Abstract
Although H5N1 influenza A viruses can cause systemic infection, their neurotropism and long-term effects on the central nervous system (CNS) are not fully understood. We assessed H5N1viral invasion of the CNS and its long-term effects in a ferret model. An H5N1 virus caused nonsuppurative encephalitis, which lasted for 3 months without neurologic signs. Further, another H5N1 virus caused nonsuppurative vasculitis with brain hemorrhage. Three-dimensional analysis of viral distribution in the brain identified the olfactory system as a major route for brain invasion. The efficient growth of virus in the upper respiratory tract may thus facilitate viral brain invasion.
TEXT
Human influenza viruses usually infect the upper respiratory system, causing sneezing, runny nose, and coughing, as well as fever, malaise, and arthralgia (22). In addition, neurologic complications have also been reported when a new subtype of influenza virus is introduced into the human population, as exemplified by the Spanish (1918) and Asian (1957) pandemics (2–4, 6, 10, 18, 22).
In 1997, H5N1 influenza virus, which originated in poultry, caused an outbreak in humans. Since then, more than 500 people have been infected with H5N1 viruses worldwide, with a mortality rate of approximately 60% (21). Human H5N1 virus infection generally manifests as severe pneumonia, which progresses to acute respiratory distress syndrome; however, some H5N1 virus victims have experienced neurologic involvement (1). H5N1 viral RNA and antigens have been detected in the brains of patients, and the virus itself has been isolated from cerebrospinal fluid (5, 7) These data suggest that some H5N1 viruses could cause encephalitis in humans, as occurred in the early phases of the Spanish and Asian pandemics.
Ferrets represent a useful mammalian model of influenza infection because they are highly susceptible to infection with influenza viruses and develop some of the symptoms of influenza that are seen in humans (8, 12, 14, 16, 19). The neuroinvasiveness of an H5N1 influenza virus following intranasal exposure has also been reported in a ferret model (23). Therefore, to study the long-term neurologic effects of H5N1 virus infection, we infected ferrets with H5N1 viruses that cause mild symptoms in these animals and observed the effects on the central nervous system (CNS) for 9 months. We also sought to elucidate the route by which these H5N1 viruses invaded the CNS.
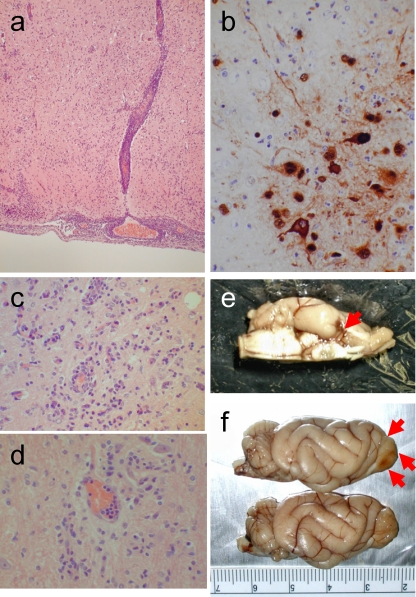
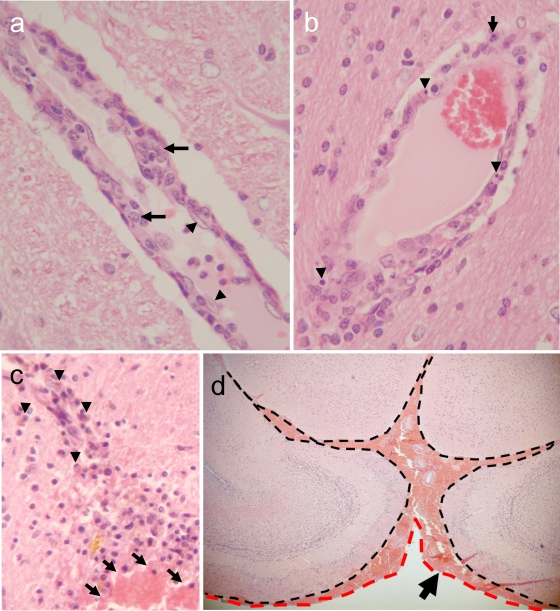
Six-month-old ferrets were inoculated intranasally with 106 PFU of A/Hong Kong/483/1997 (H5N1; HK483) or A/Hong Kong/486/1997 (H5N1; HK486) virus. These viruses were propagated in Madin-Darby canine kidney (MDCK) cells in minimal essential medium supplemented with 0.3% bovine serum albumin. On days 3, 6, and 12 and months 1, 3, 6, and 9 postinfection (p.i.), we sampled tissue specimens for virus isolation and pathological examination. The ferrets were lethargic and exhibited signs and symptoms of respiratory infection during the first 10 days p.i. but lacked appreciable neurologic signs, which is in contrast to results reported previously (23). Tissue samples from nasal turbinates, lungs, trachea, brain, liver, spleen, kidneys, heart, pancreas, and spinal cord were harvested and homogenized to a 10% suspension with phosphate-buffered saline. The virus titer in each tissue was determined by using plaque assays. The viruses replicated mainly in the nasal turbinates, with virus titers reaching 105 to 106 PFU/gram of tissue by 3 to 6 days p.i. (Table 1). Brain tissues collected from HK483- or HK486-infected ferrets were preserved in 10% neutral buffered formalin and processed for paraffin embedding. The paraffin-embedded tissues were cut into 5-mm-thick slices and stained with hematoxylin and eosin (H&E). Additional sections were cut for immunohistological staining with rabbit polyclonal antibodies against an H5 influenza virus. Histologic examination revealed neuronal invasion or damage, including inflammation of the choroid plexus (Table 2). Ferrets infected with the HK486 virus showed evidence of nonsuppurative inflammation (Fig. 1a) and viral antigen expression (Fig. 1b) that persisted for 12 days; viral antigens were not detected at 1 month p.i. The nonsuppurative encephalitis lasted for 3 months (Fig. 1c), with residual glial scarring apparent at 6 and 9 months p.i. in the areas where viral antigens were detected in the earlier phase (Fig. 1d). Further, ferrets infected with HK486 showed macroscopic injury of the olfactory system at day 12 p.i. (Fig. 1e and f). These findings suggest that while highly pathogenic H5N1 viruses affect mainly the host's airways (1), they may also produce neurologic complications. In contrast to the HK486 virus, which invaded the brain parenchyma of ferrets and produced severe parenchymal damage, the second H5N1 strain, HK483, caused severe nonsuppurative vasculitis without apparent parenchymal damage to the brains of ferrets at 6 days p.i. Although viral antigen could not be detected within the vasculature, vascular injury was evident, as characterized by endothelial swelling (Fig. 2a, arrowheads), scattered apoptotic cells (Fig. 2b, arrowheads), and intramural infiltration of macrophages (Fig. 2a, arrows) and polymorphonuclear leukocytes (Fig. 2b, arrow). Vascular lesions were found at the thalamus, at the junction between the gray and white matter, and at the brainstem. Brain specimens examined at 1 and 6 months p.i. displayed hemorrhagic lesions indicative of chronic and repeated perivascular hemorrhage (Fig. 2c) in the thalamus, cerebellum, and subarachnoid space of the fore brain (Fig. 2d). These observations are consistent with reports of influenza virus-induced hemorrhagic brain complications during annual epidemics (11). Brain vasculitis secondary to viral infection is relatively common (17) and is likely the cause of vascular wall fragility, leading to brain hemorrhage. It seems reasonable, therefore, to conclude that H5N1 virus infection in humans may cause brain hemorrhage similar to that seen upon infection with other neurotropic viruses.
Table 1.
Virus recovery from tissues of ferrets infected with the HK483 or HK486 H5N1 virus
| Virus strain | Day postinfection | Virus titer(s) in indicted organ(s) (mean log10 PFU/g ± SD)a |
||||||
|---|---|---|---|---|---|---|---|---|
| Nasal turbinates | Lungs | Trachea | Brain | Liver | Spleen | Othersb | ||
| HK483 | 1 | 3.5, 4.9 | 3.4 | — | — | — | — | — |
| 3 | 4.7 ± 0.6 | 1.0, 1.4 | 2.9 | — | — | 1.1 | — | |
| 4c | 5.0 | 5.2 | 3.6 | — | 1.9 | 2.8 | — | |
| 6 | — | — | — | — | 2.4 | — | — | |
| 12 | 2.3 | — | — | — | — | — | — | |
| 30 | — | — | — | — | — | — | — | |
| HK486 | 1 | 3.8 ± 0.5 | — | — | — | — | — | — |
| 3 | 5.7 ± 3.0 | — | — | 1.5, 2.3 | — | — | — | |
| 5c | 6.7 | 3.0 | — | — | — | — | — | |
| 6 | 2.2 | — | — | — | — | — | — | |
| 12 | — | — | — | — | — | — | — | |
| 30 | — | — | — | — | — | — | — | |
Six-month-old male ferrets (15 animals per group) were intranasally infected with virus (106 PFU/animal). Three ferrets from each group were euthanized 1, 3, 6, 12, and 30 days after inoculation, and virus in the organs was titrated by using plaque assays with MDCK cells. If viruses were not isolated from all three animals, the individual virus titers are given. —, virus was not isolated.
Kidney, heart, pancreas, and spinal cord.
Death with mucopurulent nasal discharge and dyspnea.
Table 2.
Prevalence of brain lesions in ferrets at various time points postinfection with highly pathogenic H5N1 influenza virusesa
| Time postinfection | Type(s) of brain lesion (no. of animals affected/no. of animals examined) in ferrets infected with: |
|
|---|---|---|
| HK483b | HK486 | |
| Day 3 | Choroiditis (2/3) | Choroiditis (2/3) |
| Day 6 | Vasculitis (2/3) | Encephalitis (2/3), choroiditis (1/3) |
| Day 12 | Choroiditis (2/3) | Encephalitis (3/3) |
| Mo 1 | Hemorrhage (1/3) | Encephalitis (1/3), choroiditis (1/3) |
| Mo 3 | — (0/3) | Encephalitis (2/3) |
| Mo 6 | Hemorrhage (1/3) | Glial scar (1/2) |
| Mo 9 | — (0/2) | Glial scar (1/2) |
Observation period, 9 months.
—, no brain lesion was detected.
Fig. 1.
Brain lesions in HK486 virus-infected ferrets. (a) Severe nonsuppurative encephalitis in the olfactory area at day 6 postinfection (p.i.). (b) Viral antigen expression in a brain lesion at day 12 p.i. Neuronal and glial cells are stained with anti-H5 virus antiserum. (Inset) Noninfected neurons and glia. (c) Smoldering encephalitis in brain tissue at 3 months p.i. (d) Perivascular glial scar formation at 9 months p.i. (e) Macroscopically visible brain lesion in part of the olfactory system (piriform lobe) on day 12 p.i. (f) Partial loss of an olfactory bulb (upper side of brain [red arrows]) due to viral encephalitis at 1 month p.i. Compare these images with the brain from an age-matched control (lower brain).
Fig. 2.
Brain lesions in HK483 virus-infected ferrets. (a) Prominent nonsuppurative vasculitis at day 6 p.i. Note the severe swelling of a vascular endothelial cell (arrowheads) and migration of macrophages into the vascular wall (arrows), compared with the normal appearance of the surrounding brain parenchyma. (b) Scattered apoptotic cells (arrowheads) and polymorphonuclear leukocytes (arrow) in the vascular wall on day 6 p.i. (c) Old and fresh hemorrhagic lesions in the thalamus of a ferret that underwent necropsy at 6 months p.i. (arrowheads, hemosiderin-laden macrophages in an old lesion; arrows, fresh hemorrhage with red blood cells). (d) Fresh subarachnoid hemorrhage in a ferret brain at 6 months p.i. Note the accumulation of red blood cells between leptomeninges (dashed black line) and arachnoid mater (dashed red line).
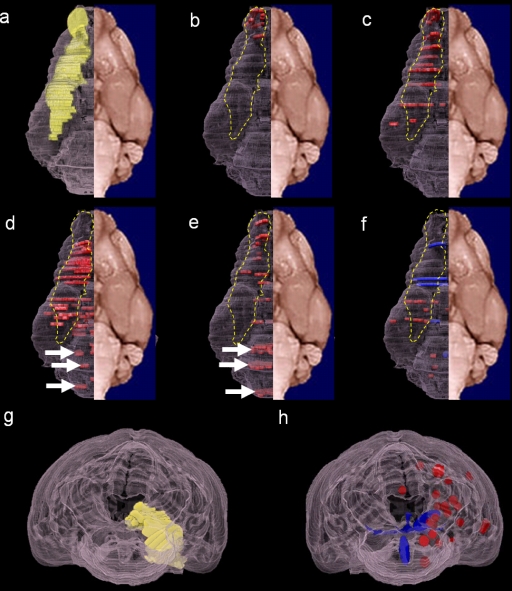
To examine the route by which H5N1 viruses invade the brains of ferrets, we analyzed the distribution of brain lesions and viral antigen expression observed in ferrets infected with the HK483, HK486, A/Hong Kong/213/2003 (HK213), A/Vietnam/1204/2004 (VN1204), and A/duck/Vietnam/NCVD-18/2004 (NCVD18) viruses. The HK213, VN1204, and NCVD18 viruses were propagated in MDCK cells in minimal essential medium supplemented with 0.3% bovine serum albumin. Six-month-old ferrets were inoculated intranasally with 106 PFU of virus. Brain tissues were collected in the acute phase, at 3 and 6 days postinfection for VN1204 and NCVD18, and additionally at 12 days postinfection for HK213. Tissues were processed for H&E staining and immunohistochemistry for histologic examination. The brain parenchymal lesions produced by VN1204, NCVD18, and HK213 were characterized by nonsuppurative encephalitis, and the expression pattern of viral antigens was essentially the same as that of HK486 (data not shown). To understand the three-dimensional (3D) distribution of the brain lesions and viral antigens, we plotted them by 3D imaging (TRI/3D-SRF2; Ratoc System Engineering Co., Ltd., Japan) of a yellow mongoose (Cynictis penicillata) brain, the architecture of which closely resembles that of the ferret brain (20). To visualize the olfactory pathway (i.e., the sensory pathway for the sense of smell), we marked the nerve pathways from the olfactory bulb to the piriform cortex on the 3D images as a frame of reference (Fig. 3a, yellow; see also Movie S1 in the supplemental material). We found three distinct patterns of brain lesions, with and without viral antigens, caused by the different H5N1 influenza viruses: those distributed only along the olfactory pathway (Fig. 3b and c, dashed yellow line; see also Movies S2 and S3) (HK213 and NCVD18); those detected along the olfactory pathway (Fig. 3d and e, dashed yellow line; see also Movies S4 and S5) and in the brain stem (Fig. 3d and e, white arrows; see also Movies S4 and S5) (HK486 and VN1204); and those in other areas (Fig. 3f; see also Movie S6) (HK483). The basis for separating the third group of lesions from the other two groups is more apparent when the lesion distributions are examined from the posterior (Fig. 3g and h, yellow versus blue versus red). These mapping results indicate that most of the H5N1 strains that we tested invaded the brain via the olfactory pathway (Fig. 3, dashed yellow line). One exception was the HK483 strain (Fig. 3f), which clearly targeted the brain vasculature in ferrets, producing lesions outside the olfactory system.
Fig. 3.
Distribution of brain lesions following infection with the HK483, HK486, HK213, NCVD18, or VN1204 strain of H5N1 virus. Brain lesions are mapped on three-dimensional images of a yellow mongoose brain. Selected parts of the brain sections were analyzed; therefore, the plots of the lesion locations are discontinuous. (a) Olfactory route (yellow). The distribution of lesions and viral antigens (red) associated with HK213 (b) or NCVD18 (c) infection follows the olfactory route (dashed yellow line). In animals infected with HK486 (d) or VN1204 (e), the lesions and viral antigens are located in the brain stem (white arrows) and the olfactory route (red plots within the dashed yellow line). The HK483 strain (f) caused severe blood vessel damage, with apparent hemorrhagic lesions (blue plots). (g) Posterior view of the olfactory route (yellow plots). (h) Posterior view of HK483-induced hemorrhagic lesions (blue) and vasculitis (red) outside the olfactory route. Panels a to f are ventral views.
Before H5N1 viruses can become pandemic, they will likely have to acquire the ability to grow well in the upper portion of the human airway, which would lead to efficient human-to-human transmission via sneezing and coughing (13, 22). Our data from ferrets suggest that if highly pathogenic H5N1 viruses do gain this foothold in humans, they could cause subclinical neurologic complications by invading the brain via the nerve pathway routes or by causing brain vasculitis. Previously, an H3N2 virus was detected in ferret brains; however, the amount of virus in the brains was low (less than 102.2 log10 50% egg-infectious doses) and no evidence of virus antigens in the brain was reported (23). We found that the 2009 pandemic H1N1 virus replicated more efficiently in the respiratory tracts of ferrets than did a human seasonal H1N1 virus (9, 13, 15) and caused nonsuppurative olfactory bulb lesions; however, we did not find virus in the brain parenchyma of these animals (data not shown). Therefore, brain parenchymal invasion, as demonstrated by the presence of viral antigens, is a characteristic of H5N1 viruses. It would, therefore, be pertinent to perform retrospective and prospective studies for subclinical neurologic complications in H5N1 virus-infected patients. Our findings raise concerns that subclinical neurologic complications could accompany severe respiratory infections upon the worldwide spread of H5N1 viruses.
Supplementary Material
Acknowledgments
We thank Susan Watson for scientific editing and Martha McGregor and Krisna Wells for excellent technical assistance.
This work was supported by a Grant-in-Aid for Specially Promoted Research from the Ministries of Education, Culture, Sports, Science, and Technology (Y.K., K.S.), by a health labor sciences research grant from the Ministry of Health Labor and Welfare of Japan (Y.K., K.S.), by the Contract Research Fund for the Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases (Y.K., K.S.), by grants-in-aid from the Ministry of Health, by ERATO (Y.K.), by a Global Center of Excellence (G-COE) program (K.S., A.M.) (Japan Science and Technology Agency), and by research grants from the National Institute of Allergy and Infectious Diseases, Public Health Service (Y.K.).
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 9 March 2011.
REFERENCES
- 1. Abdel-Ghafar A. N., et al. 2008. Update on avian influenza A (H5N1) virus infection in humans. N. Engl. J. Med. 358:261–273 [DOI] [PubMed] [Google Scholar]
- 2. Anderson W. W., Jaros R. M. 1958. Neurologic complications of Asian influenza. Neurology 8:568–570 [DOI] [PubMed] [Google Scholar]
- 3. Bell W. E., McKee A. P., Utterback R. A. 1958. Asian influenza virus as the cause of acute encephalitis. Neurology 8:500–502 [DOI] [PubMed] [Google Scholar]
- 4. Bennett A. E., Turk R. E. 1957. Acute encephalitis and death following Asian influenza. Calif. Med. 87:411–412 [PMC free article] [PubMed] [Google Scholar]
- 5. de Jong M. D., et al. 2005. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N. Engl. J. Med. 352:686–692 [DOI] [PubMed] [Google Scholar]
- 6. Flewett T. H., Hoult J. G. 1958. Influenzal encephalopathy and postinfluenzal encephalitis. Lancet ii:11–15 [DOI] [PubMed] [Google Scholar]
- 7. Gu J., et al. 2007. H5N1 infection of the respiratory tract and beyond: a molecular pathology study. Lancet 370:1137–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Herlocher M. L., et al. 2001. Ferrets as a transmission model for influenza: sequence changes in HA1 of type A (H3N2) virus. J. Infect. Dis. 184:542–546 [DOI] [PubMed] [Google Scholar]
- 9. Itoh Y., et al. 2009. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature 460:1021–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jellinger K., Seitelberger F. 1961. Findings in fatal cases of encephalitis during the 1957-1958 influenza epidemic, p. 89–95In van Bogaert L., Radermecker J., Hozay J., Lowenthal A. (ed.), Encephalitides. Elsevier Publishing Company, Amsterdam, Netherlands [Google Scholar]
- 11. Jones D. B. 1979. An association between sub-arachnoid haemorrhage and influenza A infection. Postgrad. Med. J. 55:853–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maher J. A., DeStefano J. 2004. The ferret: an animal model to study influenza virus. Lab Anim. (NY) 33:50–53 [DOI] [PubMed] [Google Scholar]
- 13. Maines T. R., et al. 2009. Transmission and pathogenesis of swine-origin 2009 A (H1N1) influenza viruses in ferrets and mice. Science 325:484–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Middleton D., et al. 2009. Evaluation of vaccines for H5N1 influenza virus in ferrets reveals the potential for protective single-shot immunization. J. Virol. 83:7770–7778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Munster V. J., et al. 2009. Pathogenesis and transmission of swine-origin 2009 A (H1N1) influenza virus in ferrets. Science 325:481–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reuman P. D., Keely S., Schiff G. M. 1989. Assessment of signs of influenza illness in the ferret model. J. Virol. Methods 24:27–34 [DOI] [PubMed] [Google Scholar]
- 17. Siva A. 2001. Vasculitis of the nervous system. J. Neurol. 248:451–468 [DOI] [PubMed] [Google Scholar]
- 18. Studahl M. 2003. Influenza virus and CNS manifestations. J. Clin. Virol. 28:225–232 [DOI] [PubMed] [Google Scholar]
- 19. Sweet C., et al. 2002. Oral administration of cyclopentane neuraminidase inhibitors protects ferrets against influenza virus infection. Antimicrob. Agents Chemother. 46:996–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Welker W., Johnson J. I., Noe A. 2007. Comparative mammalian brain collections. http://brainmuseum.org/ Accessed 15 July 2007
- 21. WHO Posting date, 5 July 2010. Global alert and response. Confirmed human cases of avian influenza A(H5N1). WHO, Geneva, Switzerland: http://www.who.int/csr/disease/avian_influenza/country/en/ [Google Scholar]
- 22. Wright P. F., Neumann G., Kawaoka Y. 2007. Orthomyxoviruses, p. 1691–1740In Knipe D. M., et al. (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 23. Zitzow L. A., et al. 2002. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J. Virol. 76:4420–4429 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.