Abstract
The Epstein-Barr virus (EBV) BZLF1 gene encodes the immediate-early (IE) protein Zta, which plays a central role in regulating the switch between viral latency and lytic replication. A silencing element, ZIIR, is located between the ZID and ZII positive regulatory elements in the BZLF1 promoter Zp. We report here the phenotypes of variants of EBV strain B95.8 containing base substitution mutations in this ZIIR element. HEK293 cells infected with ZIIR mutant (ZIIRmt) virus produced at least 20-fold more viral IE Zta and Rta and early (E) EAD protein than did cells infected with the parental wild-type (WT) virus, leading to viral DNA replication and production of infectious virus. However, ZIIR mutant virus was 1/10 as efficient as WT virus in establishing proliferating B-cell clones following infection of human primary blood B cells. The ZIIRmt-infected lymphoblastoid cell lines (LCLs) that did grow out exhibited a phenotype similar to the one observed in 293 cells, including marked overproduction of IE and E gene products relative to WT-infected LCLs and lytic replication of the viral genome. Incubation of the ZIIRmt-infected LCLs with the chemical inducer 12-O-tetradecanoyl-phorbol-13-acetate (TPA) led to much greater activation of Zp than did the same treatment of WT- or ZVmt-infected LCLs. Furthermore, a protein kinase C (PKC) inhibitor, bis-indolylmaleimide, eliminated this activation by TPA. Thus, we conclude that ZIIR is a potent silencing element of Zp; it plays a key role in establishment and maintenance of EBV latency by inhibiting activation of Zp through the PKC signal transduction pathway.
INTRODUCTION
Epstein-Barr virus (EBV), a human gammaherpesvirus, infects approximately 90% of the world's population. It is a causative agent of infectious mononucleosis and is associated with several epithelial and B-cell cancers, including Burkitt's lymphoma (BL), Hodgkin's disease, nasopharygeal carcinoma, and some gastric carcinomas (reviewed in reference 41). EBV can infect primary B lymphocytes, establishing a latent form of infection in which its genome is maintained as an episome, replicating in synchrony with its host's cellular DNA. EBV can also infect epithelial cells, leading to a latent or lytic form of infection, depending upon the specific cell type and state of cellular differentiation (reviewed in reference 28).
The switch from EBV latency to lytic replication in B cells is usually initiated via activating expression of the immediate-early (IE) BZLF1 gene (11). The BZLF1 gene encodes a sequence-specific DNA-binding protein, Zta (also called Z, Zebra, and EB1), a member of the bZIP family of leucine-zipper transactivators. The activities of Zta include direct participation in EBV replication via binding to the viral DNA origin of lytic replication, ori-lyt, downregulating the latency-associated promoters Cp and Wp, and serving as a transcriptional transactivator of its own promoter, other IE and early (E) viral promoters, including the BRLF1 promoter, Rp, and several cellular promoters (reviewed in references 26 and 31). The BRLF1 gene encodes a second viral transactivator, Rta (also called R). Acting together, Zta and Rta play multiple roles in lytic replication of EBV (17). While highly quiescent during latency, transcription from the BZLF1 promoter Zp can be activated in some cells by incubation with various inducers, including phorbol esters such as 12-O-tetradecanoyl-phorbol-13-acetate (TPA), calcium ionophores such as ionomycin, histone deacatylase (HDAC) inhibitors such as valproic acid (VPA) and sodium butyrate, transforming growth factor β (TGF-β), and anti-immunoglobulins (anti-Igs) (reviewed in references 26 and 31).
The cis-acting elements of Zp sufficient for both basal and induced activity lie within the nucleotide (nt) −221 to +12 region (mini-Zp) relative to Zp's transcription initiation site (reviewed in references 26 and 45) (Fig. 1). Three positive regulatory cis-acting elements, named ZI, ZII, and ZIII, have been identified within mini-Zp. The ubiquitous transcription factors Sp1/Sp3 can bind to the AT-rich ZIA, ZIC, and ZID elements, while the myocyte enhancer factor 2D (MEF2D) can bind to the ZIA, ZIB, and ZID elements (6, 33, 34). ZII is a cyclic AMP response element (CRE)-like motif that binds CREB, ATF family members, C/EBPs, and the AP-1 family of transcription factors (5, 6, 19, 24, 46, 50, 53). Although the ZIA-ZID and ZII elements have distinct DNA sequences and are bound by different regulatory factors, they both function as sites through which induction can be mediated by chemical inducers such as TPA and ionomycin in reporter assays (20). These inducers activate Zp through signal transduction pathways: TPA through the protein kinase C (PKC) pathway (40) with Sp1/Sp3 bound to ZIA, ZIC, and ZID, and with AP-1 bound to ZII, and ionomycin through the Ca2+-dependent signal pathway (9) with MEF2D bound to ZIA, ZIB, and ZID and with ATF bound to ZII.
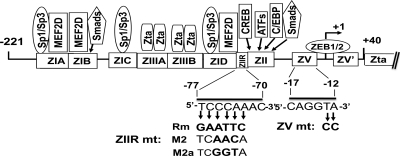
Fig. 1.
Schematic diagram indicating the cis-acting regulatory elements present within the nt −221 through +40 region of Zp relative to the transcription initiation site. Rectangles along Zp indicate approximate locations of known regulatory elements; their trans-acting factors are indicated above them. The nucleotide sequences of the ZIIR (−70 to −77) and ZV (−12 to −17) elements are shown below. The three substitution mutants in the ZIIR element correspond to the ones previously reported by Liu et al. (32). The 2-bp substitution mutant in the ZV element is the one previously reported by us (54).
Since the BZLF1 gene functions as the key switch between latent and lytic replication of EBV in most infected cell types, Zp needs to be tightly repressed to maintain latency. This silencing of expression is achieved by the presence of multiple negative regulatory elements. Three silencing elements identified within the mini-Zp region are ZIIR, HIε, and ZV/ZV′ (29, 30, 32, 42, 54). A phosphorylated form of MEF2D bound to ZIA, ZIB, and ZID can also repress Zp by recruiting HDACs to maintain chromatin in a repressed state (7). Other silencing elements of Zp, ZIV and HIα-HIδ, lie within the nt −551 to −222 region of the promoter (35, 36, 42, 48). However, they have not been extensively characterized, and their impact on Zp expression and establishment and maintenance of EBV latency remains to be determined in the context of an intact EBV genome.
Our laboratory has identified and characterized the cis-acting silencing elements ZV and ZV′, which are located between nt −12 and −17 and between nt +5 and +10, respectively, relative to the transcription initiation site of Zp (29, 30, 54; X. Yu, P. J. McCarthy, D. A. Gorlen, Z. Wang, and J. E. Mertz, unpublished data). These two elements act to enable synergistic binding of the cellular ZEB1 and ZEB2 proteins via their two zinc-finger domains (Yu et al., unpublished data). Human embryonic kidney (HEK) 293 cells infected with a variant of the B95.8 strain of EBV containing a 2-bp substitution mutation in the ZV element spontaneously reactivate into lytic replication with production of infectious virus (54). Recently, our laboratory showed that either ZEB1 or ZEB2 can play a central role in the maintenance of EBV latency, doing so in a cell-type-dependent manner (15). We also showed that addition of the cellular microRNAs (miRNAs) 200b and 429 can induce EBV lytic replication in EBV-positive cells by downregulating expression of ZEB1 and ZEB2 (16).
Liu et al. (32) identified a potent silencing element, ZIIR, located between nt −70 and −77 relative to the transcription initiation site of Zp, juxtaposed between the known positive regulatory elements ZID and ZII (Fig. 1). Using reporter assays, they showed that mutation of the ZIIR element led to increased Zp basal activity and induction by TPA and ionomycin. They also found that the ZIIR element failed to repress transcription from a heterologous promoter. Efforts by those authors to identify the ZIIR-binding protein were unsuccessful, likely due to the very close proximity of the ZID and ZII elements, which bind numerous cellular proteins.
We report here the isolation and characterization of several different variants of EBV strain B95.8 harboring base pair substitution mutations in the ZIIR element of Zp. All of these mutant EBVs were similarly defective in establishing proliferating B-cell colonies and latently infected 293 cells. The EBV-infected cells that did manage to grow spontaneously reactivated into lytic replication, doing so at a very high frequency in the presence of TPA. We conclude that binding to the ZIIR element of an as-yet-unknown cellular factor, tentatively named here ZIIR-BP, is a major contributor to the establishment and maintenance of EBV latency; the binding of this factor silences BZLF1 gene expression in part by inhibiting activation of Zp through the PKC signaling pathway.
MATERIALS AND METHODS
Cells and plasmids.
293-D, a subclone of the HEK293 cell line, was obtained from Wolfgang Hammerschmidt (13). Raji, an EBV-positive human BL cell line, and DG-75, an EBV-negative human BL cell line, were obtained from Bill Sugden. These cell lines and LCLs latently infected with EBV were maintained at 37°C in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS). The 293-D cell lines latently infected with EBV were maintained in the same medium additionally supplemented with hygromycin (100 μg/ml).
Plasmid pCMV-BZLF1 (23), encoding Zta protein, was obtained from Bill Sugden. Plasmid p2089 (13), a bacmid containing the complete genome of EBV strain B95.8, and plasmid p2670 (38), encoding EBV glycoprotein gp110, were obtained from W. Hammerschmidt. The Escherichia coli strains and plasmids used for mutagenesis of p2089 were provided by Samuel Speck. Plasmids containing the XhoI and EcoRI subfragments of EBV that correspond to the EBV sequences present at the termini of replicated linear viral genomes were provided by Nancy Raab-Traub (39).
Mutagenesis of p2089.
Base pair substitution mutations were introduced into the ZIIR element of Zp in p2089 by allelic exchange in E. coli as described by Smith and Enquist (43) and Moorman et al. (37). In brief, substitution mutations were incorporated into the ZIIR element by a two-step, PCR-based site-directed mutagenesis. A 1,100-bp EBV DNA fragment containing the mutated ZIIR element near its center was cloned into the donor plasmid, pGS284 (37). The ZIIR mutations were then recombined with the acceptor plasmid, p2089, through homologous recombination, following the conjugation of two E. coli strains harboring these two plasmids. The mutant variants of p2089 containing the ZIIR mutations were identified by a PCR-based screen (47). Presence of the desired mutations in p2089 was confirmed by DNA sequence analysis. A wild-type (WT) revertant of p2089-ZIIRmt(Rm clone 1), named p2089-ZIIRmtRev, was likewise constructed by mutagenesis of p2089-ZIIRmt.
Isolation of WT- and ZIIRmt-infected 293 cells.
The p2089-WT, p2089-ZIIRmts, and p2089ZIIRmtRev DNAs were purified by equilibrium centrifugation in CsCl-ethidium bromide gradients, introduced into 293-D cells by use of Lipofectamine 2000 (Invitrogen), and selected by incubation in the presence of 100 μg/ml hygromycin as previously described (54). Individual colonies of cells were grown and checked for (i) their ability to produce high titers of infectious virus following addition of a Zta expression plasmid, and (ii) lack of EBV genome alterations as assayed by restriction fragment patterns. Only cell lines with these properties were retained for further analysis.
Immunoblot analysis.
Viral Zta, Rta, and EAD protein levels were quantified by immunoblot analysis as previously described (54). The antibodies used were as follows: anti-Zta (Santa Cruz Biotechnology sc-53904; 1:250 dilution), anti-Rta (Argene 11-008; 1:250 dilution), anti-EAD (Vector Laboratories VP-E608; 1:250 dilution), anti-α-tubulin (Sigma T9026; 1:5,000 dilution), and anti-β-actin (Sigma A5441; 1:5,000 dilution).
IFS.
Cells were fixed and stained as previously described (54). EBV-infected 293 cells were quantified by visualization of green fluorescent protein (GFP) encoded by the bacmid. EBV-infected LCLs were quantified by visualization of nuclei after embedding in mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI). The commercial antibodies used for immunofluorescence staining (IFS) were those described above (1:30 dilution). The anti-gp350 antibody was obtained from Millipore (ZL10), and the anti-EBNA1 antibody was provided by Richard Burgess (14).
Detection of the DNA/ZIIR-BP complex.
Competition electrophoretic mobility shift assays (EMSAs) were performed essentially as previously described (30). In brief, nuclear extracts of DG-75 cells were prepared (29). The proteins were fractionated by passage through a heparin-Sepharose column. The fractions containing the peak of ZIIR-BP DNA-binding activity were pooled for use as the protein source along with radiolabeled 2× ZIIRWT DNA as probe and unlabeled 2× ZIIRWT or 2× ZIIRmt DNA as competitor.
UV cross-linking was performed as described by Chodosh (10) with some modifications. In brief, 8 μg of protein from the ZIIR-BP-enriched preparation above was mixed with 5 μg of poly(dI)n·(dC)n in gel shift buffer (10 mM HEPES [pH 7.9], 2.5 mM MgCl2, 1 mM dithiothreitol, 0.05 mM EDTA, 100 mM KCl, 2% Ficoll) in the absence or presence of 2 μg of the indicated competitor oligonucleotide. Uniformly 32P-labeled, BrdU-substituted 2× ZIIRWT probe was added to the reaction mixture. Protein-DNA cross-linking was induced by UV irradiation twice for 10 min at 2,400 kJ (UV Stratalinker 1800; Stratagene). The cross-linked adducts were digested by incubation at 37°C for 30 min with 58 units of OmniCleave endonuclease (Epicentre Biotechnologies), resolved by SDS-PAGE (150 V for 1.5 h) in a Novex Tris-glycine-gradient polyacrylamide gel (8% to 16% gradient), and detected by autoradiography. The double-stranded oligonucleotides used in the EMSAs and UV cross-linking were as follows: 2× ZIIRWT, 5′-GGGCGTCCCAAACCAGCTCGTCCCAAACCAGGG-3′; 2× ZIIRmt, 5′-GGGCTGAAACCCAGAGCTCTGAAACCCAGAGGG-3′ (italicized bases indicate the altered nucleotides).
Assays for replicated viral DNA and infectious virus.
The presence of circular and linear viral DNA in cells was determined in an EBV termini assay as previously described (39, 54). The production of infectious virus was quantified by a Raji assay (1). When the virus titer was low, the medium was concentrated by centrifugation at 22,000 rpm for 12 h at 8°C in a Beckman 75Ti rotor and resuspension in a smaller volume before infection of the Raji cells.
Human primary B-cell proliferation assay.
To make virus stocks, 293-D cells latently infected with WT-, ZIIRmt-, ZIIRmtRev-, ZVmt-, and ZVmtRev-EBV were cotransfected with Zta and gp110 expression plasmids to reactivate the virus into lytic replication and then processed as previously described (54). The virus in the culture medium was concentrated as described above and resuspended in fresh medium. The virus titers were determined using a Raji assay (1). Fresh human primary B cells from peripheral blood of healthy donors were purchased from AllCells (Emeryville, CA). The B cells were infected the day they arrived by incubation at 4°C for 2 h in parallel with WT-, ZIIRmt-, ZIIRmtRev-, ZVmt-, or ZVmtRev-EBV virus at a multiplicity of infection (MOI) equivalent to 5, 10, and 25 green Raji units (GRUs) of virus per 104 B cells and then aliquoted at 104 cells per well in 96-well plates. After incubation at 37°C for 6 weeks in RPMI 1640 medium supplemented with 10% FBS, the percentage of the wells with proliferating GFP-positive B cells was determined. The efficiency of proliferation of B cells infected with each mutant EBV relative to B cells infected in parallel with the parental WT EBV was determined by assuming a Poisson distribution for the probability that a clone arose from infection with more than 1 CFU of virus. Clones of proliferating B cells obtained from infection with the lower MOI were picked and grown at 37°C for 3 to 6 months to establish LCLs.
RESULTS
Phenotype of 293-ZIIRmt cell lines.
The ZIIR element of Zp was originally identified and characterized in transient-transfection assays with reporter plasmids (32). To determine whether ZIIR is actually a strong silencing element of Zp in the context of a whole EBV genome, we introduced into p2089, a bacmid which contains the complete B95.8 strain of EBV (13), three different sets of cluster base pair substitution mutations within the ZIIR element (Fig. 1). We constructed in E. coli three independent isolates of the Rm mutant variant and two independent isolates each of the M2 and M2a mutant variants. These bacmids were transfected into 293-D cells, and clones of latently infected cells were grown up into cell lines.
The bacmid in each cell line was thoroughly checked for extraneous mutations. First, the nt −600 to +500 region of the BZLF1 gene relative to its transcription initiation site was PCR amplified and sequenced; only the expected substitution mutations within the ZIIR element were observed. Second, the p2089 DNAs rescued from these cell lines were separately digested with EcoRI, BamHI, SalI, and HindIII restriction endonucleases; no differences were observed in any of the restriction fragment patterns compared to those of the parental p2089 DNA, except for the Rm mutation in the ZIIR element, which introduced a new EcoRI site. Third, each cell line produced infectious virus with titers ranging from 104 to 105 GRUs per ml of medium following induction by addition of a Zta expression plasmid. In addition, we constructed a wild-type revertant, named p2089-ZIIRmtRev, by using p2089-ZIIR (Rm clone 1) DNA as template for the mutagenesis; all 293 cell lines latently infected with it had the same phenotype as WT EBV (data not shown, but see Fig. 3A below). Thus, we conclude that the only significant difference between the WT and these mutant variants probably lies within the ZIIR element of Zp.
Fig. 3.
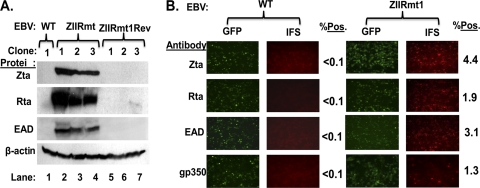
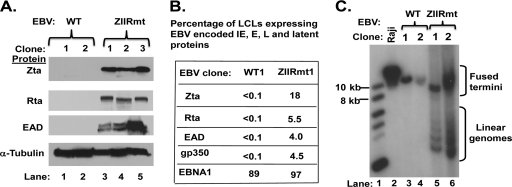
Spontaneous reactivation of EBV into lytic replication in 293-D cells latently infected with ZIIRmt, but not WT virus. (A) Immunoblots showing relative levels of EBV Zta, Rta, and EAD proteins present in 293-WT, 293-ZIIRmt(Rm), and 293-ZIIRmtRev cell lines. The cellular protein β-actin present in the same samples served as a loading control. (B) IFS of 293-WT1 and 293-ZIIRmt(Rm clone1) cells for the presence of Zta, Rta, EAD, and gp350 proteins. The primary antibodies used are indicated to the left of each row of images. Fields of cells were photographed with different filters to show the total EBV-positive ones (GFP) versus the subset of them containing the indicated EBV-encoded protein (IFS). The percentages of IFS-positive cells are indicated to the right of each field; these values were obtained by scoring 10 fields of cells.
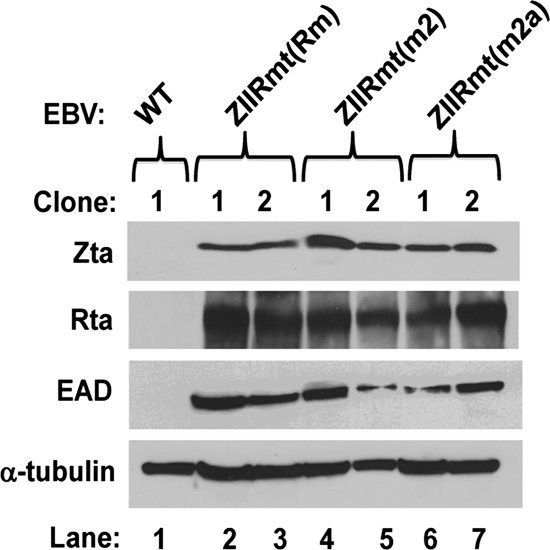
To determine the degree of latency of 293-D cells infected with the ZIIR mutants Rm, M2, and M2a, whole-cell extracts were prepared from cells infected with two completely independent isolates of each of these three ZIIR mutant variants of p2089, along with 293-D cells infected with the parental WT p2089 plasmid DNA. Immunoblot analysis indicated that each of the 293 cell lines latently infected with a ZIIR mutant virus produced at least 20-fold more IE Zta and Rta and E EAD protein than did the 293 WT-infected cells (Fig. 2, lanes 2 to 7 versus lane 1). Thus, the three different ZIIR mutations similarly abrogated ZIIR's function as a silencing element, leading to strong derepression of BZLF1 gene expression. The fact that three different cluster mutations in the ZIIR element exhibited a similar phenotype makes it extremely unlikely that these mutations fortuitously generated new binding sites in Zp for transcriptional activators. Given that their phenotypes were quite similar, we chose to work primarily with the Rm ZIIR mutant. It is referred to here simply as ZIIRmt. Thus, we conclude that substitution mutations in the ZIIR element disrupt its function as a silencer of Zp.
Fig. 2.
293-D cells latently infected with ZIIR mutants spontaneously synthesize IE and E lytic EBV proteins. Immunoblotting analyses were performed as described in Materials and Methods with whole-cell extracts prepared from independent clones of cells latently infected with the indicated wild-type and mutant variants of p2089. The cellular protein α-tubulin served as a loading control.
Spontaneous reactivation of ZIIRmt EBV in 293 cells.
Three independent clones of 293-ZIIRmt and its revertant, 293-ZIIRmtRev, were chosen to analyze in detail. First, immunoblot analysis was performed with whole-cell extracts prepared from them. Each of the 293-ZIIRmt cell lines contained at least 20-fold more Zta, Rta, and EAD protein than did either the 293-WT or 293-ZIIRmtRev cell lines (Fig. 3A, lanes 2 to 4 versus lane 1and lanes 5 to 7, respectively). This finding confirmed that the high-level expression of EBV lytic genes in the 293-ZIIRmt cell lines is, indeed, a consequence of the substitution mutation that we constructed into the ZIIR element of Zp rather than an unknown second-site mutation(s) elsewhere in the viral genome that may have arisen during the mutagenesis and selection in the 293 cells.
We also performed IFS assays with the 293-WT and 293-ZIIRmt cell lines by using antisera specific for the EBV IE Zta and Rta proteins, E EAD, and late (L) gp350 protein. All four of these EBV-encoded proteins were readily detected in all three of the 293-ZIIRmt cell lines, but not in the 293-WT and 293-ZIIRmtRev cell lines (Fig. 3B and data not shown). The lower level of gp350-positive cells observed than of Zta- or EAD-positive ones was likely due to dead and dying cells being lost during processing of the cells for the assay. Thus, we conclude that the lytic gene expression levels observed in the 293-ZIIRmt cell lines are due to their spontaneously reactivating out of latency into lytic replication at a rate of 1% to 2% per day, with most, if not all, of the Zta-positive cells progressing through the early and late stages of the viral lytic cycle.
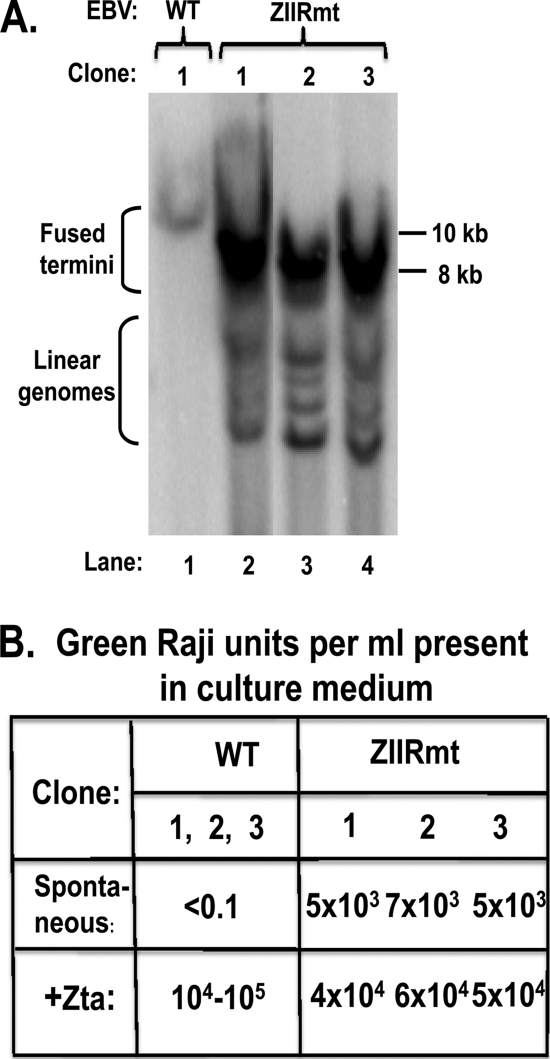
To demonstrate directly that expression of the EBV lytic IE, E, and L proteins led to replication of the EBV genome, we examined by Southern blotting the structures of the EBV genomes present in 293-ZIIRmt cells. When EBV exists in a latent state as a circular episome, the ends of its linear viral genome are fused together; cleavage with BamHI restriction endonuclease generates large terminal fragments of EBV DNA that are heterogeneous in size due to variability in the number of copies of the tandem repeat sequence present in this region of the EBV genome (39). However, in lytically infected cells, in which the EBV genome has been linearized during packaging into virion particles, cleavage with BamHI generates smaller terminal fragments of EBV DNA, which are also heterogeneous in size because of the tandem repeat sequence. As expected, the EBV DNA terminal BamHI fragments isolated from each of the 293-ZIIRmt(Rm) cell lines had sizes consistent with the presence of both circular and linear EBV genomes, while the DNA from the 293-WT cell line had sizes consistent with the presence of only circular EBV (Fig. 4A, lanes 2 to 4 versus lane 1, respectively). Thus, we conclude that viral genomes are being replicated in the 293-ZIIRmt(Rm) cell lines.
Fig. 4.
293-ZIIRmt(Rm) cell lines spontaneously produce infectious virus. (A) EBV termini assay results for the presence of linear and circular episomal EBV genomes in 293-WT and 293ZIIRmt(Rm) cell lines. The extraction, digestion, and Southern blot analysis of viral DNA were performed as previously described (39). The size markers indicated on the right are based on a DNA ladder run in the same gel. The positions of circular EBV genomes with fused termini and linear EBV genomes are indicated on the left. (B) Green Raji units/ml of infectious EBV spontaneously present in the culture medium after incubation for 3 days without or following transfection with a Zta expression plasmid. The GRU/ml values were determined as previously described (1).
We also documented by using a Raji assay the spontaneous production of infectious virus by the three 293-ZIIRmt cell lines (Fig. 4B). As expected, virus was only produced by the 293-WT and 293-ZIIRmtRev cell lines following transfection with an expression plasmid encoding Zta (Fig. 4B and data not shown). Thus, we conclude that the presence of the Rm 6-bp substitution mutation in the Zp ZIIR element leads not only to derepression of Zp but also to the entire subsequent series of events in the EBV lytic replication cycle necessary for production of infectious virus. Furthermore, the level of spontaneous virus production observed is an order of magnitude higher than the level previously reported for 293-ZVmt cell lines, in which the mutation was in the ZEB-binding ZV element of Zp (54). Therefore, we conclude that the ZIIR element plays a major role in maintaining latent infection by EBV in 293 cells.
Transformation of primary B cells by ZIIRmt EBV.
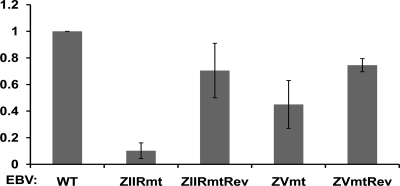
To examine the phenotype of the ZIIR mutant variant of EBV in a physiologically relevant cell type, we infected human primary blood B cells in parallel with stocks (with titers determined) of WT, ZIIRmt(Rm), ZIIRmtRev, ZVmt, or ZVmtRev virus prepared from 293 cells. The infected cells were scored for GFP-positive, proliferating B cell colonies 6 weeks after infection. Strikingly, cells infected with the ZIIRmt virus grew into colonies of proliferating B cells with an efficiency that was an order of magnitude lower than for cells infected in parallel with either the parental WT or revertant viruses (Fig. 5). Furthermore, the resulting ZIIRmt EBV-transformed B-cell lines grew out at one-third to one-fourth the rate that the WT and ZIIRmtRev EBV-transformed B-cell lines did (data not shown). By comparison, ZVmt virus was intermediate between WT and ZIIRmt in its ability to form proliferating B-cell colonies (Fig. 5). Thus, we conclude that ZIIRmt virus is highly defective in immortalizing human primary blood B cells.
Fig. 5.
ZIIRmt EBV is defective in inducing proliferation of human primary B lymphocytes. Shown here are the efficiencies relative to WT virus with means ± standard errors of the means of data obtained from three sets of infections of WT, ZIIRmt(Rm), and ZVmt virus and two sets of infections of ZIIRmtRev and ZVmtRev virus, performed in parallel on different days and starting with B cells obtained from three different donors.
Phenotype of ZIIRmt-infected LCLs.
We established multiple independent LCLs derived from the WT- and ZIIRmt-infected primary B cells. The EBV genomes present in these LCLs were rigorously checked to confirm that their entire Zp regions were identical to the infecting WT or ZIIRmt virus. Whole-cell RNA was extracted from these LCL-WT and LCL-ZIIRmt lines, reverse transcribed, and quantified by real-time quantitative PCR for expression of viral lytic genes. The LCL-ZIIRmt lines contained at least 5- to 10-fold more viral BZLF1, BRLF1, BMRF1, and BLLF1 RNA than did the LCL-WT lines (data not shown). Immunoblot analysis of the proteins present in whole-cell extracts made from the cell lines confirmed these findings: Zta, Rta, and EAD proteins were readily observed in all three of the ZIIRmt-infected LCLs, but not at detectable levels in the WT-infected LCLs (Fig. 6A). Again, the phenotype of the ZVmt-infected LCLs was intermediate between these two extremes (see Fig. 7A, below, lane 3 versus lanes 1 and 5). These findings are consistent with the ZIIR mutation leading to derepression of BZLF1 gene expression and, subsequently, spontaneous reactivation of the virus into its lytic replication cycle with lytic gene expression.
Fig. 6.
High-level spontaneous reactivation of EBV into lytic replication in LCL-ZIIRmt but not LCL-WT lines. (A) Immunoblots showing relative levels of EBV Zta, Rta, and EAD proteins present in LCLs infected with WT and ZIIRmt virus. The cellular protein α-tubulin present in the same samples served as a loading control. (B) Summary of IFS results with LCL-WT1 and LCL-ZIIRmt(Rm1) cells for the presence of Zta, Rta, EAD, gp350, and EBNA1 protein. Assays were performed as described in the legend for Fig. 3B. (C) EBV termini assay results for the presence of linear and circular EBV genomes in Raji, LCL-WT, and LCL-ZIIRmt lines. The size markers indicated on the left are based on a DNA ladder run in the same gel. The positions of circular EBV genomes with fused termini and linear EBV genomes are indicated on the right. Raji cells, known to be defective in EBV lytic replication, served as a negative control.
Fig. 7.
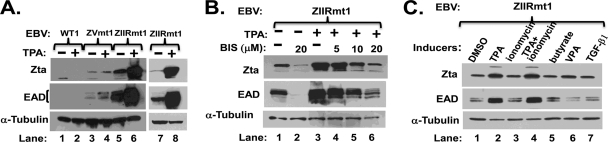
The ZIIR element affects TPA-induced activation of BZLF1 gene expression. (A) Immunoblots showing the basal and TPA-induced levels of Zta and EAD proteins in LCL-WT1, LCL-ZVmt1, and LCL-ZIIRmt(Rm1) lines (lanes 1 to 6). LCLs in logarithmic-phase growth were incubated with TPA (20 ng/ml) for 48 h before harvesting whole-cell extracts for immunoblot analyses. Lanes 7 and 8 are from a different gel with lighter exposure, using the same lysates shown in lanes 5 and 6, respectively. The cellular protein α-tubulin present in the same samples served as a loading control. (B) Immunoblots showing effects of the PKC inhibitor BIS on levels of Zta and EAD proteins in LCL-ZIIRmt(Rm1) cells. LCLs in logarithmic-phase growth were incubated with 20 ng/ml TPA alone or in combination with the indicated amount of BIS. (C) Immunoblots showing effects of TPA, ionomycin, HDAC inhibitors, and TGF-β1 on levels of Zta and EAD proteins in LCL-ZIIRmt(Rm1) cells. Concentrations of the chemicals were as follows: ionomycin, 1 μM; sodium butyrate, 3 mM; VPA, 1 mM; TGF-β1, 100 pM.
Immunofluorescence staining assays confirmed the abundant presence of Zta, Rta, EAD, and gp350 protein in the LCL-ZIIRmt lines (Fig. 6B and data not shown). An astonishing 18% of the LCL-ZIIRmt(Rm1) cells stained positive for Zta. This finding provides a likely explanation for why the ZIIR mutant-infected LCLs grew out much more slowly than did the WT-infected ones, i.e., they had a high rate of cell death due to a high rate of lytic reactivation of the virus. In contrast, none of these EBV lytic proteins was detectable in the LCL-WT lines. To rule out the possibility that the LCL-WT cells had lost their EBV genome, we also performed IFS assays for the EBV latent protein EBNA1, a protein known to be expressed in both latent and chronically lytic LCLs (51). As expected, EBNA1 was present in most LCL-WT and LCL-ZIIRmt cells. Therefore, the abundant expression of EBV IE, E, and L genes in the ZIIR mutant-infected LCLs is due to the 6-bp substitution mutation introduced into the ZIIR element of Zp.
To show that expression of EBV lytic genes led to replication of the viral genome in the LCL-ZIIRmt lines, we performed termini assays with viral DNA isolated from these lines. As observed with the 293 cell lines, replicated EBV genomes were present in the ZIIRmt- but not in the WT-infected LCLs (Fig. 6C, lanes 5 and 6 versus lanes 3 and 4). Thus, we conclude that the ZIIR element plays a crucial role in maintaining EBV latency in LCLs through silencing BZLF1 gene expression.
Effect of ZIIR mutation on EBV induction by TPA.
The ability of the ZIIR element to function as a potent silencing element has been reported to be sensitive to context, with its activity significantly diminished when either of the adjacent ZID or ZII activator elements is mutated (32). Taken together with the findings presented here, this observation suggests that the functions of Zp's ZID, ZIIR, and ZII elements are interrelated. We hypothesize that an as-yet-unknown cellular factor(s) binds the ZIIR element, repressing transcription from Zp in part by suppressing its activation, synergistically mediated through factors that bind the ZID and ZII elements.
To begin to test this possibility, we examined the effects of TPA on EBV lytic induction in LCL-WT, LCL-ZIIRmt, and LCL-ZVmt lines. We included the latter as a control since it, too, spontaneously produces Zta and EAD proteins (Fig. 7A, lane 3), but its mutated silencing element lies approximately 40 bp 3′ of the ZII element (Fig. 1). Noteworthy is the fact that the spontaneous levels of the EBV lytic proteins in the LCL-ZVmt lines were only one-third to one-fifth of the levels observed in multiple, independently isolated LCL-ZIIRmt lines (Fig. 7A, lane 3 versus lane 5, and data not shown). Thus, ZIIR is a more potent silencing element of Zp than is the ZV element in both LCLs and 293-D cells.
Incubation of the LCL-WT lines with TPA for 2 days did not lead to synthesis of detectable levels of Zta or EAD protein (Fig. 7A, lane 2 versus lane 1, and data not shown). On the other hand, incubation of ZIIRmt-infected LCLs with TPA led to a 5-fold increase in Zta and EAD protein levels compared to the levels spontaneously produced in these cells (Fig. 7A, lane 8 versus lane 7). Interestingly, incubation of ZVmt-infected LCLs with TPA induced production of Zta and EAD proteins at most 2-fold above the levels spontaneously observed in these cells (Fig. 7, lane 4 versus lane 3).
TPA-induced alteration of gene expression in mammalian cells largely occurs through activation of the PKC cellular signal transduction pathway (3). To confirm that the dramatic increase by TPA in BZLF1 and BMRF1 gene expression in the LCL-ZIIRmt line was due to activation of Zp through the PKC pathway, we also incubated these cells with bisindolymaleimide (BIS), a potent and selective inhibitor of most isoforms of PKC (49). Concurrent incubation of LCL-ZIIRmt(Rm) line 1 with BIS led to at least a 5-fold reduction in the levels of Zta and EAD proteins present in these cells, back to or even significantly below the levels observed in the uninduced cells (Fig. 7B, lanes 1 to 6). These observations held for multiple independently isolated LCL-ZIIRmt lines (data not shown).
To examine whether other known EBV lytic inducers could likewise enhance Zp activity in these LCL-ZIIRmt lines, we also incubated them with ionomycin, sodium butyrate, VPA, or TGF-β1. None of these other inducers by themselves significantly affected Zp activity in the ZIIRmt-infected LCLs (Fig. 7C, lanes 3 and lanes 5 to 7, and data not shown), even though they could reactivate EBV in some other cell lines (e.g., TGF-β1 with MutuI cells [T. Iempridee, S. Das. I. Xu, and J. E. Mertz, submitted for publication]). Incubation with ionomycin failed to enhance the effect of TPA (Fig. 7C, lane 4 versus lane 2), suggesting that these two inducers do not synergistically activate transcription from Zp in LCL-ZIIRmt cells. On the other hand, treatment of LCL-ZIIRmt cells with sodium butyrate or VPA in combination with TPA had a synergistic effect on Zp activation (data not shown). Thus, we conclude that TPA treatment specifically enhances BZLF1 gene expression in the ZIIR mutant, with both this mutant's basal and TPA-stimulated activation of Zp being mediated through the PKC signaling pathway.
A cellular factor(s) sequence specifically binds the ZIIR element.
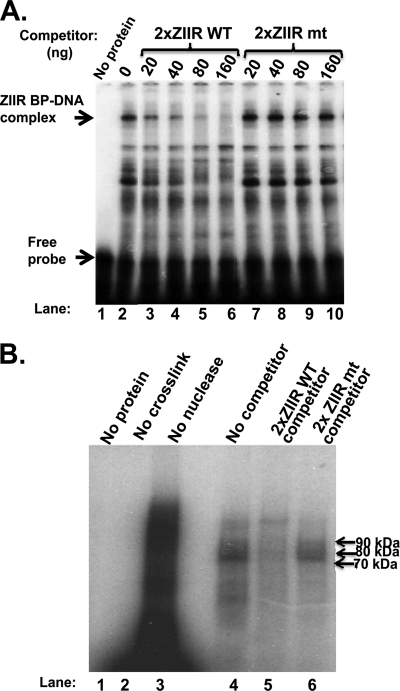
To test whether the ZIIR element is the binding site for a cellular protein(s), we performed competition EMSAs using as probe DNA a 33-bp radiolabeled oligonucleotide that contains two tandem copies of the sequence corresponding to the ZIIR element but little of the sequences corresponding to the adjacent ZID and ZII elements of Zp. We employed this probe (i) to minimize the severe problem previously reported (32) of binding of numerous factors to the ZID and ZII elements when probes corresponding to the native Zp sequence are used, and (ii) to enhance the interaction with probe DNA of the ZIIR-binding protein, here named ZIIR-BP. Using as the protein source a heparin-Sepharose fraction of a nuclear extract prepared from DG-75 cells, we readily observed a protein-DNA complex that was competed away by an oligonucleotide with the same sequence as the 2× ZIIRWT probe DNA, but not by a variant of it containing base pair substitution mutations in the two ZIIR elements (Fig. 8A, lanes 2 to 6 versus lanes 7 to 10). Preincubation of the protein extract with an antibody specific to ZEB1 had no effect on either the amount or location of this ZIIR-BP/DNA complex (data not shown). A band with this mobility was also observed when the protein source for the EMSA was a nuclear extract made from 721 LCLs established by infection of human primary B cells with the B95.8 strain of EBV, 293 cells, or mammary carcinoma C33A cells, but not from gastric cancer AGS cells or mammary carcinoma MCF-7 cells (data not shown).
Fig. 8.
A cellular factor(s) sequence specifically binds the ZIIR element of Zp. (A) Autoradiogram of competition EMSAs showing sequence-specific binding to the ZIIR element. The location of the ZIIR-BP/DNA complex and free probe are indicated by arrows. (B) Autoradiogram of the UV cross-linking experiment to estimate the size(s) of ZIIR-BP. Lanes 1 through 3 are controls with no protein added, no UV cross-linking, and no endonuclease digestion after cross-linking. Sequences of the radiolabeled probe DNAs and unlabeled 2× ZIIRWT and 2× ZIIRmt competitor DNAs are provided in Materials and Methods.
To estimate the size of ZIIR-BP, we performed a UV cross-linking experiment with the same protein source and uniformly radiolabeled, bromodeoxyuridine (BrdU)-substituted 2× ZIIRWT probe DNA. Cellular proteins migrating by SDS-PAGE in the 70- to 90-kDa size range bound the 2× ZIIRWT probe (Fig. 8B, lane 4). This binding could be competed away with unlabeled 2× ZIIRWT DNA, but not with the 2× ZIIRmt DNA (Fig. 8B, lanes 5 and 6, respectively). Thus, a protein(s) of cellular origin sequence specifically binds to the ZIIR element. While the identity of ZIIR-BP remains to be determined, it is the wrong size(s) for ZEB1 or ZEB2, proteins that migrate in SDS-PAGE at mobilities of approximately 200 to 250 kDa and 140 kDa, respectively. It is also not MEF2D, given that we omitted the nearby MEF2D-binding site from our DNA probe, which lacks a stretch of six A/T base pairs, a stringent requirement for binding by MEF2D.
DISCUSSION
We report here the phenotype of whole EBV genomes containing substitution mutations in the ZIIR element in Zp. First, we showed that 293-D cells latently infected with ZIIR mutant viruses spontaneously synthesized EBV lytic IE, E, and L proteins (Fig. 2 and 3), replicated their DNA genome (Fig. 4A), and released infectious virus into the medium (Fig. 4B) under culture conditions where WT-infected cells were highly latent. Importantly, we also found that ZIIR mutant virus was highly defective in transforming human primary blood B cells from a quiescent state into a proliferating one (Fig. 5). The ZIIR mutant-infected LCLs that appeared grew more slowly than the WT-infected LCLs, spontaneously synthesized EBV lytic proteins, and replicated their viral DNA genomes (Fig. 6). Incubation with TPA led to efficient EBV reactivation in these LCL-ZIIRmt lines, but not in the LCL-WT and LCL-ZVmt lines (Fig. 7A). Treatment of the LCL-ZIIRmt lines with the PKC inhibitor BIS led to dramatic reductions in both basal and TPA-induced reactivation (Fig. 7B). Other known chemical inducers of EBV did not exhibit this effect (Fig. 7C). Thus, we conclude that ZIIR is a potent silencing element in both 293-D and LCLs, playing a key role in establishing and maintaining EBV latency. Given that the ZIIR element binds one or more cellular factors (Fig. 8), it likely represses BZLF1 gene expression by binding a cellular factor, ZIIR-BP, that suppresses the synergistic activation of Zp through PKC signaling pathways that affect the activities of cellular proteins that bind the ZID and ZII elements.
The ZIIR element plays a crucial role in maintaining EBV latency.
While the ZIIR mutant EBVs initially established latency in 293-D cells at frequencies similar to WT EBV (data not shown), all three of the variants studied here, Rm, M2, and M2a, were defective in maintaining this latency and exhibited a similar phenotype. They spontaneously synthesized Zta protein at levels sufficient to reactivate the virus into lytic replication, with synthesis of Rta, EAD, and gp350 proteins, linear viral genomes, and infectious virus (Fig. 2 to 4 and data not shown). Strikingly, the 293-ZIIRmt cell lines spontaneously produced infectious virus with titers of approximately 5 × 103 GRU/ml of medium, only 1 order of magnitude lower than the titers of WT or ZIIRmt virus produced following exogenous addition of Zta protein (Fig. 4B).
The ZIIR element was also found to be crucial for maintaining EBV latency in B cells. The basal level of BZLF1 gene expression in the two independently isolated LCL-WT lines that we characterized in detail was extremely low; spontaneous EBV lytic reactivation was undetectable. On the other hand, all of the LCL-ZIIRmt lines we characterized spontaneously synthesized significant levels of Zta, Rta, EAD, and gp350 proteins, based on both immunoblot analysis and IFS, leading to viral DNA replication (Fig. 6). The LCL-ZIIRmt lines grew at a much slower doubling rate than the WT-infected LCLs made in parallel from the same donor cells (data not shown), probably due to their high rate of spontaneous reactivation into lytic replication leading to a high rate of cell death. Thus, the ZIIR element plays a key role in EBV latency in both 293 and B cells.
ZIIRmt EBV is defective in transforming human primary B lymphocytes.
EBV is known to infect naïve B cells, transforming them from a quiescent to active proliferating state. Some EBV latent genes, including EBNA1, EBNA2, LMP1, and LMP2A, are known to be essential for B-cell transformation by EBV (2, 8, 44, 55). Mutations in these genes can severely affect EBV's ability to transform B cells. Here, we report for the first time a mutation in EBV's Zp that can reduce by an order of magnitude EBV-induced proliferation of primary blood B cells. The ZIIRmt virus was even more defective than ZVmt virus in transforming B cells (Fig. 5). This finding is consistent with ZIIRmt virus also spontaneously reactivating at a higher frequency than ZVmt virus in LCLs (Fig. 7A, lane 5 versus lane 3, respectively) and the LCL-ZIIRmt lines doubling more slowly than the LCL-ZVmt ones (data not shown).
The BZLF1 gene of EBV is transiently expressed following infection of B cells but is normally turned off thereafter to enable establishment of latency (27, 52). We speculate that when B cells are infected with ZIIRmt virus, continued synthesis of Zta protein from the derepressed BZLF1 gene, in combination with increased BRLF1 gene expression activated by Zta, triggers the cascade of E and L gene expression events and viral replication that leads to cell death. The ZIIRmt-infected B cells that escape death are likely ones that somehow manage to sufficiently silence BZLF1 gene expression through other repressors of Zp (e.g., MEF-2D, ZEBs, YY1) or reduce the impact of the presence of Zta protein by expressing cellular factors, such as STAT3, which make the cells refractory to lytic induction (12).
Function of the ZIIR element in regulating BZLF1 gene expression.
Phorbol esters such as TPA are potent tumor promoters that induce alteration in cellular gene expression largely through activation of the PKC signal transduction pathway (3). TPA is also known to induce EBV lytic replication through activation of Zp (57), induction of phosphorylation of Zta (4), and inhibition of inducible nitric oxide synthase, leading to reduction in the level of nitric oxide, an inhibitor of EBV reactivation (21). Some EBV-infected cell lines, such as B95.8, an LCL established from a marmoset, can be induced to enter the lytic cycle and make infectious virus when treated with TPA (25). Raji cells can also be moderately induced to synthesize Zta when treated with TPA (22). However, none of the 293-WT or LCL-WT cell lines we established in parallel with the ZIIRmt-infected ones was inducible with either TPA alone or TPA in combination with other inducers, such as ionomycin or sodium butyrate (data not shown). Our finding that TPA, but not the other lytic inducers we tested, could reactivate ZIIRmt virus in the LCL-ZIIRmt lines suggests that the PKC pathway can play a key role in EBV reactivation from latency.
TPA has been shown to activate Zp via its ZI and ZII elements (20). By using artificial reporter constructs in transient-transfection assays, Liu et al. (32) studied the effects of ZIIR mutations on transcription from Zp. They found that functional ZID and ZII motifs were necessary for mutation of the ZIIR element to affect Zp activity. Liu et al. (32) proposed that the ZIIR element may be involved in modulating the synergy between MEF2D bound to ZID and transcriptional activators such as ATFs bound to ZII. Our data presented here in the context of a whole EBV genome are consistent with this hypothesis. We further extended the hypothesis by showing that TPA synergistically activates Zp through a PKC signaling pathway, with the ZIIR element likely functioning to inhibit this synergy between the ZID- and ZII-binding factors.
While we observed dramatic effects of ZIIR mutations on EBV lytic gene expression in the absence of inducers, Liu et al. (32) only observed effects of these exact same mutations in their assays when the cells were treated with TPA and ionomycin. Thus, the ZIIR element's role is much more apparent in the context of a whole EBV genome. Possible reasons for this observation include the EBV chromatin structure, promoter methylation, and EBV-encoded proteins also directly or indirectly influencing BZLF1 gene expression. Likewise, the addition of ionomycin had little, if any, synergistic effect on the TPA-induced activation of Zp we observed in 293-ZIIRmt and LCL-ZIIRmt cells (Fig. 7C and data not shown), a synergy seen by Liu et al. (32) in their assays. Ionomycin, an ionophore, activates the calcium-mediated signal transduction pathways which also affect factors that activate Zp via the ZI and ZII elements. Given our findings, we consider it unlikely that the ZIIR element suppresses the synergy between ZID and ZII elements through a calcium-mediated signal transduction pathway.
Zhang et al. (56) reported that the 993-amino-acid residue cellular protein Sμbp-2 can repress transcription from Zp, doing so at least in part via the nt −93 to −79 region of this promoter. They further showed, using reporter assays, that (i) Sμbp-2 can repress TPA-induced activation of Zp and (ii) Sμbp-2-mediated repression can be transferred to a heterologous promoter by insertion of copies of the nt −101 to −71 region of Zp. They suggested that Sμbp-2 may act in part via sequence-specific binding to the ZIIR or ZID element of Zp. Their attempts to test this hypothesis were unsuccessful due to high levels of nonspecific DNA binding by Sμbp-2. Given its size, Sμbp-2 is unlikely to be ZIIR-BP.
In summary, we conclude that cellular factors binding the ZID, ZII, and ZIIR elements probably functionally interact, with ZIIR-BP repressing BZLF1 gene expression by suppressing synergy between ZID and ZII element-binding factors activated via PKC signaling pathways. Understanding the precise molecular mechanism by which the ZIIR element functions requires knowing the identity of ZIIR-BP(s). This yet-to-be-achieved goal is an important one, given the findings presented here that indicate that the ZIIR element is an even more potent silencing element of Zp than is the ZV element, which has already been shown to be a major player in modulating EBV latency (54) via binding of ZEB1 and ZEB2 (15, 16). Possibly, knowledge about the identity of ZIIR-BP(s) and the factors that regulate its activities could lead to the development of new therapies for treating EBV-associated diseases.
ACKNOWLEDGMENTS
We thank Richard Burgess, Wolfgang Hammerschmidt, Nancy Raab-Traub, Samuel Speck, and Bill Sugden for cell lines, plasmids, antiserum, and protocols and Shannon Kenney, Stacy Hagemeier, and members of the Mertz laboratory for discussions and suggestions on the manuscript.
This work was supported by U.S. Department of Health and Human Services NIH grants AI107034, CA22443, and CA14520 and a grant from the Wisconsin Alumni Research Foundation. P.J.M. was supported in part by a Shapiro Summer Research Program grant from the UW School of Medicine and Public Health. H.-J.L. was supported by a fellowship from the government of Singapore. T.I. is a Royal Thai Government Scholar with funding from the National Science and Technology Development Agency of Thailand.
Footnotes
Published ahead of print on 9 March 2011.
REFERENCES
- 1. Altmann M., Hammerschmidt W. 2005. Epstein-Barr virus provides a new paradigm: a requirement for the immediate inhibition of apoptosis. PLoS Biol. 3:2148–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Altmann M., et al. 2006. Transcriptional activation by EBV nuclear antigen 1 is essential for the expression of EBV's transforming genes. Proc. Natl. Acad. Sci. U. S. A. 103:14188–14193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Angel P., et al. 1987. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell 49:729–739 [DOI] [PubMed] [Google Scholar]
- 4. Baumann M., et al. 1998. Activation of the Epstein-Barr virus transcription factor BZLF1 by 12-O-tetradecanoylphorbol-13-acetate-induced phosphorylation. J. Virol. 72:8105–8114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhende P. M., Dickerson S. J., Sun X., Feng W. H., Kenney S. C. 2007. X-box-binding protein 1 activates lytic Epstein-Barr virus gene expression in combination with protein kinase D. J. Virol. 81:7363–7370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borras A. M., Strominger J. L., Speck S. H. 1996. Characterization of the ZI domains in the Epstein-Barr virus BZLF1 gene promoter: role in phorbol ester induction. J. Virol. 70:3894–3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bryant H., Farrell P. J. 2002. Signal transduction and transcription factor modification during reactivation of Epstein-Barr virus from latency. J. Virol. 76:10290–10298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cahir-McFarland E., Kieff E. 2005. Epstein-Barr virus latent infection membrane protein one, p. 553–570 In Robertson E. S. (ed.), Epstein-Barr virus . Caister Academic Press, Norwich, United Kingdom [Google Scholar]
- 9. Chatila T., et al. 1997. The Epstein-Barr virus-induced Ca2+/calmodulin-dependent kinase type IV/Gr promotes a Ca2+-dependent switch from latency to viral replication. J. Virol. 71:6560–6567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chodosh L. A. 1996. UV crosslinking of proteins to nucleic acids, p. 12.5.1 In Ausubel F. M., Brent R., Kinston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl K. (ed.) Current protocols in molecular biology. John Wiley & Sons, Inc., New York, NY [Google Scholar]
- 11. Countryman J., Miller G. 1985. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc. Natl. Acad. Sci. U. S. A. 82:4085–4089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daigle D., et al. 2010. Upregulation of STAT3 marks Burkitt lymphoma cells refractory to Epstein-Barr virus lytic cycle induction by HDAC inhibitors. J. Virol. 84:993–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Delecluse H. J., Hilsendegen T., Pich D., Zeidler R., Hammerschmidt W. 1998. Propagation and recovery of intact, infectious Epstein-Barr virus from prokaryotic to human cells. Proc. Natl. Acad. Sci. U. S. A. 95:8245–8250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duellman S. J., Burgess R. R. 2009. Antigen-binding properties of monoclonal antibodies reactive with EBNA1 and use in immunoaffinity chromatography. PLoS One 4:e4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ellis A. L., Wang Z., Yu X., Mertz J. E. 2010. Either ZEB1 or ZEB2/SIP1 can play a central role in regulating the Epstein-Barr virus latent-lytic switch in a cell-type-specific manner. J. Virol. 84:6139–6152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ellis-Connell A. L., Iempridee T., Xu I., Mertz J. E. 2010. Cellular microRNAs 200b and 429 regulate the Epstein-Barr virus switch between latency and lytic replication. J. Virol. 84:10329–10343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feederle R., et al. 2000. The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators. EMBO J. 19:3080–3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feng W. H., et al. 2007. ZEB1 and c-Jun levels contribute to the establishment of highly lytic Epstein-Barr virus infection in gastric AGS cells. J. Virol. 81:10113–10122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Flamand L., Menezes J. 1996. Cyclic AMP-responsive element-dependent activation of Epstein-Barr virus zebra promoter by human herpesvirus 6. J. Virol. 70:1784–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flemington E., Speck S. H. 1990. Identification of phorbol ester response elements in the promoter of Epstein-Barr virus putative lytic gene BZLF1. J. Virol. 64:1217–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gao X., Ikuta K., Tajima M., Sairenji T. 2001. 12-O-Tetradecanoylphorbol-13-acetate induces Epstein-Barr virus reactivation via NF-κB and AP-1 as regulated by protein kinase C and mitogen-activated protein kinase. Virology 286:91–99 [DOI] [PubMed] [Google Scholar]
- 22. Gradoville L., Kwa D., El-Guindy A., Miller G. 2002. Protein kinase C-independent activation of the Epstein-Barr virus lytic cycle. J. Virol. 76:5612–5626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hammerschmidt W., Sugden B. 1988. Identification and characterization of oriLyt, a lytic origin of DNA replication of Epstein-Barr virus. Cell 55:427–433 [DOI] [PubMed] [Google Scholar]
- 24. Huang J., et al. 2006. Contribution of C/EBP proteins to Epstein-Barr virus lytic gene expression and replication in epithelial cells. J. Virol. 80:1098–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hudewentz J., Bornkamm G. W., zur Hausen H. 1980. Effect of the diterpene ester TPA on Epstein-Barr virus antigen and DNA synthesis in producer and nonproducer cell lines. Virology 100:175–178 [DOI] [PubMed] [Google Scholar]
- 26. Israel B. F., Kenney S. C. 2005. EBV lytic infection, p. 571–611 In Robertson E. S. (ed.), Epstein-Barr virus. Caister Academic Press, Norwich, United Kingdom [Google Scholar]
- 27. Kalla M., Schmeinck A., Bergbauer M., Pich D., Hammerschmidt W. 2010. AP-1 homolog BZLF1 of Epstein-Barr virus has two essential functions dependent on the epigenetic state of the viral genome. Proc. Natl. Acad. Sci. U. S. A. 107:850–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kieff E., Rickinson A. B. 2007. Epstein-Barr virus and its replication, p. 2603–2654 In Knipe D. M., Howley P. M. (ed.), Fields virology, 5th ed., vol. 2 Lippincott/Williams & Wilkins Co., Philadelphia, PA [Google Scholar]
- 29. Kraus R. J., Mirocha S. J., Stephany H. M., Puchalski J. R., Mertz J. E. 2001. Identification of a novel element involved in regulation of the lytic switch BZLF1 gene promoter of Epstein-Barr virus. J. Virol. 75:867–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kraus R. J., Perrigoue J. G., Mertz J. E. 2003. ZEB negatively regulates the lytic-switch BZLF1 gene promoter of Epstein-Barr virus. J. Virol. 77:199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin Z., Flemington E. K. 2010. Regulation of EBV latency by viral lytic proteins, p. 167–192 In Robertson E. S. (ed.), Epstein-Barr virus: latency and transformation. Caister Academic Press, Norwich, United Kingdom [Google Scholar]
- 32. Liu P., Liu S., Speck S. H. 1998. Identification of a negative cis element within the ZII domain of the Epstein-Barr virus lytic switch BZLF1 gene promoter. J. Virol. 72:8230–8239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu S., Borras A. M., Liu P., Suske G., Speck S. H. 1997. Binding of the ubiquitous cellular transcription factors Sp1 and Sp3 to the ZI domains in the Epstein-Barr virus lytic switch BZLF1 gene promoter. Virology 228:11–18 [DOI] [PubMed] [Google Scholar]
- 34. Liu S., Liu P., Borras A., Chatila T., Speck S. H. 1997. Cyclosporin A-sensitive induction of the Epstein-Barr virus lytic switch is mediated via a novel pathway involving a MEF2 family member. EMBO J. 16:143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Montalvo E. A., Cottam M., Hill S., Wang Y.-C. J. 1995. YY1 binds to and regulates cis-acting negative elements in the Epstein-Barr virus BZLF1 promoter. J. Virol. 69:4158–4165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Montalvo E. A., Shi Y., Shenk T. E., Levine A. J. 1991. Negative regulation of the BZLF1 promoter of Epstein-Barr virus. J. Virol. 65:3647–3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moorman N. J., Willer D. O., Speck S. H. 2003. The gammaherpesvirus 68 latency-associated nuclear antigen homolog is critical for the establishment of splenic latency. J. Virol. 77:10295–10303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Neuhierl B., Feederle R., Hammerschmidt W., Delecluse H. J. 2002. Glycoprotein gp110 of Epstein-Barr virus determines viral tropism and efficiency of infection. Proc. Natl. Acad. Sci. U. S. A. 99:15036–15041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raab-Traub N., Flynn K. 1986. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell 47:883–889 [DOI] [PubMed] [Google Scholar]
- 40. Reyland M. E. 2009. Protein kinase C isoforms: multi-functional regulators of cell life and death. Front. Biosci. 14:2386–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rickinson A. B., Kieff E. 2007. Epstein-Barr virus and its replication. p. 2656–2700 In Knipe D. M., Howley P. M. (ed.), Fields virology, 5th ed., vol. 2 Lippincott/Williams & Wilkins Co., Philadelphia, PA [Google Scholar]
- 42. Schwarzmann F., et al. 1994. Negatively cis-acting elements in the distal part of the promoter of Epstein-Barr virus trans-activator gene BZLF1. J. Gen. Virol. 75:1999–2006 [DOI] [PubMed] [Google Scholar]
- 43. Smith G. A., Enquist L. W. 1999. Construction and transposon mutagenesis in Escherichia coli of a full-length infectious clone of Pseudorabies virus, an alphaherpesvirus. J. Virol. 73:6405–6414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Speck S. H. 2005. Regulation of EBV latency-associated gene expression, p. 403–427 In Robertson E. S. (ed.), Epstein-Barr virus. Caister Academic Press, Norwich, United Kingdom [Google Scholar]
- 45. Speck S. H., Chatila T., Flemington E. 1997. Reactivation of Epstein-Barr virus: regulation and function of the BZLF1 gene. Trends Microbiol. 5:399–405 [DOI] [PubMed] [Google Scholar]
- 46. Sun C. C., Thorley-Lawson D. A. 2007. Plasma cell-specific transcription factor XBP-1s binds to and transactivates the Epstein-Barr virus BZLF1 promoter. J. Virol. 81:13566–13577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Swaminathan S., et al. 2001. Rapid engineering of bacterial artificial chromosomes using oligonucleotides. Genesis 29:14–21 [DOI] [PubMed] [Google Scholar]
- 48. Thomas C., Dankesreiter A., Wolf H., Schwarzmann F. 2003. The BZLF1 promoter of Epstein-Barr virus is controlled by E box-/HI-motif-binding factors during virus latency. J. Gen. Virol. 84:959–964 [DOI] [PubMed] [Google Scholar]
- 49. Toullec D., et al. 1991. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J. Biol. Chem. 266:15771–15781 [PubMed] [Google Scholar]
- 50. Wang Y.-C. J., Huang J.-M., Montalvo E. A. 1997. Characterization of proteins binding to the ZII element in the Epstein-Barr virus BZLF1 promoter: transactivation by ATF1. Virology 227:323–330 [DOI] [PubMed] [Google Scholar]
- 51. Weigel R., Fischer D. K., Heston L., Miller G. 1985. Constitutive expression of Epstein-Barr virus-encoded RNAs and nuclear antigen during latency and after induction of Epstein-Barr virus replication. J. Virol. 53:254–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wen W., et al. 2007. Epstein-Barr virus BZLF1 gene, a switch from latency to lytic infection, is expressed as an immediate-early gene after primary infection of B lymphocytes. J. Virol. 81:1037–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wu F. Y., et al. 2004. CCAAT/enhancer binding protein α binds to the Epstein-Barr virus (EBV) ZTA protein through oligomeric interactions and contributes to cooperative transcriptional activation of the ZTA promoter through direct binding to the ZII and ZIIIB motifs during induction of the EBV lytic cycle. J. Virol. 78:4847–4865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yu X., Wang Z., Mertz J. E. 2007. ZEB1 regulates the latent-lytic switch in infection by Epstein-Barr virus. PLoS Pathog. 3:e194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zetterberg H., Rymo L. 2005. EBNA2 transcriptional regulation in EBV latency, p. 439–462 In Robertson E. S. (ed.), Epstein-Barr virus, Caister Academic Press, Norwich, United Kingdom [Google Scholar]
- 56. Zhang Q., Wang Y.-C. J., Montalvo E. A. 1999. Sμbp-2 represses the Epstein-Barr virus lytic switch promoter. Virology 255:160–170 [DOI] [PubMed] [Google Scholar]
- 57. zur Hausen H., O'Neill F. J., Freese U. K., Hecker E. 1978. Persisting oncogenic herpesvirus induced by the tumour promoter TPA. Nature 272:373–375 [DOI] [PubMed] [Google Scholar]