Abstract
Six poliovirus-neutralizing Fabs were recovered from a combinatorial Fab phage display library constructed from bone marrow-derived lymphocytes of immunized chimpanzees. The chimeric chimpanzee-human full-length IgGs (hereinafter called monoclonal antibodies [MAbs]) were generated by combining a chimpanzee IgG light chain and a variable domain of heavy chain with a human constant Fc region. The six MAbs neutralized vaccine strains and virulent strains of poliovirus. Five MAbs were serotype specific, while one MAb cross-neutralized serotypes 1 and 2. Epitope mapping performed by selecting and sequencing antibody-resistant viral variants indicated that the cross-neutralizing MAb bound between antigenic sites 1 and 2, thereby covering the canyon region containing the receptor-binding site. Another serotype 1-specific MAb recognized a region located between antigenic sites 2 and 3 that included parts of capsid proteins VP1 and VP3. Both serotype 2-specific antibodies recognized antigenic site 1. No escape mutants to serotype 3-specific MAbs could be generated. The administration of a serotype 1-specific MAb to transgenic mice susceptible to poliovirus at a dose of 5 μg/mouse completely protected them from paralysis after challenge with a lethal dose of wild-type poliovirus. Moreover, MAb injection 6 or 12 h after virus infection provided significant protection. The MAbs described here could be tested in clinical trials to determine whether they might be useful for treatment of immunocompromised chronic virus excretors and for emergency protection of contacts of a paralytic poliomyelitis case.
INTRODUCTION
Poliomyelitis is an infectious neurological disease that is caused by polioviruses of three distinct serological types. Two highly effective vaccines, one prepared from formalin-inactivated virulent virus and another from live attenuated strains administered orally, were developed in the 1950s (35, 36). Their worldwide use resulted in almost complete eradication of the disease, with only a few countries remaining in which it is endemic and a few thousand paralytic cases annually. This dramatic success diminished interest in the development of new protective measures, as complete eradication of poliomyelitis was perceived to be very close. However, the original eradication target date of 2000 was missed by at least 10 years due to a variety of scientific, logistical, and political obstacles (13). Therefore, in recent years, the setbacks in the global efforts to eradicate poliomyelitis, newly recognized challenges of the final phases of the WHO-coordinated campaign, and the need to prevent the reemergence of poliomyelitis in the posteradication period led to renewed efforts to develop more efficient vaccines, new strategies for their use, and other tools to protect against poliomyelitis (17, 27, 31). They include licensure of more potent monovalent and bivalent oral polio vaccines (mOPV and bOPV, respectively), the development of a new generation of inactivated poliovirus vaccines for use in the posteradication period (8, 14), and the development of drugs effective against poliovirus (10, 29).
The use of OPV is associated with a small risk of vaccine-associated paralytic poliomyelitis (VAPP) in vaccine recipients and their immediate contacts (1). It has also led to the emergence of circulating vaccine-derived polioviruses (cVDPVs) (21) and immunodeficiency-associated VDPV (iVDPVs) (25). VDPVs of the first type cause outbreaks of paralytic poliomyelitis in inadequately immunized communities and are indistinguishable from wild polioviruses in their pathogenic properties. iVDPVs emerge in OPV-vaccinated individuals with primary B-cell immunodeficiencies and can establish chronic infection and be excreted into the environment for several years (20, 24). Besides the immediate danger to the chronic carriers (some of the patients eventually become paralyzed by the continuously evolving poliovirus), the presence of chronic excretors poses a serious challenge to the polio eradication campaign, providing an ample source of virulent polioviruses in the environment, making it impossible to halt immunization against poliomyelitis (12). Therefore, finding an effective treatment for these patients is an important public health objective. In 2006, the U.S. National Research Council recommended the development of at least two polio antiviral drugs to treat chronically infected individuals and to assist in the management of outbreaks in the posteradication period (10).
Early work by Hammond at al. showed gamma globulin to be effective for the prevention of poliomyelitis (reviewed in reference 34). Therefore, passive immunotherapy could be another way to treat chronic excretors. Even though prior attempts to use intravenous immunoglobulin (IVIG) and breast milk were unsuccessful (22), there is reason to think that higher doses of antipoliovirus antibodies could result in complete clearance of poliovirus from chronically infected individuals. In this communication, we report the development of hybrid chimpanzee-human antipoliovirus immunoglobulins that could be used in clinical trials to assess their effectiveness for the treatment of chronic poliovirus excretors, as well as for postexposure emergency prophylaxis and disease prevention if there is a reemergence of poliomyelitis in the posteradication period.
MATERIALS AND METHODS
Immunization of chimpanzees with poliovirus vaccines and construction of combinatorial Fab antibody library.
Two chimpanzees (1603 and 1609) were immunized orally with all three serotypes of the oral polio vaccine (OPV) (GlaxoSmithKline) twice at a 2-month interval and were boosted intramuscularly with inactivated polio vaccine (IPV) (GlaxoSmithKline) 7 months and 2 years after the second immunization. Bone marrow was aspirated from each chimpanzee at days 10, 20, 30, and 40 after the last immunization. Bone marrow-derived lymphocytes were prepared by centrifugation on a Ficoll-Paque gradient and were used to construct a combinatorial Fab phage display library as described previously (4, 5). A Fab phage display library containing γ1 heavy chain and κ and λ light chain with a diversity of 108 was constructed. The chimpanzee protocols were approved by the Institutional Animal Care and Use Committee, and the animals were housed in facilities accredited by AAALAC.
Panning of phage library and selection of poliovirus-specific Fabs.
Phages were produced from the library as described previously (5) and panned by affinity binding separately on serotypes 1, 2, and 3 of Sabin strains of poliovirus in a 96-well enzyme-linked immunosorbent assay (ELISA) plate. For each cycle of panning, 100 μl of OPV, which are the same viruses used for immunization, at 5 × 106 PFU/ml in phosphate-buffered saline (PBS) was used to coat each well of an ELISA plate, and specific phages were selected by incubating 1012 phages with the coated virus. The poliovirus-specific Fab clones were enriched by three cycles of panning against the three serotypes. After panning, 96 single phage-Fab clones were cultured in a 96-well plate for phage production. The resulting phages were screened for specific binding to poliovirus by phage ELISA (5). Clones that bound to the virus but not to bovine serum albumin (BSA) were scored as poliovirus-specific clones.
DNA sequence analysis of specific Fabs.
The genes coding for the variable regions of the heavy (VH) and light (VL) chains of poliovirus-specific Fabs were sequenced. Fabs with distinct VH and VL sequences were regarded as separate Fab clones. The presumed immunoglobulin family usage, germ line origin, and somatic mutations were identified by comparison with those deposited in the IMGT sequence database (http://www.imgt.org).
Production of Fabs and IgGs.
As described previously (5), histidine-tagged Fab was expressed in Escherichia coli and was affinity-purified on a nickel column. IgG was expressed in transiently transfected 293T mammalian cells and purified on a protein A column. Both Fab and IgG were further purified through a cation-exchange sulfopropyl (SP) column (GE Healthcare). The purities of the Fab and IgG were evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the protein concentrations were determined by optical density measurements at 280 nm (OD280), with the assumption that 1.35 A280 corresponds to 1.0 mg/ml.
Measurement of neutralizing activity of specific Fabs.
Purified Fabs were used in a plaque reduction neutralization test (PRNT) to determine their neutralizing activities against each of the three polio serotypes. Typically, approximately 50 PFU of Sabin poliovirus in 150 μl of serum-free Dulbecco's modified Eagle's medium (DMEM) were mixed with the same volume of serial dilutions of a Fab. The virus-Fab mixture was incubated at room temperature for 1 h and added to confluent HeLa cells in a 6-well plate in duplicate. After 1 h of incubation at room temperature, an overlay of DMEM containing 2% fetal bovine serum (FBS) and 0.8% methyl cellulose was added and the plates were incubated in a 5% CO2 incubator at 37°C for 72 h. The plaques were visualized by staining with 0.1% crystal violet for 5 min.
ELISA.
The wells in a 96-well ELISA plate were coated with 100 μl of poliovirus (OPV at a concentration of 5 × 106 PFU/ml), followed by treatment with 3% milk in PBS. After being washed, the plate was incubated with serially 3-fold-diluted antipoliovirus IgG for 2 h at room temperature. After being washed, the plate was incubated for 1 h with horseradish peroxidase-conjugated antibodies against human Fc. The color was developed by adding tetramethylbenzidine reagent (KPL; Gaithersburg, MD), and the development was stopped with H2SO4 after 10 min. The OD450 was read in an ELISA plate reader. The data were plotted, and the dose-response curves were generated with Prism software (Graphpad Software, Inc., San Diego, CA).
Blocking ELISA.
The test is based on materials and reagents developed in a previous study (32). Amounts of 0.5 to 1.0 D antigen units/ml of poliovirus were captured on 96-well ELISA plates coated with purified rabbit polyclonal serotype-specific IgG (2 μg/ml). Then, some wells containing captured antigen were incubated with serial dilutions of a monoclonal antibody (MAb) or polyclonal serum (blocking reaction), while control wells were treated with normal rabbit serum. Wells with no antigen served as a background control. Antipoliovirus antibodies reacted with their respective antigenic sites and blocked them from subsequent reaction with biotin-conjugated polyclonal antipolio IgG (0.5 μg/ml). Next, ExtrAvidin-peroxidase conjugate (1:1,000 dilution; Sigma) was added, and the reaction of bound peroxidase with tetramethylbenzidine substrate (TMB, Sigma) resulted in the development of a color reaction. After the addition of a stop reagent (Sigma), the plate was scanned at 450 nm. The OD450 in the background wells that contained no antigen corresponded to 100% blocking activity, and the OD450 for antigen-containing wells incubated with normal rabbit serum corresponded to 0% blocking activity. The percentage of blocking activity of a specific MAb or serum with a particular antigen was calculated from the difference between the average ODs of blocked and nonblocked wells containing the same antigen. The final results for blocking of reactivity were calculated from the following formula: % blocking activity = (average OD for wells containing normal rabbit serum − average OD for wells containing test serum)/(average OD for wells with normal rabbit serum − average OD for background wells with no antigen). The titer was defined as a serum dilution producing 50% blocking. Graphical linearization of S-shaped blocking ELISA titration curves was performed by plotting the logarithm of blocking activity/(1 − blocking activity) against the logarithm of dilution.
Polyclonal rabbit antisera were obtained by immunizing female rabbits with Sabin strains of poliovirus as described elsewhere (19).
Microneutralization test.
Poliovirus-neutralizing antibody titers were determined in a microneutralization test according to WHO recommendations (43), with some modifications. The MAb samples were diluted to 1 μg/ml in maintenance medium (DMEM supplemented with 2% FBS and 1% antibiotic/antimycotic solution; Invitrogen, Carlsbad, CA) and sterilized by filtration through Spin-X columns (Corning, Corning, NY). Two-fold serial dilutions of the antibodies starting at 1 μg/ml were incubated in quadruplicate for 3 h at 36°C with 100 50% tissue culture infectious doses (TCID50) of the respective poliovirus strain in an atmosphere of 5% CO2. After the incubation, 1 × 104 HEp-2c cells were added to the wells. The plates were incubated for 10 days at 36°C, 5% CO2 and evaluated microscopically, and neutralizing antibody titers were calculated using the Kärber formula.
Generation of escape mutants.
The antibodies were diluted to approximately 200 μg/ml in maintenance medium and sterilized by filtration through a Spin-X column. Four 5-fold dilutions of antibodies starting at 200 μg/ml were incubated for 1 h at room temperature with approximately 108 TCID50 of poliovirus, followed by incubation for 2 h at 36°C to allow for neutralization of susceptible virus. Monolayers of HEp-2c cells were inoculated with virus and incubated at 36°C, 5% CO2 until cytopathic effect (CPE) developed. The virus-containing cell suspension was subjected to three freeze-thaw cycles to release intracellular virus and then clarified by centrifugation, and the viral titer was determined by microtitration assay (43).
For plaque purification, HEp-2c monolayers in 6-well plates were inoculated with serial dilutions of mutant viruses (101 to 104 TCID50/ml) and incubated for 1 h at room temperature. Next, the medium was replaced with 4 ml modified Eagle's medium (MEM)–0.5% agarose overlay containing 6% FBS (Invitrogen). Plaques were picked after 48 h of incubation at 36°C, 5% CO2, transferred into 12-well plates with confluent HEp-2c monolayers, and incubated until CPE developed. Virus-containing cell suspensions were subjected to three freeze-thaw cycles to release intracellular virus and then clarified by centrifugation.
Nucleotide sequencing.
Viral RNA was isolated from virus-containing medium by using a QIAamp viral RNA minikit (Qiagen, Valencia, CA). The RNA was amplified by reverse transcription (RT)-PCR using a Qiagen OneStep RT-PCR kit (Qiagen) with type-specific primers allowing amplification of the entire capsid region of poliovirus. Reaction mixtures were assembled according to the manufacturer's recommendations. The reverse transcription step was at 55°C for 90 min, followed by 15 min of incubation at 95°C and 40 cycles of PCR as follows: 20 s at 94°C for the denaturation step, 1 min at 65°C for the primer annealing step, and 3 min at 72°C for the elongation step. The results of RT-PCRs were confirmed by agarose gel electrophoresis, and amplified DNA was purified by using a QIAquick PCR purification kit (Qiagen). Both the RNA isolation and PCR purification steps were performed with a QIAcube automation station (Qiagen). Purified PCR products were sequenced by the regular Sanger method with type-specific primers covering both strands of DNA and spaced about 600 bp apart. Four primers were used to sequence each strand of DNA in the PCR products. Overlapping reads were assembled and aligned with reference Sabin sequences, using the Macintosh MacVector program package (MacVector, Inc., Cary, NC), and used to detect mutations relative to the sequence of the parental strain.
Tg mouse challenge test.
Transgenic Tg-PVR21 mice were obtained from the Central Institute for Experimental Animals (Tokyo, Japan). Maintenance, containment, and transportation of mice were performed in accordance with the recommendations of the WHO Memorandum on transgenic mice susceptible to human viruses (2). All experiments using mice were performed in accordance with the U.S. Guide for the Care and Use of Laboratory Animals (28).
In protection experiments, groups of 10 mice (5 males and 5 females) were injected intravenously (i.v.) with 1 μg, 5 μg, or 25 μg of H2 antibody in 0.1 ml of PBS. For the control, one group of 10 animals was injected i.v. with 0.1 ml of PBS. After 24 h, all animals were challenged intramuscularly (i.m.) with 10 times the 50% paralytic dose (PD50) of wild-type poliovirus Mahoney strain. Mice were observed for signs of paresis/paralysis for 14 days.
In a postexposure treatment experiment, groups of 10 mice, equal numbers of males and females, were challenged i.m. with 5 PD50 of Mahoney virus, and 6 or 12 h later injected i.v. with 5 μg or 25 μg of H2 antibody. A control group received PBS instead of H2. Animals were observed for clinical signs for 2 weeks.
RESULTS
Isolation and characterization of poliovirus-specific Fabs.
A phage Fab display library constructed from bone marrow-derived lymphocytes of chimpanzees immunized with poliovirus was panned for three cycles against polioviruses of each serotype. The phage-Fab clones specific to each of three serotypes were identified by phage ELISA. The sequencing analysis of the variable domain of heavy (VH) and light (VL) chains showed that nine clones specific to serotype 1, three clones specific to serotype 2, and seven clones specific to serotype 3 were recovered. These positive clones were subsequently converted to produce soluble Fabs. Each soluble Fab was confirmed for its specific binding to its panning antigen (Table 1). Further measurement of their neutralization activities by PRNT showed that Fabs A12 and H2, Fabs A6 and B2, and Fabs B10, A2, E3, and C10 neutralized Sabin poliovirus serotypes 1, 2, and 3, respectively (Table 1). Six Fab clones (A12, H2, A6, B2, B10, and C10) had relatively higher neutralizing activities and, therefore, were selected for conversion to full-length IgGs (referred to hereinafter as MAbs) and further characterization. The closest human V-gene germ line usage and possible somatic mutations for each of these six poliovirus-neutralizing MAbs were identified by conducting sequence similarity searches of all of the known human Ig genes compiled in the IMGT database at http://www.imgt.org/. The analysis showed that family 3 of heavy chain genes and family 1 of kappa chain genes were overrepresented. Both VH and VL genes were highly mutated, since identities of 85 to 90% for VH and 90 to 96% for VL in comparison to germ line V-gene were observed (Table 2), suggesting that significant affinity maturation may have taken place.
Table 1.
Binding and neutralization of selected Fabs
| Fab clonea | Poliovirus serotype bound in ELISAb | In vitro neutralizing activityc |
|---|---|---|
| A7 | 1 | − |
| A12 | 1 | + |
| B7 | 1 | − |
| C1 | 1 | − |
| D2 | 1 | − |
| D4 | 1 | − |
| E1 | 1 | − |
| G1 | 1 | − |
| H2 | 1 | + |
| A1 | 2 | − |
| A6 | 2 | + |
| B2 | 2 | + |
| G6 | 3 | − |
| B10 | 3 | + |
| A2 | 3 | + |
| E3 | 3 | + |
| C2 | 3 | − |
| C10 | 3 | + |
| F5 | 3 | − |
Fab clones were defined as clones with distinct VH and VL sequences.
Binding to specific types of Sabin polioviruses in ELISA by soluble Fabs.
Neutralizing activity was measured by PRNT.
Table 2.
Assignment of poliovirus-neutralizing Fab clones to their closest human germ line counterparts, based on nucleotide sequence homologya
| Fab | Germ line gene |
||||||
|---|---|---|---|---|---|---|---|
| Heavy chain |
Light chain |
||||||
| V-gene | Identity (%)b | J-gene | D-gene | V-gene | Identity (%) | J-gene | |
| A12 | IGHV4-59*04 | 86 | IGHJ5*02 | IGHD5-12*01 | IGKV1-9*01 | 92 | IGKJ1*01 |
| H2 | IGHV3-30*03 | 88 | IGHJ4*02 | IGHD3-16*02 | IGKV6-21*01 | 96 | IGKJ1*01 |
| A6 | IGHV3-48*01 | 85 | IGHJ4*02 | IGHD4-23*01 | IGKV1-13*02 | 96 | IGLJ4*01 |
| B2 | IGHV3-30*03 | 89 | IGHJ3*01 | IGHD5-12*01 | IGLV1-44*01 | 96 | IGKJ2*01 |
| B10 | IGHV3-7*01 | 90 | IGHJ6*03 | IGHD3-3*01 | IGKV1-9*01 | 90 | IGLJ3*01 |
| C10 | IGHV3-7*01 | 93c | IGHJ6*03 | IGHD3-3*01 | IGKV1-9*01 | 90 | IGLJ3*01 |
The closest human V-gene germ lines were identified by searching the IMGT database at http://www.imgt.org/.
The VH and VL genes before the CDR3 region were used to calculate the percentage of nucleic acid identity. The mutations in the first 20 bp were excluded since these mutations could be introduced by PCR primers.
There was a 3-bp insertion in the CDR2 region in addition to the 93% identity.
Specificity of poliovirus-neutralizing MAbs.
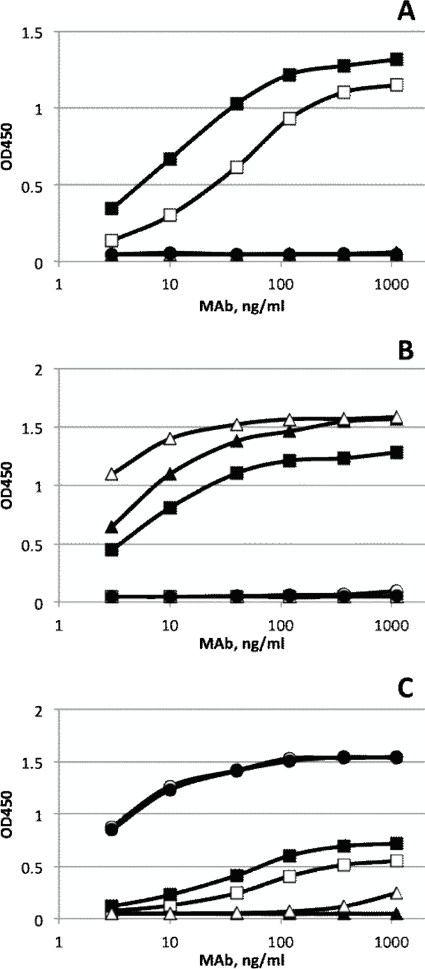
Each of the six poliovirus-neutralizing MAbs was examined by ELISA for binding to poliovirus serotype 1, 2, and 3 virions immobilized on a 96-well plate. MAbs A12 and H2 recovered from panning against type 1 poliovirus were the only ones that reacted with Sabin 1 virus (Fig. 1A). Interestingly, MAb A12 exhibited dual specificity by reacting with both Sabin 1 and Sabin 2 polioviruses (Fig. 1B). MAbs A6 and B2 that were selected by panning against type 2 poliovirus reacted well with Sabin 2 poliovirus (Fig. 1B). MAbs B10 and C10 that were recovered from panning against type 3 poliovirus reacted well with Sabin 3 (Fig. 1C). Sabin 3 virus also reacted weakly with type 1-specific MAbs A12 and H2.
Fig. 1.
Binding specificities of antipoliovirus MAbs. Binding in response to different concentrations of antipoliovirus MAbs on serotypes 1 (A), 2 (B), and 3 (C) of Sabin poliovirus was measured by ELISA with virions directly attached to the solid phase. Type 1 MAbs, H2 (□) and A12 (■); type 2 MAbs, A6 (▴) and B2 (▵); and type 3 MAbs, B10 (○) and C10 (●).
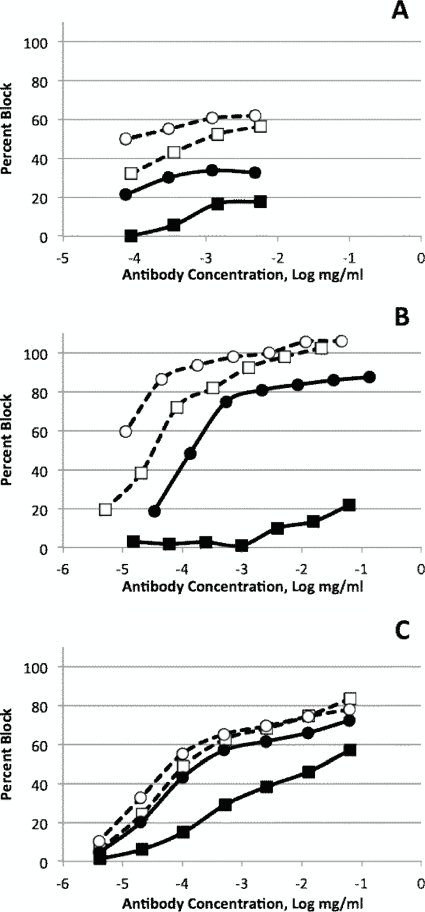
Direct ELISA may not necessarily reflect functional interactions that lead to neutralization because it can detect relatively weak binding between antibodies and antigens. Previously, we showed that the blocking ELISA procedure in which MAbs are used to compete with neutralizing polyclonal serum is much more predictive of the functional interactions and that its results correlate well with neutralization test results (32). The results in Table 3 show that, indeed, weak interactions between type 1-specific antibodies A12 and H2 and Sabin 3 poliovirus that were detected in a previous experiment did not occur in this setting. However, type 1-specific A12 antibody strongly reacted with type 2 poliovirus, suggesting that it may neutralize two serotypes of poliovirus. In another experiment, the blocking ELISA was performed with various concentrations of MAbs using both attenuated Sabin and wild polioviruses as antigens (Fig. 2). With the exception of type 3-specific MAb B10 that reacted equally well with the Sabin 3 and wild Saukett strains, all other MAbs reacted better with Sabin strains than with wild strains of the same serotype. The difference was especially pronounced for type 2-specific antibody A6 that practically did not block wild MEF-1 poliovirus.
Table 3.
Blocking ELISA reaction between human monoclonal antibodies and polioviruses
| MAb | Blocking activity (%) with antigen of typea: |
|||||
|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
||||
| Mean | SD | Mean | SD | Mean | SD | |
| H2 | 0.66 | ± 0.04 | 0 | ± 0.03 | 0 | ± 0.05 |
| A12 | 0.63 | ± 0.06 | 0.73 | ± 0.04 | 0.01 | ± 0.04 |
| A6 | 0.01 | ± 0.04 | 0.97 | ± 0.05 | 0.01 | ± 0.03 |
| B2 | −0.02 | ± 0.05 | 0.97 | ± 0.04 | 0.06 | ± 0.04 |
| B10 | −0.02 | ± 0.03 | 0.03 | ± 0.03 | 0.79 | ± 0.04 |
| C10 | −0.06 | ± 0.04 | −0.06 | ± 0.03 | 0.73 | ± 0.03 |
The values represent the relative reductions (blocking) of ELISA reactions between surface-captured antigens and polyclonal conjugates after treatment with monoclonal antibodies and reflect affinities between the antibodies and the antigens. Data in boldface show strong neutralizing interactions.
Fig. 2.
Blocking ELISA reaction of primate MAbs with wild and attenuated Sabin polioviruses. Results for reactions with type 1 (A), type 2 (B), and type 3 (C) polioviruses are shown. (A) Sabin 1/H2, ○; Mahoney/H2, ●; Sabin 1/A12, □; and Mahoney/A12, ■. (B) Sabin 2/B2, ○; MEF-1/B2, ●; Sabin 2/A6, □; and MEF-1/A6, ■. (C) Sabin 3/B10, ○; Saukett/B10, ●; Sabin 3/C10, □; and Saukett/C10, ■.
Neutralization of the vaccine and wild-type strains of poliovirus.
The results of in vitro neutralization of the six MAbs presented in Table 4 showed that all MAbs selected against each serotype effectively neutralized homotypic viruses. In addition, and consistent with the ELISA data, the A12 MAb neutralized both type 1 and type 2 polioviruses. While some MAbs, such as A12 and H2, neutralized both Sabin and wild strains of poliovirus, others exhibited a significant bias for Sabin strains. The neutralizing titer of MAb A6 tested against wild MEF-1 was more than 1,000 times lower than against the Sabin 2 strain. MAbs B2, B10, and C10 had about 10- to 100-fold lower neutralizing activities against wild-type than against Sabin strains.
Table 4.
In vitro cross-neutralization of Sabin and wild polioviruses with humanized MAbs
| MAb (1 mg/ml) | Neutralization titer (per 1 mg/ml MAb) against poliovirus strain of serotypea: |
|||||
|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
||||
| Sabin 1 | Mahoney | Sabin 2 | MEF-1 | Sabin 3 | Saukett | |
| A12 | 102,400 | 121,800 | 121,800 | 172,200 | ND | ND |
| H2 | 43,100 | 18,100 | ND | ND | ND | ND |
| A6 | ND | ND | 18,100 | <20 | ND | ND |
| B2 | ND | ND | 289,600 | 21,500 | ND | ND |
| B10 | ND | ND | ND | ND | 819,200 | 10,800 |
| C10 | ND | ND | ND | ND | 1,158,500 | 144,800 |
ND, no data.
Cross-neutralization of different serotypes of poliovirus is a relatively unknown phenomenon. It is difficult if not impossible to study it in humans because of the maternal antibodies present in newborn children and, also, because routinely used vaccines are trivalent. Therefore, we assessed whether rabbit antisera raised by immunization with purified monovalent polioviruses would demonstrate any evidence of cross-neutralization. The data in Table 5 show that there was very little cross neutralization among serotypes, suggesting that polyspecific antibodies do not represent a majority of neutralizing antibodies in rabbit polyclonal antisera.
Table 5.
Neutralization of polioviruses with rabbit hyperimmune sera
| Virus | Neutralization titer of rabbit antiserum of serotype: |
||
|---|---|---|---|
| 1 | 2 | 3 | |
| Type 1 | 38,968 | 64 | <8 |
| Type 2 | 11 | 38,968 | 362 |
| Type 3 | <11 | 91 | 28,800 |
Localization of neutralization epitopes.
To identify epitopes to which the primate MAbs bind, we selected escape mutants by treating Sabin polioviruses with the antibodies, plaque purifying the resistant strains, and sequencing their RNA to identify mutations that rendered the strains resistant to neutralization. We were able to generate escape mutants to antibodies specific to types 1 and 2 but failed to obtain resistant variants of type 3 poliovirus.
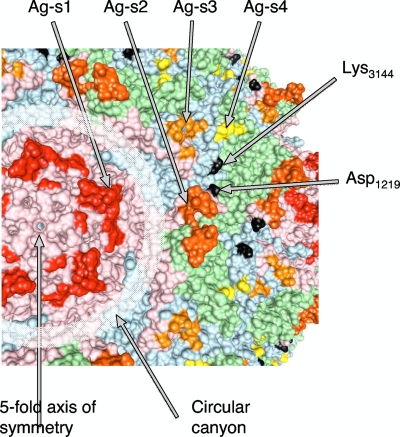
The H2-resistant mutants contained several mutations, the most frequent being D1219G and D1219E in VP1, located close to antigenic site 2. Other mutations making the virus resistant to H2 antibody were in VP3 (K3144R,E,T), in the vicinity of the first mutation but closer to antigenic site 3, also composed of VP3 (Fig. 3). Different mutations at the same amino acid site appeared to produce different effects on the blocking ELISA reactivity of the strains. For instance, the blocking activity of H2 antibody measured with D1219G virus was stronger than that measured with D1219E (8% ± 3% [mean ± standard deviation] and 20% ± 12%, respectively).
Fig. 3.
Location of the epitope to antibody H2 on the surface of type 1 poliovirus. Capsid proteins VP1, VP2, and VP3 are shown in pink, green, and blue, respectively. Antigenic sites 1, 2, 3, and 4 are shown in red, orange, gold, and yellow, respectively. Amino acids that are replaced in escape mutants are shown in black.
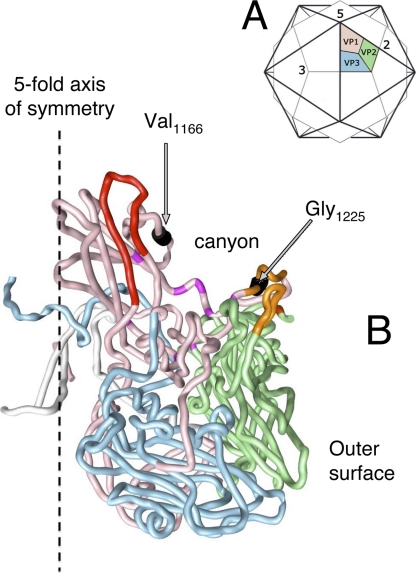
The resistant strains generated in response to the dual-reactive antibody A12 also contained an unusual pattern of mutations. All clones isolated from the Sabin 1 strain contained a V1166E mutation, while clones isolated from the Sabin 2 strain contained a G1225D mutation (Fig. 4). Some Sabin 1 escape clones also contained I1090M. It may not be necessary for the resistance, because this mutation leads to a direct reversion to the Mahoney amino acid, and Mahoney virus is sensitive to A12. Mutation V1166E was not previously identified as a part of an antigenic site 1 but is located close to it on the virion surface close to the canyon surrounding the 5-fold axis of symmetry. In contrast, mutation G1225D, which makes the Sabin 2 strain resistant to the same A12 antibody, is located on the opposite side of the canyon and is part of antigenic site 2. Thus, it appears that A12 binds the virion by interacting with both sides of the canyon, at the bottom of which the binding site for cellular receptor is located (3, 9, 42).
Fig. 4.
Location of the epitope to antibody A12 on the surface of poliovirus. (A) Scheme of poliovirus capsid showing the location of a single capsomer relative to the 5-, 3-, and 2-fold axes of symmetry. Canyon surrounds the 5-fold axis. VP1, VP2, and VP3 proteins are shown. (B) Three-dimensional structure of poliovirus capsomer. Color codes for proteins VP1, VP2, and VP3 are the same as in panel A. Antigenic sites 1 and 2 are shown in red and orange, respectively, and amino acids replaced in escape mutants are shown in black.
The escape mutants generated in response to type 2-specific antibodies A6 and B2 contained mutations R1100C,L and A1101D, respectively, both located in the B-C loop in VP1 protein that was previously defined as epitope 1.
Pre- and postexposure protection of mice from infection by virulent poliovirus.
To determine whether the MAbs can protect against poliomyelitis in vivo, we intravenously inoculated Tg-21PVR transgenic mice expressing human poliovirus receptor CD155 with various doses of H2 MAb and challenged them intramuscularly with 10 50% paralytic doses (PD50) of wild poliovirus (Mahoney strain). Table 6 shows that 1 μg of MAb protected 30% of the mice and 5 μg protected 100%. The average neutralizing titers in serum in the 5-μg and 1-μg groups were 1:32 and 1:9, respectively, consistent with the common notion that a neutralization titer of 1:8 protects against polio.
Table 6.
H2 MAb protects transgenic mice from challenge with 10 PD50 of Mahoney virus
| MAb dose (μg/mouse) | Reciprocal neutralization titer in serum | No. of mice that survived/no. challenged |
|---|---|---|
| 0 | 0 | 0/10 |
| 1 | 9 | 3/10 |
| 5 | 32 | 10/10 |
| 25 | 158 | 10/10 |
The protective effect of the MAbs is quite predictable, considering the high potency of the antibodies. It is also important to determine whether the antibodies could be used to prevent paralytic disease if administered after exposure to the virus. The results shown in Table 7 demonstrate that some mice survived challenge with a 5-PD50 dose if they were treated with 5 or 25 μg of H2 antibody at either 6 or 12 h after infection. There was a clear protective effect, even though not all mice survived. This observation suggests that these antibodies could be used for emergency prophylaxis of nonimmune individuals exposed to poliovirus.
Table 7.
H2 MAb protects transgenic mice even when administered after challenge with 5 PD50 of Mahoney virus
| Group | MAb dose (μg) | Time after challenge (h) | No. of mice that survived/no. challenged | P valuea |
|---|---|---|---|---|
| 1 | 5 | 6 | 7/10 | 0.000 |
| 2 | 25 | 6 | 4/10 | 0.010 |
| 3 | 5 | 12 | 4/10 | 0.010 |
| 4 | 25 | 12 | 3/10 | 0.031 |
| Control | 0 | 6 | 0/10 | NA |
Probability of this number of mice surviving by chance at challenge dose of 5 PD50/mouse. NA, not applicable.
DISCUSSION
Here, we describe the isolation of chimeric chimpanzee-human antipoliovirus antibodies by combining hyperimmunization of chimpanzees with phage Fab display library technology, a method previously used to isolate humanized MAbs against a variety of viral and bacterial pathogens (5–7). Compared to other methods based on human hybridoma technology (11) and B-cell immortalization with Epstein-Barr virus (37, 38), the method described in this communication has the advantage of producing cloned immunoglobulin genes that could be used directly for the production of antibodies expressed in vitro, as well as for genetic manipulations that could improve their therapeutic properties.
The antigenic structure of polioviruses has been extensively studied in the past (26). There are three distinct serotypes of poliovirus that are defined by their inability to be cross-neutralized by sera raised against the two other serotypes (30). Elucidation of the molecular structure of poliovirus and the introduction of MAb technology allowed epitopes to mouse MAbs to be mapped on the surface of polio virions. Mutations rendering poliovirus resistant to MAbs identify epitopes with which the antibodies interact. Epitopes to different MAbs are clustered into several structural regions (antigenic sites) on the surface of polio virions (18, 40). Studies with three serotypes of poliovirus identified three or four such antigenic sites. Antigenic site 1 is composed of the so-called B-C loop and the adjacent regions within VP1, while three other antigenic sites are composed of a combination of different capsid proteins, in some cases belonging to adjacent capsomers. Most poliovirus epitopes are believed to be conformational and are very sensitive to disruption of the virion structure.
Very little is known about human antipolio antibodies, but there is circumstantial evidence that their repertoire may be different from those in mice (33). The MAbs from chimpanzees, the closest relatives of humans, described in this paper support this notion. One interesting observation is the dual specificity of antibody A12. It binds and neutralizes both type 1 and type 2 polioviruses. The results of our cross-neutralization experiments with hyperimmune rabbit antisera suggest that such dual-specific antibodies may not exist in the rabbit immunoglobulin repertoire. However, there are indications that such cross-neutralizing antibodies may not be uncommon in human antisera. Uhlig and Dernick (39) isolated four immortalized clones of human B cells producing neutralizing antipoliovirus antibodies. One of the antibodies neutralized both type 1 and 2 polioviruses, and another neutralized all three serotypes, albeit at a lower potency for types 2 and 3. In another study, a mouse monoclonal antibody was shown to neutralize both type 1 and type 2 poliovirus (41). The epitope for this antibody was mapped at amino acid residues 239 to 245 of VP2, far from the binding site of A12. Therefore, the dual-reacting A12 antibody may represent a class of cross-neutralizing antibodies the role of which has been underestimated. Further studies of human antibodies are needed to assess the magnitude of this phenomenon. The method described in this communication is well suited for targeted screening for cross-neutralizing antibodies by phage display panning of Fab fragments induced by one serotype against antigens of another serotype.
The existence of cross-neutralizing antibodies goes against the common belief that underlies the classification of polioviruses into three serotypes. This classification is based on neutralization tests performed with hyperimmune animal sera that may not contain cross-neutralizing antibodies. It is very difficult to study this question in humans either epidemiologically or experimentally. Cross-reacting antibodies in monkey sera were revealed in complement fixation testing (23), and some circumstantial evidence based on the comparison of IPV-induced seroconversion of completely immunologically naïve children and children who have been exposed to heterologous wild polioviruses suggests that there may be some cross-serotypic priming effect (15).
We successfully isolated escape mutants of type 1 and type 2 poliovirus but were unable to isolate type 3 antibody-resistant strains. The reason for this is unknown and may be related to the extremely high potency of type 3 antibodies, the inability to disrupt virus-antibody interactions by one mutation, or low viability of the resulting escape mutants. Some of the epitopes that bind to the MAbs described here map to the antigenic sites previously identified for mouse antibodies, while others may have a different binding mechanism. For instance, the dual type 1-/type 2-specific antibody A12 appears to bind to both antigenic sites 1 and 2 and, thus, fills the canyon on the virion surface where the receptor-binding site is located (3, 9, 42). Interestingly, one of the antibody-resistant mutations generated in response to A12 treatment (G1225D) was previously shown to reduce binding to the cellular receptor by 7- to 14-fold (9). Therefore, it is reasonable to suggest that antibody A12 prevents virus from binding to its cellular receptor.
Comparison of the amino acid sequences and three-dimensional structures of relevant parts of Sabin 1 and Sabin 2 capsid proteins shows that they are similar, and amino acid differences in this region involve chemically similar amino acids. This provides an explanation of the dual reactivity of the A12 antibody. Another antibody, H2, binds to the space between antigenic sites 2 and 3. Therefore, parts of the virion that are immunologically important in mice and chimpanzees appear to differ. Further studies of native human antibodies to poliovirus are needed to better define antigenic sites.
The utility of passive immunotherapy for the prevention of poliomyelitis has been well documented in the past (16). Previous attempts to use intravenous immunoglobulin and breast milk were unsuccessful, even though a significant temporary decrease in shedding was observed when the patient was treated with breast milk (22). The failure to completely stop shedding may be due to insufficient levels of antipoliovirus antibodies in IVIG and breast milk. The availability of highly potent human-like antibodies to poliovirus opens the possibility of curing chronically infected individuals of their poliovirus infection, thereby stopping shedding of virus into the environment. The addition of the recombinant chimpanzee-human antibodies described here could boost neutralizing titers to extremely high levels and thereby resolve chronic infection. The efficacy of the monoclonal antibodies must be evaluated in clinical trials that are now planned.
The transgenic mouse data presented here suggest that the antibodies can protect against poliomyelitis not only when given prior to infection but also after exposure to poliovirus. This means that the antibodies can stop the development of the infectious process even after it has started. This raises the possibility of using them for emergency prophylaxis of contacts of a paralytic polio case or to prevent the spread of poliovirus should it reemerge after apparent eradication. Under these circumstances, emergency vaccinations will be performed to stop the outbreak. Adding treatment with antipoliovirus antibodies to this intervention will result in immediate protection of the individuals involved while allowing the longer-term effect of a vaccine to develop.
In conclusion, the findings presented in this communication open the possibilities of better characterizing human immune responses to poliovirus and of producing therapeutic reagents useful for polio eradication and transition to a polio-free world.
ACKNOWLEDGMENTS
The work was supported by intramural research funding from NIAID/NIH and CBER/FDA.
Footnotes
Published ahead of print on 23 February 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Alexander L. N., et al. 2004. Vaccine policy changes and epidemiology of poliomyelitis in the United States. JAMA 292:1696–1701 [DOI] [PubMed] [Google Scholar]
- 2. Anonymous 1993. Maintenance and distribution of transgenic mice susceptible to human viruses: memorandum from a WHO meeting. Bull. World Health Organ. 71:497–502 [PMC free article] [PubMed] [Google Scholar]
- 3. Belnap D. M., et al. 2000. Three-dimensional structure of poliovirus receptor bound to poliovirus. Proc. Natl. Acad. Sci. U. S. A. 97:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen Z., et al. 2007. Characterization of chimpanzee/human monoclonal antibodies to vaccinia virus A33 glycoprotein and its variola virus homolog in vitro and in a vaccinia virus mouse protection model. J. Virol. 81:8989–8995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen Z., et al. 2006. Chimpanzee/human mAbs to vaccinia virus B5 protein neutralize vaccinia and smallpox viruses and protect mice against vaccinia virus. Proc. Natl. Acad. Sci. U. S. A. 103:1882–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen Z., et al. 2009. Potent neutralization of anthrax edema toxin by a humanized monoclonal antibody that competes with calmodulin for edema factor binding. Proc. Natl. Acad. Sci. U. S. A. 106:13487–13492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen Z., et al. 2006. Efficient neutralization of anthrax toxin by chimpanzee monoclonal antibodies against protective antigen. J. Infect. Dis. 193:625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chumakov K., Ehrenfeld E. 2008. New generation of inactivated poliovirus vaccines for universal immunization after eradication of poliomyelitis. Clin. Infect. Dis. 47:1587–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Colston E., Racaniello V. R. 1994. Soluble receptor-resistant poliovirus mutants identify surface and internal capsid residues that control interaction with the cell receptor. EMBO J. 13:5855–5862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Committee on Development of a Polio Antiviral and Its Potential Role in Global Poliomyelitis Eradication, National Research Council 2006. Exploring the role of antiviral drugs in the eradication of polio. National Academies Press, Washington, DC [Google Scholar]
- 11. Dessain S. K., et al. 2004. High efficiency creation of human monoclonal antibody-producing hybridomas. J. Immunol. Methods 291:109–122 [DOI] [PubMed] [Google Scholar]
- 12. Dowdle W. R., De Gourville E., Kew O. M., Pallansch M. A., Wood D. J. 2003. Polio eradication: the OPV paradox. Rev. Med. Virol. 13:277–291 [DOI] [PubMed] [Google Scholar]
- 13. Ehrenfeld E., et al. 2008. Immunisation against poliomyelitis: moving forward. Lancet 371:1385–1387 [DOI] [PubMed] [Google Scholar]
- 14. Ehrenfeld E., Modlin J., Chumakov K. 2009. Future of polio vaccines. Expert Rev. Vaccines 8:899–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fox J. P., Hall C. E. 1980. Viruses in families: surveillance of families as a key to epidemiology of virus infections, p. 131-135 PSG Publishing Company, Inc., Littleton, MA [Google Scholar]
- 16. Hammon W. M. 1955. Passive immunization against poliomyelitis. Monogr. Ser. World Health Organ. 26:357–370 [PubMed] [Google Scholar]
- 17. Heymann D. L., Sutter R. W., Aylward R. B. 2005. A global call for new polio vaccines. Nature 434:699–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Humphrey D., Kew O. M., Feorino P. M. 1982. Neutralizing monoclonal antibodies of four different specificities for type 1 poliovirus. Infect. Immun. 36:841–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ivanov A. P., Dragunsky E. M. 2005. ELISA as a possible alternative to the neutralization test for evaluating the immune response to poliovirus vaccines. Expert Rev. Vaccines 4:167–172 [DOI] [PubMed] [Google Scholar]
- 20. Kew O. M., et al. 1998. Prolonged replication of a type 1 vaccine-derived poliovirus in an immunodeficient patient. J. Clin. Microbiol. 36:2893–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kew O. M., et al. 2004. Circulating vaccine-derived polioviruses: current state of knowledge. Bull. World Health Organ. 82:16–23 [PMC free article] [PubMed] [Google Scholar]
- 22. MacLennan C., et al. 2004. Failure to clear persistent vaccine-derived neurovirulent poliovirus infection in an immunodeficient man. Lancet 363:1509–1513 [DOI] [PubMed] [Google Scholar]
- 23. Melnick J. L.1955. Antigenic crossings within poliovirus types. Proc. Soc. Exp. Biol. Med. 89:131–133 [PubMed] [Google Scholar]
- 24. Minor P. 2001. Characteristics of poliovirus strains from long-term excretors with primary immunodeficiencies. Dev. Biol. (Basel) 105:75–80 [PubMed] [Google Scholar]
- 25. Minor P. 2009. Vaccine-derived poliovirus (VDPV): impact on poliomyelitis eradication. Vaccine 27:2649–2652 [DOI] [PubMed] [Google Scholar]
- 26. Minor P. D. 1986. Antigenic structure of poliovirus. Microbiol. Sci. 3:141–144 [PubMed] [Google Scholar]
- 27. Mohammed A. J., et al. 2010. Fractional doses of inactivated poliovirus vaccine in Oman. N. Engl. J. Med. 362:2351–2359 [DOI] [PubMed] [Google Scholar]
- 28. National Institutes of Health 1985. Guide for the care and use of laboratory animals. NIH publication no. 85-23. U.S. Department of Health and Human Services, Washington, DC [Google Scholar]
- 29. Oberste M. S., et al. 2009. In vitro antiviral activity of V-073 against polioviruses. Antimicrob. Agents Chemother. 53:4501–4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pallansch M. A., Roos R. P. 2001. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses, p. 723–775 In Knipe D. M., Howley P. M., Griffin D. E., Lamb R. A., Martin M. A., Roizman B., Straus S. E. (ed.), Fields virology, 4th ed. Lippincott Williams and Wilkins, Philadelphia, PA [Google Scholar]
- 31. Resik S., et al. 2010. Randomized controlled clinical trial of fractional doses of inactivated poliovirus vaccine administered intradermally by needle-free device in Cuba. J. Infect. Dis. 201:1344–1352 [DOI] [PubMed] [Google Scholar]
- 32. Rezapkin G. V., Dragunsky E., Chumakov K. 2005. Improved ELISA test for determination of potency of inactivated poliovirus vaccine (IPV). Biologicals 33:17–27 [DOI] [PubMed] [Google Scholar]
- 33. Rezapkin G. V., et al. 2010. Repertoire of antibodies against type 1 poliovirus in human sera. J. Virol. Methods 169:322–331 [DOI] [PubMed] [Google Scholar]
- 34. Rinaldo C. R., Jr. 2005. Passive immunization against poliomyelitis: the Hammon gamma globulin field trials, 1951-1953. Am. J. Public Health 95:790–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sabin A. B. 1957. Properties and behavior of orally administered attenuated poliovirus vaccine. JAMA 164:1216–1223 [DOI] [PubMed] [Google Scholar]
- 36. Salk J. E., et al. 1954. Formaldehyde treatment and safety testing of experimental poliomyelitis vaccines. Am. J. Public Health Nations Health 44:563–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Steinitz M., Klein G., Koskimies S., Makel O. 1977. EB virus-induced B lymphocyte cell lines producing specific antibody. Nature 269:420–422 [DOI] [PubMed] [Google Scholar]
- 38. Traggiai E., et al. 2004. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat. Med. 10:871–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Uhlig H., Dernick R. 1988. Intertypic cross-neutralization of polioviruses by human monoclonal antibodies. Virology 163:214–217 [DOI] [PubMed] [Google Scholar]
- 40. Uhlig H., Rutter G., Dernick R. 1983. Evidence for several unrelated neutralization epitopes of poliovirus, type 1, strain Mahoney, provided by neutralization tests and quantitative enzyme-linked immunosorbent assay (ELISA). J. Gen. Virol. 64:2809–2812 [DOI] [PubMed] [Google Scholar]
- 41. Uhlig J., Wiegers K., Dernick R. 1990. A new antigenic site of poliovirus recognized by an intertypic cross-neutralizing monoclonal antibody. Virology 178:606–610 [DOI] [PubMed] [Google Scholar]
- 42. Wien M. W., Curry S., Filman D. J., Hogle J. M. 1997. Structural studies of poliovirus mutants that overcome receptor defects. Nat. Struct. Biol. 4:666–674 [DOI] [PubMed] [Google Scholar]
- 43. World Health Organization 1997. Manual for the virological investigation of polio. World Health Organization, Geneva, Switzerland [Google Scholar]