Abstract
Gene transfer into quiescent T and B cells is of importance for gene therapy and immunotherapy approaches to correct hematopoietic disorders. Previously, we generated lentiviral vectors (LVs) pseudotyped with the Edmonston measles virus (MV) hemagglutinin and fusion glycoproteins (Hgps and Fgps) (H/F-LVs), which, for the first time, allowed efficient transduction of quiescent human B and T cells. These target cells express both MV entry receptors used by the vaccinal Edmonston strain, CD46 and signaling lymphocyte activation molecule (SLAM). Interestingly, LVs pseudotyped with an MV Hgp, blind for the CD46 binding site, were completely inefficient for resting-lymphocyte transduction. Similarly, SLAM-blind H mutants that recognize only CD46 as the entry receptor did not allow stable LV transduction of resting T cells. The CD46-tropic LVs accomplished vector-cell binding, fusion, entry, and reverse transcription at levels similar to those achieved by the H/F-LVs, but efficient proviral integration did not occur. Our results indicate that both CD46 and SLAM binding sites need to be present in cis in the Hgp to allow successful stable transduction of quiescent lymphocytes. Moreover, the entry mechanism utilized appears to be crucial: efficient transduction was observed only when CD46 and SLAM were correctly engaged and an entry mechanism that strongly resembles macropinocytosis was triggered. Taken together, our results suggest that although vector entry can occur through the CD46 receptor, SLAM binding and subsequent signaling are also required for efficient LV transduction of quiescent lymphocytes to occur.
INTRODUCTION
Measles virus (MV) belongs to the paramyxoviridae family and is the causative agent of measles disease. It has two envelope glycoproteins (gp's), the hemagglutinin (H) and fusion (F) glycoproteins (Hgp and Fgp, respectively), which mediate receptor binding and fusion, respectively (28, 29). Signaling lymphocyte activation molecule (SLAM) (CD150) is the receptor for both clinical isolates and vaccine strains (49, 55). However, vaccine strains like Edmonston (Edm) have gained, in addition to entry through the SLAM receptor, entry through the CD46 receptor after their adaptation in SLAM-negative cells (25, 54). Moreover, recent findings suggest the existence of a third MV receptor in epithelial cells (54). CD46 is a complement-regulatory molecule expressed on all human nucleated cells (27), whereas SLAM is constitutively expressed at the surfaces of some T and B cell subsets and upregulated upon proliferation of T and B lymphocytes and mature dendritic cells (DCs) (3, 8). The cellular distribution of SLAM determines lymphoid tropism and explains in part the immunosuppressive character of measles virus.
Importantly, even though wild-type and vaccine MV strains have been extensively studied at the levels of virulence (55), immunosuppression, and immune response (4, 21, 36) and the crystal structures of CD46 and SLAM receptor binding to MV hemagglutinin have recently been elucidated (7, 17, 41), there are still few data about the roles of the CD46 and SLAM receptors in the process of MV entry. Furthermore, although MV is thought to enter the cell by pH-independent fusion at the plasma membrane, recent findings with other paramyxoviruses, like Nipah virus, raise the possibility of macropinocytosis as an entry route (35). Moreover, other viruses, such as vaccinia virus (19, 32), HIV (53), and adenovirus 3 (Ad3) (2, 46), exploit this route for entry into target cells (33).
We recently engineered lentiviral vectors (LVs) carrying Edm Hgp and Fgp at their surfaces (H/F-LVs), which conserved the original MV Edm tropism through CD46 and SLAM receptors. These were the first LVs to allow efficient transduction of quiescent human T cells and healthy and cancer B cells without inducing entry into the cell cycle (10, 11, 26). Gene transfer into quiescent T and B cells has great potential for gene therapy and immunotherapy approaches (12). Interestingly, although all human primary lymphocytes express the CD46 receptor, H/F-LVs achieve efficient transduction only if the SLAM receptor is coexpressed on these cells. Indeed, H/F-LV transduction efficiency correlated tightly with SLAM expression levels on primary lymphocytes, as reported by us (10–12). In contrast, SLAM and CD46 coexpression is not a requirement for the transduction of human cell lines. Interestingly, we have shown that cotransduction of H/F-LVs and vesicular stomatitis virus G (VSV-G) LVs to quiescent B or T cells does not trigger or facilitate VSV-G LV entry, strongly suggesting that the two different vector pseudotypes exploit different entry mechanisms in these cells. Thus, the H/F-LVs can surmount restrictions for transduction of resting T cells that VSV-G LVs cannot (10). Since these H/F-LVs are able to transduce completely quiescent human lymphocytes, it was important to elucidate the roles of the CD46 and SLAM receptors in the transduction process of MV-LVs in these quiescent primary cells, because these are valuable tools for gene therapy (12) and they might shed light on vaccinal MV entry into these immune cells. We used vectors pseudotyped with MV glycoproteins carrying mutations in residues of the Hgp which eliminate binding to SLAM or CD46 to study the different steps of lentiviral transduction. Here, we show that both the CD46 and SLAM receptors are indispensable to achieve efficient gene transfer and integration into primary quiescent T lymphocytes. By the use of the clinical strain Hgp in which residues were mutated to gain binding to the CD46 receptor, we determined that a successful entry leading to stable integration occurs only if CD46- and SLAM-binding residues are present in cis in the Hgp. Finally, inhibition of H/F-LV entry into quiescent T cells by several drugs supported the hypothesis that entry into these cells could occur through macropinocytosis.
MATERIALS AND METHODS
Plasmids and vector production.
Edm Hgp is from the Edmonston vaccine strain. Hgps from the clinical strains C2 and D4 (H-C2 and H-D4 [also called Hclin], respectively), were used to generate the SLAM-restricted vectors (Hclin/F-LVs). CD46-blind and SLAM-blind Hgps (Ha481 and Ha533) (Fig. 1B) were generated from Edm Hgp by introduction of the Y481N and R533A mutations, respectively. The H-D4-YG gp was generated from H-D4 by PCR using primers containing the N481Y and E492G mutations. All Hgps and Fgp are inserted into pCG plasmids under the control of the cytomegalovirus early promoter. Cytoplasmic tails of all Hgps and Fgps were deleted by truncation of 24 and 30 amino acids (aa), respectively. The pCMV-G plasmid was described previously (31).
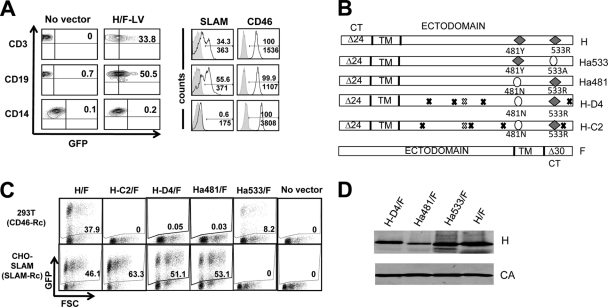
Fig. 1.
Generation of singly SLAM- and CD46-tropic measles virus gp-pseudotyped lentiviral vectors. (A) Freshly isolated unstimulated T cells (CD3+), B cells (CD19+), and monocytes (CD14+) were transduced with H/F-LVs (Edmonston strain) encoding GFP (MOI of 10). Three days posttransduction, the cells were analyzed for GFP expression by FACS (left plots). Before transduction, surface expression of the two measles virus receptors SLAM and CD46 was analyzed by FACS in T cells, B cells, and monocytes. (B) Schematic representation of the different MV hemagglutinin gp's (Hgps) and the fusion gp (Fgp). Edm Hgp contains binding residues for both SLAM and CD46 (H). CD46-tropic mutant Hgps were engineered by mutating one residue of Edm Hgp responsible for fusion to or entry through SLAM (R533A; Ha533). SLAM-tropic Hgps were derived from Edm H by ablation of one residue responsible for binding/fusion through CD46 (Y481A; Ha481) or from clinical strains (H-D4 and H-C2). In order to obtain efficient Hgp and Fgp incorporation into LV cores, 24 aa (Δ24) and 30 aa (Δ30) of the cytoplasmic tails (CT) were deleted from the Hgps and Fgp, respectively. Black and white exes indicate differences in the amino acids of H-D4 and H-C2 from those for Edmonston H. Black exes mark differences in amino acids between H-D4 and H-C2. White exes indicate identical amino acids for H-D4 and H-C2. TM, transmembrane region. (C) 293T cells, expressing the CD46 receptor (CD46-Rc), and CHO-SLAM cells, expressing the SLAM receptor (SLAM-Rc), were transduced with the different MV gp-pseudotyped LVs (H/F-, H-C2/F-, H-D4/F-, Ha481/F-, and Ha533/F-LVs; MOI = 0.3). Three days after transduction, cells were evaluated for GFP expression by FACS. FSC, forward side scatter. (D) Immunoblots of lentiviral particles displaying H-D4, Ha481, Ha533, or Edm H (H) at their surfaces. LVs were purified over a sucrose cushion by ultracentrifugation. The upper part of the membrane was stained with a monoclonal antibody against the ectodomain of H, and the lower part of the membrane was stained with anti-HIV p24 antibody directed against the capsid.
Self-inactivating HIV-1-derived vectors were generated by transient transfection of 293T cells in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) as described previously (11). Briefly, for codisplay of the different Hgps and Fgps, 3 μg of each envelope plasmid was transfected together with a Gag-Pol packaging plasmid and a plasmid encoding a lentiviral vector expressing green fluorescent protein (GFP) (self-inactivating [SIN]-HIVSFFVGFP) or murine red fluorescent protein (mRFP) (SIN-HIVSFFVmRFP). After 18 h of transfection, the medium was replaced by Opti-MEM supplemented with HEPES (Invitrogen). Viral supernatants were harvested 48 h after transfection and filtered. The vectors were concentrated at low speed by overnight centrifugation of the viral supernatants at 3,000 × g at 4°C. Infectious titers (in transduction units [TU]/ml) were determined by fluorescence-activated cell sorting (FACS) of target cells using serial dilutions of the supernatants added to the appropriate target cell (CHO-SLAM or 293T cells for SLAM- or CD46-tropic vectors, respectively).
Transduction protocols.
Adult peripheral blood samples, obtained from healthy donors after informed consent, were collected in acid citrate dextrose. Human peripheral blood T and B lymphocytes and monocytes were isolated by negative selection using Rosette Sep (Stem Cell Technologies) to avoid cell activation. Depending on the experiment, cells were immediately seeded for transduction in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal calf serum (FCS) (HyClone) and penicillin-streptomycin (Invitrogen), or they were prestimulated. B cell prestimulation consisted of the addition of Staphylococcus aureus Cowan (SAC; 0.001%; Calbiochem)–interleukin 2 (IL-2) (1 ng/ml; Sigma-Aldrich) for 24 h. T cells were either preactivated for 3 days with recombinant IL-7 (rIL-7; 10 ng/ml; BD Biosciences) or stimulated through the T cell receptor (TCR) by anti-human CD3 (anti-hCD3; 1 μg/ml; BD Biosciences)–anti-hCD28 (1 μg/ml; BD Biosciences)–IL-2 (1 ng/ml; Preprotech). For transduction, 5E4 to 1E5 cells were seeded in 48-well plates in RPMI 1640–10% FCS medium, and concentrated vector supernatants were added at multiplicities of infection (MOIs) of 5 to 10, as indicated below. The transduction efficiency of vectors (percent GFP+ cells) was assessed by FACS 72 h after transduction. For some experiments, cells were washed 3 times in phosphate-buffered saline (PBS) at day 3 of transduction and kept in culture for up to 12 days, either by the addition of rhIL-2 for T cells or by coculture of B cells with MS5 feeder cells as previously described (10).
Antibodies.
Unconjugated and phycoerythrin (PE)-labeled anti-hCD150 (clone IPO-3) were from eBioscience. Anti-hCD46-PE, anti-hCD3-allophycocyanin (APC), anti-hCD19-APC, and anti-hCD14-APC were purchased from BD Biosciences. Anti-CD46 (clone 13/42) was a kind gift from Jürgen Schneider-Schaulies (Würzburg, Germany).
DNA extraction, Q-PCR, and Alu-PCR.
DNA was extracted using the QIAamp DNA minikit (Qiagen) or with NucleoSpin tissue XS (Macherey-Nagel) for less than 5E4 cells by following the manufacturer's instructions. All primer sequences and PCR conditions for quantitative PCR (Q-PCR) and Alu-PCR (a DNA fingerprinting technique based on the simultaneous analysis of many genomic loci flanked by Alu repetitive elements) were previously described (10). Briefly, total vector LTR sequences were quantified by Sybr green Q-PCR using GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as a housekeeping gene in the StepOne Fast cycler (Applied Biosystems). The number of copies of long terminal repeats (LTRs) per cell was calculated by dividing the absolute number of LTR sequences by the number of copies of the GAPDH gene in each sample duplicate. Vector integration in the cell genome was assessed by Alu-PCR as previously described (10). Briefly, a first PCR step was performed using a forward primer that recognizes genome Alu sequences and a reverse primer that hybridizes within the vector. Then, 10 μl of the first PCR mixture was subjected to a second PCR using forward and reverse primers recognizing the vector 5′ LTR. PCR products were loaded onto 1% agarose gels and the band intensities measured in a UV transilluminator (Gene Flash; Syngene BioImaging) using Gene Tools analysis software (version 3.08E; Syngene). The GAPDH housekeeping gene was used as loading control.
Western blot analysis.
HIV-GFP vectors pseudotyped with different Hgps and Fgps were purified by ultracentrifugation over a sucrose cushion. Subsequently, viral H was detected by Western blotting with an antibody recognizing the H ectodomain; incubation with an anti-HIV p24 antibody was used to reveal HIV capsid (CA).
Receptor blocking assay.
T or B cells (1E5) were seeded in 48-well plates and incubated for 2 h with anti-CD46 (clone 13/42) and/or anti-SLAM (IPO-3; eBioscience) antibodies prior to infection. Concentrated viral supernatants were added to the cells at an MOI of 10, and 3 days later, DNA was extracted for Q-PCR and transduction efficiency was analyzed by FACS.
Transcomplementation assays.
Freshly isolated, nonstimulated T cells (1E5) were simultaneously transduced with the CD46-tropic vector (Ha533/F-LV) expressing GFP and with the SLAM-tropic vector (Hclin/F-LV) expressing mRFP, both at an MOI of 5. Three days later, the percentages of GFP- versus mRFP-positive cells were measured by FACS.
Binding assay.
A binding assay was performed on transduced unstimulated T cells or after a 3-day prestimulation of T cells with rhIL-7 (BD Biosciences) or with anti-CD3–anti-CD28–rhIL-2. Briefly, equivalent amounts of the different vectors, as determined by their p24 content, were added to 5E4 quiescent T cells for 1 h at 4°C. Cells were washed four times in PBS, and the level of vector binding to cells was determined by measuring p24 antigen by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (RetroTek- Zeptomatrix, Buffalo, NY).
BlaM-Vpr fusion assay.
The BlaM-Vpr fusion assay monitors penetration into the cytosol by pH-independent HIV-1 viral cores that contain a BlaM-Vpr fusion protein and was performed as described previously (57). The different H/F-LV pseudotypes were produced as described above, with a single modification, namely, the extra inclusion of 6 μg of plasmid encoding the BlaM-Vpr fusion protein during the transfection. Briefly, target T cells were washed with CO2-independent medium and loaded with the CCF2 fluorescent substrate (Invitrogen), washed, and then infected with the BlaM-Vpr-containing lentiviral vectors. Cytosolic delivery of BlaM-Vpr by vector-cell fusion is monitored in this assay by cleavage of the cytoplasm-trapped fluorescent substrate (CCF2) in the target cell. This results in a shift in the emission wavelength from 518 nm (green) to 447 nm (blue) after excitation at 409 nm by FACS. Virus-cell fusion is readily quantified in this assay by calculating the ratio of blue to green signals in the cells.
Entry inhibition assay.
5-(N-Ethyl-N-isopropyl) amiloride (EIPA; 100 mM in dimethyl sulfoxide [DMSO]), LY294002 (50 mM in DMSO), blebbistatin (100 mM in DMSO), cytochalasin (2.5 mM), and nocodazole (17 mM in DMSO) stock solutions were prepared (Sigma). Briefly, 1E5 freshly isolated, nonstimulated T cells were incubated with 1E−3, 1E−4, and 1E−5 dilutions of each inhibitor for 30 min at 37°C. The different vectors were added and incubated for 1 h at 37°C. Following this step, cells were washed twice with PBS and one-third of the cells were kept in RPMI 1640–10% FCS for a further 3 days to measure transduction efficiency, while two-thirds of the cells were immediately pelleted for DNA extraction and Q-PCR.
RESULTS
Transduction efficiency of MV LVs correlates with SLAM expression on human primary cells.
The Edmonston (Edm) vaccine strain of measles virus can infect target cells either through CD46 or SLAM receptors (55). By pseudotyping lentiviral vectors with Edm Hgps and Edm Fgps, we generated H/F-LVs capable of transducing cell lines expressing only the CD46 or SLAM receptor (11) (Fig. 1C). However, as we have previously reported, this is not the case for primary T and B cells, in which H/F-LVs require the presence of both CD46 and SLAM on the target cell for an efficient transduction (10, 11). Likewise, here we show the same need by H/F-LVs for both receptors in freshly isolated resting mononuclear cells. Human peripheral blood B cells, T cells, and monocytes were isolated and immediately transduced with H/F-LVs for 3 days without the addition of cytokines. H/F-LVs transduced approximately 50% of B cells and approximately 30% of T cells, coinciding with the percentages of cells expressing SLAM. In contrast, unstimulated monocytes were not transduced with H/F-LVs, coinciding with the lack of SLAM receptor expression (Fig. 1A).
In order to study the roles of SLAM and CD46 in the transduction of human lymphocytes, we generated a series of LVs pseudotyped with different Hgps (Fig. 1B): (i) Edm Hgp, which contains residues binding to both SLAM and CD46 and was previously characterized (H/F-LVs [11]); (ii) SLAM-tropic Hgps derived from clinical strains (H-C2/F-LVs and H-D4/F-LVs) (23, 37) or from Edm H by ablation of one residue responsible for binding/fusing through CD46 (Y481A; Ha481/F-LVs) (52); and (iii) a CD46-tropic H mutant in which one residue of Edm Hgp responsible for fusion/entry through SLAM was ablated (R533A; Ha533/F-LVs) (24, 34). In order to obtain efficient Hgp and Fgp incorporation into LV cores, deletions of 24 aa and 30 aa of the cytoplasmic tails of all Hgps and Fgps, respectively, were engineered (Fig. 1B). Table 1 contains the titers of all MV gp LV pseudotypes. As shown in Fig. 1C and Table 1, SLAM-tropic vectors can transduce only CHO-SLAM cells, giving infectious titers between 2.7E6 and 6.6E6 TU/ml, whereas CD46-tropic vectors (Ha533/F-LVs) gave titers only on CD46+ 293T cells ranging from 6E5 to 1E6 TU/ml. In contrast, the initial H/F-LVs efficiently transduce CHO-SLAM and 293T cell lines. Of note, SLAM- or CD46-tropic vectors showed slightly lower titers than H/F-LVs on these two cell lines. However, only Ha481 gp showed a reduced incorporation compared to that of Hgp on the lentiviral particles, as revealed by Western blot analysis (Fig. 1D).
Table 1.
Titers of MV-derived LVsa
| Vector (100× concentrated)b | Mean titer ± SD (TU/ml) on: |
|
|---|---|---|
| 293T cells (n = 6) | CHO-SLAM cells (n = 6) | |
| H/F-LV (H Δ24 aa/F Δ30 aa) | 6.5E06 ± 1.3E06 | 1.3E07 ± 3.9E06 |
| Ha533/F-LV (H Δ24 aa Ha533/F Δ30 aa) | 9.0E05 ± 3.3E05 | 0.0 ± 0.0 |
| H-C2/F-LV (H Δ24 aa-C2/F Δ30 aa) | 0.0 ± 0.0 | 9.0E06 ± 1.3E07 |
| H-D4/F-LV (H Δ24 aa-D4/F Δ30 aa) | 0.0 ± 0.0 | 7.0E06 ± 1.4E06 |
| Ha481/F-LV (H Δ24 aa-Ha481/F Δ30 aa) | 0.0 ± 0.0 | 1.7E07 ± 1.4E07 |
Supernatants of 293T producer cells were harvested and concentrated 100-fold by low-speed centrifugation. Infectious titers were assessed by the addition of serial dilutions of each vector preparation to the appropriate target cell. For each vector, the percentage of GFP+ cells was analyzed at day 3 posttransduction. The titers of the SLAM-tropic vectors H-C2/F-LV, H-D4/F-LV, and Ha481/F-LV were determined on CHO-SLAM cells, whereas the titer of the CD46-tropic vector Ha533/F-LV was determined on 293T cells. The titer of the CD46- and SLAM-tropic H/F vector was determined on both 293T and CHO-SLAM cells. Titers are expressed as transduction units per ml (TU/ml).
Hgp cytoplasmic tails carry a deletion of 24 aa; Fgps carry a 30-aa deletion.
We then used these validated CD46 and SLAM singly tropic MV LVs to dissect the role of SLAM and CD46 receptors in efficient H/F-LV transduction of quiescent lymphocytes.
The simultaneous interaction of MV LVs with both CD46 and SLAM is required to achieve efficient and stable transduction.
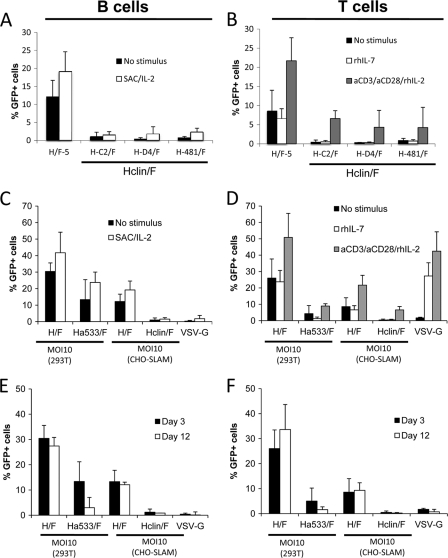
In order to investigate the contribution of each of the CD46 and SLAM receptors in H/F-LV transduction, human T and B cells were purified by negative selection from peripheral blood and prestimulated before transduction or immediately transduced with CD46-tropic, SLAM-tropic, or CD46- and SLAM-tropic H/F-LVs. As all SLAM-tropic LVs (H-C2/F, H-D4/F, and Ha481/F) gave equivalent percentages of transduction in primary resting and prestimulated B and T cells (Fig. 2A and B), we chose for clarity to represent them collectively under the name of Hclin/F-LVs. An MOI of 10 was used for all of the vectors, taking into account the difference in titers obtained with SLAM+ and CD46+ cell lines (Table 1). At day 3 after addition of the vectors, Hclin/F-LVs transduced quiescent T and B cells only very poorly, while Ha533/F (CD46-tropic)-LVs showed a important decrease in transduction compared to that of H/F-LVs, which use both receptors for transduction (Fig. 2C and D). Hclin/F-LV is shown as a representative member of SLAM-tropic vectors (Fig. 2A and B). Upon 24 h of B cell receptor (BCR) stimulation, all LV pseudotypes slightly increased the transduction levels in B cells between 1.3 and 1.8 times (Fig. 2C), coinciding with an upregulation of the SLAM molecule (10). This is in agreement with another report showing that LVs pseudotyped with clinical-strain MV gp's can transduce primary B cells to some extent (14). As expected, VSV-G LVs remained inefficient in BCR-stimulated B cells, as reported by us and others (10, 26, 45). For T cells, preactivation protocols using anti-CD3–anti-CD28–rIL-2, which induces TCR stimulation and cell proliferation, render cells permissive to VSV-G LVs and increase H/F-LV transduction 2- to 3-fold, coinciding with a surface upregulation of the SLAM receptor (Fig. 2D and reference 11). Under these conditions, SLAM-tropic (Hclin/F) vectors increase their T cell transduction efficiency 10 times, reaching GFP levels of around 7%, while the CD46-tropic (Ha533/F) vector showed almost a 2-fold-higher transduction (Fig. 2D). A milder activation protocol using rhIL-7 that induces T cell activation but no proliferation and no SLAM upregulation (11) allowed transduction with VSV-G LVs and H/F-LVs but not with Ha533/F- and Hclin/F-LVs (Fig. 2D). Of note, the same superiority of H/F-LVs compared to their singly tropic counterparts persists even at higher vector doses (data not shown). Strikingly, upon long-term culture of T and B cells, we saw 3-fold and 4.5-fold reductions of GFP levels for Ha533/F-LVs (percent GFP+ expression on day 3, 13%, and on day 12, 3%) and a decrease to nondetectable levels for Hclin/F-LVs (Fig. 2E and F). Overall, these data indicate that the singly CD46- or SLAM-tropic H/F-LVs did not result in stable transduction of quiescent T and B lymphocytes.
Fig. 2.
Singly CD46-tropic or SLAM-tropic LVs do not allow stable transduction of quiescent T and B cells. B cells (left graphs) and T cells (right graphs) were purified from peripheral blood by negative selection. (A) Freshly isolated B cells were transduced either immediately or after 24 h of stimulation with SAC/IL-2 with SLAM-tropic vectors: H-C2/F, H-D4/F, and Ha481-LVs (grouped under the name Hclin/F-LVs). (B) Unstimulated T cells, rIL-7-prestimulated T cells, or TCR (anti-CD3 [aCD3]–anti-CD28–rIL-2)-stimulated T cells were transduced with SLAM-tropic vectors: H-C2/F, H-D4/F, and Ha481-LVs (grouped under the name Hclin/F). Transduction levels were analyzed 3 days after transduction by FACS (means + standard deviations [SD]; n = 4). These unstimulated lymphocytes were immediately transduced with a CD46-tropic Ha533/F-LV, the original CD46+ SLAM-tropic H/F-LVs, and VSV-G-LVs at an MOI of 10, as determined on 293T cells. Transductions of unstimulated B cells (C) and T cells (D) were performed with H/F-LVs or SLAM-tropic Hclin/F LVs at an MOI of 10, as determined on CHO-SLAM (means + SD; n = 4). Identical transductions were performed on B cells prestimulated with SAC-rIL-2 for 48 h (C) or on T cells prestimulated with rIL-7 or through the TCR (aCD3-aCD28-rIL-2) for 3 days (D). Transduction levels were analyzed 3 days after transduction by FACS (means + SD; n = 4). (E and F) Short-term transduction (3 days) and long-term cultures (12 days) of the transductions of unstimulated B and T cells, respectively. Transduced B cells were washed twice after transduction and cultured for 9 more days on MS5 feeder cells and were then analyzed for GFP-expressing cells by FACS (E), while transduced T cells were washed twice and continued in RPMI 1640 medium supplemented with rIL-2 (10 ng/ml) for 9 more days before FACS analysis (F).
Blocking of the CD46 receptor on quiescent T cells abrogates H/F-LV entry.
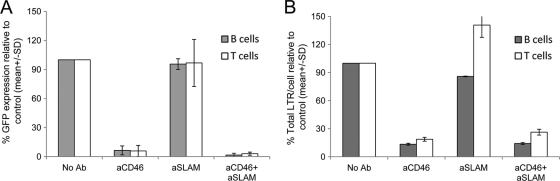
In order to get a better insight into the roles of the measles virus receptors in H/F-LV transduction, quiescent T and B cells were isolated and immediately incubated with specific monoclonal antibodies (MAbs) against CD46 and/or SLAM receptors before transduction with H/F-LVs. Of note, we had determined in previous assays that anti-SLAM antibody does not block CD46 receptor interaction and vice versa. Interestingly, the blocking of the CD46 receptor on quiescent T and B cells completely abrogated H/F-LV transduction, while blocking the SLAM receptor had no significant effect. Blocking both receptors at the same time decreased H/F-LV transduction almost completely (Fig. 3A). In addition, we evaluated the effect of blocking the CD46 or SLAM receptors at a postentry stage by quantification of reverse-transcribed vector sequences. Importantly, blocking of CD46 alone or together with SLAM led to a 90-fold reduction in total H/F-LV-retrotranscribed LTR sequences compared to levels with no antibody or with anti-SLAM antibody incubation (Fig. 3B). These results strongly suggest that interaction of H/F-LVs with the CD46 receptor is indispensable for vector entry and reverse transcription of proviral RNA in quiescent lymphocytes.
Fig. 3.
Blocking of the CD46 receptor abrogates H/F-LV transduction of quiescent T and B cells. Quiescent T and B cells were transduced with H/F-LVs (MOI, 10) in the absence or presence of anti-CD46 or anti-SLAM blocking antibodies (Ab) or a combination of both. (A) At day 2 after transduction, GFP expression in the B cell population was determined by FACS, while for T cells, transduction levels were analyzed at day 3 posttransduction. Transduction levels are presented as GFP expression relative to the H/F-LV transduction in the absence of antibody, set to 100% (means ± SD; n = 3). (B) The amount of total retrotranscribed proviral DNA measured by LTR content was determined by Q-PCR as described in Materials and Methods at 12 h posttransduction. The amount of LTR content is presented relative to the LTR content obtained upon transduction with H/F-LVs in the absence of antibody (means ± SD; n = 3).
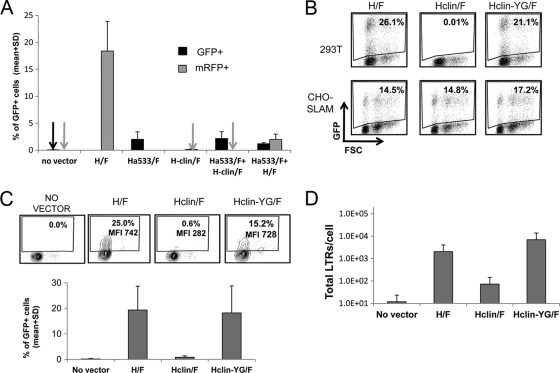
CD46-tropic MV gp LVs allow T cell binding fusion and reverse transcription (RT) but do not permit stable transduction of quiescent T cells.
To complement the receptor blocking experiment, we sought to investigate if the difference in transduction observed with singly tropic vectors developed as described above was at the level of virus-cell binding and fusion or rather due to postentry events. First, the level of binding of the different MV-derived vectors was determined in quiescent T cells by incubation of the cells with equal amounts of vectors, followed by extensive washes and detection of p24 as a measure for cell-associated vector particles (Fig. 4A). To avoid virus-cell fusion, the assay was performed at 4°C. In the case of Ha533/F-LV, we detected high p24 levels for quiescent T cells, though this was lower than for the H/F-LVs. In contrast, Hclin/F-LVs demonstrated much less cell binding. Binding of H/F-LVs in rIL7-stimulated T cells was equivalent to that in resting cells and increased in TCR-stimulated T cells, in which SLAM is upregulated (data not shown). Importantly, Hclin/F-LV significantly increased binding to TCR-stimulated T cells, while Ha533/F-LVs gave equal levels of p24 in IL-7- or TCR-stimulated T cells, in agreement with constant surface CD46 expression (data not shown).
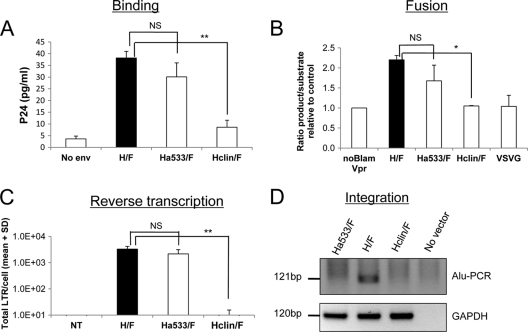
Fig. 4.
CD46-tropic H/F-LVs allow T cell binding and fusion but not stable proviral integration into the T cell genome. (A) Resting T cells were incubated with H/F-LVs, CD46-tropic LVs (Ha533/F) or SLAM-tropic LVs (Hclin/F) for 1 h at 4°C. As a control, cells were incubated with lentiviral particles not displaying gp's (no env). Cells were then washed extensively, and the amount of bound LVs was determined by measuring the p24 content associated with the cells detected by ELISA (means + SD; n = 3). (B) Resting T cells were washed with CO2-independent medium and loaded with the CCF2 fluorescent substrate, washed again, and then incubated with the BlaM-Vpr-containing lentiviral vector pseudotypes H/F-LVs, Ha533/F-LVs, Hclin/F-LVs, and VSV-G-LVs. As a control, a lentivector without incorporation of BlaM-Vpr was used (noBlam Vpr). Cytosolic delivery of BlaM-Vpr by vector-cell fusion is monitored in this assay by cleavage of the cytoplasm-trapped fluorescent substrate (CCF2) in the target cell. This results in a shift in the emission wavelength from 518 nm (green) to 447 nm (blue), analyzed by FACS. Virus-cell fusion is readily quantified in this assay by calculating the ratio of blue to green signals in the cells (means + SD; n = 3). (C) Quiescent T cells were transduced with H/F-, Ha533/F-, and Hclin/F-LVs at an MOI of 10 or incubated without a vector. The total amount of LTRs/cell in these transduced quiescent T or B cells was determined at 3 days posttransduction. The specific primers used are described in Materials and Methods. The numbers of total LTRs/cell are indicated on the y axis (means + SD; n = 3). NT, no transduction. (D) Quiescent T cells were transduced with H/F-, Ha533/F-, and Hclin/F-LVs at an MOI of 10 and washed at day 3 of transduction, and their culture was continued in the presence of rIL-2. A nested Alu-PCR of the DNA of transduced and nontransduced T cells at day 12 of culture was performed using specific primers (see Materials and Methods). The PCR-specific band corresponding to the integrated vector sequence of 121 bp is detected only in H/F-LV-transduced quiescent T cells. The PCR-specific band for the GAPDH housekeeping gene was used as the loading control. Where indicated, the results were analyzed by a paired Student t test. The levels of significance are indicated as follows: *, P < 0.05; **, P < 0,005; and NS, not significant.
Second, we investigated if all vectors could support efficient fusion with quiescent T cells by performing an assay that monitors penetration into the cytosol by HIV cores that contain a BlaM-Vpr fusion protein (57). Cytosolic delivery of BlaM-Vpr is monitored in this assay by cleavage of the cytoplasm-trapped fluorescent substrate CCF2, which results in a shift in the emission wavelength from 518 nm (green) to 447 nm (blue) after excitation at 409 nm. Therefore, viral penetration into the cytosol is readily quantified in this assay by calculating the ratio of blue to green signals in the cells. As shown in Fig. 4B, all H/F- and Ha533/F-LVs support fusion levels equivalent to those of the target T cells, while Hclin/F-LV and VSV-G-LVs gave signal intensities that could not be discriminated from that of the negative control using a vector particle not containing BlaM-Vpr.
Next, we determined the level of RT proviral DNA upon vector incubation. To evaluate the efficacy of this postentry step, genomic DNA from quiescent T cells was extracted after transduction with equivalent amounts of H/F-, Ha533/F-, and Hclin/F-LVs, and the total reverse-transcribed vector DNA (total LTRs) was quantified by real-time PCR. In agreement with transduction levels observed at day 3 posttransduction (Fig. 2A), a significantly smaller amount of total RT LTRs was detected for Hclin/F-LVs than for H/F-LVs (Fig. 4C). Intriguingly, the CD46-tropic vector gave total LTR levels similar to those of H/F-LVs, albeit with a 9- to 20-fold-lower percentage of GFP expression at day 12 than that of H/F-LVs (Fig. 2B versus 4C).
Finally, we verified if the singly tropic vectors allowed stable proviral integration into the genomes of quiescent T cells by the Alu-PCR technique. Transduction of quiescent T cells was performed at an MOI of 10. At day 3 posttransduction, cells were washed and continued in culture in the presence of rIL-2 for 9 days. As shown in Fig. 4D, only H/F-LVs gave a significant Alu-PCR signal at day 12 of culture. These findings underline the lack of integration of Hclin/F-LVs and Ha533/F-LV CD46-tropic vectors and explain the loss of GFP signal in T and B cells upon long-term culture (Fig. 2D).
Overall, these experiments show that CD46-tropic and CD46+ SLAM-tropic H/F-LVs bind more efficiently to quiescent T cells than SLAM-tropic H/F-LVs. Moreover, the reduced transduction efficiency observed with SLAM-tropic vectors is due to a defect in the fusion of the particles with the target cells. The second major finding is that only H/F- and Ha533/F-LVs can accomplish efficient entry and reverse transcription. Third, the unique interaction of the vector with CD46 does not support vector integration and therefore does not lead to a stable transduction.
Both CD46 and SLAM binding sites must be present in H in cis to allow efficient H/F-LV transduction of quiescent lymphocytes.
We next speculated that cotransduction of a CD46-tropic vector with a SLAM-tropic vector could lead to an increased and stable transduction of the CD46-tropic vector by providing SLAM downstream signaling in trans. Thus, we performed cotransduction of quiescent T cells with Hclin/F-LV expressing mRFP and Ha533/F-LV expressing GFP. The H/F-LV control vector coded for mRFP. Three and 12 days later, transduction efficiency was measured. As shown in Fig. 5A, cotransduction of quiescent T cells with CD46- or SLAM-tropic vectors resulted in a small increase in the percentage of GFP at day 12, but the transduction efficiency conferred by H/F-LVs was far from achieved. Of note, cotransduction of H/F-LVs encoding mRFP and Ha533/F-LVs encoding GFP resulted in a strong reduction in transduction by H/F-LVs, suggesting that Ha533/F-LV could act as a blocking agent for H/F-LV binding to CD46. Finally, we hypothesized that CD46 binding could stabilize SLAM interaction and vice versa as an explanation for the efficient transduction observed only with H/F-LVs. We generated a SLAM/CD46 mutant by adding the mutation N481Y (responsible for binding to CD46) to the clinical strain H-D4 (44, 47). The mutation E492G present in Edm strain's Hgp was included in addition, as it has been shown to stabilize CD46 binding, and the mutant is called Hclin-YG herein (47). Indeed, this Hclin-YG/F-LVs gained entry through the CD46 receptor expressed on 293T cells, and titers obtained were equivalent to those of H/F-LVs on these cells (Fig. 5B and data not shown).
Fig. 5.
MV hemagglutinin, able to bind both the SLAM and the CD46 receptor, allows efficient transduction of quiescent T cells. (A) Quiescent T cells were transduced with H/F-LVs or Hclin/F-LVs, both expressing mRFP, and Ha533/F-LV, expressing GFP separately. In parallel, a cotransduction of T cells was performed with Hclin/F-LVs expressing mRFP and Ha533/F-LV expressing GFP, or they were simultaneously incubated with H/F-LVs encoding mRFP and Ha533/F-LV expressing GFP. Twelve days later, transduction efficiencies were analyzed by FACS (means + SD; n = 3). (B) In the clinical strain H-D4 (Hclin), the mutations N481Y and E492G (Hclin-YG), responsible for stable CD46 binding of the H from the Edmonston strain, were introduced. Transduction efficiencies of H/F-LVs, Hclin/F-LVs, and the Hclin-YG/F-LVs on CD46 receptor-expressing (293T) cells and on CHO-SLAM cells are presented (MOI = 0.2). (C) Quiescent T cells were incubated with H/F-, Hclin/F-, and Hclin-YG/F-LVs (MOI = 10). Levels of transduction of quiescent T cells by the different pseudotypes were compared by FACS 3 days posttransduction (means + SD; n = 3). MFI, mean fluorescence intensity. (D) Quiescent T cells were transduced with H/F-, Hclin/F-, and Hclin-YG/F-LVs at an MOI of 10 or incubated without a vector. The total amount of LTRs/cell in these transduced quiescent T cells was determined at 12 h posttransduction. The specific primers used are described in Materials and Methods. The number of total retrotranscribed LTRs/cell is indicated in the y axis (means + SD; n = 3).
Remarkably, Hclin-YG/F-LVs transduced quiescent T cells at the same levels as H/F-LVs (Fig. 5C). Moreover, both Hclin-YG/F-LVs and H/F-LVs showed equal amounts of reverse-transcribed total LTR sequences (Fig. 5D), and long-term culture of transduced cells demonstrated a stable true transduction for Hclin-YG/F-LVs (Fig. 5C, lower graph).
In summary, SLAM and CD46 receptors most probably need to be stimulated by Hgp in a sterically defined manner and by SLAM- and CD46-binding residues present in cis on the Hgp. In this way, correct CD46 and SLAM downstream signaling may be triggered and efficient transduction accomplished.
Quiescent T cell entry of H/F-LVs might occur through macropinocytosis.
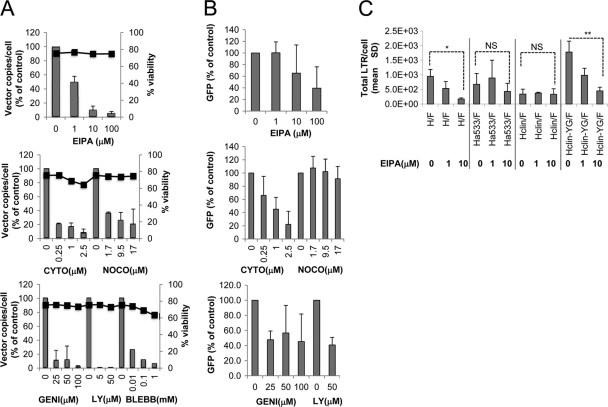
Macropinocytosis is an actin-dependent uptake mechanism physiologically exploited by several types of cells, such as dendritic cells and macrophages, which can be hijacked by pathogens (53). Several reports support the idea that macropinocytosis can act as an entry route for many pathogens, including viruses such as vaccinia virus (19, 32), adenovirus (2, 46), HIV (30, 53), and the Nipah paramyxovirus (35). In addition, it was reported that addition of soluble Edm Hgp to cells induced internalization of the Edm H receptor complex by macropinocytosis (9). In all these cases, specific vector-receptor interactions are required to trigger macropinocytosis. Thus, we explored the possibility that macropinocytosis could be the route of entry of H/F-LVs in quiescent T cells, which might offer an explanation for the efficient transduction observed in these cells exclusively with H/F-LV pseudotypes. Thus, H/F-LV transduction was performed in quiescent T cells in the presence of a series of inhibitors related to this entry pathway (Fig. 6A). The doses of each inhibitor were set up in previous experiments according to the criteria of maximum effect and minimum cellular toxicity, evaluated by cell viability (Fig. 6A). As a measure for entry, total numbers of RT proviral LTR sequences/cell were measured at 1 h posttransduction, and the percentage of GFP was detected 3 days after transduction (Fig. 6A and B). Importantly, H/F-LV entry was inhibited in a dose-dependent manner by the macropinocytosis-specific Na/H+ exchange inhibitor 5-(N-ethyl-N-isopropyl) amiloride (EIPA). Macropinocytosis requires actin polymerization to facilitate changes in the cytoskeleton. Indeed, H/F-LV entry was shown to be dependent on the actin cytoskeleton and microtubule formation, as it was inhibited by the respective inhibitors cytochalasin and nocodazole (Fig. 6A). Furthermore, inhibition of myosin II, required to effect the closure of macropinosomes, by blebbistatin, which prevents membrane blebbing, also decreased H/F-LV entry. As Ras activation by receptor tyrosine kinases initiates the phosphatidylinositol-3-kinase pathway, which modulates the closure of macropinosomes, the phosphoinositide-3 kinase (PI3K) inhibitor LY294002 should have an effect on viral entry if this occurs through macropinocytosis. Indeed, we found that LY294002 inhibited H/F-LV entry completely, in accordance with engagement of the macropinocytotic mechanism of entry (Fig. 6A). The broad-spectrum tyrosine kinase inhibitor genistein affected H/F-LV entry, suggesting that tyrosine kinase activity plays a role in H/F-LV entry in quiescent T cells. In terms of transduction, H/F-LV was also inhibited by the addition of the same drugs, albeit to a lesser extent, except that nocodazole had no effect on transduction, possibly because the inhibitory effect was lost over the following 3 days (Fig. 6B).
Fig. 6.
H/F-LVs might enter quiescent T cells through macropinocytosis. Unstimulated T cells were preincubated for 30 min at the indicated concentrations with a specific macropinocytosis inhibitor (EIPA), inhibitors of the actin cytoskeleton (cytochalasin [CYTO]) and microtubule formation (nocodazole [NOCO]), inhibitors of myosin II (blebbistatin), or an inhibitor of PI3 kinase (LY). GEN, genistein. Subsequently, the cells were incubated with H/F-LVs in the presence of the inhibitors for 1 h (MOI = 10). (A) In the left axis, the total amount of retrotranscribed LTRs/cell was measured after 1 h of incubation with the vectors, while the percentage of GFP-expressing cells was determined 3 days after transduction, as shown in panel B. The right axis represents the viability of primary T cells upon incubation with the highest inhibitor drug concentration. (C) Unstimulated T cells were preincubated for 30 min at the indicated concentrations with a specific macropinocytosis inhibitor (EIPA), followed by an incubation in the presence of the EIPA inhibitor with H/F-LVs, a CD46-tropic LV (Ha533/F-LV), a SLAM-tropic LV (Hclin/F-LV), and finally the SLAM-tropic LV that gained entry through the CD46 receptor (Hclin-YG/F-LV). The total amount of retrotranscribed LTRs/cell was measured after 1 h of incubation with the vectors. Where indicated, the results were analyzed by a paired Student t test. The levels of significance are indicated as followed: *, P < 0.05; **, P < 0,005, and NS, not significant.
Interestingly, the CD46 (Ha533)- and SLAM (Hclin)-tropic vectors did not seem to induce macropinocytosis, as their entry was not inhibited by the EIPA drug at the concentrations that affected H/F-LVs (Fig. 6C). Of importance, entry of the Hclin-YG/F-LVs, engineered to recognize CD46 in addition to SLAM, was inhibited by EIPA and demonstrated an inhibition pattern similar to that of the H/F-LVs. Of note, H/F-LV transduction was markedly reduced in Wiskott-Aldrich syndrome (WAS) patient-derived cells, compared to that in cells of healthy patients, since WAS cells are impaired in actin polymerization (48). Nevertheless, we detected 100% CD46 expression and 60 to 70% SLAM expression on WAS cells (data not shown).
Taken together, these results suggest that macropinocytosis may be a successful route of entry for H/F-LVs into quiescent T cells that is triggered only by the interaction of an MV vector displaying an Hgp carrying binding sites for both the CD46 and SLAM receptors.
DISCUSSION
We and others have previously constructed lentiviral vectors pseudotyped with H and F glycoproteins derived from the Edmonston vaccine strain of MV (H/F-LVs) (11, 13). These vectors allowed stable and efficient transduction of quiescent T and B cells, which express both measles virus receptors and are at present the only vectors capable of this effect (10–12). Here, we sought to determine the roles of the MV receptors in the H/F-LV transduction process of quiescent lymphocytes. By using CD46-tropic MV LVs and receptor blocking assays, we found that vector-T cell binding, fusion, and reverse transcription can occur by a unique interaction through CD46; however, this did not result in final proviral integration. Indeed, in addition to CD46, SLAM engagement was needed for vector integration into the host cell genome. Moreover, an efficient transduction occurs only if the SLAM and CD46 receptors bind to MV H containing binding residues for both receptors in cis. Finally, we propose that high transduction efficiency is observed only when CD46 and SLAM are correctly engaged and that this triggers an entry mechanism that strongly resembles macropinocytosis.
Our previous work already strongly suggested that H/F-LVs need both CD46 and SLAM receptor binding to achieve a stable and efficient transduction of T and B cells, which was supported by multiple findings (11, 12, 26). First, we obtained a high transduction level with H/F-LVs in IL-7-stimulated CD46+ SLAM+ adult T cells, while CD46+ cord blood naive T cells, which express SLAM at low levels, were barely transduced. Second, in TCR-stimulated T cells, which resulted in SLAM upregulation, a higher transduction level was observed. Third, we obtained a higher transduction of quiescent memory T cells than of naive T cells, which express SLAM at low levels (8). We also detected a preferential transduction of quiescent CD4+ T cells, which express higher levels of SLAM than CD8+ cells do (38). Finally, in a mixed peripheral blood mononuclear cell (PBMC) population where B cells express larger amounts of the SLAM receptor than T cells do, the former are indeed consistently better transduced by H/F-LVs, despite equivalent levels of CD46 expression (10). Here we show that, in contrast to H/F-LVs, SLAM-tropic-only or CD46-tropic-only vectors cannot efficiently transduce quiescent and activated/proliferating T and B cells. Likewise, treatment of T cells with IL-7, which induces T cell cycle entry into G1b (51) without SLAM upregulation (11), did not lead to an efficient transduction of SLAM-tropic or CD46-tropic vectors. This means that the SLAM-CD46 interaction cannot be replaced by cell cycle entry into G1b, a requirement allowing classical VSV-G LVs to transduce primary human T cells (22, 50, 51).
Moreover, the lack of T cell transduction by SLAM-tropic vectors is not exclusively due to a low level of binding to the SLAM receptor (Fig. 4A) since some binding occurs particularly upon SLAM upregulation induced by TCR engagement (data not shown), but the latter stimulation still results in poor transduction (Fig. 2B). It can rather be speculated that postentry events cannot be accomplished in the absence of CD46 interaction and that entry and fusion are less efficient (40). Indeed, SLAM-tropic Hclin/F-LVs, like VSV-G-LVs, did not fuse efficiently with quiescent T cells. For VSV-G-LVs, it has already been reported by Agosto et al. (1) that low-level binding and fusion lead to inefficient gene transfer into quiescent T cells. On the other hand, CD46-tropic Ha533/F-LVs not only efficiently bound to the target cells but also led to a high level of vector-T cell fusion and a high number of RT vector sequences, but there was a lack of proviral DNA integration. In agreement with the last finding, antibody blocking of the CD46 receptor resulted in a strong decrease of H/F-LV T cell transduction. Surprisingly, preincubation with a blocking antibody against SLAM did not reduce H/F-LV transduction, which might suggest that efficient H/F-LV transduction of quiescent lymphocytes requires other Hgp-SLAM interactions that are as yet undescribed. Importantly, transduction of the CD46-tropic vector in the presence of SLAM activation/stimulation in trans was far less than the transduction levels observed with H/F-LV, suggesting that CD46 and SLAM must be engaged at a certain conformation that would take place only when both binding sites are within the same Hgp. Recently, the crystal structure of Edm H showed that the CD46- and SLAM-binding residues conformationally overlap, so this suggests that binding of H to the receptors is probably a sequential two-step process. In our vector system, the possible stabilization of CD46 interaction by SLAM interaction and vice versa in H/F-LV is further demonstrated by efficient transduction of quiescent T cells obtained with Hclin-YG/F-LV, in which the CD46 receptor-binding residues, 481Y and 492G (44, 47), were added to only the H-D4 gp carrying SLAM-binding residues. Presumably, binding of CD46 and SLAM in such a conformation may trigger downstream signaling of both molecules, thus enabling the vector to accomplish all the postbinding events leading to genome vector integration. In addition, binding of MV gp's to SLAM/CD46 receptors on quiescent cells, together with other specific steps required for viral replication, may activate the uncoating process.
The facts that VSV-G-LVs cannot transduce quiescent cells and that the presence of Hgps and Fgps on the lentiviral surface turns the vector into a potent transduction tool for quiescent lymphocytes strongly suggest that the transduction efficiency can already be determined at the level of vector entry (12). Although MV entry is thought to occur exclusively through pH-independent fusion at the level of the plasma membrane, other paramyxoviruses can use endocytosis for cell entry (16, 39). Recently, vaccinia virus, HIV, Ad3 V (which uses CD46 as a receptor), and the Nipah paramyxovirus have been shown to use macropinocytosis for entry (33, 35).
Macropinocytosis causes cytoskeletal rearrangements to occur in cells that lead to ruffling and blebbing at the plasma membrane. These changes in actin polymerization are induced by signaling from receptor tyrosine kinases, which can be directly or indirectly activated by virus binding to a receptor or protein on the plasma membrane. When the blebs that are formed retract into the plasma membrane, they engulf the virus into a macropinosome (33). Multiple lines of evidence strongly suggest that H/F-LVs use entry into quiescent T cells via macropinocytosis: (i) IEPA inhibited H/F-LV T cell entry; (ii) H/F-LV entry was shown to be dependent on the actin cytoskeleton and microtubule formation, as it was inhibited by the inhibitors cytochalasin and nocodazole; (iii) blebbistatin, which prevents membrane blebbing, also decreased H/F-LV entry; (iv) the PI3K inhibitor LY294002 inhibited H/F-LV T cell entry completely; and (v) genistein affected H/F-LV entry, suggesting that tyrosine kinase activity plays a role in H/F-LV entry into quiescent T cells. These data are in accordance with the finding that contact of Edm H with CD46-expressing cells induces internalization of this receptor by macropinocytosis (9). It was hypothesized that MV LV infection of lymphocytes induced rearrangement of the actin network, and it was proposed that this allowed proviral transport into the nucleus in the absence of stimulation (6). This entry pathway through SLAM/CD46 may also alter trafficking of the particles through cellular compartments, protecting them for proteasome degradation (42) or inducing the uncoating process. In addition, H/F-LVs using this alternative cell entry mechanism might avoid interaction with postentry restriction factors.
We postulate that entry of H/F-LV and H-D4-YG/F-LV may occur by macropinocytosis as a result of a correct dual triggering of CD46 and SLAM downstream signaling. Indeed, SLAM was downregulated upon H/F-LV binding to quiescent T cells (11). On the one hand, CD46 engagement triggers several actin regulators, such as Rho, Vav-1, Rac1, Nck, Erk1/2, SHP-1, and mitogen-activated protein (MAP) kinases, all of which are related to actin-dependent processes, such as filopodium formation and membrane protrusion during particle engulfment (5, 18, 56). Moreover, even if the SLAM molecule regulates T cell-B cell and DC-T cell activation, it has been shown that upon CD3 engagement, SLAM becomes tyrosine phosphorylated and leads to activation of SAP, SHP, lck, and fyn (15, 18, 43), which are also closely related to actin cytoskeleton regulation and lead to expression of nuclear factor of activated T cells (NFAT), which is required for transcription of latent HIV sequences (20). In line with the macropinocytosis hypothesis, T and B cells with impaired actin cytoskeleton polymerization, like Wiskott Aldrich syndrome patient-derived cell lines, are less transduced with H/F-LV than cells from healthy patients, despite a high expression of SLAM and CD46 receptors (data not shown). However, the role of CD46 and SLAM in H/F-LVs' productive proviral integration into quiescent lymphocytes still needs to be elucidated.
In conclusion, a concerted SLAM/CD46 engagement, which might be a sequential two-step receptor attachment process, may induce macropinocytosis on the one hand as a successful entry route of MV-derived vectors into quiescent lymphocytes and, on the other hand, ensure that the vaccine-derived MV provokes a potent immune response rather than immunosuppression.
ACKNOWLEDGMENTS
This work was supported by grants from the Agence Nationale pour la Recherche contre le SIDA et les Hépatites Virales (ANRS), the Agence Nationale de la Recherche (ANR), the European Research Council (ERC-2008-AdG-233130 HEPCENT), and the European Community (FP7-Health-2007-B/222878 Persist and FP7-E-Rare Genthalther).
We declare no conflict of interest.
The corresponding authors contributed equally to this work.
We acknowledge Anais Girard for technical assistance.
Footnotes
Published ahead of print on 30 March 2011.
REFERENCES
- 1. Agosto L. M., et al. 2009. The CXCR4-tropic human immunodeficiency virus envelope promotes more-efficient gene delivery to resting CD4+ T cells than the vesicular stomatitis virus glycoprotein G envelope. J. Virol. 83:8153–8162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amstutz B., et al. 2008. Subversion of CtBP1-controlled macropinocytosis by human adenovirus serotype 3. EMBO J. 27:956–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aversa G., et al. 1997. SLAM and its role in T cell activation and Th cell responses. Immunol. Cell Biol. 75:202–205 [DOI] [PubMed] [Google Scholar]
- 4. Avota E., Gassert E., Schneider-Schaulies S. 2010. Measles virus-induced immunosuppression: from effectors to mechanisms. Med. Microbiol. Immunol. 199:227–237 [DOI] [PubMed] [Google Scholar]
- 5. Baba T., Fusaki N., Shinya N., Iwamatsu A., Hozumi N. 2003. Actin tyrosine dephosphorylation by the Src homology 1-containing protein tyrosine phosphatase is essential for actin depolymerization after membrane IgM cross-linking. J. Immunol. 170:3762–3768 [DOI] [PubMed] [Google Scholar]
- 6. Buchholz C. J., Muhlebach M. D., Cichutek K. 2009. Lentiviral vectors with measles virus glycoproteins—dream team for gene transfer? Trends Biotechnol. 27:259–265 [DOI] [PubMed] [Google Scholar]
- 7. Casasnovas J. M., Larvie M., Stehle T. 1999. Crystal structure of two CD46 domains reveals an extended measles virus-binding surface. EMBO J. 18:2911–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cocks B. G., et al. 1995. A novel receptor involved in T-cell activation. Nature 376:260–263 [DOI] [PubMed] [Google Scholar]
- 9. Crimeen-Irwin B., et al. 2003. Ligand binding determines whether CD46 is internalized by clathrin-coated pits or macropinocytosis. J. Biol. Chem. 278:46927–46937 [DOI] [PubMed] [Google Scholar]
- 10. Frecha C., et al. 2009. Efficient and stable transduction of resting B lymphocytes and primary chronic lymphocyte leukemia cells using measles virus gp displaying lentiviral vectors. Blood 114:3173–3180 [DOI] [PubMed] [Google Scholar]
- 11. Frecha C., et al. 2008. Stable transduction of quiescent T cells without induction of cycle progression by a novel lentiviral vector pseudotyped with measles virus glycoproteins. Blood 112:4843–4852 [DOI] [PubMed] [Google Scholar]
- 12. Frecha C., Levy C., Cosset F. L., Verhoeyen E. 2010. Advances in the field of lentivector-based transduction of T and B lymphocytes for gene therapy. Mol. Ther. 18:1748–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Funke S., et al. 2008. Targeted cell entry of lentiviral vectors. Mol. Ther. 16:1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Funke S., et al. 2009. Pseudotyping lentiviral vectors with the wild-type measles virus glycoproteins improves titer and selectivity. Gene Ther. 16:700–705 [DOI] [PubMed] [Google Scholar]
- 15. Gu C., et al. 2006. The X-linked lymphoproliferative disease gene product SAP associates with PAK-interacting exchange factor and participates in T cell activation. Proc. Natl. Acad. Sci. U. S. A. 103:14447–14452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gutierrez-Ortega A., Sanchez-Hernandez C., Gomez-Garcia B. 2008. Respiratory syncytial virus glycoprotein uptake occurs through clathrin-mediated endocytosis in a human epithelial cell line. Virol. J. 5:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hashiguchi T., et al. 2011. Structure of the measles virus hemagglutinin bound to its cellular receptor SLAM. Nat. Struct. Mol. Biol. 18:135–141 [DOI] [PubMed] [Google Scholar]
- 18. Howie D., et al. 2002. Molecular dissection of the signaling and costimulatory functions of CD150 (SLAM): CD150/SAP binding and CD150-mediated costimulation. Blood 99:957–965 [DOI] [PubMed] [Google Scholar]
- 19. Huang C. Y., et al. 2008. A novel cellular protein, VPEF, facilitates vaccinia virus penetration into HeLa cells through fluid phase endocytosis. J. Virol. 82:7988–7999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kinoshita S., Chen B. K., Kaneshima H., Nolan G. P. 1998. Host control of HIV-1 parasitism in T cells by the nuclear factor of activated T cells. Cell 95:595–604 [DOI] [PubMed] [Google Scholar]
- 21. Koga R., Ohno S., Ikegame S., Yanagi Y. 2010. Measles virus-induced immunosuppression in SLAM knock-in mice. J. Virol. 84:5360–5367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Korin Y. D., Zack J. A. 1998. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J. Virol. 72:3161–3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kremer J. R., et al. 2008. High genetic diversity of measles virus, World Health Organization European Region, 2005-2006. Emerg. Infect. Dis. 14:107–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leonard V. H., Hodge G., Reyes-Del Valle J., McChesney M. B., Cattaneo R. 2010. Measles virus selectively blind to signaling lymphocytic activation molecule (SLAM; CD150) is attenuated and induces strong adaptive immune responses in rhesus monkeys. J. Virol. 84:3413–3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leonard V. H., et al. 2008. Measles virus blind to its epithelial cell receptor remains virulent in rhesus monkeys but cannot cross the airway epithelium and is not shed. J. Clin. Invest. 118:2448–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levy C., et al. 2010. Lentiviral vectors and transduction of human cancer B cells. Blood 116:498–500 [DOI] [PubMed] [Google Scholar]
- 27. Liszewski M. K., Post T. W., Atkinson J. P. 1991. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu. Rev. Immunol. 9:431–455 [DOI] [PubMed] [Google Scholar]
- 28. Maisner A., et al. 2000. Recombinant measles virus requiring an exogenous protease for activation of infectivity. J. Gen. Virol. 81:441–449 [DOI] [PubMed] [Google Scholar]
- 29. Malvoisin E., Wild T. F. 1993. Measles virus glycoproteins: studies on the structure and interaction of the haemagglutinin and fusion proteins. J. Gen. Virol. 74(Part 11):2365–2372 [DOI] [PubMed] [Google Scholar]
- 30. Marechal V., et al. 2001. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J. Virol. 75:11166–11177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maurice M., et al. 2002. Efficient gene transfer into human primary blood lymphocytes by surface-engineered lentiviral vectors that display a T cell-activating polypeptide. Blood 99:2342–2350 [DOI] [PubMed] [Google Scholar]
- 32. Mercer J., Helenius A. 2008. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science 320:531–535 [DOI] [PubMed] [Google Scholar]
- 33. Mercer J., Helenius A. 2009. Virus entry by macropinocytosis. Nat. Cell Biol. 11:510–520 [DOI] [PubMed] [Google Scholar]
- 34. Navaratnarajah C. K., et al. 2008. Dynamic interaction of the measles virus hemagglutinin with its receptor signaling lymphocytic activation molecule (SLAM, CD150). J. Biol. Chem. 283:11763–11771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pernet O., Pohl C., Ainouze M., Kweder H., Buckland R. 2009. Nipah virus entry can occur by macropinocytosis. Virology 395:298–311 [DOI] [PubMed] [Google Scholar]
- 36. Pfeuffer J., Puschel K., Meulen V., Schneider-Schaulies J., Niewiesk S. 2003. Extent of measles virus spread and immune suppression differentiates between wild-type and vaccine strains in the cotton rat model (Sigmodon hispidus). J. Virol. 77:150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rogalska J., Santibanez S., Mankertz A., Makowka A., Szenborn L., Stefanoff P. 2010. Spotlight on measles 2010: an epidemiological overview of measles outbreaks in Poland in relation to the measles elimination goal. Euro Surveill. 15(17):pii=19549 [DOI] [PubMed] [Google Scholar]
- 38. Romero X., et al. 2004. Differential expression of SAP and EAT-2-binding leukocyte cell-surface molecules CD84, CD150 (SLAM), CD229 (Ly9) and CD244 (2B4). Tissue Antigens 64:132–144 [DOI] [PubMed] [Google Scholar]
- 39. San Roman K., Villar E., Munoz-Barroso I. 1999. Acidic pH enhancement of the fusion of Newcastle disease virus with cultured cells. Virology 260:329–341 [DOI] [PubMed] [Google Scholar]
- 40. Santiago C., Bjorling E., Stehle T., Casasnovas J. M. 2002. Distinct kinetics for binding of the CD46 and SLAM receptors to overlapping sites in the measles virus hemagglutinin protein. J. Biol. Chem. 277:32294–32301 [DOI] [PubMed] [Google Scholar]
- 41. Santiago C., Celma M. L., Stehle T., Casasnovas J. M. 2010. Structure of the measles virus hemagglutinin bound to the CD46 receptor. Nat. Struct. Mol. Biol. 17:124–129 [DOI] [PubMed] [Google Scholar]
- 42. Santoni de Sio F. R., Cascio P., Zingale A., Gasparini M., Naldini L. 2006. Proteasome activity restricts lentiviral gene transfer into hematopoietic stem cells and is down-regulated by cytokines that enhance transduction. Blood 107:4257–4265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schwartzberg P. L., Mueller K. L., Qi H., Cannons J. L. 2009. SLAM receptors and SAP influence lymphocyte interactions, development and function. Nat. Rev. Immunol. 9:39–46 [DOI] [PubMed] [Google Scholar]
- 44. Seki F., Takeda M., Minagawa H., Yanagi Y. 2006. Recombinant wild-type measles virus containing a single N481Y substitution in its haemagglutinin cannot use receptor CD46 as efficiently as that having the haemagglutinin of the Edmonston laboratory strain. J. Gen. Virol. 87:1643–1648 [DOI] [PubMed] [Google Scholar]
- 45. Serafini M., Naldini L., Introna M. 2004. Molecular evidence of inefficient transduction of proliferating human B lymphocytes by VSV-pseudotyped HIV-1-derived lentivectors. Virology 325:413–424 [DOI] [PubMed] [Google Scholar]
- 46. Sirena D., et al. 2004. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J. Virol. 78:4454–4462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tahara M., Takeda M., Seki F., Hashiguchi T., Yanagi Y. 2007. Multiple amino acid substitutions in hemagglutinin are necessary for wild-type measles virus to acquire the ability to use receptor CD46 efficiently. J. Virol. 81:2564–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Takenawa T., Suetsugu S. 2007. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat. Rev. Mol. Cell Biol. 8:37–48 [DOI] [PubMed] [Google Scholar]
- 49. Tatsuo H., Ono N., Tanaka K., Yanagi Y. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406:893–897 [DOI] [PubMed] [Google Scholar]
- 50. Unutmaz D., KewalRamani V. N., Marmon S., Littman D. R. 1999. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J. Exp. Med. 189:1735–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Verhoeyen E., et al. 2003. IL-7 surface-engineered lentiviral vectors promote survival and efficient gene transfer in resting primary T lymphocytes. Blood 101:2167–2174 [DOI] [PubMed] [Google Scholar]
- 52. Vongpunsawad S., Oezgun N., Braun W., Cattaneo R. 2004. Selectively receptor-blind measles viruses: identification of residues necessary for SLAM- or CD46-induced fusion and their localization on a new hemagglutinin structural model. J. Virol. 78:302–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang J. H., Wells C., Wu L. 2008. Macropinocytosis and cytoskeleton contribute to dendritic cell-mediated HIV-1 transmission to CD4+ T cells. Virology 381:143–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Watanabe A., et al. 2010. CD147/EMMPRIN acts as a functional entry receptor for measles virus on epithelial cells. J. Virol. 84:4183–4193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yanagi Y., Takeda M., Ohno S. 2006. Measles virus: cellular receptors, tropism and pathogenesis. J. Gen. Virol. 87:2767–2779 [DOI] [PubMed] [Google Scholar]
- 56. Zaffran Y., et al. 2001. CD46/CD3 costimulation induces morphological changes of human T cells and activation of Vav, Rac, and extracellular signal-regulated kinase mitogen-activated protein kinase. J. Immunol. 167:6780–6785 [DOI] [PubMed] [Google Scholar]
- 57. Zhang J. X., Diehl G. E., Littman D. R. 2008. Relief of preintegration inhibition and characterization of additional blocks for HIV replication in primary mouse T cells. PLoS One 3:e2035. [DOI] [PMC free article] [PubMed] [Google Scholar]