Abstract
In human-cytomegalovirus (HCMV)-infected cells, the localization of the viral protein pp150 to the virus assembly compartment (AC) is dependent on its direct interaction with the cellular protein Bicaudal D1 through a dynein- and microtubule-dependent mechanism. We found that the small GTPase Rab6 also interacts indirectly with pp150 through its interaction with Bicaudal D1. Inhibition of Rab6 activity in HCMV-infected cells interrupted the intracellular trafficking of pp150, significantly reducing infectious virus production without affecting the formation of the AC, arguing for an important function for this cellular GTPase in the intracellular localization of pp150 during virus assembly.
TEXT
Human cytomegalovirus (HCMV), the prototypic betaherpesvirus, is one of the most common infections in newborn infants, affecting 0.2% to 2.2% of all newborns, and is a major cause of neurological disease in infected infants (4, 11). HCMV establishes a persistent infection in the infected host, and although it is associated with asymptomatic infections in healthy individuals, it can cause clinically significant infections in immunocompromised patients, including transplant recipients and AIDS patients (2, 5, 6).
The virion consists of a nucleocapsid containing an approximately 230-kbp linear DNA genome, an envelope studded with viral glycoproteins, and a tegument layer located between the capsid and envelope (26, 28, 36). Assembly of HCMV is an incompletely understood process and involves multiple stages in different cellular compartments. HCMV infection modifies the host secretory pathway leading to the accumulation of virion tegument and envelope proteins in a perinuclear site termed the assembly compartment (AC), which has been postulated to be the site of viral assembly (16, 31).
The HCMV tegument protein pp150 is an essential betaherpesvirus-specific 1,048-amino-acid protein encoded by the UL32 open reading frame (ORF) of HCMV (19, 20, 30). It is both phosphorylated and glycosylated, although the role of these posttranslational modifications in HCMV assembly is unknown (14, 20). In primary human foreskin fibroblasts (HFFs) infected with HCMV, pp150 localizes to the AC, where it colocalizes with other viral proteins (31). Deletion of the UL32 ORF leads to loss of infectious virus production, and the protein has been reported to be important in the cytoplasmic maturation of HCMV (1, 7, 35, 40, 41).
The localization of pp150 to the AC in infected cells is presumably dependent on interactions with cellular and/or viral proteins, since it has no predicted intracellular trafficking signals. We previously reported that pp150 interacts directly with Bicaudal D1 (BicD1), an effector protein of the small GTPase Rab6 that links Rab6 activity to dynein (18). We showed that the interaction between pp150 and BicD1 was necessary for targeting pp150 to the AC by a dynein- and microtubule-dependent mechanism (18). BicD1 binds to Rab6 GTPase through its C-terminal domain and to dynein through its N-terminal domain and colocalizes with Rab6 in the trans-Golgi network (TGN) and on cytoplasmic vesicles that associate with Golgi membranes in a Rab6-dependent manner (10, 17, 25). Rab6 is required for tethering of BicD1 to vesicles, while BicD1 is essential for recruitment of the dynein-dynactin complex to Rab6-positive vesicles (17, 25, 33). Finally, we have reported that the C-terminal domain of BicD1 that interacts with Rab6 is also the binding site for pp150 (18). The finding that this domain of BicD1 is also important for the trafficking of pp150 to the viral AC in infected cells argued that Rab6 could direct the intracellular trafficking of pp150 to sites of HCMV assembly (18).
Rab6 is a Golgi network-associated GTPase that has been shown to regulate endosome-to-Golgi network and Golgi network-to-endoplasmic reticulum (ER) retrograde-transport pathways (3, 12, 13, 21, 23, 24, 29, 37, 39). Rab6 is important for the organization and function of exocytic vesicles derived from the Golgi network and targeted to the cell membrane (15). Rab6 is conserved from Saccharomyces cerevisiae to humans, and in humans there are four known isoforms: Rab6A, A′, B, and Rab6C (8, 29, 32, 34). Rab6A and A′ are ubiquitously and abundantly expressed, Rab6B is expressed in neuronal cells, and Rab6C shows tissue-specific expression, such as in brain, spinal cord, testis, and prostate (38). Rab6A′ differs from Rab6A in three amino acid substitutions, Val62 to Ile, Thr87 to Ala, and Val88 to Ala (8).
Rab6 localizes to the AC in HCMV-infected cells.
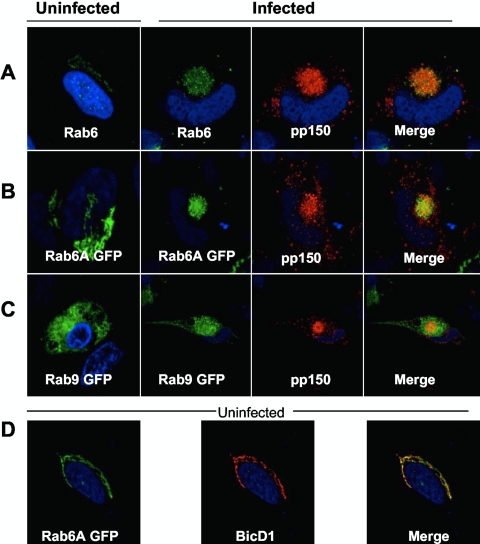
In uninfected HFFs, Rab6 is localized to a distinct perinuclear compartment that has been shown to colocalize with markers of the TGN (Fig. 1A). Upon viral infection, Rab6 relocalizes to the AC, where it colocalizes with pp150 (Fig. 1A). We investigated the trafficking of Rab6 isoforms A and A′ in infected HFFs by transfecting HFFs with green fluorescent protein (GFP)-tagged Rab6A and A′, followed by HCMV infection as described previously (18). Although only data for the Rab6A isoform are shown, both isoforms localized to the TGN in uninfected cells and trafficked to the viral AC by 5 days postinfection (dpi), where they colocalized with pp150 (Fig. 1B and data not shown). In uninfected cells, they colocalized with endogenous BicD1 in a perinuclear compartment (Fig. 1D and data not shown). As a negative control, we used GFP-tagged Rab9, which does not interact with BicD1 (25). In infected cells, Rab9 failed to localize to the AC or colocalize with pp150 (Fig. 1C). These findings demonstrated that HCMV infection resulted in the specific relocalization of Rab6 isoforms from a perinuclear distribution to the AC.
Fig. 1.
Rab6 traffics to the AC and colocalizes with pp150. (A) Rab6 expression in human foreskin fibroblasts (HFFs; uninfected and infected with HCMV). Infected cells were fixed at 5 days postinfection (dpi) and stained with rabbit anti-Rab6 antisera (green) and pp150 monoclonal antibody (MAb) (red). The anti-Rab6 antiserum is reactive with both isoforms of Rab6. (B, C) Expression of GFP-tagged Rab6A (B) and Rab9 (C) in uninfected or HCMV-infected HFFs. The cells were stained at 5 dpi with pp150 MAb (red). (D) Localization of GFP-Rab6A and endogenous BicD1 in transfected HFFs. HFFs were transfected with GFP-tagged Rab6A (green) and then stained with BicD1-specific rabbit antiserum (red). Images were collected by confocal microscopy (magnification, ×100). Nuclear dye TOTO3 was used to demarcate nuclei (blue).
Rab6 forms a ternary complex with pp150 and Bicaudal D1.
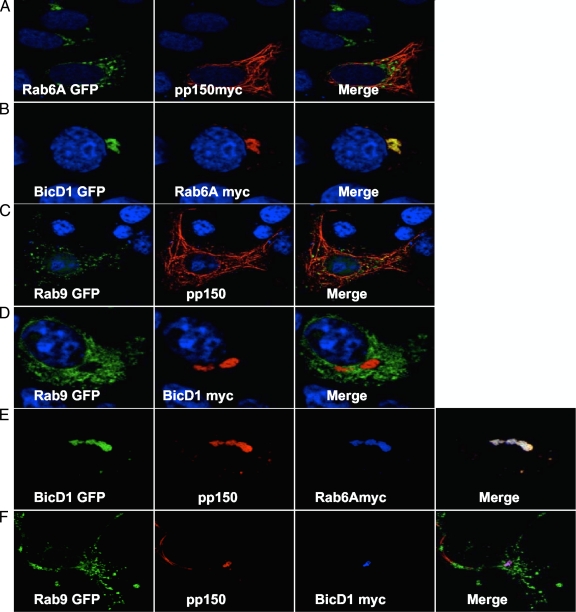
Localization of Rab6 to the AC may be secondary to its association with BicD1 or achieved by a mechanism independent of the previously reported BicD1-pp150 interaction. In transfected cells, pp150 colocalizes with microtubules (18), while in a minority of cells, it can be observed dispersed in the cytoplasm (data not shown). We found that in COS-7 cells transfected with myc-tagged pp150-expressing plasmids and GFP-tagged Rab6A-expressing plasmids, pp150 and Rab6A did not colocalize (Fig. 2A). Similarly, GFP-tagged Rab6A′ did not colocalize with myc-tagged pp150 in transfected COS-7 cells (data not shown). When GFP-tagged BicD1 and myc-tagged Rab6A were coexpressed in COS-7 cells, they colocalized (Fig. 2B). Similarly, GFP-tagged BicD1 colocalized with myc-tagged Rab6A′ (data not shown). The negative control, Rab9, did not colocalize with pp150 (Fig. 2C) or with BicD1 (Fig. 2D). We observed that when myc-tagged Rab6A was expressed together with GFP-tagged BicD1 and pp150, all three colocalized in transfected COS-7 cells and that pp150 was completely relocalized to the site of BicD1 and Rab6 expression (Fig. 2E). In transfected COS-7 cells expressing pp150, BicD1-GFP, and Rab6A′-myc, all three colocalized, suggesting that both isoforms of Rab6 form a complex with BicD1 and pp150 (data not shown). Rab9 failed to colocalize with pp150 even in the presence of overexpressed BicD1 (Fig. 2F), whereas BicD1 and pp150 were observed to colocalize (Fig. 2F).
Fig. 2.
Rab6 isoforms colocalize with pp150 only in the presence of BicD1. (A) COS-7 cells were cotransfected with plasmids encoding GFP-tagged Rab6A and myc-tagged pp150, fixed, and stained with anti-myc MAb. GFP-tagged Rab6A (green) failed to colocalize with pp150 (red) on tubular structures in the cytoplasm. (B) COS-7 cells cotransfected with GFP-tagged BicD1 and myc-tagged Rab6A plasmids were fixed and stained with anti-myc MAb. GFP-tagged BicD1 (green) colocalizes with Rab6A (red) in the perinuclear compartment. (C) COS-7 cells were cotransfected with plasmids encoding GFP-tagged Rab9 and pp150 without a tag and fixed and stained with anti-pp150 MAb. GFP-tagged Rab9 (green) failed to colocalize with pp150 (red) on tubular structures in the cytoplasm. (D) COS-7 cells cotransfected with GFP-tagged Rab9 and myc-tagged BicD1 plasmids were fixed and stained with anti-myc MAb. GFP-tagged Rab9 (green) failed to colocalize with BicD1 (red). (E) COS-7 cells cotransfected with GFP-tagged BicD1, pp150 without a tag, and myc-tagged Rab6A plasmids were fixed and stained with anti-myc and anti-pp150 antibodies. GFP-tagged BicD1 (green), pp150 (red), and Rab6A (blue) colocalized in a perinuclear compartment. Merging is indicated by the development of the white color when all three colocalized. Note that the cytoplasmic localization of pp150 is altered in the presence of BicD1 and Rab6. (F) COS-7 cells cotransfected with GFP-tagged Rab9, pp150 without a tag, and myc-tagged BicD1 plasmids were fixed and stained with anti-myc and anti-pp150 antibodies. GFP-tagged Rab9 (green), pp150 (red), and BicD1 (blue) failed to colocalize, while p150 (red) and BicD1 (blue) colocalized. All images were collected by laser scanning confocal microscopy (magnification, ×100). Nuclear dye TOTO3 was used to demarcate nuclei (blue) in panels A to D.
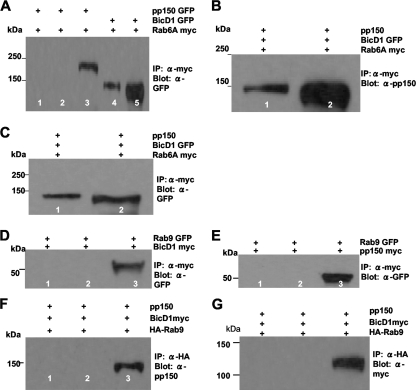
We used coimmunoprecipitation assays to determine if BicD1 interacted simultaneously with pp150 and Rab6 or if pp150 competed with Rab6 for binding sites on BicD1. In agreement with our colocalization studies, we found that myc-tagged Rab6A failed to pull down GFP-tagged pp150 when both were transiently expressed in HEK 293 cells (Fig. 3A, lane 2). Unconjugated magnetic beads used as a negative control did not pull down myc-tagged Rab6A or pp150-GFP from cell lysates (Fig. 3A, lane 1). As expected, Rab6A-myc pulled down BicD1GFP (Fig. 3A, lane 4). Similar results were obtained when myc-tagged Rab6A′ was used in these assays (data not shown). HEK 293 cells were then transfected with plasmids expressing myc-tagged Rab6A and pp150 and GFP-tagged BicD1. myc-tagged Rab6A pulled down pp150 (Fig. 3B, lane 1) while in complex with BicD1 (Fig. 3C, lane 1). In similar experiments, myc-tagged Rab6A′ was able to pull down pp150 in complex with BicD1 (data not shown). The expression of myc-tagged Rab6A and A′ was confirmed using anti-myc antibody (data not shown). The negative-control, GFP-tagged Rab9 did not pull down either BicD1 (Fig. 3D, lane 2) or pp150 (Fig. 3E, lane 2). Also, Rab9 did not pull down pp150 (Fig. 3F, lane 2) while in complex with BicD1 (Fig. 3G, lane 2). These results argued that pp150 does not prevent the binding of Rab6 to BicD1 and suggests that Rab6 indirectly interacts with pp150 through BicD1, thus providing a mechanism by which Rab6 could function to target the BicD1-pp150 complex 32 to the AC in HCMV-infected cells.
Fig. 3.
BicD1, pp150, and Rab6 form a ternary complex. (A) Coimmunoprecipitation (IP) of pp150 with Rab6A. Protein complexes from lysates of HEK 293 cells cotransfected with myc-tagged Rab6A and pp150-GFP (or Rab6A-myc and BicD1-GFP) were precipitated with anti-myc magnetic beads. Lane 1, negative control in which cell lysates were treated with unlabeled magnetic beads; lane 2, Rab6A-myc, which does not precipitate pp150-GFP; lane 3, pp150-GFP in the input lysate; lane 4, positive control showing that Rab6A-myc precipitates BicD1-GFP; lane 5, input lysate containing BicD1-GFP. The blot was probed with anti-GFP antibody. (B) Coimmunoprecipitation of pp150 and Rab6A in the presence of overexpressed BicD1. Protein complexes from lysates of HEK 293 cells cotransfected with Rab6A-myc, pp150, and BicD1-GFP were precipitated with anti-myc magnetic beads. Lane 1, Rab6A-myc precipitating pp150 when it was coexpressed with BicD1-GFP; lane 2, the input lysate containing pp150. The blot was probed with anti-pp150 MAb. (C) Protein complexes from lysates of HEK 293 cells cotransfected with Rab6A-myc, pp150, and BicD1-GFP (same as described for panel B) were precipitated with anti-myc magnetic beads. Lane 1, the precipitate is positive for BicD1-GFP; lane 2, input lysate containing BicD1-GFP. The blot was probed with anti-GFP antibody. (D) Coimmunoprecipitation of BicD1 with Rab9. Protein complexes from lysates of HEK 293 cells cotransfected with Rab9-GFP and BicD1-myc were precipitated with anti-myc magnetic beads. Lane 1, negative control in which cell lysates were treated with unlabeled magnetic beads; lane 2, BicD1-myc does not precipitate Rab9-GFP; lane 3, Rab9-GFP in the input lysate. The blot was probed with anti-GFP antibody. (E) Coimmunoprecipitation of pp150 with Rab9. Protein complexes from lysates of HEK 293 cells cotransfected with Rab9-GFP and pp150-myc were precipitated with anti-myc magnetic beads. Lane 1, negative control in which cell lysates were treated with unlabeled magnetic beads; lane 2, pp150-myc does not precipitate Rab9-GFP; lane 3, Rab9-GFP in the input lysate. The blot was probed with anti-GFP antibody. (F) Rab9 does not form a complex with BicD1 and pp150. Protein complexes from lysates of HEK 293 cells cotransfected with hemagglutinin (HA)-tagged Rab9, pp150 without a tag, and BicD1-myc were precipitated with anti-HA magnetic beads. Lane 1, negative control in which cell lysates were treated with unlabeled magnetic beads; lane 2, HA-Rab9 failing to precipitate pp150 when it was coexpressed with BicD1; lane 3, input lysate showing pp150. The blot was probed with anti-pp150 antibody. (G) Protein complexes from lysates of HEK 293 cells cotransfected with HA-Rab9, pp150, and BicD1-myc (same as described for panel F) were precipitated with anti-HA magnetic beads. Lane 1, negative control in which cell lysates were treated with unlabeled magnetic beads; lane 2, HA-Rab9 does not pull down BicD1; lane 3, input lysate showing BicD1. The blot was probed with anti-myc antibody.
Inhibition of Rab6 function inhibits pp150 trafficking to the AC and affects viral yield.
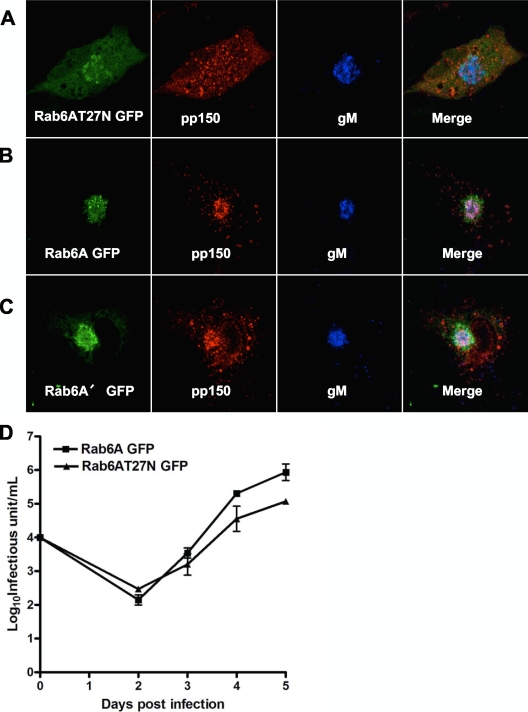
To investigate the importance of the Rab6-pp150 interaction in the trafficking of pp150 and hence in HCMV assembly, we inhibited the activity of endogenous Rab6 in HFFs by overexpressing dominant negative (DN) Rab6AT27N (27). DN Rabs are locked in the GDP conformation and sequester the guanine nucleotide exchange factors (GEFs) needed for Rab activation by competing with endogenous wild-type (WT) Rab (9). The guanosine dinucleotide (GDP)-restricted conformer binds more tightly to the GEFs than the endogenous conformer and subsequently blocks Rab activation and Rab effector recruitment (9). Rab6A and Rab6A′ utilize the same GEF for their activation (22). HFFs expressing GFP-tagged Rab6AT27N were infected with HCMV, and 5 days later the phenotype of the AC was observed by imaging. In cells expressing DN Rab6AT27N, trafficking of pp150 to the AC was inhibited and pp150 was observed to be dispersed throughout the cytoplasm, unable to localize to the AC (Fig. 4A). In contrast, trafficking of HCMV glycoprotein gM to the AC remained unaffected (Fig. 4A). To control for the specificity of the effects of DN mutants from the effects of overexpression of the respective transgenes, we directly compared GFP-tagged versions of the wild-type forms to those of the DN mutants and found that GFP-tagged Rab6A and A′ trafficked normally to the AC, where they colocalized with pp150 and the viral envelope glycoprotein gM (Fig. 4B and C).
Fig. 4.
Inhibition of Rab6 function decreases pp150 trafficking to the AC and reduces viral yield. (A) HFFs were electroporated with the GFP-tagged DN Rab6AT27N mutant form of Rab6 (green) and 24 h later were infected with HCMV. At 5 dpi, the cells were fixed and stained with anti-pp150 (red) and anti-gM (blue) MAbs. (B, C) HFFs were electroporated with GFP-tagged Rab6A (B) or Rab6A′ (C) (green) and 24 h later were infected with HCMV. At 5 dpi, the cells were fixed and stained with anti-pp150 (red) and anti-gM (blue) MAbs. Colocalization is indicated by the development of a white color in the merge channel. (D) HFFs were transfected with the GFP-tagged Rab6A or DN Rab6AT27N constructs and 24 h later were infected with HCMV at a multiplicity of infection of 0.1. The virus from the supernatant and cells was harvested at the indicated times and assayed for quantity of infectious virus. Results are expressed as a log10 infectious units/ml of sample, with error bars indicating standard deviations of the means. Images were collected by laser scanning confocal microscopy (magnification, ×100).
To test if inhibition of Rab6 activity could alter virus assembly and the yield of infectious virus, we transfected HFFs with DN Rab6AT27N and infected them a day later with HCMV. The cells were harvested at days 2, 3, 4, and 5 postinfection and assayed for the total yield (cellular and supernatant) of infectious virus. As shown in Fig. 4D, cells depleted of Rab6 activity produced about 1.0 log10 less infectious virus than cells expressing WT Rab6A. In a second experiment, HFFs were transfected with WT Rab6A or DN Rab6AT27N and infected with HCMV, and total infectious virus yield (cellular and supernatant) and infectious virus yield in the supernatant only were assayed. In addition, we also quantified viral DNA by real-time PCR. In this experiment, expression of DN Rab6AT27N inhibited total infectious virus yield by 0.7 log10 and infectious supernatant virus production by 0.75 log10 on day 7 postinfection compared to yields of virus in cells transfected with WT Rab6A. We compared the amounts of viral DNA produced in cells and in supernatants from WT-Rab6A- and DNRab6aT27N-transfected cells and found an insignificant difference (2-fold) in HCMV genome copy number between samples from WT Rab6A and DNRab6AT27N-transfected cells (data not shown). This result suggested that decreased production of infectious virus associated with the expression of DN Rab6AT27N was likely secondary to an inhibition of the assembly of virions and not secondary to the production of noninfectious particles that would be reflected by an increased genome copy number in a virus population produced from infected cells expressing DN Rab6AT27N. Taken together, these observations suggest that inhibition of Rab6 specifically altered the trafficking of the essential tegument protein pp150 to the sites of viral assembly and reduced the yield of infectious virus.
Previous studies have shown that the active-membrane-associated, GTP-bound form of Rab6 recruits BicD1 to Golgi membranes and that BicD1 recruits the dynein-dynactin complex to Rab6-positive vesicles (25). Consequently, overexpression of Rab6 T27N abolishes the association of BicD1 with Golgi membranes (25). Based on the results obtained from these and previously reported studies, we propose that the pp150-BicD1 complex is recruited to TGN-derived membranes by membrane-associated, GTP-loaded Rab6, followed by its interaction with dynein, which then moves the complex toward the AC. Consistent with this pathway are our findings that DN Rab6AT27N blocks the membrane association of the pp150-BicD1 complex and results in the complex being distributed throughout the cytoplasm (data not shown and reference 25). The mechanism by which an HCMV tegument protein utilizes components of the cellular secretory pathway to target its trafficking to the membrane-bound AC may also redirect host cell membranes to the AC to facilitate the accumulation of other virion structural proteins within this cytoplasmic site.
Acknowledgments
We thank A. Akhmanova (Erasmus Medical Center, Rotterdam, Netherlands) for her kind gifts of enhanced-GFP (EGFP)-tagged Bicaudal D1 plasmid and Bicaudal D1-specific rabbit antisera, M. A. Scidmore (Cornell University, Ithaca, NY) for her kind gifts of EGFP-tagged DN Rab6AT27N, Rab6, and Rab6A′ plasmids, and B. Goud (Institut Curie, Paris, France) for myc-tagged Rab6A and Rab6A′ plasmids.
This work was supported by grants AI035602 and AI050189 from the NIH to W.J.B.
Footnotes
Published ahead of print on 16 March 2011.
REFERENCES
- 1. AuCoin D. P., Smith G. B., Meiering C. D., Mocarski E. S. 2006. Betaherpesvirus-conserved cytomegalovirus tegument protein ppUL32 (pp150) controls cytoplasmic events during virion maturation. J. Virol. 80:8199–8210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Britt W. 2008. Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr. Top. Microbiol. Immunol. 325:417–470 [DOI] [PubMed] [Google Scholar]
- 3. Del Nery E., et al. 2006. Rab6A and Rab6A′ GTPases play non-overlapping roles in membrane trafficking. Traffic 7:394–407 [DOI] [PubMed] [Google Scholar]
- 4. Demmler G. J. 1991. Infectious Diseases Society of America and Centers for Disease Control. Summary of a workshop on surveillance for congenital cytomegalovirus disease. Rev. Infect. Dis. 13:315–329 [DOI] [PubMed] [Google Scholar]
- 5. Doerr H. W., Braun R., Munk K. 1985. Human cytomegalovirus infection: recent developments in diagnosis and epidemiology. Klin. Wochenschr. 63:241–251 [DOI] [PubMed] [Google Scholar]
- 6. Dreikorn K., Doerr H. W., Geursen R. G. 1985. Cytomegalovirus (CMV) infections in renal transplant recipients. Preliminary results of prophylaxis by an intramuscular human hyperimmune CMV IgG. Scand. J. Urol. Nephrol. Suppl. 92:15–21 [PubMed] [Google Scholar]
- 7. Dunn W., et al. 2003. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. U. S. A. 100:14223–14228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Echard A., et al. 2000. Alternative splicing of the human Rab6A gene generates two close but functionally different isoforms. Mol. Biol. Cell 11:3819–3833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feig L. A. 1999. Tools of the trade: use of dominant-inhibitory mutants of Ras-family GTPases. Nat. Cell Biol. 1:E25–E27 [DOI] [PubMed] [Google Scholar]
- 10. Fuchs E., Short B., Barr F. A. 2005. Assay and properties of rab6 interaction with dynein-dynactin complexes. Methods Enzymol. 403:607–618 [DOI] [PubMed] [Google Scholar]
- 11. Gaytant M. A., Steegers E. A., Semmekrot B. A., Merkus H. M., Galama J. M. 2002. Congenital cytomegalovirus infection: review of the epidemiology and outcome. Obstet. Gynecol. Surv. 57:245–256 [DOI] [PubMed] [Google Scholar]
- 12. Girod A., et al. 1999. Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nat. Cell Biol. 1:423–430 [DOI] [PubMed] [Google Scholar]
- 13. Goud B., Zahraoui A., Tavitian A., Saraste J. 1990. Small GTP-binding protein associated with Golgi cisternae. Nature 345:553–556 [DOI] [PubMed] [Google Scholar]
- 14. Greis K. D., Gibson W., Hart G. W. 1994. Site-specific glycosylation of the human cytomegalovirus tegument basic phosphoprotein (UL32) at serine 921 and serine 952. J. Virol. 68:8339–8349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grigoriev I., et al. 2007. Rab6 regulates transport and targeting of exocytotic carriers. Dev. Cell 13:305–314 [DOI] [PubMed] [Google Scholar]
- 16. Homman-Loudiyi M., Hultenby K., Britt W., Soderberg-Naucler C. 2003. Envelopment of human cytomegalovirus occurs by budding into Golgi-derived vacuole compartments positive for gB, Rab 3, trans-Golgi network 46, and mannosidase II. J. Virol. 77:3191–3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoogenraad C. C., et al. 2001. Mammalian Golgi-associated Bicaudal-D2 functions in the dynein-dynactin pathway by interacting with these complexes. EMBO J. 20:4041–4054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Indran S. V., Ballestas M. E., Britt W. J. 2010. Bicaudal D1-dependent trafficking of human cytomegalovirus tegument protein pp150 in virus-infected cells. J. Virol. 84:3162–3177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jahn G., et al. 1987. Map position and nucleotide sequence of the gene for the large structural phosphoprotein of human cytomegalovirus. J. Virol. 61:1358–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jahn G., Mach M. 1990. Human cytomegalovirus phosphoproteins and glycoproteins and their coding regions. Curr. Top. Microbiol. Immunol. 154:171–185 [DOI] [PubMed] [Google Scholar]
- 21. Jasmin B. J., Goud B., Camus G., Cartaud J. 1992. The low molecular weight guanosine triphosphate-binding protein Rab6p associates with distinct post-Golgi vesicles in Torpedo marmorata electrocytes. Neuroscience 49:849–855 [DOI] [PubMed] [Google Scholar]
- 22. Jiang S., Storrie B. 2005. Cisternal rab proteins regulate Golgi apparatus redistribution in response to hypotonic stress. Mol. Biol. Cell 16:2586–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martinez O., et al. 1997. GTP-bound forms of rab6 induce the redistribution of Golgi proteins into the endoplasmic reticulum. Proc. Natl. Acad. Sci. U. S. A. 94:1828–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martinez O., et al. 1994. The small GTP-binding protein rab6 functions in intra-Golgi transport. J. Cell Biol. 127:1575–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matanis T., et al. 2002. Bicaudal-D regulates COPI-independent Golgi-ER transport by recruiting the dynein-dynactin motor complex. Nat. Cell Biol. 4:986–992 [DOI] [PubMed] [Google Scholar]
- 26. Mocarski E. S., Tan Courcelle C. 2001. Cytomegaloviruses and their replication. In Knipe D. M., et al. (ed.), Fields virology, 4th ed., vol. 2 Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 27. Moorhead A. R., Rzomp K. A., Scidmore M. A. 2007. The Rab6 effector Bicaudal D1 associates with Chlamydia trachomatis inclusions in a biovar-specific manner. Infect. Immun. 75:781–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murphy E., et al. 2003. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc. Natl. Acad. Sci. U. S. A. 100:14976–14981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Opdam F. J., et al. 2000. The small GTPase Rab6B, a novel Rab6 subfamily member, is cell-type specifically expressed and localised to the Golgi apparatus. J. Cell Sci. 113:2725–2735 [DOI] [PubMed] [Google Scholar]
- 30. Roby C., Gibson W. 1986. Characterization of phosphoproteins and protein kinase activity of virions, noninfectious enveloped particles, and dense bodies of human cytomegalovirus. J. Virol. 59:714–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sanchez V., Greis K. D., Sztul E., Britt W. J. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74:975–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shan J., Yuan L., Budman D. R., Xu H. P. 2002. WTH3, a new member of the Rab6 gene family, and multidrug resistance. Biochim. Biophys. Acta 1589:112–123 [DOI] [PubMed] [Google Scholar]
- 33. Short B., Preisinger C., Schaletzky J., Kopajtich R., Barr F. A. 2002. The Rab6 GTPase regulates recruitment of the dynactin complex to Golgi membranes. Curr. Biol. 12:1792–1795 [DOI] [PubMed] [Google Scholar]
- 34. Simpson J. C., Wellenreuther R., Poustka A., Pepperkok R., Wiemann S. 2000. Systematic subcellular localization of novel proteins identified by large-scale cDNA sequencing. EMBO Rep. 1:287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tandon R., Mocarski E. S. 2008. Control of cytoplasmic maturation events by cytomegalovirus tegument protein pp150. J. Virol. 82:9433–9444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Trus B. L., Gibson W., Cheng N., Steven A. C. 1999. Capsid structure of simian cytomegalovirus from cryoelectron microscopy: evidence for tegument attachment sites. J. Virol. 73:2181–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. White J., et al. 1999. Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J. Cell Biol. 147:743–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Young J., Menetrey J., Goud B. 2010. RAB6C is a retrogene that encodes a centrosomal protein involved in cell cycle progression. J. Mol. Biol. 397:69–88 [DOI] [PubMed] [Google Scholar]
- 39. Young J., et al. 2005. Regulation of microtubule-dependent recycling at the trans-Golgi network by Rab6A and Rab6A′. Mol. Biol. Cell 16:162–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yu D., Silva M. C., Shenk T. 2003. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc. Natl. Acad. Sci. U. S. A. 100:12396–12401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zipeto D., Baldanti F., Percivalle E., Gerna G., Milanesi G. 1993. Identification of a human cytomegalovirus mutant in the pp150 matrix phosphoprotein gene with a growth-defective phenotype. J. Gen. Virol. 74:1645–1648 [DOI] [PubMed] [Google Scholar]