Abstract
Transmission between hosts is required for the maintenance of parasites in the host population and determines their ultimate evolutionary success. The transmission ability of parasites conditions their evolution in two ways: on one side, it affects the genetic structure of founded populations in new hosts. On the other side, parasite traits that increase transmission efficiency will be selected for. Therefore, knowledge of the factors and parameters that determine transmission efficiency is critical to predict the evolution of parasites. For plant viruses, little is known about the parameters of contact transmission, a major way of transmission of important virus genera and species. Here, we analyze the factors determining the efficiency of contact transmission of Tobacco mosaic virus (TMV) that may affect virus evolution. As it has been reported for other modes of transmission, the rate of TMV transmission by contact depended on the contact opportunities between an infected and a noninfected host. However, TMV contact transmission differed from other modes of transmission, in that a positive correlation between the virus titer in the source leaf and the rate of transmission was not found within the range of our experimental conditions. Other factors associated with the nature of the source leaf, such as leaf age and the way in which it was infected, had an effect on the rate of transmission. Importantly, contact transmission resulted in severe bottlenecks, which did not depend on the host susceptibility to infection. Interestingly, the effective number of founders initiating the infection of a new host was highly similar to that reported for aphid-transmitted plant viruses, suggesting that this trait has evolved to an optimum value.
INTRODUCTION
During the last 30 years, important efforts have been made to model the evolution of parasites, since understanding parasite evolution is necessary to develop sustainable strategies for the control of infectious diseases and to anticipate, prevent, and control new emergences. This is particularly so in the case of RNA viruses, which make up the largest fraction of emerging pathogens of humans, animals, and plants (6, 24, 47). Experimental work has not followed the pace of theory, and there is a paucity of information on the values of key parameters in evolutionary models, on the relationship among various parameters, and on the applicability of model assumptions. Data on parameters related to virus transmission are particularly scant, despite the fact that horizontal transmission is a critical step of the infective cycle of parasites. Indeed, between-host transmission and within-host multiplication are the two main components of parasite fitness, and thus, knowledge on the rates of transmission and multiplication and on the relationship between the two parameters is required to understand parasite evolution. Most models of parasite evolution consider that within-host multiplication and between-host transmission are linked traits, so that parasites must multiply up to a certain level for transmission to occur and that rates of transmission are positively correlated with rates of multiplication, at least within a range of values (14, 17, 32, 33). Although this is a reasonable assumption, its experimental support for plant viruses derives from a limited number of systems that represent few transmission mechanisms (see below). Also, since viruses may multiply to very high levels within the infected hosts, virus evolution has often been analyzed using purely deterministic models in which selection is considered to be the primary evolutionary factor acting on virus populations (9, 10). However, the relevant evolutionary parameter is not the total number of individuals in the population but the effective population number (Ne), which can be grossly assimilated to the number of individuals that pass their genes to the next generation. At small Ne values, random genetic drift will predominate over selection (7). The occurrence of population bottlenecks during the virus life cycle will result in the reduction of Ne, hence the interest in identifying and quantifying these bottlenecks. Population bottlenecks may occur when a new virus population is initiated by horizontal transmission, resulting in a type of genetic drift known as the founder effect, given that the new virus population is initiated from a small number of genotypes randomly sampled from the mother population. It has been shown that severe population bottlenecks may occur during horizontal transmission of plant viruses, as infection of a new host may be started by just a small number of individuals (12, 27). Once again, however, this information derives from just a few systems that represent only one of the various possible mechanisms of plant virus transmission.
In nature, plant viruses are horizontally transmitted either by contact or by means of vectors. Transmission by aphids, which vector the highest number of virus genera, has been best analyzed. The relationship between virus accumulation in the source leaf and transmissibility by aphids has been analyzed for a number of systems, showing that both traits, as assumed in evolutionary models, are positively correlated (3, 13, 16, 29). It has also been shown that nonpersistent transmission by aphids results in stochastic effects in the composition of the transmitted population (1), thus indicating the existence of founder effects due to the low number of particles transmitted. Accordingly, the effective number of founders (Nef) after aphid transmission has been estimated to be on the order of units (4, 37).
In contrast, little is known about the parameters of transmission by contact, despite the fact that this is the major way of transmission during field epidemics of important viruses in genera such as Tobamovirus, Potexvirus, and Hordeivirus (15). The little attention paid to contact transmission may be explained by the general assumption that it should not be essentially different from experimental mechanical inoculation, which is a routine technique in plant virology for transferring virus populations to new hosts. Mechanical inoculation consists of applying a suspension of viral particles or RNA (for viruses with a single-stranded RNA genome of messenger sense) on the leaf surface, in which infection occurs after the leaf is gently rubbed. Early work by plant virologists extensively analyzed the process of mechanical inoculation (reviewed in reference 20). However, no attempts have been made to analyze if these results could be applied to understand the nature of contact transmission.
The goal of this work is to characterize the evolutionarily relevant parameters of contact transmission of plant viruses. We used experimental populations of Tobacco mosaic virus (TMV) to address two major questions: (i) if the rate of contact transmission is positively correlated with virus accumulation in the source leaf and (ii) if contact transmission results in population bottlenecks leading to founder effects. Our data show that the rate of transmission does not depend primarily on the virus titer in the source leaf. We also show that contact transmission results in severe population bottlenecks.
MATERIALS AND METHODS
Biological material and virus inoculations.
Two TMV genotypes were used: wild-type TMV (wt TMV) and the coat protein mutant P20L-TMV, which has the transition C5656U, resulting in the amino acid replacement P → L at position 20 of the coat protein. These TMV genotypes were derived from biologically active cDNA clones that have been described elsewhere (8) and were a gift of W. O. Dawson and J. N. Culver. Infectious RNA was transcribed from these clones with T7 (for wt) or SP6 (for P20L mutant) RNA polymerase as previously described (8) and was inoculated into Nicotiana tabacum cv. Samsun plants suspended in 0.1 M Na2HPO4. Virus particles were purified from plants infected with RNA transcripts as described previously (5). Virus suspensions in 10 mM sodium phosphate buffer (pH 7.2) were used for further inoculations. Two tobacco genotypes were used: Samsun, which is fully susceptible to systemic infection by TMV, and Xanthi-nc, which is hypersensitively resistant to TMV infection, thus showing necrotic local lesions (nlls) around infection foci. All plants were kept in a greenhouse at 20 to 25°C with 16 h of light.
Detection of TMV genotypes and quantification of their accumulation in infected tobacco leaves.
TMV genotypes present in individual nlls were detected in lesion prints on nylon membranes that were hybridized with 5′ 32P-labeled oligonucleotide probes specific for each TMV genotype. The genotype-specific oligonucleotide probes used for these analyses and hybridization conditions have been described previously (44). Virus accumulation was quantified as viral RNA accumulation. Total RNA was extracted from 0.2 g (fresh weight) tobacco leaves (36) and resuspended in 50 μl of distilled water. Viral RNA in each sample was quantified by dot blot hybridization with genotype-specific 32P-labeled oligonucleotide probes. In each blot, internal standards for each genotype were included as a 2-fold dilution series of purified RNA (2 to 0.015 μg) in nucleic acid extracts from noninoculated tobacco plants, as described previously (44). Total RNA of mock-inoculated tobacco plants was used as a negative control. Different amounts of nucleic acid extracts of each sample were blotted to ensure that the hybridization signal was in the linear portion of the RNA concentration-versus-hybridization signal curve. The RNA hybridization signal was detected using a Typhoon 9400 scanner (GE Healthcare, Chalfont St. Giles, United Kingdom) after exposure of the labeled samples to Eu2+-containing phosphor screens and was quantitated using Image-Quant (version 5.2) software (Molecular Dynamics, GE Healthcare).
Statistical analyses.
All the statistical analyses are described in references 40 and 45. Means were compared by the nonparametric Wilcoxon signed rank test. Correlation between data was analyzed by the nonparametric Spearman rank correlation test, and significance was obtained from 1,000 random pairings of data. Analyses of variance (ANOVAs), general linear models (GLMs), and Student-Newman-Keuls post hoc contrasts of means were calculated using the software Statgraphics Centurion XV (StatPointTechnologies, Warenton, VA). Data were transformed when necessary in order to meet the assumptions for parametric analyses.
RESULTS
Efficiency of contact transmission under controlled conditions.
Contact transmission between plants was simulated by brushing one leaf of a recipient plant with an infected source leaf. Source leaves were collected from Samsun tobacco plants systemically infected after inoculation with 600 ng of wt-TMV particles per leaf. Three types of source leaves were used: inoculated leaves (L0 leaves) collected either at 4 or at 7 days postinoculation (dpi) and the systemically infected second leaf above L0 (L2 leaves) collected at 10 dpi. Five leaves of each type, each from a different infected tobacco plant, were used to inoculate two sets of 20 5-week-old Xanthi-nc tobacco plants, with the recipient leaf always being the youngest fully expanded leaf of each plant. One set of plants was inoculated by brushing the recipient leaf once with the source leaf; the other set was inoculated by brushing the recipient leaf 10 times with the source leaf. In this way, the transmission efficiency during a single contact event (1 brush) and the transmission efficiency during a more prolonged contact (10 brushes) were compared. In each source leaf, TMV accumulation in the area that had been in contact with the recipient leaf was quantified. At 4 days postinoculation, the recipient Xanthi-nc tobacco leaves were collected and local lesions were counted.
The number of plants with nlls is a measure of the number of successful transmission events. Ten brushes always resulted in more plants infected than a single brush (Table 1), and both values were positively correlated for each source leaf (Spearman correlation coefficient rS = 0.80, P = 0.003). In addition, the mean number of lesions was higher in recipient leaves brushed 10 times than in those brushed once (Wilcoxon signed rank test, P ≤ 0.004; Table 1). No correlation was found between virus accumulation in the source leaf and either the number of plants with nlls or the mean number of nlls per recipient leaf (P = 1 in both cases). The number of plants showing nlls was higher after they were brushed with the systemically infected source L2 leaves than with L0 leaves (P < 0.01; Table 1), although the viral titer in L2 leaves was significantly lower than that in L0 leaves (P = 0.03; Table 1). Therefore, the number of successful transmission events depended on the number of contact opportunities between the source and recipient leaves but not on the viral titer in the source leaf. Since systemically infected leaves were a more efficient source than inoculated leaves, factors other than contact opportunity, related to the nature of the source leaf, must also determine the efficiency of contact transmission: L2 leaves are younger than L0 leaves, which suggests that leaf age might be one such factor. Results also suggest that the mode in which the source leaf had been infected (directly inoculated or systemically infected) might be another factor.
Table 1.
Number of infected plants and mean number of nll in the recipient leaf per source leaf
| Source leaf and time postinoculationa | TMV accumulation in source leafb | No. of infected recipient plantsc |
No. of nlls per infected recipient leafd |
||
|---|---|---|---|---|---|
| 1 brush | 10 brushes | 1 brush | 10 brushes | ||
| L0 | |||||
| 4 dpi | 5.9 | 5 | 14 | 1.0 ± 0.0 | 1.4 ± 0.2 |
| 5.4 | 1 | 13 | 1.0 ± 0.0 | 2.3 ± 0.3 | |
| 5.0 | 3 | 14 | 1.0 ± 0.0 | 1.6 ± 0.3 | |
| 6.0 | 2 | 13 | 1.0 ± 0.0 | 1.6 ± 0.2 | |
| Mean ± SEe | 5.6 ± 0.2 | 2.8 ± 0.9 | 13.5 ± 0.3 | 1.0 ± 0.0 | 1.7 ± 0.1 |
| 7 dpi | 6.4 | 3 | 10 | 1.7 ± 0.5 | 3.6 ± 1.0 |
| 6.9 | 2 | 17 | 1.0 ± 0.0 | 5.2 ± 1.1 | |
| 4.7 | 14 | 19 | 3.6 ± 1.2 | 38.6 ± 6.7 | |
| 4.7 | 15 | 20 | 8.6 ± 3.6 | 33.9 ± 8.3 | |
| Mean ± SEe | 5.7 ± 0.6 | 8.5 ± 3.5 | 16.5 ± 2.3 | 5.5 ± 1.7 | 23.1 ± 3.7 |
| L2 | 2.4 | 18 | 20 | 8.5 ± 1.7 | 39.7 ± 4.9 |
| 10 dpi | 4.5 | 16 | 20 | 3.6 ± 0.9 | 25.0 ± 4.4 |
| 3.5 | 17 | 20 | 6.0 ± 1.2 | 24.5 ± 4.1 | |
| 3.5 | 20 | 19 | 31.2 ± 3.9 | 85.2 ± 12.6 | |
| Mean ± SEe | 3.5 ± 0.4 | 17.8 ± 0.9 | 19.8 ± 0.3 | 13.2 ± 1.8 | 43.2 ± 4.5 |
Time postinoculation at which the source leaf was used for inoculations. L0, inoculated leaf; L2, systematically infected second leaf above L0.
Viral accumulation in the source leaf expressed as micrograms of viral RNA per gram (fresh weight) of leaf.
Two sets of 20 Xanthi-nc tobacco plants were inoculated per source leaf by brushing the source leaf either once or 10 times.
Data are means and standard errors for the infected recipient leaves.
Data are means and standard errors for four source leaves.
Source leaf factors that determine efficiency of contact transmission.
In order to determine which factors associated with the nature of the source leaf affect its infectivity, an experiment was performed in which the effects of age and mode of infection of the source leaf (directly inoculated or systemically infected) were analyzed, together with the effect of virus titer. Two sets of three and eight plants, respectively, were inoculated (inoculation sets S1 and S2, respectively; Table 2), in order to obtain source leaves of different ages that had been infected either directly (L0 leaves) or systemically (L2 and L4 leaves, second and fourth leaves above L0, respectively). These source plants were inoculated with a lower viral dose than in the experiment described in the previous section (50 ng of virus particles of wt TMV per leaf), in order to promote variation of viral accumulation at the source leaves over a larger range of values. S2 plants were inoculated 6 days later than S1 plants, but in both cases plants were inoculated at the same age of 30 days. Since the tobacco leaf plastochron under our growing conditions is 3 days, the difference in age between two alternate leaves (L0 and L2 leaves or L2 and L4 leaves) was 6 days. This allowed us to use L0, L2, and L4 source leaves of the same and of different ages (Table 2) in a single transmission experiment, with inoculations of recipient leaves taking place at 16 and 10 dpi for S1 and S2 plants, respectively. Thus, the type of source leaf factor could be differentiated from the source leaf age factor. At the time of inoculation of source plants, L2 leaves were 4 days old and L4 leaves would appear 2 days later. Systemic infection of those leaves may have happened at any time after 3 dpi (44), when both leaves had already started expansion. Therefore, we can assume that L2 and L4 leaves have been infected for the same period of time (a maximum of 13 days for inoculation set S1 and 7 days for inoculation set S2). Each source leaf was used to inoculate a set of 10 5-week-old Xanthi-nc tobacco plants by brushing 10 times their youngest fully expanded leaf. In each source leaf, virus accumulation in the area that had been in contact with the recipient leaf was quantitated. At 4 days postinoculation, the recipient Xanthi-nc tobacco leaves were collected and local lesions were counted.
Table 2.
Relationship between transmission efficiency and age and mode of infection of the source leaf
| Type of source leaf | S1a |
S2 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ageb,c | Surfacec,d | TMV accumulationc,e | No. of infected recipient plantsc,f | No. of nlls per infected recipient leafg | Age | Surface | TMV accumulation | No. of infected recipient plants | No. of nlls per infected recipient leaf | |
| L0 | 26 | 269 ± 26* | 2.9 ± 0.3* | 6.7 ± 0.7* | 4.7 ± 0.9* | 20 | 446 ± 20* | 8.8 ± 2.6* | 8.1 ± 0.8* | 9.3 ± 0.9*** |
| L2 | 20 | 457 ± 38** | 7.9 ± 1.9** | 10.0 ± 0.0** | 24.9 ± 3.2** | 14 | 592 ± 28** | 27.7 ± 6.1** | 8.9 ± 1.1* | 14.6 ± 1.3* |
| L4 | 14 | 410 ± 23** | 19.5 ± 0.2† | 10.0 ± 0.0** | 15.7 ± 2.9** | 8 | 236 ± 19† | 40.3 ± 4.9** | 9.1 ± 0.4* | 5.6 ± 0.5** |
Two sets of three (S1) and eight (S2) Samsun tobacco plants were inoculated with a lag period of 6 days. Different symbols in each column indicate significant differences in Student-Newman-Keuls contrasts of means at 95% confidence.
Age (days) of the source leaf L0, inoculated leaf; L2 and L4, 2nd and 4th leaves above the inoculated leaf, systematically infected at the time of the transmission experiment.
Data are means ± standard errors of three (S1) or eight (S2) source leaves.
Surface (in cm2) of the source leaf at the time of the transmission experiment.
Viral accumulation in the source leaf expressed as micrograms of viral RNA per gram (fresh weight) of leaf.
Number of plants with lesions out of 10 Xanthi-nc tobacco plants inoculated with each source leaf.
Data are means ± standard errors for the infected recipient leaves.
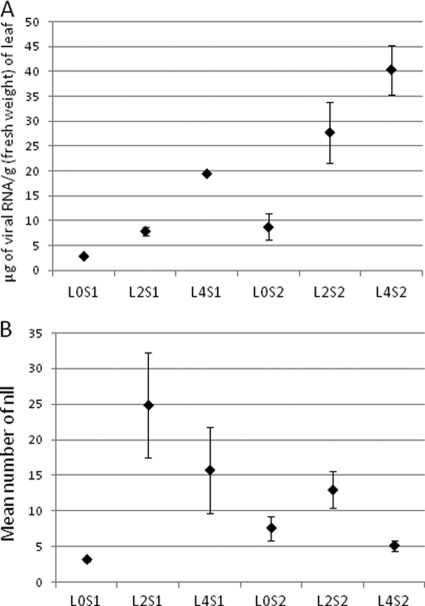
Viral accumulation in the source leaves was compared in a two-way ANOVA using type of leaf and inoculation set as factors. Viral accumulation differed significantly according to type of leaf (F2,27 = 9.77; P = 6 × 10−6), and it was lower in the inoculated L0 leaves than in the systemically infected L2 and L4 leaves (Table 2 and Fig. 1 A). Also, viral accumulation was lower in L2 leaves than in L4 leaves. Viral accumulation was also significantly different according to inoculation set, and it was higher in S2 than in S1 (F1,27 = 7.78; P = 0.01). There was no interaction between the two factors (P = 0.66).
Fig. 1.
TMV accumulation (A) and efficiency of contact transmission (B) from different types of source leaves. (A) TMV accumulation in the source leaf. (B) Mean number of nlls produced by the source leaf in 20 recipient leaves. Source leaves L0S1, L2S1, and L4S1 correspond to inoculation set S1, and source leaves L0S2, L2S2, and L4S2 correspond to inoculation set S2. Data are means and standard errors (error bars) from 3 (S1) and 8 (S2) plants.
To analyze the effects of the different factors on the efficiency of transmission, the number of inoculated plants with nlls and the mean number of nlls per recipient leaf were analyzed using GLMs, in which the factors type of leaf (L0, L2, or L4 leaves), inoculation set (S1 or S2), and viral accumulation were included. Only the factor type of leaf had a significant effect on the number of infected plants (F2,30 = 3.84; P = 0.03), and transmission was significantly less effective from inoculated leaves (L0 leaves) than from systemically infected L2 or L4 leaves (Student-Newman-Keuls contrast of means, P < 0.05). Hence, the number of infected plants was not dependent on viral accumulation or the inoculation set. The mean number of nlls in recipient leaves depended both on the type of source leaf and on viral accumulation in the source leaves, and the effect of these factors differed according to the inoculation set (F7,25 = 4.92; P = 0.001, data not shown). For S1, L0 leaves (L0S1) were the least effective source leaves (P < 0.05; Table 2 and Fig. 1B), while for S2, L4 leaves (L4S2) were the least effective ones and L2 leaves (L2S2) were the most effective ones (P < 0.05; Table 2 and Fig. 1B). When S1 and S2 data are compared, significant differences were found only with L4 leaves (P < 0.05). L4S2 leaves were the youngest and least effective source ones, despite having the highest viral content (Table 2). They were 8 days old when they were used as source leaves and not yet completely expanded, as shown by their smaller surface (Table 2). However, source leaf surface was not a significant factor on transmission efficiency when it was introduced in GLMs (data not shown).Therefore, efficiency of transmission depended on the age of the source leaf and on the way it was infected.
Estimation of effective number of founders starting an infection by contact transmission.
The estimation of the number of founders starting an infection by contact transmission was based on the probability of segregation of two alleles in demes originated from a mother deme (44). Given a source leaf that is infected by genotypes A and B with frequencies psA and psB, respectively, assuming that the probability of transmission of each genotype is given by its frequency in the source leaf and that both probabilities are independent (psA + psB = 1), the genetic composition of the transmitted viral population will be given by the binomial distribution (psA + psB)Nef. Hence, the probability P0B that a plant that has been in contact with that source leaf is infected only by genotype A is given by the equation
| (1) |
Nef can be estimated from this expression by estimating psA and P0B experimentally. Conversely, the probability P0A that a plant that has been in contact with that source leaf is infected only by genotype B is given by the expression
| (2) |
If genotypes A and B are not equally fit and selection occurs in coinfected leaves, the least-fit genotype can be lost before observation, and hence, the probability of no infection by that genotype can be overestimated. Therefore, the actual effective number of founders should lie between those obtained from equations 1 and 2. The transmission probability of each TMV genotype will depend on both its frequency in the source leaf and its transmissibility. In case both genotypes had different transmissibilities, it would be necessary to correct psA and psB accordingly (4).
Nef can also be maximum likelihood estimated by comparing the observed frequencies of plants infected with either genotype A, genotype B, or both genotypes with those expected from the binomial distribution (psA + psB)Nef.
To estimate the transmissibility of each TMV genotype, two sets of five Samsun tobacco plants were inoculated with 600 ng/leaf of either wt-TMV or P20L-TMV particles. At 4 dpi, the inoculated leaf of each plant was collected and used as the source leaf to inoculate the youngest fully expanded leaf of 20 Xanthi-nc tobacco plants by brushing them 10 times. In each source leaf, the wt-TMV or P20L-TMV accumulation in the area that had been in contact with the recipient leaf was quantified (Table 3). In the recipient leaves, nlls were counted at 4 dpi. The transmissibility for each TMV genotype was calculated as the ratio between the number of plants with lesions produced by each source leaf and the virus accumulation in that leaf and was not significantly different for the two different viral genotypes (P = 0.46; Table 3). Therefore, Nef can be calculated from equations 1 and 2 with no need for correction for transmissibility.
Table 3.
Transmissibility by contact of wt and P20L TMV genotypes
| TMV genotype inoculated in source leaf | Recipient Xanthi-nc tobacco plants |
Recipient Samsun tobacco plants |
||||
|---|---|---|---|---|---|---|
| TMV accumulation in source leafa | No. of infected recipient plantsb | Transmissibilityc | TMV accumulation in source leaf | No. of infected recipient plants | Transmissibility | |
| wt | 1.1 | 12 | 10.7 | 2.8 | 8 | 2.9 |
| 1.1 | 4 | 3.7 | 0.4 | 7 | 17.5 | |
| 0.7 | 8 | 11.0 | 1.2 | 12 | 10.0 | |
| 1.0 | 10 | 10.1 | 0.8 | 16 | 20.0 | |
| 2.4 | 9 | 3.7 | 0.9 | 20 | 22.2 | |
| Mean ± SEd | 1.3 ± 0.3 | 8.6 ± 1.3 | 7.8 ± 1.7 | 1.2 ± 0.4 | 12.6 ± 2.4 | 14.5 ± 3.6 |
| P20L | 0.3 | 9 | 29.0 | 0.9 | 6 | 6.7 |
| 1.1 | 13 | 12.1 | 0.3 | 5 | 16.7 | |
| 1.1 | 5 | 4.6 | 0.4 | 1 | 2.5 | |
| 0.2 | 2 | 9.1 | 0.3 | 2 | 6.7 | |
| 0.3 | 2 | 5.9 | 0.3 | 10 | 33.3 | |
| Mean ± SEd | 0.6 ± 0.2 | 6.2 ± 2.1 | 12.1 ± 4.4 | 0.4 ± 0.1 | 4.8 ± 1.6 | 13.2 ± 5.6 |
Viral accumulation in the source leaf expressed as μg of viral RNA per gram (fresh weight) of leaf.
Number of infected plants out of 20 plants inoculated with each source leaf.
Ratio between the number of infected plants from each source leaf and the virus accumulation in that leaf.
Data are means and standard errors for five source leaves.
To estimate P0B and P0A, eight Samsun tobacco plants were inoculated with equal amounts (600 ng/leaf) of wt-TMV and P20L-TMV particles. This inoculum dose was chosen on the basis of previous single-lesion infectivity experiments in which it was shown that the number of nlls induced in Xanthi-nc tobacco did not differ significantly between the two TMV genotypes at 300 ng/half leaf. At 4 dpi, the inoculated leaf of each plant was collected and used as the source to inoculate the youngest fully expanded leaf of 20 Xanthi-nc tobacco plants, as described above. In each source leaf, the wt-TMV and P20L-TMV accumulation in the area that had been in contact with the recipient leaf was quantified (Table 4). In the recipient leaves, nlls were counted at 4 dpi and the TMV genotype(s) present in each nll was determined. The number of plants showing nlls ranged from 3 to 13 per source leaf and was not correlated with virus accumulation in the source leaf (P = 0.09; Table 4). The number of nlls per recipient leaf ranged from 1 to 15, with a mean of 3.7 (mean numbers per source leaf are shown in Table 4). P0B and P0A were calculated as the number of plants with lesions caused only by wt-TMV and only by P20L-TMV, respectively (Table 4). The frequency of each TMV genotype in the source leaf (psA and psB) was estimated from the fraction of wt-TMV or P20L-TMV RNA in the total viral RNA in each leaf (Table 4). The effective number of founders transmitted by contact was estimated from the data in Table 4 using equations 1 and 2 and was between 1.4 and 3.6, with a maximum-likelihood estimate of 2 (P = 0.70). More than one virus particle may be involved in initiating an infection focus, as shown by the occurrence of nlls in which both TMV genotypes were present (Table 4). Applying the same model used to estimate the effective number of founders that infect a leaf to the data on frequency of lesions with only one TMV genotype, the mean number of founders initiating an infection focus was estimated to be between 0.9 and 1.4, with a maximum-likelihood estimate of 1 (P = 0.95).
Table 4.
Frequency of Xanthi-nc tobacco plants and of nlls infected with only one TMV genotype after contact transmission from double-infected source leaves
| TMV accumulation in source leafa | Proportion of each genotype in the source leafb |
No. of infected recipient plantsc | Frequency of single infected recipient plantsd |
No. of nlls per infected recipient leafe | Frequency of nlls with just one TMV genotypef |
|||
|---|---|---|---|---|---|---|---|---|
| psA | psB | P0B | P0A | PL0B | PL0A | |||
| 0.9 | 0.90 | 0.10 | 12 | 0.67 | 0.00 | 4.4 ± 1.4 | 0.74 | 0.26 |
| 0.6 | 0.94 | 0.06 | 3 | 0.67 | 0.00 | 4.0 ± 2.0 | 0.92 | 0.08 |
| 1.5 | 0.93 | 0.07 | 4 | 1.00 | 0.00 | 2.0 ± 1.0 | 1.00 | 0.00 |
| 1.3 | 0.90 | 0.10 | 9 | 0.56 | 0.00 | 4.7 ± 1.8 | 0.81 | 0.17 |
| 0.8 | 0.75 | 0.25 | 10 | 0.70 | 0.00 | 4.2 ± 1.1 | 0.86 | 0.12 |
| 0.9 | 0.87 | 0.13 | 9 | 0.44 | 0.11 | 2.3 ± 0.9 | 0.67 | 0.33 |
| 2.0 | 0.87 | 0.13 | 12 | 0.17 | 0.33 | 4.1 ± 1.0 | 0.69 | 0.31 |
| 1.5 | 0.86 | 0.14 | 5 | 0.80 | 0.00 | 4.2 ± 1.2 | 0.95 | 0.05 |
| 1.2 ± 0.2g | 0.88 ± 0.02g | 0.12 ± 0.02g | 8 ± 1g | 0.63 ± 0.09g | 0.06 ± 0.02g | 3.7 ± 0.4g | 0.83 ± 0.04g | 0.17 ± 0.04g |
Viral accumulation in the source leaf expressed as μg of viral RNA per gram (fresh weight) of leaf.
Fraction of wt-TMV (psA) or P20L-TMV (psB) RNA in the total viral RNA in each leaf.
Number of plants with lesions out of 20 plants inoculated with each source leaf.
Fraction of plants infected by only wt-TMV (P0B) or P20L-TMV (P0A).
Data are means and standard errors for the infected recipient leaves.
Fraction of nlls infected by only wt-TMV (PL0B) or P20L-TMV (PL0A).
Means ± standard error values for 8 source leaves.
In nature, virus outbreaks occur only between susceptible plants and transmissibility of a virus could be different for susceptible (e.g., Samsun tobacco for TMV) and resistant (e.g., Xanthi-nc tobacco for TMV) plants. Since there is no information on a possible relationship between host susceptibility and effective number of founders, Nef was estimated in an experiment simultaneously and in a manner similar to that described above, using Samsun tobacco as recipient plants. It was shown that transmissibility to Samsun tobacco plants of wt-TMV and of P20L-TMV did not differ (P = 0.35) and that for neither of them was it different from transmissibility to Xanthi-nc tobacco plants (P = 0.50; Table 3). The genetic composition of the virus population in the recipient leaves of Samsun tobacco plants is shown in Table 5. Once again, the number of infected plants was not correlated with the virus concentration in the source leaf (P = 0.08; Table 5). P0B and P0A were calculated as the number of plants infected only with wt-TMV and only with P20L-TMV, respectively (Table 5). Estimates of psA and psB were calculated as described above (Table 5). The effective number of founders transmitted by contact to a susceptible host estimated from the data in Table 5 according to equations 1 and 2 was between 1.3 and 3.3, with a maximum-likelihood estimate of 2 (P = 0.72). Hence, the effective number of founders did not differ for a resistant and a susceptible host, although the efficiency of transmission to the susceptible host was higher (P = 0.007; Tables 4 and 5).
Table 5.
Frequency of Samsun tobacco plant infection with only one TMV genotype after contact transmission from double-infected source leaves
| TMV accumulation in source leafa | Proportion of each genotype in source leafb |
No. of infected recipient plantsc | Frequency of single infected recipient plantsd |
||
|---|---|---|---|---|---|
| psA | psB | P0B | P0A | ||
| 0.7 | 0.88 | 0.12 | 14 | 0.50 | 0.00 |
| 0.8 | 0.88 | 0.12 | 17 | 0.24 | 0.12 |
| 1.1 | 0.91 | 0.09 | 16 | 0.50 | 0.19 |
| 0.9 | 0.88 | 0.12 | 9 | 0.89 | 0.00 |
| 0.8 | 0.90 | 0.10 | 10 | 0.80 | 0.10 |
| 1.9 | 0.87 | 0.13 | 16 | 0.94 | 0.06 |
| 0.6 | 0.90 | 0.10 | 20 | 0.70 | 0.00 |
| 2.2 | 0.88 | 0.12 | 18 | 0.83 | 0.00 |
| 1.1 ± 0.2e | 0.89 ± 0.004e | 0.11 ± 0.004e | 15 ± 1e | 0.67 ± 0.09e | 0.06 ± 0.03e |
Viral accumulation in the source leaf expressed as μg of viral RNA per gram (fresh weight) of leaf.
Fraction of wt-TMV (psA) or P20L-TMV (psB) RNA in the total viral RNA in each leaf.
Number of plants with lesions out of 20 plants inoculated with each source leaf.
Fraction of plants infected by only wt-TMV (P0B) or P20L-TMV (P0A).
Means ± standard error values for 8 source leaves.
DISCUSSION
In this work, factors involved in the efficiency of contact transmission of TMV were analyzed, as these factors may determine key parameters in virus evolution models. Direct contact between infected and noninfected hosts is an important way of transmission of animal and human viruses, and its efficiency may result in severe epidemics (11, 31, 35, 41). Therefore, parameters related to contact transmission are included in epidemiological models aimed at predicting epidemic outbreaks or at assessing the effectiveness of control strategies (30). Many plant viruses of economic importance for crops, such as Tomato mosaic virus, Pepper mild mottle virus, Potato virus X, and Pepino mosaic virus, are also transmitted by contact during the course of epidemic outbreaks, regardless of other mechanisms of vertical or horizontal transmission that may be relevant in outbreak initiation (15, 25). However, the epidemics of contact-transmitted plant viruses have been scarcely studied (27), and no attempt has been carried out to identify the relevant epidemiological and evolutionary parameters. Similarly, the analysis of the mechanisms involved in contact transmission of plant viruses has been neglected, as it has been considered a simple and passive phenomenon (20). In addition, it has been assumed that contact transmission is essentially similar to mechanical inoculation, an experimental procedure that was extensively studied in the past (20). In mechanical inoculation, the inoculum is rubbed on the leaf surface and the use of abrasives increases its efficiency. This is thought to be due to the generation of wounds that facilitate the direct contact of infectious particles with an infectible site, i.e., with a leaf cell whose transiently exposed protoplast is susceptible to infection (19, 25). Contact transmission might differ from mechanical inoculation because the recipient leaf does not enter into contact with a suspension of virus particles but enters into contact with an infected source leaf from which infectious particles must be released, and the factors that favor inoculum release are unknown. Also unknown is whether contact between the source and recipient leaves results in the generation of transiently susceptible cells, similarly to leaf rubbing in mechanical inoculation. Because of these two uncertainties, the probability that an infectious viral particle encounters an infectible site in the recipient leaf during contact transmission might be largely different from that in mechanical inoculation. In our experiments, the efficiency of contact transmission, estimated to be the number of successful transmission events from a source leaf, was positively correlated with the number of contacts between the source and recipient leaves. This result suggests that during leaf contact, microwounds are produced in the surfaces of both leaves, which result in the liberation of infectious particles from the source leaf and in the induction of infectible sites in the recipient leaf, as it has been hypothesized (19, 25). Although this result is not unexpected, it is highly significant: all epidemiological models of parasite evolution and dynamics assume that transmission rates are positively correlated with the number of contacts (direct or indirect) between infected and susceptible noninfected hosts (2, 26, 42), and our result shows that this is a realistic assumption for TMV and, possibly, other contact-transmitted plant viruses.
A second relevant result is that the efficiency of TMV contact transmission, estimated to be the fraction of infected plants, was not a function of virus titer in the source leaf for the broad range of virus titers analyzed, which covered a 14-fold increase in virus accumulation (between 2.9 and 40.3 μg TMV RNA/g fresh leaf tissue; Table 2). This is an unexpected result, and if it is general, it would set contact transmission widely apart from vector transmission. Indeed, it has been shown repeatedly that the rate of plant virus transmission by homopterous insect vectors is positively correlated with virus titer in the source leaf for virus titer ranges similar to those reported here (3, 13, 16, 29). The consequences of this result for the analysis of virus evolution are also important: if rates of transmission are independent of within-host virus multiplication, the applicability of most models of virulence evolution to contact-transmitted plant viruses could be questioned. Indeed, most of the theory on evolution of virulence rests on the trade-off hypothesis, which assumes that within-host multiplication, between-host transmission, and virulence of parasites are positively correlated traits, among which trade-offs exist (17). The underlying concept is that the parasite needs to multiply within the host to a level that allows its transmission to new hosts and that virulence would be an unavoidable consequence of the within-host multiplication of the parasite. In our case, such a correlation has not been found, although a marginal correlation (in one experiment out of four) was found between the number of infection foci in the recipient leaf and the virus titer in the source. Also, we obtained no evidence for a lower threshold of TMV accumulation below which contact transmission would not occur: efficient transmission was observed from source leaves in which the level of TMV accumulation was as low as 0.2 μg RNA/g fresh tissue (data not shown). Thus, if there is a threshold of TMV accumulation below which contact transmission does not occur, it must be very low.
Interestingly, the efficiency of TMV contact transmission seems to be dependent on unsuspected factors associated with the nature of the source leaf. One such factor was the developmental stage of the source leaf, with younger leaves being less efficient source leaves than older ones. Age-associated changes in thickness, structure, and chemical composition of the cuticle (28, 39) might render older leaves more abrasive, thus increasing the opportunities for contact transmission. Our results also show that systemically infected leaves are better sources for TMV transmission than directly inoculated leaves, which could be related to the much more even virus invasion of the mesophyll in systemically infected leaves than in directly inoculated ones (21, 43, 46, 48).
As it is the case for all other modes of viral transmission analyzed so far, contact transmission of TMV results in severe population bottlenecks. The Nef initiating a TMV infection after a contact transmission event is in the order of units (between 1 and 4), with 2 being the most likely estimate. This number imposes a severe bottleneck, since the number of TMV particles in a tobacco leaf may reach 107 to 109 (23, 34, 38). The Nef after a contact transmission is similar to that reported for nonpersistent aphid transmission (Nef of about 1 for Potato virus Y [37] and between 1 and 2 for Cucumber mosaic virus [4]) or for mite transmission (3, as estimated from reference 22). Mechanical inoculation also constitutes a severe bottleneck, with values of Nef being between 1.6 and 7, highly similar to those reported here (44). All these estimates are based on similar probabilistic considerations, and future work should reexamine Nef estimates on the basis of deep sequencing analyses of the virus population in source and recipient leaves. However, current analyses are consistent in detecting severe population bottlenecks associated with horizontal transmission, which indicate that purely deterministic models of virus and virulence evolution should be modified to integrate the effects of random genetic drift. The effective number of founders that initiate an nll was estimated to be 1. This value agrees with older results from mechanical inoculation experiments that lead to the one particle-one site hypothesis of the initiation of infection (18, 19). If an nll is initiated by a single genotype, comparison of the effective number of founders per leaf with the number of observed nlls per leaf indicates that most infection foci initiated during contact do not contribute to the genetic diversity of the TMV population within a leaf. In agreement with this hypothesis, the value of Nef did not depend on the susceptibility to transmission of the recipient host, which was much higher for Samsun than for Xanthi-nc tobacco plants, which suggests that Nef has evolved to an optimum value.
Thus, our results show unsuspected traits of contact transmission that differ widely from vector transmission: one is that the efficiency of contact transmission is not positively correlated with virus titer in the source leaf, and the other is the dependency of transmission efficiency on specific properties of the source leaf. The higher rates of transmission from mature, systemically infected leaves prompt us to speculate on a possible relationship between the rate of systemic host invasion and the rate of transmission for a contact-transmitted virus. Within the conceptual framework of the trade-off hypothesis of the evolution of virulence, which assumes that within-host multiplication and between-host transmissions are linked traits, it would thus be the rate of systemic host colonization rather than the within-leaf multiplication rate that is the within-host component of the viral fitness subject to selection. Future experiments will be required to verify this hypothesis.
ACKNOWLEDGMENTS
This work has been supported by grant AGL2008-02458/AGR from the Ministerio de Ciencia e Innovación, Spain, to F.G.-A. M.D. was supported by fellowship SENACYT-IFARHU of Panama-Programa de Investigadores.
We thank Miguel Angel Ibáñez, Department of Statistics, E.T.S.I. Agrónomos, Universidad Politécnica de Madrid, for advice with the GLM analysis.
Footnotes
Published ahead of print on 2 March 2011.
REFERENCES
- 1. Ali A., et al. 2006. Analysis of genetic bottlenecks during horizontal transmission of Cucumber mosaic virus. J. Virol. 80:8345–8350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson R., May R. 1982. Coevolution of hosts and parasites. Parasitology 85:411–426 [DOI] [PubMed] [Google Scholar]
- 3. Barker H., Harrison B. 1986. Restricted distribution of potato leafroll virus-antigen in resistant potato genotypes and its effect on transmission of the virus by aphids. Ann. Appl. Biol. 109:595–604 [Google Scholar]
- 4. Betancourt M., Fereres A., Fraile A., García-Arenal F. 2008. Estimation of the effective number of founders that initiate an infection after aphid transmission of a multipartite plant virus. J. Virol. 82:12416–12421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bruening G., Beachy R., Scalla R., Zaitlin M. 1976. In vitro and in vivo translation of ribonucleic-acids of a cowpea strain of Tobacco mosaic virus. Virology 71:498–517 [DOI] [PubMed] [Google Scholar]
- 6. Cleaveland S., Laurenson M. K., Taylor L. H. 2001. Diseases of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356:991–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crow J. F., Kimura M. 1970. An introduction to population genetics theory. Harper & Row, Publishers, Inc., New York, NY [Google Scholar]
- 8. Culver J., Dawson W. 1989. Tobacco mosaic virus coat protein—an elicitor of the hypersensitive reaction but not required for the development of mosaic symptoms in Nicotiana sylvestris. Virology 173:755–758 [DOI] [PubMed] [Google Scholar]
- 9. Domingo E. 2002. Quasispecies theory in virology. J. Virol. 76:463–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Domingo E., Holland J. 1997. RNA virus mutations and fitness for survival. Annu. Rev. Microbiol. 51:151–178 [DOI] [PubMed] [Google Scholar]
- 11. Donnelly C., et al. 2003. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet 361:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elena S., Sanjuán R. 2007. Virus evolution: insights from an experimental approach. Annu. Rev. Ecol. Evol. Syst. 38:27–52 [Google Scholar]
- 13. Escriu F., Perry K., García-Arenal F. 2000. Transmissibility of Cucumber mosaic virus by Aphis gossypii correlates with viral accumulation and is affected by the presence of its satellite RNA. Phytopathology 90:1068–1072 [DOI] [PubMed] [Google Scholar]
- 14. Ewald P. 1983. Host-parasite relations, vectors, and the evolution of disease severity. Annu. Rev. Ecol. Syst. 14:465–485 [Google Scholar]
- 15. Fauquet C. M., Mayo M. A., Maniloff J., Desselberger U., Ball L. A. 2005. Virus taxonomy: eighth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, San Diego, CA [Google Scholar]
- 16. Foxe M., Rochow W. 1975. Importance of virus source leaves in vector specificity of Barley yellow dwarf virus. Phytopathology 65:1124–1129 [Google Scholar]
- 17. Frank S. 1996. Models of parasite virulence. Q. Rev. Biol. 71:37–78 [DOI] [PubMed] [Google Scholar]
- 18. Furumoto W., Mickey R. 1967. A mathematical model for infectivity-dilution curve of Tobacco mosaic virus—experimental tests. Virology 32:224–233 [DOI] [PubMed] [Google Scholar]
- 19. Furumoto W., Mickey R. 1967. A mathematical model for infectivity-dilution curve of Tobacco mosaic virus—theoretical considerations. Virology 32:216–223 [DOI] [PubMed] [Google Scholar]
- 20. García-Arenal F., Fraile A. 2011. Population dynamics and genetics of plant infection by viruses, p. 263–281 In Caranta C., López-Moya J. J., Aranda M., Tepfer M. (ed.), Recent advances in plant virology. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 21. González-Jara P., Fraile A., Canto T., García-Arenal F. 2009. The multiplicity of infection of a plant virus varies during colonization of its eukaryotic host. J. Virol. 83:7487–7494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hall J., French R., Hein G., Morris T., Stenger D. 2001. Three distinct mechanisms facilitate genetic isolation of sympatric Wheat streak mosaic virus lineages. Virology 282:230–236 [DOI] [PubMed] [Google Scholar]
- 23. Harrison B. 1956. The infectivity of extracts made from leaves at intervals after inoculation with viruses. J. Gen. Microbiol. 15:210–220 [DOI] [PubMed] [Google Scholar]
- 24. Holmes E. C. 2009. The evolutionary genetics of emerging viruses. Annu. Rev. Ecol. Evol. Syst. 40:353–372 [Google Scholar]
- 25. Hull R. 2002. Mathews' plant virology. Academic Press, Inc., London, United Kingdom [Google Scholar]
- 26. Jeger M., Holt J., Van den Bosch F., Madden L. 2004. Epidemiology of insect-transmitted plant viruses: modelling disease dynamics and control interventions. Physiol. Entomol. 29:291–304 [Google Scholar]
- 27. Jeger M., Seal S., Van den Bosch F. 2006. Evolutionary epidemiology of plant virus disease. Plant Virus Epidemiol. 67:163–203 [DOI] [PubMed] [Google Scholar]
- 28. Jetter R., Schaffer S. 2001. Chemical composition of the Prunus laurocerasus leaf surface. Dynamic changes of the epicuticular wax film during leaf development. Plant Physiol. 126:1725–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jiménez-Martínez E., Bosque-Pérez N. 2004. Variation in Barley yellow dwarf virus transmission efficiency by Rhopalosiphum padi (Homoptera: Aphididae) after acquisition from transgenic and non transformed wheat genotypes. J. Econ. Entomol. 97:1790–1796 [PubMed] [Google Scholar]
- 30. Klinkenberg D., Fraser C., Heesterbeek H. 2006. The effectiveness of contact tracing in emerging epidemics. PloS One 1:e12 doi:10.1371/journal.pone.0000012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lange E., et al. 2009. Pathogenesis and transmission of the novel swine-origin influenza virus A/H1N1 after experimental infection of pigs. J. Gen. Virol. 90:2119–2123 [DOI] [PubMed] [Google Scholar]
- 32. Lenski R., May R. 1994. The evolution of virulence in parasites and pathogens—reconciliation between 2 competing hypotheses. J. Theor. Biol. 169:253–265 [DOI] [PubMed] [Google Scholar]
- 33. Levin B. 1996. The evolution and maintenance of virulence in microparasites. Emerg. Infect. Dis. 2:93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Malpica J., et al. 2002. The rate and character of spontaneous mutation in an RNA virus. Genetics 162:1505–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morens D. M., Fauci A. S. 2007. The 1918 influenza pandemic: insights for the 21st century. J. Infect. Dis. 195:1018–1028 [DOI] [PubMed] [Google Scholar]
- 36. Moriones E., Diaz I., Rodríguez-Cerezo E., Fraile A., García-Arenal F. 1992. Differential interactions among strains of Tomato aspermy virus and satellite RNAs of Cucumber mosaic virus. Virology 186:475–480 [DOI] [PubMed] [Google Scholar]
- 37. Moury B., Fabre F., Senoussi R. 2007. Estimation of the number of virus particles transmitted by an insect vector. Proc. Natl. Acad. Sci. U. S. A. 104:17891–17896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nixon H. 1956. An estimate of the number of Tobacco mosaic virus particles in a single hair cell. Virology 2:126–128 [DOI] [PubMed] [Google Scholar]
- 39. Pe K. 1970. Cutin biosynthesis in Vicia faba leaves—effect of age. Plant Physiol. 46:759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Quinn G. P., Keough M. J. 2002. Experimental design and data analysis for biologists. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 41. Rabinowitz P., Perdue M., Mumford E. 2010. Contact variables for exposure to avian influenza H5N1 virus at the human-animal interface. Zoonoses Public Health 57:227–238 [DOI] [PubMed] [Google Scholar]
- 42. Rhodes C., Anderson R. 2008. Contact rate calculation for a basic epidemic model. Math. Biosci. 216:56–62 [DOI] [PubMed] [Google Scholar]
- 43. Roberts A., et al. 1997. Phloem unloading in sink leaves of Nicotiana benthamiana: comparison of a fluorescent solute with a fluorescent virus. Plant Cell 9:1381–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sacristán S., Malpica J., Fraile A., García-Arenal F. 2003. Estimation of population bottlenecks during systemic movement of Tobacco mosaic virus in tobacco plants. J. Virol. 77:9906–9911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sokal R. R., Rohlf F. J. 1995. Biometry. Freeman and Company, New York, NY [Google Scholar]
- 46. Takahashi T., et al. 2007. Analysis of the spatial distribution of identical and two distinct virus populations differently labeled with cyan and yellow fluorescent proteins in coinfected plants. Phytopathology 97:1200–1206 [DOI] [PubMed] [Google Scholar]
- 47. Woolhouse M. E. J., Haydon D. E., Antia R. 2005. Emerging pathogens: the epidemiology and evolution of species jumps. Trends Ecol. Evol. 20:238–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Worley J., Schneider I. 1963. Progressive distribution of Southern bean mosaic virus antigen in bean leaves determined with a fluorescent antibody stain. Phytopathology 53:1255–1257 [Google Scholar]