Abstract
Human T-cell leukemia virus type 1 (HTLV-1) infection and transformation are associated with an incremental switch in the expression of the Src-related protein tyrosine kinases Lck and Lyn. We examined the physical and functional interactions of Lyn with receptors and signal transduction proteins in HTLV-1-infected T cells. Lyn coimmunoprecipitates with the interleukin-2 beta receptor (IL-2Rβ) and JAK3 proteins; however, the association of Lyn with the IL-2Rβ and Lyn kinase activity was independent of IL-2 stimulation. Phosphorylation of Janus kinase 3 (JAK3) and signal transducers and activator of transcription 5 (STAT5) proteins was reduced by treatment of cells with the Src kinase inhibitor PP2 or by ectopic expression of a dominant negative Lyn kinase protein.
Human T-cell leukemia virus type 1 (HTLV-1) Tax protein is necessary and sufficient for T-cell immortalization in vitro and displays transforming activity in transgenic mice (1, 8, 10, 11, 28, 31). In addition to activating virus transcription, Tax alters cell cycle regulation and cellular gene expression profiles via its interactions with transcription factors, signal transduction proteins, and cell cycle regulators (15, 35, 47). Soon after HTLV-1-immortalized cell clones are established, a switch in Src-related protein kinases occurs during the course of T-cell immortalization (1, 18, 43, 46). T cells express both Lyn and Lck protein tyrosine kinases, but after prolonged passage, the immortalized cells express only Lyn (1, 18). In HTLV-1-transformed (interleukin-2 [IL-2]-independent) cell lines, Lyn and the constitutive activation of Janus kinase 3 (JAK3) and signal transducers and activator of transcription 5 (STAT5) proteins completely replace Lck (24, 44, 49). Lck is primarily associated with the T-cell receptor (TCR) signaling complex. Although Lck has also been reported to be associated with the IL-2 receptor (IL-2R), its role in IL-2 signaling is not clear (4, 13, 14, 16, 17, 25, 26, 37). Lyn kinase is expressed in hematopoietic cells of myeloid and B-lymphoid origin but not in T cells (19, 36). Lyn has been shown to influence the phosphorylation of various signaling molecules and transcription factors, including phosphoinositol-3-kinase, Syk, MAP kinase, and the JAK and STAT proteins (2, 3, 7, 20, 32, 40, 45). Lyn was shown to mediate both positive and negative regulation, depending on the cell type and the receptor interaction, of signal transduction, cell proliferation, and apoptosis.
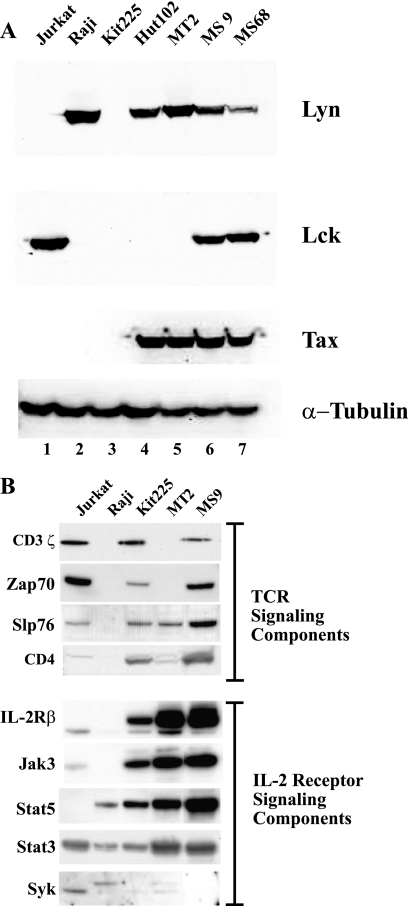
Since Lyn is not usually expressed in T cells, we were interested in identifying proteins with which Lyn interacts and in determining how Lyn influences their activities in HTLV-1-immortalized or -transformed T-cell lines. We first characterized cell lines for their expression of Tax, Lyn, Lck, and components of the TCR and IL-2R signaling pathways. The cell lines included the HTLV-1-transformed (IL-2-independent) T-cell lines Hut102 and MT2; the HTLV-1-immortalized (IL-2-dependent) T-cell lines MS9 (CD4+ CD8−) and MS68 (CD4− CD8+), which were established by infection of activated primary blood lymphocytes with the molecularly cloned provirus pHTLV-XIMT (39, 40); the uninfected T-cell lines Jurkat and IL-2-dependent Kit225; and the B-cell line Raji. All of the HTLV-1-infected cells as well as Raji cells expressed Lyn protein, while the uninfected T-cell lines Jurkat and Kit225 were negative for Lyn (Fig. 1A). Lck was detected in extracts from Jurkat, MS9, and MS68 cells (Fig. 1A). Hut102, MT2, MS9, and MS68 expressed comparable levels of Tax protein, and all of the cell lines expressed the control protein, α-tubulin, at similar levels, indicating equal loading of the proteins (Fig. 1A). Thus, MS9 and MS68 cells expressed both Lyn and Lck, while Kit225 cells expressed neither Lyn nor Lck, suggesting that an alternate Src-related kinase is expressed in these cells. The expression levels of Lyn in both MS9 and MS68 appear to be less than in Hut102 and MT2, but we did not correlate this difference with Tax expression since the amounts of Tax protein detected by immunoblotting were equivalent in Hut102, MT2, MS9, and MS68. Immunoblots probed with antibodies against components of the TCR signaling complex showed that Jurkat, Kit225, and MS9 cells expressed CD3ζ, ZAP70, Slp76, and CD4 proteins (Fig. 1B). In contrast, MT2 cells, with the exception of Slp76, no longer expressed these TCR components. As expected, Raji cell extracts were negative for these TCR components. The expression patterns for components of the IL-2 signaling pathway indicated higher levels of expression of IL-2R beta (IL-2Rβ), JAK3, STAT3, and STAT5 in HTLV-1-infected lines than in the uninfected T-cell lines Jurkat and Kit225 (Fig. 1B). These immunoblot data are in agreement with previously reported changes in protein expression patterns for HTLV-1-immortalized and -transformed cell lines (1, 43). In addition, previous studies have shown that expression of SHP-1 and CD45 is decreased and that expression of Syk is increased in HTLV-1-transformed cells (23, 43).
Fig. 1.
(A) Altered expression of Lyn and Lck in HTLV-1-infected T cells. Protein extracts from the indicated cell lines were immunoblotted with anti-Lyn, anti-Lck, anti-Tax, or anti-α-tubulin antibodies. Jurkat T cells, Kit225 T cells, and Raji B cells served as uninfected controls. The HTLV-1-transformed (IL-2-independent) cell lines Hut102 and MT2 were described previously (27, 33). The IL-2-dependent, HTLV-1-immortalized T-cell lines MS9 and MS68 were established by infection of activated primary blood lymphocytes with the molecularly cloned provirus pHTLV-XIMT (39, 40). MS9 is CD4+ CD8−, and MS68 is CD4− CD8+. The cell lines were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum, and recombinant human IL-2 (100 U/ml; PeproTech, Inc.) was added to the media for Kit225, MS9, and MS68 cells. Cellular extracts were resolved on 4 to 12% Bis-Tris NuPage gels (Invitrogen) and transferred to polyvinylidene difluoride (PVDF) membranes, which were probed with anti-Lyn (catalog no. sc-7274; Santa Cruz), anti-Lck 3A5 (catalog no. 05-435; Upstate Biotechnology, Inc.), anti-α-tubulin (catalog no. 9099; Cell Signaling Technology), or anti-Tax (21) antibody. (B) Expression of proteins involved in TCR and IL-2 receptor signal transduction. Protein extracts from the indicated cell lines were immunoblotted with antibodies to the proteins shown on the left. Anti-IL-2Rβ (catalog no. sc-1046), anti-JAK3 (catalog no. sc-513), anti-Zap70 (catalog no. sc-574), anti-CD3ζ (catalog no. sc-1239), and anti-CD4 (catalog no. sc-7219) antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-Slp76 (catalog no. 06-548) and anti-Syk (catalog no. 06-486) were obtained from Upstate Biotechnology, Inc. (Lake Placid, NY), while anti-STAT3 (catalog no. s21320-L3) and anti-STAT5 (catalog no. s21520-L4) antibodies were obtained from Transduction Laboratories.
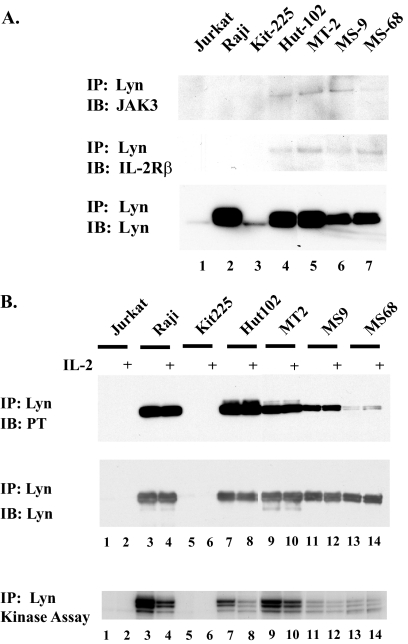
We next determined whether Lyn interacts with components of the IL-2R signaling pathway in HTLV-1-infected cell lines. Our reasoning for suspecting this involvement was based on known associations of Lyn with various cytokine receptors, including IL-2R, in other cell types (42). Cell extracts were immunoprecipitated with anti-Lyn antibody and immunoblotted with anti-JAK3, anti-IL-2Rβ, and anti-Lyn antibodies (Fig. 2A). Anti-Lyn immunoprecipitates from all four HTLV-1-transformed cell lines—HUT102, MT2, MS9, and MS68—contained both IL-2Rβ and JAK3. IL-2Rβ and JAK3 were absent in anti-Lyn immunoprecipitates from Jurkat, Raji, and Kit225 cells (Fig. 2A). We also examined whether Lyn activity is modulated by IL-2 stimulation. Uninfected and HTLV-1-infected cell lines were grown in the absence of serum and IL-2 for 16 h, and half of each culture was then stimulated with IL-2 for 5 min. Immunoprecipitation of cell extracts with anti-Lyn antibody and immunoblotting with anti-phosphotyrosine antibody revealed that Lyn was tyrosine phosphorylated independently of IL-2 stimulation (Fig. 2B, top). While relative levels of Lyn protein on the blot were nearly equivalent (Fig. 2B, middle), Lyn was most highly phosphorylated in Raji, HUT102, and MT2 cells. Assays for autokinase activity in Lyn immunoprecipitates again revealed that Lyn kinase activity was not affected by IL-2 stimulation (Fig. 2B, bottom). Although Lyn is associated with IL-2β and JAK3 of the IL-2R complex in HTLV-1-infected cell lines, the data suggest that Lyn is not activated in response to IL-2 binding. Lyn appears to behave differently in certain transformed cells, since a previous report showed that Lyn interacted with IL-2Rβ in normal human neutrophils and its association with the receptor and its kinase activity were increased in response to IL-2 stimulation (42). Cell cycle-dependent proteins have also been shown to correlate with the IL-2-dependent-to-IL-2-independent transformation of HTLV-1-positive T cells. In particular, p27Kip1 protein is lower in IL-2-independent, HTLV-1-transformed T cells than in IL-2-dependent, HTLV-1-transformed T cells (5), while p21Waf1/Cip1 expression is activated by Tax in a p53-independent manner (6, 48). It would be of interest in future studies to determine whether Lyn expression is correlated with the expression of p27Kip1 and p21Waf1/Cip1.
Fig. 2.
(A) IL-2Rβ and JAK3 are present in anti-Lyn immunoprecipitates from HTLV-1-transformed cells. Protein extracts from the indicated cell lines were immunoprecipitated with anti-Lyn antibody followed by immunoblotting with anti-JAK3 (upper), anti-IL-2Rβ (middle), or anti-Lyn (lower) antibody. (B) Lyn phosphorylation and kinase activity are independent of IL-2 stimulation. The indicated cell lines were grown in the absence of IL-2 for 16 h. Each culture was split into two samples; one was left untreated and the other was stimulated for 5 min with 300 U/ml of IL-2. (A) Immunoprecipitation with anti-Lyn antibody followed by immunoblotting with anti-phosphotyrosine (upper) or anti-Lyn (lower) antibody. (B) Anti-Lyn immunopre-cipitates bound to protein A-agarose beads were incubated with [γ-32P]ATP in kinase reaction buffer. Autophosphorylated Lyn protein was visualized after gel electrophoresis by phosphorimage analysis. IP, immunoprecipitation; IB, immunoblotting.
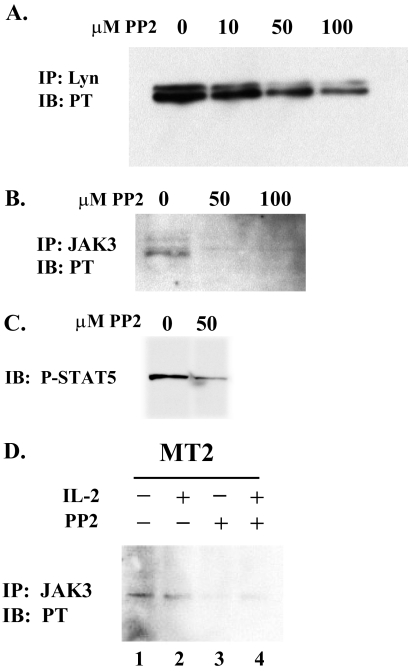
JAK3 and STAT5 are activated in T cells in response to IL-2 stimulation and are constitutively activated in HTLV-1-transformed T cells (24, 44, 49). To test whether the constitutive activation of JAK3 and STAT5 was dependent on Lyn, we treated MT2 cells with the Src kinase-specific inhibitor PP2,which is at least 1,000-fold more active against Src family kinases than other tyrosine kinases such as JAK2 or Zap70 (12). Treatment of MT2 cells with increasing concentrations of PP2 resulted in a dose-dependent decrease in Lyn tyrosine phosphorylation (Fig. 3A). At concentrations of PP2 that significantly reduced Lyn tyrosine phosphorylation, a complete loss of JAK3 tyrosine phosphorylation was observed (Fig. 3B). It should be noted that 50 μM PP2 is well below the level at which any direct effect on JAK3 occurs; therefore, a decrease in Src-related kinase activity appears to be responsible for the loss of JAK3 phosphorylation. We also observed that the constitutive phosphorylation of STAT5 in MT2 cells was inhibited by treatment with PP2 (Fig. 3C). We then examined whether the JAK3 activity is modulated by IL-2 stimulation in HTLV-1-infected cells. MT2 cells were grown in the absence of serum and IL-2 for 16 h, and half of each culture was then stimulated with IL-2 for 5 min. Immunoprecipitation of cell extracts with anti-JAK3 antibody and immunoblotting with anti-phosphotyrosine antibody revealed that JAK3 was tyrosine phosphorylated in MT2 cells independently of IL-2 stimulation and that JAK3 tyrosine phosphorylation is lost in PP2-treated MT2 cells due to a decrease in Src-related kinase activity (Fig. 3D).
Fig. 3.
Inhibition of Lyn kinase activity with PP2 diminishes activation of JAK3 and STAT5. (A through D) MT2 cells were treated for 20 min with the indicated concentrations of PP2. (A) The tyrosine phosphorylation status of Lyn was monitored by immunoprecipitation with anti-Lyn antibody followed by immunoblotting with anti-phosphotyrosine (4G10) antibody. (B) Effects of PP2 on JAK3 activation were determined by immunoprecipitation with anti-JAK3 followed by immunoblotting with 4G10 antibody. (C) Effects of PP2 on STAT5 activation were monitored by immunoblotting cell extracts with anti-phosphoSTAT5 antibody. (D) Effects of PP2 on IL-2-stimulated or unstimulated MT2 cells were determined by immunoprecipitation with anti-JAK3 followed by immunoblotting with anti-4G10. PT, phosphotyrosine.
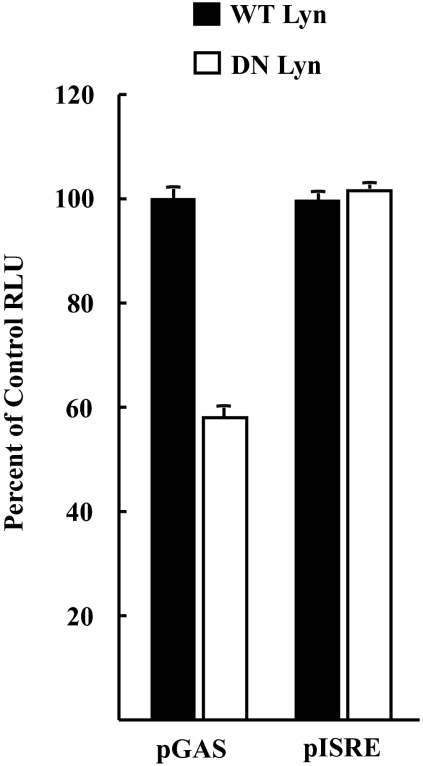
Since PP2 could have been inhibiting other protein tyrosine kinases nonspecifically, a second, more specific approach was used to test the role of Lyn on STAT5 activation in MT2 cells. Endogenous Lyn activity was inhibited by ectopic expression of a mutated Lyn protein with an inactive kinase domain that was previously shown to display a dominant negative phenotype (29). Wild-type or dominant negative Lyn expression plasmids were cotransfected into MT2 cells in combination with luciferase reporter plasmids whose promoters contained multiple copies of GAS or ISRE elements. The GAS element is responsive to STAT5, whereas the ISRE reporter is activated by a STAT1/STAT2 heterodimer (9, 22, 30, 41). The dominant negative Lyn protein inhibited expression from the STAT5-responsive pGAS reporter but had no effect on the pISRE promoter (Fig. 4), indicating that Lyn is required for optimal STAT5 activation in MT2 cells. These data are consistent with the results of PP2 inhibition experiments and support the conclusion that Lyn contributes to JAK/STAT activation in HTLV-1-transformed T cells. Thus, Lyn appears to be involved in JAK/STAT activation in MT2 cells. It is unlikely that Lyn is responsible for the constitutive activation of JAK3 and STAT5, since Lyn is expressed in HTLV-1-immortalized T cells (e.g., MS9 and MS68), where JAK3 and STAT5 are activated only in response to cytokine stimulation. However, it appears that Lyn is necessary to transduce the signals that lead to sustained JAK/STAT activation. We found another Src family kinase expressed in B cells, FynB, to be overexpressed and hyperactivated in the HTLV-1 cell line C91, although we did not examine the effect, if any, of FynB and the JAK/STAT pathway (43). The participation of Lyn in JAK/STAT signaling in HTLV-1-infected T cells in our studies provides yet another example of a more general cooperation between Src family kinases with JAK/STAT proteins (34).
Fig. 4.
Expression of dominant negative Lyn in MT2 cells inhibits STAT5-dependent promoter activity. MT2 cells were transfected with reporter plasmid pGAS-luc (STAT5 responsive) or pISRE-Luc (STAT1/STAT2 responsive) in combination with either wild-type (WT) or dominant negative (DN) Lyn expression plasmids. Luciferase activities are expressed relative to that of wild-type Lyn cotransfected with each reporter plasmid. RLU, relative light unit.
Acknowledgments
We thank Daniel McVicar for helpful discussions, Diana Linnekin for providing plasmids and for critical review of the manuscript, and Boguslawa Korona for technical assistance.
M. Shuh is grateful to the Louisiana Cancer Research Consortium and the Louisiana Board of Regents (RC/EEP-12) for funding and to Steven W. Rick for his unwavering support.
D. Derse was the original corresponding author and principal investigator of this research. He passed away on 9 October 2009 (38), and G. Heidecker is now the contact person for the Derse laboratory.
Footnotes
Published ahead of print on 23 February 2011.
REFERENCES
- 1. Akagi T., Ono H., Shimotohno K. 1995. Characterization of T cells immortalized by Tax1 of human T-cell leukemia virus type 1. Blood 86:4243–4249 [PubMed] [Google Scholar]
- 2. Alam R., Pazdrak K., Stafford S., Forsythe P. 1995. The interleukin-5/receptor interaction activates Lyn and Jak2 tyrosine kinases and propagates signals via the Ras-Raf-1-MAP kinase and the Jak-STAT pathways in eosinophils. Int. Arch. Allergy Immunol. 107:226–227 [DOI] [PubMed] [Google Scholar]
- 3. Al-Shami A., Naccache P. H. 1999. Granulocyte-macrophage colony-stimulating factor-activated signaling pathways in human neutrophils. Involvement of Jak2 in the stimulation of phosphatidylinositol 3-kinase. J. Biol. Chem. 274:5333–5338 [DOI] [PubMed] [Google Scholar]
- 4. Brockdorff J., et al. 2000. Lck is involved in interleukin-2 induced proliferation but not cell survival in human T cells through a MAP kinase-independent pathway. Eur. Cytokine Netw. 11:225–231 [PubMed] [Google Scholar]
- 5. Cereseto A., Washington Parks R., Rivadeneira E., Franchini G. 1999. Limiting amounts of p27Kip1 correlates with constitutive activation of cyclin E-CDK2 complex in HTLV-I-transformed T-cells. Oncogene 18:2441–2450 [DOI] [PubMed] [Google Scholar]
- 6. Chowdhury I. H., et al. 2003. Human T-cell leukemia virus type 1 Tax activates cyclin-dependent kinase inhibitor p21/Waf1/Cip1 expression through a p53-independent mechanism: inhibition of cdk2. Int. J. Cancer 107:603–611 [DOI] [PubMed] [Google Scholar]
- 7. Corey S., et al. 1993. Granulocyte macrophage-colony stimulating factor stimulates both association and activation of phosphoinositide 3OH-kinase and src-related tyrosine kinase(s) in human myeloid derived cells. EMBO J. 12:2681–2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coscoy L., et al. 1998. Molecular mechanism of tumorigenesis in mice transgenic for the human T cell leukemia virus Tax gene. Virology 248:332–341 [DOI] [PubMed] [Google Scholar]
- 9. Ghislain J. J., Wong T., Nguyen M., Fish E. N. 2001. The interferon-inducible Stat2:Stat1 heterodimer preferentially binds in vitro to a consensus element found in the promoters of a subset of interferon-stimulated genes. J. Interferon Cytokine Res. 21:379–388 [DOI] [PubMed] [Google Scholar]
- 10. Grossman W. J., et al. 1995. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. U. S. A. 92:1057–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hall A. P., et al. 1998. Tumours derived from HTLV-I tax transgenic mice are characterized by enhanced levels of apoptosis and oncogene expression. J. Pathol. 186:209–214 [DOI] [PubMed] [Google Scholar]
- 12. Hanke J. H., et al. 1996. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 271:695–701 [DOI] [PubMed] [Google Scholar]
- 13. Hatakeyama M., et al. 1991. Interaction of the IL-2 receptor with the src-family kinase p56lck: identification of novel intermolecular association. Science 252:1523–1528 [DOI] [PubMed] [Google Scholar]
- 14. Horak I. D., et al. 1991. T-lymphocyte interleukin 2-dependent tyrosine protein kinase signal transduction involves the activation of p56lck. Proc. Natl. Acad. Sci. U. S. A. 88:1996–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson J. M., Harrod R., Franchini G. 2001. Molecular biology and pathogenesis of the human T-cell leukaemia/lymphotropic virus type-1 (HTLV-1). Int. J. Exp. Pathol. 82:135–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karnitz L., et al. 1992. Effects of p56lck deficiency on the growth and cytolytic effector function of an interleukin-2-dependent cytotoxic T-cell line. Mol. Cell. Biol. 12:4521–4530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kobayashi N., et al. 1993. Functional coupling of the src-family protein tyrosine kinases p59fyn and p53/56lyn with the interleukin 2 receptor: implications for redundancy and pleiotropism in cytokine signal transduction. Proc. Natl. Acad. Sci. U. S. A. 90:4201–4205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koga Y., et al. 1989. Absence of transcription of lck (lymphocyte specific protein tyrosine kinase) message in IL-2-independent, HTLV-I-transformed T cell lines. J. Immunol. 142:4493–4499 [PubMed] [Google Scholar]
- 19. Korade-Mirnics Z., Corey S. J. 2000. Src kinase-mediated signaling in leukocytes. J. Leukoc. Biol. 68:603–613 [PubMed] [Google Scholar]
- 20. Kurosaki T., et al. 1994. Syk activation by the Src-family tyrosine kinase in the B cell receptor signaling. J. Exp. Med. 179:1725–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Langton B. C., et al. 1988. Development and characterization of monoclonal antibodies to the HTLV-I (P40X) protein. Med. Virol. 8:295–302 [Google Scholar]
- 22. Li X., Leung S., Burns C., Stark G. R. 1998. Cooperative binding of Stat1-2 heterodimers and ISGF3 to tandem DNA elements. Biochimie 80:703–710 [DOI] [PubMed] [Google Scholar]
- 23. Migone T. S., et al. 1998. Recruitment of SH2-containing protein tyrosine phosphatase SHP-1 to the interleukin 2 receptor; loss of SHP-1 expression in human T-lymphotropic virus type I-transformed T cells. Proc. Natl. Acad. Sci. U. S. A. 95:3845–3850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Migone T. S., et al. 1995. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science 269:79–81 [DOI] [PubMed] [Google Scholar]
- 25. Mills G. B., et al. 1992. Neither the LCK nor the FYN kinases are obligatory for IL-2-mediated signal transduction in HTLV-I-infected human T cells. Int. Immunol. 4:1233–1243 [DOI] [PubMed] [Google Scholar]
- 26. Minami Y., et al. 1993. Association of p56lck with IL-2 receptor beta chain is critical for the IL-2-induced activation of p56lck. EMBO J. 12:759–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miyoshi I., et al. 1981. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature 294:770–771 [DOI] [PubMed] [Google Scholar]
- 28. Nerenberg M. I. 1990. An HTLV-I transgenic mouse model: role of the tax gene in pathogenesis in multiple organ systems. Curr. Top. Microbiol. Immunol. 160:121–128 [DOI] [PubMed] [Google Scholar]
- 29. O'Laughlin-Bunner B., et al. 2001. Lyn is required for normal stem cell factor-induced proliferation and chemotaxis of primary hematopoietic cells. Blood 98:343–350 [DOI] [PubMed] [Google Scholar]
- 30. Ooi G. T., Hurst K. R., Poy M. N., Rechler M. M., Boisclair Y. R. 1998. Binding of STAT5a and STAT5b to a single element resembling a gamma-interferon-activated sequence mediates the growth hormone induction of the mouse acid-labile subunit promoter in liver cells. Mol. Endocrinol. 12:675–687 [DOI] [PubMed] [Google Scholar]
- 31. Ozden S., Coscoy L., Gonzalez-Dunia D. 1996. HTLV-I transgenic models: an overview. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 13:S154–161 [DOI] [PubMed] [Google Scholar]
- 32. Pleiman C. M., et al. 1993. Mapping of sites on the Src family protein tyrosine kinases p55blk, p59fyn, and p56lyn which interact with the effector molecules phospholipase C-gamma 2, microtubule-associated protein kinase, GTPase-activating protein, and phosphatidylinositol 3-kinase. Mol. Cell. Biol. 13:5877–5887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Posner L. E., et al. 1981. Natural antibodies to the human T cell lymphoma virus in patients with cutaneous T cell lymphomas. J. Exp. Med. 154:333–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reddy E. P., Korapati A., Chaturvedi P., Rane S. 2000. IL-3 signaling and the role of Src kinases, JAKs and STATs: a covert liaison unveiled. Oncogene 19:2532–2547 [DOI] [PubMed] [Google Scholar]
- 35. Ressler S., Connor L. M., Marriott S. J. 1996. Cellular transformation by human T-cell leukemia virus type I. FEMS Microbiol. Lett. 140:99–109 [DOI] [PubMed] [Google Scholar]
- 36. Scapini P., Pereira S., Zhang H., Lowell C. A. 2009. Multiple roles of Lyn kinase in myeloid cell signaling and function. Immunol. Rev. 228:23–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schmandt R., et al. 1992. T-lymphocyte proliferation: tyrosine kinases in interleukin 2 signal transduction. Baillieres Clin. Haematol. 5:551–573 [DOI] [PubMed] [Google Scholar]
- 38. Shuh M. 2009. David D. Derse, 1949–2009. Retrovirology 6:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shuh M., Hill S. A., Derse D. 1999. Defective and wild-type human T-cell leukemia virus type I proviruses: characterization of gene products and trans-interactions between proviruses. Virology 262:442–451 [DOI] [PubMed] [Google Scholar]
- 40. Simon H. U., Yousefi S., Dibbert B., Levi-Schaffer F., Blaser K. 1997. Anti-apoptotic signals of granulocyte-macrophage colony-stimulating factor are transduced via Jak2 tyrosine kinase in eosinophils. Eur. J. Immunol. 27:3536–3539 [DOI] [PubMed] [Google Scholar]
- 41. Soldaini E., et al. 2000. DNA binding site selection of dimeric and tetrameric Stat5 proteins reveals a large repertoire of divergent tetrameric Stat5a binding sites. Mol. Cell. Biol. 20:389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wei S., et al. 2000. IL-2 induces the association of IL-2Rbeta, lyn, and MAP kinase ERK-1 in human neutrophils. Immunobiology 202:363–382 [DOI] [PubMed] [Google Scholar]
- 43. Weil R., et al. 1999. Altered expression of tyrosine kinases of the Src and Syk families in human T-cell leukemia virus type 1-infected T-cell lines. J. Virol. 73:3709–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu X., et al. 1995. Constitutive activation of different Jak tyrosine kinases in human T cell leukemia virus type 1 (HTLV-1) tax protein or virus-transformed cells. J. Clin. Invest. 96:1548–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yamanashi Y., et al. 1992. Activation of Src-like protein-tyrosine kinase Lyn and its association with phosphatidylinositol 3-kinase upon B-cell antigen receptor-mediated signaling. Proc. Natl. Acad. Sci. U. S. A. 89:1118–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamanashi Y., et al. 1989. Selective expression of a protein-tyrosine kinase, p56lyn, in hematopoietic cells and association with production of human T-cell lymphotropic virus type I. Proc. Natl. Acad. Sci. U. S. A. 86:6538–6542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yoshida M. 1993. HTLV-1 Tax: regulation of gene expression and disease. Trends Microbiol. 1:131–135 [DOI] [PubMed] [Google Scholar]
- 48. Zhang L., Zhi H., Liu M., Kuo Y. L., Giam C. Z. 2009. Induction of p21(CIP1/WAF1) expression by human T-lymphotropic virus type 1 Tax requires transcriptional activation and mRNA stabilization. Retrovirology 6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang Q., et al. 1999. Differences in phosphorylation of the IL-2R associated JAK/STAT proteins between HTLV-I(+), IL-2-independent and IL-2-dependent cell lines and uncultured leukemic cells from patients with adult T-cell lymphoma/leukemia. Leuk. Res. 23:373–384 [DOI] [PubMed] [Google Scholar]