Abstract
The influenza A virus genome consists of eight RNA segments that associate with the viral polymerase proteins (PB1, PB2, and PA) and nucleoprotein (NP) to form ribonucleoprotein complexes (RNPs). The viral NS1 protein was previously shown to associate with these complexes, although it was not clear which RNP component mediated the interaction. Using individual TAP (tandem affinity purification)-tagged PB1, PB2, PA, and NP, we demonstrated that the NS1 protein interacts specifically with NP and not the polymerase subunits. The region of NS1 that binds NP was mapped to the RNA-binding domain.
TEXT
The influenza A virus genome consists of eight RNA segments that associate with the polymerase (PB1, PB2, and PA) and nucleoprotein (NP) to form ribonucleoproteins (RNPs) (reviewed in reference 3). NS1 is a small viral protein whose primary function is the suppression of host innate immune responses (reviewed in reference 8), although it has also been implicated in the regulation of viral RNA synthesis (5, 17, 24, 27, 28), possibly through interaction with the viral RNP complex. Indeed, NS1 can be coimmunoprecipitated with the polymerase proteins and NP from infected-cell lysates (15). In addition, NS1 interacts with CPSF30, a host RNA polymerase II (Pol II)-associated factor involved in the 3′-end processing of host mRNAs, in a macromolecular complex also containing viral polymerase and NP (12). Together, these studies suggest a direct interaction between NS1 and the RNP complex. However, it remains to be determined which specific component of RNPs interacts with NS1, providing a rationale for this study.
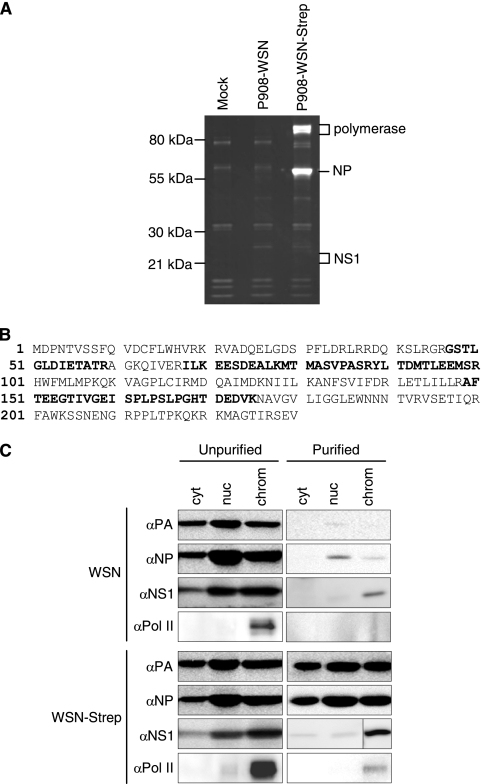
We initially confirmed that NS1 interacts with RNPs by purifying One-Strep-tagged RNP complexes (21), followed by mass spectrometry of copurifying factors. 293T cells were mock infected or infected with wild-type virus (P908-WSN) or a virus containing a One-Strep affinity purification tag on the C terminus of PB2 (P908-WSN-Strep) at a multiplicity of infection (MOI) of 2 for 6 h, followed by affinity purification. The reassortant viruses, which expressed PB1, PB2, PA, and NP derived from the A/Paris/908/97 virus in an A/WSN/33 virus background, were described previously (21). Polymerase proteins and NP were detectable in the sample obtained from P908-WSN-Strep-infected cells, confirming that RNP complexes were purified (Fig. 1A). In order to identify interacting proteins, the gel was cut into slices, and samples were sent for analysis by mass spectrometry at the University of Oxford Central Proteomics Facility. NS1 peptides, corresponding to 31% of the protein (Fig. 1B), were identified in a gel slice obtained from the P908-WSN-Strep virus sample, while no NS1 peptides were identified in the analogous gel slice for the P908-WSN virus.
Fig. 1.
The NS1 protein copurifies with One-Strep-tagged RNPs. (A) 293T cells were mock infected or infected with P908-WSN or P908-WSN-Strep virus (with a One-Strep tag on the C terminus of the PB2 protein) at an MOI of 2 for 6 h. Cell lysates were incubated with Strep-tactin beads overnight, and bound proteins were eluted in desthiobiotin elution buffer. Purified proteins were analyzed on a 12% polyacrylamide gel and stained with Sypro Ruby. Proteins from individual gel slices were analyzed by mass spectrometry. (B) NS1 peptides (covering 31% of the protein, shown in bold), were identified in a gel slice of the P908-WSN-Strep sample, indicated in panel A. (C) NS1 associates with RNP complexes in the chromatin fraction of infected cells. HeLa cells were infected with WSN or WSN-Strep virus, at an MOI of 3 for 6 h. Cells were harvested and fractionated into cytoplasmic (cyt), soluble nuclear (nuc), and chromatin (chrom) fractions. Lysates from these fractions were then used for affinity purification as described above. Fractions were analyzed by Western blotting.
To determine in which cellular compartment NS1 associates with RNPs during the viral replication cycle, lysates from cells infected with A/WSN/33 virus containing either wild-type PB2 (WSN) or a C-terminally One-Strep-tagged PB2 (WSN-Strep) were fractionated into cytoplasmic, nuclear, and chromatin fractions, as described previously (1). RNPs from these fractions were then affinity purified, followed by Western blot analysis using antibodies detecting PA, NP, NS1, and Pol II. The detection of PA and NP in samples purified from WSN-Strep-infected cells confirmed the isolation of RNP complexes. The majority of copurified NS1 was found in the Pol II-containing chromatin fraction (Fig. 1C). The viral polymerase has been shown to associate with the C-terminal domain of Pol II (4, 16, 21, 23), and this interaction is likely to be important in allowing transcribing RNPs to access Pol II-associated RNA processing factors and host nascent mRNAs for endonucleolytic cleavage. The finding that NS1 predominantly associates with RNPs in this fraction is consistent with studies suggesting that NS1 plays a role in the regulation of viral RNA synthesis (5, 17, 24, 27, 28). In addition, by forming part of a macromolecular complex containing viral RNPs, CPSF30, and Pol II, NS1 could be involved in the inhibition of cellular gene expression.
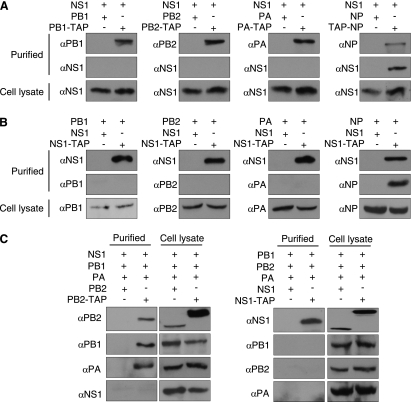
Having confirmed that NS1 specifically interacts with RNPs, we set out to identify the specific component(s) of the RNP responsible for this interaction. 293T cells were transfected with plasmids expressing either TAP (tandem affinity purification)-tagged or untagged polymerase or NP and NS1, followed by TAP purification after 48 h, as described previously (2). Briefly, TAP-tagged proteins, together with associated proteins, were purified from lysates of transfected cells using immunoglobulin G (IgG) Sepharose, which binds to the protein A component of the TAP tag. Bound proteins were then released by cleavage with tobacco etch virus protease. Western blot analysis confirmed that the polymerase proteins and NP were purified, and that NS1 specifically copurified with NP and not the individual subunits of the polymerase (Fig. 2A). A reciprocal experiment in which TAP-tagged NS1 was expressed with untagged NP, PA, PB1, or PB2 protein was then carried out, which confirmed that NS1 copurified with NP and not the polymerase proteins (Fig. 2B). The similar results obtained in the reciprocal experiments suggest that it is unlikely that the presence of the TAP tag interfered with the copurification procedure. All plasmids expressed proteins from the A/WSN/33 virus and were described previously (2, 6, 22, 25), with the exception of pcDNA-NS1-TAP, which was made by PCR amplification of the NS1 open reading frame from a pcDNA-NS1 template and subcloning into KpnI- and NotI-digested pcDNA-PA-TAP. Finally, to test the possibility that NS1 may be able to interact with the polymerase proteins only as a trimeric complex, all three polymerase proteins and NS1 were expressed together, with a TAP tag on either PB2 or NS1. Following TAP purification and Western blot analysis, it was found that NS1 did not copurify with the polymerase trimer (Fig. 2C).
Fig. 2.
The NS1 protein copurifies with NP but not the RNA polymerase. (A) TAP-tagged NP copurifies with NS1. 293T cells were transfected with plasmids expressing either TAP-tagged or untagged polymerase proteins or NP and NS1, as indicated. Cell lysates were harvested after 48 h, followed by TAP purification and Western blot analysis. (B) TAP-tagged NS1 copurifies with NP. 293T cells were transfected with plasmids expressing either TAP-tagged or untagged NS1 and various polymerase or NP proteins, as indicated, followed by TAP purification after 48 h and Western blot analysis. (C) NS1 does not copurify with the polymerase trimeric complex. 293T cells were transfected with plasmids expressing either TAP tagged or untagged proteins, as indicated, followed by TAP purification after 48 h and Western blot analysis.
The NS1 protein consists of an N-terminal RNA-binding domain (amino acids 1 to 73), which has been shown to interact with a variety of double- and single-stranded RNA species (9–11, 13, 14, 19, 20), a C-terminal effector domain (amino acids 73 to 207), and a disordered tail (amino acids 207 to 230) (reviewed in reference 8). We created a series of C-terminal-deletion mutants of NS1 (Fig. 3A) and tested their ability to interact with TAP-tagged NP. The deletion mutants were made by insertion of two consecutive stop codons into the NS1 open reading frame of the pcDNA-NS1 plasmid at the required positions by site-directed mutagenesis. Expression of the deletion mutants in the cell lysates was confirmed, and it was found that the N-terminal 73 amino acids of NS1, comprising the RNA-binding domain, was sufficient to bind NP (Fig. 3B). Copurification of the NS1 1–162 and 1–73 mutants was reduced compared to the longer 1–207 construct, and therefore the possibility that other regions of NS1 contribute to the NP interaction cannot be completely excluded. Two residues in the N terminus of NS1, an arginine at position 38 and a lysine at position 41, were previously implicated in RNA binding (26). An NS1 mutant in which these two residues were mutated to alanines by site-directed mutagenesis was unable to copurify with TAP-tagged NP (Fig. 3B). This suggested that either the RNA-binding ability of the NS1 protein was crucial for association with NP or the residues at positions 38 and 41 may be part of a binding site directly involved in the NP interaction.
Fig. 3.
The N-terminal RNA-binding domain of NS1 is required for binding NP. (A) Diagram of NS1 C-terminal deletion mutants and the RNA-binding R38A K41A NS1 mutant. (B) Analysis of the binding of mutant NS1 proteins to NP. 293T cells were transfected with plasmids expressing either TAP-tagged or untagged NP and either wild-type (wt) NS1 or NS1 mutants. Cell lysates were harvested after 48 h, followed by TAP purification and Western blot analysis. Longer film exposures were used to visualize the purified NS1 1–162 and 1–73 constructs. Background levels of NP, caused by nonspecific binding to the IgG Sepharose beads during the TAP purification procedure, are visible in lanes where untagged NP was expressed.
Next, we investigated whether the interaction between NS1 and NP was RNA dependent using an in vitro binding assay. NS1 and NP were individually expressed, and cell lysates were treated with RNase A, an endonuclease that cleaves single-stranded RNA. After the cell lysates were mixed, NS1 specifically copurified with NP in both RNase-treated and untreated samples (Fig. 4A), indicating an RNA-independent interaction. However, the level of copurified NS1 was reduced in the treated sample compared to the untreated sample, suggesting that RNA may contribute to the interaction. In addition, although analysis of RNA isolated from the cell lysates by agarose gel electrophoresis confirmed that both the 28S and 18S rRNA species were degraded in the RNase-treated samples, the possibility that short RNA fragments remained and contributed to the NP-NS1 interaction cannot be excluded. Therefore, the RNA dependence of the NS1-NP interaction was further investigated using NP mutants with reduced binding to RNA. The crystal structure of NP has been determined (18, 29), implicating a number of basic residues in RNA binding. In particular, two groups of mutations in the H5N1 NP, R74A, R75A, R174A, R175A, and R221A (group 1) and R150A, R152A, R156A, and R162A (group 2), have been shown to dramatically reduce the RNA-binding ability of NP (18). The ability of these H5N1 NP mutants to copurify with a TAP-tagged H1N1 NS1 was investigated. 293T cells were transfected with plasmids expressing either tagged or untagged NS1 protein and either wild-type or mutant NP. Cell lysates were harvested after 48 h, followed by TAP purification and Western blot analysis. Both wild-type NP and the two mutant proteins copurified with NS1, suggesting a specific protein-protein interaction (Fig. 4B).
Fig. 4.
Investigation of the RNA dependence of the NS1-NP interaction. (A) RNase treatment does not eliminate the interaction between NS1 and NP. 293T cells were transfected with plasmids expressing either TAP-tagged or untagged NP protein and NS1. Cell lysates were harvested after 48 h, during which the samples were individually treated with RNase A (Sigma) at 4°C for 30 min before being mixed together and TAP purified. Purified samples and cell lysates were analyzed by Western blotting. RNA was extracted from the remaining cell lysates using Trizol (Invitrogen) (22) and analyzed on a 1% agarose gel. (B) Analysis of the interaction of NP RNA-binding mutants with NS1. 293T cells were transfected with plasmids expressing either TAP-tagged or untagged NS1 protein and either wild-type or mutant H5N1 NP (NP group 1 contains R74A, R75A, R174A, R175A, and R221A mutations; NP group 2 has R150A, R152A, R156A, and R162A mutations). Cell lysates were harvested after 48 h and analyzed by Western blot. *, lower bands correspond to NS1-TAP.
In this study we demonstrated that NS1 interacts specifically with the nucleoprotein of RNP complexes. Our data suggest a direct protein-protein interaction between NS1 and NP; however, the interaction may be in part RNA mediated, and/or the binding of either or both NS1 and NP to RNA could result in conformational changes that stabilize the interaction between the two proteins. Analysis using highly purified proteins in the presence or absence of RNA, and a quantitative method such as surface plasmon resonance (SPR), could help to clarify the nature of the interaction further. Previous studies implicated NS1 in the regulation of viral RNA synthesis (5, 17, 24, 27, 28), and we have found that NS1 interacts with RNPs in the chromatin fraction of infected cells, possibly involving a complex also containing Pol II and CPSF30. We therefore speculate that this complex may be required for the regulation of the transcriptional activity of the viral polymerase, e.g., by enabling it to access Pol II-specific host functions (4, 16, 21, 23). However, influenza virus has previously been shown to replicate in interferon-deficient cells without NS1 (7), and therefore, the contribution of NS1 to the regulation of polymerase activity remains to be elucidated. Although we have identified two mutations in NS1 that disrupt the interaction with NP, identification of additional amino acids not implicated in RNA binding will be required to address the significance further. Structural analysis by crystallography of the interaction domains of NS1 and NP may underpin further studies of the functional significance of this interaction.
Acknowledgments
We thank Adolfo García-Sastre, Juan Ortín, and Otto Haller for antibodies, Marie-Anne Rameix-Welti and Emmanuel Dos Santos Afonso for viruses, and Benjamin Thomas for the mass spectrometry.
This work was supported by the MRC (G0700848), European Commission (FLUINNATE), and BMBF (FlueResearchNet).
Footnotes
Published ahead of print on 16 March 2011.
REFERENCES
- 1. Aygun O., Svejstrup J., Liu Y. 2008. A RECQ5-RNA polymerase II association identified by targeted proteomic analysis of human chromatin. Proc. Natl. Acad. Sci. U. S. A. 105:8580–8584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deng T., Sharps J., Fodor E., Brownlee G. G. 2005. In vitro assembly of PB2 with a PB1-PA dimer supports a new model of assembly of influenza A virus polymerase subunits into a functional trimeric complex. J. Virol. 79:8669–8674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Engelhardt O. G., Fodor E. 2006. Functional association between viral and cellular transcription during influenza virus infection. Rev. Med. Virol. 16:329–345 [DOI] [PubMed] [Google Scholar]
- 4. Engelhardt O. G., Smith M., Fodor E. 2005. Association of the influenza A virus RNA-dependent RNA polymerase with cellular RNA polymerase II. J. Virol. 79:5812–5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Falcon A. M., et al. 2004. Defective RNA replication and late gene expression in temperature-sensitive influenza viruses expressing deleted forms of the NS1 protein. J. Virol. 78:3880–3888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fodor E., et al. 2002. A single amino acid mutation in the PA subunit of the influenza virus RNA polymerase inhibits endonucleolytic cleavage of capped RNAs. J. Virol. 76:8989–9001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garcia-Sastre A., et al. 1998. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252:324–330 [DOI] [PubMed] [Google Scholar]
- 8. Hale B. G., Randall R. E., Ortin J., Jackson D. 2008. The multifunctional NS1 protein of influenza A viruses. J. Gen. Virol. 89:2359–2376 [DOI] [PubMed] [Google Scholar]
- 9. Hatada E., Fukuda R. 1992. Binding of influenza A virus NS1 protein to dsRNA in vitro. J. Gen. Virol. 73(Pt. 12):3325–3329 [DOI] [PubMed] [Google Scholar]
- 10. Hatada E., Saito S., Okishio N., Fukuda R. 1997. Binding of the influenza virus NS1 protein to model genome RNAs. J. Gen. Virol. 78(Pt. 5):1059–1063 [DOI] [PubMed] [Google Scholar]
- 11. Hatada E., Takizawa T., Fukuda R. 1992. Specific binding of influenza A virus NS1 protein to the virus minus-sense RNA in vitro. J. Gen. Virol. 73(Pt. 1):17–25 [DOI] [PubMed] [Google Scholar]
- 12. Kuo R. L., Krug R. M. 2009. Influenza a virus polymerase is an integral component of the CPSF30-NS1A protein complex in infected cells. J. Virol. 83:1611–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu Y., Wambach M., Katze M. G., Krug R. M. 1995. Binding of the influenza virus NS1 protein to double-stranded RNA inhibits the activation of the protein kinase that phosphorylates the elF-2 translation initiation factor. Virology 214:222–228 [DOI] [PubMed] [Google Scholar]
- 14. Marion R. M., Aragon T., Beloso A., Nieto A., Ortin J. 1997. The N-terminal half of the influenza virus NS1 protein is sufficient for nuclear retention of mRNA and enhancement of viral mRNA translation. Nucleic Acids Res. 25:4271–4277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marion R. M., Zurcher T., de la Luna S., Ortin J. 1997. Influenza virus NS1 protein interacts with viral transcription-replication complexes in vivo. J. Gen. Virol. 78(Pt. 10):2447–2451 [DOI] [PubMed] [Google Scholar]
- 16. Mayer D., et al. 2007. Identification of cellular interaction partners of the influenza virus ribonucleoprotein complex and polymerase complex using proteomic-based approaches. J. Proteome Res. 6:672–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Min J. Y., Li S., Sen G. C., Krug R. M. 2007. A site on the influenza A virus NS1 protein mediates both inhibition of PKR activation and temporal regulation of viral RNA synthesis. Virology 363:236–243 [DOI] [PubMed] [Google Scholar]
- 18. Ng A. K., et al. 2008. Structure of the influenza virus A H5N1 nucleoprotein: implications for RNA binding, oligomerization, and vaccine design. FASEB J. 22:3638–3647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qiu Y., Krug R. M. 1994. The influenza virus NS1 protein is a poly(A)-binding protein that inhibits nuclear export of mRNAs containing poly(A). J. Virol. 68:2425–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qiu Y., Nemeroff M., Krug R. M. 1995. The influenza virus NS1 protein binds to a specific region in human U6 snRNA and inhibits U6–U2 and U6–U4 snRNA interactions during splicing. RNA 1:304–316 [PMC free article] [PubMed] [Google Scholar]
- 21. Rameix-Welti M. A., Tomoiu A., Dos Santos Afonso E., van der Werf S., Naffakh N. 2009. Avian Influenza A virus polymerase association with nucleoprotein, but not polymerase assembly, is impaired in human cells during the course of infection. J. Virol. 83:1320–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robb N. C., Jackson D., Vreede F. T., Fodor E. 2010. Splicing of influenza A virus NS1 mRNA is independent of the viral NS1 protein. J. Gen. Virol. 91:2331–2340 [DOI] [PubMed] [Google Scholar]
- 23. Rodriguez A., Perez-Gonzalez A., Nieto A. 2007. Influenza virus infection causes specific degradation of the largest subunit of cellular RNA polymerase II. J. Virol. 81:5315–5324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shimizu K., Handa H., Nakada S., Nagata K. 1994. Regulation of influenza virus RNA polymerase activity by cellular and viral factors. Nucleic Acids Res. 22:5047–5053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vreede F. T., Brownlee G. G. 2007. Influenza virion-derived viral ribonucleoproteins synthesize both mRNA and cRNA in vitro. J. Virol. 81:2196–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang W., et al. 1999. RNA binding by the novel helical domain of the influenza virus NS1 protein requires its dimer structure and a small number of specific basic amino acids. RNA 5:195–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Z., et al. 2010. NS reassortment of an H7-type highly pathogenic avian influenza virus affects its propagation by altering the regulation of viral RNA production and antiviral host response. J. Virol. 84:11323–11335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wolstenholme A. J., Barrett T., Nichol S. T., Mahy B. W. 1980. Influenza virus-specific RNA and protein syntheses in cells infected with temperature-sensitive mutants defective in the genome segment encoding nonstructural proteins. J. Virol. 35:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ye Q., Krug R. M., Tao Y. J. 2006. The mechanism by which influenza A virus nucleoprotein forms oligomers and binds RNA. Nature 444:1078–1082 [DOI] [PubMed] [Google Scholar]