Abstract
The Epstein-Barr virus (EBV) genome is maintained as an extrachromosomal episome during latent infection of B lymphocytes. Episomal maintenance is conferred by the interaction of the EBV-encoded nuclear antigen 1 (EBNA1) with a tandem array of high-affinity binding sites, referred to as the family of repeats (FR), located within the viral origin of plasmid replication (OriP). How this nucleoprotein array confers episomal maintenance is not completely understood. Previous studies have shown that DNA replication forks pause and terminate with high frequency at OriP. We now show that cellular DNA replication fork pausing and protection factors Timeless (Tim) and Tipin (Timeless-interacting protein) accumulate at OriP during S phase of the cell cycle. Depletion of Tim inhibits OriP-dependent DNA replication and causes a complete loss of the closed-circular form of EBV episomes in latently infected B lymphocytes. Tim depletion also led to the accumulation of double-strand breaks at the OriP region. These findings demonstrate that Tim is essential for sustaining the episomal forms of EBV DNA in latently infected cells and suggest that DNA replication fork protection is integrally linked to the mechanism of plasmid maintenance.
INTRODUCTION
Epstein-Barr virus (EBV) is a human gammaherpesvirus that has been linked to several human malignancies, including Burkitt's lymphoma, nasopharyngeal carcinoma, and AIDS-associated non-Hodgkin's lymphomas (reviewed in references 34 and 55). In latently infected tumor and nontumor cells, the viral genome persists predominantly as a multicopy extrachromosomal, double-stranded, closed-circular DNA molecule of ∼170,000 bp. In proliferating cells, the viral genome is replicated by cellular enzymes and subject to many of the same cell cycle controls as the cellular chromosomal DNA (11, 17, 58, 68). Latently infected cells tend to maintain a stable copy number of viral genomes, and the newly replicated genomes are distributed faithfully to daughter cells, similar to duplicating cellular chromosomes (31, 47). Tethering of the viral episome to metaphase chromosomes has been proposed to account for this efficient maintenance and faithful segregation, but many questions regarding the molecular mechanism remain unanswered (48, 60).
Episome stability can be conferred on plasmids through the interaction of Epstein-Barr nuclear antigen 1 (EBNA1) with an ∼1.8-kb viral genetic element referred to as the origin of plasmid replication (OriP) (69, 70). OriP consists of two separable regions, the family of repeats (FR) and a dyad symmetry (DS) element, both of which bind to EBNA1 (54, 69). The FR consists of a tandem array of 30-bp elements, each of which can bind to EBNA1 with high affinity, and a minimum of 8 repeats are required to confer episomal maintenance (12, 28, 39). The DS region consists of phased EBNA1 sites juxtaposed with telomere repeat factor (TRF) binding sites, which together function as an efficient origin of DNA replication initiation (15, 16, 67). EBNA1 binding to OriP is essential for plasmid DNA replication and episome maintenance (39, 66). In addition to direct DNA binding through the C-terminal domain, EBNA1 tethers the EBV genome to metaphase chromosomes through two RGG-like motifs located amino terminal to the DNA binding domain (44, 60). The precise mechanism through which EBNA1 attaches to metaphase chromosomes and how this confers episome maintenance are not completely understood (29, 53, 60).
Although OriP can function as an efficient origin of DNA replication (11, 17, 56, 58), origin activity can be uncoupled from episomal maintenance (50–52). Early studies using two-dimensional neutral agarose gel electrophoresis demonstrated that DNA replication initiates at or near the DS element of OriP and that replication fork pausing occurs at the FR (21, 24, 42). More recent studies using single-molecule analysis of replicating DNA (SMARD) revealed that DNA replication more frequently pauses or terminates at OriP than it does initiate, at least in some cell types (18, 21, 42). These studies suggest that OriP functions predominantly as an episome maintenance element and that DNA replication is required only if replication does not initiate elsewhere. These studies also suggest that replication fork pausing at FR may play an important role in episome maintenance. Several evolutionarily conserved proteins are known to regulate replication fork pausing and termination (3, 7). The human proteins Timeless (Tim) and Tipin (Timeless-interacting protein) and their orthologues Swi1/Swi3 in fission yeast (Schizosaccharomyces pombe) and Tof1/Csm3 in budding yeast (Saccharomyces cerevisiae), have been implicated in stabilizing replication forks at repetitive and complex DNA structures (14, 20, 25, 26, 37, 49). Tim and Tipin, as well as their yeast orthologues, have also been implicated in sister chromatid cohesion and chromosome segregation (45). A more distant relationship is found between mammalian Timeless and the proteins that control circadian rhythm in flies (25). In this study, we explore the possibility that human Tim and Tipin associate with OriP and contribute to episome maintenance. We present evidence that Tim and Tipin localize at OriP in S phase and that depletion of Tim leads to the loss of DNA replication and episome maintenance and the formation of double-strand breaks at OriP.
MATERIALS AND METHODS
Cells, plasmids, and siRNA.
Raji, Mutu I, Oku I, Sav I, and Sav III cells are human EBV-positive Burkitt's lymphoma lines that were cultured in RPMI containing 10% fetal bovine serum (FBS) and supplemented with Glutamax (Invtitrogen) and antibiotics (penicillin and streptomycin). 293 is an EBV-negative human embryonic kidney cell line that was cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics. The plasmids containing OriP and EBNA1 have been described previously (16). Small interfering RNA (siRNA) studies were performed by using Dharmacon Smart Pool siRNA to Timeless (siTim; (catalog no. MQ-019488). Small hairpin RNAs (shRNAs) for Timeless (shTim) and the control (shControl) were obtained from the Sigma/TRC collection of targeted shRNA plasmid library (TRC no. 153090 and 157211), and validated clones were used to generate lentivirus particles in HEK293-derived packaging cells.
ChIP assays.
Chromatin immunoprecipitation (ChIP) assays were performed as described previously (10). Rabbit polyclonal antibodies for ChIP were either raised or were purchased for anti-IgG (Santa Cruz Biotechnology), anti-FLAG (Sigma), anti-Timeless (Bethyl), and anti-Tipin (gift of A. Gotter).
DNA replication assays.
DNA replication assays have been described previously (15). Bromodeoxyuridine (BrdU) incorporation was measured as described previously (71). BrdU combined with propidium iodide staining for fluorescence-activated cell sorter (FACS) analysis was described previously (72).
EBV episome maintenance by pulsed-field electrophoresis.
Raji Oku I, Sav I, Sav II, and Mutu I cells were infected with lentivirus expressing shTim or shControl shRNA. After 48 h postinfection, cells were resuspended in agarose plugs and incubated for 48 h at 50°C in lysis buffer (0.2 M EDTA [pH 8.0], 1% sodium sarcosyl, 1 mg/ml proteinase K). The agarose plugs were washed twice in TE buffer (10 mM Tris [pH 7.5] and 1 mM EDTA). Pulsed-field electrophoresis was performed as described previously for 23 h at 14°C with a linear ramping pulse of 60 to 120 s through 120°C (Bio-Rad CHEF Mapper) (30). DNA was transferred to nylon membranes by established methods for Southern blotting (57). The DNA was then detected by hybridization with a 32P-labeled probe specific for the EBV OriP region and visualized with a Molecular Dynamics PhosphorImager.
2D neutral agarose gel electrophoresis.
DNA for the two-dimensional (2D) gels was extracted by cetyltrimethylammonium bromide (CTAB) method as described by Allers et al. (2). Two-dimensional gel electrophoresis was performed essentially as described previously with modifications (8).
Centrifugal elutriation.
Cell cycle fractionation using centrifugal elutriation was performed with a modified Beckman JE 5.0 using counter flow rates for EBV-positive B lymphocytes as described previously (56, 71).
BrdU IP.
Asynchronously growing cells were pulse-labeled with 50 μM BrdU for 30 min and collected by centrifugation. Cells were lysed in a buffer containing 50 mM Tris (pH 8.0), 1 M NaCl, 10 mM EDTA, 0.5% SDS, and 0.2 mg/ml proteinase K for 2 h at 50°C. The DNA was extracted with phenol-chloroform and precipitated with ethanol. After the DNA had been redissolved in Tris-EDTA (TE), the tubes were sonicated and then denatured at 95°C for 5 min. A 10× concentration of immunoprecipitation (IP) buffer was then added to the sample (100 mM phosphate buffer [pH 7.0], 1.4 M NaCl and 0.5% Triton X-100). BrdU antibody (Becton Dickinson) was then added to each tube, and the tube was rotated for 1 h at room temperature. Rabbit anti-mouse IgG (Sigma) was then added to each tube and incubated again for 30 min with rotation. Samples were washed twice with 1× IP buffer and then centrifuged at 15,000 rpm for 5 min. Pellets were resuspended in 200 μl lysis buffer II (10 mM EDTA, 50 mM Tris [pH 8.8], 0.5% SDS, and 0.25 mg/ml proteinase K) overnight at 37°C. The next day, samples were treated with another 100 μl of lysis buffer II and incubated at 50°C for 1 h. Finally the samples were collected and the DNA was purified with a PCR column and used in real-time PCR with indicated primers.
BrdU FACS.
Asynchronously growing cells were pulse-labeled with 50 μM BrdU for 30 min and collected by centrifugation. Cells were then fixed with 4% paraformaldehyde in vitro for 30 min at 4°C. Following fixation, cells were washed in 1× phosphate-buffered saline (PBS) (three times for 5 min each), and the labeled cells were incubated in HCl (1 N) for 10 min on ice to denature the duplex DNA. This is followed by incubation in HCl (2 N) for 10 min at room temperature before being moved to an incubator for 20 min at 37°C. Immediately after the acid washes, borate buffer (0.1 M)was added to the cells for 15 min at room temperature. Samples were then washed in 1× PBS with 1% Triton X-100 (three times for 5 min each) at room temperature. The cells were preblocked by incubation in 1× PBS plus 1% Triton X-100 plus glycine (1 M) plus 5% bovine serum albumin (BSA) (1 h) prior to incubation overnight (at room temperature) with anti-BrdU antibodies. Following the incubation overnight, the cells were washed in 1× PBS (pH 7.4) with 1% Triton X-100 (three times for 5 min each). Samples were then incubated with a fluorescent-conjugated secondary antibody to visualize the anti-BrdU-labeled cells.
Cell cycle profile analysis.
To determine the cell cycle profiles of cells, cultures were either treated or left untreated and fixed in ice-cold 70% ethanol for at least 30 min. After fixation, cells were stained with staining solution (0.5 mg/ml propidium iodide, 100 mg/ml RNase A) for 30 min in the dark. Samples were analyzed using an EPICS XL (Beckman-Coulter, Inc., Miami, FL), and 50,000 events were recorded. For all flow cytometry experiments, the WINMDI software program (The Scripps Institute) was used to analyze the data.
TUNEL assay.
The terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay to detect apoptotic cells was performed with the Apo-BrdU TUNEL assay kit (A23210; Invitrogen) according to the manufacturer's instructions. The positive (camptothecin-treated) and negative (untreated) control cells were the fixed human lymphoma (HL60) cells provided in the kit.
RESULTS
Replication fork pausing factors Tim and Tipin are enriched at OriP.
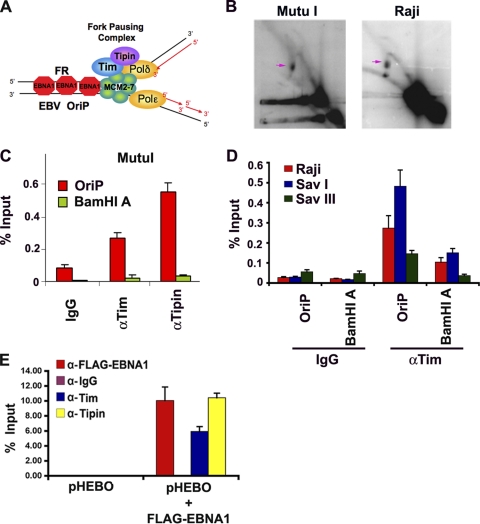
Replication pausing and termination structures have been shown to accumulate at high frequency at OriP in multiple cells carrying latent EBV (Fig. 1A and B) (24). We used two-dimensional neutral agarose gel electophoresis to confirm that replication fork pausing structures were enriched at OriP in two different cell types that maintain stable copy numbers of EBV episomes (Fig. 1B). Replication fork pausing is indicated by the appearance of accumulated DNA in Y-arcs (indicated by the pink arrows in Fig. 1B). The replication fork pausing structure was readily detected in Mutu I and Raji Burkitt's lymphoma cells that are known to maintain stable copy numbers of EBV. We also detected replication bubble arcs and X-spikes in both cell types, but these were less abundant than the fork pausing structures. To determine if known replication fork pausing factors associate with OriP in these cell types, we used chromatin immunoprecipitation (ChIP) assays with antibodies to human Tim, Tipin, or control IgG. We found that Tim and Tipin were enriched at OriP relative to the IgG control and relative to another region of the EBV genome (BamHI A) that has been implicated in replication initiation (Fig. 1C). The same pattern of enrichment was also observed in three other EBV-positive cell lines, including Raji, Sav I, and Sav III (Fig. 1D). We also tested whether Tim and Tipin association with OriP was dependent on EBNA1 binding (Fig. 1E). OriP-containing plasmid (pHEBO) was transfected into HEK293 cells with or without FLAG-EBNA1 expression vector and then assayed by ChIP for binding of Tim, Tipin, or FLAG-EBNA1. We found that Tim and Tipin bound to OriP only in cells expressing FLAG-EBNA1 (Fig. 1E). These results indicate that replisome pausing and protection factors Tim and Tipin associate with OriP in an EBNA1-dependent manner.
Fig. 1.
Replication fork pausing factors associate with the OriP region of EBV. (A) Schematic model of Tim and Tipin bound to replication fork components at EBNA1 binding sites of OriP. (B) 2D neutral agarose gel of OriP replicating DNA in Mutu I (left panel) or Raji (right panel) cells. Replication fork pause structures are indicated by the pink arrows. (C) ChIP assay of Mutu I cells with antibodies to Tim (αTim), Tipin (αTipin), or control IgG. ChIP DNA was quantified by quantitative PCR (qPCR) at OriP or the control BamHI A region. (D) ChIP assay with anti-Tim or control antibody at either OriP or the control BamHI A DNA region for either Raji (red), Sav I (blue), or Sav III (green) cells. (E) 293 cells were transfected with pHEBO with either pFLAG-EBNA1 or the pFLAG-vector control and then assayed by ChIP at 48 h posttransfection. ChIP was performed with antibodies against FLAG (red), Tim (blue), Tipin (yellow), or control IgG (purple) and assayed for OriP DNA by real-time PCR. Error bars represent standard deviations from the mean for at least three experimental replicates.
Cell cycle association of Tim and Tipin at OriP.
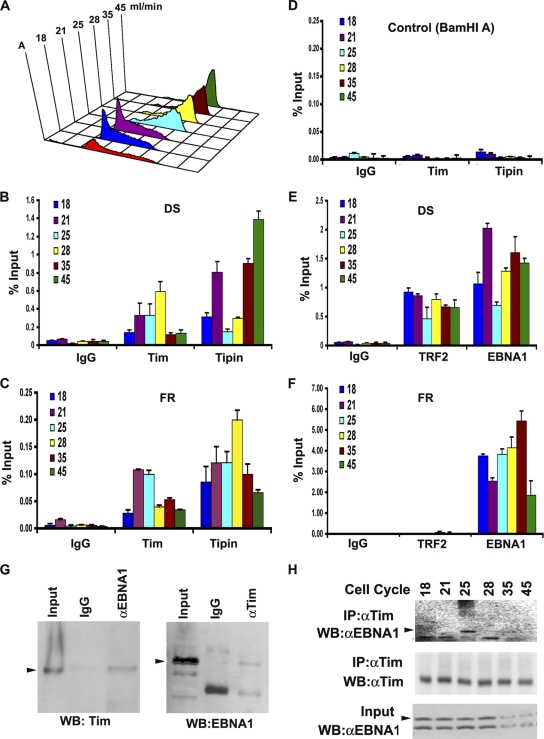
Previous studies have shown that replication factors and histone modifications at OriP are subject to cell cycle regulation (19, 71, 72). We therefore tested whether Tim and Tipin associate with OriP in a cell-cycle-dependent manner (Fig. 2). Mutu I cells were fractionated by centrifugal elutriation for separation of cell cycle stages, which were confirmed by propidium idodide staining followed by FACS analysis (Fig. 2A). Cell-cycle-fractionated cells were then subject to ChIP with antibody to Tipin, Tim, or control IgG (Fig. 2B to D) and assayed at either DS (Fig. 2B), FR (Fig. 2C), or the control region from the EBV BamHI A (Fig. 2D). Both Tim and Tipin were enriched at FR and DS in cell-cycle-dependent fashions, but were not detected at the control BamHI A region. Similar levels of enrichment were observed at DS and FR, suggesting that these two elements may function cooperatively in vivo. We also compared the levels of association of EBNA1 and TRF2 to OriP at these stages of the cell cycle (Fig. 2E and F). Consistent with previous studies, we found that EBNA1 and TRF2 binding to DS was enriched at all stages of the cell cycle (Fig. 2E), while only EBNA1, but not TRF2, bound to the FR region (Fig. 2F). We noted that binding to DS by Tipin, EBNA1, and TRF2 was reproducibly (P < 0.05) reduced in late S phase (Fig. 2B and D, fraction 25), perhaps suggesting that nucleoprotein structures are actively remodeled at DS at this stage of the cell cycle. We also detected a weak interaction between EBNA1 and Tim by coimmunoprecipitation (co-IP) from Mutu I cell extracts (Fig. 2G). Tim protein could be detected in immunoprecipitates with anti-EBNA1 antibody, but not control IgG (Fig. 2G, left panel). Similarly, EBNA1 protein could be detected in immunoprecipitates with anti-Tim antibody (Fig. 2G, right panel). The cell cycle dependence of the interaction between endogenous Tim and EBNA1 was assayed by IP using centrifugal elutriated cell cycle fractions (Fig. 2H). EBNA1 protein was most efficiently recovered from Tim immunoprecipitates derived from the late S phase (fraction 25), although total input EBNA1 protein levels were reduced in later cell cycle fractions. These findings suggest that a cell cycle interaction between EBNA1 and Tim proteins may contribute to the cell cycle enrichment of Tim at OriP observed by ChIP assay (Fig. 1).
Fig. 2.
Cell-cycle-dependent association of Tim and Tipin at OriP. (A) Mutu I cells were fractionated by centrifugal elutriation and then assayed by FACS. Elutriation fractions are indicated in ml/min. (B) ChIP assay with anti-Tim, anti-Tipin, or control IgG at the DS region for each stage of the cell cycle as indicated. (C) Same as in panel B, except for analysis at the FR region. (D) Same as in panel B, except for analysis at the control BamHI A region. (E) ChIP assay with anti-EBNA1, anti-TRF2, or control IgG at the OriP DS region for each stage of the cell cycle, as indicated. (F) Same as in panel E, except for analysis at the FR region. (G) Co-IP analysis of EBNA1 and Tim in asynchronous Mutu I cells. EBNA1 or control IgG IPs were assayed by Western blotting (WB) for Tim protein (left panel). Tim or control IgG IPs were assayed by Western blotting for EBNA1 protein (right panel). Arrowheads indicate the expected sizes of the indicated proteins. αEBNA1, anti-EBNA1; αTim, anti-Tim. (H) Cell cycle fractions from Mutu I cells were subject to IP with antibody to Tim and then assayed by Western blotting with anti-EBNA1 (top panel) or anti-Tim (middle panel). The EBNA1 input for each cell cycle fraction is shown in the lower panel.
Tim depletion inhibits OriP DNA replication.
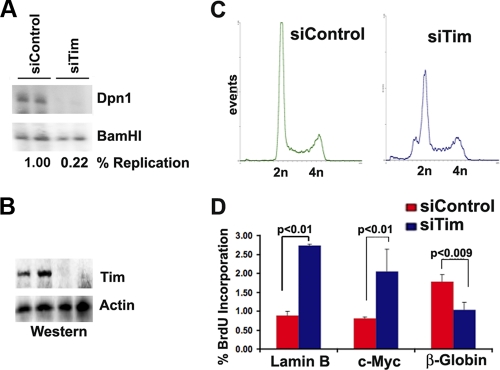
To determine if Tim has a functional role in OriP replication, we tested the effect of Tim depletion on the replication of OriP-containing plasmids (Fig. 3). HEK293 cells were transiently transfected with OriP plasmids that coexpress EBNA1 proteins and replicate efficiently in 48- to 72-h-posttransfection assays. Tim protein was depleted from 293 cells with either an siRNA specific for Tim (Fig. 3) or two different shRNA-containing lentivirus vectors that target different regions of the Tim mRNA (data not shown). We found that Tim protein could be efficiently depleted with siRNA transfection or shRNA lentivirus infection (Fig. 3B and data not shown). We found that siTim and shTim depletions led to a significant reduction of OriP replication (compare DpnI-resistant DNA relative to BamHI total DNA). Quantification of the replicate to unreplicated total DNA indicates that Tim depletion leads to an ∼4- to 5-fold reduction in OriP-dependent DNA replication at 48 h posttransfection. Tim depletion did not cause a cell cycle arrest since FACS analysis did not reveal a significant loss of cells in S phase (Fig. 3C). Analysis of BrdU incorporation at several cellular origins, including lamin B, c-Myc, and β-globin, suggests that Tim depletion does not block cellular DNA synthesis but rather alters the rate of DNA synthesis in a context-dependent manner (Fig. 3D).
Fig. 3.
Inhibition of OriP DNA replication by Tim depletion. (A) Transient DNA replication assay for OriP-EBNA1 plasmid in 293 cells cotransfected with siControl or siTim. Plasmid DNA was isolated 72 h posttransfection, and subjected to restriction enzyme digestion with DpnI-BamHI (top panel) or BamHI alone (lower panel). Quantification of ratio between DpnI-resistant and total BamHI-cut DNA is used to measure the percentage of DNA replication. (B) Western blot of cell extracts from panel A with antibodies to Tim (top panel) or actin (lower panel). (C) FACS analysis of propidium iodide-stained cells for cell cycle profile analysis of siControl- and siTim-transfected cells used for replication assays shown in panel A. (D) BrdU incorporation studies of 293 cells after transfection with siControl or siTim. BrdU incorporation was analyzed at 48 h posttransfection and analyzed for cellular origins at the lamin B, c-Myc, or β-globin loci.
Tim depletion causes a loss of episomal EBV genomes from latently infected B lymphocytes.
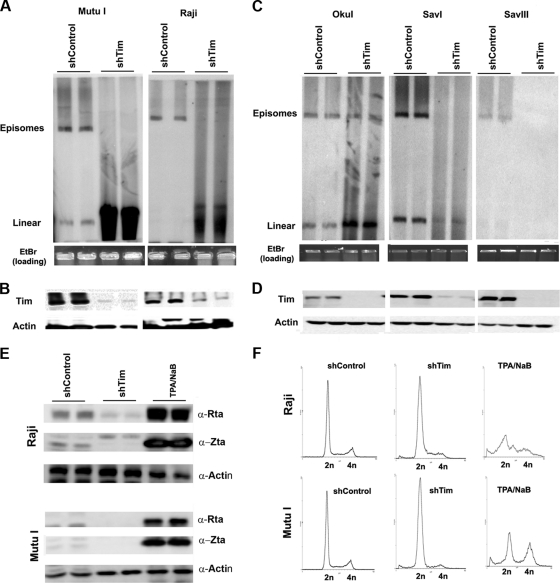
To determine if Tim played a role in EBV episomal maintenance, we assayed the effect of Tim depletion on EBV genomes in latently infected cells (Fig. 4A and C). Raji, Mutu I, Oku I, Sav I, and Sav III cells were infected with lentivirus expressing Tim shRNA or control shRNA. Tim depletion was monitored by Western blot analysis (Fig. 4B and D). To visualize EBV episomes, we assayed EBV-positive cells by pulsed-field gel electrophoresis and Southern blot analysis with a probe for EBV genomic DNA (Fig. 4A and C). DNA was isolated from agarose plugs to avoid any mechanical shearing of chromosomal DNA. Remarkably, we found that Tim depletion caused a substantial loss of the closed-circular episomal form of EBV in Mutu I, Raji, Sav I, Sav III, and, to a lesser extent, Oku I cells (Fig. 4A and C, upper DNA band). The loss of EBV episomal DNA was sometimes compensated for by a corresponding increase in the linear form of EBV DNA (Fig. 4A, lower DNA band). The linear form may be generated by a combination of double-strand breaks in the circular episomal viral genomes and some lytic replication. However, we were unable to detect lytic cycle antigens in Raji or Mutu I cells after Tim depletion (Fig. 4E). shTim depletion did not cause cell cycle arrest, although a reduction of G2 cells and an increase in sub-G1 cells was observed in both cell types, as was demonstrated by FACS analysis (Fig. 4F). These findings suggesting that Tim depletion causes a loss of EBV episomal DNA structure in a variety of cells latently infected with EBV through a process independent of viral lytic replication but potentially linked to a failure to complete DNA synthesis and enter G2/M phase.
Fig. 4.
Loss of EBV episomal forms in Burkitt's lymphoma cell lines after Tim depletion. (A) Pulsed-field electrophoresis gel analysis of Mutu I (left) or Raji (right) cells after infection with shControl or shTim lentivirus. EBV DNA was detected by Southern blotting. Episomes and linear forms of EBV genomes are indicated. EtBr, ethidium bromide. (B) Western blot of cells used for pulsed-field analysis shown in panel A. Antibodies for Tim (top panel) or actin (lower panel) are indicated. (C) Same analysis as described in panel A for Oku I, Sav I, and Sav III cells. (D) Western blots showing Tim (top) and actin (lower) levels for cells analyzed in panel C. (E) Western blot analysis of EBV lytic proteins Rta, Zta, and control actin for Raji and Mutu I cells after infection with shControl or shTim, or after treatment with tetradecanoyl phorbol acetate (TPA) and NaB for 48 h, as indicated. α-, anti-. (F) FACS analysis of cell cycle profiles for Raji and Mutu I cells after infection with shControl or shTim or after treatment with TPA and NaB for 48 h.
Tim depletion causes loss of replication fork structures at OriP.
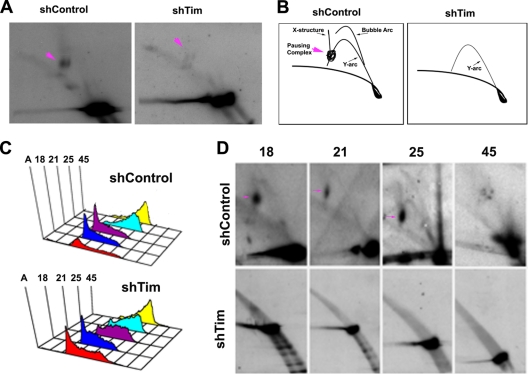
To further investigate the molecular basis for the loss of EBV episomal maintenance, we first assayed replication fork pausing structures at OriP using 2D neutral agarose gel analysis (Fig. 5). Mutu I cells were infected with shControl or shTim lentivirus and then assayed 48 h postinfection (Fig. 5A and B). In shControl cells, a robust replication fork pausing structure was observed (pink arrow, left panel), while this structure was largely absent from shTim-infected cells (right panel). shTim cells had considerably less of the replication fork structures, including Y-arc, suggesting that replication was not occurring or that replication fork structures were highly unstable. To examine the cell cycle appearance of these structures, shControl- and shTim-infected Mutu I cells were subject to centrifugal elutriation (Fig. 5C). DNA from cell cycle fractions was then analyzed by 2D neutral agarose gel for replication fork structures (Fig. 5D). In the shControl-infected Mutu I cells (top panels), we observed a robust replication fork pausing complex in early G1/S phase (pink arrow, fraction 18). Consistent with previous reports (19), a weak bubble arc and recombination-like structure were observed in late S phase (fraction 25), followed by the collapse of the replication fork in G2/M (fraction 45) (Fig. 5C, top panels). In contrast, no replication structures could be observed at OriP in shTim-infected cells at any stage of the cell cycle (Fig. 5C, lower panels). Since replication is thought to occur at some point of the cell cycle, these results suggest that replication fork structures are destabilized in Tim-depleted cells and cannot be detected by the 2D gel and DNA isolation methods employed.
Fig. 5.
Tim depletion causes a loss of replication fork structures at OriP. (A) 2D neutral agarose gel electrophoresis and Southern blot analysis of OriP DNA in Mutu I cells after infection with shControl (left panel) or shTim (right panel) lentivirus. Pink arrows indicate replication fork pausing structures. (B) Schematic interpretation of 2D neutral agarose gels shown in panel A. (C) FACS analysis of cell cycle fractions from centrifugal elutriation after shControl lentivirus infection of Mutu I cells. (B) Same as in panel A, except for shTim lentivirus infection. (D) 2D neutral agarose gel analysis of OriP DNA from shControl (top row)- or shTim (bottom row)-infected Mutu I cells. Cell cycle fractions 18, 21, 25, and 45, are indicated. Replication fork pausing structures are indicated by pink arrows.
Accelerated DNA replication through FR in Tim-depleted cells.
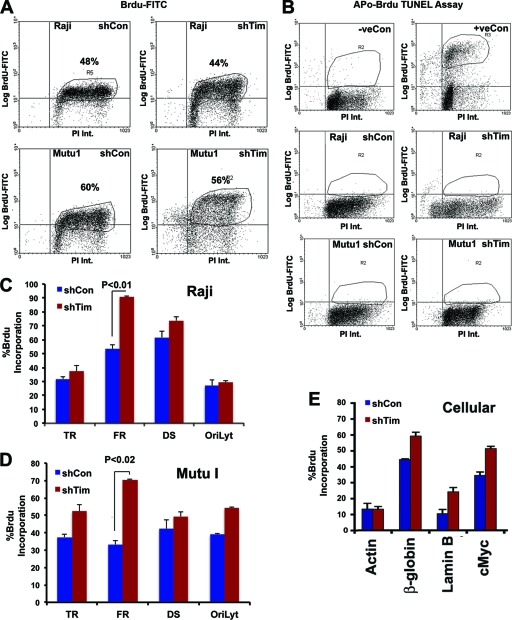
To examine the levels and rates of DNA replication at various positions in the EBV and cellular genome, we assayed BrdU incorporation by ChIP and FACS methods (Fig. 6). We first examined the global effect of Tim depletion on BrdU incorporation into total cellular DNA for both Mutu I and Raji cells (Fig. 6A). We observed that shTim caused a modest (<10%) decrease in total BrdU incorporation in both cell types. Thus, Tim depletion does not completely abrogate cellular DNA synthesis. Furthermore, we examined the effect of Tim depletion on apoptosis in Mutu I and Raji cells (Fig. 6B). Apoptosis was measured by Apo-BrdU TUNEL assay. While positive control samples for apoptosis scored highly positive for BrdU, essentially no TUNEL-positive apoptotic cells were detected in Tim-depleted or control shRNA-infected cells. Thus, Tim depletion does not cause apoptosis in Raji or Mutu I cells. To determine the effect of Tim depletion of BrdU incorporation at various EBV and cellular locations, we used BrdU-dependent IP followed by locus-specific PCR (BrdU-ChIP) (Fig. 6C to E). We found that Tim depletion in Raji (panel C) and Mutu I (panel D) cells produced only a modest increase in BrdU incorporation at several positions, including DS, while a much larger increase (∼2-fold) in BrdU incorporation was observed at FR (Fig. 6C and D). BrdU was also measured at several cellular loci known to possess origins of DNA replication, including Myc, β-globin, and lamin B (Fig. 6E). We found that Tim depletion produced an increase in BrdU incorporation at these cellular origins but not at the actin locus. Since BrdU incorporation was measured after pulse-labeling for 30 min, these results suggest that Tim is important for controlling the rate of DNA replication and that the replication rate is significantly elevated at some regions of the cellular and viral genome, like FR.
Fig. 6.
Accelerated DNA replication through FR in Tim-depleted cells. (A) FACS analysis of Raji (top panels) or Mutu I (lower panels) cells infected with shControl (left panels) or shTim (right panels) after pulse-labeling with BrdU for 30 min, followed by staining with propidium iodide (PI). BrdU intensity (Int.) was monitored by anti-BrdU-conjugated fluorescein isothiocyanate (FITC) (y axis), and PI was monitored on the x axis. (B) Apo-BrdU TUNEL assay for Raji (middle) or Mutu I (lower) after shControl (left) or shTim (right) infection. Control samples were camptothecin-treated (+) or untreated (−) HL60 cells provided by the manufacturer (top panels). (C) BrdU-ChIP assay for Raji cells after infection with shControl (blue) or shTim (red). BrdU incorporation was assayed at EBV locations for terminal repeats (TR), FR, DS, and OriLyt, as indicated. (D) Same as in panel C, except Mutu I cells were analyzed. (E) Same as in panel D, except BrdU was assayed at the cellular loci for actin, β-globin, lamin B, and c-Myc in Mutu I cells. Error bars indicate standard deviations from the mean, and P values were determined by Chi-square test.
Tim depletion causes double-strand breaks at OriP.
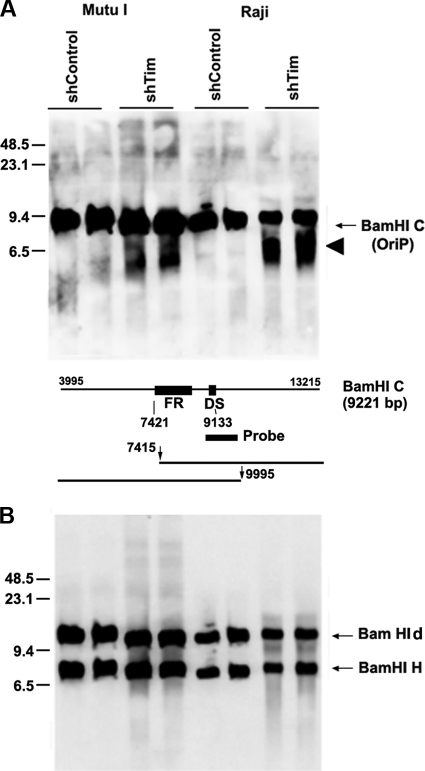
The loss of EBV circular episomes in pulsed-field gels (Fig. 4) and the failure to detect replication structures at OriP in 2D gels (Fig. 5) suggest that Tim depletion resulted in replication fork collapse at sensitive regions of the cellular and viral chromosome. Unrepaired replication fork collapse should generate double-strand breaks. To determine if replication fork collapse occurred at OriP in Tim-depleted cells, we assayed EBV DNA for double-strand break formation using one-dimensional Southern blot analysis (Fig. 7). Mutu I or Raji cells were infected with shTim or shControl lentivirus, and then total cellular DNA was digested with BamHI and analyzed by pulsed-field gel electrophoresis and Southern blot hybridization with probes for either OriP (BamHI C fragment) (Fig. 7A) or with control probes for BamHI d or BamHI H (lytic origin of replication [OriLyt] regions) (Fig. 7B). We found that DNA fragments from OriP were enriched for double-strand breaks in shTim-infected cells relative to shControl. No similar double-strand breaks were observed at BamHI d or BamHI H DNA (Fig. 7B). While the specific site of the double-strand break was not mapped precisely within the ∼9.2-kb BamHI C fragment, the fragment pattern is consistent with double-strand breaks occurring near or within the OriP. These findings suggest that double-strand breaks occur at higher frequency at some genetic loci, like OriP, in Tim-depleted cells relative to control cells.
Fig. 7.
Tim prevents the formation of double-strand breaks at OriP. (A) Mutu I cells (left) or Raji cells (right) were infected with either shControl or shTim, as indicated. Total intact DNA was extracted with agarose plugs digested with BamHI and then assayed by Southern blot analysis with probes for OriP (BamHI C) (A) or OriLyt (detecting OriLyt duplicated sequence in BamHI d and BamHI H) (B). Double-strand breaks are indicated by arrowhead. Molecular mass markers are indicated on the left. A schematic of the BamHI C probe and fragments are shown below panel A. Predicted breakpoints in the OriP region are indicated by the vertical arrow, gel fragments are indicated by horizontal lines, and numbers are derived from the EBV coordinates in GenBank accession no. AJ507799.
DISCUSSION
The molecular mechanism of EBV replication and episome maintenance is critical for understanding its role in pathogenesis and human carcinogenesis (36, 62). The prevailing models explain episome maintenance through the tethering of EBV genomes to metaphase chromosomes through the bivalent binding of EBNA1. EBNA1 binds to EBV OriP through its DNA binding domain and attaches to metaphase chromosomes through two linking regions located amino terminal to the DNA binding domain. The tethering to metaphase chromosomes is mediated by one or more interaction partners that include EBP2 (32, 48, 61), histone H1 (29), HMG2A (59, 60), p32/TAP (65), importin α (Rch1) (35), AT-rich DNA (60), and G-quadruplex RNA (53). More recent studies indicate that EBV episomes are segregated symmetrically and with relatively high fidelity to each daughter cell (31, 47). Furthermore, evidence suggests that segregation is coupled to DNA replication (47). This tendency toward bipolar segregation of newly replicated EBV genomes is not obviously explained by the random attachment to metaphase chromosomes. As an alternative, we propose that the DNA structures generated by programmed replisome pausing at OriP contributes to the chromosome segregation mechanism.
Previous studies found evidence that replication forks pause and frequently terminate at OriP. This conclusion is based on the 2D agarose gel (21, 24, 42), as well as single-molecule analysis of replicating DNA (SMARDS) (51). In addition, we have shown that replication restart and termination-associated recombination structures form at OriP in late S phase, as detected by X-spike structures detected in 2D gel analysis of cell-cycle-fractionated cells (19). Furthermore, homologous recombination repair proteins MRE11 and NBS1 associate with OriP and are required for efficient replication and episome maintenance (19). We therefore examined whether proteins known to function at replication pausing and termination sites also localize and function at OriP. We found that the replication fork pausing factors Tim and Tipin associate with OriP in several different cell types that maintain stable episomes of EBV in tissue culture (Fig. 1). Tim and Tipin bound to OriP in a cell-cycle-dependent manner consistent with their role in replication complex remodeling through the cell cycle (Fig. 2). The functional role of Tim was tested by siRNA and shRNA depletion. All si- and shRNAs that efficiently depleted Tim had a debilitating effect on OriP functions, including plasmid DNA replication (Fig. 3) and viral episome stability in latently infected B lymphocytes (Fig. 4), with no significant inhibition of global cellular DNA synthesis, as measured by the BrdU incorporation rate (Fig. 6A). We found that Tim depletion caused a loss of closed-circular forms of EBV (Fig. 4) and a loss of stable replication fork structures (Fig. 5). In addition, Tim depletion resulted in the formation of double-strand breaks near or within OriP (Fig. 7). Taken together, our data indicate that Tim and Tipin localize at OriP and that Tim plays a critical role in replication fork stability and episome maintenance mediated by OriP.
Tim is a member of a highly conserved family of proteins that have functions in DNA replication fork stability in budding and fission yeast (46). Human Tim and Tipin have been shown to form a complex with claspin, which has been implicated in the intra-S-phase DNA damage response (13, 23). Both claspin and Tim have been shown to bind to the MCM replicative helicase protein complex (23), as well as promote the chromatin binding of DNA polymerase alpha (22), and replication protein A subunits (26, 33). Replication fork monitoring and protection have been proposed for the yeast orthologues of Tim and Tipin (6, 49). Tim has also been implicated in sister chromatid cohesion in both yeast and humans (41). Swi1 and Swi3 are also known to play a role in mating-type switching and imprinting, through a mechanism that may involve the formation of an abasic or ribonucleotide insertion (27, 64). Although there is no evidence for imprinting in EBV genome, others have shown that EBV episome stability requires an infrequent epigenetic event, the molecular basis of which remains unknown (40). It is possible that Tim contributes to the formation or maintenance of the epigenetic change required for EBV maintenance. Based on studies shown here, and previous studies of recombination structures formed at OriP (19), we propose that Tim is required for the replication fork stability and formation of recombination-like structures important for EBV episome maintenance and chromosome segregation (19).
EBNA1 binds with high affinity to a tandem array of repeats (FR) at OriP. At least eight repeats are required for episome stability (12), and EBNA1 binding to FR can inhibit replication fork progression (1, 21). Replication fork blocking proteins, like budding yeast Fob1, can block replicative helicase and be bypassed by recombination-based mechanisms (9, 63). Fob1 functions at the rRNA repeats, which, like OriP, have replication origin activity. Replication fork blocking is also thought to be an important feature of the highly repetitive DNA found in centromeres and centromere-dependent chromosome segregation. Consistent with this model, shRNA depletion of Tim resulted in an increase in BrdU incorporation at FR, suggesting that replication fork blocking was inactivated. This also correlated with a decrease in full-length DNA replication of OriP (Fig. 3), loss of circular episome stability (Fig. 4), and appearance of double-strand breaks at OriP (Fig. 7). These findings suggest that replication fork blocking at FR is important for maintaining DNA stability during DNA synthesis and promoting episome maintenance. Similarities between EBNA1 and other episome maintenance protein, like human papillomavirus (HPV) E2 and Kaposi's sarcoma-associated herpesvirus (KSHV) LANA, strongly suggest that this will be a mechanism shared by these related viruses (4, 5, 38, 43). Precisely how replication fork pausing promotes sister-chromatid cohesion and segregation remains an important area of future investigation. Our findings strongly implicate Tim and Tipin proteins in the regulation of this process at OriP.
ACKNOWLEDGMENTS
We thank Anthony Gotter (Merk Research Laboratories) for the generous gift of Timeless plasmids and antibodies. We also acknowledge Andreas Wiedmer for technical support and the Wistar Institute Cancer Center Core Facilities (P30 CA10815) for Genomics, and Flow Cytometry.
This work was supported by grants from NIH (RO1CA093606 and R01DE017336) to P.M.L.
Footnotes
Published ahead of print on 13 April 2011.
REFERENCES
- 1. Aiyar A., Aras S., Washington A., Singh G., Luftig R. B. 2009. Epstein-Barr Nuclear Antigen 1 modulates replication of oriP-plasmids by impeding replication and transcription fork migration through the family of repeats. Virol. J. 6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allers T., Lichten M. 2000. A method for preparing genomic DNA that restrains branch migration of Holliday junctions. Nucleic Acids Res. 28:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bairwa N. K., Zzaman S., Mohanty B. K., Bastia D. 2010. Replication fork arrest and rDNA silencing are two independent and separable functions of the replication terminator protein Fob1 of Saccharomyces cerevisiae. J. Biol. Chem. 285:12612–12619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ballestas M. E., Chatis P. A., Kaye K. M. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641–644 [DOI] [PubMed] [Google Scholar]
- 5. Ballestas M. E., Kaye K. M. 2001. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mediates episome persistence through cis-acting terminal repeat (TR) sequence and specifically binds TR DNA. J. Virol. 75:3250–3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bando M., et al. 2009. Csm3, Tof1, and Mrc1 form a heterotrimeric mediator complex that associates with DNA replication forks. J. Biol. Chem. 284:34355–34365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Biswas S., Bastia D. 2008. Mechanistic insights into replication termination as revealed by investigations of the Reb1-Ter3 complex of Schizosaccharomyces pombe. Mol. Cell. Biol. 28:6844–6857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brewer B. J., Fangman W. L. 1987. The localization of replication origins on ARS plasmids in S. cerevisiae. Cell 51:463–471 [DOI] [PubMed] [Google Scholar]
- 9. Burkhalter M. D., Sogo J. M. 2004. rDNA enhancer affects replication initiation and mitotic recombination: Fob1 mediates nucleolytic processing independently of replication. Mol. Cell 15:409–421 [DOI] [PubMed] [Google Scholar]
- 10. Chau C. M., Lieberman P. M. 2004. Dynamic chromatin boundaries delineate a latency control region of Epstein-Barr virus. J. Virol. 78:12308–12319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chaudhuri B., Xu H., Todorov I., Dutta A., Yates J. L. 2001. Human DNA replication initiation factors, ORC and MCM, associate with oriP of Epstein-Barr virus. Proc. Natl. Acad. Sci. U. S. A. 98:10085–10089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chittenden T., Lupton S., Levine A. J. 1989. Functional limits of oriP, the Epstein-Barr virus plasmid origin of replication. J. Virol. 63:3016–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chou D. M., Elledge S. J. 2006. Tipin and Timeless form a mutually protective complex required for genotoxic stress resistance and checkpoint function. Proc. Natl. Acad. Sci. U. S. A. 103:18143–18147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dalgaard J. Z., Klar A. J. 2000. swi1 and swi3 perform imprinting, pausing, and termination of DNA replication in S. pombe. Cell 102:745–751 [DOI] [PubMed] [Google Scholar]
- 15. Deng Z., Atanasiu C., Burg J. S., Broccoli D., Lieberman P. M. 2003. Telomere repeat binding factors TRF1, TRF2, and hRAP1 modulate replication of Epstein-Barr virus OriP. J. Virol. 77:11992–12001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deng Z., et al. 2002. Telomeric proteins regulate episomal maintenance of Epstein-Barr virus origin of plasmid replication. Mol. Cell 9:493–503 [DOI] [PubMed] [Google Scholar]
- 17. Dhar S. K., et al. 2001. Replication from oriP of Epstein-Barr virus requires human ORC and is inhibited by geminin. Cell 106:287–296 [DOI] [PubMed] [Google Scholar]
- 18. Dhar V., Schildkraut C. L. 1991. Role of EBNA-1 in arresting replication forks at the Epstein-Barr virus oriP family of tandem repeats. Mol. Cell. Biol. 11:6268–6278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dheekollu J., Deng Z., Wiedmer A., Weitzman M. D., Lieberman P. M. 2007. A role for MRE11, NBS1, and recombination junctions in replication and stable maintenance of EBV episomes. PLoS One 2:e1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Egel R. 2004. DNA replication: stalling a fork for imprinting and switching. Curr. Biol. 14:R915–R917 [DOI] [PubMed] [Google Scholar]
- 21. Ermakova O. V., Frappier L., Schildkraut C. L. 1996. Role of the EBNA-1 protein in pausing of replication forks in the Epstein-Barr virus genome. J. Biol. Chem. 271:33009–33017 [DOI] [PubMed] [Google Scholar]
- 22. Errico A., et al. 2009. Tipin/Tim1/And1 protein complex promotes Pol alpha chromatin binding and sister chromatid cohesion. EMBO J. 28:3681–3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Errico A., Costanzo V., Hunt T. 2007. Tipin is required for stalled replication forks to resume DNA replication after removal of aphidicolin in Xenopus egg extracts. Proc. Natl. Acad. Sci. U. S. A. 104:14929–14934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gahn T. A., Schildkraut C. L. 1989. The Epstein-Barr virus origin of plasmid replication, oriP, contains both the initiation and termination sites of DNA replication. Cell 58:527–535 [DOI] [PubMed] [Google Scholar]
- 25. Gotter A. L. 2006. A Timeless debate: resolving TIM's noncircadian roles with possible clock function. Neuroreport 17:1229–1233 [DOI] [PubMed] [Google Scholar]
- 26. Gotter A. L., Suppa C., Emanuel B. S. 2007. Mammalian TIMELESS and Tipin are evolutionarily conserved replication fork-associated factors. J. Mol. Biol. 366:36–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holmes A. M., Kaykov A., Arcangioli B. 2005. Molecular and cellular dissection of mating-type switching steps in Schizosaccharomyces pombe. Mol. Cell. Biol. 25:303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Humme S., et al. 2003. The EBV nuclear antigen 1 (EBNA1) enhances B cell immortalization several thousandfold. Proc. Natl. Acad. Sci. U. S. A. 100:10989–10994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hung S. C., Kang M. S., Kieff E. 2001. Maintenance of Epstein-Barr virus (EBV) oriP-based episomes requires EBV-encoded nuclear antigen-1 chromosome-binding domains, which can be replaced by high-mobility group-I or histone H1. Proc. Natl. Acad. Sci. U. S. A. 98:1865–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johnson P. G., Beerman T. A. 1994. Damage induced in episomal EBV DNA in Raji cells by antitumor drugs as measured by pulsed field gel electrophoresis. Anal. Biochem. 220:103–114 [DOI] [PubMed] [Google Scholar]
- 31. Kanda T., Kamiya M., Maruo S., Iwakiri D., Takada K. 2007. Symmetrical localization of extrachromosomally replicating viral genomes on sister chromatids. J. Cell Sci. 120:1529–1539 [DOI] [PubMed] [Google Scholar]
- 32. Kapoor P., Lavoie B. D., Frappier L. 2005. EBP2 plays a key role in Epstein-Barr virus mitotic segregation and is regulated by Aurora family kinases. Mol. Cell. Biol. 25:4934–4945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kemp M. G., et al. 2010. Tipin-replication protein A interaction mediates Chk1 phosphorylation by ATR in response to genotoxic stress. J. Biol. Chem. 285:16562–16571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kieff E. 2007. Epstein-Barr virus and its replication, 5th ed Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 35. Kim A. L., et al. 1997. An imperfect correlation between the DNA replication activity of Epstein-Barr virus nuclear antigen 1 (EBNA1) and the binding to the nuclear import receptor, Rch1/importin alpha. Virology 239:340–351 [DOI] [PubMed] [Google Scholar]
- 36. Klein E., Kis L. L., Klein G. 2007. Epstein-Barr virus infection in humans: from harmless to life endangering virus-lymphocyte interactions. Oncogene 26:1297–1305 [DOI] [PubMed] [Google Scholar]
- 37. Krings G., Bastia D. 2004. swi1- and swi3-dependent and independent replication fork arrest at the ribosomal DNA of Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. U. S. A. 101:14085–14090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kung S. H., Medveczky P. G. 1996. Identification of a herpesvirus Saimiri cis-acting DNA fragment that permits stable replication of episomes in transformed T cells. J. Virol. 70:1738–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee M. A., Diamond M. E., Yates J. L. 1999. Genetic evidence that EBNA-1 is needed for efficient, stable latent infection by Epstein-Barr virus. J. Virol. 73:2974–2982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leight E. R., Sugden B. 2001. Establishment of an oriP replicon is dependent upon an infrequent, epigenetic event. Mol. Cell. Biol. 21:4149–4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leman A. R., Noguchi C., Lee C. Y., Noguchi E. 2010. Human Timeless and Tipin stabilize replication forks and facilitate sister-chromatid cohesion. J. Cell Sci. 123:660–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Little R. D., Schildkraut C. L. 1995. Initiation of latent DNA replication in the Epstein-Barr virus genome can occur at sites other than the genetically defined origin. Mol. Cell. Biol. 15:2893–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Loeb D. D., et al. 1990. Plasmid origin of replication of herpesvirus papio: DNA sequence and enhancer function. J. Virol. 64:2876–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marechal V., et al. 1999. Mapping EBNA-1 domains involved in binding to metaphase chromosomes. J. Virol. 73:4385–4392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mayer M. L., et al. 2004. Identification of protein complexes required for efficient sister chromatid cohesion. Mol. Biol. Cell 15:1736–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McFarlane R. J., Mian S., Dalgaard J. Z. 2010. The many facets of the Tim-Tipin protein families' roles in chromosome biology. Cell Cycle 9:700–705 [DOI] [PubMed] [Google Scholar]
- 47. Nanbo A., Sugden A., Sugden B. 2007. The coupling of synthesis and partitioning of EBV's plasmid replicon is revealed in live cells. EMBO J. 26:4252–4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nayyar V. K., Shire K., Frappier L. 2009. Mitotic chromosome interactions of Epstein-Barr nuclear antigen 1 (EBNA1) and human EBNA1-binding protein 2 (EBP2). J. Cell Sci. 122:4341–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Noguchi E., Noguchi C., McDonald W. H., Yates J. R., III, Russell P. 2004. Swi1 and Swi3 are components of a replication fork protection complex in fission yeast. Mol. Cell. Biol. 24:8342–8355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Norio P., Schildkraut C. L. 2004. Plasticity of DNA replication initiation in Epstein-Barr virus episomes. PLoS Biol. 2:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Norio P., Schildkraut C. L. 2001. Visualization of DNA replication on individual Epstein-Barr virus episomes. Science 294:2361–2364 [DOI] [PubMed] [Google Scholar]
- 52. Norio P., Schildkraut C. L., Yates J. L. 2000. Initiation of DNA replication within oriP is dispensable for stable replication of the latent Epstein-Barr virus chromosome after infection of established cell lines. J. Virol. 74:8563–8574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Norseen J., Johnson F. B., Lieberman P. M. 2009. Role for G-quadruplex RNA binding by Epstein-Barr virus nuclear antigen 1 in DNA replication and metaphase chromosome attachment. J. Virol. 83:10336–10346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rawlins D. R., Milman G., Hayward S. D., Hayward G. S. 1985. Sequence-specific DNA binding of the Epstein-Barr virus nuclear antigen (EBNA-1) to clustered sites in the plasmid maintenance region. Cell 42:859–868 [DOI] [PubMed] [Google Scholar]
- 55. Rickinson A. B., Kieff E. 2007. Epstein-Barr virus, 5th ed Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 56. Ritzi M., et al. 2003. Complex protein-DNA dynamics at the latent origin of DNA replication of Epstein-Barr virus. J. Cell Sci. 116:3971–3984 [DOI] [PubMed] [Google Scholar]
- 57. Sambrook J., Fritsch E. F., Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 58. Schepers A., et al. 2001. Human origin recognition complex binds to the region of the latent origin of DNA replication of Epstein-Barr virus. EMBO J. 20:4588–4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sears J., Kolman J., Wahl G. M., Aiyar A. 2003. Metaphase chromosome tethering is necessary for the DNA synthesis and maintenance of oriP plasmids but is insufficient for transcription activation by EBNA1. J. Virol. 77:11767–11780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sears J., et al. 2004. The amino terminus of Epstein-Barr virus (EBV) nuclear antigen 1 contains AT hooks that facilitate the replication and partitioning of latent EBV genomes by tethering them to cellular chromosomes. J. Virol. 78:11487–11505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shire K., Ceccarelli D. F., Avolio-Hunter T. M., Frappier L. 1999. EBP2, a human protein that interacts with sequences of the Epstein-Barr virus nuclear antigen 1 important for plasmid maintenance. J. Virol. 73:2587–2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Thorley-Lawson D. A. 2005. EBV the prototypical human tumor virus—just how bad is it? J. Allergy Clin. Immunol. 116:251–262 [DOI] [PubMed] [Google Scholar]
- 63. Torres J. Z., Bessler J. B., Zakian V. A. 2004. Local chromatin structure at the ribosomal DNA causes replication fork pausing and genome instability in the absence of the S. cerevisiae DNA helicase Rrm3p. Genes Dev. 18:498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vengrova S., Dalgaard J. Z. 2004. RNase-sensitive DNA modification(s) initiates S. pombe mating-type switching. Genes Dev. 18:794–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang Y., Finan J. E., Middledorp J. M., Hayward S. D. 1997. P32/TAP, a cellular protein that interacts with EBNA-1 of Epstein-Barr virus. Virology 236:18–29 [DOI] [PubMed] [Google Scholar]
- 66. Yates J., Camiolo S. M. 1988. Dissection of DNA replication and enhancer activation functions of Epstein-Barr virus nuclear antigen 1. Cancer Cells 6:197–205 [Google Scholar]
- 67. Yates J. L., Camiolo S. M., Bashaw J. M. 2000. The minimal replicator of Epstein-Barr virus oriP. J. Virol. 74:4512–4522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yates J. L., Guan N. 1991. Epstein-Barr virus-derived plasmids replicate only once per cell cycle and are not amplified after entry into cells. J. Virol. 65:483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yates J. L., Warren N., Reisman P., Sugden B. 1984. A cis-acting element from Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc. Natl. Acad. Sci. U. S. A. 81:3806–3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yates J. L., Warren N., Sugden B. 1985. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature 313:812–815 [DOI] [PubMed] [Google Scholar]
- 71. Zhou J., et al. 2005. Cell cycle regulation of chromatin at an origin of DNA replication. EMBO J. 24:1406–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhou J., Snyder A., Lieberman P. M. 2009. Epstein-Barr virus episome stability is coupled to a delay in replication timing. J. Virol. 83:2154–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]