Abstract
The genus Phlebovirus of the family Bunyaviridae consists of approximately 70 named viruses, currently assigned to nine serocomplexes (species) based on antigenic similarities. Sixteen other named viruses that show little serologic relationship to the nine recognized groups are also classified as tentative species in the genus. In an effort to develop a more precise classification system for phleboviruses, we are attempting to sequence most of the named viruses in the genus with the goal of clarifying their phylogenetic relationships. In this report, we describe the serologic and phylogenetic relationships of 13 viruses that were found to be members of the Candiru serocomplex; 6 of them cause disease in humans. Analysis of full genome sequences revealed branching inconsistencies that suggest five reassortment events, all involving the M segment, and thus appear to be natural reassortants. This high rate of reassortment illustrates the inaccuracy of a classification system based solely on antigenic relationships.
INTRODUCTION
The family Bunyaviridae currently includes more than 350 RNA viruses that are distributed among five genera: Orthobunyavirus, Nairovirus, Hantavirus, Phlebovirus, and Tospovirus (41). Bunyavirus genomes are comprised of three unique molecules of negative or ambisense single-stranded RNA, designated L (large), M (medium), and S (small), that total 11 to 19 kb. Within each genus, viruses share similar segment and structural protein sizes, as well as characteristic terminal sequences at the 3′ and 5′ ends of each of the three segments. Genetic reassortment among bunyaviruses has been demonstrated both in vitro and in vivo.
Most of the bunyaviruses are transmitted by arthropod vectors (mosquitoes, culicoid midges, phlebotomine sandflies, or ticks to vertebrates and thrips to plants). Hantaviruses are the exception, where transmission occurs directly from their rodent or insectivore hosts by inhalation of aerosols from infected excreta or bite. Both transovarial and venereal transmission has been demonstrated with some of the bunyaviruses in their arthropod hosts.
At present, the genus Phlebovirus is comprised of approximately 70 named viruses that are classified, based on their serological relationships, into two antigenic groups: the phlebotomus or sandfly fever group and the Uukuniemi group (41). Phleboviruses are antigenically unrelated to members of the other genera but show various degrees of antigenic cross-reaction among themselves. The lack of biochemical and genetic data for most of the phleboviruses has dictated that antigenic relationships define their taxonomy. Accordingly, the phleboviruses are currently assigned to nine complexes or species, based on their antigenic similarity in complement fixation (CF) tests (41, 59). Each species or complex currently consists of two or more named viruses that are most closely related to each other by CF and distinct by plaque reduction neutralization (PRN) tests, but their exact taxonomic status is undefined. The current recommendation of the International Committee on Taxonomy of Viruses (ICTV) is that species in the genus Phlebovirus are defined by serological relationships and are distinguishable by 4-fold differences in two-way neutralization tests (41). These criteria are no longer feasible for several reasons: (i) the growing number of new phleboviruses being discovered by molecular detection methods (12, 15, 52); (ii) some phleboviruses do not produce readable plaques under agar, making PRN tests challenging (57); (iii) while most phleboviruses grow in mammalian cells, some do not cause disease in laboratory animals or require multiple serial blind passages before they do, thus making the preparation of “clean” antisera difficult (2, 49, 57, 58); and (iv) because of the stringent biosafety and biosecurity regulations now governing work with live viruses, few laboratories have access to the more than 70 known phleboviruses for comparative studies with new isolates. A more precise and more widely available classification system for the phleboviruses is needed.
In an effort to develop a more precise taxonomic system for phleboviruses, we have attempted to sequence all of the named viruses in the genus to clarify their phylogenetic relationships. This is the first planned publication describing this work. The present communication describes the antigenic and phylogenetic relationships of 13 viruses found to be members of the Candiru complex.
MATERIALS AND METHODS
Viruses.
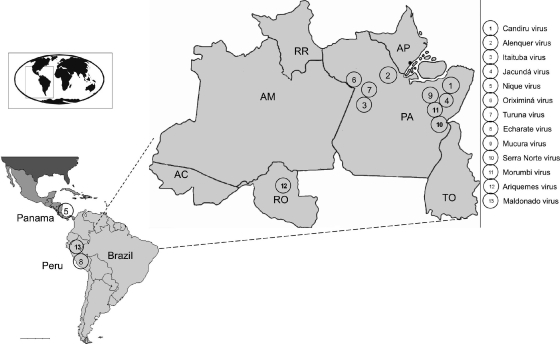
The viruses used in the present study were obtained from the World Reference Center for Emerging Viruses and Arboviruses at the University of Texas Medical Branch. All of these viruses have been described before, except for Mucura and Maldonado. Table 1 gives the names, strain numbers, sources, and dates and locality of isolation, as well as the GenBank accession numbers for the 13 phleboviruses described here (see also Fig. 1).
Table 1.
Names, abbreviations, strain numbers, sources, dates and localities of isolation, and accession numbers of the 13 Candiru complex viruses used in this study
| Virus | Abbreviation | Strain | Yr of isolation | Source of isolate | Geographical location | Accession nos. |
|---|---|---|---|---|---|---|
| Candiru | CDUV | BeH 22511 | 1960 | Human | Km 94, Belem-Brasilia Highway, Para, Brazil | HM119407-HM119409 |
| Alenquer | ALEV | BeH 301101 | 1976 | Human | Alenquer, Para, Brazil | HM119401-HM119403 |
| Itaituba | ITAV | BeAn 213452 | 1971 | Didelphis marsupialis | Itaituba, Para, Brazil | HM119416-HM119418 |
| Jacunda | JCNV | BeAn 428329 | 1984 | Myoprocta acouchy | Tucurui, Para, Brazil | HM466934-HM466936 |
| Nique | NIQV | Nique 96 | 1972 | Lutzomyia panamensis | Nique, Darien, Panama | HM119425-HM119427 |
| Oriximina | ORXV | BeAr 385309 | 1980 | Lutzomyia sp. | Oriximina, Para, Brazil | HM119434-HM119436 |
| Turuna | TUAV | BeAr 352492 | 1978 | Lutzomyia sp. | Oriximina, Para, Brazil | HM119431-HM119433 |
| Echarate | ESCV | OBS 6528 | 1998 | Human | Cusco, Peru | HM119410-HM119412 |
| Mucura | MCRV | BeAr 455230 | 1985 | Anopheles (Nys) triannulatus | Tucurui, Para, Brazil | HM119419-HM119421 |
| Serra Norte | SRNV | BeH505240 | 1991 | Human | Carajas, Para, Brazil | HM119428-HM119430 |
| Morumbi | MRMBV | BeH 475236 | 1988 | Human | Tucurui, Para, Brazil | HM119422-HM119424 |
| Ariquemes | ARQV | BeAr 485678 | 1988/1989 | Lutzomyia sp. | Ariquemes, Rondonia, Brazil | HM119404-HM119406 |
| Maldonado | MLOV | FMD 0077 | 2004 | Human | Puerto Maldonado, Madre de Dios, Peru | HM119413-HM119415 |
Fig. 1.
Geographic distribution of Candiru viruses in Central America and North America. Each number indicates a virus in the Candiru complex and the approximate geographic location of its isolation.
Antigens and antisera.
Antisera for serologic tests were prepared in adult mice, using 10% crude homogenates of infected newborn mouse brain in phosphate-buffered saline as the immunogens. The immunization schedule consisted of four intraperitoneal injections of antigen mixed with Freund adjuvant, given at weekly intervals. After the final immunization, mice were inoculated with sarcoma 180 cells, and the resulting immune ascitic fluids were collected.
Serologic tests.
CF tests were performed by the microtiter technique (4), using 2 U of guinea pig complement and overnight incubation of the antigen and antibody at 4°C. Antigens used in the CF tests were prepared from infected newborn mouse brain by the sucrose acetone extraction method (13) and were inactivated with 0.05% β-propriolactone (Sigma, St. Louis, MO). CF titers were recorded as the highest dilutions giving 3+ or 4+ fixation of complement on a scale of 0 to 4+.
Genome sequencing.
Viral stocks were extracted by using TRIzol LS (Invitrogen, Carlsbad, CA). Total RNA extracts were treated with DNase I (DNA-Free; Ambion, Austin, TX). cDNA was generated by using the Superscript II system (Invitrogen) using random hexamers linked to an arbitrary 17-mer primer sequence (45). The resulting cDNA was treated with RNase H and then randomly amplified by PCR with a 9:1 mixture of primer corresponding to the 17-mer sequence and the random hexamer-linked 17-mer primer (45). Products greater than 70 bp were selected by column chromatography (MinElute; Qiagen, Hilden, Germany) and ligated to specific adapters for sequencing on the 454 Genome Sequencer FLX (454 Life Sciences, Branford, CT) without fragmentation (17, 36, 43). Software programs accessible through the analysis applications at the GreenePortal website (http://tako.cpmc.columbia.edu/Tools/) were used for removal of primer sequences, redundancy filtering, and sequence assembly. Sequence gaps were completed by PCR using primers based on pyrosequencing data. Amplification products were size fractionated on 1% agarose gels, purified (MiniElute), and directly sequenced in both directions with ABI Prism BigDye Terminator 1.1 cycle sequencing kits on ABI Prism 3700 DNA analyzers (Perkin-Elmer Applied Biosystems, Foster City, CA). For the termini of each segment, a primer with an 8-nucleotide conserved sequence was used for a specific reverse transcription with additional arbitrary nucleotides on the 5′ end (5′-AAGCAGTGGTATCAACGCAGAGTACACACAAAG-3′; the boldface portion shows the conserved nucleotides) . This primer is designed to bind to the 3′ end of the genomic RNA and the 3′ of the mRNA. Sequences of the genomes were verified by classical dideoxy sequencing using primers designed from the draft sequence to create products of 1,000 bp with 500-bp overlaps. The assembled data revealed 13 classical phlebovirus genomes (GenBank accession numbers HM119401 to HM119436 and HM466934 to HM466936).
Phylogenetic analysis.
A set of phlebovirus sequences (66 for the L segment, 118 for the M segment, 127 for the N gene, and 94 for the NS gene) comprising all nucleotide sequences from GenBank available on 1 October 2010 were aligned by using the CLUSTAL algorithm (as implemented in the MEGA package version 3) at the amino acid level with additional manual editing to ensure the highest possible quality of alignment. We excluded the noncoding regions of the S segment, given the high variability found in those areas. Neighbor-joining (NJ) analysis at the amino acid level was performed due to the observed high variability of the underlying nucleotide sequences. The statistical significance of tree topology was evaluated by bootstrap resampling of the sequences 1,000 times. Phylogenetic analyses were performed by using MEGA software (33).
Detection of recombination events.
Systematic screening for the presence of recombination patterns was pursued by using the nucleotide alignments and the Recombination Detection Program (RDP) (37) and Bootscan (51), MaxChi (55), Chimaera (48), LARD (23), and Phylip Plot (19).
Sequence analysis.
Geneious 4.8.3 (Biomatters, Inc.) was used for sequence assembly and analysis. Topology and targeting predictions were generated by using SignalP, NetNGlyc, TMHMM (http://www.cbs.dtu.dk/services), the web-based version of TopPred2 (http://bioweb.pasteur.fr/seqanal/interfaces/toppred.html), and integrated predictions in Geneious (5, 14, 27, 28, 32).
RESULTS
Consistent with the genomic organization characteristic for the members of the genus Phlebovirus, members of the Candiru antigenic complex comprise three RNA segments, including a large (L) segment encoding the RNA polymerase, a medium (M) segment encoding a nonstructural protein (NSm) and both glycoproteins (GN and GC), and a small (S) segment encoding the nucleocapsid protein (NP) and, in an ambisense orientation, a nonstructural protein (NSs).
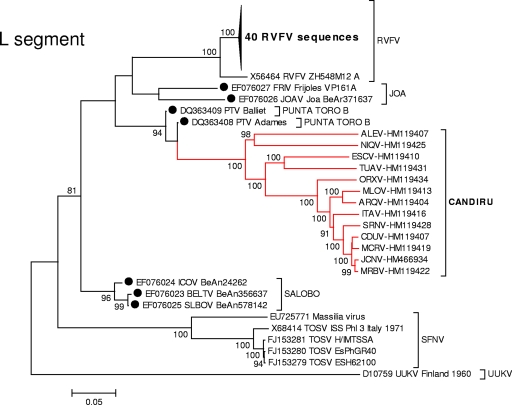
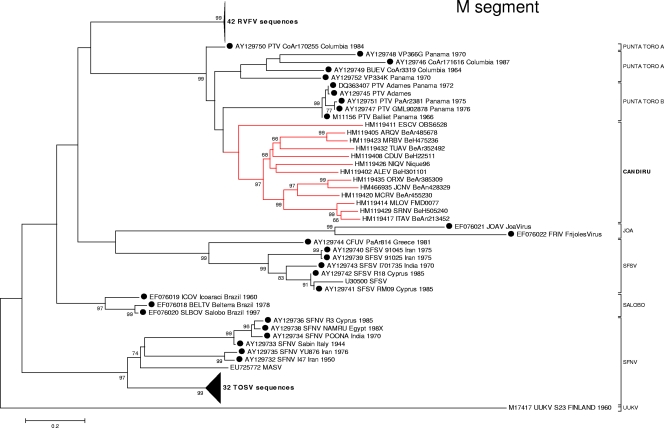
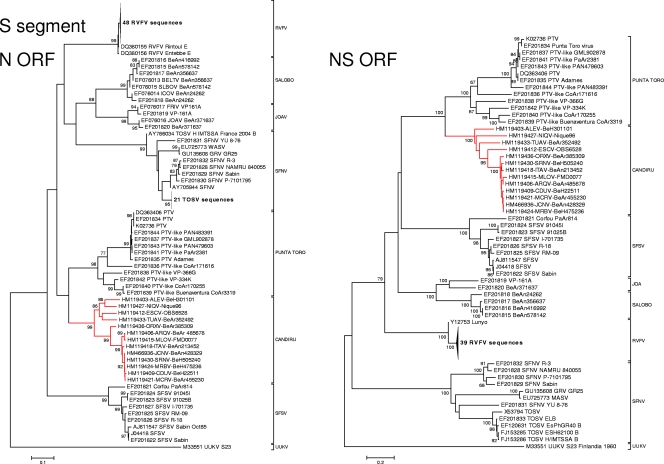
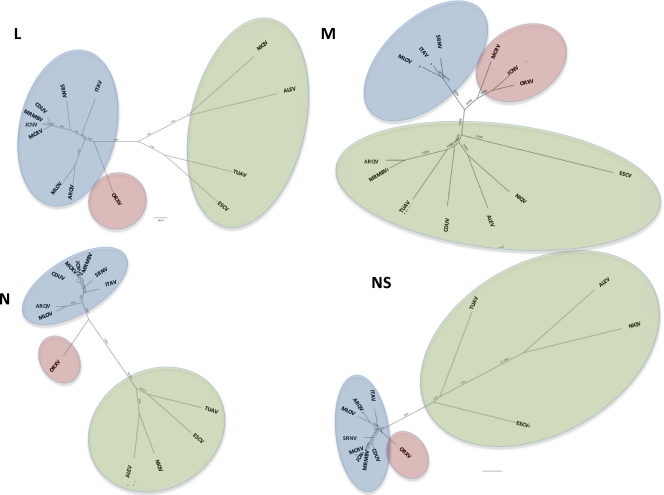
Phylogenetic analyses of the L, M, and S gene segment sequences were consistent with earlier reports indicating that viruses belonging to the same Phlebovirus complex cluster together (12, 16). As anticipated, based on their cross-reactivity in CF tests (Table 2), members of the Candiru serocomplex clustered together (Fig. 2, 3, and 4). Seven additional viruses were identified as members of the Candiru species group: (Ariquemes [ARQV], Echarate [ESCV], Jacunda [JCNV], Morumbi [MRMBV], Mucura [MCRV], and Serra Norte [SRNV]). However, the topology of the phylogenetic trees within the Candiru complex node revealed branching inconsistencies suggesting five reassortment events involving the M-segment of prototype Candiru virus (CDUV), MRMBV, MCRV, JCNV, and ARQV (Fig. 5). Analysis of reassortment using RDP, Bootscan, MaxChi, LARD, and Phylip Plot also confirmed the findings (data not shown). For purposes of analysis, we divided the Candiru complex node into three clades labeled A, B, and C (Fig. 5). Based on L- and S-segment sequences, MRMBV, JCNV, MCRV, and CDUV are closely related viruses in the clade B. Their similarities in the L, N, and NS open reading frames (ORFs) are so similar that they meet criteria for representing a single virus (Fig. 5). However, MRMBV and CDUV contain a clade-A M segment and JCNV and MCRV contain a clade-C M segment. A similar situation was observed with the pair ARQV and Maldonado virus (MLOV). Although they are very close in the L, N, and NS ORFs, the ARQV virus contains a clade-A M segment.
Table 2.
Results of complement fixation tests with 13 Candiru species complex viruses
| Virus antigen | Virus antibody CF titera |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Candiru | Alenquer | Itaituba | Nique | Oriximina | Turuna | Jacunda | Echarate | Mucura | Serra Norte | Morumbi | Ariquemes | Maldonado | |
| Candiru | ≥512/≥8 | 64/64 | 32/≥8 | 8/8 | 8/8 | 16/≥8 | 64/≥128 | 16/≥8 | 256/≥8 | 128/≥8 | 512/≥8 | 1,024/≥64 | 128/≥8 |
| Alenquer | 8/≥φ | 2,048/8 | 8/≥φ | 32/≥φ | 8/≥φ | 32/≥φ | 0 | 64/≥φ | 32/≥φ | 16/≥φ | 8/≥φ | 256/8 | ND |
| Itaituba | 64/≥8 | ≥16/≥32 | 128/≥16 | 64/≥8 | 32/≥8 | 16/≥8 | 64/≥128 | 64/16 | 256/≥8 | 256/≥8 | 1,024/≥8 | 1,024/128 | 128/≥8 |
| Nique | 8/8 | 1,024/64 | 8/8 | 128/≥8 | 8/8 | 128/≥8 | <8/<8 | 256/≥8 | 64/≥8 | 16/≥8 | 16/≥8 | 256/64 | ≥32/≥8 |
| Oriximina | ≥64/≥8 | 64/64 | 128/≥8 | 16/≥8 | 128/32 | 128/≥8 | 32/1,024 | 128/16 | 128/≥8 | 32/≥8 | ≥32/≥8 | 512/≥64 | 64/≥8 |
| Turuna | 128/≥8 | 256/64 | 16/≥8 | 32/≥8 | 0 | 256/≥8 | 8/≥128 | 256/≥8 | 64/≥8 | 32/≥8 | 16/≥8 | 512/≥64 | 64/≥8 |
| Jacunda | ≥256/128 | 64/64 | 128/≥128 | 0 | 64/64 | 128/128 | 64/≥128 | 128/64 | ≥256/≥128 | ≥256/≥128 | 128/≥128 | 1,024/128 | ≥256/≥8 |
| Echarate | 16/8 | 256/64 | 8/8 | 32/≥8 | 8/8 | 32/≥8 | 8/16 | 256/16 | 32/≥8 | 16/≥8 | 16/≥8 | 256/≥64 | ≥32/≥8 |
| Mucura | 256/≥8 | ≥16/32 | 64/≥8 | 16/≥8 | 64/≥8 | 64/≥8 | 64/≥128 | 256/≥8 | 512/128 | 128/≥8 | 512/≥8 | 1,024/≥8 | 256/≥8 |
| Serra Norte | 512/8 | 32/32 | 128/≥8 | 16/≥8 | 64/≥8 | 128/≥8 | 64/32 | 64/≥8 | 512/≥8 | 1,024/≥8 | 512/≥8 | 512/≥64 | 256/≥8 |
| Morumbi | 512/≥8 | 128/32 | 32/≥8 | 64/≥8 | 64/≥8 | 64/≥8 | 64/32 | 128/≥8 | 512/≥8 | 256/≥8 | 1,024/≥8 | 1,024/≥64 | ≥256/≥8 |
| Ariquemes | 128/8 | 64/≥256 | 64/≥8 | 8/8 | 64/≥8 | 32/≥8 | 32/128 | 64/≥8 | 256/≥8 | 32/≥8 | 64/≥8 | 1,024/≥64 | 64/≥8 |
| Maldonado | 256/≥8 | 64/≥8 | 128/≥8 | 16/≥8 | 64/≥8 | 64/≥8 | 32/≥8 | 128/≥8 | ≥256/≥8 | ≥256/≥8 | ≥256/≥8 | ≥256/≥8 | 512/≥512 |
CF titers are expressed as the highest antibody dilution/highest antigen dilution. 0 = <8/8; ND, not determined; φ, undiluted.
Fig. 2.
Phylogenetic analysis of the available sequences of phlebovirus L ORF. A set of 66 L phlebovirus sequences comprising all partial or complete sequences from GenBank available on 1 October 2010 were aligned using the CLUSTAL algorithm (as implemented in the MEGA package version 3) at the amino acid level with additional manual editing to ensure the highest possible quality of alignment. NJ analysis at the amino acid level was performed due to the observed high variability of the underlying nucleotide sequences. The statistical significance of tree topology was evaluated by bootstrap resampling of the sequences 1,000 times. Phylogenetic analyses were performed using MEGA software (33). Sequences marked with black dots corresponded to partial sequences.
Fig. 3.
Phylogenetic analysis of the available sequences of phlebovirus M ORF. A set of 118 M phlebovirus sequences comprising all partial or complete sequences from GenBank available on 1 October 2010 were analyzed under conditions identical to those for Fig. 2. Sequences marked with black dots correspond to partial sequences.
Fig. 4.
Phylogenetic analysis of the available sequences of phlebovirus S ORF. (Left panel) N ORF. A set of 127 N phlebovirus sequences comprising all partial or complete sequences from GenBank available on October 1, 2010 were analyzed under identical conditions to Fig. 2. (Right panel) NS ORF. A set of 94 NS phlebovirus sequences comprising all partial or complete sequences from GenBank available on 1 October 2010 were analyzed under conditions identical to those for Fig. 2.
Fig. 5.
Phylogenetic analysis of the complete sequences of the Candiru complex. All Candiru sequences were aligned using the CLUSTAL algorithm at the amino acid level with additional manual editing to ensure the highest possible quality of alignment. NJ analysis at the nucleotide level was performed. The statistical significance of tree topology was evaluated by bootstrap resampling of the sequences 1,000 times. Phylogenetics analyses were performed using MEGA software (33). Three clades were identified: clade A (green shading), clade B (blue), and clade C (red).
Genetic distance distribution analysis.
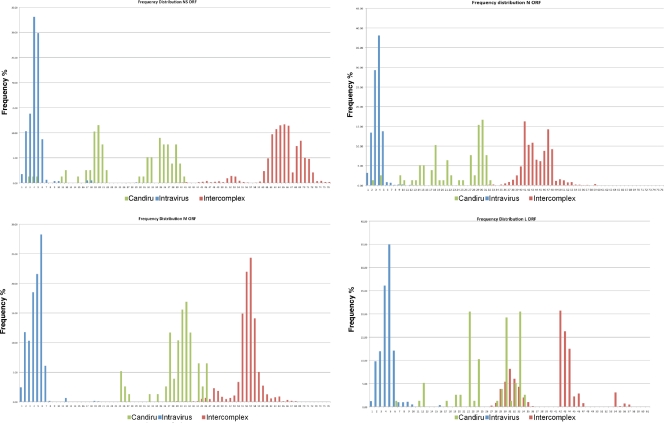
The sequences of the Candiru complex viruses were compared to all published phleboviral sequences in pairwise sequence alignments to assess the potential for establishing a simple cutoff for classification of serotypes similar to those used in the classification system created for mumps (44) and adenoviruses (11). However, given the scarcity of sequence available for phleboviruses, our conclusions can only be considered preliminary. In general, the M, N, and NS ORF similarity values cluster by species and group; however, the L-ORF similarity values are inconsistent with the two grouping levels (Fig. 6).
Fig. 6.
Pairwise sequence analysis. To establish a potential cutoff for phlebovirus classification, we pursued pairwise sequence comparison of all published phlebovirus sequences. Calculations were performed using MEGA software (33) to calculate the p-distance of each segment at the nucleotide level. p-distance values were grouped in three groups representing: blue, distances among different strains of the same virus (intraspecies); red, distances between members of different species (intergroup); and green, distances among members of the Candiru complex.
Relation between serological reactivity and genetic distance.
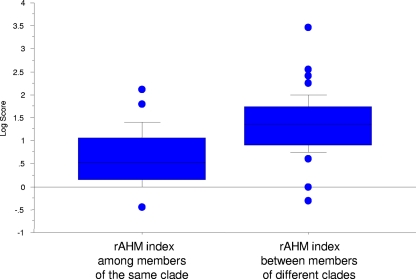
Serological comparisons among all members of the Candiru complex were done by CF test (Table 2). Selected viruses of the antigenic complex that produce consistent plaques were also evaluated by PRNT test (Table 3). To compare the serological data with the genetic distance, CF and neutralization values were transformed to rAHM, as previously described for influenza virus (40, 54). CF data correlation with amino acid p-distance data was weak: the L, M, N, and NS ORF R2 were only 0.32, 0.28, 0.34, and 0.22). The slightly higher correlation between the N ORF and the CF test is expected, since the N protein is the main determinant of the CF antigen (41). Interestingly, although the correlation was weak at the level of virus, it was stronger at the level of clades. The rAHM index among members of the same clade was significantly smaller than the values obtained between members of different clades (Fig. 7).
Table 3.
Results of plaque reduction neutralization tests with selected Candiru complex phleboviruses
| Virus | Clade on segment M | Antiserum dilutiona |
|||||
|---|---|---|---|---|---|---|---|
| Clade A |
Clade B, Itaituba | Clade C, Oriximina | |||||
| Candiru | Alenquer | Nique | Turuna | ||||
| Candiru | A | 1,280 | <10 | <10 | 10 | <10 | <10 |
| Alenquer | A | <10 | 80 | <10 | <10 | <10 | <10 |
| Nique | A | 40 | <10 | 80 | 20 | <10 | <10 |
| Turuna | A | 320 | <10 | 40 | 1,280 | <10 | 40 |
| Itaituba | B | 640 | <10 | 640 | <10 | 5,120 | 160 |
| Oriximina | C | 320 | 20 | 160 | 20 | 160 | 160 |
Reciprocal of highest antiserum dilution producing 90% plaque inhibition.
Fig. 7.
Serological distance among members of the Candiru complex as determined by CF. CF values were transformed to rAHM, as previously described for influenza virus (40, 54). rAHM values were grouped in two groups representing: distances among strains belonging to the same clade, and distances between strains of different clades. In the median log scores box plot, the whiskers represent minimum and maximum ranges. Lines in box represent the first interquartile range, followed by the median, and third interquartile range. The rAHM index among members of the same clade (n = 34) and the rAHM index between members of different clades (n = 44) are shown. The data were log transformed to better approximate a normal distribution.
Neutralization data were not available for all members of the Candiru complex. However, from the data available, we concluded that neutralization titers do not correlate with aa distance in the M gene (which is the main antigen responsible for eliciting neutralizing antibodies). For instance, Itaituba (ITAV) and Oriximina (ORXV) elicit reciprocal neutralizing antibody titers and yet have a genetic distance that is similar to those of other members of the group; conversely, ITAV and CDUV or ORXV and CDUV belong to different clades (and therefore show large genetic distance) and yet show the highest reciprocal titers among the members of the group.
ORFs. (i) Large RNA-dependent RNA polymerase (L).
Several regions of the RNA-dependent RNA polymerase overlap conserved regions found in all available phlebovirus sequences, suggesting an association with function. Region I is located in the amino terminus (aa 104 to 128) and is centered on the amino acid P111D; region II (aa 649 to 707), also located in the amino terminus, is centered around the amino acid R681Y. Regions I and II are conserved in all bunyaviruses and arenaviruses (39). Region III (aa 918 to 1195), located in the center of the protein, contains the polymerase motifs (pre-A [aa 919 to 944], A [aa 982 to 1000], B [aa 1079 to 1099], C [aa 1124 to 1137], D [aa 1168 to 1181], and E [aa 1182 to 1196]) found in all RNA-dependent polymerases from positive-, negative-, and double-stranded RNA viruses (see Fig. S1 in the supplemental material) (47, 62). Region IV (aa 1197 to 1296) identified by Aquino et al. (1) and two additional regions of high conservation (regions V [aa 1388 to 1426] and VI [aa 1518 to 1613]) are shown in Fig. S2 in the supplemental material.
(ii) Polyprotein.
Among the viruses in the Candiru complex, the length of the M polyprotein varies in length from 1,299 aa in ESCV to 1,338 aa in Alenquer (ALEV). Nique (NIQV), ALEV, and ESCV, which are phylogenetically divergent, have lengths of 1,335, 1,338, and 1,299 aa, respectively. The remaining viruses in the complex all have lengths between 1,317 and 1,320 aa. Polyproteins of Candiru complex viruses are similar in length to the M protein of Punta Toro virus (PTV) strains Adames and Balliet (1,319 aa for MLOV, 1,313 aa for PTV Adames, and 1,319 aa for PTV 1319 Balliet).
The M polyprotein is cotranslationally cleaved into three products: NSm, Gn, and Gc. The topology of the polyprotein of CDUV is similar to the predicted topologies for Toscana virus (TOSV), PTV, and Rift Valley fever virus (RVFV) (20, 22, 25, 38, 61). Signal sequences, transmembrane domains, cleavage sites for the cellular signal signalase protease and Golgi retention signals for the Gn and Gc are conserved in the majority of the viruses in the Candiru complex (see Fig. S3 in the supplemental material). There is considerable variation in the patterns of predicted glycosylation sites. Interestingly, the patterns seem to correlate with their clade placement (see Fig. S3 in the supplemental material).
(iii) Nucleocapsid protein.
The bunyavirus nucleocapsid protein (NP) is the most abundant protein in infected cells and is posited to regulate the transition between primary transcription and replication (34). The NP forms homodimers at their amino termini. In RVFV, the interacting domain is located in the N-terminal 71 residues; amino acids Tyr4, Phe11, Asp17, and Trp24 are conserved in all phleboviruses except uukuviruses (UUKV and its close relatives); the alpha-helices present in that region are crucial for this interaction (20). Although Tyr4, Asp17, or Trp24 are also conserved among the Candiru complex viruses, Phe11 is not. However, an Asp or a Glu at position 10 might serve the same function (see Fig. S4 in the supplemental material).
DISCUSSION
Segment reassortment in bunyaviruses has been reported with increasing frequency, especially in the genus Orthobunyavirus (7–10, 15, 26, 31, 42, 50, 64, 65). Nonetheless, the extent of reassortment in the Candiru complex (5 of 13 named viruses) is unprecedented. Given that phylogenetic analyses of other members of the genus are based on only single genomic segments (18, 21, 35, 63), it is possible that as additional full genomic sequences are reported, we will learn that this feature is not unique to the Candiru complex.
Interestingly, all segment reassortants reported among bunyaviruses to date involve the interchange of the M segment (7–9, 15, 42, 50, 64, 65). The M segment of phleboviruses encodes two glycoproteins: Gn and Gc, and a nonstructural protein, NSm (41). As with other bunyaviruses, the phlebovirus envelope glycoproteins are important for viral infection, pathogenesis and immunity; they serve as neutralizing and hemagglutinin-inhibiting antibody targets and are exposed to selective pressure (3, 6, 30, 46). Neutralizing epitopes typically depend on the tertiary structure of proteins, and even a single amino acid substitution may influence biological activity (24, 53). The ICTV currently distinguishes phlebovirus species based on a 4-fold difference in neutralization tests. Although neutralization assays are well-established methods for differentiating phleboviruses for taxonomic analysis, the high rate of genetic reassortment of the M segment illustrates the potential risk associated with relying on neutralization or hemagglutination inhibition assays alone. Ngari virus (NRIV) is a case in point. It has recently been associated with severe febrile illness and hemorrhagic fever among humans in East Africa (7, 8). Genetic characterization of NRIV has shown that it is a genetic reassortant with S and L RNA segments from Bunyamwera virus (BUNV) and an M RNA segment from Batai virus (BATV) (8, 65). In PRNT tests or with partial sequencing of just the M segment, NRIV virus would appear to be BATV; but in CF tests, which detect the nucleocapsid (N) protein (encoded by the S RNA segment), NRIV would appear to be BUNV.
CF assays based on using the N protein as an antigen are useful in identifying members of a given antigenic complex. However, it is less clear that reciprocal titers of CF tests are sufficient to measure distance between the members of a serological group. Although we found no correlation between genetic distance and CF titers at the level of species, we found good correlation with clade membership. Neutralization, on the other hand, does not seem to correlate with evolutionary distance. Given the low correlation observed between complement fixation titers and genetic distance, it may be prudent to embrace a system of classification of Bunyaviridae that also considers genetic data. The current system of taxonomy, which is based exclusively on antigenic classification, can be misleading given the phenomenon of segment reassortment.
It is also noteworthy that 6 of the 13 Candiru complex viruses were isolated from febrile people. The clinical manifestations of illness (29, 60) in these people were similar; nonspecific, self-limited febrile illness, indistinguishable from phlebotomus (sandfly) fever and other relatively benign arboviral illnesses (56). Based on the frequency of their isolation (most have only been isolated once), one would predict that the Candiru complex viruses are not a common cause of human illness in tropical America. However, the paucity of isolates could also be a reflection of their distribution (tropical forested regions) and their recognition (all were isolated and identified in arbovirus diagnostic or research laboratories). Because of the limited laboratory capability of most medical facilities in this geographic region and the nonspecific nature of the illness, sporadic human cases may be misdiagnosed or unrecognized. However, given their genetic diversity, geographic range and ability to evolve through segment reassortment, it seems probable that other unrecognized members of the Candiru complex now exist and that new ones will continue to evolve.
Supplementary Material
ACKNOWLEDGMENTS
We thank Charles H. Calisher for critical suggestions, corrections and comments.
This study was supported by Google.org, National Institutes of Health award AI57158 (Northeast Biodefense Center [W.I.L.]), and USAID Predict funding source code 07-301-7119-52258 (Center for Infection and Immunity) and the Department of Defense. R.T., A.T.R., and H.G. were supported by NIH contracts NO1-AI30027 and HHSN272201000040I/HHSN27200004/D04. M.R.T.N., J.P.N.-N., and P.F.C.V. were supported by CNPq grants 300460/2005-8, 483453/2007-2, and 302987/2008-8. This study was partially funded by the U.S. Department of Defense Global Emerging Infections Systems Research Program (work unit number 847705Æ82000Æ25GB.B0016).
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, the Department of Defense, or the U.S. Government. The study protocol was approved by the Ministry of Health of Peru and the Naval Medical Research Center Institutional Review Board (protocol NMRCD.2000.0006) in compliance with all applicable Federal regulations governing the protection of human subjects.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 2 February 2011.
REFERENCES
- 1. Aquino V. H., Moreli M. L., Moraes Figueiredo L. T. 2003. Analysis of oropouche virus L protein amino acid sequence showed the presence of an additional conserved region that could harbour an important role for the polymerase activity. Arch. Virol. 148:19–28 [DOI] [PubMed] [Google Scholar]
- 2. Barnett H., Suyemoto W. 1961. Field studies on sandfly fever and kala-azar in Pakistan, in Iran, and in Baltistan (Little Tibet) Kasmir. Trans. NY Acad. Sci. 23:609–617 [Google Scholar]
- 3. Battles J. K., Dalrymple J. M. 1988. Genetic variation among geographic isolates of Rift Valley fever virus. Am. J. Trop. Med. Hyg. 39:617–631 [DOI] [PubMed] [Google Scholar]
- 4. Beaty B., Calisher C. H., Shope R. E. 1989. Arboviruses, p. 979–1055 In Schmidt N. J., Emmons R. W. (ed.), Diagnostic procedures for viral, rickettsial and chlamydial infections. American Public Health Association, Washington, DC [Google Scholar]
- 5. Bendtsen J. D., Nielsen H., von Heijne G., Brunak S. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783–795 [DOI] [PubMed] [Google Scholar]
- 6. Besselaar T. G., Blackburn N. K. 1991. Topological mapping of antigenic sites on the Rift Valley fever virus envelope glycoproteins using monoclonal antibodies. Arch. Virol. 121:111–124 [DOI] [PubMed] [Google Scholar]
- 7. Bowen M. D., et al. 2001. A reassortant bunyavirus isolated from acute hemorrhagic fever cases in Kenya and Somalia. Virology 291:185–190 [DOI] [PubMed] [Google Scholar]
- 8. Briese T., Bird B., Kapoor V., Nichol S. T., Lipkin W. I. 2006. Batai and Ngari viruses: M segment reassortment and association with severe febrile disease outbreaks in East Africa. J. Virol. 80:5627–5630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Briese T., Kapoor V., Lipkin W. I. 2007. Natural M-segment reassortment in Potosi and Main Drain viruses: implications for the evolution of orthobunyaviruses. Arch. Virol. 152:2237–2247 [DOI] [PubMed] [Google Scholar]
- 10. Burt F. J., Paweska J. T., Ashkettle B., Swanepoel R. 2009. Genetic relationship in southern African Crimean-Congo haemorrhagic fever virus isolates: evidence for occurrence of reassortment. Epidemiol. Infect. 137:1302–1308 [DOI] [PubMed] [Google Scholar]
- 11. Casas I., et al. 2005. Molecular identification of adenoviruses in clinical samples by analyzing a partial hexon genomic region. J. Clin. Microbiol. 43:6176–6182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Charrel R. N., et al. 2009. Massilia virus, a novel Phlebovirus (Bunyaviridae) isolated from sandflies in the Mediterranean. Vector Borne Zoonotic Dis. 9:519–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clarke D. H., Casals J. 1958. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am. J. Trop. Med. Hyg. 7:561–573 [DOI] [PubMed] [Google Scholar]
- 14. Claros M. G., von Heijne G. 1994. TopPred II: an improved software for membrane protein structure predictions. Comput. Appl. Biosci. 10:685–686 [DOI] [PubMed] [Google Scholar]
- 15. Collao X. 2010. Granada virus: a natural phlebovirus reassortant of the sandfly fever Naples serocomplex with low seroprevalence in humans. Am. J. Trop. Med. Hyg. 83:760–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Collao X., et al. 2009. Genetic diversity of Toscana virus. Emerg. Infect. Dis. 15:574–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cox-Foster D. L., et al. 2007. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318:283–287 [DOI] [PubMed] [Google Scholar]
- 18. Elliott R. M., Dunn E., Simons J. F., Pettersson R. F. 1992. Nucleotide sequence and coding strategy of the Uukuniemi virus L RNA segment. J. Gen. Virol. 73(Pt. 7):1745–1752 [DOI] [PubMed] [Google Scholar]
- 19. Felsenstein J. 1989. PHYLIP: phylogeny inference package (version 3.2). Cladistics 5:164–166 [Google Scholar]
- 20. Gerrard S. R., Nichol S. T. 2002. Characterization of the Golgi retention motif of Rift Valley fever virus G(N) glycoprotein. J. Virol. 76:12200–12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giorgi C., et al. 1991. Sequences and coding strategies of the S RNAs of Toscana and Rift Valley fever viruses compared to those of Punta Toro, Sicilian Sandfly fever, and Uukuniemi viruses. Virology 180:738–753 [DOI] [PubMed] [Google Scholar]
- 22. Gro M. C., Di Bonito P., Fortini D., Mochi S., Giorgi C. 1997. Completion of molecular characterization of Toscana phlebovirus genome: nucleotide sequence, coding strategy of M genomic segment and its amino acid sequence comparison to other phleboviruses. Virus Res. 51:81–91 [DOI] [PubMed] [Google Scholar]
- 23. Holmes E. C. 1998. Molecular epidemiology of dengue virus: the time for big science. Trop. Med. Int. Health 3:855–856 [DOI] [PubMed] [Google Scholar]
- 24. Horling J., Lundkvist A. 1997. Single amino acid substitutions in Puumala virus envelope glycoproteins G1 and G2 eliminate important neutralization epitopes. Virus Res. 48:89–100 [DOI] [PubMed] [Google Scholar]
- 25. Ihara T., Smith J., Dalrymple J. M., Bishop D. H. 1985. Complete sequences of the glycoproteins and M RNA of Punta Toro phlebovirus compared to those of Rift Valley fever virus. Virology 144:246–259 [DOI] [PubMed] [Google Scholar]
- 26. Iroegbu C. U., Pringle C. R. 1981. Genetic interactions among viruses of the Bunyamwera complex. J. Virol. 37:383–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kahsay R. Y., Gao G., Liao L. 2005. An improved hidden Markov model for transmembrane protein detection and topology prediction and its applications to complete genomes. Bioinformatics 21:1853–1858 [DOI] [PubMed] [Google Scholar]
- 28. Kall L., Krogh A., Sonnhammer E. L. 2004. A combined transmembrane topology and signal peptide prediction method. J. Mol. Biol. 338:1027–1036 [DOI] [PubMed] [Google Scholar]
- 29. Karabatsos N. 1985. International catalogue of arbovirus, including certain other viruses of vertebrates, 3rd ed. American Society of Tropical Medicine and Hygiene, San Antonio, TX [Google Scholar]
- 30. Keegan K., Collett M. S. 1986. Use of bacterial expression cloning to define the amino acid sequences of antigenic determinants on the G2 glycoprotein of Rift Valley fever virus. J. Virol. 58:263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kondiah K., Swanepoel R., Paweska J. T., Burt F. J. 2010. A simple-probe real-time PCR assay for genotyping reassorted and non-reassorted isolates of Crimean-Congo hemorrhagic fever virus in southern Africa. J. Virol. Methods 169:34–38 [DOI] [PubMed] [Google Scholar]
- 32. Krogh A., Larsson B., von Heijne G., Sonnhammer E. L. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305:567–580 [DOI] [PubMed] [Google Scholar]
- 33. Kumar S., Tamura K., Nei M. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 5:150–163 [DOI] [PubMed] [Google Scholar]
- 34. Le May N., Gauliard N., Billecocq A., Bouloy M. 2005. The N terminus of Rift Valley fever virus nucleoprotein is essential for dimerization. J. Virol. 79:11974–11980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu D. Y., et al. 2003. Phylogenetic relationships among members of the genus Phlebovirus (Bunyaviridae) based on partial M segment sequence analyses. J. Gen. Virol. 84:465–473 [DOI] [PubMed] [Google Scholar]
- 36. Margulies M., et al. 2005. Genome sequencing in microfabricated high-density picolitre reactors. Nature 437:376–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martin D. R. E., 2000. RDP: detection of recombination amongst aligned sequences. Bioinformatics 16:562–563 [DOI] [PubMed] [Google Scholar]
- 38. Matsuoka Y., Chen S. Y., Holland C. E., Compans R. W. 1996. Molecular determinants of Golgi retention in the Punta Toro virus G1 protein. Arch. Biochem. Biophys. 336:184–189 [DOI] [PubMed] [Google Scholar]
- 39. Muller R., Poch O., Delarue M., Bishop D. H., Bouloy M. 1994. Rift Valley fever virus L segment: correction of the sequence and possible functional role of newly identified regions conserved in RNA-dependent polymerases. J. Gen. Virol. 75(Pt. 6):1345–1352 [DOI] [PubMed] [Google Scholar]
- 40. Ndifon W., Dushoff J., Levin S. A. 2009. On the use of hemagglutination-inhibition for influenza surveillance: surveillance data are predictive of influenza vaccine effectiveness. Vaccine 27:2447–2452 [DOI] [PubMed] [Google Scholar]
- 41. Nichol S. T., et al. 2005. Family Bunyaviridae. Elsevier Academic Press, London, England [Google Scholar]
- 42. Nunes M. R., Travassos da Rosa A. P., Weaver S. C., Tesh R. B., Vasconcelos P. F. 2005. Molecular epidemiology of group C viruses (Bunyaviridae, Orthobunyavirus) isolated in the Americas. J. Virol. 79:10561–10570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Palacios G., et al. 2008. A new arenavirus in a cluster of fatal transplant-associated diseases. N. Engl. J. Med. 358:991–998 [DOI] [PubMed] [Google Scholar]
- 44. Palacios G., Jabado O., Cisterna D. 2005. Molecular identification of mumps virus genotypes from clinical samples: standardized method of analysis. J. Clin. Microbiol. 43:1869–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Palacios G., et al. 2007. Panmicrobial oligonucleotide array for diagnosis of infectious diseases. Emerg. Infect. Dis. 13:73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pifat D. Y., Osterling M. C., Smith J. F. 1988. Antigenic analysis of Punta Toro virus and identification of protective determinants with monoclonal antibodies. Virology 167:442–450 [PubMed] [Google Scholar]
- 47. Poch O., Sauvaget I., Delarue M., Tordo N. 1989. Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J. 8:3867–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Posada D., Crandall K. A. 2001. Evaluation of methods for detecting recombination from DNA sequences: computer simulations. Proc. Natl. Acad. Sci. U. S. A. 98:13757–13762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sabin A. B. 1955. Recent advances in our knowledge of dengue and sandfly fever. Am. J. Trop. Med. Hyg. 4:198–207 [DOI] [PubMed] [Google Scholar]
- 50. Saeed M. F., et al. 2001. Jatobal virus is a reassortant containing the small RNA of Oropouche virus. Virus Res. 77:25–30 [DOI] [PubMed] [Google Scholar]
- 51. Salminen M. O., Carr J. K., Burke D. S., McCutchan F. E. 1995. Identification of breakpoints in intergenotypic recombinants of HIV type 1 by bootscanning. AIDS Res. Hum. Retrovir. 11:1423–1425 [DOI] [PubMed] [Google Scholar]
- 52. Sanchez-Seco M. P., et al. 2003. Detection and identification of Toscana and other phleboviruses by RT-nested-PCR assays with degenerated primers. J. Med. Virol. 71:140–149 [DOI] [PubMed] [Google Scholar]
- 53. Schmaljohn C. S., Chu Y. K., Schmaljohn A. L., Dalrymple J. M. 1990. Antigenic subunits of Hantaan virus expressed by baculovirus and vaccinia virus recombinants. J. Virol. 64:3162–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Smith D. J., et al. 2004. Mapping the antigenic and genetic evolution of influenza virus. Science 305:371–376 [DOI] [PubMed] [Google Scholar]
- 55. Smith J. 1992. Analyzing the mosaic structure of genes. J. Mol. Evol. 34:126–129 [DOI] [PubMed] [Google Scholar]
- 56. Tesh R. 2006. Sandfly fever, Oropouche fever, and other bunyavirus infections, p. 781–783 In Guerrant R. L., Walker D. H., Weller P. F. (ed.), Tropical infectious diseases: principles, pathogens, and practice, 2nd ed. Elsevier Churchill Livingston, Philadelphia, PA [Google Scholar]
- 57. Tesh R. B., et al. 1989. Characterization of five new phleboviruses recently isolated from sand flies in tropical America. Am. J. Trop. Med. Hyg. 40:529–533 [PubMed] [Google Scholar]
- 58. Tesh R. B., Chaniotis B. N., Peralta P. H., Johnson K. M. 1974. Ecology of viruses isolated from Panamanian phlebotomine sandflies. Am. J. Trop. Med. Hyg. 23:258–269 [DOI] [PubMed] [Google Scholar]
- 59. Travassos da Rosa A. P., Tesh R. B., Pinheiro F. P., Travassos da Rosa J. F., Peterson N. E. 1983. Characterization of eight new phlebotomus fever serogroup arboviruses (Bunyaviridae: Phlebovirus) from the Amazon region of Brazil. Am. J. Trop. Med. Hyg. 32:1164–1171 [DOI] [PubMed] [Google Scholar]
- 60. Travassos da Rosa J. F., et al. 1998. Arboviruses isolated in Evandro Chagas Institute, including some described for the first time in the Brazilian Amazon Region, their known hosts, and their pathology for man, p. 19–31 In Travassos da Rosa A. P., Vasconcelos P. F., Travassos da Rosa J. F. (ed.), An overview of arbovirology in Brazil and neighboring countries. Grafica e Editora Santo Antonio, Belem [Google Scholar]
- 61. Valentini M., Valassina M., Savellini G. G., Cusi M. G. 2008. Nucleotide variability of Toscana virus M segment in strains isolated from clinical cases. Virus Res. 135:187–190 [DOI] [PubMed] [Google Scholar]
- 62. Xiong Y., Eickbush T. H. 1990. Origin and evolution of retroelements based upon their reverse transcriptase sequences. EMBO J. 9:3353–3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xu F., Liu D., Nunes M. R. D. A. R. AP., Tesh R. B., Xiao S. Y. 2007. Antigenic and genetic relationships among Rift Valley fever virus and other selected members of the genus Phlebovirus (Bunyaviridae). Am. J. Trop. Med. Hyg. 76:1194–1200 [PubMed] [Google Scholar]
- 64. Yanase T., et al. 2010. Genetic characterization of Aino and Peaton virus field isolates reveals a genetic reassortment between these viruses in nature. Virus Res. 153:1–7 [DOI] [PubMed] [Google Scholar]
- 65. Yanase T., et al. 2006. Genetic characterization of Batai virus indicates a genomic reassortment between orthobunyaviruses in nature. Arch. Virol. 151:2253–2260 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.