Abstract
Plasmacytoid dendritic cells (pDCs) do not produce alpha interferon (IFN-α) unless viruses cause a systemic infection or overcome the first-line defense provided by conventional DCs and macrophages. We show here that even paramyxoviruses, whose infections are restricted to the respiratory tract, have a V protein able to prevent Toll-like receptor 7 (TLR7)- and TLR9-dependent IFN-α induction specific to pDCs. Mutational analysis of human parainfluenza virus type 2 demonstrates that the second Trp residue of the Trp-rich motif (Trp-X3-Trp-X9-Trp) in the C-terminal domain unique to V, a determinant for IRF7 binding, is critical for the blockade of TLR7/9-dependent signaling.
Plasmacytoid dendritic cells (pDCs) are unique in their capacity to rapidly secrete vast amounts of alpha interferon (IFN-α) via the Toll-like receptor 7 (TLR7)- and TLR9-dependent signaling pathway (2, 5, 11, 12, 15). The TLR7/9-dependent signaling pathway is specific to pDCs. This specificity relies on the constitutive expression of latent IFN regulatory factor 7 (IRF7) (6) and endosomal TLR7/9 (7). It was reported that measles virus (MeV) infection inhibited IFN synthesis of pDCs stimulated by the TLR7/9 agonist (29), and this inhibitory effect was exerted by viral V protein (26). Since pDCs produce IFN-α only when viruses cause a systemic infection or overcome the first-line defense provided by conventional DCs (cDCs) and macrophages (14), it is reasonable for MeV to have a strategy that antagonizes the function of pDCs. However, when virus infection was restricted in the lung, it was found that the major source of IFN-α was limited to cDCs and alveolar macrophages and that pDCs did not produce IFN-α (14). Thus, we seek to determine whether paramyxoviruses, whose infections are restricted to the respiratory tract, can block TLR7/9-dependent signaling.
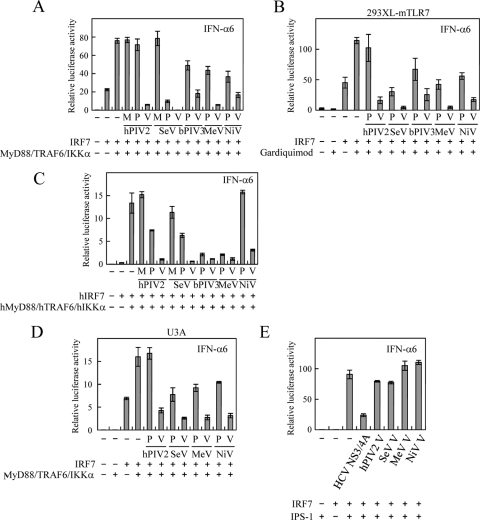
To examine the effect of V proteins from various paramyxoviruses on TLR7/9-dependent signaling, we employed a reconstitution system in 293T cells according to the procedure of Pfaller and Conzelmann (26). 293T cells were transfected with a PGV-B2 (Wako)-based luciferase reporter plasmid under the control of the mouse IFN-α6 promoter together with expression plasmids encoding a mouse TLR7/9 (mTLR7/9) downstream signaling molecule, MyD88, TRAF6, IκB kinase α (IKKα), or IRF7 in various combinations. To create these expression plasmids, each gene was subcloned into pCA7, which carries the cytomegalovirus enhancer chicken β-actin hybrid promoter (25, 30). Expression of IRF7 alone resulted in apparent induction of luciferase activity (Fig. 1A). It was enhanced 3- to 4-fold when upstream signaling molecules, MyD88, TRAF6, and IKKα, were coexpressed. This enhanced activation was significantly suppressed in the presence of any V protein of the paramyxoviruses, causing a local infection (human parainfluenza virus type 2 [hPIV2], Sendai virus [SeV], and bovine PIV3 [bPIV3]) or a systemic infection (MeV and Nipah virus [NiV]) (Fig. 1A). Essentially the same results were obtained with an established cell line, 293XL-mTLR7 (InvivoGen), which stably expresses mTLR7, when it was transfected with the IRF7 expression plasmid and stimulated with the TLR7 ligand Gardiquimod (InvivoGen) to activate TLR7-dependent signaling (22) (Fig. 1B). These results demonstrate that the ability to block TLR7/9-dependent signaling is conserved among paramyxoviruses irrespective of their infection modes. P protein showed various effects depending on virus species (Fig. 1AB). Notably, SeV P protein exhibited striking inhibition comparable to that of bPIV3 and NiV V proteins, in contrast to hPIV2 P protein, which had no effect. However, these effects varied when the transfected signaling molecules were replaced with those of human origin (Fig. 1C). Both SeV and hPIV2 P proteins showed moderate inhibition, whereas MeV and bPIV3 P proteins more potently suppressed the promoter activation. Thus, the inhibitory effects of the hPIV2, SeV, and MeV P proteins appeared to be host species specific. In contrast, the V proteins were basically inhibitory irrespective of the origin of the signaling molecules. Activation of TLR7/9-dependent signaling induces IFN-α, which in turn activates transcription of the endogenous IRF7 gene via JAK/STAT signaling (6). Accordingly, the well-known ability of V protein to block JAK/STAT signaling (8, 9, 28) may affect this reconstitution system by inhibiting endogenous IRF7 induction. However, similar results were obtained even with STAT1-deficient human fibroblasts (U3A) that have a defect in JAK/STAT signaling (20) (Fig. 1D). This demonstrates that the ability to block TLR7/9-dependent signaling is independent of the ability to block JAK/STAT signaling. IRF7 is also activated by inducible IKK (IKKi) and TBK1, whose activation is mediated by IFN-β promoter stimulator 1 (IPS-1)/Cardif/mitochondrial antiviral signaling (MAVS) protein on the TLR3, retinoic acid-inducible gene I (RIG-I), or melanoma differentiation-associated gene 5 (MDA5)-dependent signaling pathway. However, the IPS-1-mediated activation of IRF7 was not inhibited by any V proteins, unlike with hepatitis C virus (HCV) NS3/4A (Fig. 1E), which cleaves and inactivates IPS-1 (17, 21). This suggests that V protein specifically targets the activation process of IRF7 on the TLR7/9-dependent signaling pathway but not on the TLR3-, RIG-I-, or MDA5-dependent signaling pathway.
Fig. 1.
Effects of V and P proteins from different genera of the Paramyxovirinae subfamily on TLR7/9-dependent signaling. 293T (A, C, and E), 293XL-mTLR7 (B), or U3A cells (D) (∼1.5 × 105) were transfected with an IFN-α6 promoter-driven reporter plasmid (80 ng), an internal control plasmid (pRL-TK, 10 ng; Promega), and the indicated plasmids (MyD88 [25 ng], TRAF6 [25 ng], IKKα [12.5 ng], IRF7 [15 ng], IPS-1 [10 ng], M [50 ng], P [50 ng], V [50 ng], and NS3/4A [50 ng]). The total mass of transfected DNA was held constant by including the appropriate amount of the empty pCA7 plasmid. (B) 293XL-mTLR7 cells were treated with TLR7 ligand Gardiquimod (100 ng/ml) at 24 h posttransfection. Cells were lysed at 24 (A, C, D, and E) or 48 (B) h posttransfection, and activation of the IFN-α6 promoter was determined by a dual-luciferase reporter assay system (Promega). Data are derived from three independent experiments and are represented by mean values of the relative luciferase activities. Standard deviations are shown as error bars. The IFN-α6 promoter sequence and all the signaling molecule genes on the plasmids are of mouse origin unless otherwise noted. Viral sequences on the plasmids are derived from the SeV Z strain, bPIV3 910N strain, and hPIV2 Toshiba strain. hIRF7, human IRF7; hMyD88, human MyD88; hTRAF6, human TRAF6; hIKKα, human IKKα.
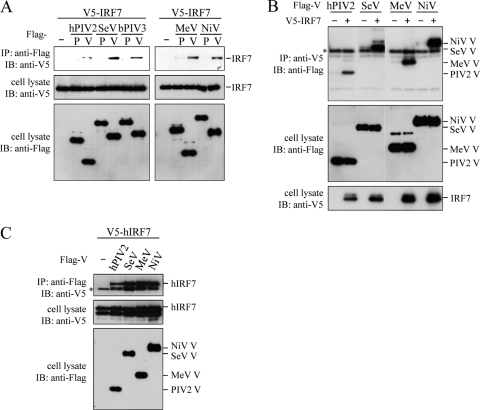
MeV V protein inhibits the phosphorylation of IRF7 (26). Unfortunately, we could not confirm the inhibition of IRF7 phosphorylation by MeV V protein, because our immunoblot analysis failed to detect phosphorylated IRF7 following overexpression of the signaling molecules, even in the absence of V protein. MeV V protein interacts with IKKα and IRF7, and the interaction with IKKα appears to be required for serving as a decoy substrate for IKKα (26). However, it is not clear whether the V-IRF7 interaction is critical for the blockade of TLR7/9-dependent signaling. To first determine whether the V-IRF7 interaction is a common feature of paramyxovirus V proteins, V5-tagged IRF7 was expressed in 293T cells in combination with Flag-tagged V or P protein and cell extracts were subjected to immunoprecipitation with anti-Flag antibody. As shown in Fig. 2A, anti-Flag antibody coprecipitated V5-tagged IRF7 in the presence of Flag-tagged V but not P protein, irrespective of virus species. Conversely, anti-V5 antibody precipitated Flag-tagged V proteins (Fig. 2B). Coprecipitation of IRF7 with V protein was also observed when mouse IRF7 was replaced with human IRF7 (Fig. 2C).
Fig. 2.
Interaction of paramyxoviral V proteins with IRF7. 293T cells (∼7.5 × 105) were transfected with the indicated plasmids. Cells were lysed in a lysis buffer (50 mM Tris-HCl, pH 7.4, 300 mM NaCl, 1% Triton X-100) at 24 h posttransfection. Flag-tagged proteins (A, C) or V5-tagged proteins (B) were immunoprecipitated (IP) with the anti-Flag antibody or anti-V5 antibody, respectively, and subjected to immunoblot analysis (IB) with the anti-V5 (A, C) or anti-Flag (B) antibody. Whole-cell lysates were also subjected to immunoblot analysis. Asterisks indicate positions of the antibody heavy chain. Plasmids expressing a tagged protein were created by appending each epitope tag to the N-terminal ends of proteins. IRF7, mouse IRF7; hIRF7, human IRF7.
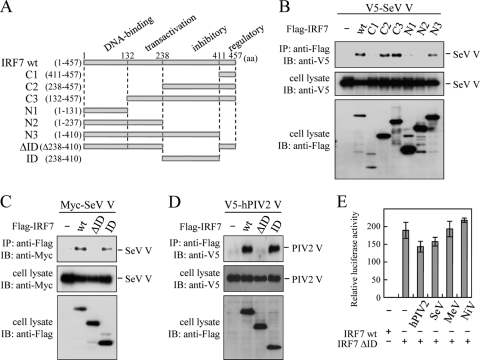
We next intended to identify a structural element in IRF7 that mediates its interaction with V protein. V5-tagged SeV V protein was coimmunoprecipitated by anti-Flag antibody from extracts of cells in which a truncated Flag-IRF7 mutant, C2 (amino acids [aa] 238 to 457), C3 (aa 132 to 457), or N3 (aa 1 to 410), was coexpressed with V5-tagged V protein (Fig. 3A and B), suggesting that the inhibitory domain (ID) (aa 238 to 410) shared by C2, C3, and N3 is responsible for the interaction with V protein. Indeed, removal of the ID from IRF7 abrogated the V-IRF7 interaction, and the ID alone could bind to V protein (Fig. 3C). Essentially the same results were obtained for hPIV2 (Fig. 3D). Expression of IRF7 ΔID alone strikingly activated the IFN-α6 promoter without upstream signaling molecules (18, 19) (Fig. 3E). However, this activation was not inhibited by any V proteins, probably due to a lack of their interaction with IRF7 ΔID.
Fig. 3.
V protein binds to the inhibitory domain of IRF7. (A) Schematic diagram of Flag-tagged truncated mutants of mouse IRF7. IRF7 C1, aa 411 to 457; IRF7 C2, aa 238 to 457; IRF7 C3, aa 132 to 457; IRF7 N1, aa 1 to 131; IRF7 N2, aa 1 to 237; IRF7 N3, aa 1 to 410; IRF7 ΔID, N2 fused to C1; IRF7 ID, aa 238 to 410. (B, C, and D) 293T cells (∼7.5 × 105) were transfected with the indicated plasmids and lysed at 24 h posttransfection. Flag-tagged proteins were immunoprecipitated with the anti-Flag antibody and subjected to immunoblot analysis with the anti-V5 (B and D) or anti-Myc (C) antibody. Whole-cell lysates were also subjected to immunoblot analysis. (E) 293T cells were transfected with an IFN-α6 promoter-driven reporter plasmid, pRL-TK, and the indicated plasmids. Cells were lysed at 24 h posttransfection. Activation of the IFN-α6 promoter was determined by dual-luciferase assay. Data are derived from three independent experiments and are represented as mean values. Standard deviations are shown as error bars.
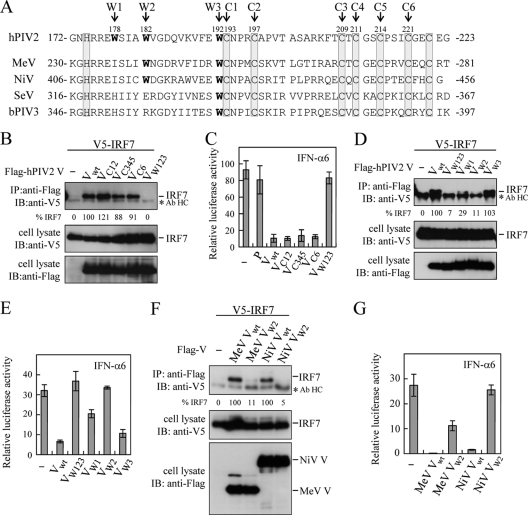
The N-terminal region of V protein is shared by P protein, whereas its C-terminal region represents the domain unique to V that is highly conserved among paramyxoviruses (16). The V-unique domain of MeV V protein mediates its interaction with IRF7 (26). This was also true for V protein of SeV and hPIV2 (data not shown). Invariantly spaced His and Cys residues in the V-unique domain form the zinc finger-like motif, and the Trp-rich motif (Trp-X3-Trp-X9-Typ), presumably implicated in protein-protein interaction (23), is located just upstream of the Cys cluster (Fig. 4A). The Trp residue at position W3 is conserved among paramyxoviruses. In contrast, the Trp residue at position W1 or W2 is limited to rubulaviruses or paramyxoviruses other than respiroviruses, respectively. To determine structural elements important for the blockade of TLR7/9-dependent signaling, we created hPIV2 V mutants in which amino acids in the Cys-rich motif or Trp-rich motif were replaced. Replacement of Cys residues at positions C1 to C6 in hPIV2 V protein affected neither IRF7 binding (Fig. 4B) nor the blockade of TLR7/9-dependent signaling (Fig. 4C), as seen in VC12 [V(C193A C197A)], VC345 [V(C209A C211A C214A)], and VC6 [V(C221A)]. In contrast, replacement of three Trp residues (W178H, W182E, and W192A) disrupted both abilities (Fig. 4BC). To specify critical Trp residues, VW1 [V(W178H)], VW2 [V(W182E)], and VW3 [V(W192A)] were examined for IRF7 binding. Of these mutants, only VW2 almost completely lost the ability to bind IRF7 (Fig. 4D). The IRF7 binding ability of VW1 strikingly decreased, whereas VW3 binds to IRF7 as efficiently as wild-type V (Vwt) (Fig. 4D). Furthermore, the magnitude of IRF7 binding closely correlated with the degree of the blockade of TLR7/9-dependent signaling (Fig. 4E). This demonstrates that the interaction of V protein with IRF7 is critical for the blockade of TLR7/9 signaling. Similar Trp V mutants were created for MeV and NiV. MeV VW2 [V(W240E)] and NiV VW2 [V(W416E)] exhibited a very low efficiency of IRF7 binding (Fig. 4F). Again, a good correlation between IRF7 binding efficiency and the degree of the blockade of TLR7/9-dependent signaling was observed (Fig. 4G). Taken together, these results demonstrate that the Trp residue at position W2 in hPIV2, MeV, and NiV V proteins is critical for IRF7 binding and the blockade of TLR7/9-dependent signaling.
Fig. 4.
The Trp-rich motif in V protein is essential for the interaction with IRF7 as well as the blockade of TLR7/9-dependent signaling. (A) Amino acid sequence alignment of the Cys-rich and Trp-rich C-terminal regions of paramyxovirus V proteins. Conserved His and Cys residues forming the zinc finger-like motif are shaded. Positions (C1 to C6) of the Cys residues are also shown. W1, W2, and W3 indicate positions of Trp residues in the Trp-rich motif. (B, D, and F) 293T cells were transfected with the indicated plasmids. Cells were lysed at 24 h posttransfection. Flag-tagged proteins were immunoprecipitated with anti-Flag antibody and subjected to immunoblot analysis with anti-V5 antibody. Relative intensities of coprecipitated IRF7 bands (%IRF7) are also shown. Whole-cell lysates were also subjected to immunoblot analysis. Asterisks indicate positions of the heavy chain of the anti-Flag antibody (Ab HC). (C, E, and G) 293T cells were transfected with expression plasmids (MyD88, TRAF6, IKKα, and IRF7), an IFN-α6 promoter-driven reporter, pRL-TK, and the indicated plasmid. Cells were lysed at 24 h posttransfection. Activation of the IFN-α6 promoter was determined by dual-luciferase assay. Data are derived from three independent experiments and are represented as mean values. Standard deviations are shown as error bars. VC12, V(C193A C197A); VC345, V(C209A C211A C214A); VC6, V(C221A); VW123, V(W178H W182E W192A); VW1, V(W178H); VW2, V(W182E); VW3, V(W192A); MeV VW2, MeV V(W240E); NiV VW2, NiV V(W416E).
The present study reveals that all paramyxoviruses examined can prevent TLR7/9-dependent signaling, suggesting their potential abilities to prevent pDCs from producing IFN-α. This finding implies that the unique immunosuppressive phenotype of MeV is not attributed only to its antagonism against the TLR7/9-dependent signaling pathway. Suppression of IFN-α production from pDCs was also reported for respiratory syncytial virus strain A2 and its clinical isolates, as well as human metapneumovirus (4, 29), whose infections are restricted to the respiratory tract. Thus, pDCs appear to play an important role in antiviral immune responses irrespective of virus infection modes. Why do paramyxoviruses causing a local infection need to antagonize the function of pDCs? Most paramyxoviruses express either C or V protein, which can block JAK/STAT signaling (3). For SeV, this ability proved to prevent alveolar macrophages and cDCs from producing IFN-α in vivo, probably due to inhibition of IFN-mediated induction of endogenous IRF7 (14). This prevention promoted the recruitment of pDCs in IFN-α production (14). Accordingly, the ability to block TLR7/9-dependent signaling may be needed to antagonize these recruited pDCs. From another standpoint, recent studies suggest that the role of pDCs in the development of antiviral adaptive immunity is independent of the mode of virus infection (10, 13). Therefore, the ability may be required for preventing the development of adaptive immunity.
The second Trp residue in the Trp-rich motif in hPIV2, NiV, and MeV V proteins was found to be critical for IRF7 interaction and the blockade of TLR7/9-dependent signaling. This characteristic discriminates the ability addressed here from other abilities of V protein, such as the blockade of MDA5-dependent signaling or JAK/STAT signaling (23, 24, 27). Since amino acid residues at positions W1 and W2 in SeV and bPIV3 V proteins are not Trp (Fig. 4A), these respiroviruses may be different from other paramyxoviruses in their blockade mechanisms. The close correlation between IRF7 binding and the blockade of TLR7/9-dependent signaling strongly suggests the significance of the interaction with IRF7 for the blockade of TLR7/9-dependent signaling. However, it remains unclear how V protein blocks TLR7/9-dependent signaling via its interaction with IRF7. It is unlikely that V protein inactivates the transcriptional activity of IRF7 itself, because V protein could not inhibit IPS-1-mediated activation of IRF7 (Fig. 1D). Thus, the V-IRF7 interaction may specifically affect TLR7/9-dependent IRF7 activation processes in which upstream signaling molecules act cooperatively. This possibility is now under investigation.
Acknowledgments
We thank Y. Yanagi (Fukuoka, Japan), H. Ogura, T. Abe, Y. Matsuura (Osaka, Japan), and L.-F. Wang (Geelong, Victoria, Australia) for providing pCA7, the MeV Edmonston strain, pCAGGs-puro/N-Flag-NS3/4A (1), and pCP721 (encoding the NiV P gene), respectively, and J. Miyazaki (Osaka, Japan) for his permission to use the CAG promoter of pCA7.
Sequence analyses were performed using the ABI Prism 310 genetic analyzer in the Central Research Laboratory of the Shiga University of Medical Science. This work was supported by grants-in-aid for scientific research from the Japan Society for the Promotion of Science and by grants from the Shiga University of Medical Science, Wajinkai, and the Yakult Foundation, Japan.
Footnotes
Published ahead of print on 23 February 2011.
REFERENCES
- 1. Abe T., et al. 2007. Hepatitis C virus nonstructural protein 5A modulates the Toll-like receptor-MyD88-dependent signaling pathway in macrophage cell lines. J. Virol. 81:8953–8966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cao W., Liu Y. J. 2007. Innate immune functions of plasmacytoid dendritic cells. Curr. Opin. Immunol. 19:24–30 [DOI] [PubMed] [Google Scholar]
- 3. Goodbourn S., Randall R. E. 2009. The regulation of type I interferon production by paramyxoviruses. J. Interferon Cytokine Res. 29:539–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guerrero-Plata A., et al. 2006. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 34:320–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Honda K., et al. 2004. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc. Natl. Acad. Sci. U. S. A. 101:15416–15421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Honda K., et al. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434:772–777 [DOI] [PubMed] [Google Scholar]
- 7. Hornung V., et al. 2002. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168:4531–4537 [DOI] [PubMed] [Google Scholar]
- 8. Horvath C. M. 2004. Silencing STATs: lessons from paramyxovirus interferon evasion. Cytokine Growth Factor Rev. 15:117–127 [DOI] [PubMed] [Google Scholar]
- 9. Horvath C. M. 2004. Weapons of STAT destruction. Interferon evasion by paramyxovirus V protein. Eur. J. Biochem. 271:4621–4628 [DOI] [PubMed] [Google Scholar]
- 10. Jung A., et al. 2008. Lymphocytoid choriomeningitis virus activates plasmacytoid dendritic cells and induces a cytotoxic T-cell response via MyD88. J. Virol. 82:196–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kawai T., Akira S. 2009. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 21:317–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kawai T., et al. 2004. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 5:1061–1068 [DOI] [PubMed] [Google Scholar]
- 13. Koyama S., et al. 2007. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J. Immunol. 179:4711–4720 [DOI] [PubMed] [Google Scholar]
- 14. Kumagai Y., et al. 2007. Alveolar macrophages are the primary interferon-alpha producer in pulmonary infection with RNA viruses. Immunity 27:240–252 [DOI] [PubMed] [Google Scholar]
- 15. Kumar H., Kawai T., Akira S. 2009. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 388:621–625 [DOI] [PubMed] [Google Scholar]
- 16. Lamb R. A., Parks G. D. 2007. Paramyxoviridae: the viruses and their replication, p. 1449–1496 In Knipe D. M., Howley P. M. (ed.), Fields virology, 5th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 17. Li X. D., Sun L., Seth R. B., Pineda G., Chen Z. J. 2005. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc. Natl. Acad. Sci. U. S. A. 102:17717–17722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin R., Mamane Y., Hiscott J. 2000. Multiple regulatory domains control IRF-7 activity in response to virus infection. J. Biol. Chem. 275:34320–34327 [DOI] [PubMed] [Google Scholar]
- 19. Marie I., Smith E., Prakash A., Levy D. E. 2000. Phosphorylation-induced dimerization of interferon regulatory factor 7 unmasks DNA binding and a bipartite transactivation domain. Mol. Cell. Biol. 20:8803–8814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McKendry R., et al. 1991. High-frequency mutagenesis of human cells and characterization of a mutant unresponsive to both alpha and gamma interferons. Proc. Natl. Acad. Sci. U. S. A. 88:11455–11459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meylan E., et al. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437:1167–1172 [DOI] [PubMed] [Google Scholar]
- 22. Ning S., Pagano J. S. 2010. The A20 deubiquitinase activity negatively regulates LMP1 activation of IRF7. J. Virol. 84:6130–6138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nishio M., Garcin D., Simonet V., Kolakofsky D. 2002. The carboxyl segment of the mumps virus V protein associates with stat proteins in vitro via a tryptophan-rich motif. Virology 300:92. [DOI] [PubMed] [Google Scholar]
- 24. Nishio M., et al. 2005. Identification of paramyxovirus V protein residues essential for STAT protein degradation and promotion of virus replication. J. Virol. 79:8591–8601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Niwa H., Yamamura K., Miyazaki J. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193–199 [DOI] [PubMed] [Google Scholar]
- 26. Pfaller C. K., Conzelmann K. K. 2008. Measles virus V protein is a decoy substrate for IkappaB kinase alpha and prevents Toll-like receptor 7/9-mediated interferon induction. J. Virol. 82:12365–12373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ramachandran A., Horvath C. M. 2010. dissociation of paramyxovirus interferon evasion activities: universal and virus-specific requirements for conserved V protein amino acids in MDA5 interference. J. Virol. 84:11152–11163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramachandran A., Horvath C. M. 2009. Paramyxovirus disruption of interferon signal transduction: STATus report. J. Interferon Cytokine Res. 29:531–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schlender J., et al. 2005. Inhibition of toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J. Virol. 79:5507–5515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takeda M., et al. 2005. Long untranslated regions of the measles virus M and F genes control virus replication and cytopathogenicity. J. Virol. 79:14346–14354 [DOI] [PMC free article] [PubMed] [Google Scholar]