Abstract
The role of the hepatitis C virus (HCV) NS3/4A protease in ablating the signaling pathway involved in the production of alpha/beta interferon (IFN-α/β) suggests a relationship between NS3/4A proteolytic activity and a patient's response to IFN-based therapy. To identify viral factors associated with the HCV treatment response, we analyzed the pretreatment NS3/4A protease gene quasispecies composition of 56 HCV genotype 1–HIV-1-coinfected patients treated in our clinic with pegylated IFN (pegIFN) plus ribavirin (RBV). The catalytic efficiency of the dominant (i.e., the most abundant) quasispecies was also assayed for Cardif cleavage and correlated with treatment outcome. A total of 1,745 clones were isolated and sequenced. Significantly less nucleotide quasispecies heterogeneity and lower Shannon entropy values were detected within the responder group (P < 0.05). A correlation was also found between the efficiency of NS3/4A protease Cardif cleavage and therapy outcome. Proteases from sustained responder patients were more efficient at processing Cardif (mean ± standard error of the mean [SEM], 0.8960 ± 0.05568; n = 19) than proteases from nonresponders (mean ± SEM, 0.7269 ± 0.05306; n = 37; P < 0.05). Finally, the amino acid p distance (the proportion [p] of nucleotide sites at which two sequences being compared are different) was significantly shorter in patients with an interleukin-28B (IL-28B) risk allele (P < 0.01), suggesting that IL-28B risk allele carriers exert a lower positive selection pressure on the NS3/4A protease. NS3/4A protease efficiency in cleaving Cardif may be associated with the pegIFN-RBV treatment response, as shown in our cohort of HIV-HCV-coinfected patients. Greater NS3/4A nucleotide heterogeneity and higher Shannon entropy values in nonresponders suggest that less HCV quasispecies complexity may favor a better response to pegIFN-RBV.
INTRODUCTION
Hepatitis C virus (HCV) nonstructural protein 3 (NS3) contains a serine protease that cleaves the virus-encoded polyprotein and inactivates cellular proteins required for innate immunity. HCV NS3/4A protease functions as an antagonist of virus-induced interferon (IFN) regulatory factor 3 activation and IFN-β expression through its ability to block retinoic acid-inducible gen I (RIG-I) and Toll-like receptor 3 signaling by cleaving caspase recruitment domain adaptor-inducing IFN-β (Cardif) and Toll/interleukin-1 (IL-1) receptor domain-containing adaptor-inducing IFN proteins, respectively. NS3/4A protease activity allows the virus to evade the cellular innate immune response, which may influence the subsequent development of adaptive immunity to HCV, virus persistence, and the response to IFN-based therapy (21).
HCV is the causal agent of chronic liver infection, which afflicts more than 170 million people worldwide (http://www.who.int/mediacentre/factsheets/fs164/en/), and one of the leading causes of liver cirrhosis and failure (9). The standard of care for patients with chronic hepatitis C is treatment with pegylated IFN-α (pegIFN-α) plus ribavirin (RBV). However, only 50 to 60% of the patients treated with pegIFN-α and RBV achieve a sustained virologic response (SVR) (18). In human immunodeficiency virus type 1 (HIV-1) patients coinfected with HCV, standard therapy elicits significantly lower rates of SVR. Among patients infected with HCV genotype 1 or 4, the SVR rate is only about 30% (5, 50). Therefore, a considerable effort has been made to develop markers associated with a better response to IFN-based therapies in HCV–HIV-1-coinfected patients.
Similar to other RNA viruses, one prominent feature of HCV is its genetic variability (30). Experimental evidence has demonstrated that HCV populations consist of a distribution of mutant genomes termed quasispecies (25). High mutation rates and the quasispecies dynamics of HCV are intimately related to both viral disease and antiviral treatment strategies (30). Several directly acting antiviral (DAA) agents for HCV infection are in phase 1 to 3 clinical trials (41). The most advanced compounds are inhibitors of the NS3/4A protease and include telaprevir and boceprevir, which are in phase 3 clinical development in combination with pegIFN-α and RBV. However, DAA therapies may be limited by the rapid selection of resistant virus unless administered in combination with pegIFN-α and RBV. Thus, pegIFN is likely to remain a basis of therapy in the near future.
We hypothesize that NS3/4A protease, by disrupting the signaling pathway involved in the production of IFN-α/β, could be implicated in the response to IFN-based therapy. Evasion of innate immune responses likely exerts a negative influence on the subsequent development of adaptive immunity to HCV and possibly contributes to virus persistence and resistance to therapy. We previously established the relationships between genotype, phenotype, and fitness within the NS3/4A quasispecies (13). In the present study, the impact of NS3/4A protease activity and quasispecies complexity on virus clearance after IFN-based therapy was examined in HCV–HIV-1-coinfected patients.
MATERIALS AND METHODS
Patients.
A total of 56 samples from 56 HCV–HIV-1-coinfected patients at our HIV clinic were analyzed (Table 1). All patients included in this study were infected with HCV genotype 1 (38 with subtype 1a and 18 with subtype 1b) and had a standard course of treatment with pegIFN-α and RBV with a known virologic response status at 24 weeks posttreatment. The duration of treatment was the same for all patients. Plasma samples were taken at the pegIFN-α–RBV treatment baseline. Patients 50 and 51 correspond to patients A and B in the study by Franco et al. (13). The HCV genotype, a marker for hepatitis B virus (HBV) infection, HCV and HIV-1 viral loads, CD4+ T cell counts, and liver enzyme levels were all determined using standard procedures. IL-28B genotyping was performed as described previously (2). Written informed consent was obtained from each patient who participated in this study, and ethics approval was obtained from our Institutional Review Board.
Table 1.
Clinical characteristics of patients with chronic HIV-1–HCV coinfection treated with pegIFN-α–RBV therapy
| Parameter | SVR | Treatment failure | P valuea |
|---|---|---|---|
| No. (%) of samples | 19 (34) | 37 (66) | |
| Mean patient age, yr (range) | 40.9 (32–53) | 39.4 (23–50) | 0.3680 |
| No. (%) of patients with IL-28B risk allele rs12979860 | 2 (11) | 24 (65) | 0.0002 |
| No. (%) of: | 0.6936 | ||
| Females | 5 (26) | 8 (22) | |
| Males | 14 (74) | 29 (78) | |
| No. (%) with HCV subtype: | 1.0000 | ||
| 1a | 13 (68) | 25 (68) | |
| 1b | 6 (32) | 12 (32) | |
| Mean CD4+ cell count/μl (range) | 553 (309–1186) | 546 (265–1165) | 0.7423 |
| Mean ALT activity (U/liter) (range) | 77.1 (13–208) | 78.9 (15–239) | 0.7033 |
| Mean AST activity (U/liter) (range) | 48.0 (19–89) | 55.2 (24–157) | 0.4516 |
| Mean HCV RNA level (log10 IU/ml) (range) | 5.75 (3.37–7.11) | 6.25 (5.04–7.10) | 0.0112 |
| No. (%) of patients with undetectable HIV-1 RNA (<50 copies/ml) | 15 (79) | 30 (81) | 1.0000 |
Age, Mann-Whitney U test; IL-28B genotype, Fisher's exact test; gender, Fisher's exact test; HCV subtype, Fisher's exact test; CD4+T cell count, Mann-Whitney U test; ALT (alanine aminotransferase) and AST (aspartate aminotransferase), Mann-Whitney U test; HCV RNA level, unpaired t test; undetectable HIV-1 RNA, Fisher's exact test.
Recovery and analysis of HCV NS3 protease sequences.
RNA extraction and amplification were performed as previously described (14, 26). Briefly, the HCV RNA was extracted from 140 μl of plasma and amplified by PCR using a nested primer set. After the viral RNA was isolated, 5 μl of resuspended RNA was reverse transcribed and PCR amplified by using the SuperScript III One-Step reverse transcription (RT)-PCR system with Platinum Taq DNA polymerase (Invitrogen) and genotype 1a oligonucleotides NS3P1A1 (sense; 5′-CAAGGGGTGGAGGTTGCTGGC-3′, residues 3389 to 3409 of the HCV-J strain with GenBank accession number D90208) and NS4B-4 (antisense; 5′-AGTACTGTATCCCGCTGATG-3′, residues 5647 to 5666 of the HCV-J strain). The oligonucleotides for subtype 1b were HCVproL1 (5′-GCAAGGGTGGCGACTCCTTGC-3′, residues 3392 to 3412 of the HCV-J strain) and NS4B-4. A nested PCR was then performed with a 5′ subtype 1a oligonucleotide (NS31a1a) containing an EcoRI site (underlined), residues 21 to 34 of NS4, and a dipeptide linker, Gly-Gly, along with residues 2 to 8 of NS3 (residues 3411 to 3431 of the HCV-J strain): 5′-GGGTTGAATTCTATGGCTCCTATTGGCTGCGTGGTCATAGTGGGCAGGATTGTTTTGTCCGGGAAGGGAGGACCCATCACGGCGTACGCCCAG-3′. The 3′ oligonucleotide (NS31ar4) was complementary to residues 3930 to 3950 of the HCV-J strain (amino acid residues 175 to 181 of NS3) and contained an in-frame stop codon (bold) flanked by an XhoI site (underlined): 5′-GGGAGGGGCTCGAGTCAGGACCTCATGGTTGTCTCTAG-3′. For subtype 1b, oligonucleotides HCVproL2 (sense, 5′-GGGTTGAATTCTATGGCTCCTATTGGATCTGTTGTTATTGTTGGAAGAATTATTTTGTCTGGAAGAGGAGGACCTATCACGGCCTACTCCCAA-3′, residues 3414 to 3434 of the HCV-J strain) and HCVproR (antisense, 5′-GGGAGGGGCTCGAGTCAAGACCGCATAGTAGTTTCCAT-3′, residues 3933 to 3952 of the HCV-J strain) were used. The PCR products were digested with EcoRI and XhoI and ligated to pBSK (Stratagene) to generate a β-gal–HCV NS32-181/421-34 protease fusion protein (pHCVNS32-181/421-34 protease). To ensure that multiple NS3/4 protease templates were present in each quasispecies that was analyzed, four different PCR amplifications were performed for each sample and pooled before cloning. Endpoint RNA limiting dilution was performed before RT-PCR to ensure that an excess of template HCV RNA was present in the PCR amplification mixtures. At least 25 RNA molecules were added in each of the four PCR amplifications. A minimum of 20 individual plasmid clones were obtained and analyzed for each sample (range, 20 to 60). The previously cloned quasispecies (13), samples 50 and 51 (96 and 97 clones, respectively), were also included in the analysis. The different proteases were sequenced with the flanking oligonucleotides T3 (5′-ATTAACCCTCACTAAAGGGA-3′) and T7 (5′-TAATACGACTCACTATAGGG-3′) using the BigDye v3.1 kit in the 3100 DNA sequencing system (Applied Biosystems). Sequence alignment and editing were performed with the Sequencer version 4.1 (GeneCodes) software program. For phylogenetic analysis, the PAUP* 4.028 software package was used with a GTR + G model of evolution. Nucleotide and amino acid p distances (the proportions [p] of nucleotide sites at which two sequences being compared are different) were calculated by the MEGA 4 software package (48). Nucleotide and amino acid heterogeneity data were obtained from the distance matrix generated by the MEGA 4 software package used to calculate p distances. The normalized Shannon entropy (Sn) value was calculated as Sn = −Σi (piln pi)/ln N, where N is the total number of sequences analyzed and pi is the frequency of each sequence in the viral quasispecies. Sn varies from 0 (no complexity) to 1 (maximum complexity) (51). To determine possible selective pressures, the proportion of synonymous substitutions to potential synonymous sites and the proportion of nonsynonymous substitutions to potential nonsynonymous sites were calculated by the SNAP software program (19). To estimate codon-specific selection pressures, we used the fixed-effects likelihood (FEL) method, which directly estimates nonsynonymous and synonymous substitution rates at each site (17). The HKY85 nucleotide substitution bias model was used with FEL.
Genetic screen for determining the catalytic efficiency of HCV NS3/4A proteases.
The catalytic efficiency of the different HCV NS3/4A proteases was determined using a previously described bacteriophage lambda (λ)-based genetic screen (13, 26). A plasmid with the Cardif NS3/4A protease cleavage site, EREVPC/HRPS, was constructed (pcI.Cardifcro). The Cardif cleavage site was introduced by using pcI.HCVNS4B/NS5Acro (13) as a template and a PCR overlap extension protocol (37). Two fragments containing the Cardif cleavage site were amplified in separate amplifications. For amplification of the 5′ fragment, the cI19 oligonucleotide (sense; 5′-CCATTAACACAAGAGCAGCTT-3′, positions 19 to 39 of the λ cI repressor) (42) was used with an antisense oligonucleotide containing the Cardif cleavage site 4B5ACARDIFR (5′-TGACGGTCGATGGCACGGTACTTCACGCTCCTCATTGCGCGCCTGAACATGAGA-3′). For amplification of the 3′ fragment, cI697R (antisense; 5′-TTCAGGCCACTGACTAGCGAT-3′, positions 679 to 699 of the λ cI repressor) was used with a sense oligonucleotide containing the Cardif cleavage site 4B5ACARDIFF (5′-GAAGTACCGTGCCATCGACCGTCACTAAGGGATGTTTGGGCGCGCATGTTCTCACCT-3′). The PCR mixture contained 20 pmol of each primer, 200 μM deoxynucleoside triphosphates, 2.5 mM MgCl2, PCR buffer (10 mM Tris-HCl, pH 8.3, and 50 mM KCl), and 0.5 U Taq polymerase (Promega) in a total reaction volume of 50 μl. Cycling parameters were 1 cycle of denaturation at 95°C for 2 min, followed by 35 cycles of 30 s at 95°C, 30 s at 55°C, and 45 s at 72°C, with a final extension step of 72°C for 7 min. The 5′ and 3′ PCR fragments were mixed with oligonucleotides cI19 and cI697R, and a second PCR was performed under the conditions described above. The resulting PCR products were digested with NsiI and HindIII and ligated to pcI.HCVNS4B/NS5Acro previously digested with NsiI and HindIII. Escherichia coli JM109 cells containing plasmid pcI.Cardifcro were then transformed with plasmid pHCVNS32-181/421-34 protease. Transformed cells were grown overnight at 30°C in the presence of 0.2% maltose–12.5 μg/ml of tetracycline–20 μg/ml of ampicillin, harvested by centrifugation, and resuspended to an optical density at 600 nm of 2.0/ml in 10 mM MgSO4. To induce the expression of HCV NS32-181/421-34 protease, cells (20 μl) were incubated in 100 μl of Luria-Bertani (LB) medium containing 12.5 μg/ml tetracycline, 20 μg/ml ampicillin, 0.2% maltose, 10 mM MgSO4, and 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 1 h. The cell cultures were then infected with 105 PFU of λ phage. After 3 h at 37°C, the titer of the resulting phage was determined by coplating the cultures with 200 μl of E. coli XL-1 Blue cells (adjusted to an optical density at 600 nm of 2.0/ml in 10 mM MgSO4) on LB plates using 3 ml of top agar containing 12.5 μg of tetracycline/ml, 0.2% maltose, and 0.1 mM IPTG. After incubation at 37°C for 6 h, the resulting phage plaques were counted in order to score growth. In the experiments in which E. coli cells express master protease sequences from study samples, λ phage replicated up to 1.45 log (range, −0.18 to 1.45)-fold more efficiently than in cells that did not express the HCV NS32-181/421-34 construct or a construct with a patient protease variant carrying the inactivating substitution S139A.
Statistical analysis.
The unpaired t test, the Mann-Whitney U test, Fisher's exact test, and linear regression analysis of covariates were performed using GraphPad Prism version 4.00 for Windows (GraphPad, San Diego, CA).
Nucleotide sequence accession numbers.
The HCV NS3 sequences obtained and characterized in this study have been submitted to the GenBank database under accession numbers HQ891983 to HQ893534.
RESULTS
Study population.
Most patients (55/56, 98%) were on antiretroviral therapy, and most patients (45/56, 80%) had controlled HIV-1 replication (below 50 copies/ml). Nineteen patients (34%) responded successfully to HCV treatment (i.e., achieved SVR). The proportion of patients infected with HCV subtype 1a who achieved SVR (32%) was not significantly different from the proportion of patients infected with subtype 1b viruses (32%, P = 1.0000, Fisher's exact test). A significantly higher HCV RNA load was measured in patients who failed treatment (P = 0.0112, unpaired t test). Likewise, IL-28B risk allele rs12979860 was significantly associated with treatment failure (P = 0.0002, Fisher's exact test). HBV infection was detected in only one sample (from patient 45). No significant differences were found between those who achieved SVR and those who did not with respect to sex, age, HIV-1 viral load, liver enzymes, or CD4+ T cell count.
HCV NS3/4A quasispecies diversity.
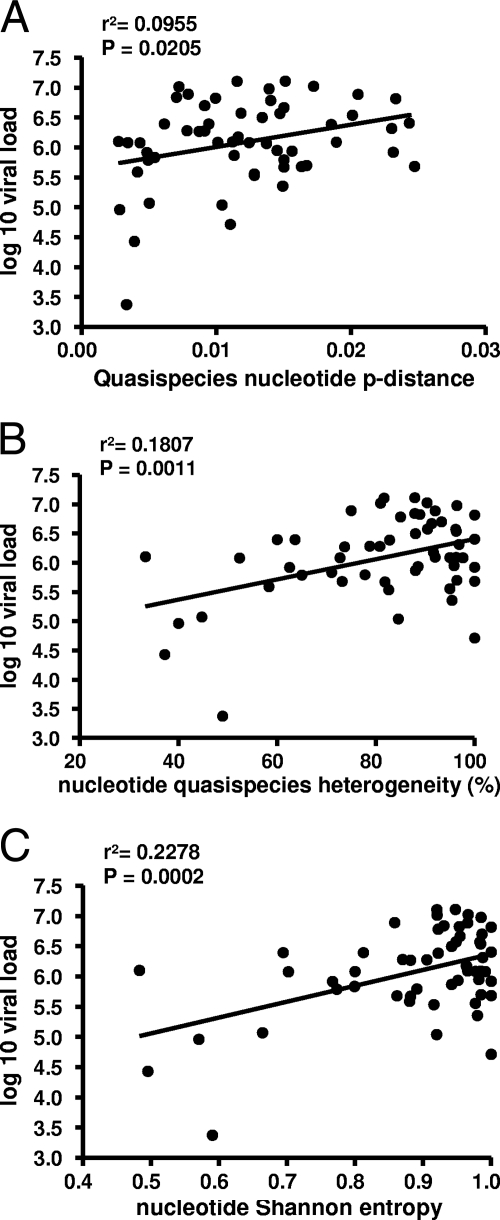
A total of 1,745 clones (an average of 31 clones per patient) were isolated, sequenced, and analyzed. Neighbor-joining phylogenetic reconstruction was performed for all NS3 protease nucleotide sequences to determine the evolutionary relationships of the different variants. Sequences from each individual produced a monophyletic group, which was supported by bootstrap analysis (data not shown). Similarly, sequences from subtype 1a and 1b viruses segregated separately. Similar numbers of clones per patient were obtained from those in the SVR and treatment failure groups (32 and 31, respectively, P = 0.8849, unpaired t test). Diversity was assessed by calculating intrasample genetic distances. Mean nucleotide p distances were higher in the group of patients who failed therapy than in the patient group with SVR (0.0127 and 0.0108, respectively; Table 2). However, these values were not significantly different (P = 0.2579, unpaired t test). A wide range of different nucleotide p distances was found within the two groups of patients (0.0034 to 0.0027 and 0.0027 to 0.0247, respectively), suggesting that different diversifying forces are acting in different individuals. Remarkably, we identified a positive relationship between the nucleotide p distance and patient viral load (P = 0.0205, linear regression; Fig. 1A). No significant difference was found between the amino acid p distance of therapy failures and SVRs (0.0072 and 0.0073, respectively, P = 0.9122, unpaired t test). Interestingly, the amino acid p distance was significantly shorter in patients with an IL-28B risk allele (0.0060 versus 0.0188, P = 0.0084, unpaired t test). This difference in the amino acid p distance was not observed at the nucleotide level (0.0136 versus 0.0150, P = 0.5172, unpaired t test). This result suggests that IL-28B risk allele carriers exert a lower positive selection pressure on the NS3/4A protease.
Table 2.
Comparison of HCV NS3 protease coding region quasispecies
| Parameter | SVR (n = 19) | Treatment failure (n = 37) | P valuea |
|---|---|---|---|
| p distance | |||
| Nucleotides | 0.0108 ± 0.0016 (0.0027–0.0247)b | 0.0127 ± 0.0009 (0.0034–0.0027)b | 0.2579 |
| Amino acids | 0.0073 ± 0.0008 (0.0020–0.0169) | 0.0072 ± 0.0006 (0.0030–0.0159) | 0.9122 |
| % Heterogeneity | |||
| Nucleotides | 74.17 ± 5.33 (33.33–100) | 85.48 ± 2.04 (52.27–100) | 0.0208 |
| Amino acids | 37.88 ± 2.93 (16.67–61.90) | 43.00 ± 3.02 (17.31–80.00) | 0.2825 |
| ds | 0.0320 ± 0.0056 (0.0046–0.0906) | 0.0412 ± 0.0032 (0.0076–0.0856) | 0.1311 |
| dn | 0.0032 ± 0.0003 (0.0009–0.0077) | 0.0031 ± 0.0003 (0.0007–0.0073) | 0.9145 |
| ds/dn ratio | 10.37 ± 1.66 (2.51–26.71) | 16.08 ± 1.72 (3.48–23.11) | 0.0383 |
| Sn value | |||
| Nucleotides | 0.8278 ± 0.0416 (0.4830–1.000) | 0.9188 ± 0.0136 (0.6942–1.000) | 0.0252 |
| Amino acids | 0.4973 ± 0.0439 (0.1978–0.8062) | 0.5525 ± 0.0339 (0.2484–0.8945) | 0.3343 |
Unpaired t test.
Values are mean ± SEM (range).
Fig. 1.
(A) Quasispecies nucleotide p distance as a function of the HCV viral load. (B) Quasispecies nucleotide heterogeneity as a function of the HCV viral load. (C) Quasispecies nucleotide Sn value as a function of the HCV viral load. In panels A, B, and C, a significant positive linear correlation was found.
The synonymous-to-nonsynonymous (ds/dn) mutation ratio, a marker of selective pressure, was compared between patients with SVR and those who failed treatment (Table 2). The ds/dn ratio was greater than 1 in the 56 quasispecies analyzed, indicating a preponderance of genetic drift over selection within the coding region studied. However, different values were found for each quasispecies, ranging from 2.51 to 26.71, demonstrating that different selective constraints may be acting on different quasispecies. Patients who failed treatment exhibited a significantly higher mean ds/dn ratio (16.08) than SVRs (10.37) (P = 0.0383, unpaired t test; Table 2). Again, a significantly lower proportion of nonsynonymous mutations to potential nonsynonymous sites (dn) was observed in patients with an IL-28B risk allele (0.0027 versus 0.0036, P = 0.0490, unpaired t test). In contrast, this difference in the dn value was not observed when the proportion of synonymous mutations to potential synonymous sites (ds) was calculated (0.0037 versus 0.0039, P = 0.7295, unpaired t test). Positive selective pressures were also evaluated using FEL, which directly estimated synonymous and nonsynonymous substitution rates at each site (17). Nine positively selected codons were identified in nine samples (see Table S1 in the supplemental material). Six of these positions, V33, T61, V83, P86, Q89, and A147, are common polymorphisms found in genotype 1 isolates (19). The other three positions, P88, G120, and F169, are highly conserved, though a few variants can be found in databases. Eight of the codons identified, V33, T61, V83, P86, P88, Q89, G120, and A147, have been described as belonging to a cytotoxic T lymphocyte (CTL) epitope (52).
When the nucleotide or amino acid p distances were grouped by subtype, 1a or 1b, independent of therapy response, no significant differences were observed (0.0125 to 0.0111, P = 0.4338, for nucleotides; 0.0068 to 0.0082, P = 0.1571, for amino acids [unpaired t test]).
Mutations conferring resistance to NS3 protease inhibitors (PIs) were also found in 31 (55%) of the study quasispecies (see Table S2 in the supplemental material). Thirty-five (2.0%) of 1,745 individual clones had one resistance substitution. Five quasispecies had the resistance substitution Q80K in the majority of their individual clones. Similarly, one quasispecies had the V55A substitution as a dominant variant. The mutations V36M/A, T54A, V55A, R155K/T, A156T/V, and V170A, which have been found in patients who fail therapy with the PIs telaprevir and boceprevir (40, 41, 47), were also observed in individual clones (see Table S2 in the supplemental material). The proportion of resistance mutations was not different between patients undergoing antiretroviral therapy including or not including HIV-1 PI (P = 0.7859, Fisher exact test).
HCV NS3/4A quasispecies complexity.
Complexity was assessed by computing the heterogeneity and Sn value of each quasispecies. Significantly greater quasispecies nucleotide heterogeneity and higher Sn values were found within the group of patients who failed therapy (Table 2). At the amino acid level, patients who failed therapy also had higher values but the difference was not significant. Similar to nucleotide p distance analysis, we identified a positive relationship between nucleotide heterogeneity and the Sn value and the patient's HCV viral load (P = 0.0011 and P = 0.0002, respectively, linear regression analysis; Fig. 1B and C). These results demonstrate that lower treatment baseline NS3 protease quasispecies complexity is associated with SVR.
When sequences were grouped by subtype, a significantly higher Sn value was observed in subtype 1b amino acid quasispecies (0.4974 and 0.6104 for subtypes 1a and 1b, respectively, P = 0.0484, unpaired t test). No significant differences were observed at the nucleotide level (0.9054 and 0.8616 for subtypes 1a and 1b, respectively, P = 0.2404, unpaired t test).
Catalytic efficiency of HCV NS3/4A protease and response to IFN-based therapy.
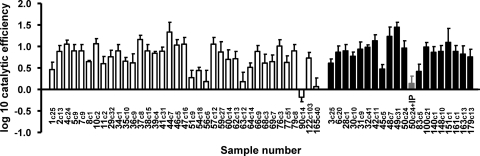
The catalytic efficiency of the dominant quasispecies of each type (Fig. 2) was assayed for Cardif cleavage using a bacteriophage λ genetic screen (13, 26). The enzymatic activities of variant proteases were evaluated by engineering the Cardif cleavage site in the λ cI repressor. The enzymatic activity was related to the activity of a patient protease variant carrying the inactivating substitution S139A. The distribution of log enzymatic activity values ranged over 2 orders of magnitude, which likely reflects the fact that mutations present in the different proteases affected their catalytic Cardif-cleaving efficiency (Fig. 3A). To further demonstrate the specificity of the genetic screen employed here, the assay was also performed in the presence of 25a, an inhibitor of the HCV NS3/4A protease (22). The enzymatic activity of one of the proteases that displayed a high efficiency level (sample 50) was inhibited by 25a (Fig. 3A). When the log enzymatic activities were grouped by patient response to IFN-based therapy, a significantly higher value was obtained in the SVR group (mean ± standard error of the mean [SEM], SVR versus treatment failure group, 0.8970 ± 0.0556 versus 0.7269 ± 0.0530; P = 0.0497, unpaired t test; Fig. 3B). Remarkably, within the treatment failure group, some proteases had no Cardif-cleaving activity (samples 90 and 156; Fig. 3A) or very low activity (samples 51, 56, and 63). Proteases with low catalytic efficiency were not observed in the SVR group. Notably, the former low-activity proteases displayed good catalytic efficiency when tested with the HCV NS5A/NS5B cleavage site (data not shown). When proteases were grouped by IL-28B genotype, those obtained from patients carrying the IL-28B risk genotype displayed a lower mean log catalytic efficiency level (mean ± SEM, 0.7013 ± 0.0856 versus 0.8175 ± 0.0456). However, this difference was not significant (P = 0.2027, unpaired t test). Overall, these results suggest that NS3/4A protease Cardif-cleaving efficiency may be associated with the pegIFN-RBV treatment response in HIV-HCV-coinfected patients.
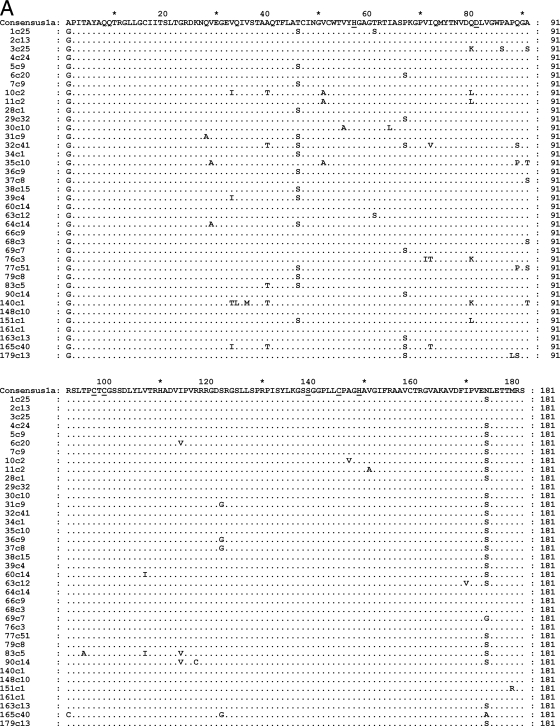
Fig. 2.
(A) Amino acid sequence alignment of the subtype 1a NS3/4A dominant quasispecies. (B) Amino acid sequence alignment of the subtype 1b NS3/4A dominant quasispecies. Amino acid changes are indicated relative to a consensus amino acid obtained from a reference database that included 307 sequences of subtype 1a or 328 sequences of subtype 1b (19). The catalytic triad of the NS3/4A protease formed by His 57, Asp 81, and Ser 139 is underlined. The Zn2+ binding site, Cys 97, Cys 99, Cys 145, and His 149, is also underlined. Dots indicate amino acid sequence identity.
Fig. 3.
Comparative catalytic efficiencies of 56 master HCV NS3/4A proteases based on Cardif cleavage. The catalytic efficiency of each protease variant was compared to that of a patient protease variant carrying the inactivating substitution S139A (0.0). White bars correspond to patients who failed therapy. Black bars correspond to patients with SVR. One gray bar corresponds to sample 50, which was tested in the presence of 20 μM 25a, an inhibitor of the HCV NS3/4A protease (22). Three independent replicates were performed for each sample. Error bars correspond to standard deviations. Catalytic efficiency distribution of 37 master NS3/4A proteases from treatment failure patients (squares) compared to the distribution of 19 proteases from patients with SVR (triangles). The horizontal continuous lines represent the mean values. NR, nonresponders.
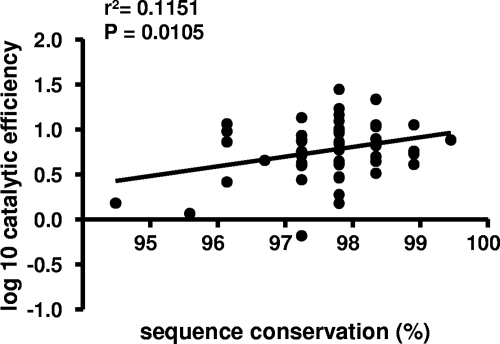
Next, the relationship between protease enzymatic activity and sequence conservation was investigated. The protease Cardif-cleaving activity was compared to how conserved the protease amino acid sequences were relative to amino acid frequencies in a reference database that included 307 sequences of subtype 1a (consensus 1a in Fig. 2A) and 328 sequences of subtype 1b (consensus 1b in Fig. 2B) (19). A positive linear relationship was found between the conservation of sequences relative to the site-specific database frequencies and their relative catalytic efficiency (r2 = 0.1151, P = 0.0105, linear regression; Fig. 4). This result indicates that selected or random deleterious mutations are imprinted in HCV NS3 protease sequences. In contrast, no correlation was found between protease activity and patient HCV viral load (r2 = 0.0008, P = 0.8284, linear regression).
Fig. 4.
HCV NS3/4A protease catalytic efficiency based on Cardif cleavage as a function of sequence conservation. The catalytic efficiency was measured for 56 master proteases obtained from 56 patients and compared to how conserved the protease amino acid sequences were relative to amino acid frequencies in a reference database that included 307 sequences of subtype 1a and 328 sequences of subtype 1b (19).
Finally, when protease catalytic efficiencies were grouped by subtype, a significantly higher activity level was observed in 1b samples (mean ± SEM, 1b versus 1a, 0.9119 ± 0.0564 versus 0.7238 ± 0.0517; P = 0.0307, unpaired t test). Dominant quasispecies were also grouped by subtype (Fig. 2). Noticeably, subtype 1b virus nucleotide and amino acid p distances were significantly greater than those of 1a viruses (0.0694 and 0.0872 for nucleotide subtypes 1a and 1b, respectively, P < 0.0001; 0.0240 and 0.0272 for amino acid subtypes 1a and 1b, respectively, P = 0.0074, unpaired t test), indicating greater nucleotide and amino intersample diversification among 1b dominant quasispecies.
DISCUSSION
Treatment-induced clearance of HCV infection can be affected by various host and viral factors. Because a significant number of patients fail to respond to current IFN-based therapy or have significant side effects, predicting the treatment response is of major interest. In addition, patients with HIV-1 coinfection, particularly those infected with HCV genotype 1, have significantly lower rates of treatment response than HCV-monoinfected patients. Significant factors associated with SVR are the baseline HCV load and genotype. Despite HCV quasispecies and treatment response being a controversial topic, HCV quasispecies complexity before therapy has been independently associated with treatment response (1, 7, 8, 10, 29, 31, 36, 43, 45). However, although prior studies have demonstrated correlations in the genetic complexity of HCV hypervariable region 1 (HVR1) at the baseline and nonresponse to IFN-based therapy (31), other studies have found that HVR1 genetic complexity or heterogeneity did not correlate with HCV susceptibility to IFN-based therapy (23, 38). Genetic studies recently identified several single nucleotide polymorphisms (SNPs) in and near IL-28B (which encodes IFN-λ3) that are strongly associated with HCV clearance and SVR after IFN-based therapy (15, 35, 46, 49). This association is highly significant for genotype 1-infected patients and more controversial for non-genotype 1 infections (27, 28, 34, 35). Importantly, HIV-1 infection does not affect the association between IL-28B SNPs and HCV clearance (2, 32, 34). However, the IL-28B genotype does not have a positive predictive value of 100% for SVR and cannot be used as the only predictor of the IFN-based treatment response (4).
In this study, low NS3 protease nucleotide quasispecies complexity and high NS3/4A protease Cardif-cleaving efficiency were significantly associated with a successful response to pegIFN-α–RBV treatment. These findings provide support for a role for the NS3/4A protease in IFN-based therapies. Though the correlation between low protease Cardif-cleaving efficiency and treatment failure is counterintuitive, this result reflects the fact that sensitivity to exogenous IFN is inversely associated with levels of IFN-stimulated genes (ISGs) (39). Moreover, individuals with IL-28B genotypes associated with SVR have lower pretreatment levels of hepatic ISG expression than individuals carrying the risk genotypes (16). Activation of the endogenous IFN system in HCV-infected patients may hamper the response to IFN-based therapy, most likely by inducing a refractory state of the IFN signaling pathway (39). Nevertheless, alternative interpretations of our findings cannot be discarded because there is a wide variation in the ISG expression and induction levels among patients during IFN-based therapy. A recent study has demonstrated that NS3/4A-mediated Cardif cleavage is an important, but not unique, determinant of activation of the IFN system in the livers of patients with chronic hepatitis C (6). We also observed significantly lower protease activity in 1a samples than in 1b samples. Similarly, subtype 1a has been associated with a weaker response to IFN-based therapy than subtype 1b (20).
Another important correlation found in this study is the positive relationship between the catalytic efficiency of the NS3/4A protease and its amino acid conservation. The precise conservation of some NS3 protease segments implies that certain amino acid changes affect enzyme viability. Indeed, mutations associated with drug resistance and CTL escape have been shown to have an impact on HCV fitness (44). Yet, little is known about the distribution of catalytic efficiency among NS3 protease mutations at the population level. Our results demonstrate how positive selection processes, random drift, or purifying negative selection of insufficiently fit variants can imprint HCV NS3 protease sequences and, as a consequence, modulate HCV fitness. Importantly, some mutations can affect the capability of the protease to process Cardif but not its ability to cleave viral polyprotein. This finding has implications not only for HCV fitness at the population level but also for therapeutic strategies targeting NS3/4A protease and aimed at limiting viral replication.
This study extends and confirms recent studies in which HCV quasispecies (43), in particular, NS3 quasispecies (8), complexity before treatment was identified as an independent predictor of SVR. A wide range of nucleotide and amino acid quasispecies diversity was observed in both patient groups, those with SVR and those who failed treatment. However, no significant differences were detected between the groups. A positive correlation was found between quasispecies nucleotide diversity and sample viral load, indicating that both high NS3 quasispecies diversity and a heavy viral load are signaling in the same direction: treatment failure.
To the best of our knowledge, this is the first study to investigate the relationship between HCV quasispecies diversity and the host IL-28B genotype. Our results provide compelling evidence of less NS3 protease amino acid quasispecies diversification in patients with an IL-28B risk allele and suggest that IL-28B risk allele carriers exert less positive selection pressure on the NS3/4 protease. Whether the lower amino acid quasispecies diversification in patients with an IL-28B risk allele is restricted to the NS3 protease coding region or affects other viral genomic regions remains to be elucidated. Lower NS3 protease amino acid quasispecies complexity (heterogeneity and Sn value) was also detected in IL-28B risk allele carriers, but this difference was not significant (data not shown). Taking into account the diversification of nucleotide quasispecies, which is essentially almost identical in the two groups of IL-28B genotype carriers, a tendency for less NS3 protease amino acid quasispecies diversification in patients carrying the IL-28B risk allele is clear. Determination of the IL-28B genotype in longitudinal HCV quasispecies previously reported in studies may provide critical insights into the relationship between IL-28B and early spontaneous virus clearance (12), IFN-associated viral clearance (8, 11, 31), HCV emergence following liver transplantation (24), and the association with progression to end-stage liver disease (33).
PI mutations observed in our study include V36A, Q41R, F43S, T54A, V55A, Q80R/K, R155K/T, A156T/V, D168G/N/E, and V170A. Three PI mutations (V36M, V55A, and Q80K) were found as dominant variants. The substitutions V36M and V55A were identified in one sample, whereas the substitution Q80K was detected in four patient samples. Remarkably, one sample (number 140) had two dominant resistance mutations: V36M and Q80K. The V36M mutation confers low-to-moderate resistance to telaprevir, boceprevir, and narlaprevir and higher resistance to telaprevir in conjunction with a R155K or A156T mutation (40). The V55A mutation confers low resistance to boceprevir (47). The Q80K mutation is associated with significantly reduced susceptibility to TMC-434350 and low-level resistance to vaniprevir and danoprevir but wild-type susceptibility to telaprevir and boceprevir (3). The other mutations associated with resistance were observed only as low-level variants, and most of them were detected in one clone per sample; only mutations Q41R and D168G were found in two clones of the same sample. The mutations R155K/T and A156T/V, which are associated with resistance to multiple PIs (41), including telaprevir and boceprevir, were also detected in our study samples. The A156S/T/V mutations are associated with high resistance to telaprevir and boceprevir (40, 47). These findings expand upon those of Chary et al. (8), confirming that the mutations R155K/T and A156T/V are minority variants in PI-naïve patients. Using an allele-specific PCR protocol that detects the A156S/T/V substitutions in at least 0.05 to 0.5% of the viral population, we observed that 65% of PI-naïve patients had at least one minor resistance variant at this NS3 position (S. Franco et al., unpublished data). Nevertheless, whether drug-resistant mutants present at low levels are associated with an increased risk of PI therapy failure remains to be elucidated.
Our study has some limitations that are worth noting. First, although our conclusions were supported statistically, they had narrow significance. Our results were likely limited by the small sample size, particularly by the small group of patients with SVR. Nevertheless, our results are in agreement with previous work and show a consistent trend. Second, the in vitro approach used to measure the capability of the protease to cleave Cardif only partially mimics what happens in vivo. However, the finding that defective or highly deleterious proteases that cleave Cardif were observed only in patients who failed therapy strongly supports the hypothesis of a role for the NS3/4A protease in the response to IFN-based therapies. Future work should include an evaluation of the pretreatment hepatic activation of endogenous IFN and whether it is related to the catalytic efficiency of the HCV NS3/4A protease in vivo.
Supplementary Material
ACKNOWLEDGMENT
This study was supported by grants from the Spanish Ministry of Science and Innovation (BFU2010-15194 and SAF2010-21617).
We thank Daria Hazuda of Merck, Sharp and Dohme for providing the Z5a protease inhibitor.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 6 April 2011.
REFERENCES
- 1. Abbate I., et al. 2004. HVR-1 quasispecies modifications occur early and are correlated to initial but not sustained response in HCV-infected patients treated with pegylated- or standard-interferon and ribavirin. J. Hepatol. 40:831–836 [DOI] [PubMed] [Google Scholar]
- 2. Aparicio E., et al. 2010. IL28B SNP rs8099917 is strongly associated with pegylated interferon-alpha and ribavirin therapy treatment failure in HCV–HIV-1 coinfected patients. PLoS One 5:e13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bae A., et al. 2010. Susceptibility of treatment-naive hepatitis C virus (HCV) clinical isolates to HCV protease inhibitors. Antimicrob. Agents Chemother. 54:5288–5297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Balagopal A., Thomas D. L., Thio C. L. 2010. IL28B and the control of hepatitis C virus infection. Gastroenterology 139:1865–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ballesteros A. L., et al. 2004. Early HCV dynamics on Peg-interferon and ribavirin in HIV/HCV co-infection: indications for the investigation of new treatment approaches. AIDS 18:59–66 [DOI] [PubMed] [Google Scholar]
- 6. Bellecave P., et al. 2010. Cleavage of mitochondrial antiviral signaling protein in the liver of patients with chronic hepatitis C correlates with a reduced activation of the endogenous interferon system. Hepatology 51:1127–1136 [DOI] [PubMed] [Google Scholar]
- 7. Chambers T. J., et al. 2005. Quasispecies heterogeneity within the E1/E2 region as a pretreatment variable during pegylated interferon therapy of chronic hepatitis C virus infection. J. Virol. 79:3071–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chary A., et al. 2010. Impact of interferon-ribavirin treatment on hepatitis C virus (HCV) protease quasispecies diversity in HIV- and HCV-coinfected patients. J. Infect. Dis. 202:889–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chevaliez S., Pawlotsky J. M. 2007. Hepatitis C virus: virology, diagnosis and management of antiviral therapy. World J. Gastroenterol. 13:2461–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Donlin M. J., et al. 2007. Pretreatment sequence diversity differences in the full-length hepatitis C virus open reading frame correlate with early response to therapy. J. Virol. 81:8211–8224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farci P., et al. 2006. Evolution of hepatitis C viral quasispecies and hepatic injury in perinatally infected children followed prospectively. Proc. Natl. Acad. Sci. U. S. A. 103:8475–8480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farci P., et al. 2000. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science 288:339–344 [DOI] [PubMed] [Google Scholar]
- 13. Franco S., Parera M., Aparicio E., Clotet B., Martinez M. A. 2007. Genetic and catalytic efficiency structure of an HCV protease quasispecies. Hepatology 45:899–910 [DOI] [PubMed] [Google Scholar]
- 14. Franco S., Tural C., Clotet B., Martinez M. A. 2007. Complete nucleotide sequence of genotype 4 hepatitis C viruses isolated from patients co-infected with human immunodeficiency virus type 1. Virus Res. 123:161–169 [DOI] [PubMed] [Google Scholar]
- 15. Ge D., et al. 2009. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 461:399–401 [DOI] [PubMed] [Google Scholar]
- 16. Honda M., et al. 2010. Hepatic ISG expression is associated with genetic variation in interleukin 28B and the outcome of IFN therapy for chronic hepatitis C. Gastroenterology 139:499–509 [DOI] [PubMed] [Google Scholar]
- 17. Kosakovsky Pond S. L., Frost S. D. 2005. Not so different after all: a comparison of methods for detecting amino acid sites under selection. Mol. Biol. Evol. 22:1208–1222 [DOI] [PubMed] [Google Scholar]
- 18. Kronenberger B., Zeuzem S. 2009. Current and future treatment options for HCV. Ann. Hepatol. 8:103–112 [PubMed] [Google Scholar]
- 19. Kuiken C., Yusim K., Boykin L., Richardson R. 2005. The Los Alamos hepatitis C sequence database. Bioinformatics 21:379–384 [DOI] [PubMed] [Google Scholar]
- 20. Legrand-Abravanel F., et al. 2009. Influence of the HCV subtype on the virological response to pegylated interferon and ribavirin therapy. J. Med. Virol. 81:2029–2035 [DOI] [PubMed] [Google Scholar]
- 21. Lemon S. M. 2010. Induction and evasion of innate antiviral responses by hepatitis C virus. J. Biol. Chem. 285:22741–22747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liverton N. J., et al. 2008. Molecular modeling based approach to potent P2-P4 macrocyclic inhibitors of hepatitis C NS3/4A protease. J. Am. Chem. Soc. 130:4607–4609 [DOI] [PubMed] [Google Scholar]
- 23. López-Labrador F. X., et al. 1999. Relationship of the genomic complexity of hepatitis C virus with liver disease severity and response to interferon in patients with chronic HCV genotype 1b infection [correction of interferon]. Hepatology 29:897–903 [DOI] [PubMed] [Google Scholar]
- 24. Lyra A. C., et al. 2002. Evolution of hepatitis C viral quasispecies after liver transplantation. Gastroenterology 123:1485–1493 [DOI] [PubMed] [Google Scholar]
- 25. Martell M., et al. 1992. Hepatitis C virus (HCV) circulates as a population of different but closely related genomes: quasispecies nature of HCV genome distribution. J. Virol. 66:3225–3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martinez M. A., Clotet B. 2003. Genetic screen for monitoring hepatitis C virus NS3 serine protease activity. Antimicrob. Agents Chemother. 47:1760–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCarthy J. J., et al. 2010. Replicated association between an IL28B gene variant and a sustained response to pegylated interferon and ribavirin. Gastroenterology 138:2307–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Montes-Cano M. A., et al. 2010. Interleukin-28B genetic variants and hepatitis virus infection by different viral genotypes. Hepatology 52:33–37 [DOI] [PubMed] [Google Scholar]
- 29. Morishima C., et al. 2006. Hepatitis C virus-specific immune responses and quasi-species variability at baseline are associated with nonresponse to antiviral therapy during advanced hepatitis C. J. Infect. Dis. 193:931–940 [DOI] [PubMed] [Google Scholar]
- 30. Pawlotsky J. M. 2006. Hepatitis C virus population dynamics during infection. Curr. Top. Microbiol. Immunol. 299:261–284 [DOI] [PubMed] [Google Scholar]
- 31. Pawlotsky J. M., et al. 1998. Genetic complexity of the hypervariable region 1 (HVR1) of hepatitis C virus (HCV): influence on the characteristics of the infection and responses to interferon alfa therapy in patients with chronic hepatitis C. J. Med. Virol. 54:256–264 [PubMed] [Google Scholar]
- 32. Pineda J. A., et al. 2010. Prediction of response to pegylated interferon plus ribavirin by IL28B gene variation in patients coinfected with HIV and hepatitis C virus. Clin. Infect. Dis. 51:788–795 [DOI] [PubMed] [Google Scholar]
- 33. Qin H., et al. 2005. HCV quasispecies evolution: association with progression to end-stage liver disease in hemophiliacs infected with HCV or HCV/HIV. Blood 105:533–541 [DOI] [PubMed] [Google Scholar]
- 34. Rallón N. I., et al. 2010. Association of a single nucleotide polymorphism near the interleukin-28B gene with response to hepatitis C therapy in HIV/hepatitis C virus-coinfected patients. AIDS 24:F23–F39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rauch A., et al. 2010. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology 138:1338–1345 [DOI] [PubMed] [Google Scholar]
- 36. Salmerón J., et al. 2008. Quasispecies as predictive factor of rapid, early and sustained virological responses in chronic hepatitis C, genotype 1, treated with peginterferon-ribavirin. J. Clin. Virol. 41:264–269 [DOI] [PubMed] [Google Scholar]
- 37. Sambrook J., Russell D. W. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor Laboratory, NY [Google Scholar]
- 38. Sandres K., et al. 2000. Genetic heterogeneity of hypervariable region 1 of the hepatitis C virus (HCV) genome and sensitivity of HCV to alpha interferon therapy. J. Virol. 74:661–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sarasin-Filipowicz M., et al. 2008. Interferon signaling and treatment outcome in chronic hepatitis C. Proc. Natl. Acad. Sci. U. S. A. 105:7034–7039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sarrazin C., et al. 2007. Dynamic hepatitis C virus genotypic and phenotypic changes in patients treated with the protease inhibitor telaprevir. Gastroenterology 132:1767–1777 [DOI] [PubMed] [Google Scholar]
- 41. Sarrazin C., Zeuzem S. 2010. Resistance to direct antiviral agents in patients with hepatitis C virus infection. Gastroenterology 138:447–462 [DOI] [PubMed] [Google Scholar]
- 42. Sauer R. T. 1978. DNA sequence of the bacteriophage gama cI gene. Nature 276:301–302 [DOI] [PubMed] [Google Scholar]
- 43. Sherman K. E., et al. 2010. Hepatitis C virus (HCV) quasispecies complexity and selection in HCV/HIV-coinfected subjects treated with interferon-based regimens. J. Infect. Dis. 201:712–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shimakami T., et al. 2011. Protease inhibitor-resistant hepatitis C virus mutants with reduced fitness from impaired production of infectious virus. Gastroenterology 140:667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shire N. J., et al. 2006. HCV kinetics, quasispecies, and clearance in treated HCV-infected and HCV–HIV-1-coinfected patients with hemophilia. Hepatology 44:1146–1157 [DOI] [PubMed] [Google Scholar]
- 46. Suppiah V., et al. 2009. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat. Genet. 41:1100–1104 [DOI] [PubMed] [Google Scholar]
- 47. Susser S., et al. 2009. Characterization of resistance to the protease inhibitor boceprevir in hepatitis C virus-infected patients. Hepatology 50:1709–1718 [DOI] [PubMed] [Google Scholar]
- 48. Tamura K., Dudley J., Nei M., Kumar S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 49. Tanaka Y., et al. 2009. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat. Genet. 41:1105–1109 [DOI] [PubMed] [Google Scholar]
- 50. Torriani F. J., et al. 2004. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N. Engl. J. Med. 351:438–450 [DOI] [PubMed] [Google Scholar]
- 51. Wolinsky S. M., et al. 1996. Adaptive evolution of human immunodeficiency virus-type 1 during the natural course of infection. Science 272:537–542 [DOI] [PubMed] [Google Scholar]
- 52. Yusim K., et al. 2005. Los Alamos hepatitis C immunology database. Appl. Bioinformatics 4:217–225 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.