Abstract
West Nile virus (WNV) replicates in the skin; however, cell targets in the skin have not been identified. In the current studies, WNV infected the epidermis and adnexal glands of mouse skin, and the epidermal cells were identified as keratinocytes by double labeling for WNV antigen and keratin 10. Inoculation of mice with WNV replicon particles resulted in high levels of replication in the skin, suggesting that keratinocytes are an initial target of WNV. In addition, primary keratinocytes produced infectious virus in vitro. In conclusion, keratinocytes are cell targets of WNV in vivo and may play an important role in pathogenesis.
TEXT
The flavivirus West Nile virus (WNV) is transmitted to vertebrates by the bite of an infected mosquito, which deposits high doses of virus extravascularly in the skin (19). The in vivo cell targets of WNV in the skin are unknown; however, evidence suggests that WNV infects Langerhan cells (LCs), the resident dendritic cells (DCs) of the skin. LCs are in vivo cell targets of dengue virus (22), another flavivirus, and DCs are susceptible to infection by WNV in vitro (6, 10, 12, 18). Following WNV inoculation of mice, LCs migrate from the skin at the inoculation site to the draining lymph nodes (DLN) (9). On the other hand, initial WNV replication after mosquito transmission or subcutaneous (s.c.) inoculation occurs in both the DLN and the skin (5, 20), suggesting that cells in the skin and lymph node are productively infected. In addition, WNV RNA persists in the skin for up to 4 months postinoculation (1). These results led us to hypothesize that WNV infects nonmigrating cells in the skin.
We examined mice inoculated with WNV to identify cell targets in the skin. Six-week-old female C3H/HeN (C3H) or C57BL/6 (B6) mice (Taconic, Germantown, NY) were inoculated s.c. in both rear footpads (5) with diluent (mock) or 105 PFU of clone-derived WNV (17), with approval from the Wadsworth Center's Institutional Animal Care and Use Committee, in accordance with the criteria established by the National Institutes of Health. Mice were sacrificed at 4 or 5 days postinoculation (dpi), and feet were fixed with 10% buffered formalin for 1 day and 70% ethanol for 6 days. The plantar skin was embedded in paraffin, and the entire block was serially sectioned (approximately 80 sections, 6 μm each), with placement of 2 to 4 sections per slide (ProbeOn Plus; Fisher Scientific, Hanover Park, IL). For immunohistochemistry (IHC) and immunofluorescence assays (IFA), tissues were deparaffinized in xylene, hydrated in graded alcohol, and microwaved in citric acid-based antigen unmasking solution (Vector Laboratories, Burlingame, CA) for 3 cycles of 5 min (800, 500, and 500 W). An M.O.M. basic kit (Vector Laboratories) was used for staining, and tissues were incubated with primary antibodies at 4°C overnight and secondary antibodies at 37°C for 1 h (detailed information on the antibodies is available from the corresponding author upon request). For IHC, tissues were also treated with peroxidase suppressor (Thermo Scientific, Rockford, IL), ImmPACT NovaRED substrate (Vector Laboratories), and hematoxylin QS (Vector Laboratories), dehydrated, and mounted with VectaMount (Vector Laboratories).
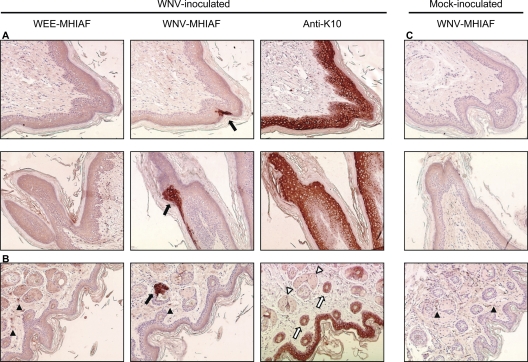
We screened alternate slides of every foot sample by IHC with mouse hyperimmune ascites fluid (MHIAF) against WNV (Centers for Disease Control and Prevention, Atlanta, GA) and detected WNV-positive cells in 17% (29/167) of the slides with positive sections in 100% of C3H (n = 4) and 75% of B6 (n = 8) mice. WNV antigen was found in focal areas of the epidermis (20/29 slides) (Fig. 1A) and in epithelial cells of adnexal glands associated with hair follicles (9/29 slides) (Fig. 1B) of WNV-inoculated mice but not in those of mock-inoculated mice (Fig. 1C). No staining was observed in the epidermis or glands with an irrelevant control (MHIAF against western equine encephalitis [WEE]; Centers for Disease Control and Prevention); however, nonspecific staining by WEE-MHIAF and WNV-MHIAF was occasionally observed in the dermis of mock- and WNV-inoculated mice (Fig. 1).
Fig. 1.
WNV-positive cells are found in the epidermis and adnexal glands of mouse skin. Representative photomicrographs of skin sections from WNV-inoculated (A and B) and mock-inoculated (C) mice are shown. Six-week-old female C3H mice were sacrificed at 5 days after s.c. inoculation with diluent (mock) or 105 PFU of WNV in both rear footpads. Footpad skin was processed for IHC, and selected adjacent sections were stained with WNV-MHIAF, WEE-MHIAF, or anti-K10. (A) WNV-positive cells were located in the epidermis (black arrows) in areas of K10-positive cells. (B) WNV-positive cells were also found in ducts of glands (black arrow), which were not positive for K10 (white arrowhead); however, hair follicles (white arrows) and epidermis were K10 positive. Nonspecific staining in the dermis (black arrowheads) was also observed in some sections, as shown in panels B and C. Magnification, ×150.
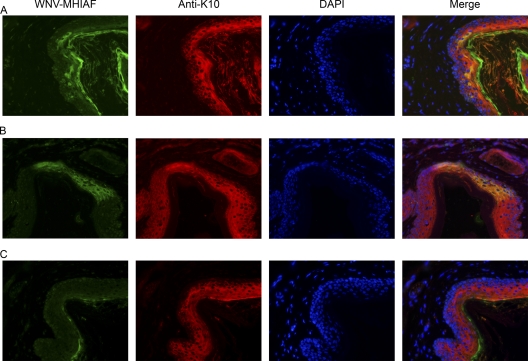
Based on the location of the WNV-positive cells in the epidermis, we hypothesized that the WNV-positive cells were keratinocytes. We performed IHC on adjacent sections of the WNV-positive tissues using antibodies against keratin 10 (K10; Covance, Emeryville, CA), a marker for keratinocytes, and found K10-positive cells in the same area as the WNV-positive cells (Fig. 1A). Double labeling by IFA, using WNV-MHIAF and anti-K10, was performed to confirm that keratinocytes are susceptible to WNV infection in vivo. WNV-positive cells were again detected in the epidermis of WNV-inoculated mice, and these cells were also positive for K10 (Fig. 2A and B). Weak, nonspecific reactivity was also observed with WNV-MHIAF in the stratum corneum of mock-inoculated (Fig. 2C) and WNV-inoculated (Fig. 2A and B) mice. In conclusion, results from the IFA and IHC demonstrate that keratinocytes are in vivo cell targets of WNV.
Fig. 2.
WNV-positive cells in the epidermis are keratinocytes. Representative photomicrographs of skin sections from WNV-inoculated (A and B) and mock-inoculated (C) mice are shown. Six-week-old female C3H mice (A and C) or B6 mice (B) were sacrificed at 4 or 5 days after s.c. inoculation with diluent (mock) or 105 PFU of WNV in both rear footpads. Footpad skin was processed for IFA, and sections were stained with WNV-MHIAF and anti-K10, followed by fluorescent conjugated secondary antibodies and Vectashield mounting medium with DAPI (4′,6-diamidino-2-phenylindole; Vector Laboratories). Tissues were observed and photographed using a fluorescence microscope (Nikon Instruments, Inc.), and the images were merged using the public domain ImageJ program (developed at the U.S. National Institutes of Health and available at http://rsb.info.nih.gov/ij/). Magnification, ×300.
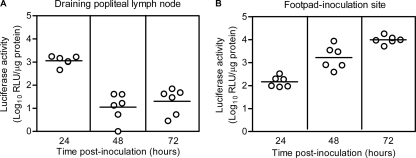
We were unable to detect WNV antigen in mice earlier than 4 dpi (data not shown); therefore, we used WNV replicon particles (16), which infect a single round of cells and encode a luciferase reporter, to examine whether skin cells are initial targets of WNV. Mice were inoculated s.c. in the left rear footpads (5) with diluent or 2 × 105 WNV replicon particles. At various time points, skin and DLN were harvested and assayed for luciferase activity, using the Renilla luciferase assay system (Promega, Madison, WI), and for protein concentration, using the Compat-Able protein assay preparation and Micro BCA protein assay kits (Thermo Scientific). Luciferase activity was detected in the DLN at 24 h postinoculation (hpi) (mean = 1,100 relative light units [RLU]/μg protein), which decreased 100-fold at 48 hpi (mean = 10 RLU/μg protein) (Fig. 3A). In the skin at the inoculation site, luciferase activity increased 65-fold between 24 hpi (mean = 150 RLU/μg protein) and 72 hpi (mean = 9,900 RLU/μg protein) (Fig. 3B), suggesting that WNV infects nonmigrating cells in the skin that continue to support virus replication. Taken together, our data strongly suggest that keratinocytes are one of the initial targets of WNV.
Fig. 3.
WNV replicon particles target the skin at the inoculation site and the draining lymph node. Six-week-old female B6 mice were inoculated s.c. in the left rear footpads with diluent (mock, n = 2) or 2 × 105 of WNV replicon particles that express luciferase (n = 6). At various time points postinoculation, mice were sacrificed, and popliteal lymph nodes (A) and footpad skin (B) were harvested, homogenized, and assayed for luciferase activity and protein concentration. The results were calculated as follows: (number of RLU/μg protein for each WNV replicon-inoculated mouse) − (average number of RLU/μg protein for mock-inoculated mice). Data points are from individual mice, and the horizontal lines represent the geometric means.
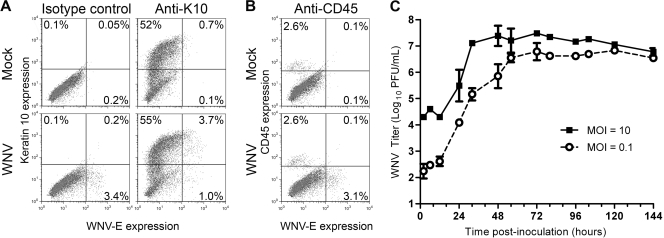
In vitro studies with primary keratinocytes were performed to confirm our in vivo results. We previously showed that WNV productively infects primary skin cells (10); however, the cell targets of WNV in these cultures were not identified. Thus, primary skin cells were harvested from mice (9) and inoculated with diluent or WNV at a multiplicity of infection (MOI) of 50. Cells were processed for flow cytometry (10), using antibodies against WNV envelope (WNV-E) (7H2; BioReliance, Rockville, MD) and either K10 or the pan-leukocyte marker CD45 (eBioscience, San Diego, CA). At 48 hpi, 3.5% of the skin cells expressed WNV-E, and 74% of these WNV-positive cells expressed K10. Thus, consistent with our IHC and IFA results (Fig. 1 and 2), keratinocytes were the major cell population susceptible to WNV infection in primary skin cells (Fig. 4A). We did not detect CD45+ WNV-E+ cells; however, small numbers of WNV-infected CD45+ cells would have been difficult to detect since only 2.7% of the skin cell population was CD45+ (Fig. 4B). WNV growth curves were performed on primary human keratinocytes (PCS-200-011; ATCC). WNV titers increased over time and plateaued at 32 and 56 hpi for MOIs of 10 and 0.1, respectively (Fig. 4C). In conclusion, WNV productively infects human and mouse keratinocytes.
Fig. 4.
Primary keratinocytes are susceptible to WNV infection and support productive WNV infection. (A and B) Primary skin cells were isolated from ears of mice and inoculated with diluent (mock) or WNV at an MOI of 50. At 48 hpi, cells were harvested, fixed, permeabilized, and incubated with antibodies against WNV-E and keratin 10 (A) or CD45 (B). Samples were analyzed on a FACScan flow cytometer (BD Biosciences, San Jose, CA) using FlowJo software (Tree Star, Inc., Ashland, OR), and results from representative dot plot analyses from two independent experiments performed in triplicate are shown. (C) Primary human keratinocytes were inoculated with WNV at an MOI of 0.1 or 10. At various time points postinoculation, medium was harvested, and viral titers were determined by a plaque assay with Vero cells. The geometric means and standard deviations for triplicate samples are shown, and data are representative of two independent experiments.
In summary, we are the first to demonstrate that WNV infects keratinocytes in vivo. In addition, our current study definitively demonstrates that cells of the skin and DLN are primary targets of replication, and one of these first cell targets is most likely keratinocytes. Consistent with a previous report on primary mouse keratinocytes (21), primary human keratinocytes supported a productive WNV infection, yielding more than 200 infectious virus particles per cell (data not shown). Thus, WNV infection of keratinocytes in vivo is likely the source of high levels of infectious virus detected in the skin of mice for up to 7 dpi following s.c. inoculation with WNV (5).
We detected WNV-positive cells only in epithelial cells of the epidermis and adnexal glands associated with hair follicles (i.e., sebaceous or apocrine glands). In other studies, epidermal cells were positive for WNV antigen in fatal cases in hawks (23), and eccrine glands of the skin were positive for WNV antigen in a fatal human case with West Nile encephalitis (2). Keratinocyte labeling was not performed in these studies; however, these results still support our findings that the epidermis and adnexal glands are targets of WNV. Furthermore, the lack of WNV-positive epidermal cells in the human cases may be due to limited sampling and/or sectioning. It is possible that infected epithelial cells in human skin are the cause of rash, which is observed in 25 to 40% of patients with West Nile encephalomyelitis (4). Other members of the Flaviviridae family, dengue (11) and bovine virus diarrhea (14) viruses, also infect epidermal keratinocytes in vivo. In addition, bovine virus diarrhea virus infects sebaceous and apocrine glands (3, 14), supporting our finding that adnexal glands are viral targets.
On the other hand, we cannot rule out that other types of skin cells may be infected at low numbers due to limitations in the sensitivity and specificity of our IHC assay. Paddock et al. observed WNV antigens in perivascular mononuclear inflammatory cells and vessel lumens in the dermis from a hemorrhagic skin lesion of a fatal human case of WNV hemorrhagic fever (15), but this is likely a rare finding associated with hemorrhaging. LCs are postulated to be cell targets of WNV, but we were unable to identify WNV-positive, K10-negative cells in the skin except for epithelial cells of adnexal glands. It is, however, possible that infected LCs migrated to the DLN and, thus, were not detected. Alternatively, small numbers of WNV-positive LCs or other K10-negative cells may have been overlooked due to the nonspecific background staining observed with WNV-MHIAF in the dermis.
Finally, keratinocytes may contribute to WNV persistence in the skin. WNV persists in the skin for up to 4 months postinoculation in mice (1) and 1 month in birds (13), and high titers of WNV were produced in primary skin cultures (10) and human keratinocytes (Fig. 4C) for at least 6 days in vitro. Epidermal keratinocytes have turnover rates of 34 to 44% per week in mice (8) and every 52 to 75 days for humans (7). Thus, it is possible that WNV persists in keratinocytes for the life of the cell without infecting new cells. In addition, the lack of inflammation in the skin (Fig. 1), which is consistent with a previous report (2), could prevent clearance of WNV in the skin. Alternatively, WNV could persist within skin glands or other cells not identified in the current study. Further studies are warranted to understand the role of keratinocytes during WNV infection and persistence.
Acknowledgments
We thank Anju Subba and Gang Zou for technical assistance and the Wadsworth Center's tissue culture core and histopathology core for cell preparation and preparation of tissue samples, respectively.
This work was supported partly by NIH/NIAID contract N01-AI25490. The BSL-3 vivarium at the Wadsworth Center was used, which is funded in part as a core facility by NIH/NIAID U54-AI057158 (Northeast Biodefense Center).
Footnotes
Published ahead of print on 2 March 2011.
REFERENCES
- 1. Appler K. K., et al. 2010. Persistence of West Nile virus in the central nervous system and periphery of mice. PLoS One 5:e10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Armah H. B., et al. 2007. Systemic distribution of West Nile virus infection: postmortem immunohistochemical study of six cases. Brain Pathol. 17:354–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baszler T. V., Evermann J. F., Kaylor P. S., Byington T. C., Dilbeck P. M. 1995. Diagnosis of naturally occurring bovine viral diarrhea virus infections in ruminants using monoclonal antibody-based immunohistochemistry. Vet. Pathol. 32:609–618 [DOI] [PubMed] [Google Scholar]
- 4. Bode A. V., Sejvar J. J., Pape W. J., Campbell G. L., Marfin A. A. 2006. West Nile virus disease: a descriptive study of 228 patients hospitalized in a 4-county region of Colorado in 2003. Clin. Infect. Dis. 42:1234–1240 [DOI] [PubMed] [Google Scholar]
- 5. Brown A. N., Kent K. A., Bennett C. J., Bernard K. A. 2007. Tissue tropism and neuroinvasion of West Nile virus do not differ for two mouse strains with different survival rates. Virology 368:422–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis C. W., et al. 2006. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J. Virol. 80:1290–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Halprin K. M. 1972. Epidermal “turnover time”—a re-examination. Br. J. Dermatol. 86:14–19 [DOI] [PubMed] [Google Scholar]
- 8. Hsieh E. A., Chai C. M., de Lumen B. O., Neese R. A., Hellerstein M. K. 2004. Dynamics of keratinocytes in vivo using HO labeling: a sensitive marker of epidermal proliferation state. J. Invest. Dermatol. 123:530–536 [DOI] [PubMed] [Google Scholar]
- 9. Johnston L. J., Halliday G. M., King N. J. 2000. Langerhans cells migrate to local lymph nodes following cutaneous infection with an arbovirus. J. Invest. Dermatol. 114:560–568 [DOI] [PubMed] [Google Scholar]
- 10. Lim P. Y., Louie K. L., Styer L. M., Shi P. Y., Bernard K. A. 2010. Viral pathogenesis in mice is similar for West Nile virus derived from mosquito and mammalian cells. Virology 400:93–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Limon-Flores A. Y., et al. 2005. Dengue virus inoculation to human skin explants: an effective approach to assess in situ the early infection and the effects on cutaneous dendritic cells. Int. J. Exp. Pathol. 86:323–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martina B. E., et al. 2008. DC-SIGN enhances infection of cells with glycosylated West Nile virus in vitro and virus replication in human dendritic cells induces production of IFN-alpha and TNF-alpha. Virus Res. 135:64–71 [DOI] [PubMed] [Google Scholar]
- 13. Nemeth N., et al. 2009. Persistent West Nile virus infection in the house sparrow (Passer domesticus). Arch. Virol. 154:783–789 [DOI] [PubMed] [Google Scholar]
- 14. Njaa B. L., Clark E. G., Janzen E., Ellis J. A., Haines D. M. 2000. Diagnosis of persistent bovine viral diarrhea virus infection by immunohistochemical staining of formalin-fixed skin biopsy specimens. J. Vet. Diagn. Invest. 12:393–399 [DOI] [PubMed] [Google Scholar]
- 15. Paddock C. D., et al. 2006. Fatal hemorrhagic fever caused by West Nile virus in the United States. Clin. Infect. Dis. 42:1527–1535 [DOI] [PubMed] [Google Scholar]
- 16. Puig-Basagoiti F., et al. 2005. High-throughput assays using a luciferase-expressing replicon, virus-like particles, and full-length virus for West Nile virus drug discovery. Antimicrob. Agents Chemother. 49:4980–4988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shi P. Y., Tilgner M., Lo M. K., Kent K. A., Bernard K. A. 2002. Infectious cDNA clone of the epidemic West Nile virus from New York City. J. Virol. 76:5847–5856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Silva M. C., Guerrero-Plata A., Gilfoy F. D., Garofalo R. P., Mason P. W. 2007. Differential activation of human monocyte-derived and plasmacytoid dendritic cells by West Nile virus generated in different host cells. J. Virol. 81:13640–13648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Styer L. M., et al. 2007. Mosquitoes inoculate high doses of West Nile virus as they probe and feed on live hosts. PLoS Pathog. 3:e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Styer L. M., et al. 2011. Mosquito saliva causes enhancement of West Nile virus infection in mice. J. Virol. 85:1517–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Welte T., et al. 2009. Toll-like receptor 7-induced immune response to cutaneous West Nile virus infection. J. Gen. Virol. 90:2660–2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu S. J., et al. 2000. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 6:816–820 [DOI] [PubMed] [Google Scholar]
- 23. Wunschmann A., et al. 2004. Pathologic findings in red-tailed hawks (Buteo jamaicensis) and Cooper's hawks (Accipiter cooper) naturally infected with West Nile virus. Avian Dis. 48:570–580 [DOI] [PubMed] [Google Scholar]