Abstract
Herpes simplex virus 1 (HSV-1) Us3 protein kinase phosphorylates threonine at position 887 (Thr-887) in the cytoplasmic tail of envelope glycoprotein B (gB) in infected cells. This phosphorylation downregulates cell surface expression of gB and plays a role in viral pathogenesis in the mouse herpes stromal keratitis model. In the present study, we demonstrated that Us3 phosphorylation of gB Thr-887 upregulated the accumulation of endocytosed gB from the surfaces of infected cells. We also showed that two motifs in the cytoplasmic tail of gB, tyrosine at position 889 (Tyr-889) and dileucines at positions 871 and 872, were required for efficient downregulation of gB cell surface expression and upregulation of accumulation of endocytosed gB in infected cells. A systematic analysis of mutations in these three sequences in gB suggested that the expression of gB on the surfaces of infected cells was downregulated in part by the increase in the accumulation of endocytosed gB, which was coordinately and tightly regulated by the three gB trafficking signals. Tyr-889 appeared to be of predominant importance in regulating the intracellular transport of gB and was linked to HSV-1 neurovirulence in mice following intracerebral infection. These observations support the hypothesis that HSV-1 evolved the three gB sequences for proper regulation of gB intracellular transport and that this regulation plays a critical role in diverse aspects of HSV-1 pathogenesis.
INTRODUCTION
Herpes simplex virus 1 (HSV-1) envelope glycoprotein B (gB) is well conserved throughout the Herpesviridae family (36). Like the gBs of other members of the Herpesviridae family, HSV-1 gB plays an essential role in viral entry. In HSV-1 entry into a cell, gB together with envelope glycoprotein gC mediates virus attachment by interacting with cell surface glycosaminoglycans, primarily heparan sulfate (19, 20). Although not essential for entry, this attachment provides a stable interaction between the virion and the cell that facilitates the next entry steps (44). These steps have been suggested to include both the binding of the envelope glycoprotein gD to one of its identified receptors and the binding of gB to one of its identified receptors (2, 15, 33, 42, 43, 45). Subsequent fusion between the virion envelope and the host cell membrane, which requires the cooperative function of a heterodimer of HSV-1 envelope glycoproteins gH/gL, gD, a gD receptor, gB, and a gB receptor, delivers the nucleocapsid into the host cell (37, 50). Another potential role of HSV-1 gB is to regulate the nuclear egress of nucleocapsids, during which nucleocapsids acquire primary envelopes by budding through the inner nuclear membrane into the space between the inner and outer nuclear membranes, followed by fusion with the outer nuclear membrane. In this process, gB has been proposed to be involved in promoting the fusion of the virion envelope with the outer nuclear membrane (12).
In general, cell membrane glycoproteins are synthesized at the endoplasmic reticulum (ER) and travel through the Golgi apparatus and trans-Golgi network (TGN); a fraction of the cell membrane glycoproteins are transported to the cell surface (16). Most enveloped viruses utilize this pathway for posttranslational modification of viral progeny envelope glycoproteins. Some viruses (e.g., human immunodeficiency virus and influenza virus) also utilize this pathway to target viral envelope glycoproteins to the cell surface in order to assemble their final envelopes (34, 38). Although HSV-1 envelope glycoproteins are expressed on the surfaces of infected cells (8), HSV-1 acquires its final envelope at cytoplasmic membranes, most likely at the TGN (17, 31, 46, 49). Therefore, the biological significance of cell surface expression of HSV-1 glycoproteins remains unclear at present, although several reports have implied a potential role(s) for herpesvirus envelope glycoproteins on the cell surface (9, 28). However, expression of HSV-1 envelope glycoproteins on the surfaces of infected cells is considered to mark those cells for the host immune response, because HSV-1 glycoproteins on the cell surface are potent inducers of the immune response (5, 6, 40, 41). In particular, it has been reported that lysis of HSV-1-infected cells by natural killer cells correlates with expression of gB on the cell surface (5). Therefore, HSV-1 presumably needs a mechanism to downregulate cell surface expression of its envelope glycoproteins in order to evade the host immune response. In support of this hypothesis, several HSV-1 envelope glycoproteins contain typical motifs, tyrosine-based YXXΦ and/or dileucine motifs in their cytoplasmic tails, which have been suggested to regulate the endocytosis of envelope glycoproteins from the surfaces of infected cells (8).
HSV-1 gB is endocytosed from the cell surface, like the gBs of other members of the Herpesviridae family, including pseudorabies virus (PRV), human cytomegalovirus (HCMV), and varicella-zoster virus (VZV) (4, 13, 18, 39). HSV-1 gB contains two motifs in its cytoplasmic tail, tyrosine at residue 889 (Tyr-889) and dileucines at residues 871 and 872 (Di-Leu-871-872), that are involved in gB endocytosis (3, 4, 8). It has been reported that the tyrosine-based motif in PRV gB is critical for its endocytosis in infected cells (14). Furthermore, potential roles in intracellular trafficking have been reported for tyrosine-based and/or dileucine motifs in the gBs of other herpesviruses, including HSV-2, VZV, and rhesus rhadinovirus (11, 18, 27). In agreement with these findings, Beitia Ortiz de Zarate et al. have reported that intracellular transport of HSV-1 gB is regulated differently by each of the two motifs (Di-Leu-871-872 and Tyr-889) (3, 4). However, the effects of these motifs on the regulation of gB intracellular transport, proper localization and accumulation of gB in infected cells, and HSV-1 pathogenesis in vivo remain largely unknown. We recently identified an additional site in the cytoplasmic tail of gB that is involved in the regulation of the intracellular transport of gB: a viral serine/threonine protein kinase, Us3, phosphorylates gB threonine at position 887 (Thr-887) in infected cells, and this phosphorylation downregulates the expression of gB on the cell surface (24). Unlike Tyr-889 and Di-Leu-871-872, Thr-887 is not conserved in the gB homologs of other alphaherpesviruses, including HSV-2, VZV, and PRV (8, 24). Us3 phosphorylation of gB Thr-887 has also been suggested to be involved in regulation of the fusion of the virion envelope with the cell's outer nuclear membrane during nuclear egress of nucleocapsids (53). Also, an amino acid replacement of gB Thr-887 significantly reduced viral replication and pathogenesis in a mouse herpes stromal keratitis (HSK) model (22), suggesting that Us3 phosphorylation of gB Thr-887 and, probably, its effects in cell cultures, including downregulation of gB cell surface expression and promotion of nuclear egress of nucleocapsids, are critical for HSV-1 infection in vivo. However, the mechanism by which Us3 phosphorylation of gB Thr-887 regulates gB cell surface expression and the relationship of the function of this phosphorylation with those of the tyrosine and dileucine motifs in gB remain to be elucidated.
In the present study, we focused on the three sequences (Di-Leu-871-872, Thr-887, and Tyr-889) in gB and examined the effects of systematic amino acid replacements of these residues on gB cell surface expression, gB intracellular transport, viral growth in cell cultures, and neurovirulence in mice. These studies suggested that accumulation of endocytosed gB in infected cells was coordinately regulated by all three gB sequences and that this regulation partially determined proper expression of gB on the surfaces of infected cells. We also present data suggesting that, of the three gB sequences, Tyr-889 played the most critical role in the regulation of gB intracellular transport, expression of gB on the surfaces of infected cells, and neurovirulence in mice.
MATERIALS AND METHODS
Cells and viruses.
Vero and rabbit skin cells have been described previously (47). The HSV-1 wild-type strain HSV-1(F), recombinant virus YK511, encoding an enzymatically inactive Us3 mutant in which lysine at Us3 residue 220 was replaced with methionine (Us3-K220M), recombinant virus YK513, in which the Us3 K220M mutation in YK511 was repaired (Us3-KM-repair), recombinant virus YK551, in which threonine at gB residue 887 was replaced with alanine (gB-T887A), recombinant virus YK553, in which the gB T887A mutation in YK551 was repaired (gB-TA-repair), recombinant virus YK555, in which threonine at gB residue 887 was replaced with aspartic acid (gB-T887D), and recombinant virus YK557, in which the gB T887D mutation in YK555 was repaired (gB-TD-repair), have been described previously (24).
Recombinant viruses YK563, YK567, and YK571 (Fig. 1), containing gB with alanine substitutions for Tyr-889 (gB-Y889A), Thr-887 and Tyr-889 (gB-T887A/Y889A), and Di-Leu-871-872 (gB-LL871-872AA), respectively, were generated by the two-step Red-mediated mutagenesis procedure using Escherichia coli GS1783 containing pYEbac102 and the primers listed in Table 1 as described previously (25). Recombinant viruses YK575, YK579, and YK583 (Fig. 1), containing gB with alanine substitutions for Di-Leu-871-872 and Thr-887 (gB-LL871-872AA/T887A), Di-Leu-871-872 and Tyr-889 (gB-LL871-872AA/Y889A), and Di-Leu-871-872, Thr-887, and Tyr-889 (gB-LL871-872AA/T887A/Y887A), respectively, were generated as described above but with E. coli containing the YK571 genome and with the primers listed in Table 1. Recombinant viruses YK565, YK569, and YK573, in which the gB Y889A, gB T887A Y889A, and gB LL871-872AA mutations in YK563, YK567, and YK571, respectively, were repaired, were generated with the primers listed in Table 1 as described previously (25). Recombinant virus YK577, in which the gB LL871-872AA T887A mutation in YK575 was repaired, was generated as described above except that the gB T887A and gB LL871-872AA mutations in the YK575 genome were sequentially repaired in E. coli using the primers listed in Table 1. Similarly, recombinant viruses YK581 and YK585, in which the gB LL871-872AA Y889A and gB LL871-872AA T887A Y887A mutations in YK579 and YK583, respectively, were repaired, were generated using the primers listed in Table 1. Recombinant virus YK587 (Fig. 1), containing gB with aspartic acid and alanine substitutions for Thr-887 and Tyr-889, respectively (gB-T887D/Y889A), was generated with the primers listed in Table 1 as described previously (25).
Fig. 1.
Schematic diagrams of the genome of wild-type YK304 virus and the relevant domains of the recombinant viruses in this study. Line 1, linear representation of the YK304 genome carrying a bacmid (BAC) in the intergenic region between UL3 and UL4. Line 2, the UL26, UL27 (gB), and UL28 open reading frames. Line 3, domain of the UL27 gene encoding gB residues 869 to 904. Line 4, amino acid sequence of gB residues 869 to 904. Lines 5 to 21, schematic diagrams of recombinant viruses used in this study. The amino acid sequences of gB residues 869 to 904 in the viruses are shown.
Table 1.
Primer sequences used for construction of recombinant viruses
| Mutation | Sequence |
|---|---|
| gB-LL871AA | 5′-AGCGCACGGAACACAAGGCCAAGAAGAAGGGCACGAGCGCGGCGGCCAGCGCCAAGGTCACCGACATAGGATGACGACGATAAGTAGGG-3′ |
| 5′-TTGCGGCGCTTGCGCATGACCATGTCGGTGACCTTGGCGCTGGCCGCCGCGCTCGTGCCCTTCTTCTCAACCAATTAACCAATTCTGATTAG-3′ | |
| gB-T887A | 5′-CCAAGGTCACCGACATGGTCATGCGCAAGCGCCGCAACGCCAACTACACCCAAGTTCCCAAGGATGACGACGATAAGTAGGG-3′ |
| 5′-GGCGTCACCGTCTTTGTTGGGAACTTGGGTGTAGTTGGCGTTGCGGCGCTTGCGCATGACCAACCAATTAACCAATTCTGATTAG-3′ | |
| gB-Y889A | 5′-AGGTCACCGACATGGTCATGCGCAAGCGCCGCAACACCAACGCCACCCAAGTTCCCAACAAAGAAGGATGACGACGATAAGTAGGG-3′ |
| 5′-TCGTCCTCGTCGGCGTCACCGTCTTTGTTGGGAACTTGGGTGGCGTTGGTGTTGCGGCGCTTGCGCACAACCAATTAACCAATTCTGATTAG-3′ | |
| gB-T887A/Y889A | 5′-AGCGCCAAGGTCACCGACATGGTCATGCGCAAGCGCCGCAACGCCAACGCCACCCAAGTTCCCAACAAAGAAGGATGACGACGATAAGTAGGG-3′ |
| 5′-TCGTCCTCGTCGGCGTCACCGTCTTTGTTGGGAACTTGGGTGGCGTTGGCGTTGCGGCGCTTGCGCATGACAACCAATTAACCAATTCTGATTAG-3′ | |
| gB-T887D/Y889A | 5′-AGCGCCAAGGTCACCGACATGGTCATGCGCAAGCGCCGCAACGACAACGCCACCCAAGTTCCCAACAAAGAAGGATGACGACGATAAGTAGGG-3′ |
| 5′-TCGTCCTCGTCGGCGTCACCGTCTTTGTTGGGAACTTGGGTGGCGTTGTCGTTGCGGCGCTTGCGCATGACAACCAATTAACCAATTCTGATTAG-3′ | |
| gB-LLAA-repair | 5′-AGCGCACGGAACACAAGGCCAAGAAGAAGGGCACGAGCGCGCTGCTCAGCGCCAAGGTCACCGACATAGGATGACGACGATAAGTAGGG-3′ |
| 5′-TTGCGGCGCTTGCGCATGACCATGTCGGTGACCTTGGCGCTGAGCAGCGCGCTCGTGCCCTTCTTCTCAACCAATTAACCAATTCTGATTAG-3′ | |
| gB-TAYA-repair | 5′-CCAAGGTCACCGACATGGTCATGCGCAAGCGCCGCAACACCAACTACACCCAAGTTCCCAAGGATGACGACGATAAGTAGGG-3′ |
| 5′-GGCGTCACCGTCTTTGTTGGGAACTTGGGTGTAGTTGGTGTTGCGGCGCTTGCGCATGACCAACCAATTAACCAATTCTGATTAG-3′ |
Antibodies.
Mouse monoclonal antibodies to gB (H1817) and VP5 (3B6) were purchased from Virusys. A monoclonal antibody against the phospho-protein kinase A (phospho-PKA) substrate (100G7), horseradish peroxidase (HRP)-conjugated streptavidin, and streptavidin agarose were purchased from Cell Signaling Technology, Invitrogen, and Novagen, respectively. A mouse monoclonal antibody that recognizes gB with phosphorylated Thr-887 (gB-T887P) has been described previously (22).
Immunoblotting.
The electrophoretically separated proteins transferred to nitrocellulose or polyvinylidene difluoride (PVDF) sheets were reacted with appropriate antibodies. The final dilutions of the antibodies used were 1:5,000 for the mouse anti-gB monoclonal antibody (H1817), 1:3,000 for the mouse monoclonal antibody to gB-T887P, and 1:1,000 for the rabbit monoclonal antibody against the phospho-PKA substrate (100G7) and the mouse anti-VP5 monoclonal antibody (3B6). To detect gB and VP5, nitrocellulose membranes were blocked with 5% skim milk in phosphate-buffered saline (PBS) containing 0.1% Tween 20 (T-PBS) for 1 h, rinsed twice, washed once for 5 min in T-PBS, and reacted for 1 h with primary antibodies in T-PBS containing 1% bovine serum albumin (BSA). The blots were then washed as before, reacted for 1 h with a 1:3,000 dilution of anti-mouse immunoglobulin G (IgG) conjugated to peroxidase (GE Healthcare) in T-PBS containing 3% skim milk, rinsed twice, washed four times, for 5 min each time, in T-PBS, and detected by using the ECL chemiluminescence reagent (GE Healthcare). To detect phosphorylated proteins, PVDF membranes were blocked with 2% BSA in T-PBS for 1 h, rinsed twice, washed once for 5 min in T-PBS, and reacted for 1 h with primary antibodies in Can Get Signal immunoreaction enhancer solution (Toyobo). The blots were then washed as before, reacted for 1 h with a 1:3,000 dilution of anti-mouse or anti-rabbit IgG conjugated to peroxidase (GE Healthcare) in Can Get Signal immunoreaction enhancer solution, rinsed twice, washed four times, for 5 min each time, in T-PBS, and detected by using the ECL chemiluminescence regent (GE Healthcare). The amount of protein in a band was quantitated using the Dolphin-Doc image capture system with Dolphin-1D software (Wealtec).
Immunofluorescence.
Vero cells cultured on glass-bottom dishes were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 in PBS, blocked with PBS containing 10% human serum, rinsed once in PBS, reacted for 2 h at room temperature with a mouse monoclonal antibody to gB, rinsed three times with PBS, reacted for 1 h with anti-mouse IgG conjugated to Alexa Fluor 488 (Invitrogen), and rinsed three times with PBS. The cells were then examined with a Zeiss LSM5 laser scanning microscope.
Immunoprecipitation.
Infected Vero cells were harvested and lysed in radioimmunoprecipitation assay (RIPA) buffer (10 mM Tris-HCl [pH 7.4], 1% NP-40, 0.1% deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 150 mM NaCl, 1 mM EDTA) or NP-40 buffer (50 mM Tris-HCl [pH 8.0], 0.5% NP-40, 120 mM NaCl, 50 mM NaF) containing a protease inhibitor cocktail (Sigma). Supernatants obtained after centrifugation of the cell lysates were precleared by being mixed with protein A Sepharose beads (GE Healthcare) and were reacted at 4°C for 2 h with an anti-gB or anti-gB-T887P monoclonal antibody. Protein A Sepharose beads were then added and allowed to react for additional 1 h. Immunoprecipitates were collected by a brief centrifugation, washed extensively with lysis buffer, and analyzed by immunoblotting.
Flow cytometry.
To analyze the expression of gB on the cell surface, infected adherent Vero cells were detached in PBS containing 0.02% EDTA and were washed once with PBS supplemented with 2% fetal calf serum (FCS) (washing buffer). To analyze the total expression of gB, infected Vero cells were detached as described above, fixed in 4% paraformaldehyde in PBS, and then permeabilized with 0.1% Triton X-100 in PBS. Cells with or without fixation were incubated with mouse monoclonal antibodies to gB in washing buffer on ice for 30 min. After the cells were washed with washing buffer, they were further incubated with anti-mouse IgG conjugated to Alexa Fluor 488 (Invitrogen). After the cells were washed again, they were analyzed with a FACSCalibur flow cytometer with CellQuest software (Becton Dickinson).
Purification of virions.
Virions were purified as described previously (26). Briefly, Vero cells were infected with the indicated virus at a multiplicity of infection (MOI) of 0.01 for 24 or 48 h. Cell culture supernatants were then harvested by low-speed centrifugation. The HSV-containing supernatant was centrifuged for 2 h at 22,000 rpm in an SRP28S rotor (Hitachi). The pellet was resuspended in 0.5 ml of TBSal (200 mM NaCl, 2.6 mM KCl, 10 mM Tris-HCl [pH 7.5], 20 mM MgCl2, 1.8 mM CaCl2), layered onto a 9-ml discontinuous sucrose gradient (30%, 40%, and 50%) in TBSal, and centrifuged for 2 h at 20,000 rpm in a P40ST rotor. Aliquots of peak virion-containing fractions were pelleted by centrifugation for 2 h at 26,000 rpm in a P40ST rotor.
Cell fusion.
Confluent Vero cells were grown in 35-mm-diameter plastic dishes and were infected with the indicated virus at an MOI of 0.01. At 72 h postinfection, cells were analyzed by phase microscopy.
Endocytosis of gB assayed by biotinylation in infected cells.
Endocytosis of gB in infected cells was assayed as described previously (30) with minor modifications. Briefly, Vero cells were infected with the indicated virus at an MOI of 3 for 18 h, and the cells were then biotinylated for 15 min at 4°C using cleavable Sulfo-N-hydroxysuccinimide (NHS)-SS biotin (Pierce). After three washes, cells were incubated at 37°C for 0 or 4 h to allow endocytosis of the biotinylated cell surface proteins. The cells were then treated twice with freshly prepared reducing solution (15.5 mg glutathione/ml, 75 mM NaCl, 0.3% NaOH, 10% calf serum) at 4°C to remove any biotin label remaining on the proteins at the cell surface. After three washes, free SH groups were quenched with 5 mg iodoacetamide/ml in phosphate-buffered saline containing 1% bovine serum albumin. The cells were then harvested and solubilized in RIPA buffer (10 mM Tris-HCl [pH 7.4], 1% NP-40, 0.1% deoxycholate, 0.1% SDS, 150 mM NaCl, 1 mM EDTA) or NP-40 buffer (50 mM Tris-HCl [pH 8.0], 0.5% NP-40, 120 mM NaCl, 50 mM NaF). The lysates were immunoprecipitated with an anti-gB antibody, an anti-gB-T887P antibody, or streptavidin agarose and were analyzed by immunoblotting with streptavidin-HRP or an anti-gB antibody.
Animal studies.
Female ICR mice were purchased from Charles River. Three-week-old mice were infected intracerebrally with 102 PFU of the indicated virus as described previously (47). Mice were monitored daily, and mortality from 1 to 14 days postinfection was attributed to the viruses inoculated. To determine viral titers in the brains, nine mice were inoculated intracerebrally with 102 PFU of the indicated virus as described previously (47). At 2 days postinfection, the mice were sacrificed, and the whole brain was removed, sonicated in 1 ml of 199 medium containing 1% FCS and antibiotics, and frozen at −80°C. Frozen samples were later thawed, and viral titers in the supernatants obtained after centrifugation of the samples were determined by standard plaque assays on Vero cells. All animal studies were carried out with the approval of the Ethical Committee for Animal Experimentation of the University of Tokyo.
RESULTS
Effect of Us3 phosphorylation of gB Thr-887 on the accumulation of endocytosed gB in infected cells.
To determine the effect of Us3 phosphorylation of gB Thr-887 on the intracellular transport of gB in infected cells, Vero cells were infected with either wild-type HSV-1(F), mutant YK551 (gB-T887A), YK555 (gB-T887D), or YK511 (Us3K220M), or the corresponding repaired virus YK553 (gB-TA-repair), YK557 (gB-TD-repair), or YK513 (Us3-KM-repair), respectively, at an MOI of 3. At 18 h postinfection, infected cells were biotinylated with cleavable biotin. After an additional 4 h of incubation at 37°C to allow endocytosis of the biotinylated cell surface proteins, infected cells were treated with glutathione to remove any remaining biotin from proteins at the cell surface. The cells were then harvested, solubilized, and precipitated with streptavidin agarose, and endocytosed gB was detected by immunoblotting with an anti-gB antibody. In this assay, the level of biotinylation of gB represented the accumulation of endocytosed gB, as determined by the balance between gB endocytosis from the cell surface and recycling to the cell surface. A biotinylated gB-avidin precipitate was detected by an anti-gB antibody in a lysate of wild-type HSV-1(F)-infected cells that were incubated at 37°C for 4 h after biotinylation and were then treated with glutathione (Fig. 2A, lane 3), but this precipitate was barely detectable in the lysate of wild-type HSV-1(F)-infected cells treated with glutathione immediately after biotinylation (Fig. 2A, lane 2). This result indicated that specific accumulation of endocytosed gB in HSV-1-infected Vero cells was detectable in these assays and that gB was endocytosed from the surfaces of infected cells as described previously (3, 22).
Fig. 2.
Effects of alanine substitutions in the Us3 phosphorylation site (Thr-887) in gB on the accumulation of endocytosed gB in infected cells. (A) Vero cells were infected with wild-type HSV-1(F) (lanes 1 to 3), YK551 (gB-T887A) (lanes 4 to 6), YK555 (gB-T887D) (lanes 7 to 9), or YK511 (Us3-K220M) (lanes 10 to 12) at an MOI of 3, biotinylated at 18 h postinfection, and incubated for 0 h (lanes 1, 2, 4, 5, 7, 8, 10, and 11) or 4 h (lanes 3, 6, 9, and 12) at 37°C. The cells were then either treated with glutathione (lanes 2, 3, 5, 6, 8, 9, 11, and 12) or left untreated (lanes 1, 4, 7, and 10), harvested, solubilized with RIPA buffer, precipitated with streptavidin agarose, and analyzed by immunoblotting with an anti-gB monoclonal antibody. The data are representative of three independent experiments. (B) Relative amounts of endocytosed gB in cells infected with wild-type HSV-1(F), YK551, YK555, or YK511, corresponding to panel A, lanes 3, 6, 9, and 12. The relative amount of endocytosed gB was calculated as follows: [(amount of biotinylated gB in infected cells incubated for 4 h at 37°C after biotinylation and treatment with glutathione) − (amount of biotinylated gB in infected cells incubated for 0 h at 37°C after biotinylation and treatment with glutathione)]/(amount of biotinylated gB in infected cells incubated for 0 h at 37°C after biotinylation and not treated with glutathione). Data are means and standard errors from three independent experiments and are expressed relative to the mean value of endocytosed gB for wild-type HSV-1(F), which was normalized to 100. (C) Vero cells were infected with HSV-1(F) (lanes 1 to 3), YK553 (gB-TA-repair) (lanes 4 to 6), YK557 (gB-TD-repair) (lanes 7 to 9), or YK513 (Us3-repair) (lanes 10 to 12) at an MOI of 3 and were assayed as described for panel A. The data are representative of three independent experiments. (D) Relative amounts of endocytosed gB in cells infected with each of the repaired viruses for which results are shown in panel C (lanes 3, 6, 9, and 12) were calculated and presented as described for panel B.
The level of intracellular accumulation of the biotinylated gB-T887A mutant from the surfaces of cells infected with YK551 (gB-T887A) was 10.6-fold less than that of biotinylated wild-type gB from the surfaces of cells infected with wild-type HSV-1(F) (Fig. 2A, lanes 3 and 6, and B). A similar decrease was observed in cells infected with YK511, which encodes a kinase-dead mutant of Us3, the major protein kinase responsible for the phosphorylation of gB Thr-887 in infected cells (22, 24), compared to cells infected with wild-type HSV-1(F) (Fig. 2A, lanes 3 and 12, and B). In contrast, accumulation of biotinylated gB-T887D, which contained a mutation predicted to mimic constitutive phosphorylation of gB at Thr-887, from the surfaces of cells infected with YK555 (gB-T887D) was significantly greater than that of biotinylated wild-type gB from the surfaces of cells infected with wild-type HSV-1(F) (Fig. 2A, lanes 3 and 9, and B). The increase in the intracellular accumulation of biotinylated gB-T887D over that of biotinylated wild-type gB was in agreement with the hypothesis that phosphorylation of Thr-887 is important for the accumulation of endocytosed gB, since our previous study showed that only a fraction (∼15%) of gB is phosphorylated in wild-type HSV-1(F)-infected cells (22), but all gB-T887D should mimic phosphorylation at Thr-887 in YK555 (gB-T887D). Wild-type phenotypes were restored in cells infected with the repaired virus YK553 (gB-TA-repair), YK557 (gB-TD-repair), or YK513 (Us3-KM-repair) (Fig. 2C and D). These results indicated that Us3 phosphorylation of gB Thr-887 promoted intracellular accumulation of endocytosed gB from the surfaces of infected cells.
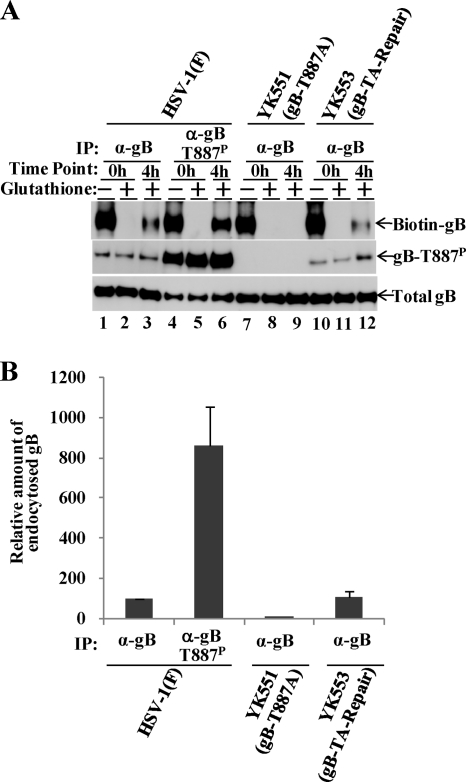
To examine directly whether phosphorylation of gB Thr-887 regulates the intracellular transport of gB in infected cells, we used an anti-gB-T887P monoclonal antibody. We reported previously that the anti-gB-T887P antibody specifically immunoprecipitated gB-T887P from lysates of wild-type HSV-1(F)-infected cells, whereas an anti-gB antibody immunoprecipitated phosphorylated gB, unphosphorylated gB, and mutant gB-T887A equivalently from the lysates of infected cells (22). Vero cells were infected with wild-type HSV-1(F), YK551 (gB-T887A), or YK553 (gB-TA-repair) and were then biotinylated, incubated at 37°C for 4 h, treated with glutathione, harvested, solubilized, and immunoprecipitated with an anti-gB or an anti-gB-T887P antibody. Approximately equal amounts of immunoprecipitated gB for each of the antibodies, obtained as described previously (22), were separated in denaturing gels, and endocytosed gB was detected with streptavidin. In these experiments, we used 4-fold more cell lysate for the anti-gB-T887P antibody immunoprecipitations than for the anti-gB antibody immunoprecipitations. In agreement with our previous report that only a fraction of total gB was phosphorylated at Thr-887 in wild-type HSV-1(F)-infected Vero cells (22), immunoprecipitates with the anti-gB-T887P antibody from Vero cells infected with wild-type HSV-1(F) contained much more gB-T887P than immunoprecipitates with the anti-gB antibody, and immunoprecipitates from cells infected with YK551 (gB-T887A) contained no gB-T887P (Fig. 3A). More of the wild-type gB that was immunoprecipitated with the anti-gB-T887P antibody than with the anti-gB antibody was biotinylated (Fig. 3A, lanes 3 and 6, and B). The relative amount of biotinylated gB in anti-gB-T887P immunoprecipitates was 8.6-fold more than that in anti-gB immunoprecipitates (Fig. 3B). Furthermore, no biotinylated gB was detected in anti-gB immunoprecipitates from cells infected with YK551 (gB-T887A) (Fig. 3A, lane 9, and B). In contrast, the relative amount of biotinylated gB in anti-gB immunoprecipitates from cells infected with YK553 (gB-TA-repair) was similar to that from wild-type HSV-1(F)-infected cells (Fig. 3A, lanes 3 and 12, and B). These results further support the conclusion that Us3 phosphorylation of gB Thr-887 promoted intracellular accumulation of endocytosed gB from the surfaces of infected cells.
Fig. 3.
Accumulation of endocytosed gB phosphorylated at Thr-887 in infected cells. (A) Vero cells infected with wild-type HSV-1(F) (lanes 1 to 6), YK551 (gB-T887A) (lanes 7 to 9), or YK553 (gB-TA-repair) (lanes 10 to 12) at an MOI of 3 were biotinylated, incubated for 0 h (lanes 1, 2, 4, 5, 7, 8, 10, and 11) or 4 h (lanes 3, 6, 9, and 12) at 37°C, harvested after treatment with glutathione (lanes 2, 3, 5, 6, 8, 9, 11, and 12) or no treatment (lanes 1, 4, 7, and 10), solubilized with the NP-40 buffer, immunoprecipitated with an anti-gB (α-gB) (lanes 1 to 3 and 7 to 12) or anti-gB-T887P (lanes 4 to 6) antibody, and then analyzed by immunoblotting with either streptavidin-HRP (top), an anti-gB-T887P antibody (center), or an anti-gB monoclonal antibody (bottom). The data are representative of three independent experiments. (B) Relative amount of endocytosed gB in cells infected with wild-type HSV-1(F), YK551, or YK553. The relative amount of endocytosed gB was calculated as follows: (amount of biotinylated gB [“Biotin-gB” in panel A] in infected cells incubated for 4 h at 37°C after biotinylation and treatment with glutathione relative to amount of total gB [“Total gB” in panel A] in infected cells treated identically − amount of biotinylated gB in infected cells incubated for 0 h at 37°C after biotinylation and treatment with glutathione relative to amount of total gB in infected cells treated identically)/(amount of biotinylated gB in infected cells incubated for 0 h at 37°C after biotinylation and not treated with glutathione relative to amount of total gB in infected cells treated identically). Data are means and standard errors for three independent experiments and are expressed relative to the mean value for cells infected with wild-type HSV-1(F) and immunoprecipitated with anti-gB, which was normalized to 100.
Characterization of recombinant viruses carrying single, double, or triple alanine substitutions in the three gB sequences (Di-Leu-871-872, Thr-887, and Tyr-889).
To examine the significance and functional relationships among the three gB sequences, we constructed a series of recombinant viruses carrying single, double, or triple alanine substitutions in the gB sequences and a series of recombinant viruses in which these mutations had been repaired (Fig. 1). Initial characterization of these mutant viruses revealed that the single, double, and triple mutations in the gB sequences had no effect on the intracellular localization of gB, as determined by immunofluorescence of infected Vero cells, and no effect on the sizes of plaques on infected Vero cells (data not shown).
Since Tyr-889 is near the Us3 phosphorylation site (Thr-887) (Fig. 1), we examined whether alanine substitutions in Tyr-889 and/or Di-Leu-871-872 affected Us3 phosphorylation of gB Thr-887. Vero cells infected with wild-type HSV-1(F), recombinant viruses carrying single, double, or triple mutations in the gB sequences, or repaired viruses were lysed, immunoprecipitated with an anti-gB antibody, and analyzed by immunoblotting with an anti-phospho-PKA substrate antibody. We reported previously that the anti-phospho-PKA substrate antibody specifically recognized phosphorylated gB Thr-887 in infected cells (24). We used the anti-phospho-PKA substrate antibody but not the anti-gB-T887P antibody in these experiments because Tyr-889 appears to be within the epitope of the anti-gB-T887P antibody. Thus, the anti-gB-T887P antibody did not react with the gB-Y889A mutant, whereas the anti-phospho-PKA substrate antibody did (data not shown). As shown in Fig. 4A, the anti-phospho-PKA substrate antibody reacted with gB from cells infected with wild-type HSV-1(F) and gB mutants that did not have the T887A mutation (Fig. 4A, lanes 2, 4, 5, and 8). However, the anti-phospho-PKA substrate antibody did not react with gB from cells infected with gB mutants with the T887A mutation (Fig. 4A, lanes 3, 6, 7, and 9) but did react with all the repaired viruses (Fig. 4B).
Fig. 4.
Characterization of recombinant viruses carrying single, double, or triple mutations in the three gB sequences (Di-Leu-871-872, Thr-887, and Tyr-889). (A) Immunoblot of electrophoretically separated lysates from Vero cells that were either mock infected (lane 1) or infected with wild-type HSV-1(F) (lane 2), YK551 (gB-T887A) (lane 3), YK563 (gB-Y889A) (lane 4), YK571 (gB-LL871AA) (lane 5), YK567 (gB-T887A/Y889A) (lane 6), YK575 (gB-LL871AA/T887A) (lane 7), YK579 (gB-LL871AA/Y889A) (lane 8), or YK583 (gB-LL871AA/T887A/Y889A) (lane 9) at an MOI of 3. The infected Vero cells were harvested at 18 h postinfection, immunoprecipitated with an anti-gB monoclonal antibody, and analyzed by immunoblotting with an anti-PKA substrate antibody (100G7) (gB-T887P) or an anti-gB monoclonal antibody (Total gB). (B) Vero cells were either mock infected (lane 1) or infected with wild-type HSV-1(F) (lane 2), YK553 (gB-TA-repair) (lane 3), YK565 (gB-YA-repair) (lane 4), YK573 (gB-LLAA-repair) (lane 5), YK569 (gB-TA/YA-repair) (lane 6), YK577 (gB-LLAA/TA-repair) (lane 7), YK581 (gB-LLAA/YA-repair) (lane 8), or YK585 (gB-LLAA/TA/YA-repair) (lane 9) at an MOI of 3. The infected Vero cells were harvested at 18 h postinfection, immunoprecipitated with an anti-gB monoclonal antibody, and analyzed by immunoblotting with an anti-PKA substrate antibody (100G7) (gB-T887P) or an anti-gB monoclonal antibody (Total gB). (C) Immunoblots of electrophoretically separated virions of wild-type HSV-1(F) (lane 1), YK551 (lane 2), YK563 (lane 3), and YK571 (lane 4) harvested at 48 h postinfection, purified by sucrose gradient centrifugation, and reacted with an antibody to gB or VP5. (D) Immunoblots of electrophoretically separated virions of wild-type HSV-1(F) (lane 1), YK567 (lane 2), YK575 (lane 3), YK579 (lane 4), and YK583 (lane 5) purified by sucrose gradient centrifugation and reacted with an antibody to gB or VP5. (E) Digital images of Vero cells infected with wild-type HSV-1(F), YK551, YK563, YK571, YK567, YK575, YK579, YK583, YK573, YK577, YK581, or YK585 at an MOI of 0.01. Live cells were examined at 72 h postinfection by phase microscopy.
We next examined the effects of single, double, and triple mutations in the gB sequences on the incorporation of gB into progeny virions by harvesting and purifying total intracellular and extracellular virions at 48 h postinfection and analyzing them by immunoblotting with an anti-gB or anti-VP5 antibody. As shown in Fig. 4C and D, the amounts of gB in single, double, and triple mutant progeny virions were similar to that in wild-type progeny virions. Similar results were also obtained with purified virions harvested at 24 h postinfection (data not shown). These results indicated that the three gB sequences were not required for efficient packaging of gB into virions.
Beitia Ortiz de Zarate et al. have reported that the LL871-872AA mutation in gB induced syncytium formation in infected COS-7 cells (3). In agreement with that report, syncytia were observed in Vero cells infected with YK571, carrying the LL871-872AA mutation in gB (Fig. 4E). Recombinant viruses YK575 (gB-LL871-872AA/T887A), YK579 (gB-LL871-872AA/Y889A), and YK583 (gB-LL871-872AA/T887A/Y889A), carrying the gB-T887A and/or gB-Y889A mutation in combination with the gB-LL871-872AA mutation, induced syncytium formation in infected cells at levels similar to that for YK571 (gB-LL871-872AA) (Fig. 4E). The wild-type phenotype was restored in cells infected with the repaired viruses (Fig. 4E). These results indicated that the LL871-872AA mutation in gB is the only mutation of those in the gB sequences that induced syncytium formation in infected cells.
Effects of single, double, or triple mutations in the three gB sequences on the accumulation of endocytosed gB in infected cells.
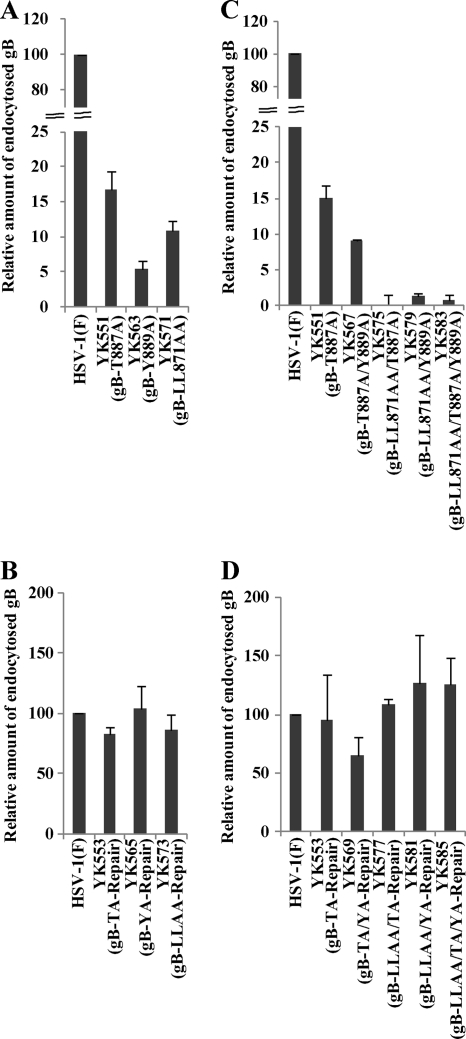
To investigate the contribution of each gB sequence to the regulation of gB intracellular transport, Vero cells infected with either wild-type HSV-1(F), mutant YK551 (gB-T887A), YK563 (gB-Y889A), or YK571 (gB-LL871-872AA), or the corresponding repaired virus were assayed for gB endocytosis. As shown in Fig. 5A, the T887A, Y889A, and LL871-872AA mutations in gB significantly decreased (6.0-, 18.8-, and 9.2-fold, respectively) the accumulation of endocytosed gB in infected cells, with the Y889A mutation having the most inhibitory effect. The wild-type phenotype was restored in cells infected with a repaired virus (Fig. 5B). These results indicated that all three gB sequences were required for regulation of the accumulation of endocytosed gB but that Tyr-889 appeared to play the most critical role in this regulation.
Fig. 5.
Effects of single, double, and triple mutations in the three gB sequences on the accumulation of endocytosed gB in infected cells. Experiments were performed as described for Fig. 2A except that recombinant viruses carrying the single, double, or triple mutations in the gB sequences were used. The relative amount of endocytosed gB in infected cells was calculated and presented as described for Fig. 2B.
To examine the functional relationships among the three gB sequences, the gB endocytosis assays were carried out using Vero cells infected with either wild-type HSV-1(F), a recombinant virus carrying single, double, or triple mutations in the gB sequences, or the corresponding repaired virus. In agreement with the results in Fig. 5A, accumulation of the endocytosed gB-T887A mutant was significantly lower (6.6-fold) than that of wild-type gB (Fig. 5C). Accumulation of the endocytosed gB-T887A/Y889A double mutant was decreased even more than that of the gB-T887A single mutant. However, the difference in the amount of endocytosed gB between the gB-T887A and gB-T887A/Y889A mutants (Fig. 5C) was comparable to that between the gB-T887A and gB-Y889A mutants (Fig. 5A). In contrast, double mutations in gB Di-Leu-871-872 and either Thr-887 or Tyr-889, as well as triple mutations in all three gB sequences, reduced the accumulation of endocytosed gB to barely detectable levels (Fig. 5C). Wild-type levels of accumulation of endocytosed gB were restored in cells infected with the repaired viruses (Fig. 5D). These results indicated that a double mutation of gB Di-Leu-871-872 and either gB Thr-887 or gB Tyr-889 had an additive inhibitory effect on the accumulation of endocytosed gB, but that the gB T887A Y889A double mutation did not, compared to the gB Y889A mutation.
Effects of single, double, or triple mutations in the three gB sequences on expression of gB on the surfaces of infected cells.
To examine the significance and functional relationships among the three gB sequences in the regulation of gB expression on the surfaces of infected cells, Vero cells infected with either wild-type HSV-1(F), recombinant viruses carrying single, double, or triple mutations in the gB sequences, or repaired viruses were analyzed by flow cytometry. As shown in Fig. 6, expression of the gB-T887A, gB-LL871-872AA, or gB-Y889A mutant on the surfaces of cells infected with YK551 (gB-T887A), YK571 (gB-LL871-872AA), or YK563 (gB-Y889A) was upregulated compared to that of wild-type gB in cells infected with wild-type HSV-1(F). Among the gB mutants with single mutations, cell surface expression of the gB-Y889A mutant was significantly higher than that of the gB-T887A or gB-LL-871-872AA mutant. In addition, cell surface expression of the gB-T887A/Y889A and gB-LL871AA/Y889A double mutants and the gB-LL871AA/T887A/Y889A triple mutant was upregulated to a level similar to that of the gB-Y889A single mutant. We noted that the levels of cell surface expression of the gB single mutants were roughly inverse to their relative levels of accumulation of endocytosed gB (Fig. 5 and 6). However, although double mutants with Di-Leu-871-872 and either Thr-887 or Tyr-889 showed little or no accumulation of endocytosed gB (Fig. 5), this double mutation had no additive effect on the cell surface expression of gB, compared to the gB-Y889A mutation (Fig. 6). The wild-type phenotype was restored in cells infected with a repaired virus (Fig. 6). These results indicated that, although all three gB sequences downregulated gB expression on the surfaces of infected cells, Tyr-889 played a prominent role in this function.
Fig. 6.
Effects of single, double, and triple mutations in the three gB sequences on the expression of gB on the surfaces of infected cells. Vero cells were either mock infected or infected with wild-type HSV-1(F), recombinant viruses carrying single, double, or triple mutations in the gB sequences, or repaired viruses at an MOI of 3. At 6 h postinfection, cell surface expression and total expression of gB in infected cells were analyzed by flow cytometry. The relative amount of expression of gB on the cell surface was calculated as follows: [(mean fluorescence intensity for gB expression on the surfaces of cells infected with the indicated virus) − (mean fluorescence intensity for gB expression on the surfaces of mock-infected cells)]/[(mean fluorescence intensity for total-gB expression in cells infected with the indicated virus) − (mean fluorescence intensity for total-gB expression in mock-infected cells)]. Data are means and standard errors for three independent experiments and are expressed relative to the mean value for wild-type HSV-1(F), which was normalized to 100.
Effects of single, double, or triple mutations in the three gB sequences on viral replication in cell cultures and neurovirulence in mice.
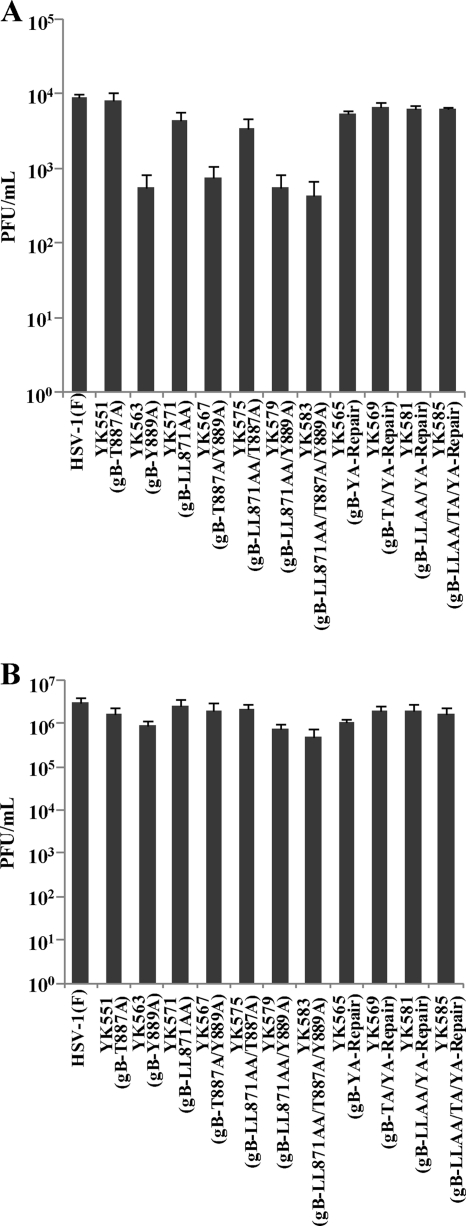
Two types of experiments were carried out to examine the significance of regulation of gB intracellular transport and cell surface expression by the three gB sequences in the HSV-1 replication cycle. In the first series of experiments, we examined the effects of mutations in the three gB sequences on progeny virus production. Preliminary experiments indicated that growth curves measuring the total intracellular and extracellular viral production of recombinant viruses carrying single, double, or triple mutations in the gB sequences infecting Vero cells at an MOI of 3 were similar to those for wild-type HSV-1(F) (data not shown). Therefore, we examined the effects of these mutations in the gB sequences on viral production of extracellular and intracellular viruses in Vero cells infected at an MOI of 3. The titers of intracellular viruses with single, double, or triple mutations in gB at 12 and 24 h postinfection were similar to those of wild-type HSV-1(F) (data not shown). In addition, the titers of extracellular viruses with these mutations in gB at 24 h postinfection were similar to those of wild-type HSV-1(F) (Fig. 7B). At 12 h postinfection, however, among viruses with a single mutation (Fig. 7A), although the titers of extracellular YK551 (gB-T887A) and YK571 (gB-LL871-872AA) were only slightly lower than that of wild-type HSV-1(F), the titer of extracellular YK563 (gB-Y889A) was more than 10-fold lower than that of wild-type HSV-1(F) and the repaired YK565 (gB-YA-repair) virus. There were no further reductions in extracellular virus titers at 12 h postinfection for cells infected by the YK567 (gB-T887A/Y889A) or YK579 (gB-LL871-872AA/Y889A) double mutant compared to cells infected by YK563 (gB-Y889A). These results were reproducible in repeated experiments (data not shown). We should note that the effects of the Y889A and LL-871-872AA mutations in gB on viral replication in Vero cells in this study are not in agreement with the previous report of Beitia Ortiz de Zarate et al. (3). That report showed that the Y889A mutation in HSV-1 strain KOS gB decreased both intracellular and extracellular virus production in 143B cells infected at an MOI of 5 and that the LL-871-872AA mutation in gB decreased intracellular but not extracellular virus production (3). Thus, the effects of the Y889A and LL-871-872AA mutations in gB on HSV-1 replication appeared to depend on the viral strain and cell type studied. It has been reported that HSV-1 infection induced dispersal of the Golgi apparatus and TGN in Vero cells and, to a much lesser extent, in 143B cells (10, 49, 52). The cell type-dependent dynamics of the Golgi apparatus and TGN induced by HSV-1 infection might account for the different effects of the Y889A and LL-871-872AA mutations in gB on HSV-1 replication in the different cell types.
Fig. 7.
Effects of single, double, and triple mutations in the three gB sequences on the production of extracellular infectious virus. Vero cells were either mock infected or infected with wild-type HSV-1(F), recombinant viruses carrying single, double, or triple mutations in the gB sequences, or repaired viruses at an MOI of 3. Extracellular infectious virus from the supernatants of infected cells was harvested at 12 h (A) and 24 h (B) postinfection and was assayed on Vero cells. Data are means and standard errors for three independent experiments.
In the second series of experiments, mice were infected intracerebrally with recombinant viruses carrying single, double, or triple mutations in the three gB sequences or with the repaired viruses, and survival was monitored. As shown in Fig. 8, there was significantly better survival in mice infected with YK563 (gB-Y889A) than in mice infected with YK565 (gB-YA-repair). Similarly increased survival was found in mice infected with the double mutant virus YK567 (gB-T887A/Y889A) or YK579 (gB-LL871-872AA/Y889A) or the triple mutant virus YK583 (gB-LL871-872AA/T887A/Y889A). In contrast, the survival of mice infected with a virus that did not carry the Y889A mutation (i.e., YK551 [gB-T887A], YK571 [gB-LL871-872AA], or YK575 [LL871-872AA/T887A]) was not significantly different from that of mice infected with the corresponding repaired virus. We also examined the effect of the Y889A mutation on viral replication in the brain following intracerebral inoculation. In agreement with the effect of the Y889A mutation in gB on the survival of mice, the titers of YK563 (gB-Y889A) were significantly (3.7-fold) lower than those of YK565 (gB-YA-repair) (Table 2).
Fig. 8.
Effects of single, double, and triple mutations in the three gB sequences on HSV-1 pathogenesis in mice following intracerebral infection. Twenty 3-week-old female ICR mice were inoculated intracerebrally with 102 PFU of a recombinant virus carrying a single, double, or triple mutation in the gB sequences or a repaired virus and were monitored for 14 days. Statistically significant differences in the survival curves of mice infected with recombinant viruses carrying the Y889A mutation in gB relative to its repaired virus are noted (*; P < 0.01). n.s.; not significant.
Table 2.
Virus titers in the brains of mice following intracerebral inoculation
| Mouse no. | Virus titer (PFU/tissue) |
|
|---|---|---|
| YK563 (gB-Y889A) | YK565 (gB-YA-repair) | |
| 1 | 8.8 × 104 | 1.5 × 105 |
| 2 | 4.3 × 104 | 1.3 × 105 |
| 3 | 3.0 × 104 | 8.0 × 104 |
| 4 | 1.5 × 104 | 1.9 × 105 |
| 5 | 7.8 × 104 | 1.7 × 105 |
| 6 | 5.3 × 104 | 5.8 × 104 |
| 7 | 1.6 × 104 | 2.3 × 105 |
| 8 | 4.3 × 104 | 1.4 × 105 |
| 9 | 2.3 × 104 | 2.8 × 105 |
| Mean ± SEa | 4.3 × 104 ± 8.1 × 103 | 1.6 × 105 ± 2.2 × 104 |
The differences between the mean titers of the YK563 and YK565 viruses are statistically significant (P < 0.05).
Taken together, these results indicated that Tyr-889 was the only one of the three gB sequences required for efficient extracellular virus production at 12 h postinfection in Vero cells, although by 24 h postinfection, the extracellular virus titer for cells infected with a Y889A mutant virus was similar to that for cells infected with the wild-type virus. Tyr-889 was also the only one of the three gB sequences required for efficient neurovirulence in mice following intracerebral infection.
DISCUSSION
In the present study, we have further analyzed the role of HSV-1 Us3 phosphorylation of gB Thr-887, which we previously showed was required for proper expression of gB on the surfaces of infected cells (24) and for efficient viral replication and pathogenesis in the mouse HSK model (22). The experiments reported here showed that Us3 phosphorylation of gB Thr-887 promoted the accumulation of endocytosed gB in infected cells. This conclusion was based on the observations that (i) the T887A mutation in gB impaired the accumulation of endocytosed gB in infected cells; (ii) the K220M mutation in Us3, which inactivates Us3 kinase activity without affecting the expression of Us3 protein (25), also impaired the accumulation of endocytosed gB in infected cells; (iii) endocytosed gB-T887P accumulated to a much greater extent than unphosphorylated gB at Thr-887; and (iv) a phosphomimetic substitution in gB Thr-887 (T887D) increased the accumulation of endocytosed gB to a level greater than that of wild-type gB, since only ∼15% of wild-type gB was phosphorylated at Thr-887 in wild-type virus-infected cells (22). In addition, we have demonstrated that two gB motifs (Tyr-889 and Di-Leu-871-872) mediated the downregulation of gB cell surface expression and promoted the accumulation of endocytosed gB in infected cells, based on results showing that mutations in these two gB motifs upregulated gB cell surface expression and impaired the accumulation of endocytosed gB in infected cells. Taken together, these results indicate that HSV-1 has evolved at least three sequences (Di-Leu-871-872, Thr-887, and Tyr-889) in the cytoplasmic tail of gB to regulate proper cell surface expression and intracellular transport of gB in infected cells.
Tyr-889 appeared to be of predominant importance in regulating the cell surface expression and intracellular transport of gB, based on the result that a mutation in gB Tyr-889 affected gB cell surface expression and the accumulation of endocytosed gB much more than mutations in the other two gB sequences (Thr-887 and LL-871/872). Tyr-889 was also found to be the only one of the three gB sequences required for efficient production of extracellular virions in Vero cells at 12 h postinfection, but not at 24 h postinfection, and for neurovirulence in mice following intracerebral inoculation. These results suggested that the regulation of gB intracellular transport mediated by Tyr-889 is required for efficient release of progeny virions in cell cultures and is critical for neurovirulence in vivo. To our knowledge, this is the first report showing that a typical endocytosis motif in the cytoplasmic tail of an HSV-1 envelope glycoprotein, which can be found in many herpesvirus envelope glycoproteins (8), played a role in viral pathogenesis in vivo. However, as is usual with such mutational analyses, we cannot discount the possibility that the Y889A mutation in gB had an effect on a gB function(s) other than the regulation of gB intracellular transport shown in this study. It is interesting that, of the viruses with mutations in one or more of the three gB sequences in this study, extracellular virion production was impaired only in cells infected with viruses carrying the Y889A mutation in gB, and a decrease in neurovirulence was observed only in mice infected with viruses carrying the gB Y889A mutation. Mature virions, which acquire the final envelope, most likely at the TGN, are secreted from cells by exocytosis (17, 32, 46, 49), and therefore, gB Tyr-889 may optimally regulate the virion secretion pathway by exocytosis from the TGN. The reduced yield of extracellular virions of recombinant viruses carrying the Y889A mutation may also in part account for their lower neurovirulence in mice. In addition, we demonstrated here that the expression of the gB-Y889A mutant on the surfaces of infected cells was significantly higher than that of the gB-LL871-872AA and gB-T887A mutants. It is well established that gB on the cell surface is a potent inducer of the immune response in vivo (5, 6, 40, 41). In fact, it has been demonstrated that lysis of HSV-1-infected cells by natural killer cells, which play critical roles in the initial defense against herpesvirus infection in the central nervous system (29), is correlated with cell surface expression of gB (5). Therefore, the Y889A mutation in gB, which remarkably increases the number of gB molecules on the cell surface exposed to the immune system, may make it easier for infected cells to be recognized and attacked by the immune system in vivo, leading to a decrease in neurovirulence. We previously reported that a mutation in the Us3 phosphorylation site (Thr-887) of gB had no effect on neurovirulence in mice following intracerebral inoculation, whereas the mutation significantly impaired viral replication in the mouse cornea and the development of herpes stromal keratitis and periocular skin disease in mice following ocular infection (22). In experimental animal models of HSV-1 infection, viral pathogenesis in sites peripheral to the initial infection site and destruction of the central nervous system caused by viral replication are semi-independent indicators of viral virulence and are tested by peripheral and intracerebral inoculation, respectively (51). These studies suggested that Tyr-889, Thr-887, and probably Di-Leu-871-872 play distinct roles in diverse aspects of HSV-1 pathogenesis in vivo, although a role(s) for Di-Leu-871-872 and Tyr-889 in viral replication and pathogenesis in mice following peripheral inoculation remains to be determined.
The expression of gB mutants on the surfaces of infected cells in this study was partially inversely related to the level of endocytosed gB accumulation, suggesting that one of the mechanisms for the downregulation of gB cell surface expression in infected cells was increased accumulation of endocytosed gB mediated by the three gB sequences. However, for some gB mutants, gB cell surface expression was not inversely related to the level of endocytosed gB accumulation in infected cells. For example, accumulation of endocytosed gB-LL871-872AA/T887A was barely detectable and much less than that of endocytosed gB-T887A/Y889A, whereas the level of cell surface expression of gB-LL871-872AA/T887A was lower than that of gB-T887A/Y889A. These observations suggested that proper expression of gB on the surfaces of infected cells was regulated not only by the efficiency of endocytosed gB accumulation but also by other intracellular trafficking events, such as gB transport to the cell membrane and gB retention in the ER, Golgi apparatus, TGN, and/or endosomes. It has been reported that a tyrosine motif in the cellular TGN38 protein functions in the promotion of both retention at the TGN and endocytosis of the protein (7). Similarly, gB Tyr-889, which seems to be the major determinant for proper gB cell surface expression and intracellular transport among the three gB sequences, as described above, might promote gB retention at the TGN as well as gB endocytosis, thereby regulating proper gB expression on the surfaces of infected cells.
Beitia Ortiz de Zarate et al. previously investigated the roles of gB motifs Tyr-889 and Di-Leu-871-872 in the intracellular transport of gB by an indirect immunofluorescence endocytosis assay in 143B cells infected with an HSV-1 KOS strain carrying a Y889A or LL871-872AA mutation in gB (3). They demonstrated that the Y889A mutation in gB inhibited gB endocytosis in infected cells. In contrast, the LL871-872AA mutation in gB induced gB endocytosis, as observed with wild-type gB, but impaired its return to the TGN. They also reported that the LL871-872AA mutation in gB enhanced the recycling of gB to the plasma membrane in transfected COS-7 cells (4), suggesting that Di-Leu-871-872 promoted the accumulation of endocytosed gB by inhibiting the recycling of gB to the plasma membrane. These imaging analyses clearly indicated that Tyr-889 and Di-Leu-871-872 in gB regulated the intracellular transport of gB differently. In the present study, systematic analysis of mutations in the three gB sequences (Di-Leu-871-872, Thr-887, and Tyr-889) showed that double mutations in Tyr-889 and Di-Leu-871-872 produced an additive effect on the accumulation of endocytosed gB in infected cells compared to single mutations in Tyr-889 or Di-Leu-871-872. This result was in agreement with the suggestion by Beitia Ortiz de Zarate et al. that gB Tyr-889 and Di-Leu-871-872 regulated the intracellular transport of gB by different pathways (3). We also obtained similar results with double mutations in gB Di-Leu-871-872 and Thr-887, raising the possibility that Us3 phosphorylation of gB Thr-887 regulated the accumulation of endocytosed gB by a pathway different from that of Di-Leu-871-872. In contrast, double mutations in Thr-887 and Tyr-889 had no additive effect on the accumulation of endocytosed gB compared to single mutations in Tyr-889. Similarly, the phosphomimetic substitution in Thr-887 in combination with the alanine substitution in Tyr-889 showed no additive effect on the accumulation of endocytosed gB compared to a single mutation in Tyr-889 (data not shown). These observations suggested that Tyr-889 and Us3 phosphorylation of gB Thr-887 regulated the accumulation of endocytosed gB in infected cells by the same pathway or a related pathway and that Us3 phosphorylation of gB Thr-887 probably upregulated the efficiency of Tyr-889-mediated gB endocytosis. Phosphorylation of gB at Thr-887 may strengthen the interaction of Tyr-889 in gB with potential endocytosis adaptor proteins. Phosphorylation of gB Thr-887 appeared to be tightly regulated in infected cells, based on our previous report that only a fraction (∼15%) of gB Thr-887 was phosphorylated in infected cells (22). Although there are other interpretations of these data with regard to the effects of double mutations in the gB sequences, these experiments suggested that the accumulation of endocytosed gB in infected cells was coordinately and tightly regulated by these three sequences and that strict regulation of gB intracellular transport is critical for the HSV-1 replication cycle. This hypothesis may explain why HSV-1 has evolved multiple sequences in the gB cytoplasmic tail that regulate the intracellular transport of gB.
It has been proposed that one of the roles of endocytosis of herpesvirus envelope glycoproteins is to recruit viral components (e.g., envelope glycoproteins) to the appropriate cellular compartment (most likely the TGN) for viral assembly (8). In support of this hypothesis, endocytosed envelope glycoproteins from the cell surface have been demonstrated to be incorporated into herpesvirus virions (30, 39). More recently, ultrastructural analyses using horseradish peroxidase as a fluid-phase marker of endocytosis showed that the final envelopes of HSV-1 and bovine herpesvirus 1 were derived from endocytosed cell membranes (21). In contrast, it has been reported that endocytosis-defective gE mutants were incorporated into virions as efficiently as wild-type gE (1, 48) and that inhibition of endocytosis of HCMV gB by using a dominant-negative dynamin I variant did not affect the production of infectious HCMV (23). In the present study, the gB-LL871-872AA/T887A, gB-LL871-872AA/Y889A, and gB-LL-871-872AA/T887A/Y889A mutations, which almost abolished the accumulation of endocytosed gB in infected cells, had no effect on the incorporation of gB into virions compared to that for the wild-type virus. These results are in agreement with a previous report that PRV gB lacking the Y-based and dileucine motifs was incorporated into virions with an efficiency equal to that of wild-type gB (35). These observations support the hypothesis that endocytosis of herpesvirus envelope glycoproteins is not required for efficient virion assembly.
ACKNOWLEDGMENTS
We thank Toshiyuki Yamaji for helpful advice on the cell surface biotinylation assays and Shihoko Koyama for excellent technical assistance.
This study was supported in part by the Funding Program for Next Generation World-Leading Researchers from the Japan Society for the Promotion of Science (JSPS), Grants for Scientific Research, a Grant for Scientific Research in Priority Areas, a contract research fund for the Program of Japan Initiative for Global Research Network on Infectious Diseases and the Global COE Program “Center of Education and Research for Advanced Genome-Based Medicine—For personalized medicine and the control of worldwide infectious diseases” from the Ministry of Education, Culture, Science, Sports and Technology (MEXT) of Japan, and grants from the Takeda Science Foundation, Astellas Foundation for Research on Metabolic Disorders, Daiichi-Sankyo Foundation of Life Science, and Uehara Memorial Foundation.
Footnotes
Published ahead of print on 9 March 2011.
REFERENCES
- 1. Alconada A., Bauer U., Sodeik B., Hoflack B. 1999. Intracellular traffic of herpes simplex virus glycoprotein gE: characterization of the sorting signals required for its trans-Golgi network localization. J. Virol. 73:377–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arii J., et al. 2010. Non-muscle myosin IIA is a functional entry receptor for herpes simplex virus-1. Nature 467:859–862 [DOI] [PubMed] [Google Scholar]
- 3. Beitia Ortiz de Zarate I., Cantero-Aguilar L., Longo M., Berlioz-Torrent C., Rozenberg F. 2007. Contribution of endocytic motifs in the cytoplasmic tail of herpes simplex virus type 1 glycoprotein B to virus replication and cell-cell fusion. J. Virol. 81:13889–13903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beitia Ortiz de Zarate I., Kaelin K., Rozenberg F. 2004. Effects of mutations in the cytoplasmic domain of herpes simplex virus type 1 glycoprotein B on intracellular transport and infectivity. J. Virol. 78:1540–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bishop G. A., Glorioso J. C., Schwartz S. A. 1983. Relationship between expression of herpes simplex virus glycoproteins and susceptibility of target cells to human natural killer activity. J. Exp. Med. 157:1544–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bishop G. A., Marlin S. D., Schwartz S. A., Glorioso J. C. 1984. Human natural killer cell recognition of herpes simplex virus type 1 glycoproteins: specificity analysis with the use of monoclonal antibodies and antigenic variants. J. Immunol. 133:2206–2214 [PubMed] [Google Scholar]
- 7. Bos K., Wraight C., Stanley K. K. 1993. TGN38 is maintained in the trans-Golgi network by a tyrosine-containing motif in the cytoplasmic domain. EMBO J. 12:2219–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brideau A. D., Enquist L. W., Tirabassi R. S. 2000. The role of virion membrane protein endocytosis in the herpesvirus life cycle. J. Clin. Virol. 17:69–82 [DOI] [PubMed] [Google Scholar]
- 9. Cai W. H., Gu B., Person S. 1988. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J. Virol. 62:2596–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campadelli G., et al. 1993. Fragmentation and dispersal of Golgi proteins and redistribution of glycoproteins and glycolipids processed through the Golgi apparatus after infection with herpes simplex virus 1. Proc. Natl. Acad. Sci. U. S. A. 90:2798–2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fan Z., et al. 2002. Truncation of herpes simplex virus type 2 glycoprotein B increases its cell surface expression and activity in cell-cell fusion, but these properties are unrelated. J. Virol. 76:9271–9283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farnsworth A., et al. 2007. Herpes simplex virus glycoproteins gB and gH function in fusion between the virion envelope and the outer nuclear membrane. Proc. Natl. Acad. Sci. U. S. A. 104:10187–10192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Favoreel H. W., Nauwynck H. J., Van Oostveldt P., Pensaert M. B. 2000. Role of anti-gB and -gD antibodies in antibody-induced endocytosis of viral and cellular cell surface glycoproteins expressed on pseudorabies virus-infected monocytes. Virology 267:151–158 [DOI] [PubMed] [Google Scholar]
- 14. Favoreel H. W., Van Minnebruggen G., Nauwynck H. J., Enquist L. W., Pensaert M. B. 2002. A tyrosine-based motif in the cytoplasmic tail of pseudorabies virus glycoprotein B is important for both antibody-induced internalization of viral glycoproteins and efficient cell-to-cell spread. J. Virol. 76:6845–6851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Geraghty R. J., Krummenacher C., Cohen G. H., Eisenberg R. J., Spear P. G. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618–1620 [DOI] [PubMed] [Google Scholar]
- 16. Glick B. S., Nakano A. 2009. Membrane traffic within the Golgi apparatus. Annu. Rev. Cell Dev. Biol. 25:113–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harley C. A., Dasgupta A., Wilson D. W. 2001. Characterization of herpes simplex virus-containing organelles by subcellular fractionation: role for organelle acidification in assembly of infectious particles. J. Virol. 75:1236–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heineman T. C., Hall S. L. 2001. VZV gB endocytosis and Golgi localization are mediated by YXXφ motifs in its cytoplasmic domain. Virology 285:42–49 [DOI] [PubMed] [Google Scholar]
- 19. Herold B. C., Visalli R. J., Susmarski N., Brandt C. R., Spear P. G. 1994. Glycoprotein C-independent binding of herpes simplex virus to cells requires cell surface heparan sulphate and glycoprotein B. J. Gen. Virol. 75(Pt 6):1211–1222 [DOI] [PubMed] [Google Scholar]
- 20. Herold B. C., WuDunn D., Soltys N., Spear P. G. 1991. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J. Virol. 65:1090–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hollinshead M., Gonzalez-Lopez C., Sayers C., Smith G. L., Elliott G. 2010. Herpes simplex virus acquires its final envelope from tubular endosomes, abstr. 5.04. 35th Annu. Int. Herpesvirus Workshop. [Google Scholar]
- 22. Imai T., Sagou K., Arii J., Kawaguchi Y. 2010. Effects of phosphorylation of herpes simplex virus 1 envelope glycoprotein B by Us3 kinase in vivo and in vitro. J. Virol. 84:153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jarvis M. A., et al. 2002. Retrieval of human cytomegalovirus glycoprotein B from cell surface is not required for virus envelopment in astrocytoma cells. J. Virol. 76:5147–5155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kato A., et al. 2009. Herpes simplex virus 1 protein kinase Us3 phosphorylates viral envelope glycoprotein B and regulates its expression on the cell surface. J. Virol. 83:250–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kato A., et al. 2008. Identification of a physiological phosphorylation site of the herpes simplex virus 1-encoded protein kinase Us3 which regulates its optimal catalytic activity in vitro and influences its function in infected cells. J. Virol. 82:6172–6189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kopp M., Klupp B. G., Granzow H., Fuchs W., Mettenleiter T. C. 2002. Identification and characterization of the pseudorabies virus tegument proteins UL46 and UL47: role for UL47 in virion morphogenesis in the cytoplasm. J. Virol. 76:8820–8833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lang S. M., Means R. E. 2010. Characterization of cytoplasmic motifs important in rhesus rhadinovirus gB processing and trafficking. Virology 398:233–242 [DOI] [PubMed] [Google Scholar]
- 28. Laquerre S., et al. 1998. Heparan sulfate proteoglycan binding by herpes simplex virus type 1 glycoproteins B and C, which differ in their contributions to virus attachment, penetration, and cell-to-cell spread. J. Virol. 72:6119–6130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lodoen M. B., Lanier L. L. 2006. Natural killer cells as an initial defense against pathogens. Curr. Opin. Immunol. 18:391–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maresova L., Pasieka T. J., Homan E., Gerday E., Grose C. 2005. Incorporation of three endocytosed varicella-zoster virus glycoproteins, gE, gH, and gB, into the virion envelope. J. Virol. 79:997–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mettenleiter T. C. 2004. Budding events in herpesvirus morphogenesis. Virus Res. 106:167–180 [DOI] [PubMed] [Google Scholar]
- 32. Mettenleiter T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Montgomery R. I., Warner M. S., Lum B. J., Spear P. G. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427–436 [DOI] [PubMed] [Google Scholar]
- 34. Nayak D. P., Hui E. K., Barman S. 2004. Assembly and budding of influenza virus. Virus Res. 106:147–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nixdorf R., Klupp B. G., Karger A., Mettenleiter T. C. 2000. Effects of truncation of the carboxy terminus of pseudorabies virus glycoprotein B on infectivity. J. Virol. 74:7137–7145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pellett P. E., Roizman B. 2007. The family Herpesviridae: a brief introduction, p. 2479–2499 In Knipe D. M., Howley P. M., Griffin D. E., Lamb R. A., Martin M. A., Roizman B., Straus S. E. (ed.), Fields virology, 5th ed. Lippincott Williams &Wilkins, Philadelphia, PA [Google Scholar]
- 37. Pertel P. E., Fridberg A., Parish M. L., Spear P. G. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313–324 [DOI] [PubMed] [Google Scholar]
- 38. Pornillos O., Garrus J. E., Sundquist W. I. 2002. Mechanisms of enveloped RNA virus budding. Trends Cell Biol. 12:569–579 [DOI] [PubMed] [Google Scholar]
- 39. Radsak K., et al. 1996. Retrieval of human cytomegalovirus glycoprotein B from the infected cell surface for virus envelopment. Arch. Virol. 141:557–572 [DOI] [PubMed] [Google Scholar]
- 40. Sanchez-Pescador L., Paz P., Navarro D., Pereira L., Kohl S. 1992. Epitopes of herpes simplex virus type 1 glycoprotein B that bind type-common neutralizing antibodies elicit type-specific antibody-dependent cellular cytotoxicity. J. Infect. Dis. 166:623–627 [DOI] [PubMed] [Google Scholar]
- 41. Sanchez-Pescador L., Pereira L., Charlebois E. D., Kohl S. 1993. Antibodies to epitopes of herpes simplex virus type 1 glycoprotein B (gB) in human sera: analysis of functional gB epitopes defined by inhibition of murine monoclonal antibodies. J. Infect. Dis. 168:844–853 [DOI] [PubMed] [Google Scholar]
- 42. Satoh T., et al. 2008. PILRα is a herpes simplex virus-1 entry coreceptor that associates with glycoprotein B. Cell 132:935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shukla D., et al. 1999. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99:13–22 [DOI] [PubMed] [Google Scholar]
- 44. Spear P. G., et al. 2006. Different receptors binding to distinct interfaces on herpes simplex virus gD can trigger events leading to cell fusion and viral entry. Virology 344:17–24 [DOI] [PubMed] [Google Scholar]
- 45. Suenaga T., et al. 2010. Myelin-associated glycoprotein mediates membrane fusion and entry of neurotropic herpesviruses. Proc. Natl. Acad. Sci. U. S. A. 107:866–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sugimoto K., et al. 2008. Simultaneous tracking of capsid, tegument, and envelope protein localization in living cells infected with triply fluorescent herpes simplex virus 1. J. Virol. 82:5198–5211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tanaka M., Kagawa H., Yamanashi Y., Sata T., Kawaguchi Y. 2003. Construction of an excisable bacterial artificial chromosome containing a full-length infectious clone of herpes simplex virus type 1: viruses reconstituted from the clone exhibit wild-type properties in vitro and in vivo. J. Virol. 77:1382–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tirabassi R. S., Enquist L. W. 1999. Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE. J. Virol. 73:2717–2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Turcotte S., Letellier J., Lippe R. 2005. Herpes simplex virus type 1 capsids transit by the trans-Golgi network, where viral glycoproteins accumulate independently of capsid egress. J. Virol. 79:8847–8860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Turner A., Bruun B., Minson T., Browne H. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Van Sant C., Kawaguchi Y., Roizman B. 1999. A single amino acid substitution in the cyclin D binding domain of the infected cell protein no. 0 abrogates the neuroinvasiveness of herpes simplex virus without affecting its ability to replicate. Proc. Natl. Acad. Sci. U. S. A. 96:8184–8189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wisner T. W., Johnson D. C. 2004. Redistribution of cellular and herpes simplex virus proteins from the trans-Golgi network to cell junctions without enveloped capsids. J. Virol. 78:11519–11535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wisner T. W., et al. 2009. Herpesvirus gB-induced fusion between the virion envelope and outer nuclear membrane during virus egress is regulated by the viral US3 kinase. J. Virol. 83:3115–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]