Abstract
Corneal neovascularization represents a key step in the blinding inflammatory stromal keratitis (SK) lesion caused by ocular infection with herpes simplex virus (HSV). In this report, we describe a novel approach for limiting the angiogenesis caused by HSV infection of the mouse eye. We show that topical or systemic administration of the Src kinase inhibitor (TG100572) that inhibits downstream molecules involved in the vascular endothelial growth factor (VEGF) signaling pathway resulted in markedly diminished levels of HSV-induced angiogenesis and significantly reduced the severity of SK lesions. Multiple mechanisms were involved in the inhibitory effects. These included blockade of IL-8/CXCL1 involved in inflammatory cells recruitment that are a source of VEGF, diminished cellular infiltration in the cornea, and reduced proliferation and migration of CD4+ T cells into the corneas. As multiple angiogenic factors (VEGF and basic fibroblast growth factor [bFGF]) play a role in promoting angiogenesis during SK and since Src kinases are involved in signaling by many of them, the use of Src kinase inhibition represents a promising way of limiting the severity of SK lesions the most common cause of infectious blindness in the Western world.
INTRODUCTION
Ocular herpes simplex virus (HSV) infection can result in blinding immunoinflammatory lesions in the cornea termed stromal keratitis (SK) (3, 25). A critical step in the pathogenesis in SK is neovascularization of the normally avascular cornea, but such vessels are leaky and permit the escape of cells and inflammatory molecules into stromal tissues, events that impair vision. Preventing or limiting neovascularization was shown in animal models of SK to be a useful means to control the severity of lesions (16, 30, 31). Many molecules may participate in causing neovascularization in the HSV-infected eye, but vascular endothelial growth factor A (VEGF-A) is the principal angiogenic factor involved (30). VEGF-A can derive from multiple sources, including endogenous production of VEGF-A, whose angiogenic function is blocked by being bound to a soluble form of one of its receptors (2). HSV infection results in the breakdown of this inhibitory interaction (26). Additional VEGF-A supplies come from newly synthesized protein by infected or cytokine-stimulated cells as well as from transportation of VEGF-A to the eye by inflammatory cells (8). Whatever the source, VEGF-A mediates ocular angiogenesis by signaling mainly through the VEGFR2 receptor, which in turn sets off a sequence of intracellular events that involve Src kinases (6, 7, 29).
Recent studies have shown that the Src family of tyrosine kinases are responsible for VEGF-mediated vascular permeability and angiogenesis in several systems (6, 11, 24). Accordingly, using inhibitors of Src kinases represents a logical approach for therapy against pathological angiogenesis such as that which occurs in SK. Approaches tested to date for inhibition of angiogenesis in the SK system have targeted either VEGF or one of its receptors, but inhibiting biochemical events set off by VEGF signaling, such as Src kinase activation, has not been evaluated. This approach could have advantages over others since Src kinases are also responsible for mediating vascular permeability and may also be involved in signaling by other angiogenic factors, such as fibroblast growth factors (FGFs) (24). The later are known to be involved in pathological angiogenesis caused by ocular HSV infection (10, 30).
Drugs that effectively inhibit one or more Src kinases and that can function to inhibit new blood vessel development and function have recently become available (5, 19, 24). One such example is the drug TG100572, shown recently to be effective at inhibiting VEGF-mediated events involved in a noninfectious vascular disease of the retina (24). A compound of particular interest is the prodrug Src kinase inhibitor TG100801, since upon topical ocular administration to the eye it converts to the active Src kinase inhibitor molecule TG100572, which inhibits VEGF signaling (24). In the present report, we demonstrate that TG100801 given topically is an effective means of inhibiting neovascularization and the subsequent severity of SK in the HSV-infected eye. The use of Src kinase inhibitors could add to the arsenal of therapeutics useful for the clinical management of SK, an important cause of impaired vision in humans.
MATERIALS AND METHODS
Mice and virus.
Female 5- to 6-week-old C57BL/6 mice and BALB/c mice were obtained from Harlan Sprague-Dawly (Indianapolis, IN). The animals were housed in the animal facility at the University of Tennessee. All manipulations were done in a laminar flow hood. All experimental procedures were in complete agreement with the Association for Research in Vision and Ophthalmology resolution on the use of animals in research. HSV-1 strain RE was propagated and titrated on Vero cells (ATCC CCL81) using standard protocols. The virus was stored in aliquots at −80°C until use.
Corneal HSV-1 infection and clinical observations.
Corneal infections of C57BL/6 mice were conducted under deep anesthesia. Mice were scarified on their corneas with 27-gauge needles, and a 3-μl drop containing the required viral dose (104 PFU of HSV RE) was applied to the eye. The eyes were examined at different time points postinfection (p.i.) with a slit lamp biomicroscope (Kowa), and the clinical severities of keratitis and angiogenesis in individually scored mice were recorded. The scoring system was as follows: 0, normal eye; 1, mild corneal haze; 2, moderate corneal opacity, iris visible; 3, severe corneal opacity, iris visible; 4, opaque cornea, ulcer formation; and 5, necrotizing SK. Similarly, the angiogenic scoring system was based on quantifying the degree of neovessel formation based on three primary parameters: (i) the circumferential extent of neovessels (as the angiogenic response is not uniformly circumferential in all cases), (ii) the centripetal growth of the longest vessel in each quadrant of the circle, and (iii) the longest neovessels in each quadrant, which were identified and graded between 0 (no neovessels) and 4 (neovessels in corneal center) in increments of nearly 0.4 mm (the radius of the cornea is 1.5 mm). According to this system, a grade of 4 for a given quadrant of the circle represents the centripetal growth of 1.5 mm toward the corneal center. The scores of four quadrants of the eye were then be summed up to derive the neovessel index (range, 0 to 16) for each eye at a given time point.
Antibodies and reagents.
For flow cytometry measurement of the infiltrating cells, 6 corneas per group were collected at the indicated time points by dissecting the corneal buttons above the limbus by a scalpel. Corneas were digested in liberase (Roche diagnostics) for 45 min at 37°C. A single-cell suspension was prepared as described elsewhere (30). The Fc receptors were blocked with unconjugated anti-CD16/32 (BD Pharmingen) for 30 min. Samples were incubated with CD4-APC (RM4-5), CD11b-peridinin chlorophyll protein (PerCP) (M1/70), F4/80-fluorescein isothiocyanate (FITC) (BM8), Gr1-phycoerythrin (PE) (RB6-8C5), CD45-allophycocyanin (APC) (LCA-Ly5 and 30-F11), CD31-PE (MEC 13.3), CD49d (MFR4.B), CD8-PerCP, gamma interferon (IFN-γ)–APC, CD44-FITC, and CD62L-FITC purchased from BD Pharmingen (San Diego, CA) for 30 min. All samples were collected on a fluorescence-activated cell sorting FACScan (BD biosciences), and data were analyzed using FlowJo software.
Immunofluorescent staining for PECAM-1 in vascular endothelium was also performed on the corneal flat mounts. Corneas of BALB/c mice infected with 5 × 105 PFU of HSV-1 RE were dissected under a stereo microscope (Leica, Wetzlar, Germany), and corneal flat mounts were rinsed in phosphate-buffered saline (PBS) for 30 min and flattened on a glass slide under the stereo microscope. Corneal flat mounts were dried and fixed in 100% acetone (Sigma, St. Louis, MO) for 10 min at −20°C. Nonspecific binding was blocked with 10% goat serum (G 9023; Sigma) for 24 h at 4°C (Invitrogen). The PECAM-1 was detected by an antibody directly labeled with conjugate (PE anti-mouse CD-31 MEC 13.3; BD Pharmingen). Each step was followed by three washes with PBS. Stained corneal flat mounts were mounted with prolong gold antifade mounting reagent (P36934; Invitrogen) and visualized with a Nikon Ti fluorescence microscope using Elements software (Nikon).
TG100801, a topically applied prodrug form of a Src kinase inhibitor, was obtained from Sanofi-Aventis (Paris)/Targegen, Inc., San Diego, CA. The active compound, TG100572 (also obtained from Targegen for in vitro use and intraperitoneal [i.p.] injections), is an ATP competitive multitargeted tyrosine kinase inhibitor, whereas the prodrug TG100801 is devoid of kinase inhibitory activity.
Treatment of animals with Src kinase inhibitor TG100801/TG100572.
Female 5- to 6-week-old C57BL/6 mice were used. Corneal infections were conducted under deep anesthesia induced by i.p. injection of Avertin (Sigma Aldrich). The mice were scarified on their corneas with a 27-gauge needle, infected with 104 PFU of HSV-1 RE per eye, and divided randomly into groups. In some groups, Src kinase inhibitor TG100801 (0.6% and 0.3%; Targegen, Inc.) was applied topically (5-μl eye drop, twice daily), and in the other group, TG100572 (0.5, 1.5, and 5 mg/kg of body weight dissolved in dimethyl sulfoxide [DMSO] was administered intraperitoneally) starting from either day 1 to day 14 postinfection or day 6 to day 14 p.i. Animals in the control group received liposomal vehicle (25% phospholipon vehicle 90 G) in the case of topical drug administration and DMSO in the intraperitoneal treatment modality in accordance with the same regimen. Mice were observed for the development and the progression of herpetic stromal keratitis (HSK) lesions and angiogenesis from day 6 until day 15 as described previously. Most of the experiments were repeated at least 3 times unless stated otherwise.
Treatment of animals with the anti-VEGF antibody bevacizumab (avastin).
The anti-VEGF antibody avastin was obtained from Genentech. Female 5- to 6-week-old C57BL/6 mice were used. Corneal infections were conducted under deep anesthesia induced by i.p. injection of Avertin (Sigma Aldrich). The mice were scarified on their corneas with a 27-gauge needle, infected with 104 PFU of HSV-1 RE per eye, and divided randomly into groups. In one group, Src kinase inhibitor TG100572 (5 mg/kg of body weight; Targegen, Inc.) was administered intraperitoneally, daily, and in the other group, avastin (5 mg/kg of body weight at days 3, 6, 9, and 12 p.i.) was given intraperitoneally. Animals in the control group received mock treatment. Mice were observed for the development and the progression of HSK lesions and angiogenesis from day 6 until day 15 as described in Materials and Methods. Most of the experiments were repeated at least 3 times unless stated otherwise.
Virus-specific CD8+ IFN-γ staining.
To determine the immune response generated in the controls and Src kinase-treated groups, intracellular cytokine staining was performed as previously described (13). A single-cell suspension of infected draining lymph node (DLN) was prepared, and 106 cells per well were cultured in 96-well U-bottom plates. Cells were left untreated/stimulated with gB peptide (1 μg/ml) for 5 h at 37°C in 5% CO2. Brefeldin A (10 μg/ml) was added to the culture for the intracellular cytokine accumulation. Cell surface marker and intracellular cytokine staining for IFN-γ was performed using a cytofix/cytoperm kit (BD Pharmingen). All samples were collected with a FACScan and were analyzed by FlowJo.
Thymidine incorporation assay.
Lymphocytes from the draining cervical lymph nodes (CLN) were obtained at day 15 p.i. and enriched for CD4+ T cell population by use of a Miltenyi biotech CD4+ T cell isolation kit. The cells were plated at a density of 5 × 105 in 96-well round-bottom tissue culture plates in a total volume of 200 μl of RPMI medium (Gibco). The cells were stimulated with anti-CD3 (1 μg/well) anti-CD28 (1 μg/well). Sixteen hours before harvest, [3H]thymidine (1 mCi/well; 1 Ci = 37 GBq) was added. The cells were harvested onto a UniFilter (PerkinElmer). [3H]thymidine incorporation was measured with a scintillation counter, and the results were expressed as mean cpm from triplicate cultures.
Western blotting.
For the detection of FAK phosphorylation, 3 corneas per group were dissected at the indicated time points in 300 μl of radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitor cocktail (aprotinin, phenylmethylsulfonyl fluoride [PMSF], and sodium orthovanadate), cell debris was removed by centrifugation, and the samples were stored at −80°C until they were used for SDS-PAGE. In brief, after 2 h of blocking with 3% nonfat milk in Tris-buffered saline (TBS), membranes were incubated with a 1:1,000 dilution of rabbit polyclonal phosphor-specific anti-FAK861 antibody, and membranes were then incubated for 1 h with secondary antibody coupled to horseradish peroxidase. Specific bands were detected with Immobilon Western blotting (Millipore). Membranes were stripped and then reprobed to detect total FAK and β-actin.
Histopathology.
For histopathological analysis, eyeballs from different groups of mice were extirpated at the indicated time points postinfection in 10% formalin. In brief, the samples were put overnight in Tissue-Tek (Sakura), which virtually removes all the moisture content from the samples and embeds it in paraffin. Tissue-Tek was automatically programmed, treating the samples sequentially with alcohol (100%), xylene (100%), and paraffin. Five-micrometer sections were then cut using a microtome and stained with hematoxylin and eosin (H&E).
Cytokine enzyme-linked immunosorbent assay (ELISA).
Six corneas per group were collected at the indicated time points. The corneas were sonicated, and the levels of the CXCL1/KC/mouse homologue of interleukin 8 (IL-8) were measured in the supernatants using a Qantikine kit per the manufacturer's protocol (R&D systems).
qPCR.
Total mRNA was isolated from corneal cells using TRIzol LS reagent (Invitrogen). The cDNA prepared using 1 μg of RNA was used for subsequent analysis. Quantitative PCR (qPCR) was done using SYBR green PCR master mix (Applied Biosystems, Foster City, CA) with an iQ5 real-time PCR detection system (Bio-Rad, Hercules, CA). The expression levels of cytokines were normalized to β-actin levels with the threshold cycle (ΔCT) method, and relative quantification between control and infected mice was performed using the 2−ΔΔCt formula. The primers used were as follows: IL-1β forward primer, CATCAACAAGAGCTTCAGGC; IL-1β reverse primer, CATCATCCCATGAGTCACAGAG; IL-6 forward primer, CCAGAGTCCTTCAGAGAGATAC; IL-6 reverse primer, CTCCTTCTGTGACTCCAGCTTATC; IFN-γ forward primer, GAACGCTACACACTGCATCT; IFN-γ reverse primer, CCAGTTCCTCCAGATATCCAAG; β-actin forward primer, CCTTCTTGGGTATGGAATCCTG; and β-actin reverse primer, GGCATAGAGGTCTTTACGGATG.
RT-PCR.
Reverse transcription-PCR (RT-PCR) for the presence of CXCL1 transcripts was done according to the manufacturer's protocol (Promega, Madison, WI). The amplified products were resolved on 1% agarose gel. The primers for RT-PCR were as follows: β-actin forward primer, CCTTCTTGGGTATGGAATCCTG; β-actin reverse primer, GGCATAGAGGTCTTTACGGATG; CXCL1 forward primer, GGGATTCACCTCAAGAACATCC; and CXCL1 reverse primer, TCTGAACCAAGGGAGCTTCA.
In vitro and in vivo virus (HSV-1)-drug interaction assay.
The effect of the Src kinase inhibitor TG100572 on viral replication (if any) was confirmed by incubating cells with inhibitor either before (pretreatment) or after (posttreatment) inoculation with the virus and then quantifying the infectious HSV Kos yield by the viral plaque assay. Vero cells were infected at a multiplicity of infection (MOI) of 1 and were incubated with 100 nM (maximum below-cytotoxicity levels) TG100572 or 0.1% DMSO either before (−1 h) or after infection. The effect of the drug (TG100572) on production of HSV-1 was determined by titrating the virus in cell culture supernatant at different time points following infection. For the in vivo assay, the virus (HSV RE) in eye swabs was quantified following HSV-1 infection in TG100801-treated and control mice and titrated by a viral plaque assay.
Statistical analysis.
Most of the analyses for determining the level of significance were performed using either-one way analysis of variance (ANOVA) (Dunnett's post hoc test) or two-way ANOVA (Bonferroni test). P values of ≤0.001, ≤0.01, and ≤0.05 were considered significant. Results are expressed as means ± standard deviations (SD).
RESULTS
Topical application of the prodrug TG100801 inhibits angiogenesis and SK.
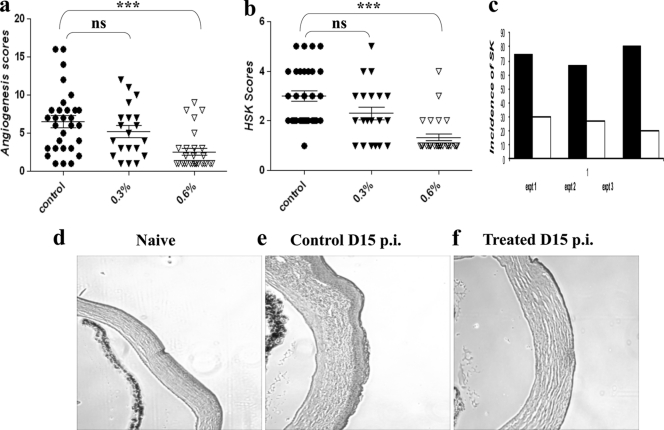
The prodrug was shown by others to convert to an active inhibitor of the Src kinase involved in VEGF signaling following topical application to the eye (5, 24). With the use of the same conditions shown by others to inhibit retinal angiogenesis and vessel permeability, the effects on responses to ocular infection with HSV were evaluated. C57BL/6 animals were infected with 104 PFU of HSV-1 and divided into 3 groups. One group served as the infected and untreated control, and the other groups were treated with TG100801 at either 0.3% or 0.6%, topically. Drug treatment was administered twice daily, with therapy begun 1 day after infection and continued until the experiments were terminated on day 14 p.i. Three separate experiments were performed, and cumulated results are shown in Fig. 1a and b. As is evident, there were significant inhibitions of both angiogenesis and SK lesion severity with the increased dose of the drug investigated compared to the level for the controls. The incidence of SK as recorded in three independent experiments was lower in the TG100801-treated group (Fig. 1c). Histological sections taken from sample animals from test and control groups revealed evident differences in ocular inflammatory responses (Fig. 1d, e, and f).
Fig. 1.
Effect of topical administration of the Src kinase inhibitor (TG100801) on the severity of SK. C57BL/6 animals infected with 104 PFU of HSV-1 were treated with TG100801 (0.3% and 0.6%, topically). TG100801 was applied topically starting at day 1 and continuing until day 14 postinfection (p.i.). (a, b) Cumulative data for angiogenesis (a) and HSK (b) scores at day 12 p.i. as measured by slit lamp biomicroscopy in three independent experiments. The levels of significance were determined by one-way ANOVA using Dunnett's post hoc setting. ***, P ≤ 0.001. (c) The bar diagram demonstrates the percent severity for each group of mice infected with 104 PFU of HSV-1 RE at day 15 p.i. in three independent experiments. The SK scores of 3 and >3 were counted as representing SK incidence (black bars represent the infected control; white bars represent TG100801-treated animals). (d to f) H&E staining of the corneal sections of naïve (d), control (e), and TG100801-treated (f) animals.
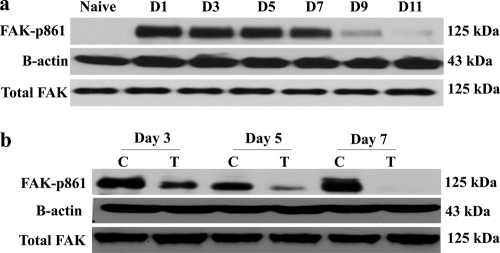
In another experiment, mice were infected with HSV-1 and divided into two groups. One group was treated with TG100801 (0.6%), a concentration that resulted in significant inhibition of both angiogenesis and SK lesion severity, and the nontreated groups receiving liposomal vehicle served as an infected control. The corneas from these mice were collected at the indicated time points postinfection and subjected to Western blotting for detection of FAK phosphorylation, an event known to be the biomarker of Src kinase activity (6, 7, 29). Whereas phosphorylated FAK was undetectable in naïve corneal lysates, it could be demonstrated in infected corneas as early as day 1 postinfection (Fig. 2a). However, there were significant inhibitions in FAK phosphorylation in TG100801-treated corneal lysates at the time points tested within the periods of elevated FAK-p861 (Fig. 2b), suggesting that an important step in VEGF signaling, i.e., Src kinase activation and FAK phosphorylation were efficiently blocked by the inhibitor (TG100801).
Fig. 2.
TG100801 inhibits FAK-y861 phosphorylation in the murine cornea. (a) Western blot analysis for phosphorylated FAK861 in corneal cell lysate at different time points following HSV-1 infection. (b) Inhibition of FAK phosphorylation. Mice were infected with 104 PFU of HSV-1 RE. Twenty four hours following infection, one group was treated topically (cornea) with a 5-μl drop of 0.6% TG100801, twice daily, until day 14 p.i. The second group received the vehicle control in the same way. Corneal cell lysate was used to detect the level of phosphorylated FAKY861 by anti-rabbit FAK antibody in the Western blot. Drug-treated mice showed inhibition of FAK phosphorylation at all time points tested within the periods of elevated phosphorylated FAK. N, naïve; C, control; T, treated.
TG100572 controls the lesion severity independent of viral replication.
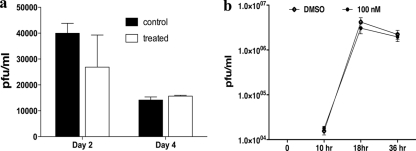
Ocular swabs from control and TG100801-treated animals from the above-mentioned experiments were taken in the early time periods to determine if the drug was inhibitory to virus replication. No significant differences were observed in the titers of virus recovered from the corneas in different groups (Fig. 3a). To measure whether the Src kinase inhibitors had any antiviral activity, we examined the effect of TG100572 on HSV infection in vitro. Vero cells were infected with HSV Kos at an MOI of 1 and treated with maximum below-cytotoxic levels (100 nM) of TG100572 or 0.1% DMSO as a control. Quantitation of viral titers in culture supernatants by a plaque assay revealed no significant effect on viral titers compared to the level for the DMSO-treated controls (Fig. 3b). Accordingly, we conclude that TG100572 does not have antiviral activity.
Fig. 3.
TG100572 controls SK lesion severity independent of viral replication. (a) Viral titers in corneal swabs in control and TG100801-treated mice at days 2 and 4 p.i. are shown. (b) Vero cells were infected at an MOI of 1 and incubated with 100 nM TG100572 (maximum below-cytotoxicity level) after infection or with 0.1% DMSO. The effect of the drug (TG100572) on production of HSV-1 was determined by titrating the virus in cell culture supernatant at different time points following infection.
Effect of active Src kinase inhibitor given systemically on ocular HSV infection.
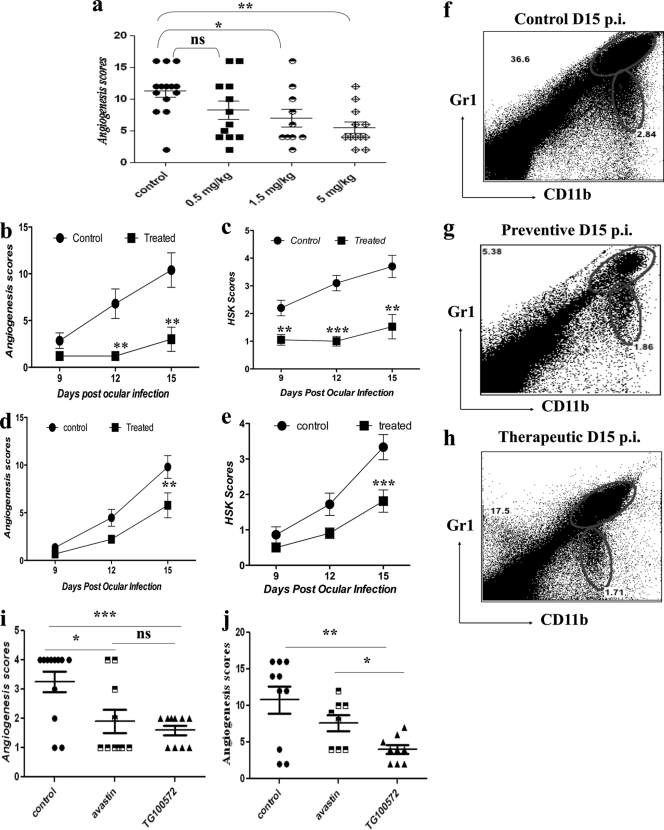
Repeatedly administering drugs topically to the infected mouse eye can be problematic and requires a general anesthetic to facilitate the procedure. In consequence, experiments for measuring the effects of drug treatment on cellular and molecular events at different time points after treatment were also done with infected animals given the active drug intraperitoneally. Animals were ocularly infected with HSV, divided into different groups, and treated with TG100572 (0.5, 1.5, or 5 mg/kg of body weight) once daily starting at 24 h after infection. The pattern of angiogenic response, compared to what was observed for sham-treated controls, was recorded at day 15 p.i. Potent effects were obtained with a dose of 5 mg/kg of body weight (Fig. 4a), with minimal effects on their general health. As shown in Fig. 4b and c, treatment that started at day 1 p.i. (preventive) resulted in major reductions in the extents of angiogenesis (which was >5-fold at day 12 postinfection) and SK (3-fold at day 12 p.i.), along with a 6-fold reduction in CD11b+ Gr1+ cells (at day 15 p.i.) (Fig. 4g), compared to the levels for the infected and untreated control (Fig. 4f). Additionally, commencing treatment at day 6 p.i. (Fig. 4d and e) also resulted in significant inhibition of angiogenesis, lesion scores, and CD11b+ Gr1+ infiltration (Fig. 4h) measured at the termination of the experiments at day 15 p.i. The visible angiogenesis and SK lesion severities were also significantly less in the treated mice (Fig. 5a and b).
Fig. 4.
Effect of systemic administration of Src kinase inhibitor (TG100572) on angiogenesis and SK lesion severity. (a) C57BL/6 animals infected with 104 PFU of HSV were treated with different concentrations of TG100572 (0.5, 1.5, and 5 mg/kg of body weight) intraperitoneally, daily, starting from either 24 h postinfection (preventive) or day 6 p.i. (therapeutic manipulation) and continuing until day 14. The comparative angiogenesis scores obtained at day 14 for HSV-infected animals treated with the indicated concentrations of TG100572 are shown. The statistical significance was determined using one-way ANOVA. (b to e) Kinetics of angiogenesis and lesion expression in control and TG100572-treated animals at 9 to 15 days postinfection (dpi) is shown. Disease progression in control and TG100572-treated mice following infection with 104 PFU of HSV-1 in C57BL/6 animals under preventive (b and c) and therapeutic (d and e) modes of treatment. The level of significance was calculated by two-way ANOVA. (i and j) Comparative angiogenesis scores for bevacizumab and TG100572-treated mice compared to the levels for infected but untreated controls at day 9 p.i. (i) and day 14 p.i. (j). The statistical significance was determined by Student's t test. (f to h) Infiltration of CD11b+ Gr1+ polymorph nuclear neutrophils in the corneas of the control group (f) and following preventive (g) and therapeutic (h) treatment at day 15 p.i. All experiments were repeated at least three times. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
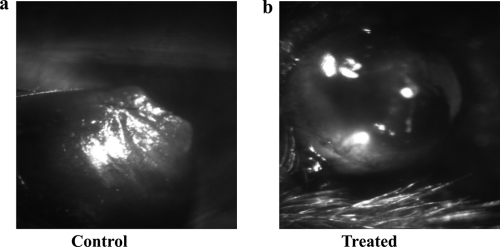
Fig. 5.
Representative eye photograph (at day 15 p.i.) of control (a) and TG100572-treated (b) mice.
To compare our novel approach (TG100572) with that involving a potential positive control, bevacizumab (a monoclonal antibody that binds to VEGF with high specificity, thereby blocking VEGF-mediated signaling pathways and thus angiogenesis) (22), experiments where mice were divided into three groups (n = 6/group) were done. One group received bevacizumab (avastin) i.p. (5 mg/kg of body weight) at days 3, 6, 9, and 12 p.i., and the other group was treated with TG100572 i.p. (5 mg/kg of body weight) starting at day 3 p.i. and continuing until day 13 p.i. The third group served as an infected and untreated control. As shown (Fig. 4i) at day 9 p.i., bevacizumab (avastin) resulted in significant inhibition of angiogenesis and SK severity (P < 0.0201), but this inhibition was less than that observed in a group of animals that received TG100572 systemically. TG100572 treatment showed a highly significant inhibition of angiogenesis. At day 14 p.i. (Fig. 4j), there were around 1.6-fold and 2.9-fold reductions in angiogenesis following bevacizumab (avastin) and TG100572 treatment, respectively, compared to the levels for the control groups.
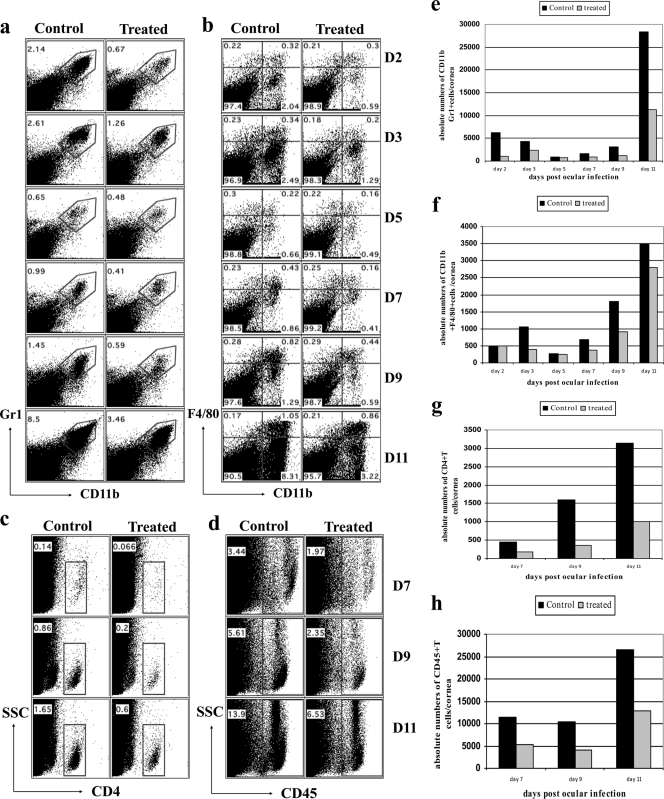
To evaluate whether the administration of the Src kinase inhibitor could influence the expression of SK, animals were treated with TG100572 either from day 1 p.i. to day 14 p.i. or from day 6 to day 14 p.i., and the extents of inflammatory ocular reactions in the treated and control animals were compared by sacrificing animals and recovering ocular cells from corneas following collagenase digestion. As is evident, there were reduced infiltrations of Gr1+ CD11b+ cells (neutrophils) in treated mice at all indicated time points postinfection (Fig. 6a). The neutrophil infiltration peaked at day 2 and day 11 postinfection in both the control and the treated mice; however, the control mice had 3-fold- and 2.5-fold-higher frequencies (Fig. 6a) and absolute numbers (Fig. 6e) of neutrophil infiltrates at day 2 and day 11 p.i., respectively, than the treated group.
Fig. 6.
Kinetics of cellular infiltration in the corneas of control and TG100572-treated mice. Effects of TG100572 treatment on kinetics of cellular infiltration in corneas of HSV-infected animals analyzed by flow cytometry. C57BL/6 mice infected with 104 PFU of HSV were either treated with TG100572 (5 mg/kg of body weight) i.p. daily starting from 24 h p.i. and continuing until day 14 p.i. or untreated controls. A single-cell suspension of the infected corneas was prepared from 6 pooled corneas (n = 3) at the indicated time points from each group of mice (TG100572 treated or control). The cells were labeled for Gr1+ CD11b+ (polymorph nuclear cells) (a, e), CD11b+ F4/80+ (macrophage) (b, f), CD4+ (c, g), and CD45+ (leukocyte common antigen; pan-leukocyte marker) (d, h). The numbers on the dot plots indicate the percentages of the cells expressing the particular markers in control and kinase inhibitor-treated mice at the indicated time points p.i. The experiment was repeated three times, and data are representative of a single experiment.
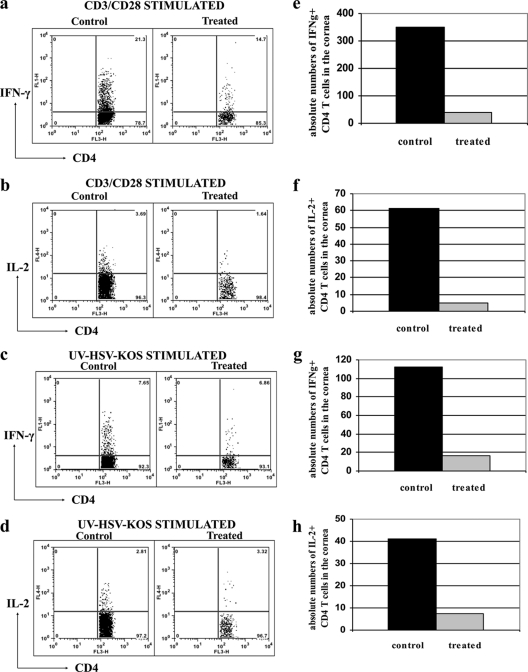
A significant reduction in the infiltration of CD11b+ F4/80+ cells (macrophages) was also observed following Src kinase inhibition when evaluated at the later time points postinfection (in the clinical phase). There were both reduced frequencies (Fig. 6b) and reduced total numbers (Fig. 6f) of F4/80 cells in treated animals compared to the levels for the infected and untreated controls. Strikingly, an approximately 4-fold decrease in the CD4+ T cell infiltration was evident in the Src kinase inhibitor-treated mice in terms of both percentages (Fig. 6c) and absolute numbers (Fig. 6g) at the clinical phase of the disease. Additionally, the frequencies and numbers of IFN-γ+ CD4+ T cells were reduced 7- to 8-fold as a consequence of TG100572 treatment along with highly significant reductions in IFN-γ- and IL-2-producing CD4+ T cells upon stimulation with anti-CD3 anti-CD28 (Fig. 7a, b, e, and f) or UV-inactivated HSV Kos (Fig. 7c, d, g, and h). A corneal single-cell suspension stained for CD45 (a pan-leukocyte marker) revealed significantly reduced frequencies (Fig. 6d) and absolute numbers (Fig. 6h) of CD45+ T cells (almost 2-fold) in the corneas of the treated mice at all indicated time points analyzed postinfection. Taken together, our data indicate that both topical and systemic administrations of small-molecule inhibitors of Src kinases result in significant reduction in both the extent of neovascularization and the severity of SK.
Fig. 7.
TG100572 treatment diminishes the infiltration of pathogenic Th1 cells in the cornea. C57BL/6 mice infected with 104 PFU of HSV-1 were treated with TG100572 (5 mg/kg of body weight) i.p. daily, staring from 24 h p.i. and continuing until the termination of the experiments. A single-cell suspension of the infected corneas was prepared from 6 pooled corneas at day 15 p.i. from each group of mice (control and treated). The cells were stimulated with either anti-CD3 anti-CD28 or UV-inactivated HSV Kos and stained for CD4+ T cells producing IFN-γ and IL-2. Frequencies (a and b) and absolute numbers (per cornea) (e and f) of IFN-γ+ and IL-2+ T cells, respectively, in the control and treated groups were observed following stimulation by anti-CD3 anti-CD28. The frequencies (c and d) and absolute numbers (g and h) of IFN-γ+ and IL-2+ T cells, respectively, in the control and treated groups following stimulation by UV-inactivated HSV Kos are shown. The experiment was repeated three times, and data are representative of a single experiment.
Inhibition of Src activity blocks CXCL1 and proinflammatory cytokines.
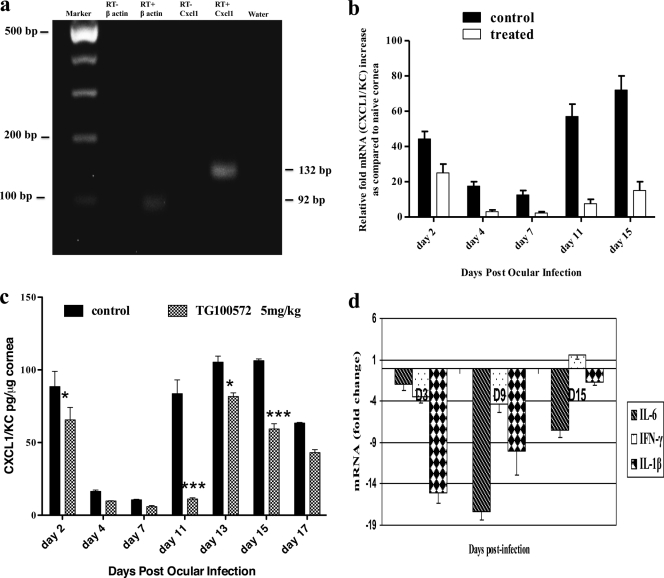
Our observation that Src kinase inhibitors caused a reduction in neutrophil infiltration could mean that the drug inhibited the expression of molecules involved in neutrophil recruitment, such as the chemokine CXCL1. In support of this, CXCL1 mRNA was present at higher levels in the infected eye than in the scarified controls. Figure 8a depicts the expression of CXCL1 in HSV-1-infected corneas as early as day 1 p.i. For the quantification of CXCL1 gene expression, mice were ocularly infected with HSV and 6 corneas from each group (control and treated) were collected, pooled, and analyzed by qPCR at the indicated time points. Corneas from Src kinase-treated mice subjected to qPCR revealed decreases in CXCL1 levels at all time points tested, with the maximum reduction observed during the clinical phase of the disease (Fig. 8b). In addition, the same situation was evident with the protein levels measured by ELISA. The corneal supernatants were assayed for CXCL1/IL-8 by ELISA as indicated in Materials and Methods. Figure 8c depicts the kinetics of CXCL1 protein expression levels in the control and Src kinase inhibitor-treated mice throughout the course of ocular infection. There was a significant reduction in the CXCL1 at day 2 and also during the clinical phase (around 8-fold reduction at day 11 p.i.) of the disease in the treated mice. Thus, our data could mean that Src kinases may regulate critical “downstream” signaling pathways that might contribute to expression of CXCL1, a proangiogenic and proinflammatory chemokine in the murine cornea. Additionally, treated mice represented diminished levels of proinflammatory cytokines, notably IL-6, IFN-γ, and IL-1β, compared to the levels for the infected and untreated controls (Fig. 8d).
Fig. 8.
TG100572 treatment results in the blockade of CXCL1 in the cornea. (a) Agarose gel analysis for CXCL1 (132 bp) (4th lane) transcripts from infected corneas is shown. The initial lane is a marker; the 2nd lane represents β-actin (92 bp). The 1st and 3rd lanes represent the RT-negative control for β-actin and CXCL1, respectively. The 5th lane represents a negative control (water). (b) Kinetic analysis for the expression of CXCL1 mRNA by qPCR at different time points p.i. after Src kinase inhibitor or mock treatment is shown. Wild-type (WT) mice were infected with HSV and mock treated or treated with TG100572. Six corneas were harvested from the respective groups at the indicated time points, pooled, and subjected to quantification by qPCR for CXCL1 mRNA. (c) Quantification of CXCL1 protein in HSV-1-infected corneas by ELISA after mock or TG100572 treatment is shown. At each time point, 6 corneas were harvested from HSV-infected mice mock treated or treated with TG100572, and levels of CXCL1 protein were determined by ELISA. The level of significance was determined using two-way ANOVA with the Bonferroni posttest. *, P ≤ 0.05; ***, P ≤ 0.001. (d) Reduction in IL-6, IFN-γ, and IL-1β after TG100572 treatment is shown. The mice infected with HSV were mock treated or treated with TG100572, and corneas collected from the respective groups at the indicated time points were subjected to qPCR for IL-6, IFN-γ, and IL-1β mRNAs. The values are represented as fold changes mRNA compared to the level for the infected control. The above-described experiments were repeated three times.
TG100572 may downregulate CD49d on CD4+ T cells in lymphoid organs, resulting in fewer cells to migrate to the ocular site.
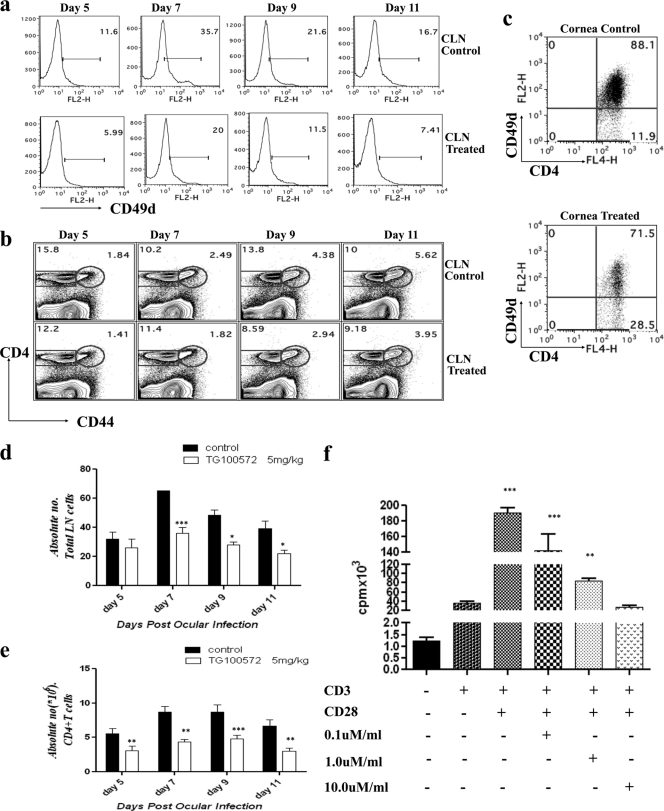
SK is well known to be orchestrated by CD4+ T cells (27). The frequencies and absolute numbers of CD4+ T cells recovered by collagenase digestion of corneas were diminished in TG100572-treated animals at all time points tested after ocular HSV infection. To address the cause of reduced CD4+ T cells in the treated mice, animals that were ocularly infected and begun TG100572 treatment i.p. at day 1 p.i. and continued daily were sacrificed and the phenotype of CD4+ T cells isolated from the DLN as well as from corneal lesions after collagenase digestion was evaluated at indicated time points (days 5, 7, 9, and 11 following HSV-1 ocular infection). In these experiments, lesion severity was greater in the control animals than in the drug-treated animals. Fluorescence-activated cell-sorting (FACS) analysis revealed that whereas there was no difference in the expression of CD62L (data not shown) and a modest difference in the expression of CD44 (Fig. 9b), interestingly, CD49d on CD4+ T cells in CLN (Fig. 9a) and corneas (Fig. 9c) was significantly downregulated at all time points in the Src kinase inhibitor recipients. This observed downregulation of CD49d on CD4+ T cells could be relevant since previous studies indicate that blocking CD49d reduces SK (27). Taken together, we interpret these observations to mean that Src kinases are involved in controlling the expression of integrin molecules such as CD49d that are involved in migration of inflammatory cells to the ocular lesion site (11), which could explain the overall impaired infiltration of CD4+ T cells in the cornea.
Fig. 9.
Src kinase inhibition may result in the attenuation of T cell function. (a) Kinetic analysis of CD49d expression on the CD4+ T cells in the control and treated animals after ocular HSV-1 infection. C57BL/6 mice were infected with 104 PFU of HSV. Mice (n = 3) were sacrificed at each indicated time point, and their draining cervical lymph nodes and corneas were analyzed for surface expression of CD4 and CD49d by flow cytometry. (a) Histograms representing the percentages of CD49d expression on CD4+ T cells in draining cervical lymph nodes at the indicated time points postinfection. Data shown are from one representative experiment. (b) Reduced expression of CD44 on CD4+ T cells in draining CLN of TG100572-treated mice. (c) FACS plots showing the percentages of CD4+ CD49d+ T cells in the corneas in control and TG100572-treated animals at day 11 postinfection. All kinetic experiments were repeated at least twice. (d, e) Absolute numbers (106) of the total lymph node cells (d) and CD4+ T cells (e) in control and treated animals are shown for the indicated time points p.i. The level of significance was determined using two-way ANOVA with the Bonferroni posttest. (f) DLN cells were enriched for CD4+ T cells and stimulated with anti-CD3 anti-CD28 in the presence or absence of drug at the indicated concentrations. Cell proliferation results are expressed as mean cpm from triplicate cultures. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
TG100572 inhibits CD4+ T cell proliferation in vitro.
Src knockout mice have shown marked reduction in inflammatory responses to a variety of physiological insults (11). Measurement of the absolute numbers of draining cervical lymph node (CLN) cells revealed diminished numbers of total lymph node cells (Fig. 9d) and also CD4+ T cells (Fig. 9e) in the kinase inhibitor (TG100572 i.p.) recipient mice at all the time points tested. This likely means that in addition to effects on VEGF, TG100572 may also interfere directly with CD4+ T cell activation and proliferation. To explore this possibility, draining CLN cells from HSV-infected mice at day 15 p.i. were enriched for CD4+ T cells and stimulated with anti-CD3 anti-CD28 (as described in Materials and Methods). In some wells, different concentrations of TG100572 (noncytotoxic concentrations) were added, and the effects on proliferation responses were recorded. Whereas a minimal proliferation was observed with anti-CD3 alone, anti-CD3 anti-CD28 resulted in a significant CD4+ T cell proliferation. TG100572 inhibited CD4+ T cell proliferation in a dose-dependent manner (Fig. 9f), suggesting that by diminishing immune activation and CD4+ T cell proliferation, TG100572 may additionally serve to limit the sizes of immunopathogenic CD4+ T cells involved in lesion expression.
DISCUSSION
Neovascularization of the otherwise avascular cornea represents a pathological hallmark of ocular HSV-1 infection. Present approaches for clinical management of corneal neovascularization rely on antivirals, corticosteroids, or anti-VEGF antibody treatment. In this study, we show that a small-molecule inhibitor of Src kinases results in suppression of angiogenesis and lesion severity in a murine model of SK. Administered topically as a prodrug (TG100801) or in the active form (TG100572) systemically, this inhibitor resulted in the inhibition of several key events in the pathogenesis of SK. These included diminished cellular infiltration of CD4+ T cells and neutrophils, the cells primarily involved in SK, in the corneas. There were also reductions in levels of the chemokine CXCL1 and proinflammatory cytokines such as IFN-γ, IL-1β, and IL-6. Importantly, treatment resulted in inhibited FAK phosphorylation in the corneal tissues, an essential step in VEGF-mediated angiogenesis (1). Additionally, TG100572 administered systemically resulted in downregulation of CD49d on CD4+ T cells in the DLN and cornea. However, the Src kinase inhibitor had no demonstrable proinflammatory effect and failed to express antiviral activity.
The current antiangiogenic approach shows effective control of newly proliferating vascular endothelial cells, and since the phosphorylation of FAK and Src activation appears to be a very early event postinfection, Src blockade by the inhibitors early during the course of infection could be advantageous for significant and complete antiangiogenic effects. Additionally, achieving efficacious drug concentration in the corneal tissues following the topical delivery route (TG100801) is generally considered a technical challenge. However, recent clinical trials with these drugs have shown that TG100801, while lacking antikinase activity of its own, quickly generated active TG100572 within the eye upon topical delivery; however, neither compound was detectable in plasma, indicating that delivery to the eye occurs by local penetration and not systemic absorption (5).
Systemic treatment with the Src kinase inhibitors also significantly reduced angiogenesis and cellular infiltration, particularly of neutrophils, one plausible explanation being that by inhibition of FAK phosphorylation, Src kinase inhibitors preserved the junctional integrity of the endothelial cells (14) and thus inhibited the paracellular transport of neutrophils. However, Src kinases were also shown to modulate the expression of the proangiogenic neutrophil attracting chemokine CXCL1/mouse KC/homologue of IL-8 (28) and that Src kinase activation correlates with the amount of IL-8 produced (14, 17, 28). Consistent with this, Src kinase inhibitors resulted in inhibition of CXCL1 expression. Thus, it is possible that significant reduction in the neutrophils in the treated group may be a consequence of reduced chemokine levels, which is the prominent regulator of neutrophil infiltration into the inflamed cornea (23).
In humans, SK may lead to permanent loss of vision, and the current treatment modalities that are used for the clinical management of SK include anti-VEGF antibody treatment, such as that using bevacizumab (4, 23), antivirals, and corticosteroids, but none of them are considered ideal. Antiviral compounds that block virus entry or reduce viral replication can be prophylactic and may not be efficacious against SK. As a result, small-molecule inhibitors targeting cellular responses that contribute to disease may have a substantial advantage over antiviral approaches. Corticosteroid therapy, on the other hand, when continued for a longer duration, might exhibit several side effects (15), and although anti-VEGF antibody (bevacizumab) was shown to reduce VEGF-induced neovascularization (22, 23), abnormal vascular permeability is frequently associated with neovascularization (24). Thus, an antivascular permeability compound that is also antiangiogenic, such as Src kinase inhibitor, should have added therapeutic benefit. Endothelial cell barrier functions are disrupted by a number of viruses, and a very recent study suggests that VEGFR2 and SFK Src family kinase inhibitors may be of therapeutic utility in stabilizing vasculature during viral infections (9). Additionally, both VEGF and FGF have been shown to be potent proangiogenic factors in HSV-1-induced corneal neovascularization. The binding of angiogenic growth factors (VEGF and basic fibroblast growth factor [bFGF]) to their receptors is known to result in activation of nonreceptor tyrosine kinases (Src kinases), which in turn regulates endothelial cell proliferation, migration, and survival (by inhibiting apoptosis of endothelial cells) (6). A compound that inhibits redundant pathways of angiogenesis has the potential of being therapeutically advantageous (24). In support of this, our results clearly indicate that bevacizumab (avastin), a VEGF antagonist, inhibits HSV-1-induced corneal neovascularization, but the levels of inhibition achieved were less than that caused by TG100572. However, we do not preclude the possibility that the observed lesser reduction in angiogenesis in the avastin-treated mice could be due to a weaker binding affinity of avastin to mouse VEGF-A (4). Our findings therefore rationalize testing of these kinase inhibitors for additional indications and clinical application in reducing HSV-1-induced immunopathology. Because VEGFR2 and SFK inhibitors are already FDA approved for use in humans (9), they could be immediately rationalized for use in clinical cases of HSK.
A significant antiangiogenic and anti-inflammatory effect was also observed following systemic treatment with TG100572. This mode of treatment also produced additional effects such as CD49d expression (on CD4+ T cells) and attenuation of T cell function. It may be argued that these effects might be due to the limited specificity of the Src kinase inhibitors (20). However, the drug was well tolerated systemically, with no adverse effects on general health except for some reduction in the body weights of the animals. Our observations that systemically administered TG100572 influences the severity of SK lesions is supported by the observation of significantly less CD4+ T cell infiltration in the cornea in the clinical phase, suggesting that TG100572 (Src kinase inhibitor, i.p.) could control the lesion development by limiting the migration of pathogenic T cells to the extralymphoid inflammatory site. The SK model represents a situation where normally immunoprotective CD4+ T cells exert an immunopathological function in the cornea of the eye (18). The integrin CD49d (VLA-4) is known to be involved in the migration of pathogenic CD4+ T cells to the ocular site, and in SK, 70 to 80% of corneal T cells in the disease express CD49d (VLA-4) early in lesion development (27). Thus, it was intriguing to observe that the CD49d integrin was expressed on a significantly lower percentage of lymphoid CD4+ T cells in the Src kinase inhibitor-treated mice than in the controls. The downregulation of CD49d in Src kinase inhibitor-treated mice was not unexpected, since there is mounting evidence that α4 integrins use the Src family kinases to transduce intracellular signals (21). Our observations could shed light on the relative merits of various cellular targets as candidates for therapeutic intervention during an ongoing immune-inflammatory reaction such as SK. Src kinase inhibition also resulted in a less activated phenotype of CD4+ T cells, as shown by decreased CD44 expression on cells in the draining CLN. Additionally, Src kinase inhibitors resulted in diminished levels of inflammatory cytokines, which is in accordance with findings that the reduction in proinflammatory cytokines resulted in impairment of Th1 differentiation (12).
In conclusion, we demonstrated that the blockade of Src kinase activation (which is an essential step in VEGF signaling) with VEGFR2/Src kinase inhibitor resulted in reduced SK lesion severity and diminished cellular infiltration, probably by inhibition of vascular leak and removal of a corneal chemokine gradient. In the present approach, which is novel for infectious ocular angiogenesis, we did achieve highly significant but not complete antiangiogenesis. We advocate that combining the Src kinase inhibitor with additional therapeutic approaches could be valuable for use in the clinic for management of herpetic ocular lesions.
ACKNOWLEDGMENTS
We sincerely acknowledge Sanofi-Aventis/Targegen for providing us with reagents for our experiments. We thank Naveen Rajasagi, Pradeep B. J. Reddy, Tamara Veiga Parga, and Gregory T. Spencer for invaluable discussions during the research and assistance in many ways during manuscript editing.
This study was supported by National Eye Institute grant EY 005093.
Footnotes
Published ahead of print on 6 April 2011.
REFERENCES
- 1. Abu-Ghazaleh R., Kabir J., Jia H., Lobo M., Zachary I. 2001. Src mediates stimulation by vascular endothelial growth factor of the phosphorylation of focal adhesion kinase at tyrosine 861, and migration and anti-apoptosis in endothelial cells. Biochem. J. 360:255–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ambati B. K., et al. 2006. Corneal avascularity is due to soluble VEGF receptor-1. Nature 443:993–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biswas P. S., Rouse B. T. 2005. Early events in HSV keratitis—setting the stage for a blinding disease. Microbes Infect. 7:799–810 [DOI] [PubMed] [Google Scholar]
- 4. Bock F., et al. 2007. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest. Ophthalmol. Vis. Sci. 48:2545–2552 [DOI] [PubMed] [Google Scholar]
- 5. Doukas J., et al. 2008. Topical administration of a multi-targeted kinase inhibitor suppresses choroidal neovascularization and retinal edema. J. Cell. Physiol. 216:29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eliceiri B. P., et al. 1999. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol. Cell 4:915–924 [DOI] [PubMed] [Google Scholar]
- 7. Eliceiri B. P., et al. 2002. Src-mediated coupling of focal adhesion kinase to integrin alpha v beta 5 in vascular endothelial growth factor signaling. J. Cell Biol. 157:149–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gong Y., Koh D. R. 2010. Neutrophils promote inflammatory angiogenesis via release of preformed VEGF in an in vivo corneal model. Cell Tissue Res. 339:437–448 [DOI] [PubMed] [Google Scholar]
- 9. Gorbunova E. E., Gavrilovskaya I., Pepini T., Mackow E. R. 2011. VEGFR2 and Src kinase inhibitors suppress ANDV induced endothelial cell permeability. J. Virol. 85:2296–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim B., et al. 2004. Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes—therapeutic strategy for herpetic stromal keratitis. Am. J. Pathol. 165:2177–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim M. P., Park S. I., Kopetz S., Gallick G. E. 2009. Src family kinases as mediators of endothelial permeability: effects on inflammation and metastasis. Cell Tissue Res. 335:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuka M., et al. 2010. Src Kinases are required for a balanced production of IL-12/IL-23 in human dendritic cells activated by Toll-like receptor agonists. PLoS One 5(7):e11491 doi:10.1371/journal.pone.0011491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumaraguru U., Rouse B. T. 2000. Application of the intracellular gamma interferon assay to recalculate the potency of CD8 (+) T-cell responses to herpes simplex virus. J. Virol. 74:5709–5711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kvietys P. R., Sandig M. 2001. Neutrophil diapedesis: paracellular or transcellular? News Physiol. Sci. 16:15–19 [DOI] [PubMed] [Google Scholar]
- 15. McGhee C. N. J., Dean S., Danesh-Meyer H. 2002. Locally administered ocular corticosteroids—benefits and risks. Drug Safety 25:33–55 [DOI] [PubMed] [Google Scholar]
- 16. Nagy J. A., Dvorak A. M., Dvorak H. F. 2007. VEGF-A and the induction of pathological angiogenesis. Annu. Rev. Pathol. 2:251–275 [DOI] [PubMed] [Google Scholar]
- 17. Natarajan K., Rajala M. S., Chodosh J. 2003. Corneal IL-8 expression following adenovirus infection is mediated by c-Src activation in human corneal fibroblasts. J. Immunol. 170:6234–6243 [DOI] [PubMed] [Google Scholar]
- 18. Newell C. K., Martin S., Sendele D., Mercadal C. M., Rouse B. T. 1989. Herpes-simplex virus induced stromal keratitis-Role of lymphocyte-T subsets in immunopathology. J. Virol. 63:769–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Noronha G., et al. 2007. Discovery of 7-(2,6-dichlorophenyl)-5-methylbenzo 1,2,4 triazin-3-yl-4-(2-pyrrolidin-1-ylethoxy)phenyl amine—a potent, orally active Src kinase inhibitor with anti-tumor activity in preclinical assays. Bioorg. Med. Chem. Lett. 17:602–608 [DOI] [PubMed] [Google Scholar]
- 20. Okutani D., Lodyga M., Han B., Liu M. Y. 2006. Src protein tyrosine kinase family and acute inflammatory responses. Am. J. Physiol. Lung Cell. Mol. Physiol. 291:L129–L141 [DOI] [PubMed] [Google Scholar]
- 21. Pereira S., Zhon M., Mocsai A., Lowell C. 2001. Resting murine neutrophils express functional alpha(4) integrins that signal through Src family kinases. J. Immunol. 166:4115–4123 [DOI] [PubMed] [Google Scholar]
- 22. Perez-Santonja J. J., Campos-Mollo E., Lledo-Riquelme M., Javaloy J., Alio J. L. 2010. Inhibition of corneal neovascularization by topical bevacizumab (anti-VEGF) and sunitinib (anti-VEGF and anti-PDGF) in an animal model. Am. J. Ophthalmol. 150:519–528 [DOI] [PubMed] [Google Scholar]
- 23. Saravia M., Zapata G., Ferraiolo P., Racca L., Berra A. 2009. Anti-VEGF monoclonal antibody-induced regression of corneal neovascularization and inflammation in a rabbit model of herpetic stromal keratitis. Graefes Arch. Clin. Exp. Ophthalmol. 247:1409–1416 [DOI] [PubMed] [Google Scholar]
- 24. Scheppke L., et al. 2008. Retinal vascular permeability suppression by topical application of a novel VEGFR2/Src kinase inhibitor in mice and rabbits. J. Clin. Invest. 118:2337–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Streilein J. W., Dana M. R., Ksander B. R. 1997. Immunity causing blindness: five different paths to herpes stromal keratitis. Immunol. Today 18:443–449 [DOI] [PubMed] [Google Scholar]
- 26. Suryawanshi A., et al. 2011. Ocular neovascularization caused by HSV-1 infection results from breakdown of binding between VEGF-A and its soluble receptor. J. Immunol. 186:3653–3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Suvas S., Azkur A. K., Kim B. S., Kumaraguru U., Rouse B. T. 2004. CD4(+) CD25(+) regulatory T cells control the severity of viral immunoinflammatory lesions. J. Immunol. 172:4123–4132 [DOI] [PubMed] [Google Scholar]
- 28. Trevino J. G., et al. 2005. Expression and activity of Src regulate interleukin-8 expression in pancreatic adenocarcinoma cells: implications for anglogenesis. Cancer Res. 65:7214–7222 [DOI] [PubMed] [Google Scholar]
- 29. Weis S. M., Cheresh D. A. 2005. Pathophysiological consequences of VEGF-induced vascular permeability. Nature 437:497–504 [DOI] [PubMed] [Google Scholar]
- 30. Zheng M., Deshpande S., Lee S., Ferrara N., Rouse B. T. 2001. Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis. J. Virol. 75:9828–9835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zheng M., Schwarz M. A., Lee S. J., Kumaraguru U., Rouse B. T. 2001. Control of stromal keratitis by inhibition of neovascularization. Am. J. Pathol. 159:1021–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]