Abstract
Egress of herpes simplex virus (HSV) and other herpesviruses from cells involves extensive modification of cellular membranes and sequential envelopment and deenvelopment steps. HSV glycoproteins are important in these processes, and frequently two or more glycoproteins can largely suffice in any step. Capsids in the nucleus undergo primary envelopment at the inner nuclear membrane (INM), and then enveloped virus particles undergo deenvelopment by fusing with the outer nuclear membrane (ONM). Capsids delivered into the cytoplasm then undergo secondary envelopment, involving trans-Golgi network (TGN) membranes. The deenvelopment step involves HSV glycoproteins gB and gH/gL acting in a redundant fashion. This fusion has features common to the fusion that occurs between the virion envelope and cellular membranes when HSV enters cells, a process requiring gB, gD, and gH/gL. Whether HSV gD also participates (in a redundant fashion with gB or gH/gL) in deenvelopment has not been characterized. Secondary envelopment in the cytoplasm is known to involve HSV gD and gE/gI, also acting in a redundant fashion. Whether gB might also contribute to secondary envelopment, collaborating with gD and gE/gI, is also not clear. To address these questions, we constructed an HSV double mutant lacking gB and gD. The HSV gB−/gD− mutant exhibited no substantial defects in nuclear egress. In contrast, secondary envelopment was markedly reduced, and there were numerous unenveloped capsids that accumulated in the cytoplasm, as well as increased numbers of partially enveloped capsids and morphologically aberrant enveloped particles with thicker, oblong tegument layers. These defects were different from those observed with HSV gD−/gE−/gI− mutants, which accumulated capsids in large, aggregated masses in the cytoplasm. Our results suggest that HSV gB functions in secondary envelopment, apparently acting downstream of gE/gI.
INTRODUCTION
Herpesvirus capsids are produced in the nucleus and must traverse the inner nuclear membrane (INM) and outer nuclear membrane (ONM) as well as cytoplasmic membranes to be released from cells. For herpes simplex virus (HSV), egress begins with the disruption of the nuclear lamina, involving HSV proteins UL34 and UL31 (reviewed in references 38 and 39). Capsids coated with a subset of tegument proteins then become wrapped with INM containing UL34/UL31 (45) and HSV membrane glycoproteins gB, gD, gH/gL, and gM (3, 18, 50, 54) so that enveloped virions bud into the perinuclear space (Fig. 1). Perinuclear, enveloped virus then undergoes deenvelopment by fusing with the ONM (reviewed in references 38 and 39). Capsids delivered into the cytoplasm are coated with additional tegument proteins and then wrapped in membranes of the trans-Golgi network (TGN) (16, 53, 55, 59). This process, known as secondary envelopment, apparently involves bridges formed between tegument proteins on the surfaces of capsids and the cytoplasmic tails of certain viral glycoproteins, as enveloped particles bud into TGN vesicles (reviewed in references 38 and 39). Enveloped virions within membrane vesicles are then transported to cell surfaces, where there is fusion between the vesicle and the plasma membrane delivering enveloped particles out of cells. This last step in virus egress follows the normal exocytic pathway, but there is evidence for redistribution of the Golgi apparatus and TGN membranes in the cell and to surface membranes (10, 37, 59). In polarized cells, this redistribution of the TGN specifically to basolateral cell surfaces promotes virus spread to other cells (29, 59). All (or most) of these alterations to cellular membranes, i.e., envelopment, deenvelopment, and exocytosis, involve effects of HSV glycoproteins (Fig. 1).
Fig. 1.
Schematic representation of HSV egress and the involvement of HSV glycoproteins in various steps. HSV capsids are assembled in the nucleus and bud into the INM by a process known as primary envelopment. No HSV glycoproteins have been shown to be essential for this step, although HSV glycoproteins are components of the INM and perinuclear virus particles. Deenvelopment, fusion with the ONM, requires HSV gB or gH/gL. Cytoplasmic capsids acquire additional tegument proteins and undergo secondary envelopment, which requires gB, gD, and gE/gI. Virions are then transported to cell surfaces in exocytic vesicles. There is evidence of directed exocytosis to specific cell surfaces in polarized cells, a process that requires gE/gI and likely other glycoproteins.
There is evidence that HSV glycoproteins gB and gH/gL are involved in the second step of nuclear egress, deenvelopment, fusion between the virion envelope and the ONM (18). This process bears structural similarities to the membrane fusion between the virion envelope and cell surface or endosomal membranes when HSV enters cells (reviewed in reference 24). HSV gB, gD, and gH/gL are all required for entry into cells, whether through fusion at cell surfaces or in low-pH endosomes (9, 20, 34, 40, 46). gD binds so-called “gD receptors,” such as nectin-1 and herpesvirus entry modulator HVEM, and then apparently triggers gH/gL and gB, which mediate entry fusion (reviewed in reference 24). The recently determined crystal structures of gB and gH/gL (11, 25) suggest that gB is the major membrane fusion-inducing protein. This conclusion was supported by recent observations that gB directly interacts with membranes (22) and evidence that gB can mediate cell-cell fusion in trans (1). Paralleling entry fusion, deenvelopment fusion involves gB and gH/gL. An HSV gB−/gH− mutant accumulated enveloped particles in the perinuclear spaces or in structures termed herniations, vesicles containing numerous enveloped virions that herniated from the INM into the nucleoplasm (18). There were also more-minor defects in nuclear egress observed with gB-null mutants but no obvious defects observed with a gH-null mutant (18). Importantly, it was necessary to delete both gB and gH in order to observe major defects in nuclear egress, although gB appears to be more important in this process. This is very different from entry fusion, which is entirely blocked when any one of the glycoproteins gB, gD, or gH/gL is deleted. gB apparently plays a direct, rather than an indirect, role in promoting fusion between the virion envelope and the ONM, because point mutations in gB affecting so-called “fusion loops” inhibited both entry and nuclear egress (61). Fusion loops are thought to promote insertion of gB into cellular membranes during the membrane fusion process (22, 23).
All herpesviruses express gB and gH/gL homologues. The involvement of gB in nuclear egress may extend to Epstein-Barr virus (EBV) and Kaposi's sarcoma herpesvirus (KSHV), because gB mutants have defects in assembly or egress (31, 33), although a KSHV gB mutant protein lacking just the gB cytoplasmic domain could apparently function in egress (52). There was also a report that pseudorabies virus (PRV) mutants lacking gB and gH and other glycoproteins were not blocked for nuclear egress (30). This might be explained by use of other herpesvirus membrane proteins in deenvelopment. Consistent with this hypothesis, the HSV gB−/gH− mutant was not entirely blocked in nuclear egress (18).
HSV capsids delivered into the cytoplasm after fusion with the ONM become enveloped at the TGN, the Golgi apparatus, or endosomal membranes (16, 53, 55, 59). Secondary envelopment involves two HSV membrane glycoproteins, gD and the heterodimer gE/gI, that act in a relatively redundant fashion. Deletion of gE, gI, and gD reduced enveloped virus particles on cell surfaces by over 80% (15). Large aggregates of capsids, apparently immersed in a layer of tegument, were observed in the cytoplasm of cells infected with gD−/gE−/gI− mutants. The phenotypes of single gD− or gE− mutants were much more subtle, involving 2- to 3-fold reductions in enveloped virions. Similarly, PRV mutants lacking both gE and gM were defective in secondary envelopment (6, 7). Thus, alphaherpesviruses utilize at least two membrane glycoproteins (for PRV, gE/gI and gM, or for HSV, gE/gI and gD) for secondary envelopment. HSV and PRV mutants lacking the cytoplasmic tail of gE, as well as gD or gM, were also defective for secondary envelopment (7, 17). These results suggest that the cytoplasmic domains of gE/gI and gD or gM function to tether tegument-coated capsids onto TGN membranes in order to promote secondary envelopment. This fits with evidence that gE/gI and gD apparently interact with tegument proteins, such as VP22 and UL11, which play important roles in secondary envelopment (2, 13, 17, 35, 41, 42, 51).
Given that HSV gD represents an essential function for entry fusion, acting upstream of gH/gL and the fusion glycoprotein gB, it was of substantial interest to characterize whether an HSV mutant lacking both gD and gB displayed defects in nuclear egress, especially the deenvelopment step. Whether gD is required to bind receptors in order to trigger gB and gH/gL for deenvelopment fusion was at issue. It was also possible that gD might promote primary envelopment, given its role in secondary envelopment. A second question focused on whether HSV gB might contribute to secondary envelopment, acting in conjunction with gD and gE/gI. HSV gB and all herpesvirus gB homologues possess large cytoplasmic domains that target the glycoproteins to the TGN or endosomes (4), sites involved in secondary envelopment. Thus, it is possible that the gB cytoplasmic tail might contribute to secondary envelopment, i.e., collaborating with other glycoproteins, such as gD. Related to observations that gD and gE/gI participate in secondary envelopment, a mutant lacking gB, gE, and gI was not substantially defective in secondary envelopment (18). Here, we constructed an HSV mutant lacking both gB and gD in order to investigate effects on different stages of virus egress. Loss of both gB and gD did not obviously reduce nuclear egress beyond the defects observed with a gB− mutant. However, the gB−/gD− double mutant exhibited substantial defects in late stages of virus egress: (i) increased numbers of unenveloped cytoplasmic capsids, (ii) more partially enveloped particles in the cytoplasm, and (iii) fully enveloped particles with aberrant structures, apparently related to increased quantities of tegument proteins. Thus, gB apparently functions in the combined processes of tegument assembly and secondary envelopment along with gD and gE/gI.
MATERIALS AND METHODS
Cells.
HaCaT cells represent a human keratinocyte line (5) and were maintained in Dulbecco's minimum essential medium (DMEM) (Invitrogen) with 8 to 10% fetal bovine serum (FBS) (ISC BioExpress, Kayesville, UT). Monkey Vero cells were maintained in DMEM with 7 to 8% FBS. VD60 cells were derived from Vero cells by transfection with HSV gD and a plasmid producing resistance to histidinol, pSV2His (34), and were propagated in DMEM lacking histidine and containing 0.2 to 0.4 mM histidinol (Sigma). A subclone of VD60 cells, denoted VD60R, was made more recently and used in subsequent experiments. VB38 cells were derived from Vero cells by transfection with a plasmid containing the gB gene and pSV2His (18) and were propagated in DMEM lacking histidine (HyClone) and containing 0.2 to 0.4 mM histidinol. Cells expressing both gB and gD were initially constructed by cotransfecting VD60R cells with a plasmid containing HSV gB coding sequences, pTAM4 (pUC19 containing a 4.7-kbp KpnI insert from the BamHI G fragment of HSV type 1 [HSV-1] strain KOS, containing 1,653 bp upstream of the gB start codon and 349 bp downstream of the stop codon), and pSV2Neo. Cell clones resistant to 150 to 300 μg/ml of G418 were picked using cloning rings and screened for their capacity to complement HSV-1 KO82, which contains a stop codon in gB coding sequences (9). A second cell line, VD60/gB47 cells, expressing both gB and gD, was constructed by cotransfecting VD60R cells with pVF30 (containing the entire BamHI G fragment of HSV-1 strain KOS, encompassing the 4,838 bp upstream of the gB start site and 492 bp downstream of the stop codon) and pSV2Neo and selecting clones in 150 to 300 μg/ml G418. VD60/gB47 and VD60/gB97 cells were propagated in DMEM lacking histidine and containing 8% FBS, 150 to 250 μg/ml G418, and 0.3 mM histidinol.
Viruses.
A bacterial artificial chromosome (BAC) clone containing HSV-1 strain F DNA in which gD coding sequences were replaced with a kanamycin resistance gene cassette, BACgD-kan, was described previously (17). A linear PCR product in which galK sequences were flanked by 50 nucleotides (nt) of DNA encompassing sequences upstream and downstream of the gB coding sequences (60) was introduced into SW102 bacteria containing BACgD-kan, and lambda Red recombination promoted replacement of wild-type gB sequences with galK as described previously (56). galK-positive BAC clones were selected using minimal medium containing galactose as described previously (56). The presence of galK within the gB gene was confirmed by PCR and by sequencing BAC DNAs upstream and downstream of the insertion points into the gB and gD genes. The resulting BACgD-kan/gB-galK DNA was transfected into VD60/gB97 cells as described previously (16), producing virus F-BAC gD-kan/gB-galK, denoted F-BAC gB−/gD− herein. Other HSV-1 mutants lacking gD, F-BAC gD-kan (17) and vRR1097 (44), were propagated on VD60R cells (34). HSV recombinants lacking gB, F-BAC gB− (18) and KO82 (9), were propagated on VB38 cells that express gB (18). F-BAC is a virus derived from a BAC that expresses all HSV glycoproteins (16, 26), considered wild type for purposes of this study, and was propagated on Vero cells.
Radiolabeling of virus-infected cells or extracellular virus particles.
Vero, VB38, or VD60/gB47 cells were infected with various HSVs; then, after 5 to 6 h, the cells were washed with DMEM lacking methionine and cysteine and then incubated in DMEM containing [35S]methionine-cysteine (50 μCi/ml) for 2 to 3 h. Cell extracts were made using 50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate containing 2 mg/ml bovine serum albumin and 0.5% phenylmethylsulfonyl fluoride (NP-40–DOC buffer) (58). To obtain radiolabeled extracellular HSV particles, HaCaT cells were infected with 10 PFU/cell of F-BAC, F-BAC gD-kan, or F-BAC gB−/gD− for 6 h; then, the cells were washed 3 times with DMEM lacking methionine and cysteine and incubated in DMEM containing [35S]methionine-cysteine (150 μCi/ml). Thirteen hours after infection, the medium was supplemented with 6.6% of the normal concentration of methionine and cysteine. Cell culture supernatants were harvested at 24 to 28 h postinfection. The cell culture supernatants were clarified by centrifugation at low speed (1,000 × g). The virus was pelleted by centrifugation through a 9-ml cushion of dextran-10 (density, 1.052 gm/cm3) in an SW32 Beckman rotor at 28,000 rpm for 60 min. Virus pellets were resuspended in NP-40–DOC buffer.
Immunoprecipitation of viral glycoproteins.
Radiolabeled supernatant virus particles or cells dissolved in NP-40–DOC buffer were centrifuged at 40,000 rpm in a Beckman benchtop ultracentrifuge for 40 min to remove insoluble proteins. gB and gD were immunoprecipitated from cell extracts using rabbit antiserum R68, specific for gB, or R45, specific for gD (27) (kindly provided by Roz Eisenberg and Gary Cohen). Detergent (NP-40–DOC) extracts of extracellular virus particles were incubated with protein A-Sepharose for 90 min to remove nonspecific proteins, and then the protein A-Sepharose was removed by centrifugation. These extracts of virus particles were then mixed with 3 anti-gH monoclonal antibodies (MAbs), LP11 (20), 52S, and 53S (48), or with 2 MAbs specific for gE, 3114 and II-481 (28, 43), for 60 to 90 min; then, protein A-Sepharose was added for an additional 2 h. The protein A-Sepharose was washed 3 times with NP-40–DOC buffer, and proteins were eluted by boiling in buffer containing 2% sodium dodecyl sulfate and 2% β-mercaptoethanol and then subjected to electrophoresis using polyacrylamide gels as described previously (58).
Electron microscopy.
HaCaT, Vero, or VD60/gB47 cells were infected using 10 to 20 PFU/cell of wild-type HSV-BAC derived from Vero cells or, in the case of gB− and gD− mutants, viruses derived from complementing cells (VD60R, VB38, or VD60/gB47 cells). After 18 to 28 h, the cells were fixed in Ito and Karnovsky's fixative (1.6% paraformaldehyde, 2.5% glutaraldehyde, and 0.5% picric acid in 0.1 M sodium cacodylate) for 20 to 30 min. The cells were washed 3 times with phosphate-buffered saline and stored overnight at 4°C. Samples were postfixed in 1.5% osmium tetroxide, rinsed, and then postfixed in 4% paraformaldehyde.
Samples were dehydrated in a graded acetone series and embedded in epoxy resin, and ultrathin sections were double stained in uranyl acetate and lead citrate and viewed with a Philips EM300 electron microscope as described previously (15).
RESULTS
Cell lines expressing both gB and gD.
HSV gB and gD are both essential for virus entry, and thus it was necessary to produce cells expressing both gD and gB in order to propagate an HSV gB−/gD− double mutant. VD60 cells had been constructed to complement HSV gD− mutants by cotransfection of Vero cells with pSV2His (conferring resistance to histidinol) and a plasmid with the gD gene (34). This particular cell clone was rare, 1 of approximately 1,000 clones that were resistant to histidinol, and contained approximately 110 copies of the gD gene. The VD60 clone was the only clone that expressed levels of gD sufficient to complement HSV gD-null mutant viruses, i.e., plaques of relatively normal size after 2 days. Other clones with only 10 copies of the gD gene did not allow plaque formation with HSV gD mutants. To construct cells capable of expressing gB and gD, VD60R cells (a subclone of VD60 cells) were initially cotransfected with pSV2Neo and a plasmid (pTAM4) including a smaller, 4.6-kbp KpnI fragment containing 1,653 bp upstream of the start codon for gB and 349 bp downstream of the stop codon. Several hundred G418-resistant clones were isolated using cloning rings and screened for the capacity to promote plaques when infected with the gB-null mutant KO82 (9) and the gD-null mutant vRR1097 (44). Several of these clones exhibited very small plaques when infected with the gB-null virus, i.e., only 5 to 10 infected cells after 4 days, compared with >300 infected cells with wild-type HSV. One clone, VD60/gB97 cells, was selected for further study. Yields of the gB-null virus grown on VD60/gB97 cells were low, <108 PFU derived from 107 cells. Later, a second cell line, denoted VD60/gB47, was constructed by cotransfecting VD60 cells with pSV2Neo and another plasmid (pVF30) containing 4,838 bp upstream of the gB start site and 492 bp downstream of the stop codon. Again, hundreds of G418-resistant cell clones were screened using gB- and gD-null HSV. Plaques formed by gB-null HSV on one of these clones, denoted VD60/gB47 cells, included approximately 50 cells after 4 days, compared with only 5 cells with VD60/gB97 cells. VD60/gB47 cells complemented gD-null viruses well. Complementation of HSV gB− mutants by VD60/gB47 cells was also better, i.e., yields of gB-null mutants were 2 × 108 to 4 × 108 PFU (produced by 107 cells), but plaques were much smaller than those of gB− mutants on VB38 cells (which express gB) or F6/gB12 cells (which express both gB and gH) (18).
Construction and characterization of an HSV gB−/gD− double mutant.
BACgD-kan is a bacterial artificial chromosome copy of HSV strain F DNA in which the gD coding sequences were replaced with a kanamycin resistance gene cassette (17). A PCR product containing galK sequences flanked by gB sequences upstream and downstream of the gB coding sequences was produced (60). This PCR product was introduced into bacteria containing BACgD-kan, and lambda Red recombination replaced the wild-type gB sequences with galK sequences in BACgD-kan, producing BACgD-kan/gB-galK. BACgD-kan/gB-galK was transfected into VD60/gB97 cells, and cytopathic effect was observed in the cells after 8 days, but this effect did not spread well. The cells were harvested and sonicated, and the virus initially expanded on VD60/gB97 cells, which showed cytopathic effect involving approximately 20% of the cells. The virus produced by this transfection was subsequently expanded using the second complementing cell line VD60/gB47, and the virus isolate F-BAC gB−/gD− formed plaques including about 50 cells on VD60/gB47 cells after 4 days.
To assess the expression of gB and gD, Vero, VD60/gB47 (which express gB and gD), and VB38 (which express gB but not gD) cells were infected with HSV gB−/gD− or F-BAC (wild-type HSV); then, the cells were labeled with [35S]methionine-cysteine, and gB or gD was immunoprecipitated using rabbit polyclonal antibodies. F-BAC expressed gB and gD in Vero, VB38, and VD60/gB47 cells, as expected (Fig. 2 A and B). There was no expression of gB and gD in F-BAC gB−/gD−-infected Vero cells (Fig. 2, lanes 5 and 11). F-BAC gB−/gD−-infected VD60/gB47 cells expressed gD (Fig. 2B, lane 10) in quantities similar to that produced in wild-type-F-BAC-infected VD60/gB47 cells (Fig. 2B, lane 7). Expression of gB in F-BAC gB−/gD−-infected VD60/gB47 cells was substantially lower (Fig. 2A, lane 4) than that of gB in F-BAC gB−/gD−-infected VB38 cells (Fig. 2A, lane 6). This observation of reduced expression of gB in VD60/gB47 cells compared with expression in the other gB complementing cell line likely explains the reduced titers and slower plaque formation of F-BAC gB−/gD− growing on VD60/gB47 cells. It was puzzling to us that we could produce VB38 and F6/gB12 cells that express gB well (18) but not cells that express both gB and gD (derived from VD60 cells). This may relate to the use of different drug selection protocols, as VB38 cells were produced by transfection with pSV2His, which provides resistance to histidinol, and there is evidence for amplification of HSV genes following application of increased histidinol concentrations (18, 34). Here, VD60 cells (which were already resistant to histidinol) were transfected with the gB gene and pSV2Neo, and it was not possible to grow these cells in increased concentrations of G418 with hopes of amplifying HSV genes. Although we characterized 400 to 500 clones, none complemented F-BAC gB−/gD− to the extent that normal-sized plaques formed, higher yields of virus were produced, or cells expressed normal levels of gB. This said, it was possible to grow stocks of F-BAC gB−/gD− with titers ranging from 1 × 108 to 4 × 108 PFU (total virus derived from 107 cells). However, note that in Vero cells infected with 10 PFU/cell of F-BAC gB−/gD− (derived from VD60/gB47 cells), the cytopathic effect was substantially slower than that in Vero cells infected with F-BAC or F-BAC gD-kan, so that most electron microscopy was done at 24 to 28 h for F-BAC gB−/gD− infection, compared with 19 to 21 h for F-BAC or F-BAC gD-kan infection.
Fig. 2.
Characterization of gB and gD expressed in noncomplementing and complementing cells. Vero cells (which do not express HSV glycoproteins), VB38 cells (which express gB) (columns labeled “38”), and VD60/gB47 cells (which express gB and gD) (columns labeled “47”) were infected with wild-type (w.t.) F-BAC or F-BAC gB−/gD−. After 5 to 6 h of infection, the cells were radiolabeled with [35S]methionine-cysteine for 2 to 3 h; then, cell extracts were made using NP-40–DOC buffer. gB or gD was immunoprecipitated from cell extracts using rabbit polyclonal antibodies (R68, specific for gB, and R45, specific for gD), and proteins were subjected to electrophoresis in 6% (gB) or 10% (gD) polyacrylamide gels. Molecular mass markers (in kilodaltons) are shown on the left side of the gels.
Defects in HSV gB−/gD− egress from Vero cells.
To investigate whether F-BAC gB−/gD− was defective in virus egress, infected Vero cells were characterized by transmission electron microscopy. At 2 to 3 h after infection, no virus particles were observed, ruling out input virus. After 24 to 28 h, in and around the nuclear envelope, no obvious defects were observed (Fig. 3 A to C and data not shown). Specifically, there were not large numbers of enveloped virus particles accumulated in the perinuclear spaces, as had been observed with Vero cells infected with the gB−/gH− mutant (15). We previously observed more-subtle defects in nuclear egress involving HSV mutants lacking just gB (18). However, with F-BAC gB−/gD−, there was no further increase in these perinuclear particles beyond that observed with a gB-null mutant (not shown).
Fig. 3.
Electron microscopy of Vero cells infected with F-BAC gB−/gD−. Vero cells were infected with F-BAC gB−/gD− for 24 to 28 h and then fixed, stained, and sectioned for electron microscopic examination. N, nucleus.
In contrast to nuclear events, egress of F-BAC gB−/gD− from the cytoplasm to cell surfaces was aberrant. Three apparently related egress defects were observed in the cytoplasm of F-BAC gB−/gD−-infected Vero cells. First, there were significantly increased numbers of unenveloped cytoplasmic capsids (Fig. 3A to C). In a minority of these cells, these capsids formed relatively large aggregates in the cytoplasm (Fig. 3C) that were similar to those previously observed in Vero cells infected with HSV mutants lacking gD, gE, and gI (15). We stress that the larger capsid aggregates were relatively rare and that, much more often, capsids appeared in groups of 2 to 10 or as single capsids often distributed uniformly in the cytoplasm and interspersed with some partially or fully enveloped particles (Fig. 3A; also see Fig. 5A and B). This was in contrast to observations with HSV gD−/gE−/gI− mutants, which accumulated almost entirely unenveloped capsids in aggregates (15). Vero cells infected with wild-type F-BAC displayed primarily enveloped particles on cell surfaces, and there were few unenveloped capsids in the cytoplasm (Fig. 4 A and B), as reported previously (15). The second phenotype observed in F-BAC gB−/gD−-infected Vero cells was the accumulation of capsids that were associated with cytoplasmic membranes, often with the membranes partially wrapped around the capsids (arrows in Fig. 5 A and B). These cytoplasmic membranes appeared similar to the Golgi apparatus or TGN, where secondary envelopment probably occurs (16, 53, 55, 59). It is relatively unusual to observe HSV cytoplasmic capsids in the process of secondary envelopment, i.e., with partial wrapping of membranes around cytoplasmic capsids. The third phenotype involved moderately increased numbers of enveloped particles in the cytoplasm of F-BAC gB−/gD−-infected Vero cells, including particles that exhibited a more densely stained and thicker tegument layer (asterisks in Fig. 5B, C, and D). Some of these F-BAC gB−/gD− enveloped virions displayed denser and misshapen tegument and envelopes that were not spherical; instead, particles were elongated, in some cases with blebs extending from one side, as if envelopment was aberrant or incomplete (Fig. 5D). F-BAC gD-kan-infected cells displayed primarily cell surface enveloped virions (Fig. 6 A and B), although increased unenveloped cytoplasmic capsids were observed in some cells (Fig. 6B).
Fig. 5.
Electron microscopy of Vero cells infected with F-BAC gB−/gD−. Vero cells were infected with F-BAC gB−/gD− for 24 to 28 h and then fixed, stained, and sectioned for electron microscopic examination. Arrows point to partially enveloped particles. Asterisks are adjacent to morphologically aberrant enveloped virus particles with denser tegument and misshapen tegument and envelopes.
Fig. 4.
Electron microscopy of Vero cells infected with wild-type (w.t.) F-BAC. Vero cells were infected with F-BAC for 19 to 21 h and then fixed, stained, and sectioned for electron microscopic examination.
Fig. 6.
Electron microscopy of Vero cells infected with F-BAC gD-kan. Vero cells were infected with F-BAC gD-kan (gD−) for 19 to 21 h and then fixed, stained, and sectioned for electron microscopic examination.
We analyzed virus particles on the surfaces of F-BAC gB−/gD−-infected Vero cells and compared them to particles on the surfaces of F-BAC- and F-BAC gD-kan-infected Vero cells. These analyses focused on the morphologies of particles at the end of the egress pathway and avoided the cytoplasm, which obscured details of morphology. The surfaces of F-BAC gB−/gD−-infected cells contained markedly reduced numbers of virus particles compared with those on the surfaces of F-BAC- and F-BAC gD-kan-infected cells. However, it was possible to find examples of F-BAC gB−/gD− cell surface particles. Some of these displayed increased tegument diameter and density and oblong shapes (Fig. 7 A, B, and C). It was difficult to quantify these changes in terms of distortion; there was a continuum of structures between normal and aberrant, but it was also clear that many F-BAC gB−/gD− particles were not obviously morphologically different from wild-type-HSV enveloped particles. In different experiments, morphologically aberrant cell surface F-BAC gB−/gD− particles ranged from 10 to 35%. Wild-type HSV (F-BAC) and the gD mutant F-BAC gD-kan produced cell surface particles with the more familiar spherical morphology and with less density associated with tegument (Fig. 7D and E).
Fig. 7.
Electron microscopy of virus particles on the surfaces of Vero cells infected with F-BAC gB−/gD−, F-BAC, or F-BAC gD-kan. Vero cells were infected with F-BAC gB−/gD−, F-BAC (w.t.), or F-BAC gD-kan (gD−) for 19 to 28 h and then fixed, stained, and sectioned for electron microscopic examination.
We counted virus particles on the surfaces of cells and in the cytoplasm to quantify these differences (Table 1). Given that there were no obvious defects in nuclear egress with the gB−/gD− mutant, the focus was on unenveloped cytoplasmic capsids, partially and fully enveloped particles in the cytoplasm, and enveloped particles on cell surfaces. Most (74%) particles observed in wild-type-F-BAC-infected Vero cells accumulated as enveloped cell surface particles. Moreover, there were fewer unenveloped (11%), partially enveloped (0.2%), and enveloped (15%) capsids in the cytoplasm of F-BAC-infected Vero cells. In contrast, F-BAC gB−/gD−-infected Vero cells contained large numbers of unenveloped cytoplasmic capsids, amounting to about half the total particles observed in the cytoplasm and on cell surfaces. There were 75 times more partially enveloped capsids in the cytoplasm in F-BAC gB−/gD−-infected cells than in F-BAC-infected cells. Cell surface particles were reduced 5-fold comparing F-BAC gB−/gD− with wild-type F-BAC. Cell surface enveloped particles made up the majority of particles in F-BAC gD-kan-infected cells, although there were increases in unenveloped, partially enveloped, and enveloped cytoplasmic capsids.
Table 1.
Numbers of unenveloped, partially enveloped, and fully enveloped particles in Vero and VD60/gB47 cells infected with F-BAC gB−/gD−a
| Cell type and virus | No. (%) of: |
Total | |||
|---|---|---|---|---|---|
| Unenveloped cytoplasmic capsids | Partially enveloped cytoplasmic capsids | Enveloped cytoplasmic particles | Enveloped cell surface particles | ||
| Vero cells | |||||
| F-BAC (w.t.) | 140 (11) | 3 (0.2) | 191 (15) | 939 (74) | 1,273 |
| F-BAC gB−/gD− | 631 (49) | 201 (15) | 271 (21) | 194 (15) | 1,297 |
| F-BAC gD− | 289 (19) | 72 (5) | 325 (21) | 836 (55) | 1,522 |
| F-BAC gB− | 149 (15) | 2 (0.2) | 141 (14) | 714 (71) | 1,006 |
| VD60/gB47 cells | |||||
| F-BAC (w.t.) | 162 (18) | 0 | 90 (10) | 647 (72) | 899 |
| F-BAC gB−/gD− | 225 (20) | 7 (0.6) | 145 (13) | 738 (66) | 1,115 |
Vero or VD60/gB47 cells were infected with wild-type (w.t.) F-BAC, F-BAC gB−/gD−, F-BAC gD-kan (F-BAC gD−), or F-BAC gB− for 19 to 28 h and then fixed and processed for electron microscopy. Virus particles were counted in 10 to 20 representative cells.
One concern with any herpesvirus mutant is that there is the potential for the accumulation of secondary mutations, separate from the gB and gD gene mutations. Often, repaired versions of virus mutants are constructed to deal with this problem. Here, we infected VD60/gB47 cells (which express both gB and gD) with F-BAC gB−/gD− and observed relatively normal egress. Enveloped cell surface F-BAC gB−/gD− particles accumulated on VD60/gB47 surfaces, and there were fewer cytoplasmic unenveloped capsids (Table 1). These results demonstrated that defects in egress were related to loss of gB and gD and not secondary mutations.
Defects in HSV gB−/gD− egress from HaCaT keratinocytes.
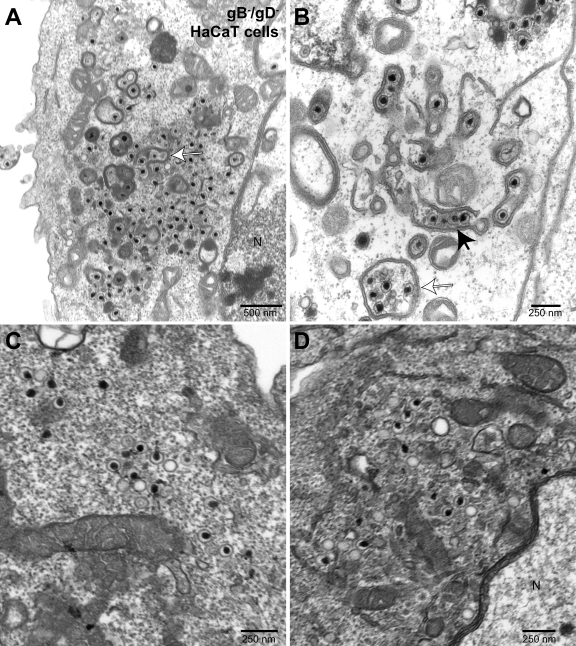
In previous studies, we observed that certain aspects of the egress of HSV mutants differed between monkey Vero cells and HEC-1A and HaCaT human epithelial cells (15, 18, 58). For example, an HSV mutant lacking both gB and gH produced numerous herniations in HaCaT cells, but these were less common in Vero cells (18). Moreover, HaCaT cells are a human keratinocyte line, making them especially appropriate for studies of HSV. We compared aspects of virus egress in HaCaT cells infected with F-BAC, F-BAC gD-kan, or F-BAC gB−/gD−. HaCaT cells infected with wild-type F-BAC displayed primarily cell surface enveloped particles (Fig. 8 A and B), although there were also some enveloped cytoplasmic particles (Fig. 8A, arrow). Unenveloped cytoplasmic capsids were relatively rare in HaCaT cells infected with wild-type F-BAC, compared with those in F-BAC-infected Vero cells (Tables 1 and 2). In HaCaT cells infected with F-BAC gB−/gD−, there were not substantial defects in nuclear egress (Fig. 9 A to D and data not shown) beyond more-minor defects we previously observed with an HSV gB− mutant (18). However, there were marked accumulations of unenveloped, partially enveloped, and fully enveloped capsids in the cytoplasm of F-BAC gB−/gD−-infected HaCaT cells (Fig. 9A to D; Table 2). Related to these observations, many fewer cell surface virions were observed (Fig. 9A; Table 2). Interestingly, in some cells there were numerous F-BAC gB−/gD− capsids bound onto the surfaces of cytoplasmic membranes that were partially wrapped around the capsids (Fig. 9B, filled arrow). In other cases, 2 to 5 capsids were enclosed by double membrane vesicles (Fig. 9A and B, open arrows). Such structures were exceedingly rare in wild-type-HSV-infected HaCaT cells but much more common in F-BAC gB−/gD−-infected HaCaT cells (Table 2). In some F-BAC gB−/gD−-infected HaCaT cells, there was accumulation of aggregates of capsids, but more often these unenveloped capsids were not massively aggregated; instead, capsids were distributed more uniformly in the cytoplasm (Fig. 9C and D). This was somewhat surprising and very unlike the distribution of cytoplasmic capsids produced by HSV gD−/gE−/gI− mutants (15). Counts of virus particles in the cytoplasm and on cell surfaces indicated a 10-fold increase in cytoplasmic capsids, a 56-fold increase in partially enveloped particles, a 3-fold increase in cytoplasmic enveloped particles, and a 6-fold decrease in cell surface enveloped particles in the gB−/gD− mutant compared with wild-type F-BAC (Table 2).
Fig. 8.
Electron microscopy of HaCaT keratinocytes infected with wild-type F-BAC. HaCaT cells were infected with F-BAC (w.t.) for 19 to 21 h and then fixed, stained, and sectioned for electron microscopy. The arrow points to an enveloped virus particle in the cytoplasm.
Table 2.
Numbers of unenveloped, partially enveloped, and fully enveloped particles in F-BAC gB−/gD−-infected HaCaT cellsa
| Virus | No. (%) of: |
Total | |||
|---|---|---|---|---|---|
| Unenveloped cytoplasmic capsids | Partially enveloped cytoplasmic capsids | Enveloped cytoplasmic particles | Enveloped cell surface particles | ||
| F-BAC (w.t.) | 51 (3.2) | 4 (0.25) | 202 (13) | 1,324 (84) | 1,581 |
| F-BAC gB−/gD− | 612 (36) | 234 (14) | 618 (36) | 243 (14) | 1,707 |
| F-BAC gD− | 227 (15) | 62 (4) | 254 (17) | 967 (64) | 1,510 |
| F-BAC gB− | 108 (9) | 2 (0.17) | 139 (11) | 963 (79) | 1,212 |
HaCaT cells were infected with wild-type (w.t.) F-BAC, F-BAC gB−/gD−, F-BAC gD-kan (F-BAC gD−), or F-BAC gB− for 19 to 28 h and then fixed and processed for electron microscopy. Virus particles were counted in 10 to 20 representative cells.
Fig. 9.
Electron microscopy of HaCaT cells infected with F-BAC gB−/gD−. HaCaT cells were infected with F-BAC gB−/gD− for 24 to 28 h and then fixed, stained, and sectioned for electron microscopy. The filled arrow in panel B points to a row of partially enveloped capsids, i.e., partially wrapped with a double membrane. Open arrows in panels A and B point to multiple capsids apparently fully wrapped in a double membrane. N, nucleus.
F-BAC gD-kan-infected HaCaT cells displayed predominantly cell surface enveloped particles (Fig. 10 A and B; Table 2). However, a fraction of these cells also showed accumulation of enveloped and, especially, unenveloped capsids in the cytoplasm (Fig. 10C; Table 2). The accumulation of unenveloped capsids in F-BAC gD-kan-infected HaCaT cells was more apparent, because F-BAC-infected HaCaT cells had relatively few unenveloped capsids (Table 2). Unenveloped cytoplasmic capsids were more common in F-BAC-infected Vero cells (Table 1). In addition, there were substantially more partially enveloped particles and slightly fewer numbers of cell surface particles in F-BAC gD-kan-infected HaCaT cells than in F-BAC-infected cells (Table 2). Similar results were observed with a second HSV gD-null mutant, vRR1097 (44) (not shown). These defects in egress of an HSV gD− mutant in HaCaT cells were larger than those previously reported for a gD-null mutant in HEC-1A human epithelial cells (15). However, it was also clear that defects in egress of F-BAC gB−/gD− in HaCaT cells were more profound than those of F-BAC gD-kan in the same cells. F-BAC gB−, a mutant lacking gB, did not show major defects in cytoplasmic egress (Table 2).
Fig. 10.
Electron microscopy of HaCaT cells infected with F-BAC gD-kan. HaCaT cells were infected with F-BAC gD-kan (gD−) for 19 to 21 h and then fixed, stained, and sectioned for electron microscopy.
We compared the morphology of F-BAC gB−/gD− particles on HaCaT cell surfaces with those of wild-type and F-BAC gD-kan cell surface particles. Figure 11 A shows the normal morphology of virus particles present on F-BAC-infected cells. Morphologically aberrant enveloped particles were observed on HaCaT cells infected with F-BAC gB−/gD− (Fig. 11B). Again, these particles exhibited oblong morphologies and denser or expanded tegument layers. As with F-BAC gB−/gD−-infected Vero cells, these morphologically aberrant F-BAC gB−/gD− particles did not represent the majority of cell surface particles; many particles appeared normal, but a significant fraction exhibited this altered morphology. F-BAC gD-kan particles were also relatively normal (Fig. 11C).
Fig. 11.
Electron microscopy of virus particles on the surfaces of HaCaT cells infected with F-BAC gB−/gD−, F-BAC gD-kan, or F-BAC. HaCaT cells were infected with F-BAC gB−/gD−, F-BAC gD-kan (gD−), or F-BAC (w.t.) for 19 to 28 h and then fixed, stained, and sectioned for electron microscopic examination.
Reduced numbers of particles secreted into cell culture supernatants and presence of gE/gI in particles.
To provide a second measure of the defects in HSV egress from F-BAC gB−/gD−-infected cells, we radiolabeled infected cells with [35S]methionine-cysteine and then collected cell culture supernatants and pelleted extracellular virus particles through dextran-10 cushions. Virus particles were disrupted with detergents, and viral proteins were analyzed by using SDS-polyacrylamide gels. When the total virus pellet was analyzed by electrophoresis, there were markedly reduced amounts of HSV proteins derived from F-BAC gB−/gD− supernatant particles, compared to both F-BAC and F-BAC gD-kan particles (Fig. 12 A). Based on the intensity of the VP5 band, we estimated that there was 4- to 5-fold less virus present in the F-BAC gB−/gD− sample. This was in line with 5-fold-reduced cell surface virus particles produced by F-BAC gB−/gD− (Table 2). F-BAC gB−/gD− extracellular particles were also analyzed for the presence of gE/gI and gH. Observations that HSV gD−/gE−/gI− mutants were defective in secondary envelopment suggested that loss of gB and gD could have indirect effects, altering the subcellular distribution of gE/gI, so that secondary envelopment was reduced. Given the decreased numbers of extracellular particles produced by F-BAC gB−/gD−-infected HaCaT cells, we loaded into the gel lanes ≈8 times more material immunoprecipitated from F-BAC gB−/gD−-infected cells than from F-BAC- and F-BAC gD-kan-infected cells. Related to this increased quantity of material loaded, we observed moderately more gH immunoprecipitated from F-BAC gB−/gD− supernatant virus particles (Fig. 12B). Importantly, the quantities of gE and gI were also moderately higher than with virus derived from F-BAC- and F-BAC gD−-infected cells (Fig. 12C). Therefore, loss of gB and gD does not significantly alter incorporation of gE/gI into enveloped virus particles.
Fig. 12.
Amounts of cell culture supernatant virus produced by F-BAC gB−/gD− and characterization of these particles for gE/gI and gH. HaCaT cells were infected with 10 PFU/cell of F-BAC gB−/gD−, F-BAC gD-kan, or F-BAC and radiolabeled from 6 to 13 h postinfection (p.i.); then, cell culture supernatants were harvested at 24 to 28 h p.i. Virus particles were partially purified by centrifugation through dextran-10 cushions, and then particles were resuspended in NP-40–DOC buffer. (A) Equal amounts of the total virus preparations were subjected to electrophoresis following addition of 2% sodium dodecyl sulfate and 2% β-mercaptoethanol and boiling. (B) gH was immunoprecipitated from NP-40–DOC extracts of the virus particles using MAbs LP11, 52S, and 53S. (C) gE/gI was immunoprecipitated from NP-40–DOC extracts of virus particles using MAbs 3114 and II-481. For panels B and C, note that 4-fold more of the supernatant was immunoprecipitated from F-BAC gB−/gD−-infected cells than from F-BAC- and F-BAC gD-kan-infected cells and 2-fold more immunoprecipitated material was loaded (totaling 8-fold more material). Molecular mass markers (in kilodaltons) are shown on the left side of the gels. The relative amounts of gE, gI, and gH are shown below panels B and C.
DISCUSSION
HSV membrane glycoproteins play important roles in several stages of virus egress, causing rearrangement of cellular membranes and promoting both envelopment and deenvelopment (Fig. 1). To date, there is not evidence that HSV glycoproteins are important for the first step in egress, primary envelopment. No single or double mutants have shown defects in primary envelopment, although one might argue that appropriate double or triple glycoprotein mutants have yet to be made. HSV glycoproteins gB and gH/gL act in a redundant fashion to promote the second step of egress, deenvelopment at the ONM (18, 60, 61). gB and gH/gL are also involved in the much better understood process of entry fusion, which also involves gD and gD receptors (1), but the role of gD in deenvelopment was unclear when we began these studies. Given the redundancy in deenvelopment (18), we constructed an HSV recombinant unable to express gB and gD. Propagating this gB−/gD− virus required complementing cells (which expressed gB and gD), and we encountered major difficulties in constructing these cells, compared with other cells that complemented gB− single or gB−/gH− double mutants (18). The HSV gB−/gD− mutant exhibited no obvious defects in nuclear egress beyond that observed with a gB-null virus. These results make it less likely that gD and gD receptors are important for deenvelopment. However, it remains possible that a gD−/gH− double mutant might be inhibited in deenvelopment.
Secondary envelopment is the third phase of HSV egress involving membrane rearrangements (Fig. 1). HSV mutants lacking gD and gE/gI or gD and just the cytoplasmic domain of gE accumulated capsids in the cytoplasm, often in large arrays that appeared to be immersed in tegument (15). One explanation for these data was that the cytoplasmic domains of gD, gE, and gI anchor tegument-coated capsids onto TGN-derived membranes to promote secondary envelopment. Arguing against this hypothesis, there was a report of an HSV mutant lacking the gD cytoplasmic domain and all of gE that did not have defects in egress (32), arguing that the gD cytoplasmic domain is not important in secondary envelopment. On the other hand, an HSV recombinant lacking just the cytoplasmic domain of gD and expressing wild-type gE/gI displayed moderately reduced replication and smaller plaques, suggesting that the gD cytoplasmic domain plays some role in HSV replication (19). Moreover, we observed more-minor or moderate defects in cytoplasmic egress from HaCaT cells with HSV gD− mutants here. There is substantial evidence that tegument proteins, such as VP22 and UL11, can interact with HSV gE/gI and gD and that interactions between the gE/gI and gD cytoplasmic domains and VP22 and UL11 might bridge capsids onto membranes (13, 17, 35, 41, 42, 51). VP22 and UL11 can also interact directly with membranes, i.e., without other HSV proteins (8, 14, 35, 36, 41, 51). Therefore, it is also possible that VP22 and UL11 can localize to TGN membranes and then subsequently interact with the cytoplasmic or transmembrane domains of gE/gI and gD. Before the present studies, we believed that it was primarily glycoproteins gD and gE/gI that mediated secondary envelopment. However, there was the possibility that other glycoproteins were also involved, because cell surface particles were observed with HSV gD−/gE−/gI− mutants, albeit reduced 5- to 10-fold (15).
HSV gB possesses a large cytoplasmic domain that is essential for membrane fusion (47) and that contains several sorting motifs promoting endocytosis and localization to the TGN (4), where secondary envelopment occurs. Previous studies of HSV gB− mutants did not describe defects in cytoplasmic stages of virus egress (9, 18). However, based on previous experience, redundancy was a real possibility. An HSV recombinant lacking gB, gE, and gI showed some modest defects in egress (18). In contrast, the HSV gB−/gD− mutant described here accumulated 5- to 12-fold more unenveloped capsids, 50- to 70-fold more partially enveloped particles, and 5-fold fewer cell surface particles than did wild-type HSV. On closer examination, there were also more-subtle defects (increased cytoplasmic and partially enveloped capsids) observed in the egress of HSV gD− mutants. However, the phenotype of the gD-null mutants was not nearly as severe as that of the gB−/gD− mutant. These observations suggest that gB contributes to some aspect of secondary envelopment, although gD appears to be able to substitute largely in this process.
One surprising result of these studies was that gE/gI was not sufficient for secondary envelopment in cells infected with the gB−/gD− mutant. Previous studies suggested that either gD or gE/gI would largely suffice for secondary envelopment, although defects with gD− or gE− mutants were noted (15). One theoretical possibility is that gB may alter the intracellular distribution or functions of gE/gI, so that the gB−/gD− mutant also has defects in gE/gI. Arguing against this, extracellular particles produced by the gB−/gD− mutant did not exhibit reduced gE/gI. However, there were markedly reduced numbers of these extracellular particles secreted from gB−/gD− mutant-infected cells. Thus, it is very possible that loss of gB could alter how gE/gI traffics, perhaps reducing TGN localization of gE/gI, so that the contribution of gE/gI to secondary envelopment is reduced substantially. Another model suggests that the cytoplasmic domain of gB directly contributes to secondary envelopment by interacting with tegument-coated capsids to anchor capsids onto membranes. In cells infected with the HSV gD−/gE−/gI− mutant, the contribution of the gB cytoplasmic domain may be insufficient for efficient secondary envelopment, or gB may be mislocalized. These models require testing by characterization of the subcellular localization of glycoproteins and by construction of other mutants lacking just the cytoplasmic domains of (i) gB and gD or (ii) gD, gE, and gI.
There were several observations involving F-BAC gB−/gD− that provide further insights into how HSV glycoproteins promote secondary envelopment. First, we observed substantial numbers of capsids (50- to 70-fold more than in the wild type) that were partially enveloped, often nestled against cytoplasmic membranes. In F-BAC gB−/gD−-infected Vero cells, these partially enveloped capsids tended to consist of a double membrane incompletely wrapped around a single capsid (Fig. 5A and B). In HaCaT cells, there were often numerous capsids that were adjacent to or partially wrapped with (what appeared to be) a single segment of the Golgi apparatus or TGN (Fig. 9B, filled arrow). In other cases, these double membranes were wrapped completely around 2 to 5 capsids (Fig. 9A and B, open arrows). We have rarely observed partially enveloped capsids in the cytoplasm of HaCaT, HEC-1A, and Vero cells infected with wild-type or mutant HSV (15, 18, 29, 34, 59). Unenveloped viral capsids accumulated with a gD−/gE−/gI− mutant without evidence of partial envelopment (15). With wild-type PRV and a frog herpesvirus, partially enveloped virus particles were observed (21, 39, 49, 57). However, these wild-type PRV particles were very different from the gB−/gD− mutant capsids shown in Fig. 9B, which consisted of capsids aberrantly wrapped in a double membrane (where the inner membrane was not tightly wrapped around the capsid). In contrast, the multiple enveloped particles observed in PRV-infected cells consisted of normally assembled enveloped virions inside a much larger membrane vesicle (see Fig. 5 in reference 21).
Second, we observed dispersed or more uniformly distributed capsids in the cytoplasm of F-BAC gB−/gD−-infected HaCaT cells (Fig. 9C and D). This was in contrast to observations involving the HSV gD−/gE−/gI− mutant (15) and the PRV gE−/gM− mutant (6, 7), which accumulated large numbers of capsids in highly aggregated arrays that were apparently immersed in a dense tegument layer. Why capsids accumulated in a more dispersed distribution was not clear. However, these observations, coupled with observations of partially enveloped capsids, suggest that HSV capsids produced by the gB−/gD− mutant may proceed farther down the egress pathway toward secondary envelopment. For example, capsids produced by the gB−/gD− mutant might acquire more of the tegument proteins necessary for envelopment than capsids produced in gD−/gE−/gI− mutant-infected cells. Reduced addition of tegument proteins onto capsids (in the case of the gD−/gE−/gI− mutant) might lead to aggregation in the cytoplasm and reduce interactions with membranes. Testing this might include detailed analyses of tegument proteins bound onto capsids purified from the cytoplasm of F-BAC gB−/gD−-infected cells, compared with capsids from gD−/gE−/gI− mutant-infected cells.
Third, we observed morphologically aberrant, enveloped HSV particles in the cytoplasm of F-BAC gB−/gD−-infected cells, as well as on cell surfaces. These altered gB−/gD− mutant particles were frequently nonspherical, with oblong membranes wrapping capsids (Fig. 5C and D), and some cell surface particles contained denser, thicker, oblong tegument layers (Fig. 7A to C). These observations suggested that tegument was aberrantly assembled with membranes, although envelopment was completed. These three sets of observations (unaggregated cytoplasmic capsids, partially enveloped capsids, and aberrantly assembled enveloped particles) supported the notion that loss of gB and gD alters the combined process of tegumentation and secondary envelopment by acting relatively late. In contrast, gD−/gE−/gI− mutants might be blocked earlier, so that capsids acquire fewer tegument proteins, are more defective for assembly, and frequently aggregate in large masses without membranes. This fits with observations that gD and gE/gI interact with VP22 (13, 17, 35, 41, 42, 51) and that gE/gI affects TGN localization and assembly of VP22 into virions (13, 17, 35, 41, 42, 51). These defects in assembling membranes onto tegument-coated capsids were unlike those of “late domain” retrovirus mutants, such as defects in separation or pinching off from cellular membranes (reviewed in reference 12). We did not frequently observe stalks or bridges between cellular and viral membranes.
In summary, HSV gB collaborates with other membrane glycoproteins, gD and gE/gI, to promote secondary envelopment. The HSV gB−/gD− double mutant exhibited increased numbers of unenveloped capsids in the cytoplasm and partially or aberrantly enveloped particles. Many of these observations were unlike those involving a gD−/gE−/gI− mutant and suggested that the gB−/gD− mutant was blocked downstream of the gD−/gE−/gI− mutant in egress.
ACKNOWLEDGMENTS
We are especially indebted to Michael Webb of the OHSU electron microscopy core facility for his extensive efforts and skill in performing the electron microscopic studies. We also thank Tiffani Howard for creating all of the figures. Adam Vanarsdall finished running all the gels.
This work was supported by grant EY018755 from the National Institutes of Health (to D.C.J.).
Footnotes
Published ahead of print on 16 March 2011.
REFERENCES
- 1. Atanasiu D., Saw W. T., Cohen G. H., Eisenberg R. J. 2010. Cascade of events governing cell-cell fusion induced by herpes simplex virus glycoproteins gD, gH/gL, and gB. J. Virol. 84:12292–12299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baines J. D., Roizman B. 1992. The UL11 gene of herpes simplex virus 1 encodes a function that facilitates nucleocapsid envelopment and egress from cells. J. Virol. 66:5168–5174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baines J. D., Wills E., Jacob R. J., Pennington J., Roizman B. 2007. Glycoprotein M of herpes simplex virus 1 is incorporated into virions during budding at the inner nuclear membrane. J. Virol. 81:800–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beitia Ortiz de Zarate I., Kaelin K., Rozenberg F. 2004. Effects of mutations in the cytoplasmic domain of herpes simplex virus type 1 glycoprotein B on intracellular transport and infectivity. J. Virol. 78:1540–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boukamp P., et al. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106:761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brack A. R., Dijkstra J. M., Granzow H., Klupp B. G., Mettenleiter T. C. 1999. Inhibition of virion maturation by simultaneous deletion of glycoproteins E, I, and M of pseudorabies virus. J. Virol. 73:5364–5372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brack A. R., et al. 2000. Role of the cytoplasmic tail of pseudorabies virus glycoprotein E in virion formation. J. Virol. 74:4004–4016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brignati M. J., Loomis J. S., Wills J. W., Courtney R. J. 2003. Membrane association of VP22, a herpes simplex virus type 1 tegument protein. J. Virol. 77:4888–4898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cai W. Z., Person S., Warner S. C., Zhou J. H., DeLuca N. A. 1987. Linker-insertion nonsense and restriction-site deletion mutations of the gB glycoprotein gene of herpes simplex virus type 1. J. Virol. 61:714–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campadelli G., et al. 1993. Fragmentation and dispersal of Golgi proteins and redistribution of glycoproteins and glycolipids processed through the Golgi apparatus after infection with herpes simplex virus 1. Proc. Natl. Acad. Sci. U. S. A. 90:2798–2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chowdary T. K., et al. 2010. Crystal structure of the conserved herpesvirus fusion regulator complex gH-gL. Nat. Struct. Mol. Biol. 17:882–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Demirov D. G., Freed E. O. 2004. Retrovirus budding. Virus Res. 106:87–102 [DOI] [PubMed] [Google Scholar]
- 13. Duffy C., et al. 2006. Characterization of a UL49-null mutant: VP22 of herpes simplex virus type 1 facilitates viral spread in cultured cells and the mouse cornea. J. Virol. 80:8664–8675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elliott G., Hafezi W., Whiteley A., Bernard E. 2005. Deletion of the herpes simplex virus VP22-encoding gene (UL49) alters the expression, localization, and virion incorporation of ICP0. J. Virol. 79:9735–9745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farnsworth A., Goldsmith K., Johnson D. C. 2003. Herpes simplex virus glycoproteins gD and gE/gI serve essential but redundant functions during acquisition of the virion envelope in the cytoplasm. J. Virol. 77:8481–8494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farnsworth A., Johnson D. C. 2006. Herpes simplex virus gE/gI must accumulate in the trans-Golgi network at early times and then redistribute to cell junctions to promote cell-cell spread. J. Virol. 80:3167–3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Farnsworth A., Wisner T. W., Johnson D. C. 2007. Cytoplasmic residues of herpes simplex virus glycoprotein gE required for secondary envelopment and binding of tegument proteins VP22 and UL11 to gE and gD. J. Virol. 81:319–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farnsworth A., et al. 2007. Herpes simplex virus glycoproteins gB and gH function in fusion between the virion envelope and the outer nuclear membrane. Proc. Natl. Acad. Sci. U. S. A. 104:10187–10192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feenstra V., Hodaie M., Johnson D. C. 1990. Deletions in herpes simplex virus glycoprotein D define nonessential and essential domains. J. Virol. 64:2096–2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Forrester A., et al. 1992. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J. Virol. 66:341–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Granzow H., et al. 1997. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J. Virol. 71:2072–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hannah B. P., et al. 2009. Herpes simplex virus glycoprotein B associates with target membranes via its fusion loops. J. Virol. 83:6825–6836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hannah B. P., Heldwein E. E., Bender F. C., Cohen G. H., Eisenberg R. J. 2007. Mutational evidence of internal fusion loops in herpes simplex virus glycoprotein B. J. Virol. 81:4858–4865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heldwein E. E., Krummenacher C. 2008. Entry of herpesviruses into mammalian cells. Cell. Mol. Life Sci. 65:1653–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heldwein E. E., et al. 2006. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217–220 [DOI] [PubMed] [Google Scholar]
- 26. Horsburgh B. C., Hubinette M. M., Qiang D., MacDonald M. L., Tufaro F. 1999. Allele replacement: an application that permits rapid manipulation of herpes simplex virus type 1 genomes. Gene Ther. 6:922–930 [DOI] [PubMed] [Google Scholar]
- 27. Isola V. J., et al. 1989. Fine mapping of antigenic site II of herpes simplex virus glycoprotein D. J. Virol. 63:2325–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnson D. C., Frame M. C., Ligas M. W., Cross A. M., Stow N. D. 1988. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J. Virol. 62:1347–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson D. C., Webb M., Wisner T. W., Brunetti C. 2001. Herpes simplex virus gE/gI sorts nascent virions to epithelial cell junctions, promoting virus spread. J. Virol. 75:821–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Klupp B., Altenschmidt J., Granzow H., Fuchs W., Mettenleiter T. C. 2008. Glycoproteins required for entry are not necessary for egress of pseudorabies virus. J. Virol. 82:6299–6309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krishnan H. H., Sharma-Walia N., Zeng L., Gao S. J., Chandran B. 2005. Envelope glycoprotein gB of Kaposi's sarcoma-associated herpesvirus is essential for egress from infected cells. J. Virol. 79:10952–10967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee H. C., Chouljenko V. N., Chouljenko D. V., Boudreaux M. J., Kousoulas K. G. 2009. The herpes simplex virus type 1 glycoprotein D (gD) cytoplasmic terminus and full-length gE are not essential and do not function in a redundant manner for cytoplasmic virion envelopment and egress. J. Virol. 83:6115–6124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee S. K., Longnecker R. 1997. The Epstein-Barr virus glycoprotein 110 carboxy-terminal tail domain is essential for lytic virus replication. J. Virol. 71:4092–4097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ligas M. W., Johnson D. C. 1988. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J. Virol. 62:1486–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Loomis J. S., Bowzard J. B., Courtney R. J., Wills J. W. 2001. Intracellular trafficking of the UL11 tegument protein of herpes simplex virus type 1. J. Virol. 75:12209–12219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. MacLean C. A., Dolan A., Jamieson F. E., McGeoch D. J. 1992. The myristylated virion proteins of herpes simplex virus type 1: investigation of their role in the virus life cycle. J. Gen. Virol. 73(Pt. 3):539–547 [DOI] [PubMed] [Google Scholar]
- 37. McMillan T. N., Johnson D. C. 2001. Cytoplasmic domain of herpes simplex virus gE causes accumulation in the trans-Golgi network, a site of virus envelopment and sorting of virions to cell junctions. J. Virol. 75:1928–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mettenleiter T. C. 2004. Budding events in herpesvirus morphogenesis. Virus Res. 106:167–180 [DOI] [PubMed] [Google Scholar]
- 39. Mettenleiter T. C., Klupp B. G., Granzow H. 2009. Herpesvirus assembly: an update. Virus Res. 143:222–234 [DOI] [PubMed] [Google Scholar]
- 40. Milne R. S., Nicola A. V., Whitbeck J. C., Eisenberg R. J., Cohen G. H. 2005. Glycoprotein D receptor-dependent, low-pH-independent endocytic entry of herpes simplex virus type 1. J. Virol. 79:6655–6663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murphy M. A., Bucks M. A., O'Regan K. J., Courtney R. J. 2008. The HSV-1 tegument protein pUL46 associates with cellular membranes and viral capsids. Virology 376:279–289 [DOI] [PubMed] [Google Scholar]
- 42. O'Regan K. J., Brignati M. J., Murphy M. A., Bucks M. A., Courtney R. J. 2010. Virion incorporation of the herpes simplex virus type 1 tegument protein VP22 is facilitated by trans-Golgi network localization and is independent of interaction with glycoprotein E. Virology 405:176–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Para M. F., Parish M. L., Noble A. G., Spear P. G. 1985. Potent neutralizing activity associated with anti-glycoprotein D specificity among monoclonal antibodies selected for binding to herpes simplex virions. J. Virol. 55:483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rauch D. A., Rodriguez N., Roller R. J. 2000. Mutations in herpes simplex virus glycoprotein D distinguish entry of free virus from cell-cell spread. J. Virol. 74:11437–11446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reynolds A. E., Wills E. G., Roller R. J., Ryckman B. J., Baines J. D. 2002. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 76:8939–8952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Roop C., Hutchinson L., Johnson D. C. 1993. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J. Virol. 67:2285–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ruel N., Zago A., Spear P. G. 2006. Alanine substitution of conserved residues in the cytoplasmic tail of herpes simplex virus gB can enhance or abolish cell fusion activity and viral entry. Virology 346:229–237 [DOI] [PubMed] [Google Scholar]
- 48. Showalter S. D., Zweig M., Hampar B. 1981. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect. Immun. 34:684–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stackpole C. W. 1969. Herpes-type virus of the frog renal adenocarcinoma. I. Virus development in tumor transplants maintained at low temperature. J. Virol. 4:75–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stannard L. M., Himmelhoch S., Wynchank S. 1996. Intra-nuclear localization of two envelope proteins, gB and gD, of herpes simplex virus. Arch. Virol. 141:505–524 [DOI] [PubMed] [Google Scholar]
- 51. Stylianou J., Maringer K., Cook R., Bernard E., Elliott G. 2009. Virion incorporation of the herpes simplex virus type 1 tegument protein VP22 occurs via glycoprotein E-specific recruitment to the late secretory pathway. J. Virol. 83:5204–5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Subramanian R., D'Auvergne O., Kong H., Kousoulas K. G. 2008. The cytoplasmic terminus of Kaposi's sarcoma-associated herpesvirus glycoprotein B is not essential for virion egress and infectivity. J. Virol. 82:7144–7154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sugimoto K., et al. 2008. Simultaneous tracking of capsid, tegument, and envelope protein localization in living cells infected with triply fluorescent herpes simplex virus 1. J. Virol. 82:5198–5211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Torrisi M. R., Di Lazzaro C., Pavan A., Pereira L., Campadelli-Fiume G. 1992. Herpes simplex virus envelopment and maturation studied by fracture label. J. Virol. 66:554–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Turcotte S., Letellier J., Lippe R. 2005. Herpes simplex virus type 1 capsids transit by the trans-Golgi network, where viral glycoproteins accumulate independently of capsid egress. J. Virol. 79:8847–8860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Warming S., Costantino N., Court D. L., Jenkins N. A., Copeland N. G. 2005. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 33:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Whealy M. E., Card J. P., Meade R. P., Robbins A. K., Enquist L. W. 1991. Effect of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J. Virol. 65:1066–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wisner T., Brunetti C., Dingwell K., Johnson D. C. 2000. The extracellular domain of herpes simplex virus gE is sufficient for accumulation at cell junctions but not for cell-to-cell spread. J. Virol. 74:2278–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wisner T. W., Johnson D. C. 2004. Redistribution of cellular and herpes simplex virus proteins from the trans-Golgi network to cell junctions without enveloped capsids. J. Virol. 78:11519–11535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wisner T. W., et al. 2009. Herpesvirus gB-induced fusion between the virion envelope and outer nuclear membrane during virus egress is regulated by the viral US3 kinase. J. Virol. 83:3115–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wright C. C., et al. 2009. Fusion between perinuclear virions and the outer nuclear membrane requires the fusogenic activity of herpes simplex virus gB. J. Virol. 83:11847–11856 [DOI] [PMC free article] [PubMed] [Google Scholar]