Abstract
Herpes simplex virus 1 (HSV-1) is a common human pathogen that causes lifelong latent infection of sensory neurons. Non-nucleoside inhibitors that can limit HSV-1 recurrence are particularly useful in treating immunocompromised individuals or cases of emerging acyclovir-resistant strains of herpesvirus. We report that chebulagic acid (CHLA) and punicalagin (PUG), two hydrolyzable tannins isolated from the dried fruits of Terminalia chebula Retz. (Combretaceae), inhibit HSV-1 entry at noncytotoxic doses in A549 human lung cells. Experiments revealed that both tannins targeted and inactivated HSV-1 viral particles and could prevent binding, penetration, and cell-to-cell spread, as well as secondary infection. The antiviral effect from either of the tannins was not associated with induction of type I interferon-mediated responses, nor was pretreatment of the host cell protective against HSV-1. Their inhibitory activities targeted HSV-1 glycoproteins since both natural compounds were able to block polykaryocyte formation mediated by expression of recombinant viral glycoproteins involved in attachment and membrane fusion. Our results indicated that CHLA and PUG blocked interactions between cell surface glycosaminoglycans and HSV-1 glycoproteins. Furthermore, the antiviral activities from the two tannins were significantly diminished in mutant cell lines unable to produce heparan sulfate and chondroitin sulfate and could be rescued upon reconstitution of heparan sulfate biosynthesis. We suggest that the hydrolyzable tannins CHLA and PUG may be useful as competitors for glycosaminoglycans in the management of HSV-1 infections and that they may help reduce the risk for development of viral drug resistance during therapy with nucleoside analogues.
INTRODUCTION
Herpes simplex virus 1 (HSV-1) is an alphaherpesvirus that typically causes mucocutaneous lesions in oral, perioral, and other mucosal sites in the body (58). The virus commonly uses the oropharyngeal mucosa as a port of entry, and after primary infection, establishes a lifelong latent state in the host's trigeminal ganglia sensory neurons. Sporadic recurring infections occur when HSV-1 is reactivated by various stimuli, such as sunlight, immunosuppression, menstruation, fever, or stress (23). Although primary or reactivated HSV-1 infections can be subclinical or manifested by mild and self-limited diseases, severe cases of this viral infection may lead to complications such as keratoconjunctivitis, meningitis, and encephalitis (3, 5). Importantly, corneal HSV-1 infection can lead to stromal keratitis, which remains one of the leading causes of blindness in developing countries (37). More aggressive diseases due to HSV-1 are common in immunocompromised individuals (3, 5, 23). To date, no treatment has been identified that eradicates or resolves latent infections by this ubiquitous pathogen.
HSV-1 viral entry into cells is initiated by interaction of viral envelope glycoproteins (gB and gC) with host cell surface proteoglycans (PGs) conjugated to glycosaminoglycans (GAGs) containing heparan sulfate (HS) or chondroitin sulfate (CS) moieties. These initial interactions are sufficient for viral adsorption but not viral entry (67). Subsequently, higher affinity interaction of gD with its receptors including herpesvirus entry mediator (HVEM; a member of tumor necrosis factor receptor family), nectin-1 and nectin-2 (two members of the immunoglobulin superfamily), and/or 3-O-sulfated HS, leads to fusion of the viral membrane with either the plasma or endosomal membranes of the cell through further interactions with gB, gH, and gL (29, 57, 67). Initial interaction of HSV-1 with GAGs ensures a highly efficient infection, but infection of cells deficient in HS or CS can still be achieved via the high-affinity receptors, albeit at lower efficiency. After transport of the viral capsid to the nucleus, where the HSV-1 genome is released, viral products are then expressed in a sequential and coordinated fashion and are divided into three groups of virus-specific proteins designated as immediate-early (α) (ICP0 and ICP4)-, early (β) (ICP8, UL42, and thymidine kinase)-, and late (γ) (gB, gC, gD, and gH)-phase proteins (73). Although cellular innate immunity is activated upon virus infection, HSV-1 can produce one or more proteins that counteract the host antiviral response (46, 49, 50).
Most anti-herpes drugs target the viral DNA polymerase and include nucleoside or pyrophosphate analogues. Acyclovir (ACV), a guanosine analogue, has been the most important clinical drug for the prophylactic or therapeutic treatment of HSV infections, and represents the gold standard for anti-HSV therapy (4, 62). However, extensive use of this drug has led to clinical problems with the emergence of ACV-resistant virus strains, particularly in immunocompromised patients, including those who have had transplantation surgery or have been infected by the human immunodeficiency virus (HIV) (9, 21, 24, 48, 70, 79). Subsequent management of ACV-resistant patients using a different class of DNA polymerase-targeting inhibitor, such as foscarnet, has also been hindered by drug resistance (21, 59). There is a need to identify alternative antiviral therapies with different modes of action to improve the treatment and control of HSV infections, especially in immunocompromised individuals.
Terminalia chebula Retz. (T. chebula), a member of the Combretaceae family, is a traditional medicinal plant that is native to India and Southeast Asian countries. The dried ripened fruit of T. chebula (Fructus Chebulae), often referred to as “myrobalans,” contains antioxidants (15) and is commonly used as a broad-spectrum medicinal agent for the treatment of dysentery, asthma, cough, sore throat, bloody stools, and diseases of the heart and bladder (30). T. chebula is rich in tannins, which are polyphenolic secondary metabolites found in higher plants (27, 32, 36). Tannins are characterized by their relatively high molecular mass (500 to 20,000 Da) and the unique ability to form insoluble complexes with proteins, carbohydrates, nucleic acids, or alkaloids (27, 55, 63). The hydrolyzable class of tannins possesses structures that generally consist of gallic or ellagic acid esters conjugated to a sugar moiety (28, 36). These polyphenols have high affinity for proteins and polysaccharides and are thought to be the major bioactive compounds found in the leaves and the fruit of T. chebula.
Antiviral activities from hydrolyzable tannins are well documented and are generally thought to target viral adsorption to the host cell membrane (for HSV and HIV), as well as reverse transcriptase activity of HIV (reviewed in references 8 and 63). We have previously identified several tannins of various plant sources that exhibit potent antiviral activities against HSV-1 and HSV-2. These include 1,3,4,6-tetra-O-galloyl-β-d-glucose (77), casuarinin (10), ent-epiafzelechin-(4α→R8)-epiafzelechin (17), excoecarianin (16), geraniin (77), hippomanin A (76), prodelphinidin B-2 3′-O-gallate (11), prodelphinidin B-2 3,3′-di-O-gallate (12), pterocarnin A (13), and putranjivain A (14). Studies from other laboratories have also reported a series of tannins and related compounds capable of inhibiting HSV infections (25, 56, 64, 71, 72). These earlier reports provide strong precedent for our studies and suggest that the tannins constitute an excellent focus for antiviral discovery, particularly in the field of HSV therapeutics.
Identification of multiple drugs that can act on different phases of the viral life cycle can be particularly useful in managing HSV-1 infection or reactivation in either immunocompromised individuals or cases of ACV resistance. To pursue this goal, we extended our previous studies and concentrated our efforts on four chemically defined hydrolyzable tannins (Fig. 1), including chebulagic acid (CHLA), chebulinic acid (CHLI), punicalagin (PUG), and punicalin (PUN), which are present in T. chebula (39, 40, 78). Although an effect against HSV-1 has been previously reported for CHLA, the mechanism of its activity was not elucidated (71). In the present study we report that two of the tannins tested, specifically CHLA and PUG, were found to be most effective against HSV-1. Detailed studies into their inhibitory action revealed that both drugs specifically target HSV-1 particles, block virus entry into the cell, inhibit cell-to-cell spread of the virus, and reduce secondary infection from released virions. The antiviral mechanism is attributed to the binding of CHLA and PUG to viral glycoproteins that interact with cell surface GAGs. Their ability to effectively control viral entry and spread, underscore the potential of these two hydrolyzable tannins for treating HSV-1 infection and/or recurrence.
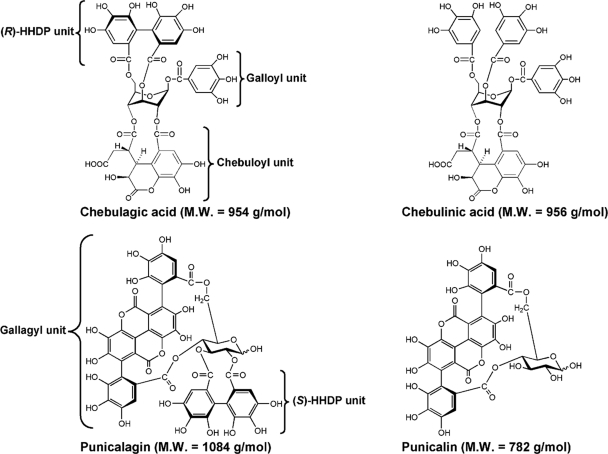
Fig. 1.
Chemical structures of chebulagic acid (CHLA), chebulinic acid (CHLI), punicalagin (PUG), and punicalin (PUN). Components of the tannins including galloyl, hexahydroxydiphenoyl (HHDP), gallagyl, and chebuloyl units are indicated.
MATERIALS AND METHODS
Chemicals and reagents.
Dulbecco modified Eagle medium (DMEM) and fetal calf serum (FCS) were supplied by Wisent Scientific (St-Bruno, Quebec, Canada). Gentamicin and amphotericin B (Fungizone) were purchased from Gibco-Invitrogen (Carlsbad, CA). ACV (acycloguanosine) was obtained from Calbiochem (EMD Biosciences, Darmstadt, Germany). Foscarnet (FOS; sodium phosphonoformate tribasic hexahydrate), dimethyl sulfoxide (DMSO), and an in vitro toxicology assay kit (XTT-based) were purchased from Sigma (St. Louis, MO).
Test compounds.
Fructus Chebulae and dried leaves from T. chebula were commercially obtained from Uchida Wakanyaku Co. (Tokyo, Japan) and an herbal market in Ping-Tung, Taiwan, respectively. Prior to extraction, both materials were anatomically authenticated by C.-C. Lin. CHLA, CHLI, and PUG were extracted from Fructus Chebulae, and PUN was derived from the leaves of T. chebula. The tannins were isolated and purified as described previously (39, 40). Before use, the structure of each compound was further confirmed by HPLC-UV/ESI-MS analyses, and their purities were checked by using high-pressure liquid chromatography with photodiode array detection (HPLC-PDA) as previously reported (34, 35). CHLA, CHLI, PUG, PUN, and ACV were dissolved in DMSO. FOS was dissolved in sterile double-distilled H2O. All compounds were diluted with cell culture medium before use. The final concentration of DMSO in the drug solution was below or equal to 1% at the effective doses used.
Plasmids.
The pCAGGS/MCS vector and its derivative plasmids expressing HSV-1 gB (pPEP98), gD (pPEP99), gH (pPEP100), and gL (pPEP101) (54) were generously provided by Patricia G. Spear and Richard Longnecker (Northwestern University, Chicago, IL).
Cells and viruses.
Vero (African green monkey kidney cells; ATCC CCL 81), HEL (human embryonic lung fibroblast; ATCC CCL 137), and A549 (human lung carcinoma; ATCC CCL-185) cells were obtained from the American Type Culture Collection (ATCC; Rockville, MD) and cultured in DMEM supplemented with 10% FCS, 50 μg of gentamicin/ml, and 0.5 μg of amphotericin B/ml at 37°C in a 5% CO2 incubator. Mouse L cells (provided by Bruce W. Banfield, Queen's University, Kingston, Ontario, Canada) and its mutant derivative cell lines gro2C (26) (obtained from Gary H. Cohen and Roselyn J. Eisenberg, University of Pennsylvania, Philadelphia, PA) and sog9 (6) were cultured as described above. Sog9-EXT1 cells were established as previously described (45) by transfecting sog9 cells with plasmid expressing the exostosin-1 (EXT1) gene and selecting in medium containing 700 μg of G418/ml. HSV-1 KOS strain (a gift from James R. Smiley, University of Alberta, Edmonton, Alberta, Canada), HSV-1 KOS strain with green fluorescent protein tag (HSV-1-GFP; provided by Karen L. Mossman, McMaster University, Hamilton, Ontario, Canada) (47), and vesicular stomatitis virus with green fluorescent protein tag (VSV-GFP; Indiana serotype, a gift from Brian D. Lichty, McMaster University, Hamilton, Ontario, Canada) (69) were propagated in Vero cells. HSV-1-GFP and VSV-GFP exhibit similar infectivity as their nontagged wild-type counterparts. Virus titers were determined by standard plaque assay. Overlay media containing 0.1% Gamunex (purified clinical human IgGs, provided by Andrew C. Issekutz, Dalhousie University, Halifax, Nova Scotia, Canada) or 2% methylcellulose were used for determination of virus titer for HSV-1 and VSV, respectively. The basal medium for the antiviral assays consisted of DMEM plus 2% FCS with antibiotics.
Cytotoxicity assay.
The cytotoxic effects of CHLA, CHLI, PUG, and PUN on the different cell types used in the present study were measured by the calorimetric XTT (2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-5-phenylamino)-carbonyl]-2H-tetrazolium hydroxide) assay as described previously with some modifications (16). Briefly, cells were seeded in 96-well plates (104 cells per well) and incubated overnight to form a monolayer. Increasing concentrations of the test compounds were then applied to the culture wells in triplicate. After incubation at 37°C for 72 h, the medium on the plate was discarded, and the cells were washed twice with phosphate-buffered saline (PBS). A volume of 100 μl of assaying solution from the in vitro XTT-based toxicology assay kit was added to each well. The plates were incubated for another 3 h to allow XTT formazan production. The absorbance was determined with an ELx800 Microplate reader (Bio-Tek Instrument, Inc., Winooski, VT) at a test wavelength of 492 nm and a reference wavelength of 690 nm. The data were calculated as percentage of surviving cells using the following formula: cell viability (%) = At/As × 100%, where “At” and “As” refer to the absorbances of the test compounds and the solvent control (DMSO), respectively. The concentration of 50% cellular cytotoxicity (CC50) of the test compounds was determined as the drug concentration that yielded 50% cell death as previously described (19).
Antiviral plaque assay and drug dose-response analysis.
A549 cells seeded in 12-well plates (5 × 105 cells per well) were treated with serial dilutions of the test compounds for 15 min at 37°C and then challenged with HSV-1 (50 PFU/well) for 1 h. The inocula and drugs were subsequently removed from the wells, and the cells were washed with PBS twice and overlaid again with different dilutions of the test compounds. After further incubation for 48 h, the supernatant was removed, and the wells were fixed with methanol and stained with Giemsa stain solution (Sigma). Viral inhibition (%) was calculated as follows: [1 − (number of plaques)exp/(number of plaques)control] × 100%, where “(number of plaques)exp” indicates the plaque counts from virus infection with test compound treatment and “(number of plaques)control” indicates the number of plaques derived from virus-infected cells with control treatment (HSV-1 with DMSO only) (10). The 50% effective concentration (EC50) for antiviral activity was defined as the concentration of antiviral compound that produced 50% inhibition of the virus-induced plaque formation (19).
For dose-response determination, A549 cells seeded in 96-well plates were infected with HSV-1-GFP (MOI = 1) in the presence or absence of the test compounds at various concentrations (0, 10, 20, 40, and 60 μM) for 24 h. The plates were then scanned by the Typhoon 9410 variable mode imager (Amersham Biosciences; Baie d'Urfe, Quebec, Canada), and the data were analyzed by using ImageQuant TL software (Amersham Biosciences). Viral infection (%) was calculated as follows: (fluorescenceexp – fluorescencecell control)/(fluorescencevirus control − fluorescencecell control) × 100%, where “fluorescenceexp” indicates the GFP expression value from the virus-infected wells with drug treatment, “fluorescencecell control” signifies the GFP expression value of the cell control (DMSO only), and “fluorescencevirus control” indicates the GFP expression value derived from virus-infected cells with control treatment (HSV-1-GFP with DMSO only). Values were obtained from three independent experiments with each sample assay performed in triplicate. A standard curve was also generated to ensure linear correlation between virus infection and GFP expression at the multiplicity of infection (MOI) assessed.
Assays for effect of tannin treatment at different times.
The effect of drug addition over time was assessed according to a previously reported method with some modifications (41). To assess the effect of pretreating cells with tannins, A549 cell monolayers seeded in 12-well plates were treated with CHLA (60 μM) and PUG (40 μM) for 24 h (long term) or 1 h (short term) and then washed with PBS before challenge with HSV-1 (50 PFU/well) in DMEM containing 2% FCS. To study the effect of adding tannins and virus concurrently, A549 cells were treated simultaneously with HSV-1 (50 PFU/well) and CHLA (60 μM) or PUG (40 μM). After incubation for 1 h at 37°C, the virus-drug mixture was removed, and cells were washed prior to overlay with media. To evaluate whether the tannins had any effects after viral entry, A549 cells were challenged with HSV-1 (50 PFU/well) for 1 h, and after removal of the virus inoculum, infected cells were washed and subsequently overlaid with media containing CHLA (60 μM) or PUG (40 μM). For the continuous drug treatment, cells were pretreated for 1 h with the tannins, challenged with HSV-1 in the presence of the drugs, and overlaid with media containing the test compounds after viral entry. For all of the above experiments, viral plaques were stained and counted following a total incubation of 48 h postinfection (p.i.). DMSO (0.1%) treatment was included as control in each condition.
VSV plaque reduction assay for host innate immunity.
To evaluate whether CHLA and PUG induced host innate immune response, a VSV plaque reduction assay was performed. Briefly, A549 cells were seeded in 12-well plates (5 × 105 cells per well) and then pretreated with CHLA (60 μM), PUG (40 μM), or alpha interferon (IFN-α) from human leukocytes (1,000 U/ml; Sigma) or with medium and DMSO (0.1%) controls for 24 h. Cell monolayers were washed with PBS twice and subsequently infected with VSV-GFP at an MOI of 0.01 for 1 h before the overlay media containing 2% FCS and 2% methylcellulose were applied. The plates were scanned by a Typhoon 9410 variable mode imager to visualize fluorescent plaques at 48 h p.i.
Viral inactivation assay.
A viral inactivation assay was performed as previously described with some modifications (41). HSV-1 (104 PFU/ml) was mixed with CHLA (60 μM) or PUG (40 μM) and then incubated at 37°C for 1 h. The test compound-virus mixture was then diluted 50-fold (final virus concentration, 50 PFU/well) with DMEM containing 2% FCS to yield a subtherapeutic concentration of the drug, and the virus inocula were subsequently added to monolayers of A549 cells seeded in 12-well plates. As a comparison, HSV-1 was mixed with test compounds, diluted immediately to 50-fold (no incubation period), and added to A549 cells for infection. The 50-fold dilution served to titrate the drugs below their effective doses and prevent meaningful interactions with the host cell surface. After adsorption for 60 min at 37°C, the diluted inocula were discarded, and the cells were washed with PBS twice. An overlay medium (DMEM containing 2% FCS) was applied to each well, and the plates were further incubated at 37°C for 48 h before being subjected to plaque assay as described above. Viral plaques were counted, and plaque numbers obtained from infections in the presence of drug compounds were compared to the 0.1% DMSO control.
Viral attachment assay by ELISA and flow cytometry.
Viral attachment to cell surface was assessed at 4°C, which allows for HSV-1 binding but excludes entry (41), using cellular enzyme-linked immunosorbent assay (ELISA), as well as flow cytometry. The ELISA binding assay was performed as previously described with some modifications (2, 31). Briefly, 96-well plates were seeded with A549 cells (2 × 104 cells per well) and allowed to grow overnight. The cell monolayers were prechilled at 4°C for 1 h and subsequently challenged with HSV-1 (MOI = 5) inoculum in the presence of CHLA, PUG, or the DMSO (1%) and heparin controls at various concentrations for 3 h at 4°C. Cells inoculated with DMSO (1%) alone served as a mock control. After infection, the wells were washed twice with ice-cold PBS to remove unbound virus, fixed with prechilled 4% paraformaldehyde (PFA) in PBS for 1 h on ice, and then blocked with 5% bovine serum albumin (BSA) at 4°C. To detect bound virus by ELISA, samples were incubated at 37°C for 1 h with a polyclonal rabbit anti-HSV-1 antibody (Dako Canada, Inc., Mississauga, Ontario, Canada) at a 1:7,500 dilution in a PBS solution with 0.1% Tween 20 (PBST) and containing 5% BSA. The wells were subsequently washed two times with PBST containing 5% BSA and two times with PBST only, with each wash performed at 5-min interval on a plate shaker to minimize background effects. The secondary antibody consisted of a goat anti-rabbit IgG conjugated with horseradish peroxidase (HRP; Invitrogen) and was added to the samples at a 1:100,000 dilution in PBST containing 5% BSA. After incubation at 37°C for 1 h, the wells were washed as previously described, and developed with a TMB (3,3′,5,5′-tetramethylbenzidine) two-component microwell peroxidase substrate kit (KPL, Gaithersburg, MD) at room temperature for 20 min before stopping the reaction with 1 M phosphoric acid (H3PO4). The absorbance was immediately determined at 450 nm with an ELx800 Microplate reader. Values are expressed as the fold change of absorbance relative to the mock infection control.
For the viral binding assay using flow cytometry analysis, A549 cells (106 cells per well) were first dissociated using a nonenzymatic cell dissociation buffer (Sigma). Cells were infected with HSV-1 (MOI = 1) in the presence or absence of CHLA (60 μM) or PUG (40 μM) for 3 h at 4°C. DMSO (0.1%) was used as a negative control and heparin (100 μg/ml) was included as a positive control. Cells were subsequently washed twice with ice-cold fluorescence-activated cell sorting (FACS) buffer (1× PBS, 2% FCS, and 0.1% sodium azide), blocked with 5% FCS for 30 min on ice, and then stained with a fluorescein isothiocyanate (FITC)-conjugated polyclonal rabbit anti-HSV-1 antibody (1:500; Dako Canada, Inc). Stained samples were washed twice with FACS buffer and then fixed with 1% PFA before being subjected to standard flow cytometry analysis. Normal rabbit serum was included in the experiments as an isotype control (1:250; Abcam). The data acquisition and flow cytometry analysis were performed on a Cyan flow cytometer (Dako Canada, Inc.).
Viral penetration assay.
The viral penetration assay was performed as previously described (41) with minor modifications. A549 cell monolayers grown in 12-well plates were prechilled at 4°C for 1 h and subsequently incubated with HSV-1 (100 PFU/well) for 3 h at 4°C to allow for viral adsorption. The infected cell monolayers were then incubated in the presence of CHLA (60 μM), PUG (40 μM), heparin (100 μg/ml), or DMSO (0.1%) for an additional 20 min at 37°C to facilitate HSV-1 penetration. At the end of the incubation, extracellular virus was inactivated by citrate buffer (pH 3.0) (18) for 1 min, and then cells were washed with PBS and overlaid with DMEM containing 2% FCS. After 48 h of incubation at 37°C, viral plaques were stained and counted.
Effect of tannin addition on viral RNA expression at different times postentry.
The effects of tannin addition on HSV-1 RNA expression within the cell was performed by reverse transcriptase PCR (RT-PCR) analysis. A549 cells were infected with HSV-1 (MOI = 1) for 1 h and then treated with low pH citrate buffer (pH 3.0) to inactivate extracellular viral particles. Cells were then overlaid with medium containing CHLA (60 μM), PUG (40 μM), or DMSO control (0.1%). At 4, 8, and 12 h p.i., total cellular RNA was isolated by using TRIzol Reagent (Invitrogen), treated with DNase I (Qiagen, Inc., Mississauga, Ontario, Canada) to remove genomic DNA, and purified by phenol-chloroform according to the protocols of the manufacturers. Aliquots of 1 μg of RNA were used to generate cDNA with a high-capacity cDNA reverse transcription kit (ABI, Foster City, CA). The cDNA (10%) was then subjected to standard PCR amplification using the following primers against HSV-1 immediate-early (ICP27), early (TK), and late (gD) genes and also against the cellular glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene: ICP27 forward primer, 5′-GCCGCGACGACCTGGAATCG-3′; ICP27 reverse primer, 5′-TGTGGGGCGCTGGTTGAGGATC-3′ (216 bp); TK forward primer, 5′-AGGTATCGCGCGGCCGGGTAG-3′; TK reverse primer, 5′-ATGGCTTCGTACCCCTGCCA-3′ (533 bp); gD forward primer, 5′-ATGGGAGGCAACTGTGCTATCC-3′; gD reverse primer, 5′-CTCGGTGCTCCAGGATAAAC-3′ (250 bp); GAPDH forward primer, 5′-GCCTCCTGCACCACCAACTG-3′; and GAPDH reverse primer, 5′-ACGCCTGCTTCACCACCTTC-3′ (349 bp). For comparison, A549 cells were also infected with HSV-1 (MOI = 1) in the presence of CHLA (60 μM), PUG (40 μM), or DMSO (0.1%) at zero time (co-addition). After incubation for 1 h at 37°C, the inocula and tannins were removed, and the cells were washed with PBS before being overlaid with DMEM containing 2% FCS. Again, at 4, 8, and 12 h p.i., total cellular RNA was harvested and subjected to RT-PCR analysis as described above.
Effect of tannins on HSV-1 secondary infection and cell-to-cell spread.
To assess the effect of the tannins on secondary viral infections, confluent A549 cells were seeded in 12-well plates and then infected with HSV-1-GFP (200 PFU/well) for 1 h. After virus adsorption and penetration, extracellular virus was inactivated by citrate buffer (pH 3.0) for 1 min, followed by PBS washes. The cells were then treated with CHLA (60 μM), PUG (40 μM), heparin (100 μg/ml), or DMSO (0.1%) following one round of HSV-1 replication (12 h). Any increase in plaque formation due to released virions from secondary infections could be monitored over the subsequent incubation period. At 24 and 48 h after the initial infection, the plates were scanned by using a Typhoon 9410 variable mode imager, and fluorescent viral plaques were counted by using ImageQuant TL software to determine whether any new plaques were formed due to virus progeny. Values were obtained from three independent experiments with each sample being performed in duplicate and plotted on a bar graph.
For the analysis of virus cell-to-cell spread, experiments were performed as described above, except that the drugs were added to HSV-1-GFP-infected-cells at 24 h p.i. The overlay media also contained neutralizing Gamunex antibodies (0.1%), which were used to prevent secondary infection from released HSV-1 virions. The antibodies do not affect lateral spread of virus between cells via intercellular junctions. This allowed the monitoring of cell-to-cell spread of virus in the presence or absence of tannins. Drugs were added at 24 h p.i., and a visual comparison of viral plaque size was made at the endpoint of the experiment (48 h p.i.). Photomicrographs were taken at ×100 magnification (Leica Microsystems, Wetzlar, Germany) between 24 and 48 h p.i.
Virus-free cell fusion assay.
To examine whether the compounds interacted with HSV-1 glycoproteins to inhibit glycoprotein-mediated fusion events, a virus-free cell fusion assay was performed (54). A549 cells were seeded in six-well dishes and transfected with plasmid DNA expressing the individual HSV-1 glycoproteins (gB, gD, gH, and gL) or with the control vector using Arrest-In (Open Biosystems, Huntsville, AL). The total amount of plasmid DNA per well was 4 μg. After 6 h of incubation at 37°C, the transfection mixture was discarded, and the cells were washed with PBS before treatment with CHLA (60 μM), PUG (40 μM), heparin (100 μg/ml), or DMSO (0.1%). After incubation for another 24 h, cells were fixed with methanol and then stained with Hoechst dye (Sigma) or Giemsa stain solution. Photomicrographs were then taken at ×200 magnification (Leica Microsystems). Polykaryocytes with >10 nuclei from the dishes were also counted.
Effect of tannins using plaque assays in GAG-deficient cell lines.
For antiviral analysis in GAG mutant cell lines, confluent mouse L, gro2C, sog9, and sog9-EXT1 cells in 12-well dishes were infected with HSV-1-GFP at 200 PFU/well (MOI = 0.0004), reflecting virus titers obtained in each cell line, in the presence of CHLA (60 μM), PUG (40 μM), or DMSO (0.1%). After incubation for 1 h at 37°C, the virus-drug mixture was removed, and the cells were washed with PBS before overlaying them with DMEM containing 2% FCS. After further incubation for 48 h, fluorescent plaques were scanned as described above and counted using the ImageQuant TL software. The data were expressed as the percent viral infection = [(number of plaques)exp/(number of plaques)control] × 100%.
RESULTS
Inhibition of HSV-1 infection by the hydrolyzable tannins.
CHLA, CHLI, PUG, and PUN (Fig. 1) have been reported to exhibit antiviral activities. We investigated whether these hydrolyzable tannins could inhibit HSV-1 infection. In order to ensure that the tannin concentrations were not cytotoxic, a toxicity analysis was carried out in A549 cells by using a XTT cell viability assay. Our results indicated that these four tannins did not have apparent cytotoxic effects below 100 μM in A549 cells, while a dose-dependent cytotoxic effect was seen when concentration >100 μM was used (data not shown). The 50% cellular toxicity indices (CC50) of CHLA, CHLI, PUG, and PUN were 316.87 ± 9.01, 330.83 ± 9.07, 318.84 ± 4.98, and 310.85 ± 1.99 μM, respectively (summarized in Table 1). For comparison, toxicity in primary human fibroblast (HEL) cells was also assessed, and similar results were observed (data not shown).
Table 1.
Cytotoxicity and anti-HSV-1 activity of CHLA, CHLI, PUG, and PUN in A549 cellsa
| Compound | Mean CC50b (μM) ± SEM | Anti-HSV-1 effect |
|
|---|---|---|---|
| Mean EC50c (μM) ± SEM | SId | ||
| CHLA | 316.87 ± 9.01 | 17.02 ± 2.82 | 18.62 |
| CHLI | 330.83 ± 9.07 | 20.85 ± 2.40 | 15.87 |
| PUG | 318.84 ± 4.98 | 10.25 ± 1.13 | 31.11 |
| PUN | 310.85 ± 1.99 | 21.33 ± 1.77 | 14.57 |
| ACV | >2,000 | 14.45 ± 1.71 | >138.41 |
| FOS | >2,000 | 183.37 ± 25.18 | >10.91 |
The values shown are means from three independent experiments with each treatment performed in triplicate.
Cytotoxic effects were evaluated by XTT assay to determine the concentration of 50% cellular cytotoxicity (CC50) of the tested compounds.
Antiviral effects were evaluated by plaque assay to determine the effective concentration that achieved 50% inhibition (EC50) against HSV-1 infection.
SS, selectivity index. SI = CC50/EC50.
We then evaluated the antiviral effects of these four natural compounds against HSV-1 infection by using a plaque assay. ACV and FOS were used as positive controls, and DMSO was included as a negative control. FOS is the treatment of choice in the clinical setting if resistance develops against ACV. All four tannins could inhibit viral plaque formation, after inoculation of 50 PFU, in a dose-dependent manner, and their 50% effective concentration (EC50) values were 17.02 ± 2.82 (CHLA), 20.85 ± 2.40 (CHLI), 10.25 ± 1.13 (PUG), and 21.33 ± 1.77 μM (PUN) (Table 1). The selectivity index (SI), which measures the preferential antiviral activity of a drug in relation to its cytotoxicity, was calculated according to their CC50 and EC50. The SIs of CHLA, CHLI, PUG, and PUN were 18.62, 15.87, 31.11, and 14.57, respectively (Table 1). Given their higher SI values, CHLA and PUG were chosen for subsequent analyses.
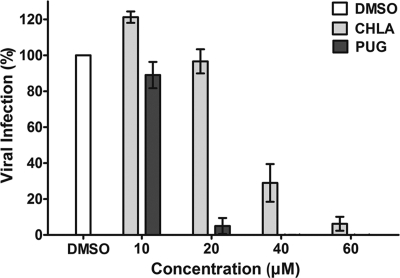
To obtain a more accurate dose-response curve for these two hydrolyzable tannins, A549 cells were infected with HSV-1-GFP (MOI = 1) in the presence of the tannins, and fluorescent signals were quantified. The HSV-1-GFP was susceptible to the antiviral effects of the tannins. Both CHLA and PUG displayed anti-HSV-1 activity in a dose-dependent manner (Fig. 2), and the concentrations of CHLA at 60 μM and PUG at 40 μM, which provided near complete protection against the virus infection at an MOI of 1, were chosen for all subsequent experiments.
Fig. 2.
Dose response for inhibition of HSV-1 infection in A549 cells by CHLA and PUG. A549 cells were seeded into 96-well plates and then infected with HSV-1-GFP (MOI = 1) in the presence or absence of the tannins at various concentrations (0, 10, 20, 40, and 60 μM) for 24 h. DMSO (0.1%) served as a negative control. Viral infection was quantified by measuring GFP fluorescence using a Typhoon 9410 variable mode imager. The data shown are means ± the standard errors of the mean (SEM) of three independent experiments, with each tannin treatment being performed in triplicate.
Antiviral activities of CHLA and PUG depend upon the presence of HSV-1, and inhibition is not due to activation of host cell innate immunity.
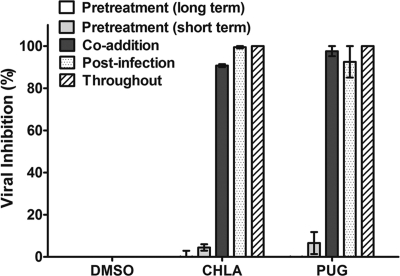
To better understand the antiviral mechanism and the stage of HSV-1 infection affected by these two T. chebula tannins, we added the compounds at different times of the virus life cycle (pre-entry, entry, and postentry). In order to study pre-entry, more specifically the effect of the compounds on the cell itself prior to virus addition, A549 cells were pretreated with CHLA and PUG for long-term (24 h) or short-term (1 h) periods and then washed prior to HSV-1 infection. For effects on the viral entry stage, virus and the drugs were simultaneously applied to the cells. To investigate events after virus entry, A549 cells were first infected with HSV-1 for 1 h and then treated with the tannins. For comparison, the tannins were also maintained throughout the experimental period.
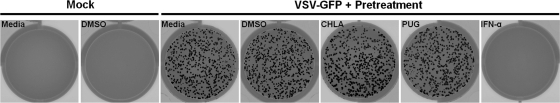
Pretreating A549 cells with CHLA and PUG (both long term and short term) did not protect against HSV-1 infection. Both tannins were effective in preventing plaque formation when added during virus adsorption, immediately after viral entry, and throughout multiple cycles of virus replication (Fig. 3). The data indicate that HSV-1 infection is severely impaired only if the drugs are present at the time of infection or during viral spread and that it is unlikely that the antiviral activity is due to direct effects on the cells (such as masking cellular receptors or entry factors for HSV-1). To confirm that neither of the tannins activated host cell innate immunity and induced production of antiviral cytokines such as interferons (IFNs), a VSV plaque reduction assay was performed. VSV replication is extremely sensitive to cellular IFN production. IFN-α, a potent inhibitor of VSV, was included as a positive control. In line with the above observation, neither CHLA nor PUG pretreatments protected A549 cells from VSV infection, whereas IFN-α treatment produced an intact cell monolayer (Fig. 4). Moreover, neither of the tannins induced IFN-stimulated genes in the A549 cells (data not shown). These results suggest that the anti-HSV-1 activities produced by CHLA and PUG (i) are unlikely to be mediated by effects through binding to the cellular receptors for HSV-1 or in triggering antiviral innate immunity and (ii) absolutely require the presence of HSV-1.
Fig. 3.
Effect of time of CHLA and PUG addition on plaque formation by HSV-1. A549 cells were seeded in 12-well plates and then treated with CHLA (60 μM) or PUG (40 μM) at various stages of HSV-1 infection (50 PFU/well). For pretreatment, A549 cells were incubated with the test compounds for 24 h (long term) or 1 h (short term) and then washed once before infection with HSV-1. For the co-addition experiment, the tannins and virus inoculum were added simultaneously to cells, incubated for 1 h, and then washed. In the postinfection experiment, the cells were infected with HSV-1 for 1 h, washed, and then treated with the tannins. In addition, cells were also incubated in the continuous presence of the test compounds from pretreatment to postinfection stages. After an additional 48 h of incubation, viral plaques were stained and counted. DMSO (0.1%) was included as control. The data shown are the means ± the SEM of three independent experiments with each treatment performed in duplicate.
Fig. 4.
CHLA and PUG do not induce IFN-mediated immune response against VSV infection. A549 cells were seeded in 12-well plates and then pretreated with CHLA (60 μM), PUG (40 μM), or IFN-α (1,000 U/ml) or with media and DMSO (0.1%) controls for 24 h. Cell monolayers were washed with PBS twice and then infected with VSV-GFP (MOI = 0.01) for 1 h before overlay media containing 2% FCS and 2% methylcellulose were applied. After an additional 48 h of incubation, the plates were scanned for visualization of fluorescent plaques. The data shown are representative images from one of two independent experiments.
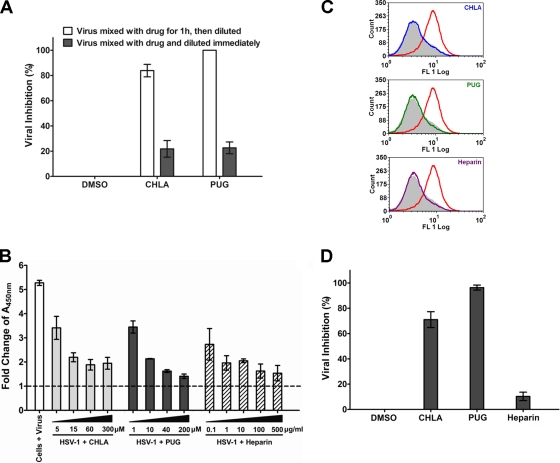
CHLA and PUG block HSV-1 entry by inactivating virus particles and preventing virus attachment and penetration into A549 cells.
In order to evaluate the antiviral mechanism of CHLA and PUG, we investigated their effects on the virus particles themselves. Tannin compounds were preincubated with virus particles and then diluted to a subtherapeutic concentration prior to infection. Both CHLA and PUG could interact with virus particles, irreversibly, to prevent infection (Fig. 5A). This suggests that CHLA and PUG can bind to virus particles and neutralize virus infectivity.
Fig. 5.
CHLA and PUG inhibit HSV-1 entry by inactivating viral particles and preventing virus binding and internalization into A549 cells. (A) Viral inactivation assay. HSV-1 (104 PFU/ml) was mixed with CHLA (60 μM) or PUG (40 μM) for 1 h at 37°C and then diluted 50-fold (final virus concentration, 50 PFU/well) before infecting A549 cells. As a control, the same amount of virus was also mixed with the tannin, but diluted immediately and applied to the A549 cells. After a 48-h incubation period, viral plaques were stained and counted. DMSO (0.1%) was included as a negative control. The data shown are means ± the SEM of three independent experiments with each treatment performed in duplicate. (B) Viral attachment analysis using ELISA. Adherent A549 cell monolayers in 96-well plates were prechilled at 4°C for 1 h and then inoculated with HSV-1 (MOI = 5) in the presence of CHLA, PUG, or the DMSO (1%) and heparin controls at various concentrations for another 3 h at 4°C. Wells were washed to remove unadsorbed virus, subsequently fixed with 4% PFA, and then blocked with 5% BSA. ELISA was performed with a primary polyclonal rabbit anti-HSV-1 antibody (1:7,500) and a secondary goat anti-rabbit IgG conjugated with HRP (1:100,000), followed by development with TMB substrate and reaction termination with 1 M H3PO4. The absorbance was immediately determined at 450 nm. Values are expressed as the fold change of absorbance relative to the mock infection control (cells + 1% DMSO), which is indicated by the dashed line. The data shown are means ± the SEM of three independent experiments with each treatment performed in triplicate. (C) Viral binding assay using flow cytometry analysis. Dissociated A549 cells were infected with HSV-1 (MOI = 1) in the presence or absence of 60 μM CHLA or 40 μM PUG for 3 h at 4°C. Cells were then washed, blocked, and stained with FITC-conjugated polyclonal rabbit anti-HSV-1 antibody (1:500). Stained samples were washed and fixed with 1% PFA before being subjected to standard flow cytometry analysis. DMSO (0.1%) was used as a negative control, and heparin (100 μg/ml) was included as a positive control. Color indication for different treatments: gray, mock + DMSO; red, HSV-1 + DMSO; blue, HSV-1 + CHLA; green, HSV-1 + PUG; and purple, HSV-1 + heparin. The data shown are representative of three independent experiments. (D) Viral penetration analysis by plaque assay. A549 cells were prechilled at 4°C for 1 h before inoculation with HSV-1 (100 PFU/well) for 3 h at 4°C. The cells were then treated in the presence or absence of CHLA (60 μM), PUG (40 μM), or heparin (100 μg/ml) and further incubated for an additional 20 min with the temperature shifted to 37°C to facilitate viral penetration. At the end of the incubation, extracellular virus was inactivated by citrate buffer (pH 3.0) for 1 min and then washed with PBS twice before overlaying with medium. After 48 h of incubation at 37°C, viral plaques were stained and counted. DMSO (0.1%) was included as a negative control. The data shown are means ± the SEM of three independent experiments with each treatment performed in duplicate.
We next assessed the ability of CHLA and PUG to affect viral attachment and penetration. The attachment assay was carried out at 4°C, which allows for virus binding but prevents entry (41). Using ELISA to detect bound virus on the adherent cells, both tannin compounds were observed to inhibit HSV-1 attachment to the A549 cell surface in a dose-dependent manner (Fig. 5B). An alternative binding assay was also performed using virus-specific antibodies to detect bound HSV-1 particles by flow cytometry. Again, CHLA and PUG could prevent HSV-1 binding to the surface of the target A549 cells (Fig. 5C). Heparin, a competitive HSV binding inhibitor, was included as a positive control in both experiments. These results suggest that CHLA and PUG might interact with viral glycoprotein(s) and/or cellular receptor(s) during the virus attachment phase. To further assess the effects of CHLA and PUG on virus penetration step, HSV-1 particles were allowed to first bind to A549 cells at 4°C and were subsequently allowed to fuse with and penetrate the host cell membrane by shifting the temperature to 37°C in the presence or absence of the tannins. As shown in Fig. 5D, CHLA retained most of its antiviral activity even during the viral penetration phase and PUG could completely abrogate virus penetration into the A549 cells, resulting in a protected monolayer. In contrast, heparin, which is effective at blocking HSV-1 adsorption, did not prevent subsequent virus penetration (42). The data indicate that CHLA and PUG impair viral receptor attachment and penetration functions during the HSV-1 infection.
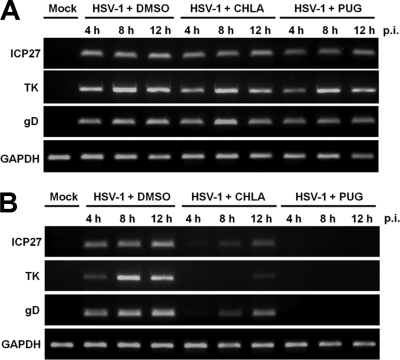
CHLA and PUG do not affect HSV-1 transcription after entry but limit secondary viral infection and cell-to-cell transmission.
The observation in Fig. 3 that CHLA and PUG inhibited HSV-1 plaque formation when treatment was initiated immediately after the virus had entered the cell suggested that the two tannins may block HSV-1 replication cycle or inhibit HSV-1 secondary infection and/or cell-to-cell spread in the ensuing incubation period. To specifically address these possibilities, we first evaluated the effects of CHLA and PUG on HSV-1 mRNA expression after virus entry. A549 cells were infected with HSV-1 for 1 h; extracellular virus was then inactivated by citrate buffer treatment and washed away; and CHLA, PUG, or DMSO was subsequently added to the cells. For comparison, CHLA and PUG were also added simultaneously with HSV-1. Total cellular RNA was isolated from all samples at various time points after viral infection. Our results clearly indicated that CHLA and PUG did not affect HSV-1 mRNA expression after virus penetration, since levels of immediate-early (ICP27), early (TK), and late (gD) viral gene transcripts were unaffected by the compounds (Fig. 6A). On the other hand, both tannins suppressed HSV-1 mRNA synthesis when added together with the virus at the same time (Fig. 6B). These findings suggest that neither CHLA nor PUG inhibit HSV-1 transcription following penetration of the host cell.
Fig. 6.
CHLA and PUG do not affect HSV-1 transcription, following entry into the host cell. (A) A549 cells were infected with HSV-1 (MOI = 1) for 1 h, treated with low-pH citrate buffer (pH 3.0) to inactivate noninternalized extracellular viral particles, and subsequently overlaid with medium containing CHLA (60 μM), PUG (40 μM), or DMSO control (0.1%). At 4, 8, and 12 h p.i., total cellular RNA was isolated, subjected to first-strand synthesis by reverse transcription, and then amplified by standard PCR procedures with primers against HSV-1 immediate-early (ICP27), early (TK), and late (gD) gene products. GAPDH was included as a control. (B) A549 cells were coincubated with HSV-1 (MOI = 1) and CHLA (60 μM), PUG (40 μM), or DMSO control (0.1%) for 1 h. Cells were washed with PBS before overlay media without the tannins was applied. Total cellular RNA was isolated for RT-PCR analysis as in panel A. Representative data shown are from one of two independent experiments.
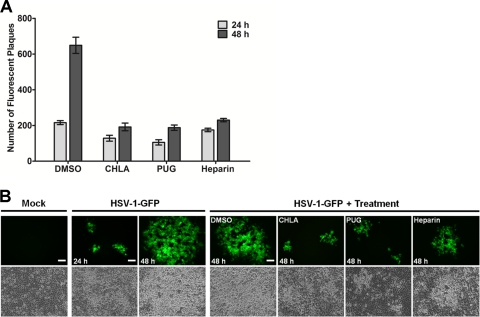
We next examined whether CHLA and PUG inhibited HSV-1 secondary infection and/or cell-to-cell spread. A fluorescent plaque assay was performed using HSV-1-GFP. After viral inoculation of A549 cells, CHLA and PUG were added to the overlay media at 12 or 24 h p.i. in the presence or absence of HSV-1 neutralizing antibodies, and fluorescent viral plaques were quantified or photographed over the subsequent course of infection (total of 48 h after the initial challenge). In this assay, neutralizing antibodies coat viral particles released from infected cells and prevent secondary infection of surrounding uninfected cells. Thus, the only route of cell-to-cell transmission in the presence of neutralizing antibody is via intercellular junctions between infected and uninfected cells. Furthermore, prior to drug addition (12 and 24 h p.i.), HSV-1 was permitted to complete at least one round of replication cycle, allowing drug effects on postentry infection to be monitored in the ensuing incubation period. As expected, in the absence of neutralizing antibodies, there was an increase in viral plaques in the DMSO control group due to secondary infections (Fig. 7A). However, addition of CHLA, PUG, or heparin substantially reduced the number of viral plaques formed and limited its increase in comparison to DMSO (Fig. 7A). Similarly, with respect to viral spread, treatment with CHLA and PUG prevented viral plaque growth. Incubation with the tannins yielded plaques with considerably reduced size compared to the DMSO control after the 48 h of infection (Fig. 7B). Interestingly, heparin, which has limited inhibitory activity on HSV-1 postattachment, also exhibited some protective effect against viral spread albeit at a lower efficiency compared to the tannins. Taken together, the data indicated that once HSV-1 entered the cells and completed at least one cycle of infection (12 to 24 h), any subsequent de novo infections and viral spread via intercellular contacts were restricted upon addition of CHLA and PUG. Tannin-mediated inhibition of viral attachment and fusion, as observed earlier (Fig. 5B to D), confirmed these results, and may be responsible for their effects in neutralizing secondary infections and restricting cell-to-cell spread, respectively.
Fig. 7.
CHLA and PUG can limit HSV-1 secondary infection and cell-to-cell spread of the virus. A549 cells were infected with HSV-1-GFP (200 PFU/well) for 1 h and then treated with citrate buffer (pH 3.0) to inactivate noninternalized extracellular viral particles. Cells were overlaid with medium alone (A) or medium containing 0.1% neutralizing antibody (B). After a p.i. incubation period of 12 h (A) or 24 h (B), the infected cells were treated with CHLA (60 μM), PUG (40 μM), heparin (100 μg/ml), or DMSO (0.1%), before further incubation for a total of 48 h after the initial infection. Over the course of infection subsequent to the drug addition, the plates were scanned and quantified for fluorescent viral plaques (A) or photographed using an inverted fluorescence microscope at ×100 magnification (B). (A) Number of fluorescent plaques counted between 24 and 48 h p.i. with drug treatment initiated at 12 h after viral challenge. The data shown are means ± the SEM of three independent experiments with each treatment performed in duplicate. (B) Comparison of viral plaque size between 24 and 48 h p.i. with drug treatment initiated at 24 h after viral challenge. Scale bars, 100 μm. Representative images are from one of two independent experiments.
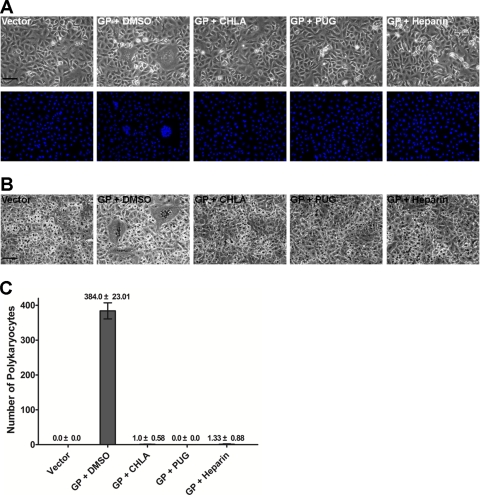
CHLA and PUG target HSV-1 glycoproteins that mediate glycosaminoglycan-specific interactions.
HSV-1 viral glycoproteins are known to mediate HSV-1 binding, internalization, and cell-to-cell spread. From the preceding data, it appears that the hydrolyzable tannins CHLA and PUG target viral glycoprotein(s), which would explain the need for virus to be present during inhibition and their effect on virus entry and spread. In an attempt to elucidate the underlying mechanism, we checked whether the two tannins interacted with HSV-1 glycoproteins in order to block entry-associated events. Using a virus-free system, we overexpressed HSV-1 glycoproteins that have been shown to mediate cell fusion (occurs during entry and cell-to-cell spread) by transfecting the individual gB, gD, gH, and gL genes into A549 cells, followed by treatment with the tannins. Expression of all four genes induced cell fusion resulting in polykaryocyte formation (>10 nuclei), which is absent after transfection with the empty vector control. The two tannins and heparin each blocked polykaryocyte formation, suggesting that CHLA and PUG interact with HSV-1 glycoproteins to prevent virus attachment, entry, and cell-to-cell spread (Fig. 8).
Fig. 8.
CHLA and PUG can prevent HSV-1 glycoprotein-mediated cell fusion events. A549 cells were transfected with plasmids expressing the individual HSV-1 glycoproteins (gB, gD, gH, and gL). After 6 h of transfection, cells were washed with PBS and treated with CHLA (60 μM), PUG (40 μM), heparin (100 μg/ml), or DMSO (0.1%). After further incubation for 24 h, cells were fixed with methanol and stained with Hoechst dye (A) or Giemsa (B). Photomicrographs were then taken at ×200 magnification. (A) Phases (upper panels) and respective fluorescence pictures displaying the Hoechst dye-stained nuclei (bottom panels). (B) Giemsa-stained cells in a similar experiment. Representative pictures shown are from one of three independent experiments. Vector, empty vector; GP, HSV-1 glycoproteins; scale bars, 100 μm. (C) The total number of polykaryocytes (>10 nuclei) from each treatment was quantified. The data shown are means ± the SEM of three independent experiments.
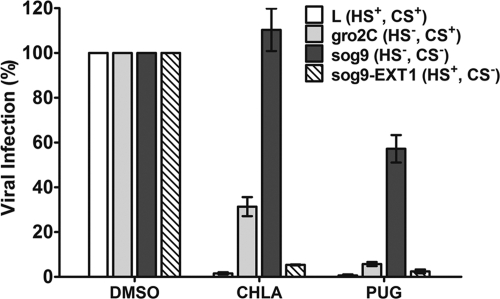
Several HSV-1 glycoproteins are known to interact with cell surface GAGs. To further explore the virus-host interactions that are being targeted by the tannins, we used a series of cell lines known to possess defects in surface HS and CS synthesis. The relative infectivities of HSV-1 (KOS) are ca. 10% for HS-deficient gro2C cells and 0.5% for HS/CS-deficient sog9 cells compared to parental mouse L cells (6). Stable expression of the EXT1 gene in sog9 cells (sog9-EXT1) restores HS biosynthesis and susceptibility to HSV-1 infection (43). To evaluate the effects of the drugs in the presence or absence of GAG expression, each cell line was infected with different dilutions of HSV-1 sufficient to achieve 200 PFU/well (MOI = 0.0004) in the presence of the tannins. CHLA and PUG effectively protected the parental mouse L cells and sog9-EXT1 cells from infection, but antiviral effects were diminished in HS-deficient gro2C cells, and almost completely abolished in HS/CS-deficient sog9 cells (Fig. 9). Similar results were obtained in experiments using different MOIs (data not shown). These observations strongly suggest that CHLA and PUG target interactions between HSV-1 glycoproteins and GAGs. CHLA inhibition also appeared to be more sensitive to cell surface deficiency in GAGs compared to that of PUG.
Fig. 9.
Anti-HSV-1 effects mediated by CHLA and PUG are impaired in GAG-deficient cells and are rescued by restoration of heparan sulfate biosynthesis through EXT1 gene expression. Confluent cells in 12-well plates were infected with HSV-1-GFP (200 PFU/well reflecting virus titers determined in each cell line) concurrently in the presence or absence of CHLA (60 μM), PUG (40 μM), or DMSO (0.1%) for 1 h. The plates were washed with PBS before applying overlay media. After an additional 48 h of incubation, fluorescent viral plaques were scanned and quantified. Values obtained were compared against each cell line's respective DMSO control for HSV-1 infection which was considered to be 100%. The respective statuses of the heparan sulfate (HS) and chondroitin sulfate (CS) GAG synthesis in the different cell lines are indicated in parentheses. The data shown are means ± the SEM of three independent experiments, with each treatment being performed in duplicate.
Taken together, the data indicate that CHLA and PUG function as GAG competitors to inhibit the initial events of HSV-1 infection (adsorption and penetration) and the cell-to-cell spread of virus. The interaction of HSV-1 glycoproteins with cellular GAGs plays a critical role in viral infections, and the hydrolyzable tannins could offer a primary means of defense against HSV-1 infections.
DISCUSSION
There is currently no cure that completely resolves latent infections caused by alphaherpesviruses. Therefore, the development of small molecules capable of inhibiting infection by reactivated virus represents an attractive therapeutic strategy, particularly in immunocompromised individuals who are often at risk of generating ACV-resistant HSV-1 strains. In a search for such molecules, we report that CHLA and PUG, two hydrolyzable tannins isolated from the fruits of T. chebula, effectively inhibited HSV-1 infection in A549 cells without significantly reducing cell viability. In addition, our results suggest that CHLA and PUG specifically targeted HSV-1 particles by binding to viral glycoproteins that interact with cellular GAGs, rendering the virus incapable of adsorbing, penetrating, and spreading throughout the cell monolayer. These features underscore the potential of tannins as HSV-1 entry inhibitors.
Our data show that entry events, including primary and secondary infection, viral attachment and/or penetration, and cell-to-cell spread are inhibited only when the tannins and HSV-1 glycoproteins are in contact with each other. Pretreatment of host cells with the tannins, followed by washes to remove unadsorbed compounds, had no effects upon HSV-1 replication. This indicated that masking cell surface receptors or entry factors for HSV-1 by the tannins is unlikely. Viral binding assays using ELISA and flow cytometry analyses revealed that the tannins blocked viral attachment to the host cell. While CHLA and PUG could inactivate the HSV-1 particles, we do not believe that a direct lysis effect of the viral membrane is responsible for their effects, since HSV-1 infection of GAG-deficient mutant cell lines was still observed, even in the presence of these compounds (Fig. 9). Given their large molecular weights (CHLA, 954; and PUG, 1,084) and high affinity for proteins and sugars, the two hydrolyzable tannins are thought to bind to HSV-1 glycoproteins on the infectious virions making them inert, impairing glycoprotein function, and preventing successful attachment and entry of the host cell. These tannins could also bind to viral glycoproteins on the infected-cell surface, rendering them unavailable to mediate the cell-to-cell spread of virus.
HSV-1 entry into epithelial cells, which express the cellular receptors (HS, HVEM, nectin-1, and nectin-2) for HSV (67), requires an ordered and concerted effort from the viral glycoproteins. Specifically, the glycoproteins gB, gC, gD, gH, and gL interact with host cell receptors and are involved in penetration of the plasma membrane through a membrane fusion process (29, 57, 67). While viral entry and spread require a particular combination of viral surface proteins, several HSV-1 glycoproteins are repeatedly involved in both processes. Importantly, gB and gD function in viral binding and fusion and are also engaged during cell-to-cell transmission in cultured epithelial cells (22, 29, 33, 38, 57, 60, 61, 67). The associations between viral glycoproteins that mediate HSV-1 attachment and entry represent a complex scenario when considering CHLA and PUG and their mechanism of action. The candidate targets of the tannins likely involve viral glycoproteins that interact with host cell surface GAGs and participate in adsorption, membrane fusion, and cell-to-cell spread. The observation that both tannins blocked virus attachment to cells, as did heparin, suggests that interaction of gC and gB with heparan sulfate proteoglycans (HSPGs) is targeted. However, the drugs also prevented virus internalization into cells in the postbinding phase. At this point, the interaction with HSPGs should be irrelevant, since virions now interact with a gD receptor and become resistant to removal by heparin (42) (Fig. 5D). One explanation is that the tannins bind to gB and block its interaction with HSPG while also interfering with its subsequent role in membrane fusion during virus entry (in which gC is not involved). Alternatively, the tannins may be impeding the activity of additional glycoproteins (gD and/or gH/gL) or working by some other mechanism(s) to prevent successful entry into the A549 cells. Finally, there is the possibility that viral glycoproteins may still be accessible to the tannins, even when these viral proteins are bound to the host cell or are expressed in the intercellular junctions. This could explain why the considerably larger heparin (molecular weight, 17,000 to 19,000) can bind free gB but is unable to interact efficiently with the shielded glycoprotein that is needed in order to inhibit viral penetration or cell-to-cell transmission. Additional binding experiments using glycoprotein-deficient HSV-1 mutants, as well as soluble recombinant HSV-1 glycoproteins could help elucidate the targeting specificity of the tannins. We speculate that the two natural compounds can bind to all GAG-interacting glycoproteins, including gB, gC, and/or gD, and neutralize their functions. The ability of CHLA and PUG to effectively block virus membrane penetration, as well as virus attachment, could explain their higher efficacy in restricting the spread of HSV-1 compared to the inhibitory effects of heparin.
In the case of HSV-1, the interactions between several of its glycoproteins and cell surface GAGs are critical for ensuring efficient viral entry, as well as viral spread (6, 26, 44, 52, 53, 65, 66, 68). CS can confer susceptibility to HSV-1 infection in the absence of HS (7, 26), but HS is still the preferred substrate for viral attachment. GAG deficiency renders cell surfaces relatively resistant to HSV-1 binding and residual infectivity relies on stable attachment receptors (6). Earlier studies have shown that the HS/CS-deficient sog9 cells are insensitive to inhibitory effect of soluble HS on HSV-1 infection (6). We observed that whereas the absence of HS in gro2C cells weakened the tannins' inhibitory effects, the absence of HS and CS on sog9 cells severely limited their antiviral activities (Fig. 9). Overexpression of the EXT1 gene, which restores HS biosynthesis in EXT1 mutant sog9 cells, rescued the antiviral effects from both CHLA and PUG to >90%. The results suggest that the tannins interfere with GAG interactions with viral glycoproteins which can involve attachment and may also affect downstream penetration of the host cell. Additional experiments are required to clarify whether the drugs are able to inhibit binding and/or fusion of HSV-1 on the GAG-deficient cells.
All four hydrolyzable tannins investigated in this report are composed of a glucopyranose core linked with galloyl derivatives, including hexahydroxydiphenoyl (HHDP; C-C coupling between galloyl moieties), gallagyl, and chebuloyl units (Fig. 1) (39, 78). Only CHLA and PUG possess the HHDP unit, with an (R) configuration (linked to the glucose core at the 3,6 positions) and (S) configuration (linked via the 2,3 or 4,6 positions of their glucose residue), respectively (36, 78). The anti-herpes activities of hydrolyzable tannins are thought to be dependent on the number of galloyl or HHDP groups, irrespective of the sugar core (71). Structures containing HHDP unit have also been valuable pharmacophores for inhibiting HIV enzymatic activities (20, 75). Indeed, anti-HIV activities from CHLA, CHLI, PUG, and PUN have been reported to prevent binding of recombinant HIV gp120 to CD4 and to exert inhibitory effects on HIV-1 RT and integrase (1, 51, 74). The ability of these natural agents to inhibit both HSV-1 and HIV-1 underscores their potential value in the treatment of AIDS patients who also exhibit HSV-1-related symptoms.
Use of these tannins could improve the prognosis of anti-HSV-1 therapy in immunosuppressed individuals and help to reduce the risk of ACV resistance by lowering the ACV dose required. Since Fructus Chebulae contains both CHLA and PUG, inclusion of purified extracts from this plant in topical creams or microbicides would be a feasible method for controlling recurrent HSV-1 infections. Future studies will determine whether these natural products are effective against additional members of the herpesvirus family and other enveloped viruses. Our preliminary studies have shown that both CHLA and PUG inhibit the growth of HSV-2 and human cytomegalovirus (L.-T. Lin and T.-Y. Chen, unpublished data). Other viruses known to use GAGs as entry factors, such as measles virus and human respiratory syncytial virus, are also inhibited by these tannins, reinforcing our discovery that these compounds act as GAG competitors that inhibit viral glycoprotein-cell receptor interactions (Lin and Chen, unpublished). Further studies with the tannins derived from T. chebula may provide new ways to inhibit recurrent HSV-1 infections and control the reemergence of this virus in immunocompromised patients.
ACKNOWLEDGMENTS
We thank James R. Smiley, Gary H. Cohen, Roselyn J. Eisenberg, Bruce W. Banfield, Karen L. Mossman, Brian D. Lichty, and Andrew C. Issekutz for reagents.
L.-T.L. is a recipient of a National CIHR Research Training Program in Hepatitis C (NCRTP-HepC) fellowship. C.-C.L. is supported by a research grant from the National Science Council of Taiwan (NSC 96-2320-B-037-016). C.D.R. is supported by an operating grant from the Canadian Institutes of Health (CIHR-MOP-10638).
We have declared that no competing interests exist.
L.-T.L., T.-Y.C., C.-C.L., and C.D.R. conceived and designed the experiments. L.-T.L. and T.-Y.C. performed the experiments. L.-T.L., T.-Y.C., C.M., C.-C.L., and C.D.R. analyzed the data. L.-T.L., T.-Y.C. C.-Y.C., R.S.N., C.M., T.B.G., T.-C.L., G.-H.W., C.-C.L., and C.D.R. contributed reagents, materials, and technical support. L.-T.L., T.-Y.C., C.-C.L., and C.D.R. wrote the paper.
Footnotes
Published ahead of print on 9 February 2011.
REFERENCES
- 1. Ahn M. J., et al. 2002. Inhibition of HIV-1 integrase by galloyl glucoses from Terminalia chebula and flavonol glycoside gallates from Euphorbia pekinensis. Planta Med. 68:457–459 [DOI] [PubMed] [Google Scholar]
- 2. Akkarawongsa R., Potocky T. B., English E. P., Gellman S. H., Brandt C. R. 2008. Inhibition of herpes simplex virus type 1 infection by cationic beta-peptides. Antimicrob. Agents Chemother. 52:2120–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arduino P. G., Porter S. R. 2008. Herpes simplex virus type 1 infection: overview on relevant clinico-pathological features. J. Oral Pathol. Med. 37:107–121 [DOI] [PubMed] [Google Scholar]
- 4. Arduino P. G., Porter S. R. 2006. Oral and perioral herpes simplex virus type 1 (HSV-1) infection: review of its management. Oral Dis. 12:254–270 [DOI] [PubMed] [Google Scholar]
- 5. Aurelian L. 2008. Herpes simplex viruses: general features, p. 383–397 In Mahy B. W. J., van Regenmortel M. H. V. (ed.), Encyclopedia of virology, 3rd ed., vol. 2 Elsevier, Amsterdam, Netherlands [Google Scholar]
- 6. Banfield B. W., Leduc Y., Esford L., Schubert K., Tufaro F. 1995. Sequential isolation of proteoglycan synthesis mutants by using herpes simplex virus as a selective agent: evidence for a proteoglycan-independent virus entry pathway. J. Virol. 69:3290–3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Banfield B. W., et al. 1995. Evidence for an interaction of herpes simplex virus with chondroitin sulfate proteoglycans during infection. Virology 208:531–539 [DOI] [PubMed] [Google Scholar]
- 8. Buzzini P., et al. 2008. Antimicrobial and antiviral activity of hydrolysable tannins. Mini Rev. Med. Chem. 8:1179–1187 [DOI] [PubMed] [Google Scholar]
- 9. Chen Y., et al. 2000. Resistant herpes simplex virus type 1 infection: an emerging concern after allogeneic stem cell transplantation. Clin. Infect. Dis. 31:927–935 [DOI] [PubMed] [Google Scholar]
- 10. Cheng H. Y., Lin C. C., Lin T. C. 2002. Antiherpes simplex virus type 2 activity of casuarinin from the bark of Terminalia arjuna Linn. Antivir. Res. 55:447–455 [DOI] [PubMed] [Google Scholar]
- 11. Cheng H. Y., Lin C. C., Lin T. C. 2002. Antiviral properties of prodelphinidin B-2 3′-O-gallate from green tea leaf. Antivir. Chem. Chemother. 13:223–229 [DOI] [PubMed] [Google Scholar]
- 12. Cheng H. Y., et al. 2003. In vitro antiviral activity of prodelphinidin B-2 3,3′-di-O-gallate from Myrica rubra. Planta Med. 69:953–956 [DOI] [PubMed] [Google Scholar]
- 13. Cheng H. Y., Lin T. C., Yang C. M., Wang K. C., Lin C. C. 2004. Mechanism of action of the suppression of herpes simplex virus type 2 replication by pterocarnin A. Microbes Infect. 6:738–744 [DOI] [PubMed] [Google Scholar]
- 14. Cheng H. Y., et al. 2004. Putranjivain A from Euphorbia jolkini inhibits both virus entry and late stage replication of herpes simplex virus type 2 in vitro. J. Antimicrob. Chemother. 53:577–583 [DOI] [PubMed] [Google Scholar]
- 15. Cheng H. Y., Lin T. C., Yu K. H., Yang C. M., Lin C. C. 2003. Antioxidant and free radical scavenging activities of Terminalia chebula. Biol. Pharm. Bull. 26:1331–1335 [DOI] [PubMed] [Google Scholar]
- 16. Cheng H. Y., et al. 6 October 2009. Excoecarianin, isolated from Phyllanthus urinaria Linnea, inhibits herpes simplex virus type 2 infection through inactivation of viral particles. Evid. Based Complement Alternat. Med. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheng H. Y., Yang C. M., Lin T. C., Shieh D. E., Lin C. C. 2006. ent-Epiafzelechin-(4α→8)-epiafzelechin extracted from Cassia javanica inhibits herpes simplex virus type 2 replication. J. Med. Microbiol. 55:201–206 [DOI] [PubMed] [Google Scholar]
- 18. Cheshenko N., Liu W., Satlin L. M., Herold B. C. 2007. Multiple receptor interactions trigger release of membrane and intracellular calcium stores critical for herpes simplex virus entry. Mol. Biol. Cell 18:3119–3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chiang L. C., Chiang W., Liu M. C., Lin C. C. 2003. In vitro antiviral activities of Caesalpinia pulcherrima and its related flavonoids. J. Antimicrob. Chemother. 52:194–198 [DOI] [PubMed] [Google Scholar]
- 20. Cos P., Maes L., Vlietinck A., Pieters L. 2008. Plant-derived leading compounds for chemotherapy of human immunodeficiency virus (HIV) infection: an update (1998–2007). Planta Med. 74:1323–1337 [DOI] [PubMed] [Google Scholar]
- 21. Danve-Szatanek C., et al. 2004. Surveillance network for herpes simplex virus resistance to antiviral drugs: 3-year follow-up. J. Clin. Microbiol. 42:242–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dingwell K. S., et al. 1994. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J. Virol. 68:834–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fatahzadeh M., Schwartz R. A. 2007. Human herpes simplex labialis. Clin. Exp. Dermatol. 32:625–630 [DOI] [PubMed] [Google Scholar]
- 24. Field H. J. 2001. Herpes simplex virus antiviral drug resistance–current trends and future prospects. J. Clin. Virol. 21:261–269 [DOI] [PubMed] [Google Scholar]
- 25. Fukuchi K., et al. 1989. Inhibition of herpes simplex virus infection by tannins and related compounds. Antivir. Res. 11:285–297 [DOI] [PubMed] [Google Scholar]
- 26. Gruenheid S., Gatzke L., Meadows H., Tufaro F. 1993. Herpes simplex virus infection and propagation in a mouse L cell mutant lacking heparan sulfate proteoglycans. J. Virol. 67:93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haslam E. 1989. Plant polyphenols: vegetable tannins revisited. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 28. Haslam E. 2007. Vegetable tannins: lessons of a phytochemical lifetime. Phytochemistry 68:2713–2721 [DOI] [PubMed] [Google Scholar]
- 29. Heldwein E. E., Krummenacher C. 2008. Entry of herpesviruses into mammalian cells. Cell Mol. Life Sci. 65:1653–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hsu H. Y., et al. 1985. Chinese materia medica: a concise guide. Modern Drug Press, Taipei, Taiwan [Google Scholar]
- 31. Huang K., Incognito L., Cheng X., Ulbrandt N. D., Wu H. 2010. Respiratory syncytial virus-neutralizing monoclonal antibodies motavizumab and palivizumab inhibit fusion. J. Virol. 84:8132–8140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Iason G. 2005. The role of plant secondary metabolites in mammalian herbivory: ecological perspectives. Proc. Nutr. Soc. 64:123–131 [DOI] [PubMed] [Google Scholar]
- 33. Johnson D. C., Huber M. T. 2002. Directed egress of animal viruses promotes cell-to-cell spread. J. Virol. 76:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Juang L. J., Sheu S. J. 2005. Chemical identification of the sources of commercial Fructus Chebulae. Phytochem. Anal. 16:246–251 [DOI] [PubMed] [Google Scholar]
- 35. Juang L. J., Sheu S. J., Lin T. C. 2004. Determination of hydrolyzable tannins in the fruit of Terminalia chebula Retz. by high-performance liquid chromatography and capillary electrophoresis. J. Sep. Sci. 27:718–724 [DOI] [PubMed] [Google Scholar]
- 36. Khanbabaee K., van Ree T. 2001. Tannins: classification and definition. Nat. Prod. Rep. 18:641–649 [DOI] [PubMed] [Google Scholar]
- 37. Knickelbein J. E., Hendricks R. L., Charukamnoetkanok P. 2009. Management of herpes simplex virus stromal keratitis: an evidence-based review. Surv. Ophthalmol. 54:226–234 [DOI] [PubMed] [Google Scholar]
- 38. Laquerre S., et al. 1998. Heparan sulfate proteoglycan binding by herpes simplex virus type 1 glycoproteins B and C, which differ in their contributions to virus attachment, penetration, and cell-to-cell spread. J. Virol. 72:6119–6130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin T. C., Chien S. C., Chen H. F., Hsu F. L. 2000. Tannins and related compounds from Combretaceae plants. Chin Pharm. J. 52:1–26 [Google Scholar]
- 40. Lin T. C., Nonaka G., Nishioka I., Ho F. C. 1990. Tannins and related compounds. CII. Structures of terchebulin, an ellagitannin having a novel tetraphenylcarboxylic acid (terchebulic acid) moiety, and biogenetically related tannins from Terminalia chebula Retz. Chem. Pharm. Bull. 38:3004–3008 [Google Scholar]
- 41. Madan R. P., et al. 2007. Molecular umbrellas: a novel class of candidate topical microbicides to prevent human immunodeficiency virus and herpes simplex virus infections. J. Virol. 81:7636–7646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McClain D. S., Fuller A. O. 1994. Cell-specific kinetics and efficiency of herpes simplex virus type 1 entry are determined by two distinct phases of attachment. Virology 198:690–702 [DOI] [PubMed] [Google Scholar]
- 43. McCormick C., Duncan G., Goutsos K. T., Tufaro F. 2000. The putative tumor suppressors EXT1 and EXT2 form a stable complex that accumulates in the Golgi apparatus and catalyzes the synthesis of heparan sulfate. Proc. Natl. Acad. Sci. U. S. A. 97:668–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McCormick C., Duncan G., Tufaro F. 2000. Herpes simplex virus: discovering the link between heparan sulfate and hereditary bone tumours. Rev. Med. Virol. 10:373–384 [DOI] [PubMed] [Google Scholar]
- 45. McCormick C., et al. 1998. The putative tumour suppressor EXT1 alters the expression of cell-surface heparan sulfate. Nat. Genet. 19:158–161 [DOI] [PubMed] [Google Scholar]
- 46. Melroe G. T., DeLuca N. A., Knipe D. M. 2004. Herpes simplex virus 1 has multiple mechanisms for blocking virus-induced interferon production. J. Virol. 78:8411–8420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Minaker R. L., Mossman K. L., Smiley J. R. 2005. Functional inaccessibility of quiescent herpes simplex virus genomes. Virol. J. 2:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morfin F., et al. 2004. HSV excretion after bone marrow transplantation: a 4-year survey. J. Clin. Virol. 30:341–345 [DOI] [PubMed] [Google Scholar]
- 49. Mossman K. L., et al. 2001. Herpes simplex virus triggers and then disarms a host antiviral response. J. Virol. 75:750–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nicholl M. J., Robinson L. H., Preston C. M. 2000. Activation of cellular interferon-responsive genes after infection of human cells with herpes simplex virus type 1. J. Gen. Virol. 81:2215–2218 [DOI] [PubMed] [Google Scholar]
- 51. Nonaka G., et al. 1990. Anti-AIDS agents. 2. Inhibitory effects of tannins on HIV reverse transcriptase and HIV replication in H9 lymphocyte cells. J. Nat. Prod. 53:587–595 [DOI] [PubMed] [Google Scholar]
- 52. Nyberg K., et al. 2004. The low molecular weight heparan sulfate-mimetic, PI-88, inhibits cell-to-cell spread of herpes simplex virus. Antivir. Res. 63:15–24 [DOI] [PubMed] [Google Scholar]
- 53. O'Donnell C. D., Shukla D. 2008. The importance of heparan sulfate in herpesvirus infection. Virol. Sin. 23:383–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pertel P. E., Fridberg A., Parish M. L., Spear P. G. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313–324 [DOI] [PubMed] [Google Scholar]
- 55. Porter J. L. 1989. Methods in plant biochemistry: plant phenolics, vol. 1 Academic Press, London, England [Google Scholar]
- 56. Quideau S., et al. 2004. Main structural and stereochemical aspects of the antiherpetic activity of nonahydroxyterphenoyl-containing C-glycosidic ellagitannins. Chem. Biodivers. 1:247–258 [DOI] [PubMed] [Google Scholar]
- 57. Reske A., Pollara G., Krummenacher C., Chain B. M., Katz D. R. 2007. Understanding HSV-1 entry glycoproteins. Rev. Med. Virol. 17:205–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Roizman B., Knipe D. M., Whitley R. J. 2007. Herpes simplex viruses, p. 2501–2601 In Knipe D. M., et al. (ed.), Fields virology, 5th ed. Lippincott-Raven Publishers, Philadelphia, PA [Google Scholar]
- 59. Safrin S., et al. 1991. A controlled trial comparing foscarnet with vidarabine for acyclovir-resistant mucocutaneous herpes simplex in the acquired immunodeficiency syndrome. N. Engl. J. Med. 325:551–555 [DOI] [PubMed] [Google Scholar]
- 60. Sakisaka T., et al. 2001. Requirement of interaction of nectin-1α/HveC with afadin for efficient cell-cell spread of herpes simplex virus type 1. J. Virol. 75:4734–4743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Satoh T., et al. 2008. PILRα is a herpes simplex virus-1 entry coreceptor that associates with glycoprotein B. Cell 132:935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schleiss M. R. 2009. Persistent and recurring viral infections: the human herpesviruses. Curr. Probl. Pediatr. Adolesc. Health Care 39:7–23 [DOI] [PubMed] [Google Scholar]
- 63. Serrano J., Puupponen-Pimia R., Dauer A., Aura A. M., Saura-Calixto F. 2009. Tannins: current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 53(Suppl. 2):S310–S329 [DOI] [PubMed] [Google Scholar]
- 64. Shahat A. A., et al. 2002. Antiviral and antioxidant activity of flavonoids and proanthocyanidins from Crataegus sinaica. Planta Med. 68:539–541 [DOI] [PubMed] [Google Scholar]
- 65. Shieh M. T., Spear P. G. 1994. Herpesvirus-induced cell fusion that is dependent on cell surface heparan sulfate or soluble heparin. J. Virol. 68:1224–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shieh M. T., WuDunn D., Montgomery R. I., Esko J. D., Spear P. G. 1992. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J. Cell Biol. 116:1273–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Spear P. G. 2004. Herpes simplex virus: receptors and ligands for cell entry. Cell Microbiol. 6:401–410 [DOI] [PubMed] [Google Scholar]
- 68. Spear P. G., Shieh M. T., Herold B. C., WuDunn D., Koshy T. I. 1992. Heparan sulfate glycosaminoglycans as primary cell surface receptors for herpes simplex virus. Adv. Exp. Med. Biol. 313:341–353 [DOI] [PubMed] [Google Scholar]
- 69. Stojdl D. F., Lichty B. D., tenOever B. R., et al. 2003. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4:263–275 [DOI] [PubMed] [Google Scholar]
- 70. Stranska R., et al. 2005. Survey of acyclovir-resistant herpes simplex virus in the Netherlands: prevalence and characterization. J. Clin. Virol. 32:7–18 [DOI] [PubMed] [Google Scholar]
- 71. Takechi M., Tanaka Y., Takehara M., Nonaka G. I., Nishioka I. 1985. Structure and antiherpetic activity among the tannins. Phytochemistry 24:2245–2250 [Google Scholar]
- 72. Tian L. W., Zhang Y. J., Qu C., Wang Y. F., Yang C. R. 2010. Phloroglucinol glycosides from the fresh fruits of Eucalyptus maideni. J. Nat. Prod. 73:160–163 [DOI] [PubMed] [Google Scholar]
- 73. Wagner E. K., Sandri-Goldin R. M. 2008. Herpes simplex viruses: molecular biology, p. 397–405 In Mahy B. W. J., van Regenmortel M. H. V. (ed.), Encyclopedia of virology, 3rd ed., vol. 2 Elsevier, Amsterdam, Netherlands [Google Scholar]
- 74. Weaver J. L., et al. 1992. Prevention of binding of rgp120 by anti-HIV active tannins. Biochem. Pharmacol. 43:2479–2480 [DOI] [PubMed] [Google Scholar]
- 75. Xie L., et al. 1995. Anti-AIDS (acquired immune deficiency syndrome) agents. 17. New brominated hexahydroxybiphenyl derivatives as potent anti-HIV agents. J. Med. Chem. 38:3003–3008 [DOI] [PubMed] [Google Scholar]
- 76. Yang C. M., Cheng H. Y., Lin T. C., Chiang L. C., Lin C. C. 2007. Hippomanin A from acetone extract of Phyllanthus urinaria inhibited HSV-2 but not HSV-1 infection in vitro. Phytother. Res. 21:1182–1186 [DOI] [PubMed] [Google Scholar]
- 77. Yang C. M., Cheng H. Y., Lin T. C., Chiang L. C., Lin C. C. 2007. The in vitro activity of geraniin and 1,3,4,6-tetra-O-galloyl-β-d-glucose isolated from Phyllanthus urinaria against herpes simplex virus type 1 and type 2 infection. J. Ethnopharmacol. 110:555–558 [DOI] [PubMed] [Google Scholar]
- 78. Yoshida T., Amakura Y., Yoshimura M. 2010. Structural features and biological properties of ellagitannins in some plant families of the order Myrtales. Int. J. Mol. Sci. 11:79–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ziyaeyan M., et al. 2007. Frequency of acyclovir-resistant herpes simplex viruses isolated from the general immunocompetent population and patients with acquired immunodeficiency syndrome. Int. J. Dermatol. 46:1263–1266 [DOI] [PubMed] [Google Scholar]