4E) or a luciferase control (shLuc; L). Four days after lentivirus infection, the cells were serum starved for 24 h and then infected with HCMV (MOI = 3). In samples treated with Torin1, the drug was either added at 2 hpi and maintained throughout or added for 24 h before harvest (48 to 72, 72 to 96, and 96 to 120 hpi). Cells were collected at 24, 48, 72, 96, and 120 hpi for determination of viral titer by use of the TCID50 method or for Western analysis of 4E-BP1 and actin. Bars represent the standard deviation.
In the growth analysis, the shLuc control with no Torin1 shows a normal growth curve. Depletion of 4E-BP1 by use of sh4E-BP1 (Fig. 5, No Torin) caused a 3- to 5-fold decrease in titer at every time point. As previously discussed, Torin1 treatment from 2 hpi was very deleterious to viral growth in the control shLuc-treated cells, and depletion of 4E-BP1 did not rescue HCMV growth in the presence of Torin1. In the samples where Torin1 was added 24 h before harvest, the HCMV titer was decreased at every time point in the shLuc control cells and the Torin1-induced decrease was greater in the 4E-BP1-depleted cells. Thus, in contrast to what was found for the MCMV system, the depletion of 4E-BP1 did not rescue the inhibition of HCMV growth in the presence of Torin1; in fact, viral growth in the 4E-BP1-depleted, Torin1-treated cells was consistently less than in the control, Torin1-treated cells. The moderate loss of titer upon 4E-BP1 depletion, as well as the failure of 4E-BP1 depletion to rescue Torin1 inhibition of viral growth, suggests that 4E-BP1 and mTOR kinase have additional functions during HCMV infection. Overall, the results of the above-mentioned studies demonstrate that, unlike the MCMV-MEF system, mTOR kinase has more functions in HCMV-infected HFs than just to phosphorylate and inactivate 4E-BP1.
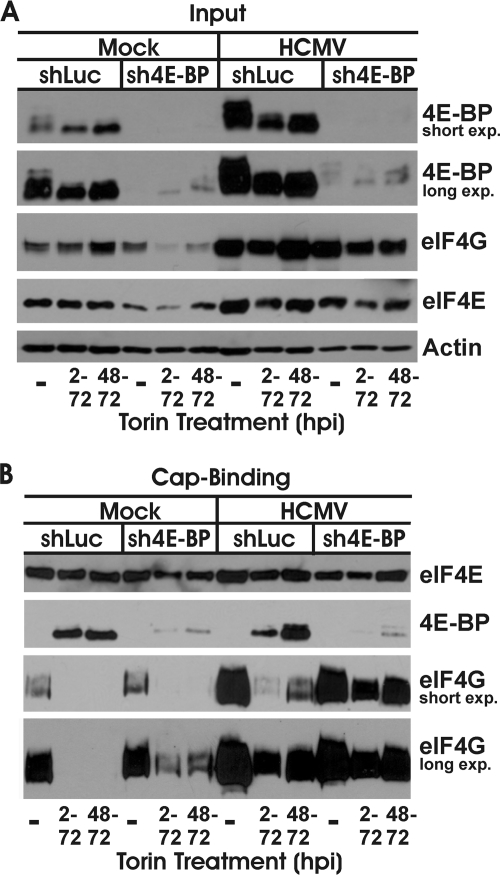
An important difference to note in our studies versus the MCMV studies is that the studies using MEFs with MCMV utilized 4E-BP1−/− cells (24), so there was no 4E-BP1 expressed. In contrast, in our shRNA depletion studies, there could be a small amount of residual 4E-BP1. In order to show that the residual 4E-BP1 was not sufficient to inhibit cap-dependent translation in Torin1-treated, HCMV-infected cells, we performed cap-binding studies. In these experiments, the cap-binding protein, eIF4E, is bound to a cap analog, 7-methyl GTP, bound to Sepharose beads. Under normal conditions, when cap-dependent translation is active, the bound eIF4E will have associated eIF4G, the scaffolding protein of the eIF4F cap-binding complex. Under these conditions, 4E-BP will be hyperphosphorylated by mTOR kinase and unable to bind eIF4E. However, under conditions where mTOR kinase is inhibited, for example, upon Torin1 treatment, 4E-BP will be hypophosphorylated and will readily bind eIF4E and preclude eIF4G binding, thus inhibiting the formation of the eIF4F complex and inhibiting cap-dependent translation. Figure 6A shows the input controls under each experimental condition; all samples were harvested at 72 hpi. Short and long exposures of Western analyses of 4E-BP1 are provided for clarity. In serum-stimulated, mock-infected cells expressing the control shLuc lentivirus, and in the absence of Torin1 treatment, 4E-BP1 is hyperphosphorylated. When Torin1 was added from 2 to 72 hpi or from 48 to 72 hpi, 4E-BP1 became hypophosphorylated. Under these same conditions, with the use of the sh4E-BP1-expressing lentivirus, significant depletion of 4E-BP1 was observed. In HCMV-infected cells, we see a significant increase in total 4E-BP1 levels in the shLuc-expressing cells (shLuc), and this 4E-BP1 is present in its hyperphosphorylated state. 4E-BP1 becomes hypophosphorylated upon Torin1 treatment from 2 to 72 or 48 to 72 hpi. In HCMV-infected cells expressing sh4E-BP1, we again observed significant depletion of 4E-BP1. Thus, the controls establish that 4E-BP1 depletion and Torin1 treatment were effective. We also examined the expression of eIF4E and eIF4G in the input samples. Here, we reiterate previous findings that both of these proteins are increased in HCMV-infected cells under normal conditions (27). In addition, the data suggest that the levels of both eIF4E and eIF4G are diminished in mock-infected cells as a result of 4E-BP1 depletion and Torin1 treatment. This can also be noted for infected cells but is substantially compensated for by the increased production of both proteins.
Fig. 6.
Residual 4E-BP in 4E-BP-depleted cells is not sufficient to block binding of eIF4G to eIF4E. HFs were infected with lentiviral vectors encoding shRNAs specific for 4E-BP1 (sh4E-BP) or a control luciferase (shLuc). Four days after lentivirus infection, the cells were serum starved for 24 h and infected with HCMV (MOI = 3). In cells treated with Torin1, the drug was added at 2 hpi or 48 hpi; all samples were collected at 72 hpi. Whole-cell lysate was separated by SDS-PAGE and subjected to Western blot analysis (A), or a cap-binding assay was performed, followed by Western blot analysis (B). Levels of eIF4E, 4E-BP1, and eIF4G were examined.
Figure 6B shows the results of the cap-binding assay. Analysis of eIF4E shows that relatively equivalent amounts of the cap-binding protein were bound by the 7-methyl GTP beads in each sample. As expected, eIF4E was bound to significant levels of eIF4G and little to no 4E-BP1 in samples not treated with Torin1. In shLuc-expressing, mock-infected cells treated with Torin1, 4E-BP1 is the predominant eIF4E-binding partner, displacing eIF4G. In mock-infected cells expressing sh4E-BP1 and treated with Torin1, there is enough residual 4E-BP1 to displace much, but not all, of the eIF4G from eIF4E (compare the short and long exposures of the eIF4G Western blot in Fig. 6B). In HCMV-infected cells which were not treated with Torin1, there is a significant increase in binding of eIF4G to eIF4E compared to the level for the equivalent mock-infected samples. This may result, in part, from the increased levels of eIF4G in infected cells (Fig. 6A); however, it may also indicate a virally induced modification that increases the avidity or stability of the eIF4G-eIF4E interaction. Upon Torin1 treatment of the shLuc-expressing, HCMV-infected cells, there is an increase in binding of 4E-BP1 to eIF4E, which displaces much, but not all, of the eIF4G. Thus, in infected cells treated with Torin1, where there is a full complement of hypophosphorylated 4E-BP1, it is not enough to completely displace all of the eIF4G. In the HCMV-infected cells expressing sh4E-BP1, the decreased 4E-BP1 levels result in significantly increased binding of eIF4G to eIF4E, even in the presence of Torin1. This is particularly apparent in the sample treated with Torin1 from 48 to 72 hpi where the infection was allowed to establish before mTOR kinase inhibition by Torin1 and where the pulse-labeling study shows that Torin1 had less effect on viral protein synthesis (Fig. 4A and B). These results show that residual 4E-BP1 in the sh4E-BP1-expressing, Torin1-treated, HCMV-infected cells was not sufficient to inhibit the formation of the eIF4F cap-binding complex needed for cap-dependent translation.
DISCUSSION
The data presented examine the roles of mTOR kinase in mTORC1 and mTORC2 during HCMV infection. Our previous data suggested that infection may change the functionality of the two complexes. In particular, these data suggested that 4E-BP1 and S6K became substrates of either mTORC1 or mTORC2 during an HCMV infection (20) but that they are substrates of only mTORC1 in uninfected cells. This was shown by the maintenance of phosphorylation of 4E-BP1 and S6K throughout infection when either raptor or rictor was depleted using shRNAs, thus inactivating mTORC1 or mTORC2, respectively. In the present studies, we reiterate these conclusions and extend the observations. A potential explanation for the maintenance of 4E-BP1 and S6K phosphorylation during infection was that a kinase other than mTOR may be involved during infection. However, using the mTOR kinase-specific inhibitor Torin1, we confirmed that mTOR kinase is responsible for 4E-BP1, S6K, and Akt phosphorylation in infected cells (Fig. 1A).
Although both rictor-depleted and raptor-depleted conditions could maintain 4E-BP1 and S6K phosphorylation, there were significant differences noted between the two depletion conditions. For example, depletion of raptor resulted in a significant lowering of total 4E-BP1 levels. The results of studies conducted with uninfected and herpes simplex 1-infected cells have demonstrated that 4E-BP1 hyperphosphorylation is followed by proteasome-mediated degradation (4, 10, 34). As shown in Fig. 2A, in HCMV-infected cells, the level of hyperphosphorylated 4E-BP1 is significantly increased during infection, suggesting that proteasome-mediated degradation of hyperphosphorylated 4E-BP1 does not occur in HCMV-infected cells. The finding that the depletion of raptor, and subsequent loss of mTORC1 activity, results in a lowering of total 4E-BP1 levels suggests that 4E-BP1 phosphorylated by mTORC1 may be more capable of avoiding proteasomal degradation than 4E-BP1 phosphorylated by mTORC2.
Differential effects of raptor depletion, compared to those of rictor depletion, are also noted in the expression of viral proteins (Fig. 1A). Raptor depletion resulted in lower expression of viral proteins representative of the immediate-early, early, and late temporal classes of HCMV genes. Similar lowering in the levels of these proteins was seen upon Torin1 inhibition of mTOR kinase. Thus, depletion of raptor and inhibition of mTOR kinase present similar phenotypes of viral protein expression. This suggests that mTORC1, where raptor and mTOR kinase function together, is involved in the maintenance of the levels of all temporal classes of HCMV proteins. These effects on immediate-early and early gene expression are more pronounced than those in a previous study which reported only modest effects on the expression of representative immediate-early and early genes (24).
Studies using MCMV showed that Torin1 inhibition of MCMV growth could be rescued in 4E-BP1−/− MEFs (24). This suggests that the primary reason for maintaining mTOR kinase activity in MCMV-infected MEFs is to hyperphosphorylate 4E-BP1 and maintain it in an inactive state. Our data show that depletion of 4E-BP1 in HCMV-infected HFs does not rescue Torin1 inhibition of viral growth, suggesting that mTOR kinase performs additional functions in HCMV-infected human cells (Fig. 5). A significant difference between these experiments is that the MCMV study used 4E-BP1−/− MEFs and our studies depleted 4E-BP1 from HFs by use of shRNAs. As shown in Fig. 5, shRNA knockdown of 4E-BP1 was very effective; however, it can be argued that a very small amount of residual 4E-BP1 may be enough to inhibit HCMV growth. Stoichiometrically, this would require eIF4E to be present in small amounts in order for the residual 4E-BP1 to significantly displace eIF4G from eIF4E. Previous data (35) show that during HCMV infection the levels of eIF4E and eIF4G both increase significantly during infection, and our data agree with this (Fig. 6). Thus, the argument that a very small amount of residual hypophosphorylated 4E-BP1 can inhibit cap-dependent translation and HCMV growth seems unlikely. Indeed, recent evidence (3) argues against the idea that small amounts of residual 4E-BP1 are able to bind all of the eIF4E. These data suggested that HCMV protein pUL69 interacts directly with eIF4A1 in the eIF4F cap-binding complex, thereby excluding 4E-BP1 from the cap-binding complex. These data suggest that the UL69 mechanism would be able to readily maintain cap-dependent translation under conditions of significant 4E-BP1 depletion. Nevertheless, we performed an in vitro cap-binding experiment to confirm that the residual 4E-BP1 in our experiments was not in sufficient quantity to displace all the eIF4G from eIF4E when Torin1 is added. For all of these reasons, we conclude that mTOR kinase activity is needed in HCMV-infected human cells for purposes other than to hyperphosphorylate 4E-BP1 and maintain it in an inactive state. We are not disputing that the function of mTOR kinase in MCMV-infected MEFs may be limited to 4E-BP phosphorylation; however, we suggest that HCMV and MCMV infections in their natural hosts are significantly different with respect to mTOR kinase utilization.
Our data show that depletion of 4E-BP1 with shRNAs moderately inhibited HCMV growth (Fig. 5). This seems counterintuitive; one might expect that the depletion of a protein that the virus strives to maintain in an inactive state would either have no effect or be beneficial. That the depletion was moderately deleterious suggests that 4E-BP1 has a unique function in HCMV-infected cells. In considering this possibility, we observed that the level of total 4E-BP1 is dramatically increased in infected cells. This puts increased burden on the infected cells, and mTOR kinase, to maintain the additional 4E-BP1 in the hyperphosphorylated, inactive state. It is not clear why the virus would do this unless hyperphosphorylated 4E-BP1 performs an alternative function during infection.
The most intriguing observation in our data is that the inhibitory effect of Torin1 on viral protein synthesis decreases with time of infection such that translation of viral protein was hardly affected by mTOR kinase inhibition after 48 hpi (Fig. 4). This insensitivity was not due to a loss of the effectiveness of Torin1 later in infection. Thus, the data suggest that, in infected cells, inhibition of mTOR kinase is deleterious to viral replication when it occurs early, before an infection can be established. However, as the infection proceeds and is allowed to establish, mTOR kinase inhibition has progressively less effect on the synthesis of viral proteins. Hence, it appears that during the course of infection, translational mechanisms that are resistant to inhibition by hypophosphorylated 4E-BP1 and resistant to the loss of S6K activity are activated.
A significant question is whether viral mRNAs are translated preferentially under these conditions. The data in Fig. 4A may suggest that there is a preference for translation of viral mRNAs when Torin1 is added after 48 hpi (compare lanes 3 and 6 in Fig. 4A); however, a definitive answer to this question requires a very careful study of the synthesis of a large number of cellular proteins, in comparison to viral protein, at different time points in infection. One possibility for a virus-specific mechanism is the effect of pUL69 described above; however, one would expect this mechanism to recover all protein synthesis. Alternatively, the viral RNAs may be able to utilize other translational initiation mechanisms when needed, for example, internal ribosome entry sites (IRESs). Recent evidence has suggested that IRES utilization is activated during HCMV infection (7). Overall, these observations suggest that HCMV may have a means to provide preferential translation of viral mRNAs. In this regard, it is intriguing to compare viral protein synthesis between HCMV and herpes simplex virus (HSV). HSV encodes a well-characterized virion host shutoff protein (vhs) which mediates shutoff of host protein synthesis, disruption of preexisting polysomes, and degradation of host mRNAs, thus giving translational advantage to viral mRNA (reviewed in reference 32). Such a mechanism does not exist in HCMV, presumably because the very long HCMV life cycle requires the host cell to remain relatively healthy and operational for a much longer time than does HSV. However, our data suggest that HCMV can mediate preferential translation of its mRNAs under specific conditions. In this regard, recent studies have suggested that an alternative and stress-inducible strategy of translation initiation ensures expression of HCMV pUL138 under a variety of cellular contexts (11).
Our data suggest a relationship between HCMV and mTOR kinase which changes during the course of the infection. Clearly, the inhibition of mTOR kinase by Torin1 decreases HCMV yield when it is added at any time in infection. Additionally, the loss of mTORC1 activity due to raptor depletion is deleterious to the infection. The present data suggest that mTOR kinase activity is most important for the establishment and early stages of infection; however, as the infection proceeds, these functions become less significant. This is suggested not only by the increased resistance of translation to Torin1 inhibition but also by an increased resistance to rapamycin as the infection proceeds (18). The function of mTORC2 in phosphorylating Akt also appears to be transient, being seen early in infection but dwindling as a normal infection proceeds (18). Thus, HCMV and mTOR kinase have a changing relationship over the course of the lytic infection.
ACKNOWLEDGMENTS
We thank all the members of the Alwine laboratory for support and critical evaluation of the experiments and data and David Sabatini (Whitehead Institute) for Torin1.
A.J.C. was supported by Training in Tumor Virology grant T32 CA115299-04, awarded to Erle Robertson. This work was supported by NIH grant R01 CA028379-29, awarded to J.C.A.
Footnotes
Published ahead of print on 9 February 2011.
REFERENCES
- 1. Alessi D. R., Pearce L. R., García-Martínez J. M. 2009. New insights into mTOR signaling: mTORC2 and beyond. Sci. Signal. 2:pe27. [DOI] [PubMed] [Google Scholar]
- 2. Alwine J. C. 2008. Modulation of host cell stress responses by human cytomegalovirus, p. 263–279 In Shenk T. E., Stinski M. F. (ed.), Current topics in microbiology and immunology, human cytomegalovirus, vol. 325 Springer, New York, NY: [DOI] [PubMed] [Google Scholar]
- 3. Aoyagi M., Gaspar M., Shenk T. E. 2010. Human cytomegalovirus UL69 protein facilitates translation by associating with the mRNA cap-binding complex and excluding 4EBP1. Proc. Natl. Acad. Sci. U. S. A. 107:2640–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Braunstein S., Badura M. L., Xi Q., Formenti S. C., Schneider R. J. 2009. Regulation of protein synthesis by ionizing radiation. Mol. Cell. Biol. 29:5645–5656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bresnahan W. A., Hultman G. E., Shenk T. 2000. Replication of wild type and mutant human cytomegalovirus in life-extended human diploid fibroblasts. J. Virol. 74:10816–10818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buchkovich N. J., Maguire T. G., Paton A. W., Paton J. C., Alwine J. C. 2008. Human cytomegalovirus specifically controls the levels of the endoplasmic reticulum chaperone BiP/GRP78 which is required for virion assembly. J. Virol. 82:31–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchkovich N. J., Yu Y., Pierciey F. J., Alwine J. C. 2010. Human cytomegalovirus induces the endoplasmic reticulum chaperone BiP through increased transcription and activation of translation by using the BiP internal ribosome entry site. J. Virol. 84:11479–11486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buchkovich N. J., Yu Y., Zampieri C. A., Alwine J. C. 2008. The TORrid affairs of viruses: effects of mammalian DNA viruses on the PI3K-Akt-mTOR signaling pathway. Nat. Rev. Microbiol. 6:266–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Child S. J., Hakki M., De Niro K. L., Geballe A. P. 2004. Evasion of cellular antiviral responses by human cytomegalovirus TRS1 and IRS1. J. Virol. 78:197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elia A., Constantinou C., Clemens M. J. 2008. Effects of protein phosphorylation on ubiquitination and stability of the translational inhibitor protein 4E-BP1. Oncogene 27:811–822 [DOI] [PubMed] [Google Scholar]
- 11. Grainger L., et al. 2010. Stress-inducible alternative translation initiation of human cytomegalovirus latency protein pUL138. J. Virol. 84:9472–9486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hakki M., Marshall E. E., De Niro K. L., Geballe A. P. 2006. Binding and nuclear relocalization of protein kinase R by human cytomegalovirus TRS1. J. Virol. 80:11817–11826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harel N. Y., Alwine J. C. 1998. Phosphorylation of the human cytomegalovirus 86-kilodalton immedicate early protein IE2. J. Virol. 72:5481–5492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Isler J. A., Maguire T. G., Alwine J. C. 2005. Production of infectious HCMV virions is inhibited by drugs that disrupt calcium homeostasis in the endoplasmic reticulum. J. Virol. 79:15338–15397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Isler J. A., Skalet A. H., Alwine J. C. 2005. Human cytomegalovirus infection activates and regulates the unfolded protein response. J. Virol. 79:6890–6899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jacinto E., et al. 2004. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 6:1122–1128 [DOI] [PubMed] [Google Scholar]
- 17. Kim D. H., et al. 2002. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110:163–175 [DOI] [PubMed] [Google Scholar]
- 18. Kudchodkar S., Yu Y., Maguire T., Alwine J. C. 2004. Human cytomegalovirus infection induces rapamycin insensitive phosphorylation of downstream effectors of mTOR kinase. J. Virol. 78:11030–11039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kudchodkar S. B., Del Prete G. Q., Maguire T. G., Alwine J. C. 2007. AMPK-mediated inhibition of mTOR kinase is circumvented during immediate-early times of human cytomegalovirus infection. J. Virol. 81:3649–3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kudchodkar S. B., Yu Y., Maguire T. G., Alwine J. C. 2006. Human cytomegalovirus infection alters the substrate specificities and rapamycin sensitivities of raptor- and rictor-containing complexes. Proc. Natl. Acad. Sci. U. S. A. 103:14182–14187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mamane Y., Petroulakis E., LeBacquer O., Sonenberg N. 2006. mTOR, translation initiation and cancer. Oncogene 25:6416–6422 [DOI] [PubMed] [Google Scholar]
- 22. Mohr I. 2006. Phosphorylation and dephosphorylation events that regulate viral mRNA translation. Virus Res. 119:89–99 [DOI] [PubMed] [Google Scholar]
- 23. Moorman N. J., et al. 2008. Human cytomegalovirus protein UL38 inhibits host cell stress responses by antagonizing the tuberous sclerosis protein complex. Cell Host Microbe 3:253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moorman N. J., Shenk T. 2010. Rapamycin-resistant mTORC1 kinase activity is required for herpesvirus replication. J. Virol. 84:5260–5269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murphy E., Rigoutsos I., Shibuya T., Shenk T. E. 2003. Reevaluation of human cytomegalovirus coding potential. Proc. Natl. Acad. Sci. U. S. A. 100:13585–13590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murphy E., et al. 2003. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc. Natl. Acad. Sci. U. S. A. 25:14976–14981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perez C., McKinney C., Chulunbaatar U., Mohr I. 2011. Translational control of the abundance of cytoplasmic poly(A) binding protein in human cytomegalovirus-infected cells. J. Virol. 85:156–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reiling J. H., Sabatini D. M. 2006. Stress and mTORture signaling. Oncogene 25:6373–6383 [DOI] [PubMed] [Google Scholar]
- 29. Sarbassov D. D., et al. 2004. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14:1296–1302 [DOI] [PubMed] [Google Scholar]
- 30. Sarbassov D. D., Ali S. M., Sabatini D. M. 2005. Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 17:596–603 [DOI] [PubMed] [Google Scholar]
- 31. Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307:1098–1101 [DOI] [PubMed] [Google Scholar]
- 32. Smiley J. R. 2004. Herpes simplex virus virion host shutoff protein: immune evasion mediated by a viral RNase? J. Virol. 78:1063–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thoreen C. C., Sabatini D. M. 2009. Rapamycin inhibits mTORC1, but not completely. Autophagy 5:725–726 [DOI] [PubMed] [Google Scholar]
- 34. Walsh D., Mohr I. 2004. Phosphorylation of eIF4E by Mnk-1 enhances HSV-1 translation and replication in quiescent cells. Genes Dev. 18:660–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walsh D., Perez C., Notary J., Mohr I. 2005. Regulation of the translation initiation factor eIF4F by multiple mechanisms in human cytomegalovirus-infected cells. J. Virol. 79:8057–8064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xuan B., Qian Z., Torigoi E., Yu D. 2009. Human cytomegalovirus protein pUL38 induces ATF4 expression, inhibits persistent JNK phosphorylation, and suppresses endoplasmic reticulum stress-induced cell death. J. Virol. 83:3463–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu Y., Alwine J. C. 2008. Interaction between simian virus 40 large T antigen and insulin receptor substrate 1 is disrupted by the K1 mutation, resulting in the loss of large T antigen-mediated phosphorylation of Akt. J. Virol. 82:4521–4526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu Y., Kudchodkar S. B., Alwine J. C. 2005. Effects of simian virus 40 large and small tumor antigens on mammalian target of rapamycin (mTOR) signaling: small tumor antigen mediates hypophosphorylation of eIF4E-binding protein 1 late in infection. J. Virol. 79:6882–6889 [DOI] [PMC free article] [PubMed] [Google Scholar]