Abstract
Ld652Y cells from Lymantria dispar readily undergo apoptosis upon infection with a variety of nucleopolyhedroviruses (NPVs), while L. dispar multicapsid NPV (LdMNPV) infection of Ld652Y cells results in the production of a high titer of progeny viruses. Here, we identify a novel LdMNPV apoptosis suppressor gene, apsup, which functions to suppress apoptosis induced in Ld652Y cells by infection with vAcΔp35, a p35-defective recombinant Autographa californica MNPV. apsup also suppresses apoptosis of Ld652Y cells induced by actinomycin D and UV exposure. Apsup is expressed in LdMNPV-infected Ld652Y cells late in infection, and RNA interference-mediated apsup ablation induces apoptosis of LdMNPV-infected Ld652Y cells.
TEXT
IPLB-Ld652Y (Ld652Y) cells derived from the gypsy moth, Lymantria dispar (8), exhibit unique responses to nucleopolyhedrovirus (NPV) infection and provide an excellent system for investigating interactions between insect cells and NPVs (13–15). Ld652Y cells are permissive for homologous L. dispar multicapsid NPV (LdMNPV) (20, 23–25) and heterologous Orgyia pseudotsugata MNPV (OpMNPV) (3), yielding high titers of progeny viruses, while they are nonpermissive for NPVs of Spodoptera exigua, Spodoptera litura, and Autographa californica (AcMNPV) (13, 17, 18, 26). Infection of Ld652Y cells with AcMNPV results in global protein synthesis shutdown, in which viral DNA replication and viral mRNA transcription occur normally but protein synthesis is completely shut down, yielding no progeny viruses (9, 10, 19, 21). This global protein synthesis shutdown in AcMNPV-infected Ld652Y cells can be precluded by the LdMNPV-encoded hrf-1 gene (25), and recombinant AcMNPV harboring hrf-1 successfully multiplies to a high titer in Ld652Y cells (6, 7). Studies have demonstrated that HRF-1 is an essential viral factor for productive infection of various NPVs in Ld652Y cells (14, 15).
We have previously shown that Ld652Y cells readily undergo apoptosis upon infection with various NPVs, while LdMNPV infection of Ld652Y cells results in the production of a high titer of progeny viruses without the induction of apoptosis (13). In addition, we have found that neither ld-iap2 nor ld-iap3 carried on the LdMNPV genome is capable of suppressing the apoptosis of Sf9 cells triggered by infection with vP35delBsu36IGus (vAcΔp35) (4), a recombinant AcMNPV that is defective in the p35 gene (unpublished data). In the present study, we identify a novel antiapoptotic gene, apsup, which functions to suppress apoptosis induced not only by NPV infection but also by exposure to actinomycin D and UV.
To identify an apoptosis suppressor gene carried by LdMNPV, Ld652Y cells cultured in TC100 medium (JRH Biosciences) supplemented with 0.26% (wt/vol) tryptose broth (Sigma) and 10% fetal bovine serum were transfected with each of seven cosmid fragments that covers the entire LdMNPV genome (16, 25), along with the hrf-1 expression plasmid pHyHr6IE1/HA-HRF-1 (14), and then infected with vAcΔp35 at 24 h posttransfection. At 72 h postinfection, the suppression of vAcΔp35-induced apoptosis was assessed by the production of polyhedrin and polyhedra. In this experimental system, if a cosmid fragment provides an apoptosis suppressor, the global protein synthesis shutdown of Ld652Y cells triggered by vAcΔp35 infection is precluded due to HRF-1, and vAcΔp35 successfully multiplies in Ld652Y cells, producing polyhedrin and polyhedra.
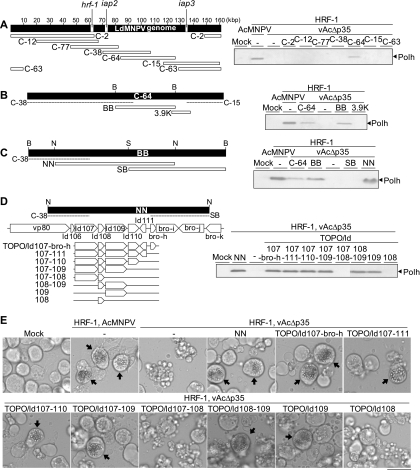
Only the cells transfected with cosmid C-64 produced both polyhedrin and polyhedra (Fig. 1 A; data not shown for polyhedra), indicating that this cosmid carries an apoptosis suppressor gene. Cosmid C-64 was then digested with BamHI to yield the BB fragment, and the BB fragment was cloned into pBlueScript II KS+ (Stratagene). The plasmid containing the BB fragment provided an apoptosis suppressor such that polyhedrin and polyhedra were produced in vAcΔp35-infected Ld652Y cells (Fig. 1B). To cover the gap between the BB fragment and cosmid C-15, a DNA fragment (ca. 3.9 kbp) amplified by PCR from C-64 using the paired primers 5′-CATTTGGACATGCCGTACGC-3′ and 5′-CGGTCTCTCGTTCAGCAAGC-3′ was cloned into pCR4-TOPO (Invitrogen). Ld652Y cells transfected with the 3.9-kbp DNA fragment did not produce polyhedrin or polyhedra (Fig. 1B).
Fig. 1.
Identification of an LdMNPV gene that is responsible for the suppression of apoptosis in Ld652Y cells infected with vAcΔp35. (A) Seven cosmid clones (C-2, C-12, C-77, C-38, C-64, C-15, and C-63), which cover the entire LdMNPV genome (left), were each transfected into Ld652Y cells along with the plasmid pHyHr6IE1/HA-HRF-1 harboring the hrf-1 gene. Twenty-four hours after transfection, the cells were infected with vAcΔp35 at a multiplicity of infection of 0.1. The transfected and infected cells were analyzed by immunoblot analysis with antipolyhedrin antibody for polyhedrin (Polh) production at 72 h postinfection (right). The polyhedrin signals were visualized by using horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG antibody (Zymed) and a Konica immunostaining HRP-1000 kit (Konica). An LdMNPV genomic map with scales of kilobase pairs (kbp) is presented, together with the locations of the hrf-1, iap2, and iap3 genes. (B) The BB fragment, which was derived from cosmid C-64 through digestion with BamHI, and the PCR-amplified 3.9K DNA fragment (left) were cloned into pBlueScript and transfected into Ld652Y cells together with hrf-1. The transfection-infection experiments were performed as described for panel A for analysis of polyhedrin production (right). (C) The NN and SB fragments, which were derived from the BB fragment by digestion with NotI or SmaI (left), were similarly tested for polyhedrin production (right). (D) Eight expression plasmids harboring one or multiple gene(s) carried on the NN fragment (TOPO/ld107-bro-h, TOPO/ld107-111, TOPO/ld107-110, TOPO/ld107-109, TOPO/ld107-108, TOPO/ld108-109, TOPO/ld109, and TOPO/ld108) (left) were similarly tested for polyhedrin production (right). (E) Images obtained by microscopy showing vAcΔp35-infected Ld652Y cells previously cotransfected with the plasmid pHyHr6IE1/HA-HRF-1 harboring the hrf-1 gene and each of the expression plasmids containing subsets of the six putative viral genes carried by the NN fragment, presented in the left side of panel D. The arrows indicate the cells containing polyhedra. The scale bar indicates 30 μm. Restriction endonuclease sites in panels B to D are as follows: B, BamHI; N, NotI; S, SmaI.
The BB fragment was digested with NotI or SmaI, and the resulting NN and SB fragments were tested for their ability to suppress apoptosis. Only the NN fragment was capable of suppressing apoptosis (Fig. 1C), indicating that a possible apoptosis suppressor gene was located in the NN fragment, which contained nine putative complete genes, ld106, ld107, ld108, ld109, ld110, ld111, bro-h, bro-i, and bro-j (Fig. 1D) (16).
To map the gene encoding the apoptosis suppressor, expression plasmids that contained subsets of five different viral genes (ld107, ld108, ld109, ld110, and ld111) contained in the NN fragment but not the SB fragment or cosmid C-38 were constructed by using pCR4-TOPO (Fig. 1D). These expression plasmids were cotransfected into Ld652Y cells, together with the plasmid harboring hrf-1. The transfected cells were infected with vAcΔp35 at 24 h posttransfection. At 72 h postinfection, the cells were examined for the production of polyhedrin and polyhedra. Only the Ld652Y cells transfected with expression plasmids containing ld109 produced polyhedrin and polyhedra, and ld109 alone was sufficient for the production of polyhedrin and polyhedra (Fig. 1D and E), suggesting that ld109 provided the apoptosis suppressor.
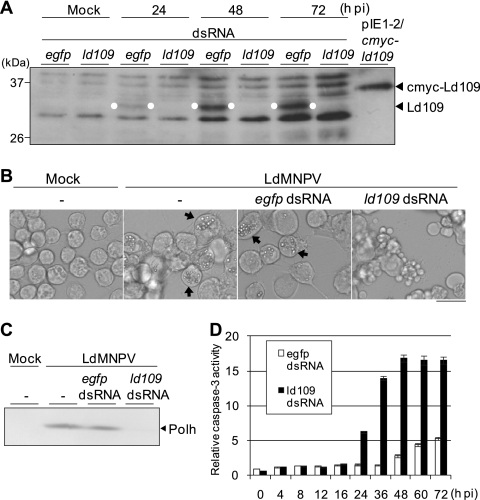
To confirm that ld109 was an apoptosis suppressor gene, RNA interference (RNAi)-mediated silencing experiments were performed (Fig. 2). Double-stranded RNAs (dsRNAs) against ld109 were synthesized by using a MEGAscript T7 kit (Ambion) and the DNA fragment which was amplified by PCR from the TOPO/ld107-110 plasmid using primers containing the T7 RNA polymerase binding site (underlined), 5′-TAATACGACTCACTATAGGGTCAGTCGCAGACATGGCCGA-3′ and 5′-TAATACGACTCACTATAGGGAATCAAACGACGGTGTGCGT-3′ (Fig. 1D and 3A). Similarly, dsRNAs against the enhanced green fluorescent protein (EGFP) gene were synthesized by using the EGFP gene-containing plasmid pIE1-2/egfp (12) and primers containing the T7 RNA polymerase binding site (underlined), 5′-TAATACGACTCACTATAGGGCCATGTCTGCGACTGAGGCG-3′ and 5′-TAATACGACTCACTATAG GGTTCTCTAACGAGCGGCGCA-3′. Polyclonal antibody against Ld109 was raised in a rabbit against two partial amino acid sequences of the Ld109 protein, 70-EQWDRRGRIDALQTTAS-86 and 320-DRYQFLLPVRYFSKNKL-336. Ld652Y cells were transfected with dsRNAs against ld109 or the EGFP gene, infected with LdMNPV at 24 h posttransfection, and subjected to immunoblot analysis with anti-Ld109 antibody at different times postinfection. The dsRNAs against ld109 but not those against the EGFP gene suppressed the expression of a polypeptide that migrated at the approximate molecular weight of 32,000 (32K), which was about 2.8K lower than that of cMyc-Ld109 expressed upon transfection with pIE1-2/cmyc-ld109 (Fig. 2A and 4 A). The cells transfected with ld109 dsRNA underwent apoptosis, producing no detectable polyhedra or polyhedrin, while mock-transfected cells and cells transfected with EGFP dsRNA produced both polyhedra and polyhedrin (Fig. 2B and C). In ld109 dsRNA-transfected cells, caspase-3-like protease activity, assayed as described previously (13), increased from 24 h postinfection and peaked at 48 h postinfection, while only a small increase in caspase-3-like protease activity occurred in the cells transfected with EGFP dsRNA from 48 to 72 h postinfection (Fig. 2D). These results indicate that LdMNPV infection causes the apoptosis of Ld652Y cells, which can be suppressed by Ld109 protein.
Fig. 2.
Effects of RNAi-mediated silencing of ld109 on the induction of apoptosis in Ld652Y cells infected with LdMNPV. (A) RNAi silencing of Ld109 protein. Monolayer cultures of 1 × 106 Ld652Y cells in 35-mm culture dishes were transfected with 1 μg of dsRNAs against ld109 and the EGFP gene. At 24 h posttransfection, the cells were infected with LdMNPV at a multiplicity of infection of 1. At 24, 48, and 72 h postinfection (h pi), these cells were examined for the expression of Ld109 by immunoblot analysis with anti-Ld109 antibody as the primary antibody and HRP-conjugated goat anti-rabbit IgG antibody as the secondary antibody. Polypeptides from LdMNPV-infected Ld652Y cells were resolved on a 12.5% SDS–polyacrylamide gel and blotted onto an Immobilon-P transfer membrane (Millipore). The Ld109 protein signals were visualized by enhanced chemiluminescence Western blotting detection reagents (Amersham Biosciences) and are highlighted by the white dots. The mock-infected cells previously transfected with dsRNAs against ld109 and the EGFP gene and the cells transfected with pIE1-2/cmyc-ld109 expressing cMyc-Ld109 protein were also analyzed. BenchMark prestained protein ladder (Invitrogen) was used as the protein size marker. (B) Images obtained by microscopy show the apoptosis induced by RNAi silencing of ld109 in LdMNPV-infected Ld652Y cells. Cells were observed at 96 h posttransfection (72 h postinfection) with dsRNAs against ld109 and the EGFP gene. The arrows indicate the cells containing polyhedra, and the scale bar indicates 30 μm. (C) Immunoblot analysis of polyhedrin (Polh) production. The transfected and infected cells were harvested at 72 h postinfection and analyzed by using antipolyhedrin antibody as the primary antibody and HRP-conjugated goat anti-rabbit IgG antibody as the secondary antibody. Polypeptides were resolved on a 12.5% SDS–polyacrylamide gel and blotted on a nitrocellulose membrane (Advantec Toyo). The polyhedrin signals were visualized by using a Konica immunostaining HRP-1000 kit. (D) Caspase-3-like protease activity of the cells transfected with ld109 dsRNA. The LdMNPV-infected cells previously transfected with dsRNAs against ld109 and the EGFP gene were harvested at the indicated times postinfection, and the caspase-3-like protease activities of these cells were determined by using Ac-DEVD-AMC (N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin) as the substrate. The caspase-3-like protease activities are presented as the ratios to the activity of mock-infected cells at 0 h postinfection. Vertical bars indicate standard deviations of averages from three determinations.
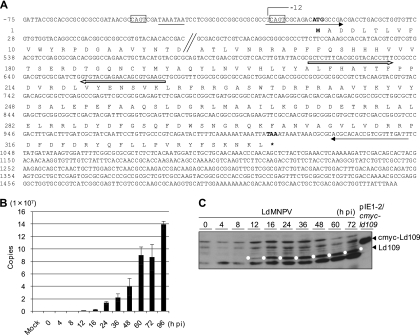
Fig. 3.
Identification of transcripts and temporal expression analysis of ld109 in the Ld652Y cells infected with LdMNPV. (A) Nucleotide and deduced amino acid sequences of ld109 transcripts and Ld109 protein. Total RNA was extracted from LdMNPV-infected Ld652Y cells at 48 h postinfection by using an RNeasy minikit. RLM-RACE for the ld109 gene was performed by using a FirstChoice RLM-RACE kit. The suggested start (ATG) and stop codons (TAA) are shown by boldface. The transcriptional initiation site (−12) is indicated. The CAGT motifs are boxed by a solid line, and the TATA box-like sequence TAAATAA is underlined. The solid and open arrows indicate the regions used for the preparation of primers for dsRNAs and qRT-PCR analyses, respectively. (B) Temporal analysis of ld109 transcripts. Ld652Y cells were infected with LdMNPV at a multiplicity of infection of 1, and RNA was extracted at the indicated times postinfection by using a Power SYBR green Cells-to-CT kit. These RNAs were treated with DNase I using an RNAqueous-4PCR kit (Ambion) and subjected to reverse transcription. The qRT-PCR analyses of ld109 were performed by using specific primers for ld109 as indicated in panel A. (C) Temporal analysis of Ld109 protein. Polypeptides from LdMNPV-infected Ld652Y cells were analyzed at the indicated times postinfection, and Ld109 protein was probed with the anti-Ld109 antibody. The Ld109 proteins are highlighted by the white dots. For details, see the legend to Fig. 2A.
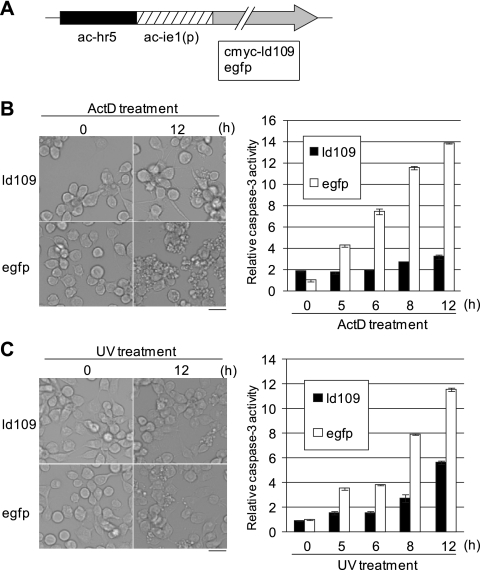
Fig. 4.
Antiapoptotic activity of Ld109 against apoptosis of Ld652Y cells induced by exposure to actinomycin D and UV. (A) Schematic representation of pIE1-2/cmyc-ld109 and pIE1-2/egfp. (B) Antiapoptotic activity of Ld109 against actinomycin D-treated cells. Ld652Y cells were transfected with 1 μg of pIE1-2/cmyc-ld109 and pIE1-2/egfp. Forty-eight h posttransfection, they were treated with TC100 medium containing 0.5 μg/ml actinomycin D. The cells were examined for apoptosis under the microscope (left), and caspase-3-like protease activity was determined at the indicated times after actinomycin D treatment (right). (C) Antiapoptotic activity of Ld109 against apoptosis induced by UV irradiation. The Ld652Y cells were transfected with 1 μg of pIE1-2/cmyc-ld109 and pIE1-2/egfp, and at 48 h posttransfection, they were exposed to UV (254 nm) irradiation at 430 μW/cm2 for 30 s by using a UVGL-58 UV lamp (Ultra-Violet Products). These cells were examined for apoptosis and caspase-3-like protease activity as described for panel B. The caspase-3-like protease activities were determined by using Ac-DEVD-AMC (N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin) as the substrate and are presented as the ratios to the activity of mock-treated cells at 0 h posttreatment (48 h posttransfection). The scale bars in the left panels indicate 30 μm. The error bars in the right panels indicate standard deviations of averages from three determinations.
To provide evidence that ld109 was expressed in LdMNPV-infected Ld652Y cells, total RNA was extracted from LdMNPV-infected Ld652Y cells by using an RNeasy minikit (Qiagen). The 5′- and 3′-RNA ligase-mediated rapid amplification of cDNA ends (RLM-RACE) was performed by using a cDNA library from LdMNPV-infected Ld652Y cells and a FirstChoice RLM-RACE kit (Ambion). Sequencing of the PCR products demonstrated that the ld109 transcript comprised 1,564 nucleotides (nt), consisting of a 1,008-nt open reading frame (ORF) and 12 and 544 nt of the 5′ and 3′ noncoding regions, respectively (Fig. 3A). The ld109 ORF encodes a predicted polypeptide of 336 amino acid residues with a molecular weight of 39,333. Examination of the regulatory region revealed that the transcription initiation site of ld109 was located 1 nt upstream from the CAGT RNA polymerase II start site motif located about 30 nt downstream from the TATA box-like sequence TAAATAA, suggesting that ld109 is an early gene.
To examine ld109 transcripts during LdMNPV infection, quantitative reverse transcription-PCR (qRT-PCR) was performed by using a Power SYBR green Cells-to-CT kit (Applied Biosciences) and primers 5′-GCTCTTTCACGCGTACACCTTT-3′ and 5′-GCTTCACGCTGTTCTCGTACAC-3′ (Fig. 3A). The ld109 transcripts were first detected at 12 h postinfection and increased continuously thereafter (Fig. 3B). Consistent with the qRT-PCR results, immunoblot analysis with anti-Ld109 antibody demonstrated that Ld109 protein expression was detected in LdMNPV-infected Ld652Y cells from 12 h postinfection and increased until 72 h postinfection (Fig. 3C), suggesting that Ld109 served as an apoptosis suppressor late in virus infection.
To determine whether Ld109 also suppressed apoptosis induced by nonviral stimuli, Ld652Y cells were transfected with pIE1-2/cmyc-ld109, the cMyc-tagged Ld109-expressing plasmid, driven by the AcMNPV ie1 gene (ac-ie1) promoter and enhanced by ac-hr5 (Fig. 4A). Forty-eight hours after transfection, cells were exposed to actinomycin D or UV and examined for the induction of apoptosis and caspase-3-like protease activity. Upon exposure to either actinomycin D or UV, the induction of apoptosis and caspase-3-like protease activity were less extensive in Ld109-expressing Ld652Y cells than in EGFP-expressing Ld652Y cells (Fig. 4B and C), indicating that Ld109 was capable of suppressing apoptosis and caspase-3-like protease activation of Ld652Y cells induced by actinomycin D and UV.
A database search revealed that ld109 homologues were found in nine NPVs, four granuloviruses, and one poxvirus. The Ld109 protein shared amino acid sequence identities ranging from 23.3% with fowlpox virus FPV217 (1) to 86.0% with Lymantria xylina MNPV ORF109 (22). A domain search revealed no characteristic domains of the Ld109 protein that were linked to possible functions, and functional analyses have not been conducted for any of these Ld109 homologues. We recently performed a transient expression assay and found that Ac112/113 (2, 11), the AcMNPV homologue of Ld109, which shares 30.2% amino acid sequence identity with Ld109, was unable to suppress the apoptosis of Ld652Y cells triggered by actinomycin D and UV (unpublished data). These results indicate that ld109 or its homologue is a crucial antiapoptotic gene for LdMNPV, which lacks p35 and functional iap genes, but not for AcMNPV, which possesses the potent antiapoptotic gene p35, suggesting that individual baculoviruses have evolved unique and divergent antiapoptotic genes and mechanisms for the regulation of apoptosis of infected cells (5). We designated the ld109 gene apsup, for apoptosis-suppressor.
Acknowledgments
We thank T. Yaginuma and T. Niimi of the Laboratory of Sericulture and Entomoresources, Nagoya University, Japan, for their helpful discussions during this study.
This work was supported in part by grants-in-aid (grants 19208006 and 20380034) from the Japan Society for the Promotion of Science.
Footnotes
Published ahead of print on 16 March 2011.
REFERENCES
- 1. Afonso C. L., et al. 2000. The genome of fowlpox virus. J. Virol. 74:3815–3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ayres M. D., Haward S. C., Kuzio J., Lopez-Ferber M., Possee R. D. 1994. The complete DNA sequence of Autographa californica nuclear polyhedrosis virus. Virology 202:586–605 [DOI] [PubMed] [Google Scholar]
- 3. Bradford M. B., Blissard G. W., Rohrmann G. F. 1990. Characterization of the infection cycle of the Orgyia pseudotsugata multicapsid nuclear polyhedrosis virus in Lymantria dispar cells. J. Gen. Virol. 71:2841–2846 [DOI] [PubMed] [Google Scholar]
- 4. Clarke T. E., Clem R. J. 2003. In vivo induction of apoptosis correlating with reduced infectivity during baculovirus infection. J. Virol. 77:2227–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clem R. J. 2007. Baculovirus and apoptosis: a diversity of genes and responses. Curr. Drug Targets 8:1069–1074 [DOI] [PubMed] [Google Scholar]
- 6. Du X., Thiem S. M. 1997. Responses of insect cells to baculovirus infection: protein synthesis shutdown and apoptosis. J. Virol. 71:7866–7872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Du X., Thiem S. M. 1997. Characterization of host range factor 1 (hrf-1) expression in Lymantria dispar M nucleopolyhedrovirus- and recombinant Autographa californica M nucleopolyhedrovirus-infected IPLB-Ld652Y cells. Virology 227:420–430 [DOI] [PubMed] [Google Scholar]
- 8. Goodwin R. H., Tompkins G. J., McCawley P. 1978. Gypsy moth cell lines divergent in viral susceptibility. I. Culture and identification. In Vitro 14:485–494 [DOI] [PubMed] [Google Scholar]
- 9. Guzo D., Dougherty E. M., Lynn D. E., Braun S. K., Weiner R. M. 1991. Changes in macromolecular synthesis of gypsy moth cell line IPLB-Ld652Y induced by Autographa californica nuclear polyhedrosis virus infection. J. Gen. Virol. 72:1021–1029 [DOI] [PubMed] [Google Scholar]
- 10. Guzo D., Rathburn H., Guthrie K., Dougherty E. 1992. Viral and host cellular transcription in Autographa californica nuclear polyhedrosis virus-infected gypsy moth cell lines. J. Virol. 66:2966–2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harrison R. L., Bonning B. C. 2003. Comparative analysis of the genomes of Rachiplusia ou and Autographa californica multiple nucleopolyhedroviruses. J. Gen. Virol. 84:1827–1842 [DOI] [PubMed] [Google Scholar]
- 12. Ikeda M., Yanagimoto K., Kobayashi M. 2004. Identification and functional analysis of Hyphantria cunea nucleopolyhedrovirus iap genes. Virology 321:359–371 [DOI] [PubMed] [Google Scholar]
- 13. Ishikawa H., et al. 2003. Induction of apoptosis in an insect cell line, IPLB-Ld652Y, infected with nucleopolyhedroviruses. J. Gen. Virol. 84:705–714 [DOI] [PubMed] [Google Scholar]
- 14. Ishikawa H., Ikeda M., Felipe Alves C. A., Thiem S. M., Kobayashi M. 2004. Host range factor 1 from Lymantria dispar nucleopolyhedrovirus (NPV) is an essential viral factor required for productive infection of NPVs in IPLB-Ld652Y cells derived from L. dispar. J. Virol. 78:12703–12708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ishikawa H., Ogasawara T., Ikeda M., Kobayashi M. 2006. A recombinant Bombyx mori nucleopolyhedrovirus possessing hrf-1 gene replicates in nonpermissive Lymantria dispar IPLB-Ld652Y cell line. J. Insect Biotechnol. Sericol. 75:31–38 [Google Scholar]
- 16. Kuzio J., et al. 1999. Sequence and analysis of the genome of a baculovirus pathogenic for Lymantria dispar. Virology 253:17–34 [DOI] [PubMed] [Google Scholar]
- 17. Laviña B. A., et al. 2001. Biological characterization of a nucleopolyhedrovirus of Spodoptera litura (Lepidoptera: Noctuidae) isolated from the Philippines. Biol. Contr. 20:39–47 [Google Scholar]
- 18. Laviña-Caoili B. A., Kamiya K., Kawamura S., Ikeda M., Kobayashi M. 2001. Comparative in vitro analysis of geographic variants of nucleopolyhedrovirus of Spodoptera litura isolated from China and the Philippines. J. Insect Biotechnol. Sericol. 70:199–209 [Google Scholar]
- 19. McClintock J. T., Dougherty E. M. 1987. Superinfection of baculovirus-infected gypsy moth cells with the nuclear polyhedrosis viruses of Autographa californica and Lymantria dispar. Virus Res. 7:351–364 [Google Scholar]
- 20. McClintock J. T., Dougherty E. M., Weiner R. M. 1986. Protein synthesis in gypsy moth cells infected with a nuclear polyhedrosis virus of Lymantria dispar. Virus Res. 5:307–322 [Google Scholar]
- 21. McClintock J. T., Dougherty E. M., Weiner R. M. 1986. Semipermissive replication of a nuclear polyhedrosis virus of Autographa californica in a gypsy moth cell line. J. Virol. 57:197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nai Y., et al. 2010. Genomic sequencing and analyses of Lymantria xylina multiple nucleopolyhedrovirus. BMC Genomics 11:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Slavicek J. M., Podgwaite J., Lanner-Herrera C. 1992. Properties of two Lymantria dispar nuclear polyhedrosis virus isolates obtained from the microbial pesticide Gypchek. J. Invertebr. Pathol. 59:142–148 [Google Scholar]
- 24. Slavicek J. M., Mercer M. J., Kelly M. E., Hayes-Plazolles N. 1996. Isolation of a baculovirus variant that exhibits enhanced polyhedra production stability during serial passage in cell culture. J. Invertebr. Pathol. 67:153–160 [Google Scholar]
- 25. Thiem S. M., Du X., Quentin M. E., Berner M. M. 1996. Identification of a baculovirus gene that promotes Autographa californica nuclear polyhedrosis virus replication in a nonpermissive insect cell line. J. Virol. 70:2221–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu F. Q., et al. 2000. Cloning and biological characterization of Spodoptera exigua nucleopolyhedroviruses isolated in China. J. Sericult. Sci. Jpn. 69:177–189 [Google Scholar]