Abstract
Vertical transmission of viruses in breast milk can expose neonates to infectious pathogens at a time when the capacity of their immune system to control infections is limited. We developed a mouse model to study the outcomes of acquisition of murine cytomegalovirus (MCMV) when neonates are breastfed by mothers with acute or latent infection. Breast milk leukocytes collected from lactating mice were examined for the presence of MCMV IE-1 mRNA by reverse transcription-PCR (RT-PCR) with Southern analysis. As determined by this criterion, breast milk leukocytes from both acute and latent mothers were positive for MCMV. This mimics the outcome seen in humans with latent cytomegalovirus infection, where reactivation of virus occurs specifically in the lactating mammary gland. Interestingly, intraperitoneal injection of breast milk collected from mothers with latent infection was sufficient to transfer MCMV to neonatal mice, demonstrating that breast milk was a source of virus. Furthermore, we found that MCMV was transmitted from infected mothers to breastfed neonates, with MCMV IE-1 mRNA or infectious virus present in multiple organs, including the brain. In fact, 1 day of nursing was sufficient to transmit MCMV from latent mothers to breastfed neonatal mice. Together, these data validate this mouse model of vertical transmission of MCMV from mothers with acute or latent MCMV infection to breastfed neonates. Its relevance to human disease should prove useful in future studies designed to elucidate the immunological and pathological ramifications of neonatal infection acquired via this natural route.
INTRODUCTION
Human cytomegalovirus (HCMV) is a leading viral cause of congenital birth defects, infecting 0.5 to 2% of newborns throughout the world. While the majority of newborns are free of symptoms at birth, approximately 10% exhibit evidence of infection, including microcephaly, jaundice, and hepatosplenomegaly (9, 59). Furthermore, 10% of newborns that are asymptomatic at birth develop neurological problems later on, most notably sensorineural hearing loss (15). The impact of HCMV infection on infants, as well as on members of immunocompromised groups such as the elderly, HIV-infected patients, or transplant recipients, emphasizes the need for the development of an effective vaccine to prevent HCMV infection (2, 62).
In addition to congenital infection, HCMV can be transmitted from seropositive mothers to newborn infants during breastfeeding. Isolation of HCMV from human breast milk was first reported in the late 1960s and has been routinely documented thereafter (11, 14, 21, 61, 69). Results of a study by Hayes et al. (23) showed that the incidence of HCMV in breast milk does not correlate with viral shedding in urine, suggesting that reactivation of HCMV may be specific for the lactating mammary gland as opposed to being systemic in nature. Interestingly, transmission of HCMV from breast milk, even in the presence of maternal neutralizing antibodies, occurs in 25 to 50% of term infants (11, 14, 21, 61, 69). However, even at this high rate of transmission, no evidence of HCMV-related illness at birth or within a 4-year follow-up period has been noted. In contrast, transmission of HCMV via breast milk in some low-birth-weight (less than 1,500 g) infants leads to the development of severe sepsis-like disease (3, 11, 12, 14, 21, 38, 40, 41, 61, 69). Thus, transmission of the virus via breast milk poses a risk to preterm infants and must be carefully weighed against the nutritional, immunological, psychological, and developmental benefits of breastfeeding.
Children infected with HCMV early in life tend to shed virus for extended periods, in some cases up to 5 years after the initial infection (1, 58). This increases horizontal transmission of HCMV from child to child in the close interactive setting of day care centers. In addition, this represents a new source of infection for seronegative parents, particularly targeting women of childbearing age. Indeed, 50% of all seronegative mothers acquire HCMV from their infected infant, and transmission of HCMV from child to mother to unborn fetus is known to occur (46, 71). Thus, the efficient transfer of HCMV via breast milk to infants may enhance continuous viral shedding in young children and indirectly increase the risk of congenital HCMV transmission. Alternatively, virus acquired via this natural route of infection could elicit immune control sufficient to protect the individual and, more importantly, to interrupt spread of the virus to at-risk individuals.
Murine cytomegalovirus (MCMV) shares several hallmarks with HCMV, making it a useful model for examining viral infection within its natural host. MCMV has provided a wealth of information concerning viral infection in adult mice, and yet studies of neonatal mice have been limited. Intraperitoneal (i.p.) infection of BALB/c mice with MCMV at less than 24 h after birth, even at a low viral dose of 200 PFU, results in increased neonatal mortality and high viral loads in peripheral organs (52). Surviving mice display characteristics of growth retardation and persistent viral shedding in salivary glands for up to 6 months, in similarity to descriptions of HCMV shedding in infants and young children (1, 58). After that time the virus becomes latent, maintaining a higher copy number of latent genomes in organs than is seen in mice infected with MCMV as adults (52). Intraperitoneal inoculation of neonatal mice with MCMV also leads to dissemination of the virus from peripheral organs to focal regions within the brain and is associated with the recruitment of mononuclear cells to these areas (29). A similar pattern of scattered focal infection with infiltrating mononuclear cells occurs in infants with congenital HCMV infection and is witnessed upon direct intracerebral infection of newborn mice with MCMV (30, 37, 48, 68). However, neither intracerebral nor i.p. inoculation with MCMV models a natural route of HCMV infection in human infants.
We have developed an animal model to study the outcomes of MCMV infection transmitted in breast milk. MCMV was detected in breast milk leukocytes collected from lactating mice with acute or latent infection. The reactivation of MCMV in the lactating mammary gland of mice with latent infection mimicked the process known to occur in humans. Furthermore, MCMV was transmitted from infected mothers to breastfed neonates, with MCMV IE-1 mRNA or infectious virus present in multiple organs, including the brain. Interestingly, nursing for 1 day on mothers with latent MCMV infection was sufficient to allow transmission of MCMV to both susceptible (BALB/c) and resistant (C57BL/6) strains of neonatal mice. On the basis of these findings, we believe that this model provides a novel means to examine the mechanisms controlling vertical transmission of virus in breast milk. Neonatal infection with MCMV in the presence of maternal antibodies may inhibit or enhance the antiviral immune response in neonates, either of which would have a significant impact on vaccination strategies. Thus, this model should prove useful in future studies designed to elucidate the immunological and pathological outcomes of neonatal infection acquired via this natural route.
MATERIALS AND METHODS
Viral inoculation of mice.
Stocks of MCMV strain Smith (ATCC VR-1399) and MCMV-GFP strain Smith (a generous gift from John Hamilton, Department of Veterans Affairs, Durham, NC [24]) were propagated in mouse embryonic cells (MEC) and purified as described previously (7). Virus titers were determined by standard plaque assays. C57BL/6 mice, purchased from The Jackson Laboratory (Bar Harbor, ME), were infected by i.p. injection with 300 to 30,000 PFU of MCMV as indicated in the text. Infected mice housed for 16 weeks were considered to have latent MCMV infection (26). Pregnant or lactating mice with latent MCMV infection were termed “latent mothers,” whereas lactating female mice infected (i.p.) 6 days after the birth of their litters were termed “acute mothers.” Oral infection of 6-day-old pups was performed by administering 1,000 PFU of MCMV Smith in a 25-μl final volume through soft polyethylene tubing (Becton Dickinson, Sparks, MD) (outer diameter, 1.09 mm; inner diameter, 0.38 mm) attached to a 28-gauge needle. Virus was suspended in sterile phosphate-buffered saline (PBS) containing 0.1% green food color (McCormick & Company, Sparks, MD) to aid in visualization of virus delivery into the stomach. Newborn BALB/c (Thy1.1) mice, used in foster nursing experiments, were from colonies maintained at the University of Connecticut Health Center (the colonies were originally obtained from Scripps Research Institute, La Jolla, CA). All mice were fed sterile food and water and housed in microisolators under specific pathogen-free conditions. Their care was in accordance with institutional and Office of Laboratory Animal Welfare guidelines.
Collection and analysis of breast milk.
Lactating female mice were anesthetized with ketamine or xylazine 11 to 14 days after birth of their litters and then treated by i.p. injection of 2 IU of oxytocin (Sigma-Aldrich, St. Louis, MO) to induce milk “let down.” Individual teats were milked with a suction apparatus attached to plastic pipet tips and a collection trap. The suction flow rate was typically set at 1.5 liters/min of air to produce a continual stream of milk and to minimize trauma to teats. Typically, 100 to 200 μl of milk was collected per nursing mother. A 1-ml volume of (calcium- and magnesium-free) Dulbecco's PBS (DPBS) was added to each sample prior to centrifugation at 4°C and 100 × g in an Eppendorf 5415R centrifuge. Using this approach, we were able to collect approximately 1 × 106 cells (primarily macrophages) from breast milk samples for isolation of RNA to evaluate MCMV gene expression by reverse transcription-PCR (RT-PCR) with Southern analysis.
Neonatal CD-1 mice were injected with breast milk collected from 3 individual latent mothers. Milk samples from each mother were diluted 1:2 in sterile DPBS and injected (i.p.) into two 6-day-old pups. Milk-injected neonatal pups, along with 2 siblings that were not injected and that served as controls, continued to nurse on their uninfected mothers. Seven days later, the injected and control siblings were sacrificed and RNA prepared from their brain, liver, lung, spleen, salivary glands, and kidney was examined for MCMV IE-1 gene expression by RT-PCR with Southern analysis.
Isolation of RNA for RT-PCR and nested PCR analysis.
Mice were sacrificed at various times (when mice were 1 to 22 days of age) as indicated in the text. Brain, liver, lung, spleen, kidney, and salivary gland tissues were harvested, immediately frozen in liquid nitrogen, and stored at −80°C. With the exception of one experiment, total RNA was isolated from organs by the use of an RNA extraction kit containing DNase I (Absolutely RNA Miniprep kit; Stratagene, Inc., La Jolla, CA). In one experiment (Fig. 1), nucleic acid was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA). In addition, RNA was isolated from blood leukocytes by the use of a PureLink Total RNA blood purification kit (Invitrogen). RT-PCR analysis was performed using 3 μg of total RNA and a OneStep RT-PCR kit (Invitrogen) with primers specific for MCMV IE-1 (forward, 5′-CCTCGAGTCTGGAACCGAAA-3′; reverse, 5′-TACAGGACAACAGAACGCTC-3′; designed by Kurz et al. [32]) and the reference gene β-actin (forward, 5′-GATGCCACAGGATTCCATA-3′; reverse, 5′-AGAGGGAAATCGTGCGTGAC-3′) or GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (forward, 5′-ATGTTCCAGTATGACTCCACT-3′; reverse, 5′-CCACAATGCCAAAGTTGTCAT-3′). Annealing occurred at 55°C, and after 35 cycles, the resulting PCR products were resolved on 3% NuSieve 3:1 agarose gels (Cambrex Corp., East Rutherford, NJ), transferred to nylon membranes (Nytran Plus; Scheicher & Schuell, Keene, NH), and hybridized with a 32P-labeled probe specific for MCMV IE-1. The probe contained the entire spliced open reading frame of MCMV IE-1 (from plasmid GS62-pp89; generous gift of Deborah Spector, University of California, San Diego, San Diego, CA). For size markers, peritoneal exudate cells (PerC) were collected by lavage from an adult mouse 3 days after MCMV i.p. infection (30,000 PFU). Total RNA, isolated from PerC in the absence of DNase I treatment, allowed the distinction between genomic DNA (310 bp) and spliced mRNA (188 bp) in amplified IE-1 products to be determined by RT-PCR with Southern analysis as described by Kurz et al. (32).
Fig. 1.
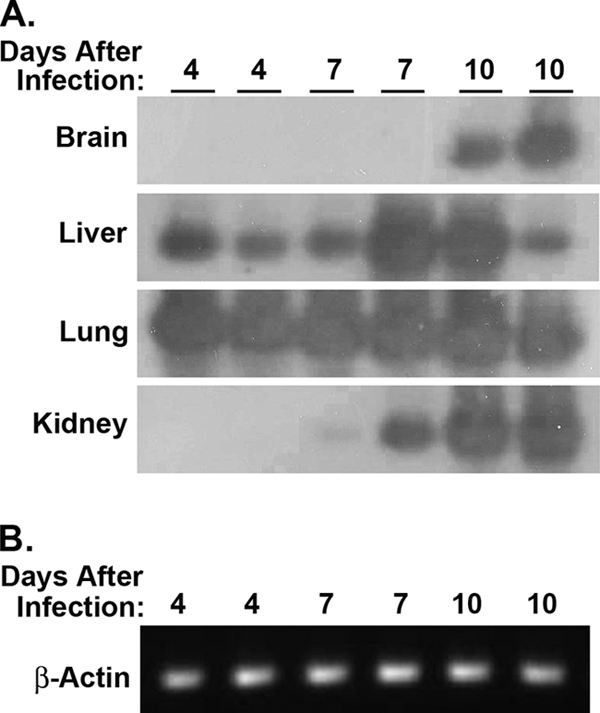
Oral infection of neonates with MCMV. (A) Six-day-old C57BL/6 pups were infected with 1,000 PFU of MCMV (Smith) by gavage using soft polyethylene tubing. At 4, 7, or 10 days after infection, total RNA was isolated from the brain, liver, lung, kidney, spleen, and salivary glands of individual mice and examined for MCMV IE-1 gene expression. Detection of MCMV IE-1 mRNA was performed by RT-PCR with Southern analysis, where spliced IE-1 RNA (188 bp) was distinguished from contaminating IE-1 genomic DNA (310 bp) on the basis of size. (B) The same RNA preparations were analyzed by RT-PCR for expression of the reference gene β-actin (representative results are shown for lung samples). Similar results were obtained in an independent experiment in which total RNA isolated from the lung and spleen of neonates orally infected with MCMV was evaluated at 8 and 15 days after infection (data not shown).
If no amplified product was observed after the first RT-PCR with Southern blotting, in some cases (as noted in the text) a nested PCR was performed using 3 to 5 μl of amplified product and nested primers for MCMV IE-1 (forward, 5′-CGTCCGCTGTGACCTGACTC-3′; reverse, 5′-AGCATGCTTGATGGCCATGT-3′). After 35 cycles, Southern analysis was performed as detailed above.
Detection of infectious virus in neonatal mice.
Lactating latent mothers nursed pups from their own litters or age-matched BALB/c neonatal mice (referred to as “foster-nursed”). After 12 to14 days, suckling neonates were sacrificed for collection of brain, liver, lung, and kidney tissue. A 10% (wt/vol) organ homogenate was prepared in Dulbecco's modified Eagle's medium (DMEM)–10% fetal calf serum (FCS)–10% dimethyl sulfoxide (DMSO), sonicated for 30 s, and then centrifuged to remove cellular debris. The resulting organ homogenates were diluted 1:5 in DMEM–10% FCS and used to infect monolayers of MEC in a 48-well plate. Monolayers were monitored daily for 1 to 22 days for signs of cytopathic effect (CPE), with the medium being changed every 5 days. To confirm that the homogenized organ had no inhibitory effect on viral CPE, 10 PFU of MCMV Smith was added to 50 μl of each sample prior to incubation on MEC and results were compared to those obtained with MEC infected with 10 PFU of MCMV in medium alone.
Seroconversion.
C57BL/6 MEC monolayers were infected with MCMV Smith at a multiplicity of infection (MOI) of 0.1 or mock infected. When CPE reached 80%, cellular monolayers were harvested, clarified by centrifugation at 400 × g for 10 min, and suspended in 1/10 the original volume of DPBS. Proteins from infected or uninfected whole-cell lysates were separated by polyacrylamide gel electrophoresis (PAGE), transferred to Protran nitrocellulose membranes (Whatman Inc., Piscataway, NJ), and probed with a 1:200 dilution of serum from pups (nursed by latent mothers) 4 months after birth. Detection was performed with goat anti-mouse IgG-horseradish peroxidase (HRP) (1:5,000) and a Western blot amplification kit (Bio-Rad). Serum from MCMV-infected and uninfected mice served as positive and negative controls, respectively.
Histological evaluation.
Newborn mice were sacrificed after 7 days of nursing on acute mothers. Age-matched newborn mice, nursing on uninfected mothers, were used as controls. Livers were removed, fixed with 10% buffered formalin, and embedded in paraffin using conventional methods. Sections were stained with hematoxylin and eosin (H&E) for histological examination and were read without previous knowledge of experimental conditions by Faripour Forouhar (Department of Pathology, University of Connecticut Health Center).
Statistical analysis.
Analysis of data from experiments with three or more groups was performed using a using one-way analysis of variance (ANOVA), followed by a Newman-Keuls post hoc test. All tests were performed using Prism 5.0 software (GraphPadSoftware, La Jolla, CA), with a probability of P < 0.05 considered to be significant.
RESULTS
Neonatal mice were susceptible to oral infection with MCMV.
To develop a model to study the outcome of MCMV infection transmitted by breast milk, we first determined whether orally administered MCMV could infect neonatal mice. Six-day-old pups were inoculated with MCMV (Smith; 1,000 PFU) by gavage using soft polyethylene tubing. At 4, 7, or 10 days after feeding, RNA was prepared from brain, liver, lung, spleen, kidney, and salivary gland tissue and examined for the presence of MCMV IE-1 mRNA by RT-PCR with Southern analysis. As shown in Fig. 1, MCMV IE-1 mRNA was detected in the lung and liver of pups at 4, 7, and 10 days after infection. In addition, MCMV IE-1 mRNA was present in the kidney after 7 and 10 days, as well as in the brain after 10 days. MCMV IE-1 mRNA was also detected in the spleen and salivary gland tissue from the same mice at all time points (data not shown). Thus, ingestion of MCMV can result in viral infection accompanied by dissemination of virus to multiple organs in neonatal mice.
MCMV disseminated to the lactating mammary gland following acute infection was transmitted to breastfed neonatal mice.
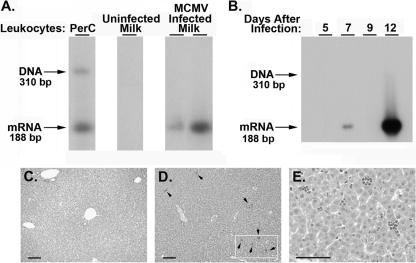
Six days after the birth of their litters, lactating mice were infected (i.p.) with 300 PFU of MCMV-GFP (“acute mothers”). Leukocytes were collected from the milk 5 days after infection and examined for MCMV IE-1 mRNA by RT-PCR with Southern analysis. As presented in Fig. 1 (and throughout this paper), we used a strategy, previously described by Kurz et al. (32), whereby forward and reverse primers selected for RT-PCR analysis are located within the third and fourth exons of the IE-1 gene, respectively. This approach distinguishes mRNA (188 bp) encoded by the virus from genomic DNA (310 bp). This was illustrated using total RNA (in the absence of DNase treatment) isolated from leukocytes collected from the peritoneal cavity (PerC) of an MCMV-infected (i.p.) adult mouse (Fig. 2A), where both IE-1 genomic DNA (310 bp) and IE-1 mRNA (188 bp) were amplified. Interestingly, MCMV IE-1 mRNA was detected in total RNA isolated from breast milk leukocytes collected from two acute mothers but not in RNA from an uninfected mother. These findings demonstrated that primary MCMV infection, initiated by i.p. inoculation during lactation, resulted in dissemination of cell-associated virus into breast milk.
Fig. 2.
Vertical transmission of MCMV following acute infection. (A) Lactating mice were infected (i.p.) with 300 PFU of MCMV-GFP at 6 days after birth of the litter. At 11 days of lactation, breast milk leukocytes were collected from an uninfected control mother or from two infected lactating mothers (5 days after virus infection). Total RNA was examined for MCMV IE-1 gene expression by RT-PCR with Southern analysis. The IE-1 primers bracket an intron, allowing a distinction between spliced viral mRNA (188 bp) and contaminating genomic DNA (310 bp) (confirmed by analysis of total RNA extracted from peritoneal cavity [PerC] exudate cells without DNase treatment from an acutely infected mouse). (B) At 5, 7, 9, or 12 days after maternal infection, nursing pups were sacrificed and lungs from individual neonatal mice were examined for expression of MCMV IE-1 mRNA by RT-PCR with Southern analysis. (C to E) Histological evaluation of liver sections from neonates nursed for 1 week by an uninfected mother (C) or an acutely infected mother (D and E). Sections were stained with hematoxylin and eosin. Arrows indicate inflammatory foci (D); an enlargement of the marked area is shown in panel E. Scale bar, 50 μm.
To demonstrate the feasibility of MCMV transmission from infected mothers to nursing pups, the following experiment was performed. Neonates, breastfed by acute mothers, were sacrificed after various times of nursing and examined for evidence of viral infection. As MCMV was readily detected in neonatal lungs after oral infection (Fig. 1), we examined RNA prepared from this organ as an indicator of the presence of ingested MCMV. By the use of the strategy described above, pups nursed by acute mothers were sacrificed 5, 7, 9, or 12 days after maternal infection. RT-PCR with Southern analysis demonstrated that MCMV IE-1 mRNA was present in lungs from pups on days 7 and 12 after maternal infection (Fig. 2B).
An additional criterion indicative of MCMV infection is virus-induced pathology in the liver (44, 45). To evaluate this parameter in our model, livers prepared from neonatal mice breastfed for 7 days by an uninfected mother, or by a mother with acute MCMV infection, were subjected to histological evaluation. In contrast to pups nursed by uninfected mothers (Fig. 2C), livers from pups nursed by acute mothers had inflammatory foci, comprising small discrete clusters of monocytes and lymphocytes, scattered throughout the liver (Fig. 2D and E). This pathology is indicative of lobular hepatitis, a well-documented nonspecific outcome of viral infections, following MCMV infection in adult mice (44, 45). In addition, hepatocytes containing mitotic figures were readily detected in livers from pups nursed by acute mothers (data not shown), suggesting the induction of cellular repair processes, perhaps in response to viral-induced organ damage. These pathological findings substantiated the molecular evidence of MCMV transmission from virally infected mothers to breastfed neonatal mice.
Reactivation of MCMV in the lactating mammary gland, with transmission to breastfed neonatal mice.
The incidence of HCMV in breast milk, along with the high rate of vertical transmission from mothers to breastfed infants, has previously been documented (11, 14, 21, 61, 69). Neonatal acquisition of HCMV infection via this common route could occur following a primary acute infection or when virus reactivates from latency during lactation (14, 58). Therefore, to mimic the human condition of mothers with latent HCMV infection, we modified our model to evaluate whether mothers with latent MCMV infection could represent a source of virus transmissible to neonatal mice. To this end, we infected female C57BL/6 mice with various doses (300 PFU, 3,000 PFU, or 30,000 PFU) of MCMV strain Smith by i.p. injection and waited at least 16 weeks to establish viral latency (26). Mice with latent MCMV infection were bred; those that were pregnant or lactating are referred to as latent mothers. Leukocytes from breast milk or peripheral blood were collected from latent mothers 11 to 14 days after the birth of their litters, and RNA prepared from these samples was analyzed for the presence of MCMV IE-1 gene expression by RT-PCR with Southern analysis. As shown in Fig. 3A, MCMV IE-1 mRNA was detected in breast milk leukocytes from two latent mothers infected with 30,000 PFU but was not detected in their peripheral blood leukocytes. These findings are consistent with human data demonstrating that reactivation of latent HCMV is specific for the lactating mammary gland and is not systemic in nature (23). In our studies, collection of breast milk was a one-time event, because it placed survival of the litter in jeopardy. Thus, milk collection was performed only in those experiments in which pups were coordinately sacrificed. Even though the number of breast milk leukocyte samples was limited, we found that 50% of the samples tested were positive for MCMV IE-1 gene expression. Specifically, 1 of 2 samples from latent mothers infected with 300 PFU of MCMV, 3 of 8 samples from latent mothers infected with 3,000 PFU of MCMV, and 2 of 2 samples from latent mothers infected with 30,000 PFU of MCMV gave positive results.
Fig. 3.
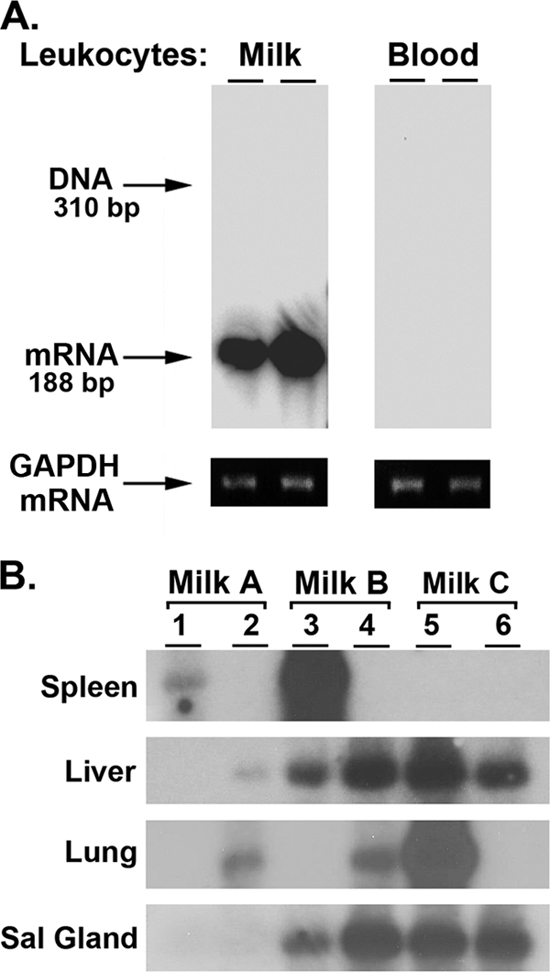
Breast milk collected from latent mothers was sufficient to transfer MCMV infection to neonatal mice. (A) Female C57BL/6 mice were infected with 30,000 PFU of MCMV; after 16 weeks, when MCMV is latent, mice were bred. Twelve days after the birth of the litter, leukocytes were collected from breast milk or blood from two nursing mothers. Total RNA was analyzed for evidence of reactivation of latent MCMV during lactation by determining MCMV IE-1 gene expression by RT-PCR with Southern analysis. The same RNA preparations were analyzed for expression of the reference gene GAPDH by RT-PCR. (B) Thirteen to 14 days after the birth of their litters, 3 latent mothers, infected with 3,000 PFU of MCMV Smith, were anesthetized and treated with 2 IU of oxytocin. Total volumes of 80 μl, 150 μl, and 150 μl of breast milk were collected from latent mothers A, B, and C, respectively. Each milk sample was diluted 1:2 in sterile DPBS and injected (i.p.) into two 6-day-old CD-1 pups. Seven days after injection, pups were sacrificed and total RNA was prepared from brain, liver, lung, spleen, kidney, and salivary glands from individual pups and examined for MCMV IE-1 gene expression by RT-PCR with Southern analysis. It was interesting that MCMV IE-1 mRNA was not detected in the brain or kidney of neonates infected with breast milk via the i.p. route.
To determine whether breast milk from latent mothers was sufficient to transmit MCMV to neonates, breast milk from 3 individual lactating latent mothers was collected and directly administered to neonatal mice. A final volume of 80 μl (milk A) or 150 μl (milk B or C) of breast milk was collected from each latent mother, diluted 1:2 with DPBS, and then injected (i.p) into two 6-day-old CD-1 pups. These pups, along with 2 uninfected siblings, continued to nurse on the uninfected mother for 7 days. At this time, brain, liver, lung, spleen, kidney, and salivary glands were collected from all nursing pups and total RNA was analyzed by RT-PCR with Southern analysis for MCMV IE-1 gene expression. As shown in Fig. 3B, evidence of MCMV infection was detected in organs from all six pups, with the liver and salivary glands being positive in 5 of 6 and 4 of 6 pups, respectively. No evidence of MCMV IE-1 mRNA was found in the brain of neonatal mice i.p. injected at 6 days of age with breast milk from lactating latent mothers. In addition, no evidence of MCMV IE-1 gene expression was noted in the 2 uninfected siblings nursed by the same uninfected mother (data not shown). Thus, breast milk (containing reactivated MCMV) from latent mothers was sufficient to transmit MCMV infection upon injection (i.p.) into neonatal mice. Furthermore, latent mothers A, B, and C vertically transmitted virus to their own suckling pups and/or foster-nursed neonatal mice (Table 1, experiment 4).
Table 1.
Transmission of MCMV from latent mothers to nursing pups
| Latent mother (n = 24) (inoculant PFU) | No. of live births | No. of pups live after 3 daysa (% survival rate) | No. of foster-nursed pups | No. of pups with evidence of MCMV vs no. of pups examined (% infection rate) | Means of analysis |
|---|---|---|---|---|---|
| Expt 1 (300) | |||||
| A | 0b | ||||
| B | 7 | 7 (100) | 0 | 4 of 6c | Seroconversion |
| C | 5 | 5 (100) | 0 | 3 of 4c | Seroconversion |
| Expt 2 (3,000) | |||||
| A | 7 | 7 (100) | 3 | 6 of 6c | RT-PCR/Southern |
| B | 7 | 3 (43) | 0 | NDd | |
| C | 6 | 2 (33) | 0 | ND | |
| Expt 3 (3,000) | |||||
| A | 5 | 5 (100) | 2 | 7 of 7 | Nested RT-PCR/Southern |
| B | 5 | 0 | 0 | ||
| C | 4 | 0 | 0 | ||
| D | 9 | 5 (56) | 0 | 5 of 5 | Nested RT-PCR/Southern |
| E | 7 | 7 (100) | 0 | 7 of 7 | Nested RT-PCR/Southern |
| F | 3 | 0 | 0 | ||
| G | 0b | 0 | |||
| H | 6 | 2 (33) | 0 | 2 of 2 | Nested RT-PCR/Southern |
| I | 0b | 0 | |||
| J | 4 | 3 (75) | 0 | 3 of 3 | Nested RT-PCR/Southern |
| Expt 4 (3,000) | |||||
| A | 7 | 0 | 2 | 1 of 2 | Viral CPE |
| B | 8 | 0 | 2 | 2 of 2 | Viral CPE |
| C | 5 | 4 (80) | 4e | 8 of 8 | RT-PCR/Southern |
| 4e | 3 of 4 | Viral CPE | |||
| D | 5 | 2 (40) | 2 | 3 of 4 | Viral CPE |
| E | 8 | 0 | 0 | ||
| F | 1 | 0 | 0 | ||
| G | 4 | 0 | 0 | ||
| H | 5 | 0 | 0 | ||
| Total | 118 | 52 (44) | 54 of 60 (90) |
Pups surviving 3 days exhibited long-term survival.
All pups were born dead or cannibalized.
Not all pups in the litter were examined for MCMV.
ND, not done.
A total of 8 BALB/c pups were foster nursed by latent mother C.
Widespread tissue distribution of MCMV in neonatal mice nursed by latent mothers.
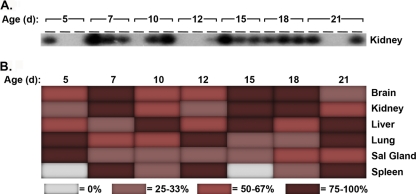
Dissemination of MCMV in adult mice has been thoroughly characterized and is dependent upon the route of infection. Typically, MCMV replication occurs at the site of entry and then spreads to other peripheral target organs. No information is available concerning MCMV distribution and spread upon oral infection, particularly for neonatal mice. Therefore, we bred 10 female mice latently infected with MCMV and examined their offspring for evidence of virus (see Table 1, experiment 3). All of the pups from five latent mothers (mothers B, C, F, G, and I) died at birth or within the first 3 days postpartum. The surviving pups were nursed by their latent mothers for 5, 7, 10, 12, 15, 18, or 21 days; at each time point, 3 neonates were sacrificed for analysis (with 4 neonatal mice sacrificed on day 21). RNA was prepared from various organs and evaluated by nested RT-PCR with Southern analysis. Representative raw data, depicting MCMV IE-1 mRNA expression in kidneys from pups of various days of age, are shown in Fig. 4A. Here, 100% (3 of 3) of neonatal mice were positive on days 7, 15, and 18, while 33% of neonates (1 of 3) were positive on days 5 and 12. A summary of the findings was expressed as a heat map, illustrating the percentages of mice with MCMV IE-1 mRNA in each organ at each day after birth (Fig. 4B). Although we could not control for viral dosing in this study (e.g., levels of reactivation in different lactating mothers, level of MCMV in breast milk, or volume of milk consumed), MCMV IE-1 mRNA was expressed in tissues of two or more organs from all surviving neonatal mice (total of 22 pups). Overall, these findings demonstrated that reactivation of MCMV in the lactating mammary gland of latent mothers resulted in a widespread tissue distribution of MCMV gene expression in neonatal mice.
Fig. 4.
Temporal distribution of MCMV IE-1 gene expression in neonates nursed by mothers with latent MCMV infection. (A) Neonates, nursed by latent mothers, were sacrificed 5, 7, 10, 12, 15, 18, or 21 days after birth. Total RNA was prepared from brain, liver, lung, spleen, kidney and salivary glands from individual neonatal mice, with 3 or 4 neonates analyzed at each time point for expression of MCMV IE-1 mRNA by nested RT-PCR with Southern analysis. A sample of the raw data obtained for neonatal kidneys is shown here. (B) The data compiled from all organs analyzed (typically 2 to 3 times per sample) are represented by a heat map, where each color depicts the percentage of samples positive for expression of MCMV IE-1 mRNA in each organ at each time point.
Data from 4 independent experiments analyzing transmission of MCMV Smith from latent mothers to nursing pups are shown in Table 1. Here, the size of the litter at birth, the neonatal survival rate, and the efficiency of MCMV transmission to nursing pups were followed for the litters from 24 latent mothers infected with either 300 PFU (experiment 1) or 3,000 PFU (experiments 2 to 4) of MCMV. While these findings cannot account for neonatal death in utero or determine the number of pups born dead and cannibalized, 52 of 118 live births exhibited long-term survival (44% survival rate). Of interest, 54 of the 60 pups nursed (or foster nursed) by latent mothers demonstrated evidence of MCMV infection. Therefore, vertical transmission of MCMV from latent mothers to nursing pups appeared to be an efficient process.
Nursing for 1 day was sufficient to transmit MCMV to neonatal mice.
We were surprised to find widespread distribution of MCMV IE-1 mRNA in multiple organs (i.e., brain, liver, lung, kidney, and salivary glands; Fig. 4B) after only 5 days of breastfeeding by latent mothers. We wondered whether pups nursed by latent mothers for shorter periods would also display signs of infection. To test this idea, we examined both C57BL/6 pups and age-matched, foster-nursed BALB/c pups after 1 day of breastfeeding by latent mothers. Strain-dependent susceptibility to MCMV has been previously reported in a study of adult mice in association with natural killer cell function, but its role in neonatal virus infection is not well understood (19). Our first experiment assessed two pups, one from the mother's own litter (C57BL/6) and one foster-nursed pup (BALB/c). Both pups gave positive results, with the C57BL/6 and BALB/c pups demonstrating MCMV IE-1 mRNA in the liver and brain, respectively (data not shown). To confirm these results, we repeated this experiment examining 4 nursing pups, 2 from the mother's own litter (Fig. 5; lanes 1 and 2) and 2 foster-nursed pups (lanes 3 and 4). After 1 day of breastfeeding, 2 of 4 pups expressed MCMV IE-1 mRNA in either liver or brain. In addition, MCMV IE-1 mRNA was detected in the brain of the C57BL/6 pup (lane 1) upon overexposure of the Southern blot (data not shown). These findings indicated that 1 day of breastfeeding was sufficient to transfer virus from latent mothers to nursing neonates of either mouse strain.
Fig. 5.

Acquisition of MCMV by neonates after 1 day of nursing by latent mothers. One day after birth, C57BL/6 neonates from the mother's own litter (lanes 1 and 2) or age-matched, foster-nursed BALB/c neonates (lanes 3 and 4) were sacrificed. Total RNA from the brain (right panel) and liver (left panel) of individual neonatal mice were collected and analyzed for MCMV IE-1 gene expression by RT-PCR with Southern analysis. In addition, MCMV IE-1 mRNA was detected in lane 1 from brain tissue upon overexposure of the Southern blot. Similar results were obtained in an independent experiment, where expression of MCMV IE-1 mRNA was detected in 2 neonates (1 from the mother's own litter and 1 fostered) nursed by a latent mother.
Transmission of infectious virus from latent mothers to breastfed neonates.
Expression of MCMV IE-1 mRNA does not necessarily indicate production of infectious virus, as abortive MCMV infection may exhibit a similar outcome. As our initial attempts to detect infectious virus in peripheral organs from breastfed pups by standard plaque assays were unsuccessful, we utilized a more sensitive in vitro tissue culture assay developed by Pollock et al. (51). This approach examines the presence of MCMV in homogenized tissue samples in the absence of methyl cellulose, allowing the detection of low (1 PFU) levels of virus by cytopathic effect (CPE). Four latent mothers breastfed a total of 12 pups for 12 to 14 days (Table 2; also Table 1, experiment 4, mothers A, B, C, and D). Each mother nursed or fostered 2 to 4 neonatal mice from either their own C57BL/6 litter or from an age-matched uninfected BALB/c litter. Organ homogenates were prepared from the brain, liver, lung, or kidney from breastfed neonates and used to inoculate monolayers of MEC, which were observed daily for 22 days for the presence of CPE. As summarized in the Venn diagram (Fig. 6), infectious virus was detected in at least one organ, including the lung (6 of 12 pups), kidney (3 of 12 pups), and brain (2 of 12 pups), in 9 of 12 breastfed neonatal mice. No infectious virus was detected in the liver. Importantly, all latent mothers were capable of transmitting infectious virus to their breastfed offspring. To confirm that the homogenized organs themselves were free of any nonspecific inhibitory effect on MCMV infection in this assay, 10 PFU of MCMV was added to uninfected organ samples prior to incubation in culture. No differences in CPE between MCMV in homogenized organs versus MCMV in medium alone were noted. To estimate the levels of infectious virus present in individual organ samples, separate monolayers were infected with 0.1, 1, and 10 PFU of MCMV. Ten PFU of virus was consistently detected after 3 days of infection, while 1 and 0.1 PFU of MCMV were sporadically observed. CPE in neonatal homogenates appeared between 10 and 15 days, suggesting a level of infectious virus of less than 10 PFU.
Table 2.
Transmission of infectious virus from latent mothers to nursing neonatesa
| Tissue/organ | Presence or absence of infectious virus in neonateb no. |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mother A |
Mother B |
Mother C |
Mother D |
|||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Brain | − | − | − | − | − | − | + | + | − | − | − | − |
| Kidney | − | − | + | + | + | − | − | − | − | − | − | − |
| Lung | − | + | + | − | − | − | − | + | + | + | + | − |
| Liver | − | − | − | − | − | − | − | − | − | − | − | − |
Evidence of infectious virus in pups after 12 to 14 days of nursing, as determined by CPE in MEC monolayers inoculated with individual neonatal tissue homogenates.
All neonates were foster nursed by the indicated latent mother, except neonates 9 and 10, who were born to mother D.
Fig. 6.
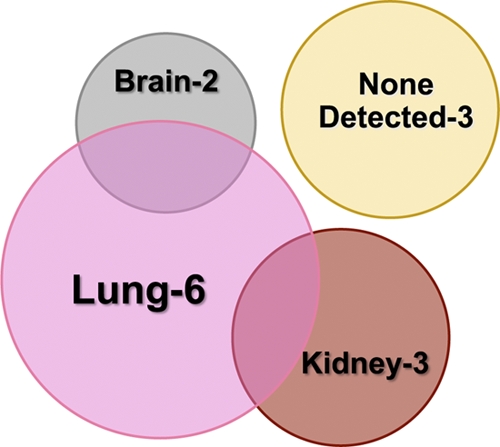
Vertical transmission of infectious virus to breastfed neonates. Twelve pups were breastfed by 4 latent mothers for 12 to 14 days. Each latently infected mother nursed (or fostered) 2 to 4 neonates either from her own C57BL/6 litter or from an uninfected, age-matched BALB/c litter. Organ homogenates prepared from brain, liver, lung, and kidney tissue from individual neonatal mice were used to inoculate MEC. The monolayers were observed daily for 22 days for signs of CPE, and a summary of the results is presented using a Venn diagram, with additional details given in Table 2. The area of each circle is proportional to the number of neonatal mice with evidence of infectious virus in each organ, with the overlapping intersections illustrating the number of mice with evidence of infectious virus in multiple organs.
Results from recent studies by Slavuljica et al. (56) demonstrate that MCMV-specific maternal antibodies are passively transferred to neonates in utero and can afford protection against viral challenge at birth. To confirm the presence of antiviral antibodies in latently infected females, we examined neutralizing activity in serum by a standard plaque reduction assay. Sera, collected from 10 individual latently infected females (including mothers A, B, C, and D [Table 2]) prior to mating, showed significantly reduced viral titers, at levels 64% and 44% of those seen with medium alone and with sera from naïve mice, respectively. Taken together, these results suggest that, in this model of natural infection, neutralizing antibodies in mothers with latent MCMV infection were insufficient to prevent transmission of infectious virus to nursing neonatal pups.
DISCUSSION
The American Academy of Pediatrics recommends breastfeeding for infants during the first 12 months of life, emphasizing nutritional, immunological, psychological, and developmental benefits (17). Despite such benefits, breastfeeding can also be a means of transporting bacterial and viral pathogens from infected mother to infant, as has been documented for HCMV during primary and nonprimary infection. Our goal was to develop a mouse model for vertical transmission of MCMV during breastfeeding that mimicked nonprimary infection in humans, with reactivation of latent MCMV as the source of virus. Such a model would support future studies examining the character and effectiveness of the immune response to MCMV that develops in neonates infected under these conditions. Neonatal infection with MCMV in the presence of maternal antibodies (from latent mothers) may inhibit or enhance antiviral immune responses in pups (10, 54, 55), thereby having the potential to significantly impact vaccination strategies. Additional analogies to the human situation reflected in this mouse model could be studied to decipher mechanisms to interrupt viral reactivation during maternal lactation that could be exploited to prevent vertical transmission of virus to breastfed neonates. Furthermore, since CMV is a component of the “immune risk profile” for enhanced morbidity and mortality in the elderly, this natural infection model could provide a means for determining the long-term effects of viral reactivation and their impact on immunosenescence and disease.
Using a strategy developed by Kurz et al. (32) to distinguish genomic DNA from active viral transcription, we detected MCMV IE-1 mRNA in breast milk leukocytes from mothers latently infected with MCMV. By the same criterion, we observed transmission of MCMV from latent mothers to nursing neonates, with viral gene expression detected in the brain, liver, lung, spleen, kidney, and salivary glands. Vertical transmission from latent mother to offspring was confirmed by the presence of infectious MCMV in the lung, kidney, and/or brain of nursing pups. One of the objectives of this model was to demonstrate that breast milk from infected mothers was sufficient to transmit MCMV to nursing pups. Such evidence was acquired by injecting breast milk, collected from latent mothers, into uninfected pups, resulting in the successful infection of 100% of these neonatal mice. In this approach, the evidence of viral gene expression in neonates served as a sensitive biomarker for the presence of infectious virus in breast milk and supported the concept that reactivation of latent MCMV occurs in the lactating mammary gland in mice, similar to human conditions of HCMV reactivation. In humans, the rate of congenital infection cannot account for the levels of HCMV infection witnessed during the first year of life, implicating postnatal infection during breastfeeding as a likely candidate to explain this discrepancy. Indeed, virolactia (viral DNA in breast milk), but not maternal shedding of HCMV in saliva or urine, corresponds to postnatal HCMV infection in newborns (23, 61). It is known that both latent HCMV and latent MCMV reside in myeloid cells (6, 20, 22, 27, 28, 42, 50), with cellular differentiation leading to reactivation of the virus (57, 64). Interestingly, recruitment of monocytes and macrophages is required for the normal branching morphogenesis and ductal outgrowth that occurs in the mammary gland immediately prior to lactation (34). Monocyte recruitment and macrophage differentiation continues during lactation, making these leukocytes a principle constituent in milk (49) and a potential source of infectious virus specific to mammary tissue during lactation.
Our studies revealed the presence of MCMV in the brains of pups after they were nursed by latent mothers for only 1 day (Fig. 5), as well as in pups that continually nursed for 5 to 21 days (Fig. 4). The results of in vitro studies by van den Pol et al. (67) demonstrate the opportunistic nature of MCMV in neonatal brains of mice, where viral infection occurs in neurons as well as in a wide range of nonneuronal cells. Neuronal infection during the embryonic or neonatal stages in mice persists after infection has cleared in peripheral organs, suggesting that this may be a site of chronic infection (66). Furthermore, susceptibility of the brain to MCMV infection is age dependent, with virus detected in brains of fetuses after placental infection and in neonatal pups after i.p. infection at less than 24 h after birth but not in immunocompetent adults (29, 33, 53, 65). Differences in the maturity of the innate and adaptive immune system could contribute to these findings; however, the developing brain itself appears more susceptible to the virus. Infection of brain slice cultures from 1-, 8-, and 12-day-old pups and adults revealed a striking decrease in the number of MCMV-GFP-infected cells in the hippocampus, cortex, corpus callosum, hypothalamus, and striatum with increasing age that was independent of an immune response (68). This predilection for infecting immature neuronal cells may in part explain the virus's ability to attack and damage the central nervous system. Indeed, infection with MCMV results in brain anomalies, including disturbances in neuronal migration and cerebellum abnormalities, that are also found in infants with congenital HCMV infection (5, 29, 36, 48, 63).
While examining the expression of MCMV IE-1 mRNA in various organs from neonatal pups breastfed by latent mothers, we noticed a high rate of mortality in pups less than 3 days of age (Table 1). However, all of the surviving pups that nursed for 5 to 21 days demonstrated evidence of viral infection in two or more organs. Although models of oral MCMV infection represent a natural, biologically relevant means of exposure to the pathogen in neonatal mice, little information collected using this approach has been available to date. The results of one study show that ∼3 weeks after oral infection of 1- to 5-day-old pups, MCMV can disseminate to the salivary gland, although a great deal of variation was observed within and among experiments (13). Most studies of neonatal MCMV infection follow the virus after i.p. infection of mice that are less than 1 day old, where infection causes mortality in pups at rates similar to what we observed after oral infection in our model (4, 8, 29, 31, 52). Viral dissemination in these mice displays a distinct pattern in which infection begins in the spleen and liver and then spreads to the brain and lung (4, 8, 29, 31, 52). Upon resolution of acute infection in neonates, MCMV persists in the salivary gland for up to 6 months; this is similar to the long-term viral shedding observed in children infected with HCMV early in life (1, 52, 58).
In our study, it appeared that there was a lack of temporal dissemination in neonates infected with MCMV while nursed by latent mothers. This could be because the times of virus infection are not synchronous in this model and/or because levels of maternal antibodies (and whether they are neutralizing or nonneutralizing) differ between latent mothers. It is known that virus-specific antibodies are not necessary for resolution of acute infection in adult mice, although they do restrict cell-to-cell spread during primary infection (25, 70). Passive treatment of infected neonatal pups with MCMV-immune sera or virus-specific antibodies exhibits a protective effect against the virus, significantly reducing viral titers and disease pathology in the brain (8). Furthermore, a recent study demonstrated that pups born to mothers infected with MCMV 2 weeks prior to mating have undetectable levels of infectious virus in organs, suggested by the authors to be the result of maternal antibody transport across the placenta (56); however, maternal antibodies absorbed during nursing could also contribute to this result (39). It is known from the guinea pig CMV (GPCMV) model that newborns nursed (or foster nursed) by GPCMV-positive mothers are protected from virus infection (18). Preconception immunity against HCMV has been reported; such immunity results in a lower rate of congenital infection and lessens the severity of disease sequelae (16, 60). The means by which preexisting maternal immunity affords some protection are unknown, although the presence of high-avidity virus-specific IgG1 in HCMV-seropositive mothers restricts viral replication in the placenta (43, 47). However, low-avidity virus-specific maternal antibodies may enhance transplacental transfer of HCMV through the neonatal FcRn-transcytosis of maternal antibody-coated virus (35). This dichotomy in the relationship between the virus, the immune system, and FcRn becomes problematic when considering the best vaccination strategy for this at risk population.
In conclusion, neonatal mice represent a suitable model for studying certain aspects of CMV disease, demonstrating signs of extended viral shedding and neural tropism that may reflect a lack of immune control over the virus. The current studies characterized the development of a model for maternal transmission of MCMV by breastfeeding. This approach will allow us to discern whether infection of neonates by this natural route has consequences on the development of immunity. It may represent a means of natural immunization against the virus, or early exposure to pathogens may play a role in the development of tolerance, with potential for detrimental outcomes in later life.
ACKNOWLEDGMENTS
We thank Adam Matson for his protocol on collecting breast milk from lactating mice. We are grateful to Ann Hill, David Woodland, Kamal Khanna, John Shanley, and Joshua Obar for helpful advice and thoughtful discussions throughout the project.
Funding for these studies was provided by NIH grant AI078192 (L.P. and C.A.W.) and NIH grant AI051583 (L.L.).
Footnotes
Published ahead of print on 2 March 2011.
REFERENCES
- 1. Adler S. P. 1991. Molecular epidemiology of cytomegalovirus: a study of factors affecting transmission among children at three day-care centers. Pediatr. Infect. Dis. J. 10:584–590 [PubMed] [Google Scholar]
- 2. Arvin A. M., Fast P., Myers M., Plotkin S., Rabinovich R. 2004. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin. Infect. Dis. 39:233–239 [DOI] [PubMed] [Google Scholar]
- 3. Ballard R. A., Drew W. L., Hufnagle K. G., Riedel P. A. 1979. Acquired cytomegalovirus infection in preterm infants. Am. J. Dis. Child. 133:482–485 [DOI] [PubMed] [Google Scholar]
- 4. Bantug G. R., et al. 2008. CD8+ T lymphocytes control murine cytomegalovirus replication in the central nervous system of newborn animals. J. Immunol. 181:2111–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barkovich A. J., Lindan C. E. 1994. Congenital cytomegalovirus infection of the brain: imaging analysis and embryologic considerations. AJNR Am. J. Neuroradiol. 15:703–715 [PMC free article] [PubMed] [Google Scholar]
- 6. Brautigam A. R., Dutko F. J., Olding L. B., Oldstone M. B. 1979. Pathogenesis of murine cytomegalovirus infection: the macrophage as a permissive cell for cytomegalovirus infection, replication and latency. J. Gen. Virol. 44:349–359 [DOI] [PubMed] [Google Scholar]
- 7. Brune W., Hengel H., Koszinowski U. H. 2001. A mouse model for cytomegalovirus infection. Curr. Protoc. Immunol. 19.7.1–19.7.13. doi:10.1002/0471142735.im1907s43 [DOI] [PubMed] [Google Scholar]
- 8. Cekinović D., et al. 2008. Passive immunization reduces murine cytomegalovirus-induced brain pathology in newborn mice. J. Virol. 82:12172–12180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Demmler G. J. 1996. Congenital cytomegalovirus infection and disease. Adv. Pediatr. Infect. Dis. 11:135–162 [PubMed] [Google Scholar]
- 10. den Haan J. M., Bevan M. J. 2002. Constitutive versus activation-dependent cross-presentation of immune complexes by CD8(+) and CD8(−) dendritic cells in vivo. J. Exp. Med. 196:817–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diosi P., Babusceac L., Nevinglovschi O., Kun-Stoicu G. 1967. Cytomegalovirus infection associated with pregnancy. Lancet ii:1063–1066 [DOI] [PubMed] [Google Scholar]
- 12. Doctor S., et al. 2005. Cytomegalovirus transmission to extremely low-birthweight infants through breast milk. Acta Paediatr. 94:53–58 [DOI] [PubMed] [Google Scholar]
- 13. Doom C. M., Hill A. B. 2008. MHC class I immune evasion in MCMV infection. Med. Microbiol. Immunol. 197:191–204 [DOI] [PubMed] [Google Scholar]
- 14. Dworsky M., Yow M., Stagno S., Pass R. F., Alford C. 1983. Cytomegalovirus infection of breast milk and transmission in infancy. Pediatrics 72:295–299 [PubMed] [Google Scholar]
- 15. Fowler K. B., Dahle A. J., Boppana S. B., Pass R. F. 1999. Newborn hearing screening: will children with hearing loss caused by congenital cytomegalovirus infection be missed? J. Pediatr. 135:60–64 [DOI] [PubMed] [Google Scholar]
- 16. Fowler K. B., Stagno S., Pass R. F. 2003. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA 289:1008–1011 [DOI] [PubMed] [Google Scholar]
- 17. Gartner L. M., et al. 2005. Breastfeeding and the use of human milk. Pediatrics 115:496–506 [DOI] [PubMed] [Google Scholar]
- 18. Griffith B. P., Lavallee J. T., Jennings T. A., Hsiung G. D. 1985. Transmission of maternal cytomegalovirus-specific immunity in the guinea pig. Clin. Immunol. Immunopathol. 35:169–181 [DOI] [PubMed] [Google Scholar]
- 19. Grundy J. E., Mackenzie J. S., Stanley N. F. 1981. Influence of H-2 and non-H-2 genes on resistance to murine cytomegalovirus infection. Infect. Immun. 32:277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hahn G., Jores R., Mocarski E. S. 1998. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc. Natl. Acad. Sci. U. S. A. 95:3937–3942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamprecht K., et al. 2001. Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet 357:513–518 [DOI] [PubMed] [Google Scholar]
- 22. Hayashi K., Saze K., Uchida Y. 1985. Studies of latent cytomegalovirus infection: the macrophage as a virus-harboring cell. Microbiol. Immunol. 29:625–634 [DOI] [PubMed] [Google Scholar]
- 23. Hayes K., Danks D. M., Gibas H., Jack I. 1972. Cytomegalovirus in human milk. N. Engl. J. Med. 287:177–178 [DOI] [PubMed] [Google Scholar]
- 24. Henry S. C., et al. 2000. Enhanced green fluorescent protein as a marker for localizing murine cytomegalovirus in acute and latent infection. J. Virol. Methods 89:61–73 [DOI] [PubMed] [Google Scholar]
- 25. Jonjić S., et al. 1994. Antibodies are not essential for the resolution of primary cytomegalovirus infection but limit dissemination of recurrent virus. J. Exp. Med. 179:1713–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jordan M. C., Shanley J. D., Stevens J. G. 1977. Immunosuppression reactivates and disseminates latent murine cytomegalovirus. J. Gen. Virol. 37:419–423 [DOI] [PubMed] [Google Scholar]
- 27. Koffron A. J., et al. 1998. Cellular localization of latent murine cytomegalovirus. J. Virol. 72:95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kondo K., Kaneshima H., Mocarski E. S. 1994. Human cytomegalovirus latent infection of granulocyte-macrophage progenitors. Proc. Natl. Acad. Sci. U. S. A. 91:11879–11883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koontz T., et al. 2008. Altered development of the brain after focal herpesvirus infection of the central nervous system. J. Exp. Med. 205:423–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kosugi I., et al. 2000. Cytomegalovirus infection of the central nervous system stem cells from mouse embryo: a model for developmental brain disorders induced by cytomegalovirus. Lab. Invest. 80:1373–1383 [DOI] [PubMed] [Google Scholar]
- 31. Krmpotic A., et al. 1999. The immunoevasive function encoded by the mouse cytomegalovirus gene m152 protects the virus against T cell control in vivo. J. Exp. Med. 190:1285–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kurz S. K., et al. 1999. Focal transcriptional activity of murine cytomegalovirus during latency in the lungs. J. Virol. 73:482–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li R. Y., Tsutsui Y. 2000. Growth retardation and microcephaly induced in mice by placental infection with murine cytomegalovirus. Teratology 62:79–85 [DOI] [PubMed] [Google Scholar]
- 34. Lin E. Y., Gouon-Evans V., Nguyen A. V., Pollard J. W. 2002. The macrophage growth factor CSF-1 in mammary gland development and tumor progression. J. Mammary Gland Biol. Neoplasia 7:147–162 [DOI] [PubMed] [Google Scholar]
- 35. Maidji E., McDonagh S., Genbacev O., Tabata T., Pereira L. 2006. Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am. J. Pathol. 168:1210–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Malinger G., et al. 2003. Fetal cytomegalovirus infection of the brain: the spectrum of sonographic findings. AJNR Am. J. Neuroradiol. 24:28–32 [PMC free article] [PubMed] [Google Scholar]
- 37. Malm G., Grondahl E. H., Lewensohn-Fuchs I. 2000. Congenital cytomegalovirus infection: a retrospective diagnosis in a child with pachygyria. Pediatr. Neurol. 22:407–408 [DOI] [PubMed] [Google Scholar]
- 38. Maschmann J., Hamprecht K., Dietz K., Jahn G., Speer C. P. 2001. Cytomegalovirus infection of extremely low-birth weight infants via breast milk. Clin. Infect. Dis. 33:1998–2003 [DOI] [PubMed] [Google Scholar]
- 39. Matson A. P., Thrall R. S., Rafti E., Lingenheld E. G., Puddington L. 2010. IgG transmitted from allergic mothers decreases allergic sensitization in breastfed offspring. Clin. Mol. Allergy 8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Meier J., et al. 2005. Human cytomegalovirus reactivation during lactation and mother-to-child transmission in preterm infants. J. Clin. Microbiol. 43:1318–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miron D., et al. 2005. Incidence and clinical manifestations of breast milk-acquired cytomegalovirus infection in low birth weight infants. J. Perinatol. 25:299–303 [DOI] [PubMed] [Google Scholar]
- 42. Mitchell B. M., Leung A., Stevens J. G. 1996. Murine cytomegalovirus DNA in peripheral blood of latently infected mice is detectable only in monocytes and polymorphonuclear leukocytes. Virology 223:198–207 [DOI] [PubMed] [Google Scholar]
- 43. Nozawa N., Fang-Hoover J., Tabata T., Maidji E., Pereira L. 2009. Cytomegalovirus-specific, high-avidity IgG with neutralizing activity in maternal circulation enriched in the fetal bloodstream. J. Clin. Virol. 46(Suppl. 4):S58–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Olver S. D., Price P., Shellam G. R. 1994. Cytomegalovirus hepatitis: characterization of the inflammatory infiltrate in resistant and susceptible mice. Clin. Exp. Immunol. 98:375–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Orange J. S., Salazar-Mather T. P., Opal S. M., Biron C. A. 1997. Mechanisms for virus-induced liver disease: tumor necrosis factor-mediated pathology independent of natural killer and T cells during murine cytomegalovirus infection. J. Virol. 71:9248–9258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pass R. F., Little E. A., Stagno S., Britt W. J., Alford C. A. 1987. Young children as a probable source of maternal and congenital cytomegalovirus infection. N. Engl. J. Med. 316:1366–1370 [DOI] [PubMed] [Google Scholar]
- 47. Pereira L., Maidji E., McDonagh S., Genbacev O., Fisher S. 2003. Human cytomegalovirus transmission from the uterus to the placenta correlates with the presence of pathogenic bacteria and maternal immunity. J. Virol. 77:13301–13314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Perlman J. M., Argyle C. 1992. Lethal cytomegalovirus infection in preterm infants: clinical, radiological, and neuropathological findings. Ann. Neurol. 31:64–68 [DOI] [PubMed] [Google Scholar]
- 49. Pitt J. 1979. The milk mononuclear phagocyte. Pediatrics 64:745–749 [PubMed] [Google Scholar]
- 50. Pollock J. L., Presti R. M., Paetzold S., Virgin IV H. W. 1997. Latent murine cytomegalovirus infection in macrophages. Virology 227:168–179 [DOI] [PubMed] [Google Scholar]
- 51. Pollock J. L., Virgin IV H. W. 1995. Latency, without persistence, of murine cytomegalovirus in the spleen and kidney. J. Virol. 69:1762–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reddehase M. J., et al. 1994. The conditions of primary infection define the load of latent viral genome in organs and the risk of recurrent cytomegalovirus disease. J. Exp. Med. 179:185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reuter J. D., Gomez D. L., Wilson J. H., N. van den Pol A. 2004. Systemic immune deficiency necessary for cytomegalovirus invasion of the mature brain. J. Virol. 78:1473–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schuurhuis D. H., et al. 2002. Antigen-antibody immune complexes empower dendritic cells to efficiently prime specific CD8+ CTL responses in vivo. J. Immunol. 168:2240–2246 [DOI] [PubMed] [Google Scholar]
- 55. Siegrist C. A., et al. 1998. Influence of maternal antibodies on vaccine responses: inhibition of antibody but not T cell responses allows successful early prime-boost strategies in mice. Eur. J. Immunol. 28:4138–4148 [DOI] [PubMed] [Google Scholar]
- 56. Slavuljica I., et al. 2010. Recombinant mouse cytomegalovirus expressing a ligand for the NKG2D receptor is attenuated and has improved vaccine properties. J. Clin. Invest. 120:4532–4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Söderberg-Nauclér C., et al. 2001. Reactivation of latent human cytomegalovirus in CD14(+) monocytes is differentiation dependent. J. Virol. 75:7543–7554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stagno S., Cloud G. A. 1994. Working parents: the impact of day care and breast-feeding on cytomegalovirus infections in offspring. Proc. Natl. Acad. Sci. U. S. A. 91:2384–2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stagno S., Pass R. F., Dworsky M. E., Britt W. J., Alford C. A. 1984. Congenital and perinatal cytomegalovirus infections: clinical characteristics and pathogenic factors. Birth Defects Orig. Artic. Ser. 20:65–85 [PubMed] [Google Scholar]
- 60. Stagno S., et al. 1982. Congenital cytomegalovirus infection: the relative importance of primary and recurrent maternal infection. N. Engl. J. Med. 306:945–949 [DOI] [PubMed] [Google Scholar]
- 61. Stagno S., Reynolds D. W., Pass R. F., Alford C. A. 1980. Breast milk and the risk of cytomegalovirus infection. N. Engl. J. Med. 302:1073–1076 [DOI] [PubMed] [Google Scholar]
- 62. Stratton K., Durch J., Lawrence R. 2001. Vaccines for the 21st century: a tool for decision making. National Academy Press, Washington, DC: [PubMed] [Google Scholar]
- 63. Tassin G. B., Maklad N. F., Stewart R. R., Bell M. E. 1991. Cytomegalic inclusion disease: intrauterine sonographic diagnosis using findings involving the brain. AJNR Am. J. Neuroradiol. 12:117–122 [PMC free article] [PubMed] [Google Scholar]
- 64. Taylor-Wiedeman J., Sissons P., Sinclair J. 1994. Induction of endogenous human cytomegalovirus gene expression after differentiation of monocytes from healthy carriers. J. Virol. 68:1597–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Trgovicich J., Pernjak-Pugel E., Tomac J., Koszinowski U., Jonjic S. 1998. Pathogenesis of murine cytomegalovirus infection in neonatal mice, p. 42–53 In Scholz M., Rabenau H. F., Doerr H. W., Cinatl J., Jr. (ed.), CMV-related immunopathology. Monographs in virology, vol. 21 Karger, Basel, Switzerland [Google Scholar]
- 66. Tsutsui Y., Kashiwai A., Kawamura N., Aiba-Masago S., Kosugi I. 1995. Prolonged infection of mouse brain neurons with murine cytomegalovirus after pre- and perinatal infection. Arch. Virol. 140:1725–1736 [DOI] [PubMed] [Google Scholar]
- 67. van den Pol A. N., Mocarski E., Saederup N., Vieira J., Meier T. J. 1999. Cytomegalovirus cell tropism, replication, and gene transfer in brain. J. Neurosci. 19:10948–10965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. van den Pol A. N., Reuter J. D., Santarelli J. G. 2002. Enhanced cytomegalovirus infection of developing brain independent of the adaptive immune system. J. Virol. 76:8842–8854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vochem M., Hamprecht K., Jahn G., Speer C. P. 1998. Transmission of cytomegalovirus to preterm infants through breast milk. Pediatr. Infect. Dis. J. 17:53–58 [DOI] [PubMed] [Google Scholar]
- 70. Wirtz N., et al. 2008. Polyclonal cytomegalovirus-specific antibodies not only prevent virus dissemination from the portal of entry but also inhibit focal virus spread within target tissues. Med. Microbiol. Immunol. 197:151–158 [DOI] [PubMed] [Google Scholar]
- 71. Yeager A. S. 1983. Transmission of cytomegalovirus to mothers by infected infants: another reason to prevent transfusion-acquired infections. Pediatr. Infect. Dis. 2:295–297 [DOI] [PubMed] [Google Scholar]