Abstract
The prion agent is the infectious particle causing spongiform encephalopathies in animals and humans and is thought to consist of an altered conformation (PrPSc) of the normal and ubiquitous prion protein PrPC. The interaction of the prion agent with the immune system, particularly the humoral immune response, has remained unresolved. Here we investigated the immunogenicity of full-length native and infectious prions, as well as the specific biological effects of the resulting monoclonal antibodies (MAbs) on the binding and clearance of prions in cell culture and in in vivo therapy. Immunization of prion knockout (Prnp0/0) mice with phosphotungstic acid-purified mouse prions resulted in PrP-specific monoclonal antibodies with binding specificities selective for PrPSc or for both PrPC and PrPSc. PrPSc-specific MAb W261, of the IgG1 isotype, reacted with prions from mice, sheep with scrapie, deer with chronic wasting disease (CWD), and humans with sporadic and variant Creutzfeldt-Jakob disease (CJD) in assays including a capture enzyme-linked immunosorbent assay (ELISA) system. This PrPSc-specific antibody was unable to clear prions from mouse neuroblastoma cells (ScN2a) permanently infected with scrapie, whereas the high-affinity MAb W226, recognizing both isoforms, PrPSc and PrPC, did clear prions from ScN2a cells, as determined by a bioassay. However, an attempt to treat intraperitoneally prion infected mice with full-length W226 or with a recombinant variable-chain fragment (scFv) from W226 could only slightly delay the incubation time. We conclude that (i) native, full-length PrPSc elicits a prion-specific antibody response in PrP knockout mice, (ii) a PrPSc-specific antibody had no prion-clearing effect, and (iii) even a high-affinity MAb that clears prions in vitro (W226) may not necessarily protect against prion infection, contrary to previous reports using different antibodies.
INTRODUCTION
Prions (PrPSc), the abnormal conformational isoforms of the cellular prion protein (PrPC), are responsible for invariably fatal neurodegenerative diseases of humans and animals (1, 35). Because they are transmissible and thereby induce characteristic morphological changes in the brains of affected individuals, these diseases have been termed transmissible spongiform encephalopathies (TSE). The current “protein-only” hypothesis postulates conformational changes of PrPC resulting in the infectious, abnormal, β-sheet-rich, insoluble, multimeric pathological isoform PrPSc in the absence of a specific nucleic acid or any other known infectious particle (39). Recent experiments with recombinant prion protein biochemically manipulated to result in the formation of infectious PrPSc strongly support the “protein-only” hypothesis (10, 22, 23).
Even though the primary amino acid sequence is the same for both the cellular and pathogenic PrP isoforms (5, 29), the two isoforms of PrP show considerable differences in their folding (31), leading to different biochemical properties that can be used to distinguish PrPC from PrPSc (7, 24). In particular, the partial resistance of PrPSc to treatment with proteinase K (PK), which contrasts with the sensitivity of the cellular form PrPC to this enzyme, has been used in the past to distinguish between these two isoforms (24). However, this indirect detection method for PrPSc has many disadvantages, because, for example, PK digestion of PK-sensitive prions may lead to false-negative results (38).
The generation of antibodies specific for the pathological isoform PrPSc was greatly accelerated by the availability of prion protein knockout (Prnp0/0) mice (9, 36). So far, various antigens had been used to generate monoclonal antibodies (MAbs), including PrP-encoding DNA and RNA (20, 21), recombinant PrP (19, 34, 42), synthetic peptides (2, 15, 30, 45), and scrapie-infected tissue culture cells (28). The first monoclonal antibody showing specificity for PrPSc, 15B3, had been obtained after immunization of Prnp0/0 mice with recombinant bovine PrP (19). Immunization of mice with a sequence comprising repetitions of the short peptide motif YYR yielded MAbs exclusively precipitating PrPSc from sheep with scrapie cases and from Creutzfeldt-Jakob disease (CJD) patients (32). Finally, Moroncini and coworkers cloned recombinant antibodies with specificity for PrPSc by grafting a prion protein peptide into an IgG antibody against HIV-1 Env (25, 26, 40).
In spite of the publication of several PrPSc-specific monoclonal antibodies in recent years (19, 25, 26, 32, 40), the therapeutic potential of such antibodies has not yet been reported.
Here we investigated the antibody immune response to sodium phosphotungstic acid (NaPTA)-precipitated full-length infectious PrPSc and evaluated the resulting antibodies by conformation specificity and their ability to cure prion infections in vitro and in vivo.
MATERIALS AND METHODS
Mice.
tga20 (13), Prnp0/0 (9), Tg33 (37), and BALB/c mice were bred at the Friedrich-Loeffler-Institut (FLI), Tübingen, Germany. Transgenic mice were a kind gift from C. Weissmann and A. Aguzzi. Infected mice were monitored twice a week initially and then daily after the development of clinical symptoms. Mice were terminated in the end stage of disease. The number of mice and the experimental design had been approved by local authorities (Regional Board; permission numbers FLI 204/02 and FLI 216/04). All experiments were conducted in compliance with German and European laws and guidelines for animal protection.
TSE strains.
An RML strain seed of the scrapie agent was kindly provided by A. Aguzzi, Zürich, Switzerland, and was propagated in CD1 mice. The 263K hamster scrapie strain was a kind gift from M. Beekes, Berlin, Germany. The sheep scrapie agent originated from diseased sheep, originally infected with brain material from a natural case of scrapie, kindly provided by U. Agrimi and F. Scholl, Italy. Samples from mock-infected white-tailed deer (infected with a control brain homogenate) (samples 103 and 123) and samples from white-tailed deer with chronic wasting disease (CWD) (samples 106, 112, and 121) were a kind gift from E. Hoover, Colorado State University. Variant CJD (vCJD) samples used for immune precipitations were provided by James Ironside, Edinburgh, United Kingdom, and vCJD samples for the capture enzyme-linked immunosorbent assay (ELISA) consisted of two reference probes from the WHO (NHBY0/0003 and NHBY0/0014). Samples from cases of sporadic CJD and genetic CJD were obtained from the German TSE Forum. Experiments with scrapie strains and CWD strains were performed at the biosafety level 2 laboratories of the FLI. Experiments using various CJD strains were conducted at the Institute of Neuropathology of the University of Düsseldorf or at Prionics AG, Schlieren, Switzerland, in authorized laboratories.
Cell lines.
Bos2 cells (8) were a kind gift from A. Aguzzi, and ScN2a cells were obtained from S. Prusiner, San Francisco, CA.
Immunization with native NaPTA-precipitated RML.
For immunization, 250 μl of a 10% brain homogenate of terminally scrapie infected CD1 mice, infected with the RML strain, was precipitated with NaPTA as described previously (43). The resulting pellet was dissolved in phosphate-buffered saline (PBS) and was mixed with an adjuvant. The first and second immunizations were performed intraperitoneally (i.p.) using the ABM-ZK adjuvant (Linaris, Germany), whereas for booster immunizations, the ABM-N adjuvant (Linaris, Germany) was used. Mice were immunized four times with NaPTA precipitates equivalent to 250 μl of 10% brain homogenate of terminally scrapie diseased CD1 mice. The last booster injection was performed intravenously (i.v.) without adjuvant 4 days prior to fusion. Mouse sera were monitored for PrP immunoreactivity by Western blotting. Spleen cells were fused to P3X63Ag8.653 cells by using a modified protocol of Köhler and Milstein (17).
Cultivation of hybridomas.
Hybridomas were grown in 96-well plates using minimal essential medium (MEM) with nonessential amino acids (NEAA) (Gibco, Invitrogen, Karlsruhe, Germany) and 10% fetal calf serum (FCS), and antibody production was monitored by use of an immunoglobulin-detecting ELISA.
Screening for PrP-specific antibodies.
First, an ELISA using recombinant mouse prion protein determined antibody specificity for PrP. All reacting hybridoma supernatants were then verified by Western blotting on RML-infected and control brain homogenates.
The epitopes of the MAbs generated were identified using a membrane-bound peptide library (Pep-Scan; JPT Peptide Technologies, Berlin, Germany) consisting of 13-amino-acid (aa) peptides spanning the prion protein from aa 23 to 230, with an 11-aa overlap between neighboring peptides.
To identify PrPSc-specific MAbs, an ELISA using mouse brain homogenates of scrapie-affected and control mice was employed. Ten percent homogenates of scrapie brain or a control homogenate was generated in 0.32 M sucrose–0.5% sodium deoxycholate–0.5% NP-40 using a ribolyzer (FastPrep FP120; Thermo Savant/Bio101). Homogenates were precleared by centrifugation for 15 min at 15,300 rpm in a Sigma 2-16K centrifuge at 4°C. Supernatants were pooled and stored at −70°C until use. The pooled supernatant was diluted 1:100 in carbonate buffer for the coating of ELISA plates (Multisorb; Nunc, Wiesbaden, Germany), which was performed overnight at 4°C. After blocking at room temperature (RT) with PBS containing 0.2% bovine serum albumin (BSA)–0.05% Tween 20 for 1 h, undiluted hybridoma supernatants were added for 1 h at RT. The ELISA was developed using horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Dianova, Hamburg, Germany) in combination with 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS; Roche, Mannheim, Germany), and absorbance was measured in a Sunrise ELISA reader (Tecan, Crailsheim, Germany).
Hybridoma supernatants showing a stronger reaction with the scrapie brain homogenate than with the control brain homogenate were further tested by immunoprecipitation.
Immunoprecipitation of prion protein.
For immunoprecipitation, 1% brain homogenate in Tris-buffered saline (TBS) with 0.3% sarcosyl was incubated with a MAb overnight at 4°C. Precipitation was performed using formalin-fixed Staphylococcus aureus for IgM or Pansorbin cells for IgG isotypes (Calbiochem, Merck, Darmstadt, Germany). After a wash, precipitates were boiled and then centrifuged for 1 min at 13,000 rpm, and the complete supernatant was used for Western blotting.
SPR analysis.
Binding kinetics was determined on a Biacore 1000 instrument (Biacore AB, Uppsala, Sweden). Recombinant mouse PrP (1 μM) was diluted in 10 mM sodium acetate (NaOAc) (pH 4.5) and was immobilized on a 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC)–N-hydroxysuccinimide (NHS)-activated CM5 chip (Biacore) at 5 μl/min. After immobilization and blocking with ethanolamine, the chip was washed with 50 mM NaOH until a steady signal was obtained. The final surface density was about 3,500 response units (RU). All kinetic surface plasmon resonance (SPR) analyses were run at a 5-μl/min PBS flow, and the antibody was injected at different concentrations ranging from 10 to 200 nM. Association and dissociation were each recorded for 180 s. After each cycle, the surface was regenerated with 50 mM NaOH. Kinetic data were calculated using BIAevaluation, version 4.1, software according to the bivalent analyte model.
Immunohistological staining of PrP.
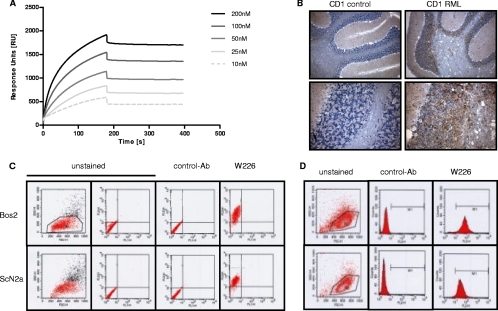
Brains of infected and control mice were fixed in 4% formalin and subsequently were treated with concentrated formic acid for 1 h. After a wash with PBS, the brains were postfixed again using 4% formalin. Sections (thickness, 4 μm) were autoclaved in citrate buffer and were treated with proteinase K for 3 min at 37°C using a Digest-All kit (Zymed). Slices were stained using an HRP-conjugated secondary antibody (SuperPicture-HRP Polymer Conjugate kit; Zymed, Invitrogen, Karlsruhe, Germany) for 10 min. Signal amplification was performed using the Tyramide Signal Amplification (TSA) kit (Perkin-Elmer, Rodgau, Germany), and the signal was developed using diaminobenzidine (DAB substrate kit for peroxidase; Vector Laboratories, Peterborough, United Kingdom). Brain slices were counterstained using hematoxylin.
Treatment of ScN2a cells.
ScN2a cells were cultured in the presence of a monoclonal antibody at various concentrations. At the indicated time points (see Fig. 5), cells were again subcultured in the presence or absence of the antibody. One subculture was treated with monoclonal antibody W226 for 31 days, and then all cultures were continued in the absence of the antibody. For bioassays, mice were injected intracerebrally (i.c.) with 2 × 104 cells per mouse. The infectious titer of inoculated cells was calculated according to reference 16. Cultures of ScN2a cells were treated in the presence of antibody W261 for as long as 104 days. For cell blot analysis, all cells were seeded in parallel on glass coverslips in 24-well plates and were cultured for a further two days. Cell blot analysis for the detection of scrapie prion protein was performed as described elsewhere (8).
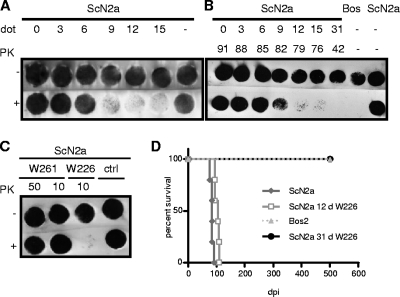
Fig. 5.
Cell blot immunoassays of scrapie-infected and antibody-treated ScN2a cells tested for the presence of the pathological prion protein PrPSc by PK digestion and immunostaining with MAb W226. (A) Reduction of the PrPSc load in ScN2a cells after various days of treatment (dot) with MAb W226. (B) Reduction and absence of PrPSc in ScN2a cells after treatment with W226 for the indicated numbers of days (0 to 31 days) (upper row) and following incubation for various periods in the absence of the antibody (42 to 92 days) (lower row). (C) Treatment of scrapie-infected ScN2a cells with the PrPSc-specific MAb W261 did not alter the amount of PrPSc. ScN2a cells were cultured with the indicated amounts of MAb W261 for 104 days and were subsequently tested for the presence of the pathological prion protein PrPSc. Analysis revealed no differences between proteinase K-treated and untreated cells, indicating that cultivation with MAb W261 had no influence on the PrPSc level in ScN2a cells. ctrl, control. (D) Absence of disease transmission to tga20 mice from ScN2a cells treated for 31 days with W226. Cell blotting was performed as described previously (8).
ELISA with MAb W261 and 6H4-HRP.
A 25-μg/ml portion of MAb W261 was diluted into PBS and applied to 96-well Nunc MaxiSorp plates for 2 h at room temperature. After three washes with PBS-Tween (PBST), wells were blocked with 0.1% BSA in TBS for 1 h. Ten percent brain homogenates in homogenization buffer (Prionics AG) were diluted 1:100, 1:500, and 1:1,000 in immunoprecipitation buffer (Prionics AG) and were incubated for 2 h at room temperature. After three washes with PBST, bound PrPSc was denatured for 15 min using conditioning buffer (Prionics AG). Prion protein was detected using 10 ng/ml MAb 6H4 conjugated to HRP (Prionics AG) diluted in PBS containing 0.05% Tween 20 and 0.1% BSA. Unbound detection antibody was washed away with PBST, and a luminescence signal was generated using the SuperSignal ELISA Femto maximum-sensitivity substrate (Pierce). The readout was performed using the MPL2 microplate luminometer (Berthold).
Production of antibody W226 and scFv W226 for therapy.
Large amounts of MAb W226 were generated by culture of hybridoma cells in cell spin bottles using MEM-NEAA (Gibco, Invitrogen, Karlsruhe, Germany) with 10% ultralow IgG FCS (PAN Biotech, Aidenbach, Germany) to prevent contamination with bovine IgG molecules. Cultures were grown at 37°C under 5% CO2 until the amount of hybridoma cells started to decrease. The culture medium was harvested, and cells and cell debris were separated from the antibody-containing medium by centrifugation at 1,200 rpm in a Beckman TJ-6 centrifuge. Supernatants were stored at −20°C until the antibody was purified by affinity chromatography using protein A Sepharose (Amersham, Freiburg, Germany). scFv W226 was expressed as described previously (27).
Statistical analysis.
Survival times for different groups of mice in treatment experiments were compared using GraphPad Prism, version 5, software. Groups were compared by using the Mann-Whitney two-tailed t test.
RESULTS
Antigen preparation and screening of monoclonal antibodies.
As an immunogen, an enriched and highly purified preparation of PrPSc obtained by NaPTA precipitation of mouse scrapie strain RML (RML6) was obtained by a method described by others (43). Since, to our knowledge, the infectivity of NaPTA-precipitated pathological prion protein for mice had not been reported previously, we first analyzed this biological property of the purified antigen by inoculating the precipitated antigen i.c. or i.v. into tga20 indicator mice. Infectivity was demonstrated in both inoculation paradigms after 60 or 191 to 212 days, respectively, and prion disease was confirmed by PK digestion and Western blotting (data not shown). These results indicated that after the NaPTA precipitation, the disease-associated PrPSc conformation should be largely preserved.
For immunization, NaPTA-precipitated PrPSc in PBS was mixed with an antibody multiplier adjuvant (ABM-ZK; Linaris, Germany) without further treatment in order to preserve the conformation. A serum antibody response in Prnp0/0 mice was detected by Western blotting as early as 2 weeks after the first immunization and increased in intensity after additional booster injections (data not shown).
Five antibodies produced from hybridoma fusion recognized the prion protein by Western blotting (W123, W187, W226, W756, and W827). Species specificity experiments with Western blotting revealed that most of the antibodies recognized only the mouse and hamster prion proteins (W123, W226, W756, and W827) (Table 1), whereas W187, recognizing the octarepeat motif, also reacted with prion proteins from various animal species and humans. In addition, clones from the same fusion were screened by selective recognition of PrPSc versus PrPC by immunoprecipitation of prion-infected mouse brain homogenates versus normal mouse brain homogenates, respectively. This screening revealed that antibodies W68 and W261 are immunoreactive to PrP from scrapie-infected homogenates but not from normal brain homogenates (Fig. 1A), and additional digestion of these precipitates with proteinase K revealed the presence of proteinase K-resistant prion protein (Fig. 1B and C). Furthermore, when PK-digested prion-infected mouse brain was used for immune precipitation, MAb W261 also proved specific for PrPSc, and its conformational epitope was then inferred to be within the PK-resistant core (Fig. 1D).
Table 1.
Binding of monoclonal antibodies generated in this study to PrP of different speciesa
| Species | Bindingb of: |
||||
|---|---|---|---|---|---|
| W123 | W187 | W226 | W756 | W827 | |
| Cats | − | − | − | − | − |
| Cattle | − | + | − | − | − |
| Deer | + | + | + | +/− | + |
| Dogs | − | + | − | − | − |
| Hamsters | + | + | + | + | + |
| Llamas | − | + | − | − | − |
| Humans | − | + | − | − | − |
| Mice | + | + | + | + | + |
| Mouflons | − | −/+ | − | − | − |
| Pigs | − | −/+ | − | − | − |
| Sheep | − | + | − | − | − |
The specificity of the antibodies was determined using Western blotting.
−, no binding; −/+, background binding; +/−, weak binding; +, strong binding.
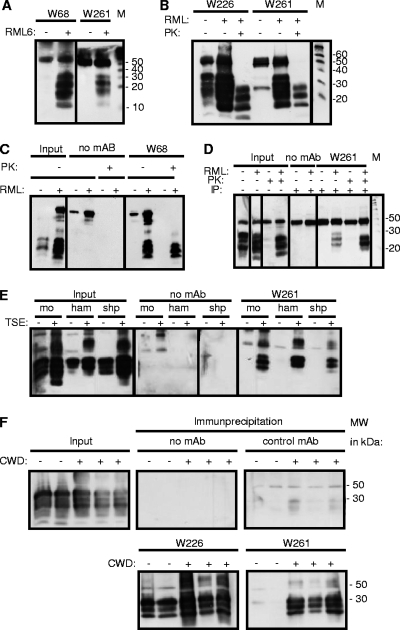
Fig. 1.
Reaction patterns of PrPSc-specific MAbs in immunoprecipitation (IP). (A) MAbs W68 and W261 react exclusively with the PrPSc isoform. (B and C) Treatment of precipitates with PK reveals the presence of PK-resistant PrPSc in MAb-precipitated material for W261 (B) and W68 (C). (D) Treatment of samples with PK prior to immunoprecipitation with MAb W261 reveals the interaction of W261 with the PK-resistant core of PrPSc. (E) (Right) Specific immunoprecipitation of scrapie PrPSc from mouse (mo), hamster (ham), and sheep (shp) samples with MAb W261. (Left) The presence of PrPC and PrPSc is shown in parallel. (F) Specific immunoprecipitation from brain samples of deer with CWD. The presence of PrP is demonstrated by the use of W226 (lower left). W261 reacts with CWD-affected brain samples but not with control brain samples, kindly provided by E. Hoover. Immunoprecipitations were performed using 200 μl of 1% brain homogenate, and complete supernatants of boiled precipitates were analyzed by Western blotting. For input material, 30 μl of 1% brain homogenate was loaded per lane. Western blots using mouse tissue were developed using antibody W226. Prion proteins on immune blots with human tissue were detected using MAb 6H4 (19). For the detection of prion proteins of animal species other than mouse, antibody POM-1 was used (34). The signal at 50 kDa corresponds to the heavy chain of mouse immunoglobulin.
Biochemical characteristics and detection abilities of PrPSc-specific MAbs.
Since W68 and W261 essentially yielded the same results, W261 was chosen for all further experiments because of its preferable IgG isotype. MAb W261 was then used for detecting reactivities against PrPSc from different animal species. W261 pulled down PrPSc exclusively from brain tissue homogenates of scrapie-diseased mice, hamsters, and sheep, not from those of control animals (Fig. 1E).
Additional experiments examined the specificity of MAb W261 for CWD of white-tailed deer. As shown in Fig. 1F, MAb W261 specifically precipitated PrPSc from the brains of three CWD-diseased white-tailed deer, but not from mock-infected controls. In contrast, MAb W226, used as a control, was able to detect both isoforms of the deer prion protein in uninfected and infected brain samples.
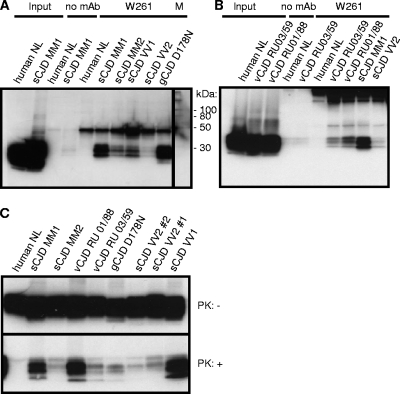
For human prion diseases, MAb W261 specifically recognized all pathological isoforms of CJD strains but did not react with human control brains (Fig. 2A and B). The amount of immunoprecipitated material from brain homogenates with different CJD strains was variable but, with the exception of genetic CJD, consistent with the different amounts of PrPSc present in the homogenates used for immunoprecipitation, as verified by proteinase K digestion and detection by antibody 6H4 (Fig. 2C).
Fig. 2.
Immunoprecipitation of PrPSc from CJD samples with MAb W261. (A) Detection of PrPSc in various sporadic (s) cases and one genetic (g) case of CJD. (B) Detection of PrPSc in various CJD cases, including vCJD. (C) Demonstration of various amounts of PrPSc in spontaneous, genetic, and variant CJD cases tested in panels A and B by Western blotting. Immunoprecipitations were performed using 1 ml of 1% brain homogenate, and complete supernatants of boiled precipitates were analyzed by Western blotting. For input material, 30 μl of 1% brain homogenate was loaded per lane. The signal at 50 kDa corresponds to the heavy chain of mouse immunoglobulin.
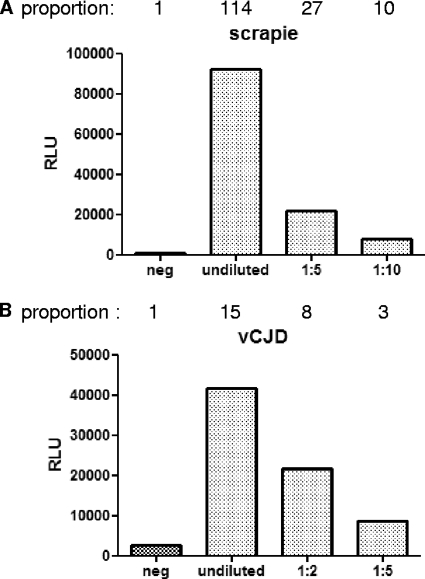
In order to investigate the suitability of MAb W261 for high-throughput serial testing without the need for centrifugation steps, we established a sandwich ELISA with MAb W261 as the capture antibody to retain the pathological prion protein PrPSc. MAb W261-mediated binding of PrPSc was detected by antibody 6H4-POD (19) in combination with chemiluminescence. Using this assay, only prion proteins from infected sheep brains, not that of a control brain homogenate, were detected (Fig. 3A). Similar discrimination could be observed for human vCJD samples, confirming the specificity of MAb W261 for sheep and human prions (Fig. 3B).
Fig. 3.
ELISA with MAb W261 as the capture antibody for the detection of PrPSc. (A) Detection of sheep PrPSc; (B) detection of vCJD PrPSc. The specificity of this capture ELISA is demonstrated for sheep scrapie (A) and for vCJD (B). For each species, a control homogenate from normal brains was only weakly recognized, whereas the homogenate from scrapie- or vCJD-affected brains was clearly detected in a dose-dependent manner. In conclusion, this sandwich ELISA using MAb W261 is able to distinguish between TSE-infected and uninfected tissue homogenates of sheep or humans without the need for proteinase K digestion, demonstrating its usefulness for diagnostic assays. Negative controls (neg) are uninfected sheep or human brain homogenates. One microliter of 10% or further diluted brain homogenate was used per sample in a volume of 200 μl. The results are given as relative light units (RLU). The proportions above the graphs represent the values obtained divided by the value for the negative control.
Conformation-insensitive specificity of MAb W226.
Within the same fusion, PrPSc-specific antibodies W68 and W261 were identified, along with antibodies recognizing PrPC and PrPSc equally well (W123, W187, W226, W756, and W827). For one of the latter (W226), Biacore analysis demonstrated an exceptionally strong dissociation constant for recombinant mouse prion protein, 523 × 10−12 M (Fig. 4A). Relative conformation insensitivity was determined by immunoprecipitation (data not shown), immunohistochemistry under denaturing conditions (Fig. 4B), and comparable detection of surface and intracellular PrP on both infected and uninfected mouse neuroblastoma cells (Fig. 4C and D), showing that this antibody detected PrP by all assays used, including denatured Western blotting, independent of the PrP conformation.
Fig. 4.
Characteristic properties of MAb W226. (A) Biacore analysis of W226 reveals a dissociation constant of 523 × 10−12 M. (B) Immunohistochemical staining of mouse brain tissue. W226 stains PrPSc in tissues of RML-diseased mice. Magnifications, ×10 (upper panels) and ×40 (lower panels). (C) Detection of surface PrP in uninfected Bos2 (top) and ScN2a (bottom) cells by flow cytometry. (D) Detection of intracellular PrP in permeabilized Bos2 (top) and ScN2a (bottom) cells. Flow cytometry was performed using a FACSCalibur flow cytometer (Becton Dickinson [BD], Heidelberg, Germany) with secondary antibodies from BD and a Cytofix/Cytoperm kit (BD) for intracellular staining.
Differential biological activity of PrPSc-specific and universal PrP antibodies in permanently scrapie infected cell lines.
The capacities of the MAbs resulting from immunizations with purified infectious prions for shielding prions during cellular replication were assessed in cell models of prion infection. We tested whether the PrPSc-specific MAb W261 was also able to reduce PrPSc levels in ScN2a cells. ScN2a cells were cultivated in the presence of MAb W261 for more than 100 days and were regularly tested for the presence of PrPSc by cell blot assays (Fig. 5C). Culture of ScN2a cells in the presence of even large amounts of MAb W261 (50 μg/ml) did not reduce PrPSc contents in these cells at any time point analyzed, demonstrating the absence of therapeutic potential of the PrPSc-specific MAb W261.
In contrast, as demonstrated by cell blot analysis, culture of cells for 9 days or more in the presence of 10 μg/ml MAb W226 resulted in a significant reduction in the level of PrPSc after proteinase K treatment (Fig. 5A). In an experiment where antibody treatment was discontinued after 31 days and cells were passaged for 42 to 88 days, no PrPSc reappeared, proving complete clearance of prions (Fig. 5B).
To confirm the efficiency of MAb W226 in curing cells from PrPSc, antibody-treated cells were tested in bioassays by i.c. inoculation into tga20 mice. Whereas mice inoculated with untreated ScN2a control cells succumbed to scrapie after 84 ± 5 days, corresponding to 2.56 log LD50 (50% lethal dose), those mice injected with cells treated for 12 days with MAb W226 had to be sacrificed after 102 ± 8 days, corresponding to 0.94 log LD50, and treatment for 31 days did not result in disease and death, even after an observation period of >500 days (Fig. 5D). From these experiments we concluded that MAb W226 is capable of curing scrapie-infected cells and therefore might be a potential therapeutic antibody for curing prion infection in vivo.
Treatment of scrapie-infected mice with monoclonal antibody W226 and scFvW226.
In their landmark paper, White et al. (44) demonstrated that an exogenously administered antibody was able to prevent an intraperitoneal prion infection, suggesting that postexposure treatment of prion infection was possible. We therefore tested the high-affinity MAb W226, which binds to the same epitope as a MAb active against prions from the study by White et al., in exactly the same experimental setup. In addition, we cloned a recombinant variable-chain fragment (scFv) from W226 into Escherichia coli and demonstrated its in vitro antiprion activity by a bioassay (27).
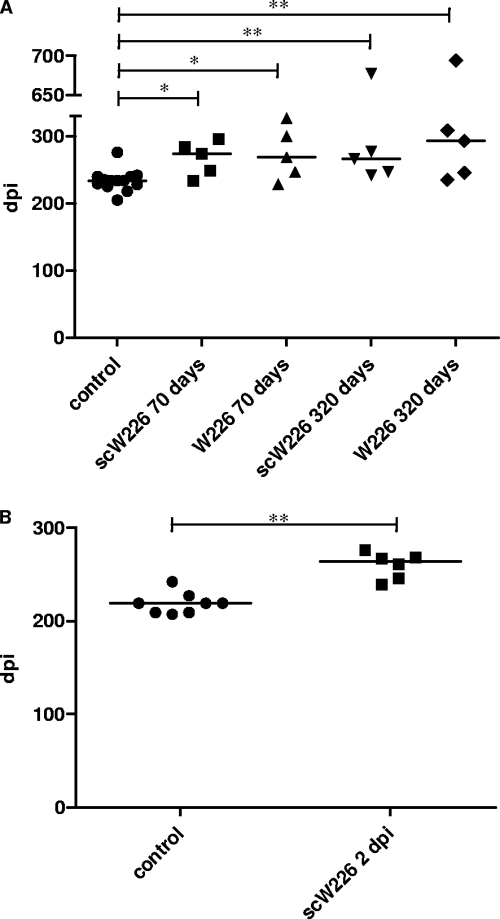
C57BL/6 mice were infected intracerebrally and intraperitoneally with a 10−5 or 10−3 dilution of a 20% brain homogenate of the RML strain of the mouse-adapted scrapie agent. The infectious titer of RML had been determined to be high enough to lead to prion disease for all mice inoculated. Twenty-eight days after infection, treatment of infected mice with a monoclonal antibody or single-chain antibody fragment was started, using 2 mg/mouse/day (W226) or 1 mg/mouse/day (scW226) twice a week by intraperitoneal injection. Treatment of mice infected via the i.c. route did not exhibit any prolongation in the incubation period at all (data not shown), consistent with previous data (44). Mice infected i.p. demonstrated minimal prolongation of the incubation period when treated for 10 weeks (Fig. 6A). Most of the mice treated for a much longer period also exhibited minimal prolongation of the incubation time, but one mouse in each group did not develop symptoms of scrapie for 677 (scW226) or 694 (W226) days postinfection (dpi), even when treatment was terminated at 320 dpi. Differences in mean incubation time between all treated groups and the untreated group were statistically significant (Fig. 6A) but did not result in the highly efficient therapeutic impact, securing the survival of all peripherally infected mice, that was described previously (44). Since differences between mice treated for 70 or 320 days were not significant (P, 0.8413 for W226 and 1 for scW226), and since there was no statistically significant difference between groups treated with scW226 or W226 (P, 1 for 70 days and 0.8413 for 320 days of treatment), we conducted a second treatment experiment using scW226 with a reduced incubation time. In the second experiment, the impact of starting antibody treatment of mice at 2 dpi was studied, and mice were treated with scW226 for 66 days. This treatment also resulted only in a minimal, though highly significant (P, 0.0035), increase in the incubation time and therefore confirmed the results of the earlier trial (Fig. 6B).
Fig. 6.
Treatment of scrapie-infected mice with MAb W226 or scW226 results in marginal prolongation of the incubation period after peripheral prion inoculation. Mice were infected i.p. (A) Treatment with W226 or scW226 was started at 28 dpi and continued for 10 or 46 weeks. Treatment of mice (n = 5) resulted in only moderate but statistically significant prolongation of the incubation period. The P values for the differences between treated groups and the untreated group are as follows: for mice treated with scW226 for 70 days, 0.0198; for mice treated with W226 for 70 days, 0.0335; for mice treated with scW226 for 320 days, 0.0048; and for mice treated with W226 for 320 days, 0.0088. (B) Treatment of mice from 2 dpi with scW226 for 66 days (n = 6) resulted in only a marginal increase in the incubation time over that for untreated controls (n = 8). The P value for the difference between the untreated and scW226 groups is highly significant (0.0035). Mice were treated with 2 mg/mouse (W226) or 1 mg/mouse (scW226) twice a week. Symbols represent individual mice, and horizontal lines indicate the median incubation times. C57BL/6 mice were inoculated using dilutions of a 20% homogenate: 30 μl of a 10e−5 dilution for i.c. inoculation and 100 μl of a 10e−3 dilution for i.p. inoculation. The endpoint titer of the scrapie pool used for the inoculation of mice was determined by i.c. inoculation of tga20 mice and was calculated to be 10.6 log LD50 per g of brain according to the method of Reed and Muench (37a).
The limited impact of treatment with MAb W226 or scW226 on the development of disease by peripherally infected mice, in view of the strong effect in clearing prions from ScN2a cells (Fig. 5), contrasts sharply with the great therapeutic benefit of antibody therapy reported previously (44).
DISCUSSION
Our findings can be summarized in three major points, as follows. (i) Full-length infectious prions are immunogenic in PrP knockout mice and lead to an antibody response that includes the generation of PrPSc-specific antibodies, as well as universal PrP-recognizing antibodies. (ii) A highly PrPSc specific IgG1 subtype MAb, W261, was unable to cure ScN2a cells. (iii) A high-affinity anti-PrP antibody, W226, or its recombinant scFv was able to cure ScN2a cells but unable to protect from an intraperitoneal prion infection when administered in vivo.
The availability of PrPSc-specific antibodies for diagnostic and therapeutic purposes has been a long-sought goal. Here we could show that PrPSc conformation specificity is part of an immune response elicited in prion-immunized mice, although in this study, PrP knockout mice with an enlarged epitope space for PrP were used. NaPTA-precipitated pathological prion protein is highly polymeric, as demonstrated by electron microscopy (38). It is therefore reasonable to argue that the polymeric nature of the NaPTA-precipitated antigen is responsible for the induction of a strong antibody response, as has been exemplified by the work of Bachmann et al. with polymers of the viral glycoprotein (GP) of vesicular stomatitis virus (VSV), showing stronger B-cell responses to polymers than to monomers of the GP (3). This stronger stimulation of the immune response is thought to be induced by B-cell receptor cross-linking through the highly ordered GP that is found on the viral surface of VSV (4). Therefore, we assume that the polymeric nature of the NaPTA-precipitated PrPSc is also able to induce a strong B-cell response against PrPSc.
The potential usefulness of W261 for diagnostic purposes was demonstrated by the establishment of an ELISA with W261 as the capturing antibody (Fig. 3).
A recently published study demonstrated the detection of infectious and noninfectious aggregates of the prion protein by PrPsc-specific monoclonal antibodies, suggesting that these MAbs recognize structural elements common to infectious and noninfectious aggregates of the prion protein (6). Since in our study the interactions of the PrPsc-specific antibodies generated with noninfectious prion protein aggregates were not analyzed, we cannot exclude the possibility that these antibodies might also bind to noninfectious aggregates of the prion protein in addition to binding to PK-resistant, supposedly infectious prions (Fig. 1B).
The intuitive concept of shielding the causative agent of a disease by highly specific immune binding, e.g., neutralization of a virus by antibodies, has long been held up for prion diseases. However, early studies argued that antibodies binding to PrPC in addition to PrPSc had improved prion clearance in permanently scrapie infected cells (5a). Here we demonstrate the complete lack of PrP conversion inhibition by PrPSc-specific MAb W261 in vitro, even if treatment of cells was continued for more than 3 months, whereas under the same conditions, the universal MAb W226, lacking conformation specificity, was effective (Fig. 5; see also below). The reasons for the absence of antiprion activity for MAb W261 may include the localization of PrPSc in cellular compartments that are not accessible to PrPsc-specific antibodies. If so, there would be no interference with the templating function of PrPSc in the process of prion replication and/or prion storage, and thus, PrPsc with a long half-life would be present. It is not certain whether a generalization that all PrPSc-specific MAbs lack antiprion activity can therefore be inferred, but the fact that no such effect has been published so far could point to this possibility.
Interestingly, the only published PrPsc-specific peptide ligand showing antiprion activity in cell culture is a retroinverso d-peptide of the complementarity-determining region (CDR) of the MAb W226 heavy chain that, in contrast to full-length W226, proved PrPSc specific, although the d-peptide has to be used at relatively high concentrations (27).
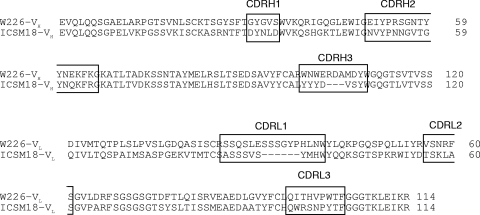
In contrast to W261, the universal MAb W226, recognizing both PrPC and PrPSc, cleared prions from ScN2a cells, as validated by a bioassay (Fig. 5D). This effect has also been shown by many other universal anti-PrP MAbs targeting helix 1 of PrP (12, 33). MAb W226 was therefore chosen for a therapeutic trial against prion infection. So far, the successful trial of White et al. with antibodies ICSM18 and ICSM35 leading to complete protection from peripheral prion infection stands alone as the only published report of successful administration of antibodies against prion disease. Here we precisely copied the treatment protocol of White et al. except that the mouse strain inoculated was C57BL/6 rather than FVB and the antibody was different (Fig. 7), although it binds to a similar region in PrP (Table 2) (44). In a separate therapeutic approach, we also included recombinant scFv W226, which had equally been proven by Müller-Schiffmann et al. to clear prions in vitro (27), in order to provide a smaller ligand with potentially easier passage through the blood-brain barrier.
Fig. 7.
Sequence alignment of the variable heavy (VH) and light (VL) chains of W226 and ICSM18 by MUSCLE (Multiple Sequence Comparison by Log Expectation) (11). The CDRs are boxed. BLAST analysis revealed 59% homology between W226 and ICSM18.
Table 2.
Isotypes and epitopes of the monoclonal antibodies generated in this studya
| Antibody | Isotype | Epitope |
|---|---|---|
| W123 | IgG2b | 145-WEDRYYREN-153 |
| W187 | IgG2b | WGQPHGG (octarepeat motif) |
| W226 | IgG1 | 145-WEDRYYREN-153 |
| W261 | IgG1 | Unknownb |
| W756 | IgG2a | 145-WEDRYYREN-153 |
| W827 | IgG1 | 145-WEDRYYREN-153 |
Amino acids are numbered according to the hamster prion protein sequence.
The epitope of W261 is thought to be a conformational epitope located in the PK-resistant core of PrPsc.
Starting antibody administration either 28 days or 2 days after i.p. inoculation did not lead to protection against prion disease to the degree published by White et al., although a single mouse in each of two long-term treatment groups survived for more than 600 days (Fig. 6A). Of note, the avidity of bivalent W226 compared to that of monovalent scW226 did not seem to play a major role with regard to the antiprion effects. From these experiments, and considering the similarity of experimental protocols, we draw the important conclusion that the therapeutic success of antibodies against peripheral prion infection must be influenced either by the strain of mouse inoculated, the specific pharmacodynamic or pharmacokinetic characteristics of the antibody itself, or perhaps both. A sequence comparison between the antibodies reveals significant differences between W226 and ICSM18, especially in the hypervariable CDR regions (Fig. 7). These sequence differences could account for differences in the half-life and distribution of the antibody in vivo; different affinities or other binding characteristics could equally make a decisive difference. In summary, the ability of an antibody to cure ScN2a cells, even if confirmed by a bioassay, does not predict successful application in a therapeutic trial. A similar finding was recently reported for quinacrine, a small molecule with clear antiprion activity in ScN2a cells (18), which also failed in a therapeutic trial (14). This failure, despite the fact that pharmacokinetic obstacles such as the multidrug resistance complex were circumvented successfully, was attributed to an evolution of quinacrine-resistant prions (14). It remains to be shown whether such an explanation could also account for the inefficiency of W226 versus ICSM18 or if different factors account for the divergent results between these antibodies.
ACKNOWLEDGMENTS
This work was supported by the TSE program Baden-Württemberg (grant 729.59-7/1, to L.S.), by EU-Europrion FP5 (to L.S.), by EU-FP6 Anteprion (LSHB-CT-2006-019090) and EU-FP7 Priority (grant 222887) to L.S. and C.K., and by EU-FP6 StrainBarrier (Food 3B 023183, to C.K.). The brain bank in the National CJD Surveillance Unit, Edinburgh, United Kingdom, is supported by the Medical Research Council.
We thank Ulrich Wulle, Silke Gaedt, and Jacqueline Schmid for excellent technical assistance, Simone Nebrich for the histological staining of brain sections, Adriano Aguzzi and Magda Polymenidou (Zürich, Switzerland) for providing the POM-1 antibody and the tga20 and Prnp0/0 mice, Edward Hoover (Fort Collins, CO) for kindly providing CWD brain material, Michael Beekes for providing the 263K strain, Eberhard Burkhardt (Giessen, Germany) for kindly providing control animal brain tissue, and Björn Schröder for critical reading of the manuscript.
Footnotes
Published ahead of print on 23 February 2011.
REFERENCES
- 1. Aguzzi A., O'Connor T. 2010. Protein aggregation diseases: pathogenicity and therapeutic perspectives. Nat. Rev. Drug Discov. 9:237–248 [DOI] [PubMed] [Google Scholar]
- 2. Arbel M., Lavie V., Solomon B. 2003. Generation of antibodies against prion protein in wild-type mice via helix 1 peptide immunization. J. Neuroimmunol. 144:38–45 [DOI] [PubMed] [Google Scholar]
- 3. Bachmann M. F., Hengartner H., Zinkernagel R. M. 1995. T helper cell-independent neutralizing B cell response against vesicular stomatitis virus: role of antigen patterns in B cell induction? Eur. J. Immunol. 25:3445–3451 [DOI] [PubMed] [Google Scholar]
- 4. Bachmann M. F., Zinkernagel R. M. 1997. Neutralizing antiviral B cell responses. Annu. Rev. Immunol. 15:235–270 [DOI] [PubMed] [Google Scholar]
- 5. Basler K., et al. 1986. Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene. Cell 46:417–428 [DOI] [PubMed] [Google Scholar]
- 5a. Beringue V., et al. 2004. PrPSc binding antibodies are portent inhibitors of prion replication in cell lines. J. Biol. Chem. 17:39671–39676 [DOI] [PubMed] [Google Scholar]
- 6. Biasini E., et al. 2008. Non-infectious aggregates of the prion protein react with several PrPSc-directed antibodies. J. Neurochem. 105:2190–2204 [DOI] [PubMed] [Google Scholar]
- 7. Bolton D. C., McKinley M. P., Prusiner S. B. 1982. Identification of a protein that purifies with the scrapie prion. Science 218:1309–1311 [DOI] [PubMed] [Google Scholar]
- 8. Bosque P. J., Prusiner S. B. 2000. Cultured cell sublines highly susceptible to prion infection. J. Virol. 74:4377–4386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Büeler H., et al. 1992. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature 356:577–582 [DOI] [PubMed] [Google Scholar]
- 10. Deleault N. R., Harris B. T., Rees J. R., Supattapone S. 2007. Formation of native prions from minimal components in vitro. Proc. Natl. Acad. Sci. U. S. A. 104:9741–9746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edgar R. C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Enari M., Flechsig E., Weissmann C. 2001. Scrapie prion protein accumulation by scrapie-infected neuroblastoma cells abrogated by exposure to a prion protein antibody. Proc. Natl. Acad. Sci. U. S. A. 98:9295–9299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fischer M., et al. 1996. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 15:1255–1264 [PMC free article] [PubMed] [Google Scholar]
- 14. Ghaemmaghami S., et al. 2009. Continuous quinacrine treatment results in the formation of drug-resistant prions. PLoS Pathog. 5:e1000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harmeyer S., Pfaff E., Groschup M. H. 1998. Synthetic peptide vaccines yield monoclonal antibodies to cellular and pathological prion proteins of ruminants. J. Gen. Virol. 79(Pt 4):937–945 [DOI] [PubMed] [Google Scholar]
- 16. Kaeser P. S., Klein M. A., Schwarz P., Aguzzi A. 2001. Efficient lymphoreticular prion propagation requires PrPC in stromal and hematopoietic cells. J. Virol. 75:7097–7106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Köhler G., Milstein C. 1975. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495–497 [DOI] [PubMed] [Google Scholar]
- 18. Korth C., May B. C., Cohen F. E., Prusiner S. B. 2001. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc. Natl. Acad. Sci. U. S. A. 98:9836–9841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Korth C., et al. 1997. Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature 390:74–77 [DOI] [PubMed] [Google Scholar]
- 20. Krasemann S., Groschup M., Hunsmann G., Bodemer W. 1996. Induction of antibodies against human prion proteins (PrP) by DNA-mediated immunization of PrP0/0 mice. J. Immunol. Methods 199:109–118 [DOI] [PubMed] [Google Scholar]
- 21. Krasemann S., Jurgens T., Bodemer W. 1999. Generation of monoclonal antibodies against prion proteins with an unconventional nucleic acid-based immunization strategy. J. Biotechnol. 73:119–129 [DOI] [PubMed] [Google Scholar]
- 22. Legname G., et al. 2004. Synthetic mammalian prions. Science 305:673–676 [DOI] [PubMed] [Google Scholar]
- 23. Legname G., et al. 2005. Strain-specified characteristics of mouse synthetic prions. Proc. Natl. Acad. Sci. U. S. A. 102:2168–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McKinley M. P., Bolton D. C., Prusiner S. B. 1983. A protease-resistant protein is a structural component of the scrapie prion. Cell 35:57–62 [DOI] [PubMed] [Google Scholar]
- 25. Moroncini G., et al. 2004. Motif-grafted antibodies containing the replicative interface of cellular PrP are specific for PrPSc. Proc. Natl. Acad. Sci. U. S. A. 101:10404–10409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moroncini G., et al. 2006. Pathologic prion protein is specifically recognized in situ by a novel PrP conformational antibody. Neurobiol. Dis. 23:717–724 [DOI] [PubMed] [Google Scholar]
- 27. Müller-Schiffmann A., et al. 2009. Complementarity determining regions of an anti-prion protein scFv fragment orchestrate conformation specificity and antiprion activity. Mol. Immunol. 46:532–540 [DOI] [PubMed] [Google Scholar]
- 28. Nakamura N., et al. 2003. Generation of antibodies against prion protein by scrapie-infected cell immunization of PrP0/0 mice. Hybrid. Hybridomics 22:263–266 [DOI] [PubMed] [Google Scholar]
- 29. Oesch B., et al. 1985. A cellular gene encodes scrapie PrP 27–30 protein. Cell 40:735–746 [DOI] [PubMed] [Google Scholar]
- 30. O'Rourke K. I., et al. 1998. Monoclonal antibody F89/160.1.5 defines a conserved epitope on the ruminant prion protein. J. Clin. Microbiol. 36:1750–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pan K. M., Stahl N., Prusiner S. B. 1992. Purification and properties of the cellular prion protein from Syrian hamster brain. Protein Sci. 1:1343–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paramithiotis E., et al. 2003. A prion protein epitope selective for the pathologically misfolded conformation. Nat. Med. 9:893–899 [DOI] [PubMed] [Google Scholar]
- 33. Peretz D., et al. 2001. Antibodies inhibit prion propagation and clear cell cultures of prion infectivity. Nature 412:739–743 [DOI] [PubMed] [Google Scholar]
- 34. Polymenidou M., et al. 2008. The POM monoclonals: a comprehensive set of antibodies to non-overlapping prion protein epitopes. PLoS One 3:e3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prusiner S. B. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216:136–144 [DOI] [PubMed] [Google Scholar]
- 36. Prusiner S. B., et al. 1993. Ablation of the prion protein (PrP) gene in mice prevents scrapie and facilitates production of anti-PrP antibodies. Proc. Natl. Acad. Sci. U. S. A. 90:10608–10612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Raeber A. J., et al. 1999. Ectopic expression of prion protein (PrP) in T lymphocytes or hepatocytes of PrP knockout mice is insufficient to sustain prion replication. Proc. Natl. Acad. Sci. U. S. A. 96:3987–3992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37a. Reed L., Muench H. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493–497 [Google Scholar]
- 38. Safar J., et al. 1998. Eight prion strains have PrPSc molecules with different conformations. Nat. Med. 4:1157–1165 [DOI] [PubMed] [Google Scholar]
- 39. Safar J. G., et al. 2005. Search for a prion-specific nucleic acid. J. Virol. 79:10796–10806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Solforosi L., et al. 2007. Toward molecular dissection of PrPC-PrPSc interactions. J. Biol. Chem. 282:7465–7471 [DOI] [PubMed] [Google Scholar]
- 41. Reference deleted.
- 42. Thackray A. M., et al. 2003. Detection of bovine spongiform encephalopathy, ovine scrapie prion-related protein (PrPSc) and normal PrPC by monoclonal antibodies raised to copper-refolded prion protein. Biochem. J. 370:81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wadsworth J. D., et al. 2001. Tissue distribution of protease resistant prion protein in variant Creutzfeldt-Jakob disease using a highly sensitive immunoblotting assay. Lancet 358:171–180 [DOI] [PubMed] [Google Scholar]
- 44. White A. R., et al. 2003. Monoclonal antibodies inhibit prion replication and delay the development of prion disease. Nature 422:80–83 [DOI] [PubMed] [Google Scholar]
- 45. Yuan F. F., et al. 2005. Detection of prion epitopes on PrPC and PrPSc of transmissible spongiform encephalopathies using specific monoclonal antibodies to PrP. Immunol. Cell Biol. 83:632–637 [DOI] [PubMed] [Google Scholar]