Abstract
We analyzed the ability of a vaccine vector based on vesicular stomatitis virus (VSV) to induce a neutralizing antibody (NAb) response to avian influenza viruses (AIVs) in rhesus macaques. Animals vaccinated with vectors expressing either strain A/Hong Kong/156/1997 or strain A/Vietnam/1203/2004 H5 hemagglutinin (HA) were able to generate robust NAb responses. The ability of the vectors to induce NAbs against homologous and heterologous AIVs after a single dose was dependent upon the HA antigen incorporated into the VSV vaccine. The vectors expressing strain A/Vietnam/1203/2004 H5 HA were superior to those expressing strain A/Hong Kong/156/1997 HA at inducing cross-clade NAbs.
The reemergence in 2003 of highly lethal avian influenza virus (AIV) infections in humans (4) generated concern about a pandemic caused by an H5N1 virus (http://www.who.int/csr/disease/avian_influenza/en/). Traditional influenza vaccine technologies have several disadvantages when it comes to AIV vaccines. The typical development time of an influenza vaccine, 6 to 9 months, would be a serious drawback in the event of a fast-spreading AIV pandemic. Additionally, biosafety and biocontainment risks arise with AIVs requiring biosafety level 3 (BSL3) laboratories. Furthermore, the use of eggs to grow the AIVs to generate the vaccines is problematic, as many of the strains with predicted pandemic potential are highly lethal to chicken eggs. Thus, reverse genetic techniques are needed to engineer viruses that are not embryo lethal and can be used in BSL2 containment. Therefore, vaccine platforms that can avoid such shortcomings are in demand.
Our laboratory and others have generated effective experimental vaccines against a number of viral diseases using recombinant vesicular stomatitis virus (rVSV). These include the respiratory diseases caused by severe acute respiratory syndrome (SARS) coronavirus (7, 8), respiratory syncytial virus (RSV) (6), influenza virus (12, 13), and AIV (16, 17). VSV is an ideal AIV vaccine vector because it can replicate to high titers and in large quantities in cell lines already approved for human vaccine production and can be delivered intranasally (i.n.). It requires minimal biosafety levels for production and expresses foreign antigens at high levels, leading to potent immune responses in the absence of adjuvant.
Nonhuman primate model.
Previously, we generated rVSV vectors expressing the influenza virus strain A/Hong Kong/156/1997 (HK/156) H5 hemagglutinin (HA) gene from the first [VSV-HK156(1)] (17) or fifth (16) genomic position of VSV. Our analyses showed that both of these vaccine vectors were 100% effective against homologous and heterologous AIV challenge in mouse models (16, 17). To further assess the potential of these vectors as human AIV vaccines, we employed a nonhuman primate vaccination model. Immune responses obtained using nonhuman primates are more likely to be predictive of immune responses in humans than those obtained using mice.
Vaccination of macaques with VSV vectors expressing AIV HK/156 HA.
Five rhesus macaques (CD02, EH71, EK39, FC29, and FG66), bred and housed at the Tulane National Primate Research Center (TNPRC), a USDA-inspected and Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-approved facility, were inoculated i.n. and intramuscularly (i.m.) with a total of 4 × 107 PFU of VSV-HK156(1) expressing the AIV HK/156 HA (17). Six control group animals (CV03, DF82, DN48, EH41, EJ48, and EK32) were given VSV vectors expressing unrelated genes from a simian immunodeficiency virus (SIV). At 2 months postprime, the vaccine group animals were boosted with 4 × 107 PFU of VSV-NJG-HK156(1) (17), which has the VSV New Jersey (NJ) serotype G gene replacing the VSV Indiana G gene present in the priming vector. This serotype switch increases the efficacy of boosting by circumventing neutralizing antibodies (NAbs) developed to the VSV G protein present in the priming vector (14). Control group animals received boosts with serotype switch vectors expressing SIV antigens. All animal experiments were performed under protocols approved by the animal care and use committee of the TNPRC.
NAb responses to VSV vectors expressing AIV HK/156 HA.
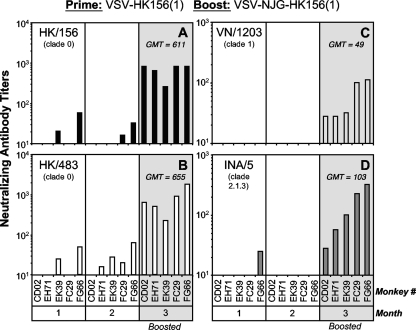
Sera collected from individual animals were analyzed for the presence of NAbs against homologous and antigenically distinct H5N1 AIVs using a stringent microneutralization assay as previously described (16–18). After the prime administration, 40% (2 of 5) of the animals made a detectable NAb response against the homologous HK/156 (Fig. 1A, left and middle panels), while 80% (4 of 5) had a detectable NAb response by 2 months postprime against the closely related A/Hong Kong/483/1997 (HK/483) (Fig. 1B, left and middle panels) clade 0 strain. One month after boosting, all animals had high NAb titers to both clade 0 strains (Fig. 1A and B, right panels). After priming, the animals did not generate detectable NAbs against the more divergent H5N1 strains, A/Vietnam/1203/2004 (VN/1203) (Fig. 1C) and A/Indonesia/5/2005 (INA/5) (Fig. 1D), with the exception of one animal that had NAbs against INA/5 (Fig. 1D, left panel). After boosting, however, the animals generated significant levels of NAbs against VN/1203 (Fig. 1C, right panel) and INA/5 (Fig. 1D, right panel), although the levels were lower than those in response to the clade 0 strains (Fig. 1A and B, right panels). The geometric mean titers (GMTs) after boosting (3 months postprime) against each AIV are shown in Fig. 1. The magnitudes of the homologous and heterologous NAb responses after boosting were similar to those seen for mice given the same vectors (17). The strong NAb responses in the macaques after boosting are clear evidence of effective priming in all animals.
Fig. 1.
Neutralization of AIV strains by sera from monkeys vaccinated with VSV-based vectors expressing the HK/156 H5 HA. Five rhesus macaques (TNPRC numbers CD02, EH71, EK39, FC29, and FG66) were vaccinated i.n. and i.m. with a total of 4 × 107 PFU of VSV-HK156(1) or negative-control vectors (data not shown). At 2 months postprime, all animals were boosted i.n. and i.m. with a total of 4 × 107 PFU of VSV-NJG-HK156(1) or negative-control vectors (data not shown). At the indicated months postprime, serum from individual monkeys was collected and analyzed for a NAb response against the homologous clade 0 strain HK/156 (black bars) (A) or the heterologous strains HK/483 (clade 0; white bars) (B), VN/1203 (clade 1; light gray bars) (C), or INA/5 (clade 2.1.3; dark gray bars) (D) as described previously (16, 17). Each bar represents the NAb titer from an individual monkey as determined by 100% inhibition of cytopathic effect (CPE) in a microneutralization assay (18). There were no detectable NAbs in pooled sera from 6 monkeys (TNPRC numbers CV03, DF82, DN48, EH41, EJ48, and EK32) vaccinated with the negative-control vectors. Undetectable titers are represented by a titer of 10. The geometric mean titers (GMTs) after boosting are shown.
VSV vectors expressing a more recent AIV HA protein.
In order to create an effective pandemic AIV vaccine, it is important that the vaccine protect against AIVs across clades and/or subtypes. In the studies described above and in our earlier mouse studies, we used an HA derived from an H5N1 virus isolated during the initial human infections in 1997. Although this antigen expressed in VSV vectors did induce cross-NAbs and cross-protection to later strains, from 2003 and 2004, we also wanted to evaluate the efficacy of an rVSV vector expressing an H5 HA from a more recent AIV strain to determine if it might be more effective at generating cross-NAbs. We therefore generated VSV priming and boosting vectors expressing a gene encoding the AIV VN/1203 H5 HA protein. The VN/1203 strain is an H5N1 AIV that emerged in humans in Vietnam in 2004. A codon-optimized VN/1203 HA gene was purchased from Blue Heron Biotechnology (Bothell, WA). This gene was cloned into the fifth genomic position of a full-length VSV plasmid (15) to generate pVSV-VN1203(5). The serotype switch boosting vector was constructed by replacing the G gene (Indiana serotype) of pVSV-VN1203(5) with the G gene from the NJ serotype to generate pVSV-NJG-VN1203(5). The rVSVs, VSV-VN1203(5) and VSV-NJG-VN1203(5) (Fig. 2A), were recovered as described previously (10, 16, 17).
Fig. 2.
Recombinant VSV vectors expressing the VN/1203 H5 HA from the fifth genomic position of VSV. (A) Each diagram shows the fifth position insertion site of the VN/1203 H5 HA gene into the recombinant WT Indiana background [VSV-VN1203(5)] or into a NJ serotype switch boosting vector [VSV-NJG-VN1203(5)]. (B) Western blot analysis of BHK-21 whole-cell extracts either mock infected or infected with the indicated viruses [WT, recombinant wild-type Indiana strain; HK156(1), VSV-HK156(1) (17); NJG-HK156(1), VSV-NJG-HK156(1) (17); VN1203(5), VSV-VN1203(5); NJG-VN1203(5), VSV-NJG-VN1203(5)] was performed as described previously (16, 17) using the polyclonal antibody NR-665 specific for the HK/156 H5 HA. The genomic position at which the HA gene is inserted is indicated by the number in parentheses. The full-length (HA0) or cleaved H5 HA isoforms (HA1, HA2) are indicated by arrows to the right of the blot.
Expression of VN/1203 H5 HA.
To ensure that the VN/1203 H5 HA was expressed from the vectors, we examined protein accumulation by Western blot analysis in infected BHK-21 cells as previously described (16, 17). Figure 2B shows that both the full-length (HA0) and cleaved isoforms (HA1, HA2) of VN/1203 H5 HA accumulate in cells infected with either VSV-VN1203(5) or VSV-NJG-VN1203(5). For comparison, Fig. 2B also shows the accumulation of the full-length and cleaved isoforms of HK/156 H5 HA in cells infected with the first-position HK/156 H5 HA vectors. It appeared that the VN/1203 H5 HA expressed from the fifth genomic position of VSV accumulated to lower levels than the HK/156 H5 HA expressed from the first position (Fig. 2B). The reduced expression was expected because of the attenuation of VSV transcription at each gene junction (5). The apparent reduced expression may also result in part from use in the blot of an antibody generated to the HK/156 H5 HA. This antibody may have less reactivity for the VN/1203 H5 HA than for the homologous HK/156 H5 HA. The VN/1203 H5 HA protein is diverged from HK/156 H5 HA at ∼4% of the amino acids. The sequence differences result in slightly different mobilities for the HA proteins by SDS-PAGE (Fig. 2B).
Neutralizing antibody responses to VSV vectors expressing AIV VN/1203 HA.
We next assessed the ability of the VSV vectors expressing VN/1203 H5 HA to elicit a NAb response in rhesus macaques just as in the initial experiments described above. Interestingly, detectable NAb responses against the homologous VN/1203 AIV were seen for all 6 monkeys (EN82, FT80, N288, FG05, DD04, and EP84) after the first dose with VSV-VN1203(5) (Fig. 3C, left and middle panels). Large increases in NAb titers were seen after boosting with VSV-NJG-VN1203(5) (Fig. 3C, right panel). In contrast, the vectors expressing the HK/156 HA only induced detectable NAbs to the homologous AIV in 2 of 5 animals after the first dose (Fig. 1A).
Fig. 3.
Neutralization of AIV strains by sera from monkeys vaccinated with VSV-based vectors expressing the VN/1203 H5 HA. Six rhesus macaques (TNPRC numbers EN82, FT80, N288, FG05, DD04, and EP84) were vaccinated i.n. and i.m. with 4 × 107 PFU total of VSV-VN1203(5) or negative-control vectors (data not shown). At 2 months postprime, all animals were boosted i.n. and i.m. with 4 × 107 PFU total of VSV-NJG-VN1203(5) or negative-control vectors (data not shown). At the indicated months postprime, serum from individual monkeys was collected and analyzed for a NAb response against the homologous clade 1 strain VN/1203 (light gray bars) (C) or the heterologous strains HK/156 (clade 0; black bars) (A), HK/483 (clade 0; white bars) (B), or INA/5 (clade 2.1.3; dark gray bars) (D) as described previously (16, 17). Each bar represents the NAb titer from an individual monkey as determined by 100% inhibition of CPE in a microneutralization assay (18). Pooled sera from 6 monkeys (TNPRC numbers CJ98, DG21, DT03, CM17, FP72, and DF38) vaccinated with the negative-control vectors had no detectable NAb titers (data not shown), which is represented by a titer of 10 in this assay. The geometric mean titers (GMTs) after boosting are shown.
We then examined the ability of the vectors expressing VN/1203 H5 HA to induce a NAb response against antigenically distinct AIVs. While NAbs against INA/5 were not detected after priming alone (Fig. 3D, left and middle panels), the VSV-VN/1203(5) was able to induce NAbs against two clade 0 viruses, HK/156 (Fig. 3A, left and middle panels) and HK/483 (Fig. 3B, left and middle panels), after the priming alone in 80 to 100% of the animals. In contrast, the VSV HK/156 vaccine did not induce cross-clade NAbs after the priming (Fig. 1C and D, left and middle panels). After boosting with the serotype switch vector, VSV-NJG-VN1203(5), the cross-clade NAb titers increased substantially against HK/156 (Fig. 3A, right panel), HK/483 (Fig. 3D, right panel), and INA/5 (Fig. 3D, right panel). The GMTs after boosting (3 months postprime) against each AIV are shown in Fig. 3. Interestingly, the NAb titer levels achieved against the heterologous clade 0 strains (Fig. 3A and B) were similar to the levels achieved using the homologous HK/156 vaccine vectors (Fig. 1A and B).
Rodent models have been an invaluable resource for studying the efficacy of vaccines, but the results obtained may not be predictive of results in humans. For example, results obtained for mice (18) with a live-attenuated cold-adapted AIV vaccine did not correlate well with results obtained for humans (9). Nonhuman primate animal models often need to be utilized in the preclinical setting, as they more closely mimic human biology and disease pathogenesis. Here, we have shown that our VSV-based AIV vaccines are able to induce a robust NAb response against homologous and heterologous AIV strains in all of the macaques after boosting. Many of the currently approved human AIV vaccines induce immune responses predictive of protection in only 40 to 70% of individuals (1–3, 11, 19). Although the protective NAb titer for AIVs is not known, there is a correlation between serum hemagglutination inhibition and NAb titers in humans who have received inactivated vaccines against H5N1 viruses with and without adjuvants (11, 19), and a NAb titer of 1:40 is believed to represent a protective titer.
Our experiments also show that obtaining optimal cross-clade NAbs in nonhuman primates is dependent upon which HA gene is used. Although the VN/1203 HA protein appeared to be expressed at lower levels than the HK/156 HA protein, it was able to induce stronger cross-clade NAb titers than the HK/156 HA protein. Induction of broader cross-NAbs by the VN/1203 HA protein could be due to better exposure of one or more epitopes shared by divergent H5 proteins. The VN/1203 vaccine vector was able to elicit a more robust cross-NAb response against HK/156 (Fig. 3A) than that elicited by INA/5 (Fig. 3D) despite the fact that the VN/1203 HA is about 96% identical to HK/156 HA and to INA/5 HA. This result suggests better exposure of cross-reactive epitopes on the HK/156 HA protein than on the INA/5 HA protein. The four AIV strains tested here are 96 to 98% identical. Moreover, even broader coverage might be obtained through expression of multiple AIV HAs in VSV vectors. Because the vaccinated animals in this study were also controls in an SIV vaccine study and were challenged with SIV, we were not able to assess the ability of the vaccine to protect from challenge. However, the NAb responses elicited would be expected to provide strong protection against AIV challenge.
Acknowledgments
This research was supported in part by NIH grants RO1AI080781, RO1AI45510, and U54 AI057158, TNPRC base grant RR000164, and the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID), NIH.
Nancy Cox and Alexander Klimov (Influenza Branch, Centers for Disease Control and Prevention, Atlanta, GA) provided the AIV strains A/Hong Kong/156/1997, A/Hong Kong/483/1997, A/Vietnam/1203/2004, and A/Indonesia/5/2005. The NR-665 antibody was obtained through the NIH Biodefense and Emerging Infectious Research Repository (NIAID, NIH). Celia Santos (NIAID, NIH) provided technical support.
Footnotes
Published ahead of print on 16 February 2011.
REFERENCES
- 1. Banzhoff A., et al. 2009. MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS One 4:e4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carter N. J., Plosker G. L. 2008. Prepandemic influenza vaccine H5N1 (split virion, inactivated, adjuvanted) [Prepandrix]: a review of its use as an active immunization against influenza A subtype H5N1 virus. BioDrugs 22:279–292 [DOI] [PubMed] [Google Scholar]
- 3. Ehrlich H. J., et al. 2008. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N. Engl. J. Med. 358:2573–2584 [DOI] [PubMed] [Google Scholar]
- 4. Gillim-Ross L., Subbarao K. 2006. Emerging respiratory viruses: challenges and vaccine strategies. Clin. Microbiol. Rev. 19:614–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iverson L. E., Rose J. K. 1981. Localized attenuation and discontinuous synthesis during vesicular stomatitis virus transcription. Cell 23:477–484 [DOI] [PubMed] [Google Scholar]
- 6. Kahn J. S., Roberts A., Weibel C., Buonocore L., Rose J. K. 2001. Replication-competent or attenuated, nonpropagating vesicular stomatitis viruses expressing respiratory syncytial virus (RSV) antigens protect mice against RSV challenge. J. Virol. 75:11079–11087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kapadia S. U., et al. 2005. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology 340:174–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kapadia S. U., Simon I. D., Rose J. K. 2008. SARS vaccine based on a replication-defective recombinant vesicular stomatitis virus is more potent than one based on a replication-competent vector. Virology 376:165–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karron R. A., et al. 2009. Evaluation of two live attenuated cold-adapted H5N1 influenza virus vaccines in healthy adults. Vaccine 27:4953–4960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lawson N. D., Stillman E. A., Whitt M. A., Rose J. K. 1995. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl. Acad. Sci. U. S. A. 92:4477–4481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leroux-Roels I., et al. 2009. Humoral and cellular immune responses to split-virion H5N1 influenza vaccine in young and elderly adults. Vaccine 27:6918–6925 [DOI] [PubMed] [Google Scholar]
- 12. Roberts A., Buonocore L., Price R., Forman J., Rose J. K. 1999. Attenuated vesicular stomatitis viruses as vaccine vectors. J. Virol. 73:3723–3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roberts A., et al. 1998. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J. Virol. 72:4704–4711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rose N. F., Roberts A., Buonocore L., Rose J. K. 2000. Glycoprotein exchange vectors based on vesicular stomatitis virus allow effective boosting and generation of neutralizing antibodies to a primary isolate of human immunodeficiency virus type 1. J. Virol. 74:10903–10910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schnell M. J., Buonocore L., Whitt M. A., Rose J. K. 1996. The minimal conserved transcription stop-start signal promotes stable expression of a foreign gene in vesicular stomatitis virus. J. Virol. 70:2318–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schwartz J. A., et al. 2007. Vesicular stomatitis virus vectors expressing avian influenza H5 HA induce cross-neutralizing antibodies and long-term protection. Virology 366:166–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schwartz J. A., et al. 2010. Potent vesicular stomatitis virus-based avian influenza vaccines provide long-term sterilizing immunity against heterologous challenge. J. Virol. 84:4611–4618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suguitan A. L., Jr., et al. 2006. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 3:e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Treanor J. J., Campbell J. D., Zangwill K. M., Rowe T., Wolff M. 2006. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N. Engl. J. Med. 354:1343–1351 [DOI] [PubMed] [Google Scholar]