Abstract
HIV-1 is neutralized by a class of antibodies that preferentially recognize a site formed on the assembled viral spike. Such quaternary structure-specific antibodies have diverse neutralization breadths, with antibodies PG16 and PG9 able to neutralize 70 to 80% of circulating HIV-1 isolates while antibody 2909 is specific for strain SF162. We show that alteration between a rare lysine and a common N-linked glycan at position 160 of HIV-1 gp120 is primarily responsible for toggling between 2909 and PG16/PG9 neutralization sensitivity. Quaternary structure-specific antibodies appear to target antigenic variants of the same epitope, with neutralization breadth determined by the prevalence of recognized variants among circulating isolates.
The extraordinary diversity of HIV-1 suggests that a vaccine serotype strategy, such as the approach used to provide broad protection against the various serotypes of poliovirus or human papillomavirus (1, 19), may have limited applicability to HIV-1. One potential alternative is to focus on conserved sites of HIV-1 vulnerability to antibody-mediated neutralization. If a limited number of immunological variants (immunotypes) exist for a given neutralization site, then a “site-of-vulnerability serotype” vaccine strategy may be possible. Several sites of HIV-1 vulnerability have been identified on the assembled envelope (Env) spike, which is composed of three gp120 exterior units and three gp41 transmembrane molecules. These sites include the initial site of viral attachment to the cellular receptor CD4 (4, 29–31), a glycan site recognized by the 2G12 antibody (22, 23), the V3 loop (6, 9, 10, 32), and the external region of gp41 proximal to the membrane (25, 33). Recently, an epitope composed of quaternary structure-dependent interactions of the V2 and V3 loops of gp120 was also identified (7, 8, 11, 21, 27, 28).
The first HIV-1 quaternary structure-specific antibody isolated was the human monoclonal antibody (MAb) 2909, which displays neutralization limited to strain SF162 (8, 11). In contrast, the quaternary structure-specific and clonally related human MAbs PG16 and PG9 neutralize 70 to 80% of circulating HIV-1 isolates (27). Interestingly, recognition by the PG antibodies was found to require an N-linked glycan at position 160 (based on HXB2 numbering) in the V2 region of gp120 (7, 27), and this glycan was shown previously to knock out 2909 recognition (8, 11). An asparagine at position 160 is conserved in over 90% of HIV-1 strains (http://www.hiv.lanl.gov/content/sequence/NEWALIGN/align.html). In contrast, SF162 contains a rare lysine at this position (11). The recent atomic-level structural analysis of antibodies PG16 and 2909 shows that both utilize protruding anionic 3rd complementarity-determining regions of the heavy chain (CDR H3) for recognition (5, 17, 18). The structural similarities of these antibodies, together with their distinct neutralization sensitivities associated with the alteration of a single residue in the V2 region of gp120, lead us to hypothesize that the PG and 2909 antibodies may target closely related variants of the same epitope on the HIV-1 viral spike. Specifically, we hypothesized that the presence or absence of the N-linked glycan in V2 at position 160 was a primary determinant of antibody recognition and that variation at this position defined two immunological variants (immunotypes) of this epitope.
To test the breadth of neutralization by MAbs 2909, PG16, and PG9, we selected 80 genetically defined HIV-1 envelopes (20 from clade A, 30 from clade B, and 30 from clade C) to use as DNA templates for the N160K point mutation (Fig. 1). In several cases (those of viruses 89.6, 6101.10, QH0692.42, and BR07), the natural residue at position 160 was neither N nor K and, hence, both 160N and 160K mutations were tested. For viruses that contained a natural 160K residue (SF162, BL01, and ZM214.15), the converse K160N mutation was tested. The full-length HIV-1 rev-env expression plasmids were described previously (2, 12–14, 20, 24, 29), and site-directed mutagenesis was performed using the QuikChange mutagenesis kit (Agilent Technologies, Santa Clara, CA). The individual wild-type and mutant HIV-1 rev-env expression plasmids were used to cotransfect 293T cells with an HIV-1 SG3Δenv plasmid to make Env pseudoviruses, and neutralization was measured using TZM-bl target cells as described previously (24, 29). Upon the introduction of N160K or other indicated mutations, some Env pseudoviruses displayed modestly reduced titers compared to the wild-type Env pseudoviruses; however, mutant virus entry was sufficient for the neutralization assay, in which the virus entry levels were normalized to generate 20,000 to 200,000 relative light units in a luciferase assay (Promega, Madison, WI). One clade B Env pseudovirus, BG1168.1, lost entry with the N160K mutation; it was therefore removed from further analysis. All MAbs used in this study were produced by transient transfection using a mammalian expression system as described previously (5). Among the 80 wild-type Env pseudoviruses, MAb 2909 neutralized only SF162 (Tables 1, 2, and 3 and Fig. 1, top left) whereas MAb PG16 neutralized 62 pseudoviruses (78%), including 17 (85%) of 20 clade A, 18 (60%) of 30 clade B, and 27 (90%) of 30 clade C isolates (Tables 1 to 3 and Fig. 1, bottom left). Antibody PG9 displayed a neutralization breadth similar to that of PG16 (Tables 1 to 3). These results are consistent with the original reports on these antibodies (8, 11, 27). Thus, for Env pseudoviruses carrying the wild-type HIV-1 envelopes, MAb 2909 was SF162 specific and MAbs PG16 and PG9 were broadly neutralizing, with the greatest coverage against clades A and C and somewhat less coverage against clade B. Although the frequency of the 160N residue among clade B isolates (91%) is lower than that among clade A (97%) and C (94%) isolates, the more limited neutralization coverage of clade B by PG16/PG9 did not appear to be due to the inclusion of the few non-160N-containing strains because the 160N versions of these viruses were also tested and generally remained resistant to PG16/PG9 neutralization (Table 2).
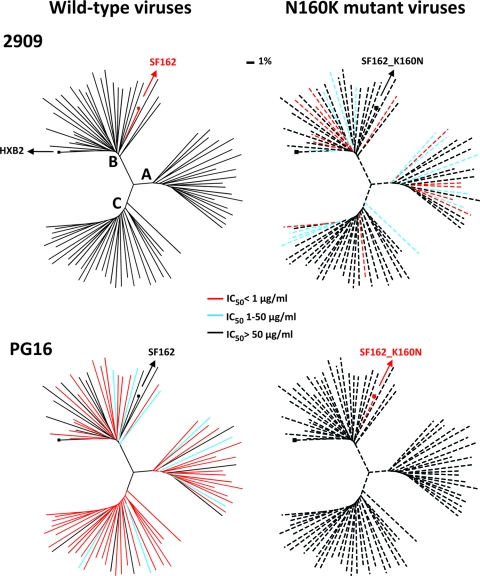
Fig. 1.
Analysis of 2909 and PG16 neutralization of 80 wild-type (left; solid lines) and N160K mutant (right; dashed lines) HIV-1 Env pseudoviruses from clades A, B, and C. Dendrograms, made by the neighbor-joining method, show the wild-type envelope protein sequence distance. The clade B strain HXB2 was used to root the tree. The clades of HIV-1 are indicated, and the amino acid distance scale is indicated with a value of 1% distance as shown. The neutralization potencies of 2909 and PG16 are indicated by the color of the branch for each virus. The 160K mutant data for viruses 89.6, 6101.10, QH0692.42, and BR07 are shown in the N160K mutant dendrograms, although these isolates did not have an asparagine at position 160. Viruses SF162, BL01, and ZM214.15 each have a wild-type 160K residue, and they are included among the viruses in the wild-type dendrograms; the data for the converse K160N mutation are shown in the N160K mutant dendrograms. For JR-FL variants, the JR-FL_E168K data are shown in the wild-type dendrograms and the JR-FL_N160K_E168K data are shown in the N160K mutant dendrograms.
Table 1.
Neutralization concentrations of MAbs 2909, PG16, and PG9 against 20 HIV-1 clade A Env pseudoviruses, either with wild-type Env or with the specific amino acid mutation indicated
| Virus designationa | Residueb at position: |
IC50 (μg/ml) of: |
IC80 (μg/ml) of: |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 156 | 160 | 165 | 168 | 2909 | PG16 | PG9 | 2909 | PG16 | PG9 | |
| Q259.17 | N | N | L | K | >50 | 0.030 | 0.041 | >50 | 0.488 | 0.166 |
| Q259.17_N160K | N | K | L | K | 0.006 | >50 | >50 | 1.2 | >50 | >50 |
| Q23.17 | N | N | L | K | >50 | 0.001 | 0.002 | >50 | 0.003 | 0.005 |
| Q23.17_N160K | N | K | L | K | 0.047 | >50 | >50 | 1.1 | >50 | >50 |
| KNH1209.18 | N | N | L | K | >50 | 0.283 | 0.167 | >50 | >50 | 19.1 |
| KNH1209.18_N160K | N | K | L | K | 0.151 | >50 | >50 | 2.0 | >50 | >50 |
| Q842.d12 | N | N | L | K | >50 | 0.009 | 0.019 | >50 | 0.031 | 0.070 |
| Q842.d12_N160K | N | K | L | K | 0.289 | >50 | >50 | 6.3 | >50 | >50 |
| DJ263.8 | N | N | L | K | >50 | 8.2 | 0.218 | >50 | >50 | 2.6 |
| DJ263.8_N160K | N | K | L | K | 0.620 | >50 | >50 | 6.3 | >50 | >50 |
| BS208.B1 | N | N | L | K | >50 | 0.003 | 0.005 | >50 | 0.008 | 0.049 |
| BS208.B1_N160K | N | K | L | K | 1.1 | >50 | >50 | 44.5 | >50 | >50 |
| UG037.8 | N | N | L | K | >50 | 0.003 | 0.011 | >50 | 0.011 | 0.044 |
| UG037.8_N160K | N | K | L | K | 8.4 | >50 | >50 | >50 | >50 | >50 |
| Q168.a2 | N | N | L | K | >50 | 0.019 | 0.045 | >50 | 0.078 | 0.173 |
| Q168.a2_N160K | N | K | L | K | 12.8 | >50 | >50 | >50 | >50 | >50 |
| KER2018.11 | N | N | L | K | >50 | 0.004 | 0.010 | >50 | 0.011 | 0.033 |
| KER2018.11_N160K | N | K | L | K | 25.4 | >50 | >50 | >50 | >50 | >50 |
| KER2008.12 | N | N | L | K | >50 | 0.008 | 0.017 | >50 | 0.051 | 0.068 |
| KER2008.12_N160K | N | K | L | K | 27.4 | >50 | >50 | >50 | >50 | >50 |
| 0330.v4.c3 | N | N | L | R | >50 | 0.001 | 0.004 | >50 | 0.007 | 0.019 |
| 0330.v4.c3_N160K | N | K | L | R | >50 | >50 | >50 | >50 | >50 | >50 |
| Q769.h5 | N | N | L | K | >50 | 0.009 | 0.009 | >50 | 0.067 | 0.033 |
| Q769.h5_N160K | N | K | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| 3718.v3.c11 | N | N | L | K | >50 | 0.012 | 0.088 | >50 | 0.136 | 0.328 |
| 3718.v3.c11_N160K | N | K | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| 3415.v1.c1 | N | N | Q | K | >50 | 0.017 | 0.116 | >50 | 0.358 | 0.814 |
| 3415.v1.c1_N160K | N | K | Q | K | >50 | >50 | >50 | >50 | >50 | >50 |
| RW020.2 | N | N | L | K | >50 | 0.037 | 0.052 | >50 | 0.385 | 0.269 |
| RW020.2_N160K | N | K | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| Q461.e2 | N | N | L | K | >50 | 2.7 | 1.5 | >50 | >50 | 10.9 |
| Q461.e2_N160K | N | K | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| 0260.v5.c1 | D | N | L | K | >50 | 3.2 | 1.4 | >50 | >50 | 31.9 |
| 0260.v5.c1_N160K | D | K | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| QH209.14 M.A2 | N | N | V | R | >50 | >50 | >50 | >50 | >50 | >50 |
| QH209.14 M.A2_N160K | N | K | V | R | >50 | >50 | >50 | >50 | >50 | >50 |
| 398-F1-F6-20 | N | N | L | R | >50 | >50 | >50 | >50 | >50 | >50 |
| 398-F1-F6-20_N160K | N | K | L | R | >50 | >50 | >50 | >50 | >50 | >50 |
| 0439.v5.c1 | N | N | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| 0439.v5.c1_N160K | N | K | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| Neutralization breadthc for: | ||||||||||
| Wild-type viruses (n = 20) | 0 (0%) | 17 (85%) | 17 (85%) | 0 (0%) | 13 (65%) | 17 (85%) | ||||
| Mutant viruses (n = 20) | 10 (50%) | 0 (0%) | 0 (0%) | 6 (30%) | 0 (0%) | 0 (0%) | ||||
The viruses are ordered first by the 2909 IC50, then by the PG16 IC50, and finally by the PG9 IC50. Mutant viruses are designated by the corresponding wild-type strain name followed by the relevant amino acid mutation.
Residues at positions 156, 160, 165, and 168 (based on HXB2 numbering) with potential importance in the PG and 2909 epitope are shown.
Neutralization breadth was calculated as the number (percentage) of viruses neutralized with an antibody IC50 or IC80 of ≤50 μg/ml.
Table 2.
Neutralization concentrations of MAbs 2909, PG16, and PG9 against 30 HIV-1 clade B Env pseudoviruses, either with wild-type Env or with the specific amino acid mutation(s) indicated
| Virus designationa | Residueb at position: |
IC50 (μg/ml) of: |
IC80 (μg/ml) of: |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 156 | 160 | 165 | 168 | 2909 | PG16 | PG9 | 2909 | PG16 | PG9 | |
| SF162 | N | K | I | K | 0.015 | >50 | >50 | 0.804 | >50 | >50 |
| SF162_K160N | N | N | I | K | >50 | 0.002 | 0.003 | >50 | 0.016 | 0.012 |
| AC10.29 | N | N | M | K | >50 | 0.009 | 0.012 | >50 | 0.038 | 0.073 |
| AC10.29_N160K | N | K | M | K | 0.001 | >50 | >50 | 0.004 | >50 | >50 |
| JR-FL | N | N | I | E | >50 | >50 | >50 | >50 | >50 | >50 |
| JR-FL_N160K | N | K | I | E | >50 | >50 | >50 | >50 | >50 | >50 |
| JR-FL_E168K | N | N | I | K | >50 | 0.003 | 0.008 | >50 | 0.015 | 0.055 |
| JR-FL_N160K_E168K | N | K | I | K | 0.001 | >50 | >50 | 0.005 | >50 | >50 |
| 7165.18 | N | N | I | K | >50 | 0.426 | >50 | >50 | >50 | >50 |
| 7165.18_N160K | N | K | I | K | 0.017 | >50 | >50 | 0.077 | >50 | >50 |
| WITO.33 | N | N | I | K | >50 | 0.002 | 0.005 | >50 | 0.006 | 0.009 |
| WITO.33_N160K | N | K | I | K | 0.105 | >50 | >50 | 0.411 | >50 | >50 |
| HT593.1 | N | N | I | K | >50 | 0.056 | 0.214 | >50 | 2.5 | 2.1 |
| HT593.1_N160K | N | K | I | K | 0.109 | >50 | >50 | 0.793 | >50 | >50 |
| YU2 | N | N | I | K | >50 | 0.258 | 3.86 | >50 | >50 | >50 |
| YU2_N160K | N | K | I | K | 0.492 | >50 | >50 | 2.3 | >50 | >50 |
| ADA | N | N | I | K | >50 | 0.012 | 0.128 | >50 | 0.060 | 5.2 |
| ADA_N160K | N | K | I | K | 1.24 | >50 | >50 | 50.0 | >50 | >50 |
| SC422.8 | N | N | I | K | >50 | >50 | 0.238 | >50 | >50 | >50 |
| SC422.8_N160K | N | K | I | K | 4.5 | >50 | >50 | >50 | >50 | >50 |
| TRO.11 | N | N | I | K | >50 | 0.077 | 29.4 | >50 | >50 | >50 |
| TRO.11_N160K | N | K | I | K | 5.0 | >50 | >50 | 50.0 | >50 | >50 |
| REJO.67 | N | N | P | K | >50 | 0.005 | 0.003 | >50 | 0.024 | 0.015 |
| REJO.67_N160K | N | K | P | K | 6.7 | >50 | >50 | >50 | >50 | >50 |
| JR-CSF | N | N | I | K | >50 | 0.001 | 0.002 | >50 | 0.008 | 0.008 |
| JR-CSF_N160K | N | K | I | K | >50 | >50 | >50 | >50 | >50 | >50 |
| 3988.25 | N | N | I | K | >50 | 0.005 | 0.016 | >50 | 0.022 | 0.062 |
| 3988.25_N160K | N | K | I | K | >50 | >50 | >50 | >50 | >50 | >50 |
| 5768.4 | N | N | L | K | >50 | 0.008 | 0.031 | >50 | 0.580 | 1.3 |
| 5768.4_N160K | N | K | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| SS1196.1 | K | N | I | K | >50 | 0.020 | 0.074 | >50 | 0.179 | 0.695 |
| SS1196.1_N160K | K | K | I | K | >50 | >50 | >50 | >50 | >50 | >50 |
| RHPA.7 | N | N | I | K | >50 | 0.334 | 10.0 | >50 | 3.5 | >50 |
| RHPA.7_N160K | N | K | I | K | >50 | >50 | >50 | >50 | >50 | >50 |
| THRO.18 | N | N | V | K | >50 | 0.498 | 13.2 | >50 | 50.0 | >50 |
| THRO.18_N160K | N | K | V | K | >50 | >50 | >50 | >50 | >50 | >50 |
| BaL.01 | N | N | I | K | >50 | 0.993 | 0.033 | >50 | >50 | 1.1 |
| BaL.01_N160K | N | K | I | K | >50 | >50 | >50 | >50 | >50 | >50 |
| TRJO.58 | N | N | T | K | >50 | 2.7 | 1.9 | >50 | >50 | 42.7 |
| TRJO.58_N160K | N | K | T | K | >50 | >50 | >50 | >50 | >50 | >50 |
| PVO.4 | N | N | I | R | >50 | 6.6 | 4.3 | >50 | >50 | 34.5 |
| PVO.4_N160K | N | K | I | R | >50 | >50 | >50 | >50 | >50 | >50 |
| CAAN.A2 | N | N | M | K | >50 | 25.0 | 14.4 | >50 | >50 | >50 |
| CAAN.A2_N160K | N | K | M | K | >50 | >50 | >50 | >50 | >50 | >50 |
| 6535.3 | N | N | R | K | >50 | >50 | 0.056 | >50 | >50 | 0.411 |
| 6535.3_N160K | N | K | R | K | >50 | >50 | >50 | >50 | >50 | >50 |
| 89.6 | N | Y | I | K | >50 | >50 | >50 | >50 | >50 | >50 |
| 89.6_Y160N | N | N | I | K | >50 | >50 | 0.140 | >50 | >50 | >50 |
| 89.6_Y160K | N | K | I | K | >50 | >50 | >50 | >50 | >50 | >50 |
| R2 | N | N | I | K | >50 | >50 | >50 | >50 | >50 | >50 |
| R2_N160K | N | K | I | K | >50 | >50 | >50 | >50 | >50 | >50 |
| R2_A162T | N | N | I | K | >50 | >50 | 10.3 | >50 | >50 | >50 |
| R2_N160K_A162T | N | K | I | K | >50 | >50 | >50 | >50 | >50 | >50 |
| HXB2 | N | N | I | K | >50 | >50 | >50 | >50 | >50 | >50 |
| HXB2_N160K | N | K | I | K | >50 | >50 | >50 | >50 | >50 | >50 |
| 6101.10 | N | D | I | K | >50 | >50 | >50 | >50 | >50 | >50 |
| 6101.10_D160N | N | N | I | K | >50 | >50 | >50 | >50 | >50 | >50 |
| 6101.10_D160K | N | K | I | K | >50 | >50 | >50 | >50 | >50 | >50 |
| QH0692.42 | N | S | I | K | >50 | >50 | >50 | >50 | >50 | >50 |
| QH0692.42_S160N_P162T | N | N | I | K | >50 | >50 | >50 | >50 | >50 | >50 |
| QH0692.42_S160K_P162T | N | K | I | K | >50 | >50 | >50 | >50 | >50 | >50 |
| BL01 | N | K | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| BL01_K160N | N | N | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| BR07 | N | H | R | K | >50 | >50 | >50 | >50 | >50 | >50 |
| BR07_H160N | N | N | R | K | >50 | >50 | >50 | >50 | >50 | >50 |
| BR07_H160K | N | K | R | K | >50 | >50 | >50 | >50 | >50 | >50 |
| QH0515.1 | N | N | I | R | >50 | >50 | >50 | >50 | >50 | >50 |
| QH0515.1_N160K | N | K | I | R | >50 | >50 | >50 | >50 | >50 | >50 |
| Neutralization breadthc for: | ||||||||||
| Wild-type viruses (n = 30) | 1 (3%) | 18 (60%) | 20 (67%) | 1 (3%) | 11 (37%) | 13 (43%) | ||||
| Mutant viruses (n = 30) | 10 (33%) | 2 (7%) | 3 (10%) | 8 (27%) | 2 (7%) | 2 (7%) | ||||
The viruses are ordered first by the 2909 IC50, then by the PG16 IC50, and finally by the PG9 IC50.
Residues at positions 156, 160, 165, and 168 (based on HXB2 numbering) with potential importance in the PG and 2909 epitope are shown.
Neutralization breadth was calculated as the number (percentage) of viruses neutralized with an antibody IC50 or IC80 of ≤50 μg/ml.
Table 3.
Neutralization concentrations of MAbs 2909, PG16, and PG9 against 30 HIV-1 clade C Env pseudoviruses, either with wild-type Env or with the specific amino acid mutation(s) indicated
| Virus designationa | Residueb at position: |
IC50 (μg/ml) of: |
IC80 (μg/ml) of: |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 156 | 160 | 165 | 168 | 2909 | PG16 | PG9 | 2909 | PG16 | PG9 | |
| ZM233.6 | I | N | L | K | >50 | 0.001 | 0.002 | >50 | 0.002 | 0.007 |
| ZM233.6_N160K | I | K | L | K | 0.004 | >50 | >50 | 2.2 | >50 | >50 |
| Du422.1 | N | N | L | K | >50 | 0.042 | 0.178 | >50 | 0.924 | 2.0 |
| Du422.1_N160K | N | K | L | K | 0.843 | >50 | >50 | 12.1 | >50 | >50 |
| CAP45.G3 | N | N | L | K | >50 | 0.002 | 0.003 | >50 | 0.007 | 0.014 |
| CAP45.G3_N160K | N | K | L | K | 2.17 | >50 | >50 | 21.7 | >50 | >50 |
| ZM249.1 | N | N | L | K | >50 | 0.009 | 0.031 | >50 | 0.192 | 0.162 |
| ZM249.1_N160K | N | K | L | K | 3.73 | >50 | >50 | 22.7 | >50 | >50 |
| Du156.12 | N | N | L | K | >50 | 0.002 | 0.035 | >50 | 0.019 | 0.109 |
| Du156.12_N160K | N | K | L | K | 7.28 | >50 | >50 | >50 | >50 | >50 |
| Du123.6 | N | N | I | K | >50 | 0.011 | 0.027 | >50 | 0.050 | 0.138 |
| Du123.6_N160K | N | K | I | K | 8.8 | >50 | >50 | >50 | >50 | >50 |
| ZM176.66 | N | N | L | K | >50 | 0.002 | 0.011 | >50 | 0.006 | 0.036 |
| ZM176.66_N160K | N | K | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| Du151.2 | N | N | I | R | >50 | 0.004 | 0.012 | >50 | 0.016 | 0.054 |
| Du151.2_N160K | N | K | I | R | >50 | >50 | >50 | >50 | >50 | >50 |
| 25710-2.43 | N | N | L | K | >50 | 0.004 | 0.014 | >50 | 0.098 | 0.080 |
| 25710-2.43_N160K | N | K | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| BR025.9 | N | N | V | K | >50 | 0.004 | 0.018 | >50 | 0.019 | 0.089 |
| BR025.9_N160K | N | K | V | K | >50 | >50 | >50 | >50 | >50 | >50 |
| SO18.18 | N | N | I | K | >50 | 0.004 | 0.031 | >50 | 0.057 | 0.106 |
| SO18.18_N160K | N | K | I | K | >50 | >50 | >50 | >50 | >50 | >50 |
| TV1.29 | N | N | L | K | >50 | 0.005 | 0.007 | >50 | 0.147 | 0.036 |
| TV1.29_N160K | N | K | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| ZM53.12 | N | N | L | K | >50 | 0.009 | 0.092 | >50 | 0.031 | 0.330 |
| ZM53.12_N160K | N | K | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| 286.36 | N | N | L | K | >50 | 0.012 | 0.084 | >50 | 0.043 | 0.390 |
| 286.36_N160K | N | K | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| TZBD.02 | N | N | L | K | >50 | 0.013 | 0.211 | >50 | 0.101 | 1.1 |
| TZBD.02_N160K | N | K | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| CAP244.D3 | N | N | L | K | >50 | 0.014 | 0.082 | >50 | 0.048 | 0.341 |
| CAP244.D3_N160K | N | K | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| 25711-2.4 | N | N | L | K | >50 | 0.015 | 0.468 | >50 | 0.101 | 4.7 |
| 25711-2.4_N160K | N | K | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| CAP210.E8 | I | N | L | K | >50 | 0.021 | 0.080 | >50 | 0.159 | 0.438 |
| CAP210.E8_N160K | I | K | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| TZA125.17 | N | N | I | K | >50 | 0.023 | 0.149 | >50 | 0.367 | 0.721 |
| TZA125.17_N160K | N | K | I | K | >50 | >50 | >50 | >50 | >50 | >50 |
| Du172.17 | N | N | I | K | >50 | 0.023 | 0.240 | >50 | 0.147 | 0.952 |
| Du172.17_N160K | N | K | I | K | >50 | >50 | >50 | >50 | >50 | >50 |
| 288.38 | N | N | V | K | >50 | 0.083 | 0.610 | >50 | 22.5 | >50 |
| 288.38_N160K | N | K | V | K | >50 | >50 | >50 | >50 | >50 | >50 |
| ZM106.9 | N | N | I | K | >50 | 0.129 | 0.234 | >50 | >50 | 3.6 |
| ZM106.9_N160K | N | K | I | K | >50 | >50 | >50 | >50 | >50 | >50 |
| ZM55.4a | N | N | L | I | >50 | 0.308 | 4.2 | >50 | 14.5 | 41.2 |
| ZM55.4a_N160K | N | K | L | I | >50 | >50 | >50 | >50 | >50 | >50 |
| ZA012.29 | N | N | I | K | >50 | 0.414 | 4.6 | >50 | >50 | >50 |
| ZA012.29_N160K | N | K | I | K | >50 | >50 | >50 | >50 | >50 | >50 |
| ZM197.7 | D | N | V | R | >50 | 0.765 | 0.287 | >50 | >50 | 2.5 |
| ZM197.7_N160K | D | K | V | R | >50 | >50 | >50 | >50 | >50 | >50 |
| ZM109.4 | H | N | V | R | >50 | 9.8 | 0.235 | >50 | >50 | 3.7 |
| ZM109.4_N160K | H | K | V | R | >50 | >50 | >50 | >50 | >50 | >50 |
| 16845-2.22 | N | N | L | K | >50 | 23.9 | 7.3 | >50 | >50 | >50 |
| 16845-2.22_N160K | N | K | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| ZM215.8 | N | N | V | K | >50 | >50 | 0.025 | >50 | >50 | 0.437 |
| ZM215.8_N160K | N | K | V | K | >50 | >50 | >50 | >50 | >50 | >50 |
| ZM135.10a | N | N | L | K | >50 | >50 | 2.8 | >50 | >50 | >50 |
| ZM135.10a_N160K | N | K | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| ZM214.15 | N | K | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| ZM214.15_K160N | N | N | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| ZM214.15_N162T | N | K | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| ZM214.15_K160N_N162T | N | N | L | K | >50 | >50 | >50 | >50 | >50 | >50 |
| Neutralization breadthc for: | ||||||||||
| Wild-type viruses (n = 30) | 0 (0%) | 27 (90%) | 29 (97%) | 0 (0%) | 22 (73%) | 25 (83%) | ||||
| Mutant viruses (n = 30) | 6 (20%) | 0 (0%) | 0 (0%) | 4 (13%) | 0 (0%) | 0 (0%) | ||||
The viruses are ordered first by the 2909 IC50, then by the PG16 IC50, and finally by the PG9 IC50.
Residues at positions 156, 160, 165, and 168 (based on HXB2 numbering) with potential importance in the PG and 2909 epitope are shown.
Neutralization breadth was calculated as the number (percentage) of viruses neutralized with an antibody IC50 or IC80 of ≤50 μg/ml.
Among the Env pseudoviruses containing a wild-type or mutant 160K residue, MAb 2909 neutralized 10 (50%) of 20 clade A, 11 (37%) of 30 clade B, and 6 (20%) of 30 clade C viruses (Fig. 1, top right, and Tables 1 to 3). In total, 27 (34%) of 80 160K-containing Env pseudoviruses were sensitive to 2909 neutralization. Remarkably, PG16 and PG9 neutralized none of the N160K mutant viruses (Fig. 1, bottom right, and Tables 1 to 3). Because wild-type SF162 had a lysine at position 160, we mutated this residue to asparagine, thereby restoring the N-linked glycan site. As described previously, the K160N mutation resulted in complete resistance to MAb 2909 while the PG MAbs were now able to neutralize the glycan-containing mutant of SF162 (21, 27). In total, these data indicate that a single glycan-specific mutation at position 160 in V2 can flip the overall neutralization landscape of antibodies 2909 and PG16/PG9 (Fig. 1 and Tables 1 to 3). Additionally, these neutralization profiles were mutually exclusive; there was no case of a virus that was sensitive to both 2909 and PG16/PG9 neutralization (Fig. 1 and Tables 1 to 3).
We next used contingency tables to categorize the 80 viral strains based on their neutralization sensitivities to PG16 and 2909 (Fig. 2A, upper panel) and to PG9 and 2909 (Fig. 2A, lower panel). As noted above, 27 of 80 Env pseudoviruses became sensitive to 2909 in the setting of the appropriate 160K sequence. Among these 27 160N-containing (mostly wild-type) viruses, all were sensitive to either PG16 or PG9. There was only one 160N wild-type virus (SC422.8) that was resistant to PG16 and one (7165.18) that was resistant to PG9. Conversely, among the 10 viruses resistant to both PG9 and PG16, none became 2909 sensitive upon 160K mutation. These data suggest that the 2909 epitope is generally not present or exposed properly among strains resistant to PG16 and PG9. Among the 64 PG16-sensitive viruses, 26 (41%) became 2909 sensitive in the setting of the appropriate 160K sequence, which was statistically significant (P = 0.008; Fisher's exact test). Likewise, 26 (38%) of 69 PG9-sensitive viruses became 2909 sensitive (P = 0.088; Fisher's exact test).
Fig. 2.
Analysis of correlations between 2909 and PG16 or PG9 neutralization sensitivities among 80 HIV-1 Env pseudoviruses. (A) Contingency tables show the relationships between viral sensitivities to 2909 and PG16 (upper panel) and to 2909 and PG9 (lower panel). The viruses were tested with 160N for PG16 and PG9 sensitivity and with 160K for 2909 sensitivity. A virus with a 50% inhibitory concentration (IC50) of ≤50 μg/ml was considered to be sensitive. Fisher's exact test was used to determine the significance of the correlations. (B) Neutralization IC50 values of PG16 (left) and PG9 (right) are plotted for 2909-sensitive (n = 23) and 2909-resistant (n = 57) strains. The geometric mean for each group is indicated with a horizontal bar. The unpaired Student t test was used to determine the significance of the difference between the geometric means for the 2909-sensitive and 2909-resistant strains.
To further assess the relationship between the PG and 2909 epitopes, we categorized the 160K versions of the 80 Env pseudoviruses as either 2909 sensitive or 2909 resistant. We then compared the neutralization sensitivities of these two categories of viruses to PG9 and PG16. The 160N versions of the 2909-sensitive viruses displayed significantly greater neutralization sensitivity to PG16 (Fig. 2B, left) and to PG9 (Fig. 2B, right) than the 160N versions of the 2909-resistant viruses. Taken together, the ability of a single mutation to alter neutralization sensitivity between PG and 2909 MAbs and the greater PG neutralization sensitivity among 2909-sensitive 160K mutants suggest that the 2909 and PG MAbs target antigenic variants of the same quaternary structure-specific epitope.
In addition to the residues at position 160, we reviewed naturally occurring sequence variations at positions 156, 165, and 168 in or adjacent to V2 that were described previously to be important for PG or 2909 binding (Tables 1 to 3). Prior studies showed that mutating the well-conserved N-glycan at position 156 to alanine in virus strain JR-CSF causes a decrease in neutralization potency for PG9 and PG16 (7, 27). However, we found that this glycan is not required for PG or 2909 antibody recognition because six isolates that did not contain the N-glycan at position 156 were sensitive to the PG antibodies and one of them (ZM233.6) was sensitive to 2909 (in the context of the N160K mutation). An analysis of residues at position 165 suggested that an isoleucine might be important for MAb 2909 but not PG9 or PG16 recognition, as an I165A mutation rendered SF162 resistant to 2909 but did not alter the PG antibody neutralization of JR-CSF (28). However, we observed two viruses (AC10.29 and REJO.67) with residues at position 165 differing from the consensus isoleucine or leucine that were neutralized by 2909 (in the setting of the appropriate N160K mutation). Thus, antibody 2909 appears to tolerate some amino acid variation at this site. Finally, the virus JR-FL contains an unusual glutamic acid at position 168 rather than the more common lysine. As described previously (7, 27), this 168E residue accounts for the JR-FL resistance to the PG MAbs. As expected, we found that the E168K mutant version of JR-FL was sensitive to the PG antibodies and that the subsequent N160K mutation altered viral sensitivity from the PG antibodies to antibody 2909 (Table 2). In total, these data suggest that the contact sites for antibodies PG16/PG9 and 2909 share some common features among these residues in or adjacent to V2. Specifically, both the PG and 2909 MAbs do not require an asparagine at position 156 for neutralization, both the PG and 2909 antibodies tolerate amino acid variation at position 165, and neither the PG nor the 2909 MAb could tolerate a glutamic acid at position 168.
Other MAbs dependent on the quaternary interaction of V2 and V3 have been isolated from rhesus monkeys infected with the chimeric simian-human immunodeficiency virus SHIVSF162p4 (21). Like MAb 2909, these rhesus quaternary structure-specific MAbs neutralized SF162 potently but could not neutralize other HIV-1 wild-type isolates. To examine if these MAbs target the same epitope as MAb 2909, we selected SF162 and 13 N160K mutant strains with high-level sensitivity to 2909 (Table 4). Of the 13 viruses, only 1 (AC10.29_N160K) was neutralized by some of the rhesus monkey quaternary structure-specific MAbs (Table 4). These data are consistent with the recent finding that these rhesus monkey MAbs recognize an epitope partially overlapping the 2909 epitope but display different amino acid and glycosylation pattern requirements for recognition of the Env trimer (21).
Table 4.
IC50 values of six rhesus monkey quaternary structure-specific MAbs against a selected panel of 14 HIV-1 clade A, B, and C Env pseudoviruses sensitive to MAb 2909, either with wild-type Env or with the N160K or K160N (for SF162) mutation
| Virus clade (no. of wild-type viruses) and designation | IC50 (μg/ml) of: |
|||||||
|---|---|---|---|---|---|---|---|---|
| 2909 | PG16 | 2.2G | 2.3E | 2.5B | 1.8E | LW10E | 1.6F | |
| Clade B (5) | ||||||||
| SF162 | 0.015 | >50 | <0.003 | 0.029 | <0.003 | <0.003 | <0.003 | <0.003 |
| SF162_K160N | >50 | 0.002 | >50 | >50 | >50 | >50 | >50 | >50 |
| AC10.29 | >50 | 0.009 | >50 | >50 | >50 | >50 | >50 | >50 |
| AC10.29_N160K | 0.001 | >50 | 0.009 | 0.272 | >50 | 5.4 | >50 | 0.022 |
| 7165.18 | >50 | 0.426 | >50 | >50 | >50 | >50 | >50 | >50 |
| 7165.18_N160K | 0.017 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| WITO.33 | >50 | 0.002 | >50 | >50 | >50 | >50 | >50 | >50 |
| WITO.33_N160K | 0.105 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| YU2 | >50 | 0.258 | >50 | >50 | >50 | >50 | >50 | >50 |
| YU2_N160K | 0.492 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| Clade A (5) | ||||||||
| Q259.17 | >50 | 0.030 | >50 | >50 | >50 | >50 | >50 | >50 |
| Q259.17_N160K | 0.006 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| Q23.17 | >50 | 0.001 | >50 | >50 | >50 | >50 | >50 | >50 |
| Q23.17_N160K | 0.047 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| KNH1209.18 | >50 | 0.283 | >50 | >50 | >50 | >50 | >50 | >50 |
| KNH1209.18_N160K | 0.151 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| DJ263.8 | >50 | 8.2 | >50 | >50 | >50 | >50 | >50 | >50 |
| DJ263.8_N160K | 0.620 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| BS208.B1 | >50 | 0.003 | >50 | >50 | >50 | >50 | >50 | >50 |
| BS208.B1_N160K | 1.1 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| Clade C (4) | ||||||||
| ZM233.6 | >50 | 0.001 | >50 | >50 | >50 | >50 | >50 | >50 |
| ZM233.6_N160K | 0.004 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| Du422.1 | >50 | 0.042 | >50 | >50 | >50 | >50 | >50 | >50 |
| Du422.1_N160K | 0.843 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| CAP45.G3 | >50 | 0.002 | >50 | >50 | >50 | >50 | >50 | >50 |
| CAP45.G3_N160K | 2.17 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| ZM249.1 | >50 | 0.009 | >50 | >50 | >50 | >50 | >50 | >50 |
| ZM249.1_N160K | 3.73 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
The analysis of antibody specificities in sera from HIV-1-infected donors shows that approximately 20% of highly selected, broadly neutralizing sera contain quaternary structure-specific antibodies sensitive to the N160K mutation (16, 28). Additionally, other quaternary structure-specific neutralizing antibodies have recently been reported (3). The observations that quaternary structure-specific antibodies may be generated with reasonable frequency during natural HIV-1 infection and that such antibodies can be broadly reactive with diverse HIV-1 isolates have focused attention on position 160 as a site of HIV-1 vulnerability (15, 26). While the atomic-level structures of PG16 and 2909 Fabs have recently been published (5, 17, 18), the glycan and quaternary requirements for binding to the Env trimer make liganded structures of these antibodies difficult to attain. Hence, nonstructural studies that provide an improved understanding of the antigenic characteristics of this epitope may foster improvements in the design of Env-based vaccine immunogens. Our data strongly suggest that the epitopes defined by 2909 and the PG MAbs are antigenic variants of the same region of the Env trimer. Hence, these antibodies define alternative variants of an immunogenic region in the quaternary structure formed by the V2 and V3 loops. Ongoing efforts seek to find forms of gp120 or full-length HIV-1 Env that can be used as vaccine immunogens to elicit antibodies to this region of vulnerability on the HIV-1 Env. The data reported herein regarding the antigenic properties of the PG and 2909 epitope will help focus design efforts on immunogens that can better elicit the more broadly reactive forms of these quaternary structure-specific antibodies.
Acknowledgments
Support for this work was provided by the Intramural Research Program of the Vaccine Research Center, NIAID, NIH, by the International AIDS Vaccine Initiative, by grants from the NIH (AI36085, HL59725, and AI27742), and by research funds from the Department of Veterans Affairs.
Footnotes
Published ahead of print on 16 February 2011.
REFERENCES
- 1. Ada G. 2001. Vaccines and vaccination. N. Engl. J. Med. 345:1042–1053 [DOI] [PubMed] [Google Scholar]
- 2. Blish C. A., et al. 2009. Cross-subtype neutralization sensitivity despite monoclonal antibody resistance among early subtype A, C, and D envelope variants of human immunodeficiency virus type 1. J. Virol. 83:7783–7788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonsignori M., et al. 2010. Immunoregulation of HIV-1 broadly neutralizing antibody response: deciphering maturation paths for antibody induction. AIDS Res. Hum. Retroviruses 26:A-153 [Google Scholar]
- 4. Burton D. R., et al. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024–1027 [DOI] [PubMed] [Google Scholar]
- 5. Changela A., et al. 2011. Crystal structure of human antibody 2909 reveals conserved features of quaternary structure-specific antibodies that potently neutralize HIV-1. J. Virol. 85:2524–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conley A. J., Gorny M. K., Kessler J. A., II, et al. 1994. Neutralization of primary human immunodeficiency virus type 1 isolates by the broadly reactive anti-V3 monoclonal antibody, 447-52D. J. Virol. 68:6994–7000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doores K. J., Burton D. R. 2010. Variable loop glycan dependency of the broad and potent HIV-1-neutralizing antibodies PG9 and PG16. J. Virol. 84:10510–10521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gorny M. K., et al. 2005. Identification of a new quaternary neutralizing epitope on human immunodeficiency virus type 1 virus particles. J. Virol. 79:5232–5237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gorny M. K., et al. 2006. Cross-clade neutralizing activity of human anti-V3 monoclonal antibodies derived from the cells of individuals infected with non-B clades of human immunodeficiency virus type 1. J. Virol. 80:6865–6872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hioe C. E., et al. 2010. Anti-V3 monoclonal antibodies display broad neutralizing activities against multiple HIV-1 subtypes. PLoS One 5:e10254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Honnen W. J., et al. 2007. Type-specific epitopes targeted by monoclonal antibodies with exceptionally potent neutralizing activities for selected strains of human immunodeficiency virus type 1 map to a common region of the V2 domain of gp120 and differ only at single positions from the clade B consensus sequence. J. Virol. 81:1424–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li M., et al. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108–10125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li M., et al. 2006. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J. Virol. 80:11776–11790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Long E. M., Rainwater S. M., Lavreys L., Mandaliya K., Overbaugh J. 2002. HIV type 1 variants transmitted to women in Kenya require the CCR5 coreceptor for entry, regardless of the genetic complexity of the infecting virus. AIDS Res. Hum. Retroviruses 18:567–576 [DOI] [PubMed] [Google Scholar]
- 15. McElrath M. J., Haynes B. F. 2010. Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity 33:542–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mikell I., et al. 2011. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog. 7:e1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pancera M., et al. 2010. Crystal structure of PG16 and chimeric dissection with somatically related PG9: structure-function analysis of two quaternary-specific antibodies that effectively neutralize HIV-1. J. Virol. 84:8098–8110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pejchal R., et al. 2010. Structure and function of broadly reactive antibody PG16 reveal an H3 subdomain that mediates potent neutralization of HIV-1. Proc. Natl. Acad. Sci. U. S. A. 107:11483–11488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Plotkin S. A. 2008. Vaccines: correlates of vaccine-induced immunity. Clin. Infect. Dis. 47:401–409 [DOI] [PubMed] [Google Scholar]
- 20. Rainwater S. M., et al. 2007. Cloning and characterization of functional subtype A HIV-1 envelope variants transmitted through breastfeeding. Curr. HIV Res. 5:189–197 [DOI] [PubMed] [Google Scholar]
- 21. Robinson J. E., et al. 2010. Quaternary epitope specificities of anti-HIV-1 neutralizing antibodies generated in rhesus macaques infected by the simian/human immunodeficiency virus SHIVSF162P4. J. Virol. 84:3443–3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sanders R. W., et al. 2002. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J. Virol. 76:7293–7305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scanlan C. N., et al. 2002. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1→2 mannose residues on the outer face of gp120. J. Virol. 76:7306–7321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seaman M. S., et al. 2010. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J. Virol. 84:1439–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stiegler G., et al. 2001. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses 17:1757–1765 [DOI] [PubMed] [Google Scholar]
- 26. Walker L. M., Burton D. R. 2010. Rational antibody-based HIV-1 vaccine design: current approaches and future directions. Curr. Opin. Immunol. 22:358–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walker L. M., et al. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walker L. M., et al. 2010. A limited number of antibody specificities mediate broad and potent serum neutralization in selected HIV-1 infected individuals. PLoS Pathog. 6:e1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu X., et al. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou T., et al. 2010. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 329:811–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou T., et al. 2007. Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature 445:732–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zolla-Pazner S. 2004. Identifying epitopes of HIV-1 that induce protective antibodies. Nat. Rev. Immunol. 4:199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zwick M. B., et al. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 75:10892–10905 [DOI] [PMC free article] [PubMed] [Google Scholar]