Abstract
Enteroviruses (EVs), members of the family Picornaviridae, are a genetically and antigenically diverse range of viruses causing acute infections in humans and several Old World monkey (OWM) species. Despite their known wide distribution in primates, nothing is currently known about the occurrence, frequency, and genetic diversity of enteroviruses infecting apes. To investigate this, 27 chimpanzee and 27 gorilla fecal samples collected from undisturbed jungle areas with minimal human contact in Cameroon were screened for EVs. Four chimpanzee samples were positive, but none of the gorilla samples were positive. Genetic characterization of the VP1, VP4, and partial VP2 genes, the 5′ untranslated region, and partial 3Dpol sequences enabled chimpanzee-derived EVs to be identified as (i) the species A type, EV76, (ii) a new species D type assigned as EV111, along with a human isolate from the Democratic Republic of Congo previously described by the International Committee on the Taxonomy of Viruses, and (iii) a new species B type (assigned as EV110) most closely related to, although a distinct type from, the SA5 isolate recovered from a vervet monkey. The identification of EVs infecting chimpanzees related to those circulating in human and OWM populations provides evidence for cross-species transmission of EVs between primates. However, the direction of transfer and the existence of primate sources of zoonotic enterovirus infections in humans require further investigation of population exposure and more extensive characterization of EVs circulating in wild ape populations.
INTRODUCTION
The genus Enterovirus within the family Picornaviridae is comprised of more than 300 serotypes, most of which are known human pathogens and circulate widely worldwide. The Enterovirus genus comprises at least 10 species, including 3 species of human rhinoviruses and 4 species of human enteroviruses (HEV) (A to D) along with further species infecting pigs and cows (bovine and porcine enteroviruses) and nonhuman primates (10, 35). Isolates within the four enterovirus species were originally classified by their antigenic and pathogenic properties in humans and laboratory animals: this divided them into polioviruses (PV) (3 serotypes), coxsackie A viruses (CAV) (23 serotypes), coxsackie B viruses (CBV) (6 serotypes), echoviruses (E) (28 serotypes), and enteroviruses 68 to 71. More recently, it has been shown that VP1 sequences are predictive of enterovirus serotype and have enabled serotype identification through genetic comparisons instead of often laborious serological classification (22, 24). Indeed, the sequence of VP1 has been considered adequate for designation of new types in the absence of serological data by the Internal Committee for Taxonomy of Viruses (ICTV) Picornavirus Study Group (PSG) (35); this has enabled several more enteroviruses to be formally classified into species and types (enterovirus 73 onwards) without there necessarily being information on antigenic cross-reactivity to assign serotype status. These genetically characterized types have been numbered chronologically (33 by the end of July 2010), leading to a combined total of 97 (sero)types.
As well as infecting humans, a series of enterovirus species A isolates have been recovered from Old World monkey (OWM) species, primarily macaques (A13, SV19, SV43, SV46, and EV92) (4, 6–8, 23). Further isolates have been described that are more similar to human species B viruses (SA5 [19]) or are genetically distinct and are classified provisionally into two separate (simian) enterovirus species, SEV-A and -B (8, 9, 23, 26, 27, 29, 30). These OWM-derived enteroviruses were originally isolated in the 1950s to 1960s from primate cell cultures during the development of tissue culture methods or from clinical specimens obtained from captive or wild-caught primates extensively used in biomedical research. Source OWM species included Macaca mulatta (rhesus macaque), Macaca fascicularis (cynomolgus monkey), Chlorocebus aethiops (African green monkey or vervet), and Papio cynocephalus (baboon). More recently, two new enterovirus types were identified in fecal samples obtained from Macaca mulatta (rhesus macaque), Macaca nemestrina (pigtail macaques), and Cercocebus atys (sooty mangabeys) with diarrheal disease at a U.S. primate center (21).
Although simian enteroviruses have been occasionally associated with acute gastroenteritis in monkeys, it is unclear whether these viruses have been the primary cause of clinical illness (7, 21). Similarly, almost no systematic information on their infection frequencies, epidemiology, or genetic diversity has been obtained in the period since the original discovery of simian viruses in cell cultures in the 1950s. Our current lack of knowledge extends to enteroviruses that might infect chimpanzees, gorillas, and other nonhuman apes. This is of concern given the possibility that our closest primate relatives may indeed harbor enteroviruses capable of cross-species transmission and thus acting as a potential source of new human epidemics.
To address this fundamental gap in current knowledge, we investigated species and serotypes of enteroviruses detected in fecal samples obtained from wild chimpanzees and gorillas in Cameroon. Our findings of viruses within species A, B, and D and detection of a serotype (EV76; species A) widespread in human populations provides evidence for cross-species transmission of enteroviruses and heightens concerns about the primate reservoirs as a potential source of emerging enterovirus infections.
MATERIALS AND METHODS
Samples.
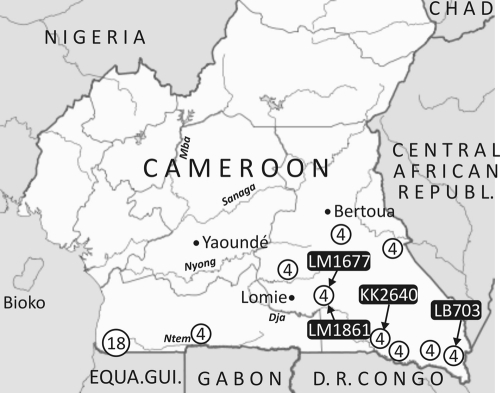
A total of 27 chimpanzees (Pan troglodytes troglodytes) and 27 gorillas (Western lowland gorilla; Gorilla gorilla gorilla) stool samples previously collected for simian immunodeficiency virus (SIV) screening were studied (13, 20, 36). Samples were collected from wild-living apes at 10 forest sites located in the southern part of Cameroon (Fig. 1); at each site equal numbers of samples from chimpanzees and gorillas were tested. Fecal samples were collected primarily around night nests or feeding sites from the jungle floor of remote, sparsely populated forested areas of Cameroon with minimal human presence. Samples were collected by experienced trackers, preserved in RNAlater (Ambion, Austin, TX), stored at base camps at room temperature for a maximum of 20 days, and subsequently transported to a central laboratory in Yaounde. The species origin of the fecal samples was determined by mitochondrial DNA analysis as previously described (36).
Fig. 1.
Chimpanzee and gorilla sample locations showing the combined number from each collection site (sites with 18 samples had 9 chimpanzee and 9 gorilla samples; sites with 4 samples had 2 chimpanzee and 2 gorilla samples). The positive chimpanzee samples are indicated.
Sample extraction and amplification.
RNA was extracted from all fecal samples as previously described (31) and subjected to cDNA synthesis using the random hexamer primers according to the manufacturer's instructions (Promega, United Kingdom). Primers targeting the 5′ untranslated region (5′UTR) conserved across the Enterovirus genus were used for initial screening, and additional primer sets were designed to obtain sequence data from VP4, VP1, and 3Dpol regions from positive samples. Amplification of the 5′UTR used three sets of overlapping primers (listed in the following format: target virus, region position [numbered according to the poliovirus Leon serotype 3 sequence, accession number K01392], orientation [s, sense; a, antisense], sequence [5′ to 3′ direction]).
Set 1: see reference 2. Set 2: EV_5′UTR_1s, TTA AAA CAG CCT GTG GGT TG; EV_5′UTR_1s, TTA AAA CAG CTC TGG GGT TG; EV_5′UTR_379a, GGY WGG CCG CCA ACG CAG; EV_5′UTR_264a, TGN TAC TAG GYT TCY CGA AGT A. Set 3: EV_5′UTR_0070s, GGN AYY YTW GTR CGC CTG TTT T; EV_5′UTR_309s, TGT AGH TYW GGT CGA TGA GTC; EV_5′UTR_370s, GGC TGC GYT GGC GGC CTR C.
The VP4 region from all species was amplified using previously described primers and conditions (2). VP1 amplification required two sets of overlapping primers spanning the coding region from each species, and additional primers specific to individual serotypes or strains (as indicated in primer name).
Species A.
Set 1: EVA_VP1_2268s, CCN TGG ATH AGY AAC CAN CAY T; EVA_VP1_2332s, TNA SNA TYT GGT AYC ARA CAN AYT; EVA_VP1_3016a, GAN GGR TTN GTN GKN GTY TGC CA; EVA_VP1_3109a, GGR TAN CCR TCR TAR AAC CAY TG. Set 2: LM1677_VP1_2925s, CAG ACA ATG CTG CAG TAC ATG TAT GT; LM1677_VP1 2865s, GCT GAG TTC ACC TTT GTC GCC ACA; EV76_VP1_3475a, TCC CAC ACY AAR TTY TCC CAR TC; EV76_VP1_3604a, CCR AAR GTG ACN GGG TAR TGY YT.
Species B.
Set 1: SA5_VP1_2265s, GTN CCN TGG ATY AGY CAR ACM CAY TA; SA5_VP1_2325s, GGN TAY ATT ACN TGY TGG TAY CAR AC; SA5_VP1_3016a, CTG GGR TTN GTD GAN GAY TGC CA; SA5_VP1_3043a, GGN GCR TTN CCY TCN GTC CAR AA. Set 2: SA5_VP1_2994s, TGG CAR TCN TCN ACN AAY CCC AG; SA5_VP1_3021s, TTN TGG ACN GAR GGN AAY GCN CC; SA5_VP1_3493a, AGG TCY CTR TTV YAR TCY TCC CA; SA5_VP1_3610a, GGS CCY TGR AAN GAV ACN GGG TA.
Species D.
Set 1: EVD_VP1_2926s, AYY TGA RAC TMC AAG CNA TGT WTG TWC C; EVD_VP1_3057s, ATA CCW TTM ATG WGC ATN AAC TCM GCW TAT; EVD_VP1_3623a, CCA YTG RAT YCC WGG NCC WTC AAA RC; EVD_VP1_3847a, TTY TGN ATG TAA TCW GTN ATA CCY TGT TCC AT. Set 2: EVD_VP1_2331s, GTN ACM TGT TTY ATG CAR ACM AAC CT; EVD_VP1_2313s, AAT GCW AAT GTM GGN TAT GTN ACM TG; KK2640_VP1_3213a, TGA CCA CTC TGA GAC ACA GAT TGC C; KK2640_VP1_3185a, CAT GGT GTT GGC TGG ATT GAT TCC.
The 3Dpol region was amplified by nested primers specific for each EV species.
Species A.
EVA_3Dpol_6850s, CNG GHA CHT CCA THT TYA AYT CVA TG; EVA_3Dpol_6942s, AAH ATG GTN GCY TAY GGR GAY GAY GT; EVA_3Dpol 7405as, ACG ACC AGA YYH CTR GTG GGG TT; EVA_3Dpol_7448as, GCT ATT CTG GTT ATA ACA AAT TTA CCC.
Species B.
For species B, see reference 1.
Species D.
HEVD_3Dpol_7227a, GGA TCT MAC ATG RTC WTG WGT GTT TC; HEVD_3Dpol_7175a, TCA TGD ATY TCY TTC ATT GGC ATN ACT GG; HEVD_3Dpol_6193s, GAG TAY ATG GAG GAR GCN GTG GA; HEVD_3Dpol_6168s, GGT AAC AAA AYY ATG YTA ATG GAT GAG.
For each PCR, 2 μl of cDNA was used for first-round PCR amplification, and 1 μl of the first-round reaction was used for the nested PCR with second-round primers. PCR amplification included 30 cycles of denaturation (94°C, 20 s), annealing (50°C, 18 s), and elongation (72°C, 90 s) in a thermal cycler.
Direct sequencing of PCR products and sequence analysis.
Positive second-round PCR amplicons were sequenced in both directions using the inner sense and inner antisense primers used in the second round of amplification. Sequencing was carried out using BigDye Terminator v3.1 (Applied Biosystems) according to manufacturer's instructions. Amplicons were directly sequenced using Big Dye (ABI) and inner-sense primers. Nucleotide sequences were annotated and aligned using the Simmonics sequencing package, version 1.9 (http://www.virus-evolution.org/Downloads/).
Phylogenetic trees were constructed by the neighbor-joining method from 100 bootstrap samplings of maximum composite likelihood (MCL), Jukes-Cantor (J-C) corrected nucleotide or uncorrected translated amino acid pairwise distances using the MEGA 4.0 software package with pairwise deletion for missing data.
Nucleotide sequence accession numbers.
Sequences obtained in this study have been assigned the following GenBank accession numbers: JF416927 to JF416938.
RESULTS
RNA extracted from the chimpanzee and gorilla fecal samples was screened using 5′UTR primers targeting a highly conserved region between known enterovirus species. Of 27 chimpanzee samples, 4 were positive, while all 27 samples from gorillas collected from the same sites were negative (Fig. 1). To genetically characterize the enteroviruses infecting the chimpanzees, sequences were amplified from the whole VP1 gene, VP4 and part of VP2, most of the 5′UTR, and regions of the 3D polymerase (3Dpol) gene (Fig. 2). Sequences from each region were obtained from three of the four screening-positive samples; repeated attempts to amplify coding sequences from LB703 were unsuccessful. The LB703 5′UTR sequence between positions 360 and 557 showed 91.8% sequence identity to that of LM1861. This is a substantially greater degree of similarity than with other species B types (data not shown), although determining whether it is the same type as LM1861 would require analysis of coding regions.
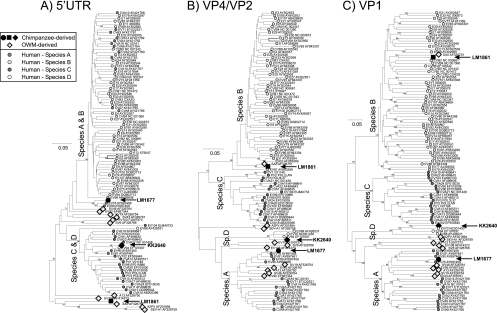
Fig. 2.
Phylogenetic comparisons of 5′UTR (positions 177 to 672, excluding the unalignable hypervariable region) (A), VP4 and partial VP2 (positions 744 to 1147) (B), and VP1 (positions 2457 to 3377) (C) from study samples and a representative sequence from each currently classified human enterovirus (sero)type and the currently available 11 (near)-complete genome sequences of enteroviruses isolated from nonhuman primates. Trees were constructed by neighbor joining using J-C corrected nucleotide pairwise distances (5′UTR) or translated amino acid pairwise distances (VP4/VP2, VP1 regions), with 100-bootstrap resampling to demonstrate robustness of groupings; values of ≥70% are shown.
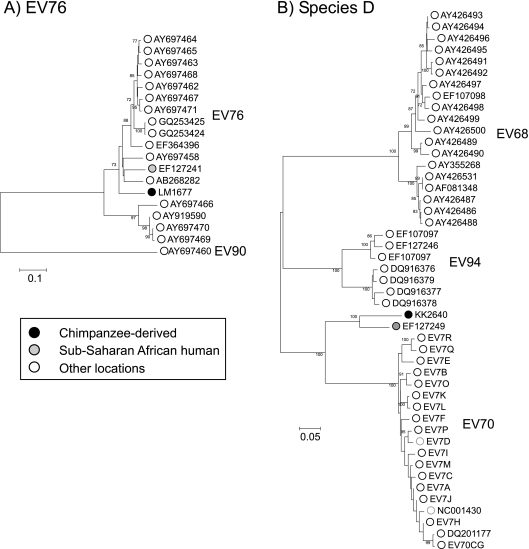
Using the sequence of the complete VP1-coding region, the chimpanzee-derived EVs showed contrasting sequence relationships to previously characterized human and OWM enteroviruses. LM1677 clustered closely with EV76 in the VP1 region (Fig. 2C and Fig. 3B), with nucleotide and translated amino acid distances within the intraserotype range (13.3% and 4.5% to AY919528, respectively, compared to type assignment thresholds of 25% and 15%; Table 1). LM1677 also grouped closely with EV76 in the VP4/VP2 region (along with EV89, EV90, and EV91; Fig. 2B) and the 3Dpol partial region (Fig. 4B). Clustering with EV76 and EV89 but not EV90 and EV91 (which group with species C and D) was additionally evident in the 5′UTR (Fig. 2A).
Fig. 3.
(A) Phylogenetic analysis of the VP1 region LM1677 and all available (>90% complete in VP1 gene) sequences of EV76 and a representative EV91 sequence as an outgroup. (B) KK2640 and all available species D sequences. Trees were constructed by neighbor joining using maximum composite likelihood nucleotide pairwise distances with 100-bootstrap resampling to demonstrate robustness of groupings; values of ≥70% are shown.
Table 1.
Pairwise distances of ape-derived viruses to the most closely simian human and nonhuman primate EVs
| Chimpanzee sample | Virus species | Type | Isolate/strain | Rank | Divergencea |
|
|---|---|---|---|---|---|---|
| Nucleotide | Amino acid | |||||
| LM1677 | A | EV76 | AY919528 | 1 | 13.3% | 4.5%a |
| A | EV76 | AB268282 | 2 | 13.5% | 4.4% | |
| LM1861 | B (simian) | SA5 | AF326751 | 1 | 32.3% | 28.2% |
| B | CVB2 | NC_000881 | 2 | 35.4% | 30.4% | |
| KK2640 | D | EV111b | EF127249 | 1 | 15.0% | 8.1% |
| D | EV70 | EV7E (D17599) | 2 | 25.3% | 16.4% | |
25% (nucleotide)/15% (amino acid) divergence threshold used for designating enterovirus types.
Formerly classified as EV70.
Fig. 4.
Phylogenetic analysis of partial 3Dpol gene sequences of chimpanzee-derived enteroviruses and related viruses from species A, B, and D and those from OWMs. (A) LM1861, SA5, and species B types between positions 5873 and 6419. (B) LM1677, OWM-derived viruses and species A types between positions 6957 and 7175. (C) KK2640 and species D between positions 6252 and 7157. Trees were constructed by neighbor joining using J-C corrected nucleotide pairwise distances with 100-bootstrap resampling to demonstrate robustness of groupings; values of ≥70% are shown.
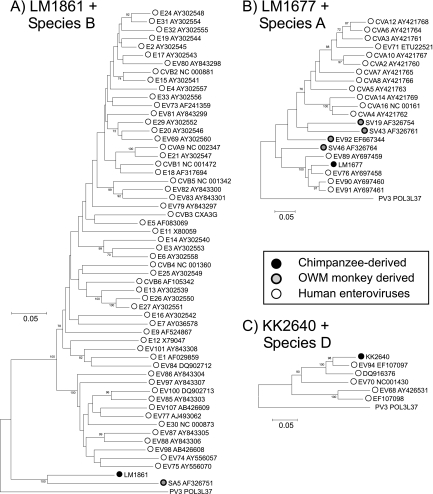
The chimpanzee-derived LM1861 showed greatest similarity to the vervet monkey-derived isolate SA5 in the VP1 region (Fig. 2C), although pairwise distances (32.3% [nucleotide] and 28.2% [amino acid]) were substantially greater than the nucleotide and amino acid type assignment thresholds, indicative of its classification as a new enterovirus type. Although both LM1861 and SA5 fell within the species B clade in the VP1 region, this sequence pair was increasingly divergent from other species B types in the VP4/VP2 region (Fig. 2B), 3Dpol (Fig. 4), and 5′UTR regions (Fig. 2A). Sequences from the latter region were indeed quite distinct from those of human enteroviruses along with several other simian viruses: A13 (species A in coding regions), EV108 (outgroup to species A in coding regions), and the A2/SEV-A1 types classified into a separate enterovirus species (27). Based on sequence divergence in VP1, LM1861 has been formally designated by the ICTV PSG as a new enterovirus type, EV110 in species B (N. J. Knowles et al., personal communication).
The chimpanzee-derived KK2640 grouped within species D, which contains the human enteroviruses EV68, EV70, and EV94 (Fig. 2; Fig. 4). Phylogenies in different genome regions showed minor inconsistencies: KK2640 grouped closely with EV94 in the 5′UTR, showed an outgroup relationship with other species D types in VP4/VP2, and grouped with EV70 in VP1 and with EV94 in 3Dpol. In the VP1 region, nucleotide pairwise distances between KK2640 and other (non-African) EV70 strains ranged from 25.3% (16.4% amino acid) to 28.4% (21.8% amino acid), marginally above the specified 25% (and 15% amino acid) values. However, it showed much closer sequence identity to an enterovirus isolate from the Democratic Republic of the Congo (17-04; GenBank accession number EF127249) previously classified as EV70 (12), with pairwise distances in VP1 of 15.0% (8.1% amino acid, well below the type threshold). The isolate 17-04 also showed a VP1 distance above 25% in VP1 from EV70, indicating that it, along with KK2640, should be assigned a separate type status contrary to its original classification. Based on these findings, the ICTV PSG has assigned formal type status to KK2640, along with 17-04, as enterovirus 111 (Knowles et al., personal communication).
DISCUSSION
The findings in the current study provide new information on the existence and genetic characteristics of enteroviruses infecting chimpanzees and their relatedness to human viruses described from Central Africa and elsewhere. Although limited by survey sample numbers, the findings of the current study provide the first information on the circulation, genetic diversity, and relationship to human and monkey viruses and provide evidence for the occurrence of cross-species transmission between humans and chimpanzees.
Classification of chimpanzee enteroviruses.
Because of extensive recombination in enterovirus genomes (17, 18, 28, 32) and the predominant determining role of the VP1 capsid region in determining neutralization susceptibility (24), both genotypic identification and assignment of types are based on this genomic region (35). The type identification and new enterovirus type assignments have therefore been based on the complete VP1 sequences for three of the four chimpanzee EVs characterized in the current study.
By this means, it was possible to classify LM1677 as a strain of EV type 76, although showing at least 13% sequence divergence from currently described EV76 strains and branching basally in the VP1 tree, along with the only available sub-Saharan human EV76 isolate described to date (12) and the more divergent strains from China and Bangladesh (2, 25). LM1861 showed greatest similarity to an enterovirus first described in 1963 after isolation from a cell culture derived from a vervet monkey (Chlorocebus pygerythrus) (19). However, its 32% nucleotide (28.2% amino acid) divergence from SA5 and greater than 35% (30% amino acid) divergence from species B human enterovirus serotypes support its assignment as a new (species B) serotype, EV110. As with several other simian enteroviruses, sequence relationships in other genomes were inconsistent, falling as an outgroup to species B (along with SA5) in other coding regions and possessing a quite distinct 5′UTR sequence from the species A/B clade formed by human viruses (Fig. 2A).
Finally, KK2640 showed nucleotide sequence distances to EV70 and other species D members in VP1 close to the 25% (15% amino acid) assignment threshold; it, along with the Congolese isolate 17-04 (15% [8.1% amino acid]) previously classified as EV70 (12), have now been assigned as EV111.
Relationship to human enteroviruses.
The discovery of chimpanzee viruses that correspond to existing human serotypes (LM1677 and KK2640, the latter specifically associated with Central African populations) or more distantly to OWM viruses (LM1861 and LB703) provides evidence for cross-species transmission of enteroviruses between primate species and families. Enteroviruses might therefore be added to the growing list of viruses known to be transmitted between humans and primates, including herpesvirus B, monkeypox virus, Ebola/Marburg viruses, and human immunodeficiency virus (1, 3, 5, 13).
Supporting the hypothesis for direct cross-species transmission of enteroviruses between apes and OWMs is the observation of some commonality between chimpanzee serotypes detected in the current study and those known, from rather fragmentary current data, in human populations in Central Africa and from African OWM species. EV76 (together with EV89, EV90, and EV91) was originally isolated from stool specimens obtained from patients presenting with acute flaccid paralysis in Bangladesh (25), although it has also been shown to circulate in the Democratic Republic of the Congo (DRC) (12). Both represent regions where interaction between human and primate populations is frequent and where conditions are favorable for transmission of viruses (11). EV76 (from both chimpanzees and humans), EV89, EV90, and EV91 additionally cluster closely together with the currently described OWM HEV-A serotypes (A13, SV19, SV43, SV46, and EV92); three of these were originally isolated from Asian primates of the genus Macaca, one from African Papio, and one from a captive macaque in a primate center in the United States (21, 23, 27). Although no data exists to indicate whether OWMs in Bangladesh/South Asia or humans in Cameroon currently harbor enteroviruses resembling the EV76/EV89/EV90/EV91 cluster, the finding of EV76 in a chimpanzee provides evidence that this geographical overlap may indeed result in cross-species transmissions of enteroviruses.
A similar overlap is apparent for species D enteroviruses. Sample KK2640, now classified as EV111, was most closely related to 17-04, isolated in the DRC on 2001 (12). EV70 was indeed first discovered in Ghana in 1969 during an acute hemorrhagic conjunctivitis epidemic (15) and has subsequently caused two global human pandemics with over 30 million cases reported worldwide (14), consistent with a sub-Saharan origin for this virus. A putative African origin for EV94 in species D was suggested by its initial detection in acute flaccid paralysis patients in the DRC and its recovery from wastewater in Egypt as part of a poliovirus surveillance program (12, 34). Furthermore, neutralizing antibodies against EV70 have been detected in the sera of African cattle, sheep, swine, chickens, goats, and dogs (16), implying a much wider host range than typically found in other enterovirus species and consistent with in vitro observations of its ability, along with EV94 to replicate in the cells derived from a wide range of mammalian species (33, 34, 37). The finding of a further species D enterovirus in chimpanzees is thus entirely consistent with widespread circulation in a range of mammalian species in sub-Saharan Africa.
Two samples, LB703 and LM1861, grouped with the African vervet monkey-derived isolate SA5 (19), classified as a divergent member of species B (27). Although the distribution of Chlorocebus species is typically in African savannah habitats, it is possible that this and related enteroviruses are widely distributed in a range of OWM species in sub-Saharan Africa that represent potential sources of chimpanzee infections detected in the current study.
Future, larger-scale sampling of remaining wild populations of chimpanzees and gorillas and other nonhuman primates is required for a better understanding of the epidemiology and direction of transmission of enteroviruses between ape, human, and OWM species. Wild-living chimpanzees are highly endangered and typically live in remote jungle areas with minimal human contact. The number of chimpanzees has fallen from an estimated one million 50 years ago to approximately 200,000 individuals to date; this population size and now highly fragmented habitat may not be large enough to maintain enterovirus circulation within the species. Population sizes of gorillas would be even more restrictive for circulations of enteroviruses and other acute virus infections. Although it is possible that chimpanzee viruses detected in the current study may be indigenous to chimpanzees, we also have to consider the alternative hypothesis that they may have been directly acquired though human- or monkey-contaminated environmental sources. To resolve the direction of transmission between humans and apes and to explore the transmission dynamics between nonhuman primates requires further investigations of enterovirus exposure in apes, different monkey species, and humans in these areas.
ACKNOWLEDGMENTS
We thank the staff from PRESICA for logistical support in Cameroon and the Cameroonian Ministries of Health, Environment and Forestry, and Research for permission to collect fecal samples in Cameroon.
The fecal sample collection was supported by grants from the National Institutes of Health (R01 AI50529), the Agence National de Recherches sur le SIDA, France (ANRS 12125 and ANRS 12182), and the Institut de Recherche pour le Développement (IRD).
Footnotes
Published ahead of print on 23 February 2011.
REFERENCES
- 1. Bailes E., et al. 2003. Hybrid origin of SIV in chimpanzees. Science 300:1713. [DOI] [PubMed] [Google Scholar]
- 2. Bingjun T., et al. 2008. Molecular typing and epidemiology of non-polio enteroviruses isolated from Yunnan Province, the People's Republic of China. J. Med. Virol. 80:670–679 [DOI] [PubMed] [Google Scholar]
- 3. Brown D. W. 1997. Threat to humans from virus infections of non-human primates. Rev. Med. Virol. 7:239–246 [DOI] [PubMed] [Google Scholar]
- 4. Fuentes-Marins R., Rodriguez A. R., Kalter S. S., Hellman A., Crandell R. A. 1963. Isolation of enteroviruses from the “normal” baboon (Papio doguera). J. Bacteriol. 85:1045–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gonzalez J. P., Pourrut X., Leroy E. 2007. Ebolavirus and other filoviruses. Curr. Top. Microbiol. Immunol. 315:363–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heberling R. L., Cheever F. S. 1965. Some characteristics of the simian enteroviruses. Am. J. Epidemiol. 81:106–123 [DOI] [PubMed] [Google Scholar]
- 7. Hoffert W. R., Bates M. E., Cheever F. S. 1958. Study of enteric viruses of simian origin. Am. J. Hyg. (Lond.) 68:15–30 [DOI] [PubMed] [Google Scholar]
- 8. Hull R. N., Minner J. R., Mascoli C. C. 1958. New viral agents recovered from tissue cultures of monkey kidney cells. III. Recovery of additional agents both from cultures of monkey tissues and directly from tissues and excreta. Am. J. Hyg. (Lond.) 68:31–44 [DOI] [PubMed] [Google Scholar]
- 9. Hull R. N., Minner J. R., Smith J. W. 1956. New viral agents recovered from tissue cultures of monkey kidney cells. I. Origin and properties of cytopathogenic agents S.V.1, S.V.2, S.V.4, S.V.5, S.V.6, S.V.11, S.V.12 and S.V.15. Am. J. Hyg. (Lond.) 63:204–215 [DOI] [PubMed] [Google Scholar]
- 10. Hyypia T., Hovi T., Knowles N. J., Stanway G. 1997. Classification of enteroviruses based on molecular and biological properties. J. Gen. Virol. 78:1–11 [DOI] [PubMed] [Google Scholar]
- 11. Jones-Engel L., Engel G. A. 2006. Disease risk analysis: a paradigm for using health-based data to inform primate conservation and public health. Am. J. Primatol. 68:851–854 [DOI] [PubMed] [Google Scholar]
- 12. Junttila N., et al. 2007. New enteroviruses, EV-93 and EV-94, associated with acute flaccid paralysis in the Democratic Republic of the Congo. J. Med. Virol. 79:393–400 [DOI] [PubMed] [Google Scholar]
- 13. Keele B. F., et al. 2006. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science 313:523–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kew O. M., Nottay B. K., Hatch M. H., Hierholzer J. C., Obijeski J. F. 1983. Oligonucleotide fingerprint analysis of enterovirus 70 isolates from the 1980 to 1981 pandemic of acute hemorrhagic conjunctivitis: evidence for a close genetic relationship among Asian and American strains. Infect. Immun. 41:631–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kono R., Sasagawa A., Ishii K., Sugiura S., Ochi M. 1972. Pandemic of new type of conjunctivitis. Lancet i:1191–1194 [DOI] [PubMed] [Google Scholar]
- 16. Kono R., et al. 1981. Seroepidemiologic studies of acute hemorrhagic conjunctivitis virus (enterovirus type 70) in West Africa. III. Studies with animal sera from Ghana and Senegal. Am. J. Epidemiol. 114:362–368 [DOI] [PubMed] [Google Scholar]
- 17. Lindberg A. M., Andersson P., Savolainen C., Mulders M. N., Hovi T. 2003. Evolution of the genome of human enterovirus B: incongruence between phylogenies of the VP1 and 3CD regions indicates frequent recombination within the species. J. Gen. Virol. 84:1223–1235 [DOI] [PubMed] [Google Scholar]
- 18. Lukashev A. N., et al. 2003. Recombination in circulating enteroviruses. J. Virol. 77:10423–10431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malherbe H., Harwin R. 1963. The cytopathic effects of vervet monkey viruses. S. Afr. Med. J. 37:407–411 [PubMed] [Google Scholar]
- 20. Neel C., et al. 2010. Molecular epidemiology of simian immunodeficiency virus infection in wild-living gorillas. J. Virol. 84:1464–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nix W. A., Jiang B., Maher K., Strobert E., Oberste M. S. 2008. Identification of enteroviruses in naturally infected captive primates. J. Clin. Microbiol. 46:2874–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Norder H., Bjerregaard L., Magnius L. O. 2001. Homotypic echoviruses share aminoterminal VP1 sequence homology applicable for typing. J. Med. Virol. 63:35–44 [PubMed] [Google Scholar]
- 23. Oberste M. S., Jiang X., Maher K., Nix W. A., Jiang B. 2008. The complete genome sequences for three simian enteroviruses isolated from captive primates. Arch. Virol. 153:2117–2122 [DOI] [PubMed] [Google Scholar]
- 24. Oberste M. S., et al. 1999. Typing of human enteroviruses by partial sequencing of VP1. J. Clin. Microbiol. 37:1288–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oberste M. S., et al. 2005. Enteroviruses 76, 89, 90 and 91 represent a novel group within the species human enterovirus A. J. Gen. Virol. 86:445–451 [DOI] [PubMed] [Google Scholar]
- 26. Oberste M. S., Maher K., Pallansch M. A. 2002. Molecular phylogeny and proposed classification of the simian picornaviruses. J. Virol. 76:1244–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oberste M. S., Maher K., Pallansch M. A. 2007. Complete genome sequences for nine simian enteroviruses. J. Gen. Virol. 88:3360–3372 [DOI] [PubMed] [Google Scholar]
- 28. Oprisan G., et al. 2002. Natural genetic recombination between co-circulating heterotypic enteroviruses. J. Gen. Virol. 83:2193–2200 [DOI] [PubMed] [Google Scholar]
- 29. Poyry T., Kinnunen L., Hovi T., Hyypia T. 1999. Relationships between simian and human enteroviruses. J. Gen. Virol. 80:635–638 [DOI] [PubMed] [Google Scholar]
- 30. Rodriguez A. R., Kalter S. S., Heberling R. L., Helmke R. J., Guajardo J. E. 1977. Viral infections of the captive Kenya baboon (Papio cynocephalus): a five-year epidemiologic study of an outdoor colony. Lab. Anim. Sci. 27:356–371 [PubMed] [Google Scholar]
- 31. Sharp C. P., et al. 2010. Widespread infection with homologues of human parvoviruses B19, PARV4, and human bocavirus of chimpanzees and gorillas in the wild. J. Virol. 84:10289–10296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Simmonds P., Welch J. 2006. Frequency and dynamics of recombination within different species of human enteroviruses. J. Virol. 80:483–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smura T., et al. 2010. Cellular tropism of human enterovirus D species serotypes EV-94, EV-70, and EV-68 in vitro: implications for pathogenesis. J. Med. Virol. 82:1940–1949 [DOI] [PubMed] [Google Scholar]
- 34. Smura T. P., et al. 2007. Enterovirus 94, a proposed new serotype in human enterovirus species D. J. Gen. Virol. 88:849–858 [DOI] [PubMed] [Google Scholar]
- 35. Stanway G., et al. 2005. Family Picornaviridae, p. 757–778 In Fauquet C. M., Mayo M. A., Maniloff J., Desselberger U., Ball L. A. (ed.), Virus taxonomy. Eighth report of the International Committee on Taxonomy of Viruses. Elsevier/Academic Press, London, United Kingdom [Google Scholar]
- 36. Van Heuverswyn F., et al. 2006. Human immunodeficiency viruses: SIV infection in wild gorillas. Nature 444:164. [DOI] [PubMed] [Google Scholar]
- 37. Yoshii T., Natori K., Kono R. 1977. Replication of enterovirus 70 in non-primate cell cultures. J. Gen. Virol. 36:377–384 [DOI] [PubMed] [Google Scholar]