Abstract
Seasonal influenza epidemics recur due to antigenic drift of envelope glycoprotein antigens and immune evasion of circulating viruses. Additionally, antigenic shift can lead to influenza pandemics. Thus, a universal vaccine that protects against multiple influenza virus strains could alleviate the continuing impact of this virus on human health. In mice, accelerated clearance of a new viral strain (cross-protection) can be elicited by prior infection (heterosubtypic immunity) or by immunization with the highly conserved internal nucleoprotein (NP). Both heterosubtypic immunity and NP-immune protection require antibody production. Here, we show that systemic immunization with NP readily accelerated clearance of a 2009 pandemic H1N1 influenza virus isolate in an antibody-dependent manner. However, human immunization with trivalent inactivated influenza virus vaccine (TIV) only rarely and modestly boosted existing levels of anti-NP IgG. Similar results were observed in mice, although the reaction could be enhanced with adjuvants, by adjusting the stoichiometry among NP and other vaccine components, and by increasing the interval between TIV prime and boost. Importantly, mouse heterosubtypic immunity that had waned over several months could be enhanced by injecting purified anti-NP IgG or by boosting with NP protein, correlating with a long-lived increase in anti-NP antibody titers. Thus, current immunization strategies poorly induce NP-immune antibody that is nonetheless capable of contributing to long-lived cross-protection. The high conservation of NP antigen and the known longevity of antibody responses suggest that the antiviral activity of anti-NP IgG may provide a critically needed component of a universal influenza vaccine.
INTRODUCTION
Seasonal influenza virus infections hospitalize 200,000 and kill 36,000 Americans annually (28, 45, 56). More recently, a novel H1N1 swine-origin influenza virus acquired the ability to be transmitted from human to human at a rate declared by the WHO to be pandemic in early June 2009 (16, 32). Seasonal influenza vaccines induce antibodies against the external viral proteins hemagglutinin (H) and neuraminidase (N). Such antibodies bind to influenza virions and either prevent virus entry into host cells (true neutralization) or prevent the release of new virions (7, 23, 45, 51). However, H and N antigens (Ags) continuously change. Consequently, antibodies induced by a given vaccine may not recognize or protect against viruses that circulate in subsequent seasons (30), and recent evidence suggests that such neutralizing antibodies can promote immune evasion by selecting for variant viruses that avidly bind to host cells (21). This failure of cross-protection burdens the American and global health care systems with the costs of annual vaccine reformulation, readministration, and caring for individuals who fail to be revaccinated or fail to be protected by mismatched vaccines. To reduce these costly circumstances and future pandemics, an influenza vaccine more cross-protective than the current formulations that depend on neutralizing antibodies is needed.
Unlike HN external Ags, the internal nucleoprotein (NP) is greater than 90% conserved among all influenza A virus strains (37, 42), including highly pathogenic H5N1 avian viruses (e.g., GenBank DQ493166), and the 2009 H1N1 pandemic virus (e.g., GenBank ACP41106). NP vaccination accelerates viral clearance and prevents mortality in mice subsequently challenged with various influenza A virus strains (9, 12, 14, 17, 27, 41, 48, 50, 53, 54, 57). Thus, unlike inactivated virus vaccines, NP immunization provides cross-protection similar to that induced by prior influenza virus infection (heterosubtypic immunity [Het-I]) (12, 14, 17, 27, 53, 54, 57). Importantly, Het-I is poor in B cell-deficient mice (39) and in mice with limited antibody diversity (33), suggesting that antibody responses to conserved Ags, such as NP, make a major contribution to Het-I. In fact, accelerated viral clearance induced by NP protein immunization requires antibody production (3).
Despite the importance of antibody for Het-I and for NP-immune protection in mice (3, 33), the presence of NP-reactive IgG in potentially susceptible individuals has raised concern about the utility of these antibodies in humans (10, 47, 58). However, such observations do not exclude the possibility that NP-immune antibody or NP-immune memory T cells (each of which is induced by prior infection and could be enhanced with proper boosting) can or do provide antiviral immunity. In fact, animal studies show that NP-immune antibody (3) and NP-immune T cells (17, 54) can each transfer protective antiviral effects. Here, we show that NP in the context of trivalent inactivated influenza virus vaccine (TIV) has relatively poor immunogenicity in humans and in mice. However, in the latter case, NP-reactive antibody induction can be influenced by adjuvants, by the relative ratio of NP to other vaccine components, and by the interval between TIV injections. Importantly, either passively or actively boosting anti-NP IgG in influenza virus-immune mice significantly enhances clearance of a secondary challenge with an HN-distinct virus. These findings have important implications for understanding the role of anti-NP IgG in Het-I and clearly show the benefit and potential of inducing such antibodies in humans by modifying existing vaccine regimens.
MATERIALS AND METHODS
Animals and viruses.
C57BL/6 and AID.μS (generated as described previously [3]) mice were bred and maintained in the University of Rochester vivarium. Because AID−/− mice cannot undergo antibody class switching and μS mice cannot secrete IgM, AID.μS mice lack all secreted antibody (data not shown). All animal procedures were approved by the University of Rochester University Committee on Animal Resources. H3N2 A/X31 (X31), H1N1 A/PR8/34 (PR8), and novel pandemic H1N1 A/California/04/09 (A/Ca; a gift from David Topham, University of Rochester) influenza viruses were grown in embryonated hen's eggs as described previously (39). Mice were infected intranasally (i.n.) with influenza virus in 100 μl phosphate-buffered saline (PBS) under isoflurane anesthesia. Viral stock titers were determined using an MDCK cell focus assay to determine viral focus units (VFUs) per ml of stock allantoic fluid. Fifty percent lethal dose (LD50) values for each viral stock were determined by infecting groups of 5 C57BL/6 mice with various VFU doses. The dose resulting in ∼50% death was considered the LD50.
Measuring NP in TIV.
To quantitate the NP content of TIV by enzyme-linked immunosorbent assay (ELISA), 96-well flexiplates were coated with 2 μg/ml anti-NP IgG2b clone 3C7. Washed and blocked wells were then incubated with serial dilutions of 2008/09 TIV (Sanofi Pasteur) or with serial dilutions of recombinant purified NP (3) as a standard. Bound NP was probed with biotinylated polyclonal mouse NP-immune IgG purified from mouse serum. Signal was detected with streptavidin-horseradish peroxidase (SA-HRP) followed by ABT [2,2′-azinobis(3-ethylbenzthiazoline)] substrate. Separately, recombinant NP (rNP) and TIV were electrophoresed under reducing conditions using a NuPage 4 to 12% polyacrylamide gel, blotted onto nitrocellulose, and probed with the biotinylated polyclonal anti-NP IgG, followed by SA-HRP and enhanced chemiluminescence (ECL) detection.
TIV immunization.
For human study 1, plasma samples were collected from healthy human subjects at the Naval Health Research Center (San Diego, CA) prior to and after intramuscular (i.m.) vaccination with 2008/09 trivalent inactivated influenza virus vaccine (TIV; Fluzone). Study 1 was approved by the Institutional Review Board of the Naval Health Research Center. For human study 2, serum was collected at the indicated time points prior to and after i.m. vaccination with 2003/04 TIV (Evans, Aventis, or Fluviron). Study 2 was approved by the Research Subjects Review Board, University of Rochester Medical Center. Endpoint ELISA was performed by coating polyvinyl plates with 2 μg/ml recombinant, purified influenza virus NP prepared as described previously (3). After being washed and blocked in bovine serum albumin (BSA)-PBS-Tween, serial dilutions of plasma or serum were added. Bound antibody was detected by biotinylated goat anti-human IgG, followed by streptavidin-HRP. The reaction was detected with nitroblue tetrazolium (NBT) substrate.
Mouse vaccinations and antibodies.
Eight- to 12-week-old mice were immunized intraperitoneally (i.p.) with lipopolysaccharide (LPS) with or without purified recombinant influenza virus NP (generated as described previously [3]) using doses and time points indicated in the figure legends. 2008/09 TIV (Fluzone; a gift from Sanofi Pasteur) was injected i.p. at the indicated time points. TIV doses for immunization are given as μg hemagglutinin (HA) equivalent (eq), represented by the H1, H3, and influenza B virus hemagglutinins contained in the vaccine. Sera were collected by mandibular bleeding and analyzed for NP-reactive IgG as described previously (3). For TIV-reactive IgG, plates were coated with 2 μg/ml total TIV protein, and then the procedure described above was followed using anti-mouse IgGHRP instead of the anti-human reagent. NP-specific IgG-secreting hybridomas (clones IC5-2A10-G1 [anti-NP IgG1], IC6-1H5-G2a [anti-NP IgG2a], and H19-L2-1-G2b [anti-NP IgG2b]) were a gift from Walter Gerhard (Wistar Institute). Low-endotoxin monoclonal antibody (MAb) was purified from hybridoma supernatants by BioXcell (Lebanon, NH). Irrelevant isotype-matched control antibodies (MOPC-21, MPC-11, and Cl.18) were purchased from BioXcell.
Preparation of lung tissue for determining viral load.
Influenza virus-infected mice were sacrificed, and lungs were homogenized in PBS with penicillin-streptomycin. Serial dilutions of lung homogenate were incubated on MDCK monolayers in the presence of 4 μg/ml trypsin. Detection of viral foci was performed as described previously (3).
Statistical analysis.
Statistical analysis was performed using Prism 5.0a software. P values were calculated by Student's t test, except in the experiment shown in Fig. 1B, which used the nonparametric Mann-Whitney test to analyze non-Gaussian data.
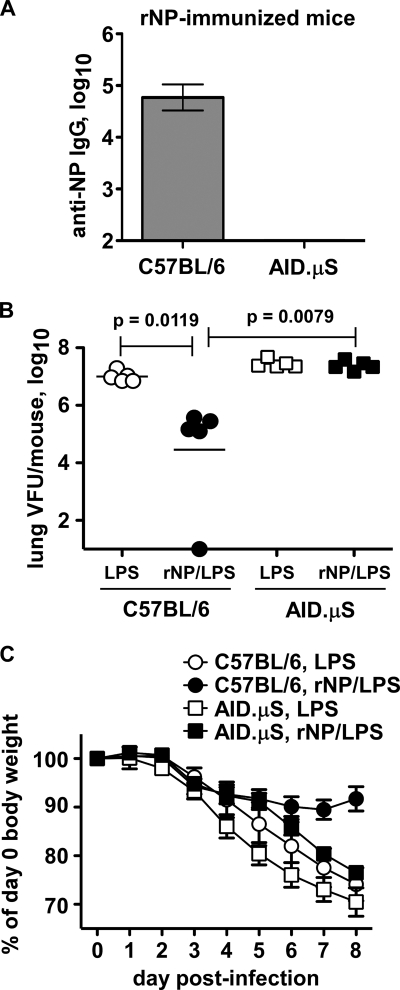
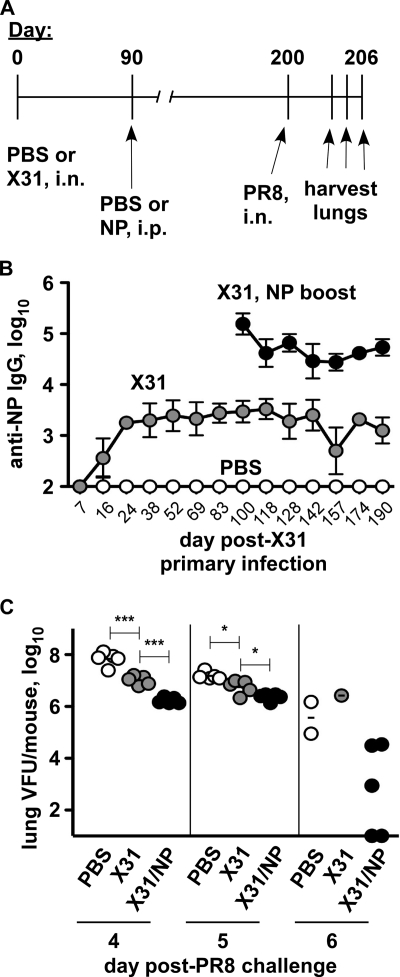
Fig. 1.
NP-immune antibody promotes pandemic H1N1 influenza virus clearance. The indicated mice were immunized i.p. with 30 μg LPS with or without 50 μg rNP (PR8 sequence) on days 0, 10, 20, and 30. (A) NP-reactive serum IgG on day 123 postprime. Mean ± standard deviation of 5 mice/group. (B) On day 124, the mice were challenged i.n. with 2.5 LD50 of pandemic H1N1 influenza virus A/CA/04/2009. Shown are lung viral titers at day 8 postinfection. (C) Body weights on the indicated days postinfection. Mean ± standard deviation of 5 mice/group.
RESULTS
NP immunization accelerates clearance of novel pandemic H1N1 influenza virus.
Influenza vaccination needs to be improved to provide cross-protection against drifting viruses, as well as unpredicted, potentially pandemic strains. NP immunization of mice accelerates virus clearance and improves survival after challenge with multiple viral serotypes (12, 14, 17, 27, 53, 54, 57) by a mechanism requiring antibody production (3). To determine whether the 2009 pandemic H1N1 virus is susceptible to antibody-mediated NP immunity, we first vaccinated C57BL/6 and antibody-deficient, AID.μS mice with recombinant purified influenza virus NP, based on the PR8 amino acid sequence, as described previously (3). Because LPS was used as an adjuvant, control mice were mock vaccinated with LPS alone. NP/LPS-immunized (NP-immune) C57BL/6 but not AID.μS mice sustained a high titer of NP-reactive IgG in the serum (Fig. 1A) as expected from previous studies (3). When challenged with the 2009 pandemic H1N1 human isolate A/California/04/09 (A/Ca) virus, mock-immunized (LPS) mice had a high titer of virus in the lung on day 8 postinfection (Fig. 1B). Compared with LPS controls, NP-immune C57BL/6 mice had significantly reduced or cleared virus at this time point (Fig. 1B). Thus, NP immunization can promote the clearance of this novel human pandemic isolate. In contrast, NP-immune AID.μS mice had no change in viral titer from that of controls (Fig. 1B). Additionally, NP immunization of C57BL/6 mice reduced weight loss after pandemic virus infection, but this was much less apparent in AID.μS mice (Fig. 1C). These results indicate that antibody production is required for NP immunization to accelerate pandemic virus clearance.
Anti-NP IgG levels after vaccination with inactivated virus.
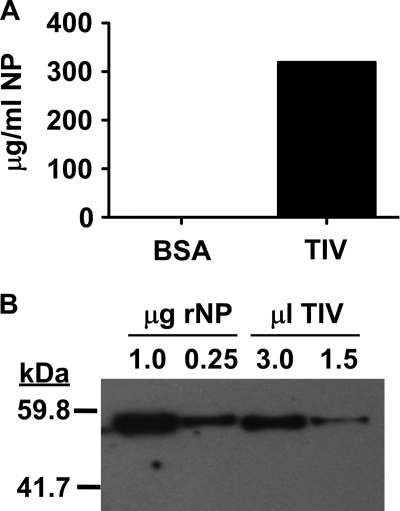
Poor viral clearance in NP-immune AID.μS mice strongly suggests that NP-specific antibody has significant antiviral activity. In fact, transfer of NP-immune C57BL/6 (but not AID.μS) mouse serum (3) or NP-specific MAb (25a) accelerates viral clearance in previously naive recipient mice. Seasonal trivalent inactivated influenza virus vaccine (TIV) is known to contain NP (4, 18, 19). It is also known that the amount of NP can vary somewhat among TIV preparations (4), and it is unclear how much of this antigen is available to B cells in a typical vaccine dose. To determine how much NP was contained in the 2008/09 Fluzone preparation used prior to the 2009 H1N1 influenza virus pandemic, we performed a capture ELISA using a combination of monoclonal and polyclonal anti-NP antibodies and recombinant purified NP as a standard. The results suggest that this preparation had as much as 300 μg/ml NP (Fig. 2A), which would amount to 150 μg per 0.5-ml human dose. To validate these findings, we performed Western blot analysis of known quantities of purified NP and TIV side by side (Fig. 2B). From this analysis, we estimate the TIV preparation to have ∼100 μg NP/ml or 50 μg per adult human dose. Although we do not know which assay is more quantitatively accurate, it is clear from these results that this antigen is at least as abundant as HA, which is 15 μg per strain (45 μg total influenza A plus influenza B virus HAs) per dose.
Fig. 2.
NP content of 2008/09 TIV. (A) Capture ELISA to measure NP in TIV as described in Materials and Methods. (B) Western blot analysis of the indicated μg rNP compared with the indicated μl TIV as described in Materials and Methods.
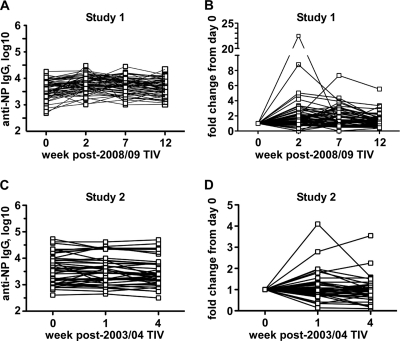
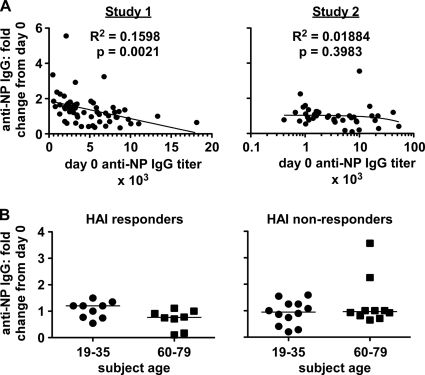
Because NP antigen is readily available in seasonal TIV (Fig. 2) (4, 18, 19), we asked whether anti-NP antibody is induced by vaccination. Two human sample sets were analyzed: plasma from before and after 2008/09 TIV immunization (study 1) and serum from different individuals before and after 2003/04 TIV immunization (study 2). All subjects appeared to have detectable NP-reactive IgG at study initiation (Fig. 3A and C), consistent with previous reports (10, 47, 58). After TIV immunization, little if any stable change was detected in these titers in most subjects (Fig. 3 and Table 1). However, 1 or 2 individuals in each study appeared to have a spike of increased anti-NP IgG 1 to 2 weeks postvaccination, which subsequently returned to prevaccination levels. One or two additional individuals had an increase in anti-NP IgG of 2- to 6-fold that remained stable over the observation period (12 weeks in study 1 and 4 weeks in study 2). Regression analysis of study 1 showed a minor inverse correlation between the day 0 titer of NP-reactive IgG and the fold changes in this titer detected at 7 and 12 weeks (Fig. 4A and data not shown). No such correlation was observed in study 2, even when subjects were analyzed separately by age cohorts (Fig. 4A and data not shown). No significant difference in the populations was observed when the subjects in study 2 were grouped by age and by hemagglutination inhibition (HAI) response (Fig. 4B). Age and HAI data were unavailable for study 1. From both studies, <10% of participants had a ≥2-fold rise and 1% had a 4-fold rise in NP-reactive antibody (Table 1). Therefore, inactivated influenza virus vaccine had little consistent effect on anti-NP IgG titers in human subjects in these studies.
Fig. 3.
TIV rarely changes anti-NP IgG titers in humans. Samples were collected from healthy human subjects on the indicated days relative to i.m. vaccination with TIV. NP-reactive IgG was detected by endpoint ELISA. (A and B) Study 1. Plasma from 64 subjects pre- and post-2008/09 TIV. (C and D) Study 2. Serum from 43 subjects pre- and post-2003/04 TIV. (A and C) Anti-NP IgG titers. (B and D) Fold change of anti-NP IgG titer from that at day 0 (prevaccination).
Table 1.
Number of human subjects with change in anti-NP IgG levels after TIV
| Group | No.of subjects with fold change/total no. of subjects (%)a |
||
|---|---|---|---|
| ≥2-fold | ≥3-fold | ≥4-fold | |
| Study 1 (12 wk) | 7/56 (12.5) | 3/56 (5.4) | 1/56 (1.8) |
| Study 2 (4 wk) | 2/40 (5.0) | 1/40 (2.5) | 0/40 (0) |
| Total | 9/96 (9.4) | 4/96 (4.2) | 1/96 (1.04) |
Stable fold change in anti-NP IgG levels after TIV immunization. Levels in plasma(study 1) and in serum(study 2) were determined as described in Materials and Methods.
Fig. 4.
Anti-NP IgG response in humans does not consistently correlate with starting titer or with HAI response. (A) Regression analysis derived from human ELISA data in Fig. 3. (B) Fold change in anti-NP IgG in study 2 serum according to HAI response (left; individuals with ≥4-fold change in HAI titer) or no response (right), as well as age (in years). Bars show median fold changes for each group.
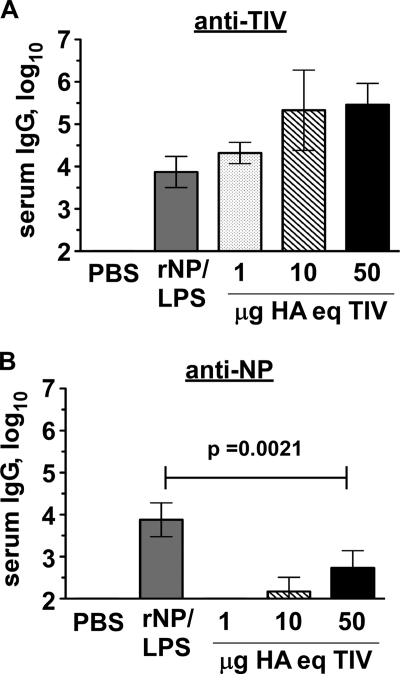
It is unknown why the NP contained in TIV (Fig. 2) (4, 18, 19) does not consistently induce or boost titers of specific antibody in humans. The regression analysis of study 1 suggested that starting titers of anti-NP IgG (and/or influenza virus experience in general) might influence whether TIV affects anti-NP IgG production. Therefore, we asked whether TIV could induce a more substantial response in “naive” specific-pathogen-free (SPF) mice with no prior influenza virus experience. Influenza virus-naive C57BL/6 mice were injected i.p. with 2008/09 TIV on days 0 and 10, and serum was analyzed on day 26. As a positive control, additional mice were immunized with recombinant purified NP in the presence of LPS adjuvant. Notably, NP-immune serum from these latter mice reacted with TIV on the ELISA plate, confirming the presence of antibody-accessible NP within the immunogen (Fig. 5A). Immunization with instead 1 to 50 μg HA equivalent of TIV resulted in little to no NP-reactive IgG (Fig. 5B). Thus, despite the immunogenicity of total vaccine content and despite the presence of NP in the vaccine, TIV induced relatively little NP-reactive antibody in influenza virus-naive mice, suggesting that a lack of (or minimal) response in humans is not likely explained solely by prior influenza virus/NP experience.
Fig. 5.
Poor induction of anti-NP IgG in TIV-immunized mice. Influenza virus-naive C57BL/6 mice were injected i.p. as indicated on days 0 and 10. Sera were collected at day 26 and analyzed by endpoint ELISA. rNP/LPS, 50 μg rNP plus 20 μg LPS. TIV was unadjuvanted and dosed as μg HA equivalent (HA eq). (A) IgG reactive with total TIV protein. (B) NP-reactive IgG. Results representative of at least 3 similar experiments. Mean ± standard deviation of 5 mice/group.
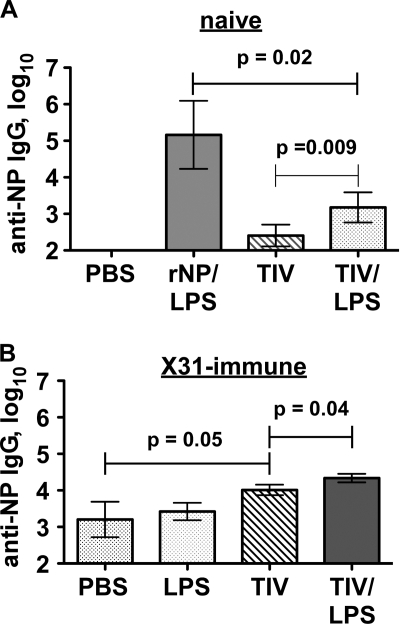
One obvious difference between vaccinations with TIV and those with our purified NP protein was the presence of adjuvant in the latter case. We therefore performed TIV immunizations in the presence and absence of LPS. This addition modestly increased TIV-induced anti-NP IgG in naive and in influenza virus X31-immune C57BL/6 mice (Fig. 6). Similar results were observed with complete Freund's adjuvant (CFA) (not shown). Interestingly, adjuvant was not required for the anti-TIV responses (Fig. 5A), indicating some kind of internal adjuvanticity that is poorly conferred on NP. The reason for this effect is unclear, but it might reflect an amount of NP contained in the vaccine different from that in our recombinant NP/LPS immunizations.
Fig. 6.
Adjuvanting TIV can enhance anti-NP IgG responses in mice. (A) Influenza virus-naive C57BL/6 mice were immunized i.p. with 100 μg rNP and 20 μg LPS or with 5 μg HA eq of 2008/09 TIV with or without 20 μg LPS on days 0, 10, and 20. Serum was collected for analysis on day 27. (B) C57BL/6 mice were infected i.n. with 0.25 LD50 of influenza virus X31. On day 30 postinfection, the mice were immunized i.p. with 20 μg LPS, 1 μg HA eq of TIV, or both. Similar results were observed when 10 μg HA eq of TIV was used. Mean ± standard deviation of 3 to 5 mice/group.
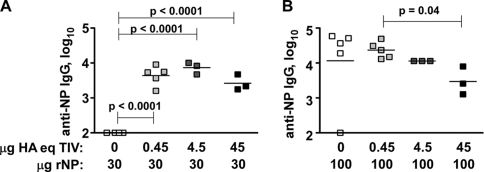
We thus tested whether the abundance of NP relative to that of other TIV components affects B cell responses to NP. In the presence of LPS, a low dose (30 μg) of purified rNP was insufficient to induce antibody; however, when combined with as little as 0.45 μg HA equivalents of TIV, 30 μg rNP induced a 4-log titer of NP-reactive IgG (Fig. 7A). Based on the results in Fig. 2, we estimate this dose of TIV (1% of that given to humans) to contain no more than ∼1.5 μg NP, suggesting that the amount of antigen per se (30 μg versus 31.5 μg NP) is unlikely to account for the difference in these mice. These results suggest that the vaccine contains intrinsic adjuvant activity that can be conferred on reactions against NP. Intriguingly, when 100 μg of purified rNP was used, the high titer of antibody induced was modestly but significantly inhibited by high-dose TIV (Fig. 5B). Although this dose of vaccine in mice likely exceeds the equivalent of what would be needed in humans, the result demonstrates that the stoichiometry of NP relative to other vaccine components may influence whether or not an NP-reactive antibody response is readily induced. This result may reflect a poorly defined mechanism in which reactions to one antigen can be compromised when delivered in the presence of another antigen (8).
Fig. 7.
The stoichiometry of NP and other vaccine components influences anti-NP IgG responses to TIV. Influenza virus-naive C57BL/6 mice were injected i.p. as indicated on days 0 and 10. Sera were collected at day 34 and analyzed by endpoint ELISA. (A) Mice injected with 30 μg rNP plus 20 μg LPS with or without the indicated HA eq doses of 2008/09 TIV. (B) Mice injected with 100 μg rNP plus 20 μg LPS with or without the indicated HA eq doses of 2008/09 TIV. Results representative of 2 similar experiments.
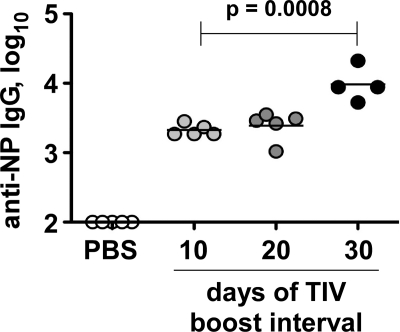
Since TIV is given annually, it is likely that NP-reactive memory B cells are reengaged by this conserved antigen, despite the changes in HA and NA content from year to year. Previous studies showed that immunization with protein antigen results in a poor boost if the interval between vaccinations is relatively short (15). We therefore extended the time between (unadjuvanted) TIV immunizations. Whereas a 10-day interval between prime and boost resulted in a 3.5-log titer of serum anti-NP IgG, a 30-day interval significantly enhanced this level by 5-fold (Fig. 8). Collectively, our results suggest that, in mice, factors affecting the immunogenicity of NP within TIV can include a lack of associated adjuvanticity and the stoichiometry between NP and other vaccine components, as well as the interval between prime and boost. It remains to be determined whether these effects or other factors contribute to the poor NP immunogenicity of TIV in humans. For example, the form of NP within the vaccine (monomeric or aggregated, RNA associated, epitopes damaged by fixation, etc.) compared with purified rNP is unknown.
Fig. 8.
A longer prime-boost interval enhances anti-NP IgG response to TIV in mice. Influenza virus-naive C57BL/6 mice were immunized i.p. with PBS alone or with 10 μg HA eq of TIV on day 0. On the indicated days, different groups of TIV-primed mice were boosted with TIV. Sera were collected on day 50 for ELISA. Results representative of 2 similar experiments.
Boosting anti-NP IgG can enhance long-term Het-I.
The presence of NP-reactive antibody in humans and the presence of NP in seasonal TIV have led to the speculation that such antibody provides little antiviral cross-protection. However, this vaccine rarely affects human anti-NP IgG levels (Fig. 3 and Table 1), a fact which does not exclude the possibility that high-titer anti-NP IgG can or does alter the course of infection. In fact, B cell and antibody responses to conserved antigens are required for mouse Het-I (33, 39; also data not shown), correlating with the presence of high-titer anti-NP IgG in mouse serum (39). Furthermore, anti-NP IgG can rescue poor Het-I in B cell-deficient μMT mice (25a). Thus, in some humans, anti-NP IgG might accelerate virus clearance and go undetected. In other individuals, however, existing levels of anti-NP IgG may be insufficient to promote virus clearance, particularly if other mechanisms associated with Het-I have decreased over time (26, 29).
To address this issue, we infected C57BL/6 mice with H3N2 influenza virus X31 and, after 90 days, gave an i.p. injection with rNP antigen (Fig. 9A). Compared with mice treated only with PBS at each time point, X31-immune mice had a serum NP-reactive IgG titer of 3 to 4 logs, which remained stable for at least 6 months post-primary infection (Fig. 9B). X31-immune mice boosted with rNP at day 90 subsequently had a 10-fold-higher level of anti-NP IgG, which remained stable for 100 days past the boost (Fig. 9B). We then challenged the mice i.n. with a lethal dose of H1N1 influenza virus PR8 on day 200 (6.7 months) after primary infection. Mice previously treated only with PBS had high titers of virus in the lung on days 4 to 6 post-PR8 challenge (Fig. 9C). X31-immune mice challenged after 6 months had begun to reduce viral load at days 4 and 5 (Fig. 9C). However, as early as day 4 postchallenge, X31-immune mice boosted with NP antigen had significantly reduced viral loads compared with the loads in those given X31 alone, leading to further reductions and, in two mice, viral clearance by day 6 (Fig. 9C). At this time, the remaining 3 mice in the NP-boosted group had variably lower viral titers than did the surviving mice in each control group. In one of three such experiments, PBS- and X31-immune mice began to succumb to the infection by day 6, whereas NP-boosted X31-immune mice survived. In two of three experiments, no mortality was observed in any group prior to tissue harvest. However, NP boosting significantly reduced viral load in all three experiments. Overall, existing levels of NP-reactive antibody can readily be boosted by systemic protein injection, correlating with significantly enhanced heterosubtypic virus clearance.
Fig. 9.
Protein boosting increases anti-NP IgG and enhances long-term Het-I. (A) Experimental design. C57BL/6 mice were infected i.n. with 0.25 LD50 of influenza virus X31 or PBS alone on day 0. On day 90, half of the X31-immune mice were boosted with 100 μg rNP i.p. On day 200, all mice were challenged with 2.5 LD50 of influenza virus PR8 i.n. (Not shown is that sera were collected at regular intervals prior to PR8 challenge.) (B) NP-reactive IgG in mouse sera prior to PR8 challenge. Mean and standard deviation of 5 mice/group. (C) Lung viral load at the indicated day post-PR8 challenge. *, P = 0.01; ***, P < 0.0001. Results representative of 3 similar experiments.
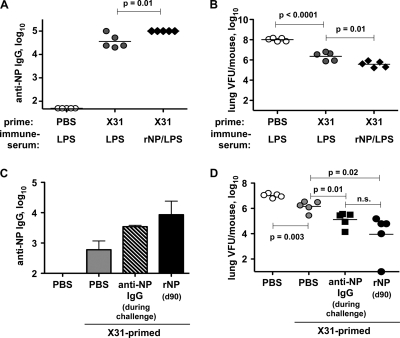
To determine the contribution of high-titer NP-reactive antibody in this system, we prepared NP/LPS-immune serum as described previously (3). Serum from mice mock vaccinated with the adjuvant (LPS) alone was used as a negative control (LPS-immune). These sera were then i.p. injected into day 239 X31-immune mice before and during lethal PR8 challenge infection. This treatment resulted in a serum titer of NP-reactive IgG similar to that of NP-boosted X31-immune mice, which in each case was higher than that induced by X31 alone (Fig. 10A). On day 6 post-PR8 challenge, NP-immune serum-treated X31-immune mice had a significant reduction in lung viral load compared with that of X31-immune mice treated with control serum (Fig. 10B). A similar result was observed if purified NP-specific monoclonal antibodies were used instead of serum (Fig. 10C and D), indicating that donor serum components such as cytokines and complement do not likely contribute to this effect. These results demonstrate that the increased antibody titer in the NP-boosted mice likely accounts for at least some of the enhanced antiviral effects.
Fig. 10.
Anti-NP IgG enhances long-term Het-I. C57BL/6 mice were infected with 0.25 LD50 of influenza virus H3N2 X31 as in Fig. 9. On days 239 to 242, the indicated mice were injected i.p. with PBS, with the indicated serum (A and B), or with the indicated MAb (C and D). All mice were infected i.n. on day 241 with 2.5 LD50 of influenza virus H1N1 PR8 and then bled on day 243 (1 day after last injection). (A and B) Mice injected with 400 μl of the indicated immune sera. (A) NP-reactive IgG in recipients' sera. (B) Lung viral titer on day 247 (6 days post-PR8 challenge). (C and D) Mice injected with PBS or a mixture of 300 μg anti-NP IgG1, IgG2a, and IgG2b (striped bar in panel C and filled squares in panel D) during PR8 challenge. The black bar in panel C and filled circles in panel D indicate an additional group of mice that were instead previously boosted on day 90 with 100 μg purified rNP. (C) Serum anti-NP IgG on day 243. (D) Lung viral titer on day 247 (6 days post-PR8 challenge).
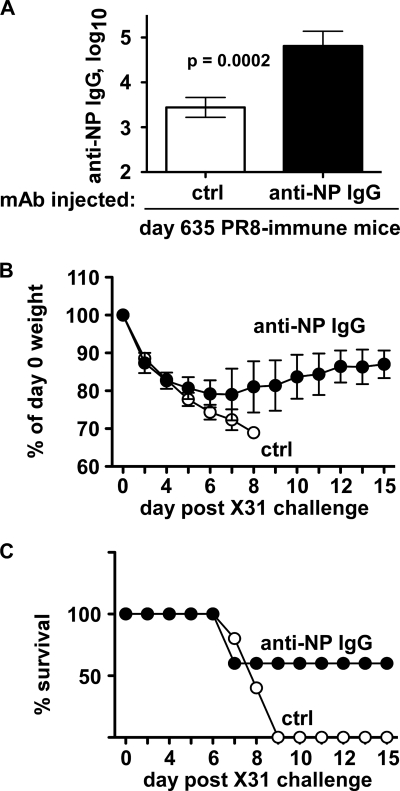
Although reductions in viral load could make a significant contribution in reducing hospitalizations, lost workdays, and viral spread from person to person, we were also interested to know whether anti-NP antibodies could improve mortality and morbidity rates, particularly after heterosubtypic immunity has extensively waned (26). We therefore injected anti-NP or control IgG into mice primed with H1N1 virus PR8 21 months (1.7 years) before. These mice were then challenged with a lethal dose of H3N2 virus X31, confirmed for increased anti-NP IgG (Fig. 11A), and then monitored for weight loss and survival. Between days 0 and 5 postchallenge, both groups of mice lost ∼20% of their initial body weights (Fig. 11B). However, whereas mice given control IgG continued to lose weight and succumb to the infection, recipients of anti-NP IgG had stopped losing weight after day 6 and 60% of these mice survived for at least 2 weeks (Fig. 11B and C). Therefore, together with accelerating viral clearance, NP-specific IgG can protect mice from lethal influenza virus challenge, even more than 1 year postpriming.
Fig. 11.
NP-specific IgG protects long-term heterosubtypic immune mice. C57BL/6 mice were infected with 0.25 LD50 of H1N1 influenza virus PR8 i.n. on day 0. On days 631 to 634, the mice were injected with control (ctrl) or anti-NP IgG (300-μg mixtures of IgG1, IgG2a, and IgG2b) i.p. On day 633, the mice were challenged i.n. with 2.5 LD50 of H3N2 influenza virus X31 i.n. (A) Serum NP-reactive IgG on day 635 (1 day after last injection). Mean ± standard deviation of 5 mice/group. (B) Relative body weights on indicated day postchallenge. Mean ± standard deviation of 5 mice/group. (C) Survival on indicated day postchallenge. Mice losing 30% of body weight were humanely euthanized and scored as nonsurvivors.
Overall, our results demonstrate that the activity of NP-immune antibody can promote clearance of a novel pandemic strain of human influenza virus. However, current systemic seasonal vaccination does little to stimulate production of this antiviral antibody. Our results in mice show that NP immunogenicity can be enhanced by optimizing vaccine context and scheduling. Most importantly, when existing levels of this antibody are successfully boosted, long-term Het-I was significantly enhanced. Together with the highly cross-protective nature of NP (9, 12, 14, 17, 27, 41, 48, 50, 53, 54, 57) and the known longevity of serum antibody in humans (1, 20), our observations suggest that inducing antibody responses against this highly conserved antigen could improve existing regimens of influenza virus immunization to provide a more universal influenza vaccine.
DISCUSSION
Current influenza virus immunization strategies need to be improved to provide long-lived cross-protection that reduces the impact of seasonal epidemics as well as pandemic influenza. Here, we show that NP immunization can promote clearance of novel H1N1 2009 pandemic influenza virus in an antibody-dependent manner, correlating with high titers of NP-reactive IgG in the serum. Although very little anti-NP antibody is induced by human TIV immunization, TIV-based NP immunogenicity in mice can be improved using adjuvants, altering the stoichiometry between NP and other vaccine components, and by extending the interval between prime and boost. Importantly, existing levels of anti-NP IgG induced by prior virus infection of mice can readily be boosted by immunization with purified NP, correlating with enhanced long-term Het-I. This effect was recapitulated by injection of purified anti-NP IgG MAbs. The conservation of NP protein and the known longevity of humoral immune responses suggest that inducing high titers of anti-NP antibodies could be a very effective way of eliciting long-term cross-protection against influenza virus.
The search for universal influenza vaccines has focused on exploiting the mechanisms of Het-I, which has been studied extensively in mice (13, 25, 26, 33, 38–40, 52). There is evidence for Het-I in humans as well, defined as reductions in hospitalization and in mortality (30, 31, 34, 35, 44) and by a lower infection rate (11). Het-I likely provides some level of protection to the population as a whole but does not completely prevent seasonal epidemics or influenza pandemics. One possible reason for this effect may be poor longevity of the memory T cell response (26, 29). Our results in mice demonstrate that long-term Het-I can be enhanced by injection of anti-NP IgG or by actively boosting NP-reactive serum antibody. Therefore, vaccination strategies to induce a similar antibody boost in humans might compensate for the variable levels of Het-I.
Heterosubtypic immunity in mice can be conferred by mucosal vaccination with a live-attenuated influenza virus vaccine (38), similar to that contained in FluMist, which has shown efficacy in humans (35). An older study using immunodiffusion did not detect changes in serum NP-reactive antibody in humans given a 1975 live-attenuated isolate (10). However, given our results, it would be worthwhile to use more current technologies to revisit the possibility that such vaccines may induce mucosal anti-NP antibody in mice and humans. These studies would help to determine whether such an antibody contributes to the efficacy of attenuated vaccines and whether supplementation with mucosal or systemic NP boosts would provide more optimal protection.
Influenza virus-experienced humans have both anti-NP antibody (6, 10, 47, 58) and antiviral (including NP-specific) memory T lymphocytes (4, 5, 10, 29), likely induced by prior influenza virus infection (6). The difficulty of performing cause-and-effect experiments in humans leaves it uncertain as to whether one or both can mediate cross-protection. Doubt about anti-NP antibody in particular has been raised, given the presence of NP in seasonal inactivated influenza virus vaccines (4, 18, 19), which are considered to be poorly cross-protective (2). This doubt is based upon the assumption that the NP in the vaccines induces high titers of specific antibody that is ineffective against virus encounter. However, previous studies suggest that NP reactivity is affected very little after vaccination with inactivated or live-attenuated virus (10). Our own analyses showed that among 96 human subjects, <10% had a 2-fold change in anti-NP IgG titer and only 1% had a 4-fold change after TIV immunization (Table 1). Thus, in most individuals, seasonal TIV is not currently inducing or boosting this antibody. Therefore, antiviral activity of anti-NP IgG in humans cannot be excluded. Extensive analyses of NP-immune antibody titers compared with laboratory-confirmed infections and viral shedding will be required to determine whether or not this is the case. Such assessment will be challenging, considering the possibility that persons with high titers of this antibody may be protected and not seek professional health care, essentially going undetected. Alternatively or additionally, the titers of anti-NP IgG induced by prior infection may be insufficient in some individuals.
In a few human subjects, we found transient responses to NP after TIV immunization. The cause of such an effect in these few individuals is unknown, although a transient (e.g., T cell-independent) response to the vaccine or to a concurrent influenza virus infection cannot be excluded. Other rare individuals had more stable increases in anti-NP IgG. In study 2, the 2 individuals with the greatest stable increase (2.3- and 3.6-fold) by 4 weeks were 76 and 74 years old (respectively) at study initiation. Since three other individuals of a similar age did not have such a response (not shown), it is unlikely that age alone or age per se contributed to this effect. However, persons of advanced age are likely to have a diverse array of health and immune experiences that are not appreciated by our current analysis.
Regression analysis to compare starting anti-NP antibody titers and increases in antibody levels was inconsistent between the two human studies. These results suggest that prior B cell reactions to NP—or immune experience with influenza virus in general—may differentially affect subsequent anti-NP antibody responses and are unlikely to be the only factor determining whether a response occurs. In mice, influenza virus-naive status did not appear to allow for additional reactogenicity compared with influenza virus-experienced mice (Fig. 6, compare panels A and B).
In human study 2, increases in HAI of ≥4-fold (against one or both constituent influenza A viruses) were observed in >40% of subjects (Fig. 4B, compare left and right graphs), confirming vaccine immunogenicity. In each age group, the median fold change in anti-NP IgG titer did not differ between those who did and those who did not have an increase in HAI titer (Fig. 4B). However, the two subjects with 2- to 4-fold increases in anti-NP IgG in the 60- to 79-year-old participant group did not show an HAI response, whereas two individuals with HAI increases had lower anti-NP IgG levels by 4 weeks. Although this observation possibly suggests an inverse relationship between immune responses to different vaccine components, additional analyses with greater statistical power are needed to confirm the validity of such an effect. Our observations in mice suggest that the stoichiometry of different TIV components can indeed influence whether or not a robust anti-NP IgG response is induced (Fig. 7).
Recently, antibodies that bind to a conserved region of the influenza virus HA stalk have been identified (46, 55). Such antibodies to H1 stalk broadly neutralize various heterologous H1 strains, and antibodies to H3 stalk can do the same for multiple H3 strains. However, anti-H1 stalk antibodies are poorly effective against H3 viruses. Importantly, up to 16 HA subtypes exist in animal reservoirs (49). At least two of these (H9 in H9N2 influenza virus and H7 in H7N7 influenza virus) have already infected humans (24, 36). Therefore, a substantial potential for reassortment and zoonotic transfer remains possible for future pandemics, which will unlikely be contained by anti-HA stalk antibodies alone. Our results very strongly suggest that inducing long-lived antibody against NP could provide a supplemental level of protection for such unpredictable outbreaks.
Annual influenza virus epidemics burden the American economy with the costs of health care, lost workdays, and annual production of vaccines that do not protect for multiple years. Furthermore, the unpredictable zoonotic transfer of animal-origin viruses to humans has resulted in devastating pandemics in the past (45, 56) and is responsible for the recent pandemic (16, 22, 32, 43). Thus, we urgently need a vaccine that provides broadly reactive and long-lasting immunity to a wide range of influenza viruses, regardless of subtype or serotype. Our findings that anti-NP antibodies promote viral clearance is a significant step toward such a vaccine, because NP proteins are highly conserved among strains and subtypes of influenza virus and rarely accumulate mutations. Furthermore, antibody responses can last for the lifetime of an individual. Thus, the combination of a highly conserved viral target antigen and potential for humoral longevity suggests that induction of anti-NP antibodies by vaccination of humans would be ideal for cross-protecting against changing and unexpected virus strains.
ACKNOWLEDGMENTS
This study was funded by NIH grant AI079537 (parent and ARRA supplement) to D.A.K.; grants AI61511 and AI72689 to T.D.R.; grant AI68056-05S1 to F.E.L.; and grants HHSN2662005500029C (N01-AI50029), K23 AI67501-01A1, N01-AI-50020, and R24 AO054953 to F.E.-H.L. and by House Resolution 3222 (Department of Defense funding) to L.H.
We also thank Elizabeth LaMere for animal husbandry, Louise Hartson and Ho-Tak Lam for technical assistance, Deanna Maffett for clinical study assistance, and David Topham for A/California/04/2009 H1N1 influenza virus. 2008/09 TIV was a gift from Sanofi Pasteur. NP-specific IgG hybridomas were provided by Walter Gerhard and Krystyna Mozanowska. We thank Javier Rangel-Moreno and André Ballesteros-Tato for critical discussions and John Treanor for reading the manuscript.
Footnotes
Published ahead of print on 2 March 2011.
REFERENCES
- 1. Amanna I. J., Carlson N. E., Slifka M. K. 2007. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 357:1903–1915 [DOI] [PubMed] [Google Scholar]
- 2. Bodewes R., et al. 2010. Vaccination with whole inactivated virus vaccine affects the induction of heterosubtypic immunity against influenza virus A/H5N1 and immunodominance of virus-specific CD8+ T-cell responses in mice. J. Gen. Virol. 91:1743–1753 [DOI] [PubMed] [Google Scholar]
- 3. Carragher D. M., Kaminski D. A., Moquin A., Hartson L., Randall T. D. 2008. A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus. J. Immunol. 181:4168–4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Co M. D., et al. 2009. In vitro evidence that commercial influenza vaccines are not similar in their ability to activate human T cell responses. Vaccine 27:319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Co M. D., et al. 2008. Discordance between antibody and T cell responses in recipients of trivalent inactivated influenza vaccine. Vaccine 26:1990–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cretescu L., Beare A. S., Schild G. C. 1978. Formation of antibody to matrix protein in experimental human influenza A virus infections. Infect. Immun. 22:322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Du L., Zhou Y., Jiang S. 2010. Research and development of universal influenza vaccines. Microbes Infect. 12:280–286 [DOI] [PubMed] [Google Scholar]
- 8. Eide D. M., Adnoy T., Larsen H. J. 1992. Selection for immune response in goats: the effect of immunization procedure on antibody response to diphtheria toxoid and human serum albumin. J. Anim. Sci. 70:1432–1438 [DOI] [PubMed] [Google Scholar]
- 9. Endo A., et al. 1991. Homotypic and heterotypic protection against influenza virus infection in mice by recombinant vaccinia virus expressing the haemagglutinin or nucleoprotein of influenza virus. J. Gen. Virol. 72:699–703 [DOI] [PubMed] [Google Scholar]
- 10. Ennis F. A., Yi-Hua Q., Schild G. C. 1982. Antibody and cytotoxic T lymphocyte responses of humans to live and inactivated influenza vaccines. J. Gen. Virol. 58:273–281 [DOI] [PubMed] [Google Scholar]
- 11. Epstein S. L. 2006. Prior H1N1 influenza infection and susceptibility of Cleveland Family Study participants during the H2N2 pandemic of 1957: an experiment of nature. J. Infect. Dis. 193:49–53 [DOI] [PubMed] [Google Scholar]
- 12. Epstein S. L., et al. 2005. Protection against multiple influenza A subtypes by vaccination with highly conserved nucleoprotein. Vaccine 23:5404–5410 [DOI] [PubMed] [Google Scholar]
- 13. Epstein S. L., et al. 1997. Mechanisms of heterosubtypic immunity to lethal influenza A virus infection in fully immunocompetent, T cell-depleted, b2-microglobulin-deficient, and J chain-deficient mice. J. Immunol. 158:1222–1230 [PubMed] [Google Scholar]
- 14. Epstein S. L., et al. 2000. Vaccination with DNA encoding internal proteins of influenza virus does not require CD8(+) cytotoxic T lymphocytes: either CD4(+) or CD8(+) T cells can promote survival and recovery after challenge. Int. Immunol. 12:91–101 [DOI] [PubMed] [Google Scholar]
- 15. Fecsik A. I., Butler W. T., Coons A. H. 1964. Studies on antibody production. XI. Variation in the secondary response as a function of the length of the interval between two antigenic stimuli. J. Exp. Med. 120:1041–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fraser C., et al. 2009. Pandemic potential of a strain of influenza A (H1N1): early findings. Science 324:1557–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fu T. M., et al. 1999. Dose dependence of CTL precursor frequency induced by a DNA vaccine and correlation with protective immunity against influenza virus challenge. J. Immunol. 162:4163–4170 [PubMed] [Google Scholar]
- 18. Garcia-Canas V., Lorbetskie B., Bertrand D., Cyr T. D., Girard M. 2007. Selective and quantitative detection of influenza virus proteins in commercial vaccines using two-dimensional high-performance liquid chromatography and fluorescence detection. Anal. Chem. 79:3164–3172 [DOI] [PubMed] [Google Scholar]
- 19. Garcia-Canas V., Lorbetskie B., Girard M. 2006. Rapid and selective characterization of influenza virus constituents in monovalent and multivalent preparations using non-porous reversed-phase high performance liquid chromatography columns. J. Chromatogr. A 1123:225–232 [DOI] [PubMed] [Google Scholar]
- 20. Hammarlund E., et al. 2003. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 9:1131–1137 [DOI] [PubMed] [Google Scholar]
- 21. Hensley S. E., et al. 2009. Hemagglutinin receptor binding avidity drives influenza A virus antigenic drift. Science 326:734–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Itoh Y., et al. 2009. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature 460:1021–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karlsson Hedestam G. B., et al. 2008. The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nat. Rev. Microbiol. 6:143–155 [DOI] [PubMed] [Google Scholar]
- 24. Koopmans M., et al. 2004. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet 363:587–593 [DOI] [PubMed] [Google Scholar]
- 25. Kreijtz J. H., et al. 2007. Primary influenza A virus infection induces cross-protective immunity against a lethal infection with a heterosubtypic virus strain in mice. Vaccine 25:612–620 [DOI] [PubMed] [Google Scholar]
- 25a. LaMere M. W., et al. 2011. Contributions of antinucleoprotein IgG to heterosubtypic immunity against influenza virus. J. Immunol. 186:4331–4339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liang S., Mozdzanowska K., Palladino G., Gerhard W. 1994. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J. Immunol. 152:1653–1661 [PubMed] [Google Scholar]
- 27. Lo C. Y., et al. 2008. Comparison of vaccines for induction of heterosubtypic immunity to influenza A virus: cold-adapted vaccine versus DNA prime-adenovirus boost strategies. Vaccine 26:2062–2072 [DOI] [PubMed] [Google Scholar]
- 28. Maines T. R., et al. 2008. Pathogenesis of emerging avian influenza viruses in mammals and the host innate immune response. Immunol. Rev. 225:68–84 [DOI] [PubMed] [Google Scholar]
- 29. McMichael A. J., Gotch F. M., Dongworth D. W., Clark A., Potter C. W. 1983. Declining T-cell immunity to influenza, 1977-82. Lancet ii:762–764 [DOI] [PubMed] [Google Scholar]
- 30. Mossad S. B. 2008. 2008–2009 influenza update: a better vaccine match. Cleve. Clin. J. Med. 75:865–870 [DOI] [PubMed] [Google Scholar]
- 31. Mossad S. B. 2007. Influenza update 2007–2008: vaccine advances, pandemic preparation. Cleve. Clin. J. Med. 74:889–894 [DOI] [PubMed] [Google Scholar]
- 32. Neumann G., Noda T., Kawaoka Y. 2009. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 459:931–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nguyen H. H., et al. 2007. Heterosubtypic immunity to influenza A virus infection requires a properly diversified antibody repertoire. J. Virol. 81:9331–9338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nichol K. L., Nordin J. D., Nelson D. B., Mullooly J. P., Hak E. 2007. Effectiveness of influenza vaccine in the community-dwelling elderly. N. Engl. J. Med. 357:1373–1381 [DOI] [PubMed] [Google Scholar]
- 35. Ohmit S. E., et al. 2006. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N. Engl. J. Med. 355:2513–2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peiris M., et al. 1999. Human infection with influenza H9N2. Lancet 354:916–917 [DOI] [PubMed] [Google Scholar]
- 37. Portela A., Digard P. 2002. The influenza virus nucleoprotein: a multifunctional RNA-binding protein pivotal to virus replication. J. Gen. Virol. 83:723–734 [DOI] [PubMed] [Google Scholar]
- 38. Powell T. J., et al. 2007. Priming with cold-adapted influenza A does not prevent infection but elicits long-lived protection against supralethal challenge with heterosubtypic virus. J. Immunol. 178:1030–1038 [DOI] [PubMed] [Google Scholar]
- 39. Rangel-Moreno J., et al. 2008. B cells promote resistance to heterosubtypic strains of influenza via multiple mechanisms. J. Immunol. 180:454–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sambhara S., et al. 2001. Heterosubtypic immunity against human influenza A viruses, including recently emerged avian H5 and H9 viruses, induced by FLU-ISCOM vaccine in mice requires both cytotoxic T-lymphocyte and macrophage function. Cell. Immunol. 211:143–153 [DOI] [PubMed] [Google Scholar]
- 41. Scheepers K., Becht H. 1994. Protection of mice against an influenza virus infection by oral vaccination with viral nucleoprotein incorporated into immunostimulating complexes. Med. Microbiol. Immunol. 183:265–278 [DOI] [PubMed] [Google Scholar]
- 42. Shu L. L., Bean W. J., Webster R. G. 1993. Analysis of the evolution and variation of the human influenza A virus nucleoprotein gene from 1933 to 1990. J. Virol. 67:2723–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smith G. J. D., et al. 2009. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza epidemic. Nature 459:1122–1125 [DOI] [PubMed] [Google Scholar]
- 44. Sonoguchi T., Naito H., Hara M., Takeuchi Y., Fukumi H. 1985. Cross-subtype protection in humans during sequential, overlapping, and/or concurrent epidemics caused by H3N2 and H1N1 influenza viruses. J. Infect. Dis. 151:81–88 [DOI] [PubMed] [Google Scholar]
- 45. Subbarao K., Murphy B. R., Fauci A. S. 2006. Development of effective vaccines against pandemic influenza. Immunity 24:5–9 [DOI] [PubMed] [Google Scholar]
- 46. Sui J., et al. 2009. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 16:265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sukeno N., et al. 1979. Anti-nucleoprotein antibody response in influenza A infection. Tohoku J. Exp. Med. 128:241–249 [DOI] [PubMed] [Google Scholar]
- 48. Tamura S., et al. 1996. Acceleration of influenza virus clearance by Th1 cells in the nasal site of mice immunized intranasally with adjuvant-combined recombinant nucleoprotein. J. Immunol. 156:3892–3900 [PubMed] [Google Scholar]
- 49. Taubenberger J. K., Kash J. C. 2010. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe 7:440–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tite J. P., et al. 1990. Anti-viral immunity induced by recombinant nucleoprotein of influenza A virus. II. Protection from influenza infection and mechanism of protection. Immunology 71:202–207 [PMC free article] [PubMed] [Google Scholar]
- 51. Tosh P. K., Jacobson R. M., Poland G. A. 2010. Influenza vaccines: from surveillance through production to protection. Mayo Clin. Proc. 85:257–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tumpey T. M., Renshaw M., Clements J. D., Katz J. M. 2001. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J. Virol. 75:5141–5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ulmer J. B., et al. 1993. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259:1745–1749 [DOI] [PubMed] [Google Scholar]
- 54. Ulmer J. B., et al. 1998. Protective CD4+ and CD8+ T cells against influenza virus induced by vaccination with nucleoprotein DNA. J. Virol. 72:5648–5653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wei C. J., et al. 2010. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science 329:1060–1064 [DOI] [PubMed] [Google Scholar]
- 56. Whitley R. J., Monto A. S. 2006. Seasonal and pandemic influenza preparedness: a global threat. J. Infect. Dis. 194(Suppl. 2):S65–S69 [DOI] [PubMed] [Google Scholar]
- 57. Wraith D. C., Vessey A. E., Askonas B. A. 1987. Purified influenza virus nucleoprotein protects mice from lethal infection. J. Gen. Virol. 68:433–440 [DOI] [PubMed] [Google Scholar]
- 58. Yamane N., Odagiri T., Arikawa J., Ishida N. 1981. Reversed single-radial-immunodiffusion test: the method for the assay of the antibody to influenza A nucleoprotein. Tohoku J. Exp. Med. 133:245–255 [DOI] [PubMed] [Google Scholar]