Abstract
The global transcriptional program of murine cytomegalovirus (MCMV), involving coding, noncoding, and antisense transcription, remains unknown. Here we report an oligonucleotide custom microarray platform capable of measuring both coding and noncoding transcription on a genome-wide scale. By profiling MCMV wild-type and immediate-early mutant strains in fibroblasts, we found rapid activation of the transcriptome by 6.5 h postinfection, with absolute dependency on ie3, but not ie1 or ie2, for genomic programming of viral gene expression. Evidence is also presented to show, for the first time, genome-wide noncoding and bidirectional transcription at late stages of MCMV infection.
TEXT
Murine cytomegalovirus (MCMV) is a ubiquitous betaherpesvirus with a 235-kbp genome transcribed in a classical cascade fashion (55). The genome sequence of MCMV has been available for some time (89), and yet a systematic study of temporal gene expression during MCMV infection has been lacking. Double-stranded PCR-based cDNA microarrays were used once previously (105) to validate the expression of a subset of predicted MCMV open reading frames (ORFs) (15) at a single time point (24 h postinfection [hpi]). More-advanced microarray technology based on oligonucleotide probes, affording increased specificity to distinguish RNA polarity, has not been reported. For human cytomegalovirus (HCMV), abundant antisense (AS) transcription has been observed (120), raising the possibility that aspects of the CMV life cycle are influenced or regulated by noncoding transcripts. For MCMV, small virus-encoded microRNAs (miRNAs) (19, 20, 33) and larger double-stranded RNAs (21, 110) have been reported to be transcribed from multiple loci; however, the frequency and abundance of noncoding transcripts throughout the MCMV genome have not yet been measured systematically on a genome-wide scale and at multiple stages of infection.
Here we have investigated the global transcriptional program of MCMV by constructing a microarray capable of measuring sense (S) and AS transcripts. Microarrays were designed using 55-mer oligonucleotide probes in sense and antisense orientations to each of the 170 viral ORFs predicted in the MCMV genome (89). One hundred ninety-two positive-control probes were designed against stably expressed mouse genes for normalization purposes, and 97 negative-control probes were designed against Saccharomyces cerevisiae sequences with no homology to mouse or MCMV genomes (for probe sequences, see Table S1 in the supplemental material). The 55-mer oligonucleotide probes were diluted to a concentration of 60 μM and inkjet printed (Arrayjet, United Kingdom) onto amino silane-coated glass slides, with each microarray consisting of six identical subarrays. Probes were printed in triplicate per array and have the capacity for developing a total of 18 measurements per probe per sample to ensure high technical replication. Target RNA was extracted from infected fibroblasts using PureLink RNA minikits with on-column DNase I treatment (Invitrogen, CA). Purified RNA (700 ng for each sample) was labeled for microarray analysis using the Agilent low-input fluorescent linear amplification protocol (Agilent, CA), with 3 μg of Cy5-labeled target cRNA hybridized per sample. Hybridized microarrays were washed and subsequently scanned using an Agilent (CA) G2505B scanner.
To perform a systematic analysis of genome-wide transcription in MCMV, we infected NIH 3T3 fibroblasts with the parental MCMV strain at a multiplicity of infection (MOI) of 1 and performed DNA microarray analysis on total RNA harvested from duplicate cultures at 0.5, 6.5, 24, and 48 hpi. Individual probe signals were background subtracted, median summarized, and log base 2 transformed to form raw data points (see Table S2 in the supplemental material). Raw data were quality controlled, and normalization between samples was performed based on a subset of 44 positive-control probes highly correlated across the data set (Pearson r of >0.90). Normalized expression data (see Table S3 in the supplemental material) were subjected to a statistically rigorous threshold detection methodology for providing on/off calls for each probe based on a receiver operating characteristic (ROC) (12). From these ROC analyses, we evaluated specificity levels corresponding to given sensitivities of 70%, 80%, and 90%. At a moderate sensitivity of 70%, we were able to obtain an average specificity of 93%, and this was chosen as affording an optimal balance between identifying true positives and excluding true negatives with stringency (for ROC plots, see Fig. S1 and S2 in the supplemental material). Accordingly, we detected 297 total probes having “on” calls and 163 probes for coding MCMV ORFs, making 87.6% of the MCMV genome detectable at 48 hpi (for a list of genes detected, see Table S4 in the supplemental material).
To account for experimental variation, statistical testing (empirical Bayes moderated t test) was applied between mock-infected and infected groups to identify differential expression of only the most highly significant MCMV ORFs. By use of this more stringent approach, 119 ORFs were found to be significantly activated to a confidence level of P ≤ 0.05 above mock-infected levels at all time points (Table 1). These included the DNA polymerase subunit M54 (59), known inhibitors of major histocompatibility complex class I (MHC-I) surface expression m04 (gp34) (51) and m06 (gp48) (90), and the Fc receptor m138 (108). After a single round of replication at 24 hpi, a total of 111 MCMV ORFs were detected at the high significance level. To further validate these findings, a subset of MCMV ORFs were subjected to quantitative reverse transcription-PCR (qRT-PCR) analysis (for primer sequences, see File S1 in the supplemental material), and in agreement with the microarray results, each test case showed that ORF expression was also detectable by qRT-PCR (Fig. 1a).
Table 1.
High-confidence MCMV microarray sense probesa
| Unique probe IDb | ORF | Strand | MCMV name | HCMV name | Time onc (hpi) | Protein type | Annotationd (reference[s]) | Virion associatione |
|---|---|---|---|---|---|---|---|---|
| vMC132 | m128 Ex3 | C | ie2 | US22 (GF2) | 0.5 | Immediate early | Spliced m128 (ie2) gene has sequence similarity to members of the US22 family of HCMV (77) | |
| vIE1rem | m123 Ex4 | C | ie1 | IE1 | 6.5 | Immediate early | ie1 exon 4, with mRNA terminating at base 179544 (53–55); total length of Ex2 plus Ex3 plus Ex4 is 595 aa, total molecular size is 66.7 kDa | |
| vMC003 | m03 | 6.5 | Glycoprotein m02 | Sequence variation and early transcriptional kinetics found in wild-type-derived MCMV isolates; m03-encoded protein could be found on cell surface (28) | ||||
| vMC004 | m04 | gp34 | 6.5 | Glycoprotein m02 | m04 gene product (gp34) forms a complex with MHC-I (51), which reaches the cell surface (46) and is required for Ly49P recognition of infected cells (57); m04/gp34 also antagonizes the effect of m152 (84) | |||
| vMC006 | m06 | gp48 | 6.5 | Glycoprotein m02 | m06 gene product (gp48) downmodulates the levels of MHC-I proteins in infected cells (50) and binds to the ternary complex of assembled MHC-I with antigenic peptide in the ER, redirecting this complex to the lysosome for degradation; unbound gp48 is destroyed by the proteasome (18) | |||
| vMC008 | m08 | 6.5 | Glycoprotein m02 | Glycoprotein family m02 | ||||
| vMC010 | m10 | 6.5 | Glycoprotein m02 | Glycoprotein family m02 | ||||
| vMC016 | m16.2 | 6.5 | Glycoprotein m02 | Glycoprotein family m02, probe overlaps with newly predicted ORF m16.2 (105) | ||||
| vMC017 | m17 | C | 6.5 | Membrane glycoprotein | ||||
| vMC018 | m18 | C | 6.5 | Early | Highly antigenic early gene (44) | VAP | ||
| vMC027 | m25.1 | C | UL23 (GF2) | 6.5 | US22 family homolog | Mutant showed no obvious growth phenotype (75) | ||
| vMC030 | M28 | C | UL28 | 6.5 | ||||
| vMC037 | m34.2 | UL34 | 6.5 | Reannotations of the MCMV genome have identified three putative M34-overlapping ORFs (m33.1, m34.1, and m34.2); this microarray probe overlaps with newly predicted ORF m34.2 (105); an M34 mutant virus which interrupted all three m34 ORFs had attenuated replication both in tissue culture and in SCID mice (10) | ||||
| vMC040 | M36 Ex2 | C | UL36 exon 2 | 6.5 | US22 family homolog | Possible transcriptional regulator (89), implicated in blocking apoptosis via inhibition of caspase-8 (FLICE) activation (100); growth of M36 mutant was attenuated in vitro and in vivo (27); HCMV vICA/pUL36 protects cells from extrinsic apoptosis induced via death receptors in TNFR1, Fas/CD95, or Trail | ||
| vMC041 | M37 | C | UL37 | 6.5 | Glycoprotein | Glycoprotein, vMIA; M37 mutant is severely attenuated in growth and virulence in vivo (63); homolog of HCMV UL37 that inhibits mitochondrial megapore activation in a manner similar to members of the antiapoptotic Bcl family (37); may also be a transcriptional regulator (58) | ||
| vMC045 | m41 | C | 6.5 | Putative antiapoptotic function (79) | ||||
| vMC046 | m42 | C | 6.5 | Putative glycoprotein | ||||
| vMC047 | M43 | C | UL43 | 6.5 | US22 family homolog | Antiapoptotic (79), immunoregulatory gene that modulates T helper cell response (99); found to be nonessential for viral growth in vitro and in vivo and dispensable for virulence in killing SCID mice (117) | VAP | |
| vMC049 | M45 | C | UL45 (RRL) | 6.5 | Ribonucleotide reductase | Antiapoptotic (17) homologue of the large subunit of ribonucleotide reductase (85); blocks NF-κB activation as a result of its inhibitory effect on RIP1 signaling (71, 109) | VAP | |
| vMC055 | m48.2 | C | 6.5 | Capsid | Smallest capsid protein (14) | VAP | ||
| vMC061 | M54 | C | DNApol | UL54 (DNApol) | 6.5 | DNA polymerase | DNA polymerase delta subtype (59) | VAP |
| vMC064 | M57 | C | UL57 (MDBP) | 6.5 | DNA binding | Major ssDNA binding protein (3, 4) | VAP | |
| vMC079 | M80 | UL80 (AP) | 6.5 | Assembly protein, protease | Assembly protein and protease (11, 69) that conserves the domain structure and cleavage sites present in HCMV UL80 | VAP | ||
| vMC080 | M82 | C | pp71 | UL82 (pp71) | 6.5 | Upper matrix phosphoprotein | Encodes a structural protein unique to the betaherpesvirus group, also known as pp71; same family as M83 (91) | VAP |
| vMC092 | M94 | UL94 | 6.5 | Virion associated | Virion-associated, tegument/second envelopment protein (112) | VAP | ||
| vMC098 | M99 | UL99 (pp28) | 6.5 | Phosphoprotein | Encodes small structural phosphoprotein unique to the betaherpesvirus group, shown to be around 16 kDa in size and to be associated with the virion (30, 78) | VAP | ||
| vMC100 | M102 | UL102 (HP) | 6.5 | Helicase-primase | Part of the helicase-primase complex of three proteins (M70, M102, and M105) (70, 97) | VAP | ||
| vMC103 | M105 | UL105 (Hel) | 6.5 | DNA helicase | DNA helicase; part of the helicase-primase complex of three proteins (M70, M102, and M105) (70, 97) | VAP | ||
| vMC114 | M118 | C | UL118 | 6.5 | Possible alternate splice to m119, as for HCMV UL118 (62, 88) | |||
| vMC117 | m119.2 | C | sgg1 | 6.5 | Exon 2 of M133 (sgg1) (61, 73) | |||
| vMC136 | m132 Ex2 | C | 6.5 | |||||
| vMC141 | m137 | C | 6.5 | Putative glycoprotein | ||||
| vMC142 | m138 | C | Fcgr | fcr1 | 6.5 | Glycoprotein | Encodes the 88-kDa Fc receptor glycoprotein (107); Fc receptor-specific (m138) deletion mutants show unexpected alterations in virulence and are attenuated in normal and immunosuppressed adult mice (31); m138 downmodulates the NKG2D ligands MULT-1, H60 (65), and RAE-1 epsilon (8) | |
| vMC146 | m142 | C | US26 (GF2) | 6.5 | US22 family homolog | Required to block PKR-mediated shutdown of cellular protein synthesis and associated antiviral response (21, 110) | ||
| vMC151 | m147 | C | 6.5 | Possible membrane-spanning protein | Spliced gene m147.5 selectively targets CD86 expression on APCs (68) | VAP | ||
| vMC156 | m152 | C | gp40 | 6.5 | Glycoprotein | m152 gene product (gp40) is a member of the MGP family that downmodulates MHC-I proteins in infected cells (50), disrupts export of MHC-I complexes from pre-Golgi compartments to the Golgi compartment via a luminal domain (122), and modulates NK cell response (inhibition), antigen presentation, and T cell response (40); viruses lacking gp40 show increased susceptibility to CTL killing in mice (43) | ||
| vMC158 | m154 | C | 6.5 | Glycoprotein | Threonine-serine-rich glycoprotein of MGP family m145, some homology to EHV1 g(X) (106) | |||
| vMC164 | m160 | C | 6.5 | Putative membrane glycoprotein | ||||
| vMC167 | m163 | C | 6.5 | Putative membrane glycoprotein | VAP | |||
| vMC168 | m164 | C | gp36.5 | 6.5 | Putative membrane glycoprotein | MCMV viral carrier protein gp36.5 (64); m164-derived peptide 257-AGPPRYSRI-265 is presented by the MHC-I molecule D(d) (45) | ||
| vMC170 | m166 | C | 6.5 | Putative membrane glycoprotein | Required for MCMV virulence in killing SCID mice and for optimal viral growth in vivo (121) | |||
| vMC173 | m169 | C | 6.5 | |||||
| vIE3rem | M122 Ex5 | C | ie3 | UL122 (IE2) | 24 | Immediate early | ie3 exon 5, with mRNA terminating at base 177817 (76); total length of Ex2 plus Ex3 plus Ex5 is 611 aa, molecular size is 68.1 kDa | |
| vMC011 | m11 | 24 | Glycoprotein m02 | |||||
| vMC013 | m13 | 24 | Glycoprotein m02 | |||||
| vMC015 | m15 | 24 | Glycoprotein m02 | |||||
| vMC020 | m20 | C | 24 | Reported irregularities in coding potential have suggested possible sequence errors in the 3′-proximal end (49) | ||||
| vMC023 | M23 | C | UL23 (GF2) P | 24 | US22 family homolog | |||
| vMC026 | M25 | UL25 (GF1) | 24 | Tegument | UL25 family homologue (26, 32); encodes a component of the MCMV tegument (116) | VAP | ||
| vMC028 | M26 | C | UL26 | 24 | ||||
| vMC029 | M27 | C | UL27 | 24 | Plays important role in MCMV growth and virulence (2) and inhibits IFN-γ signaling (56) via selective binding and downregulation of Stat2 (123) | |||
| vMC032 | m29.1 | C | 24 | |||||
| vMC033 | m30 | 24 | ||||||
| vMC034 | M31 | UL31 | 24 | Reported irregularities in coding potential have suggested possible sequence errors in the 3′-proximal end (49) | ||||
| vMC035 | M32 | C | UL32 (pp150) | 24 | Phosphoprotein (tegument) | Encodes large phosphoprotein homologous to HCMV tegument protein UL32 (pp150) (47, 48) | VAP | |
| vMC038 | M35 | UL35 (GF1) | 24 | Virion associated | Virulence factor and HCMV UL25 family homologue (26, 32); M35 insertional mutant is defective in growth in vivo (103) | VAP | ||
| vMC039 | M36 Ex1 | UL36 exon 1 | 24 | US22 family homolog | ||||
| vMC042 | M38 | C | UL38 | 24 | ||||
| vMC050 | m45.1 | C | 24 | |||||
| vMC053 | M48 | UL48 (Teg) | 24 | Tegument | Large tegument protein (101) | VAP | ||
| vMC056 | M49 | C | UL49 | 24 | ||||
| vMC057 | M50 | C | UL50 | 24 | Nuclear export | Conserved herpesvirus protein that forms a complex essential for egress of nucleocapsids from the nucleus (94); M50/p35 inserts into the inner nuclear membrane and is aggregated by M53/p38 to form the capsid docking site (82) | ||
| vMC059 | M52 | UL52 | 24 | |||||
| vMC060 | M53 | UL53 | 24 | Nuclear export | Conserved herpesvirus protein that forms a complex essential for egress of nucleocapsids from the nucleus (94); M50/p35 recruits cellular PKC for phosphorylation and dissolution of the nuclear lamina (82) | |||
| vMC062 | M55 | C | gB | UL55 (gB) | 24 | Glycoprotein | Glycoprotein B (29, 87) | VAP |
| vMC063 | M56 | C | UL56 (NM) | 24 | Tegument | Tegument protein and homologue of HCMV terminase subunit and HSV ICP18.5 (13, 38) | VAP | |
| vMC067 | M69 | C | UL69 | 24 | Tegument | Tegument protein similar to HCMV transactivator UL69 (113), which in HCMV induces a G1 block (42) | VAP | |
| vMC071 | M72 | C | UL72 (dUTPase) | 24 | Putative dUTPase | Homology to HCMV UL72, a putative dUTPase enzyme required for nucleotide metabolism, replication, and/or repair (86) | VAP | |
| vMC072 | M73 | UL73 | 24 | |||||
| vMC077 | M78 | UL78 | 24 | G protein-coupled receptor | G protein-coupled receptor homologue, same family as M33 (38); has subcellular trafficking properties (96); M78 mutants exhibit reduced replication in cultured cells as well as severe attenuation in the infected host (83) | |||
| vMC078 | M79 | C | UL79 | 24 | ||||
| vMC081 | M83 | C | UL83 (pp65) | 24 | Virion associated | Homologue of HCMV virion-associated factor with IFN repressor function (pp65) (1, 16, 74); M83 mutant has attenuated viral growth and virulence in SCID mice (119) | VAP | |
| vMC083 | M85 | C | UL85 | 24 | Capsid | Homologue of HCMV minor capsid protein (9) | VAP | |
| vMC084 | M86 | C | UL86 (MCP) | 24 | Capsid | Homologue of HCMV major capsid protein (25) | VAP | |
| vMC086 | M88 | UL88 | 24 | Virion associated | Homologue of HCMV virion protein (9) | VAP | ||
| vMC091 | M93 | UL93 | 24 | |||||
| vMC093 | M89 Ex1 | C | UL89 | 24 | ||||
| vMC094 | M95 | UL95 | 24 | |||||
| vMC095 | M96 | UL96 | 24 | |||||
| vMC096 | M97 | UL97 (PK) | 24 | Phosphotransferase | Homologue of HCMV UL97 phosphotransferase gene, whose product phosphorylates ganciclovir in HCMV-infected cells (67, 102) | VAP | ||
| vMC097 | M98 | UL98 (DNase) | 24 | Exonuclease | Alkaline exonuclease (DNase) gene (89) | VAP | ||
| vMC099 | M100 | C | gM | UL100 (gM) | 24 | Glycoprotein | Glycoprotein M with seven hydrophobic stretches that are potential membrane-spanning regions (66, 93) | VAP |
| vMC101 | M103 | C | UL103 | 24 | ||||
| vMC104 | m106 | C | 24 | |||||
| vMC105 | m107 | 24 | ||||||
| vMC106 | m108 | C | 24 | |||||
| vMC107 | M112 Ex1 | e1 | UL112 | 24 | Early | Exon 1 of e1 (22); total e1 length is 330 aa, molecular size is 36.4 kDa; IE3 and the early M112/113 gene products colocalize and coimmunoprecipitate (104); neuron-specific activation of the e1 promoter observed in transgenic mice (7) | ||
| vMC109 | M114 | C | UL114 (UNG) | 24 | Glycosylase | Uracil DNA glycosylase enzyme homolog (114) found in herpesviruses, required for nucleotide metabolism, replication, and/or repair | ||
| vMC110 | M115 | C | gL | UL115 (gL) | 24 | Glycoprotein | M115 (gL) (52) contains five potential glycosylation sites, has significant amino acid similarity to gL homologs in HCMV and HHV-6, and has previously been shown to be glycosylated in virions (118) | |
| vMC115 | m119 | 24 | ||||||
| vMC116 | m119.1 | 24 | Potential glycoprotein | |||||
| vMC119 | m119.4 | 24 | Predicted MCMV homologue of HCMV spliced ORF (89) | |||||
| vMC120 | m119.5 | 24 | ||||||
| vMC121 | m120 | C | 24 | |||||
| vMC123 | M122 Ex5 | C | ie3 | UL122 (IE2) | 24 | Immediate early | ie3 exon 5, with mRNA terminating at base 177817 (76); total length of Ex2 plus Ex3 plus Ex5 is 611 aa, molecular size is 68.1 kDa | |
| vMC127 | m124 | 24 | ORFs m124, m124.1, and m125 located within the enhancer region are nonessential for MCMV growth in vitro (6) | |||||
| vMC137 | m132 Ex1 | C | sgg1 | 24 | Exon 2 of M133 (sgg1) (61, 73) | |||
| vMC143 | m139 | C | US22 (GF2) | 24 | US22 family homolog | |||
| vMC149 | m145 | 24 | Glycoprotein | Downmodulates expression of cellular MULT-1 (60) and is a member of MGP family m145 | ||||
| vMC152 | m148 | 24 | ||||||
| vMC159 | m155 | 24 | Glycoprotein | Member of MGP family m145 that modulates NK cell (inhibition) and T cell response and impedes an NKG2D ligand (H60) (41, 79) | ||||
| vMC163 | m159 | C | 24 | Putative membrane glycoprotein | ||||
| vMC165 | m161 | C | 24 | Putative glycoprotein | Putative membrane glycoprotein | |||
| vMC172 | m168 | 24 | ||||||
| vMC174 | m170 | C | 24 | |||||
| vMC005 | m05 | 48 | Glycoprotein m02 | |||||
| vMC012 | m12 | 48 | Glycoprotein m02 | |||||
| vMC014 | m14 | 48 | Glycoprotein m02 | |||||
| vMC043 | m39 | C | 48 | |||||
| vMC066 | m59 | 48 | Potential ORF located within the origin of replication (89) | |||||
| vMC073 | m74 | C | UL74 P | 48 | Putative glycoprotein | Functional homolog of HCMV gO, has key role in determining the entry pathway of MCMV (95) | VAP | |
| vMC108 | M113 | UL113 P | 48 | Potential alternative splice to M112 Ex1 (e1) (22), as found for HCMV UL112/UL113 (115) | ||||
| vMC118 | m119.3 | 48 | Predicted MCMV homologue of HCMV spliced ORF | |||||
| vMC133 | m129 | C | 48 | m131/129 is a chemokine homolog and determinant of viral pathogenicity (35) and may modulate cytokine signaling (79) |
Microarray probes for coding MCMV ORFs found to be significantly upregulated in MCMV-infected cells versus mock-infected cells to a high confidence level (P ≤ 0.05 by empirical Bayes analysis).
Probe ID, probe identifier.
Time on, time detected.
aa, amino acids; vICA, viral inhibitor of caspase-8 activation; vMIA, viral mitochondrial inhibitor of apoptosis; ER, endoplasmic reticulum; RIP1, receptor-interacting protein kinase 1; ssDNA, single-stranded DNA; PKR, protein kinase R; APCs, antigen-presenting cells; MGP, membrane glycoprotein; CTL, cytotoxic T lymphocyte; IFN-γ, gamma interferon; HHV-6, human herpesvirus 6.
Virion-associated proteins (VAPs), based on reference 49.
Fig. 1.
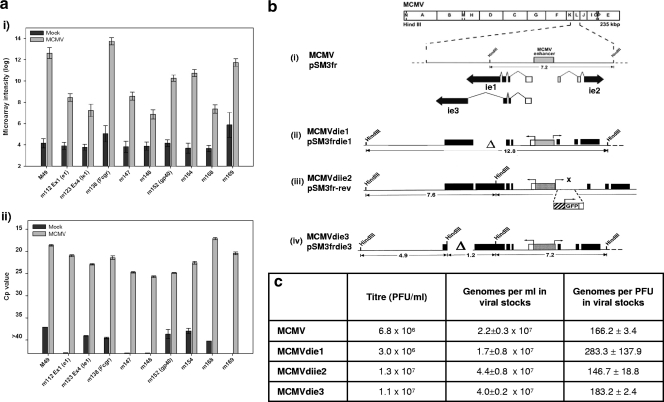
MCMV ORF detection and characterization of MCMV IE deletion mutant strains. (a) (Panel i) Microarray signals for 10 MCMV ORFs in mock- and MCMV-infected cells. (Panel ii) qRT-PCR validation of the 10 MCMV ORFs at 24 hpi. The y axis represents crossing point (Cp) values from qPCR amplification curves, with low Cp values indicating high transcript abundance. Error bars represent standard errors of the mean. (b) Schematic representations of recombinant MCMV IE deletion mutants. The map of the parental MCMV genome is shown at the top, with structures of the ie1, ie2, and ie3 transcripts below. Coding exons are shown in black, with arrows indicating the directions of transcription, and white triangles represent deleted loci. The gray box marks the MCMV ie1/ie3 promoter enhancer (MIEP). (Line iii) The MCMVdie3 revertant strain was renamed MCMVdiie2 in this study, as the HCMV MIEP is inserted between two HpaI sites spanning the transcription start site of the ie2 gene, disrupting ie2 expression (marked with ×). GFP, green fluorescent protein. (c) Viral titers and genome particle/PFU equivalences of the four MCMV strains, as determined by qPCR.
As previously noted for HCMV microarray analysis, there is no overt positional bias toward expression of coding ORFs based on genomic location that could be linked to the patterns of gene expression observed during infection. MCMV ORFs were annotated based on Rawlinson et al. (89) and updated with details from additional publications wherever possible. MCMV ORFs from recent predictions (15, 105) were aligned against 55-mer probe sequences, identifying five probes overlapping with newly predicted ORFs (m107-m107.2, m16-m16.2, m22-m22.1, M34-m34.2, and M58-m58.1), which were reannotated accordingly.
As a result of the statistical cutoff (P ≤ 0.05), MCMV probes for ORFs M44, M70, M75, m135, m143, m144, m153, and m157 failed to be included, although these genes have been reported to be expressed in previous MCMV studies or are homologues of HCMV genes reported to be expressed (21, 29, 34, 79, 80, 89, 92, 110, 118). It is most likely that the specific probes for these genes exhibit false-negative results. Nevertheless, in this study, we aimed to purposefully avoid false positives at the sacrifice of capturing a modest level of false negatives. For this reason, we also did not detect ie1 or ie3 expression until 24 hpi based on the statistical cutoff; however, these genes are detectable as early as 0.5 hpi and 2.5 hpi by use of a more sensitive qRT-PCR approach (see Fig. S3 in the supplemental material).
To gain further insight into the transcriptional programming of MCMV, we next sought to profile gene expression from three well-characterized MCMV mutants (5, 23, 36, 72) alongside the parental MCMV strain (111) (for schematics of strains, see Fig. 1b). To characterize the mutant strains before microarray analysis, we sought to (i) determine equivalent infectious doses at the genomic level by measuring genome/PFU ratio for each stock, (ii) ensure that generating the ie3 deletion mutant (MCMVdie3) in a complementing cell line did not drastically alter the infectious-particle ratio, (iii) ensure that viral growth phenotypes were consistent with those previously published (5, 36, 72), and (iv) ensure that no viral transcription was occurring from deleted loci. On the basis of quantitative PCR (qPCR), we detected equivalent numbers of MCMV genome copies per PFU for each viral strain (Fig. 1c), using as a calibrator a reference plasmid containing the m115 (gL) gene (nucleotides [nt] 166387 to 167208; GenBank accession no. NC_004065) (for a detailed account of this approach, see reference 98). Equivalent numbers of MCMV genomes were also found at 2 hpi inside cells infected with different MCMV strains (data not shown). Multistep growth curves confirmed viral growth phenotypes (see Fig. S4 in the supplemental material), and qRT-PCR (Qiagen 1-step kit; Germany) confirmed that transcription was not detectable from the respective deleted loci for ie1, ie2, and ie3 (data not shown). These data show that the four MCMV strains are experimentally comparable for downstream gene expression analysis.
Based on previous virologic characterization of these MCMV immediate-early (IE) mutants, we expected to observe different gene expression profiles for the MCMV strains. For example, given that removal of the ie2 gene causes no reported change in phenotype (45), we anticipated few gene expression changes in the MCMVdiie2 strain relative to the parental MCMV strain. Alternatively, given that ie3 has an indispensable regulatory function and is essential for viral growth (5), we expected little or no viral gene expression to be detectable from the MCMVdie3 strain. However, for the ie1 deletion mutant (MCMVdie1), the expectation was less clear, given that this strain has wild-type growth characteristics and yet the IE1 protein is well known to have transcriptional regulatory activity (24, 39, 76) and is further known to interact with cellular host factors (81, 104). In order to profile the gene expression of each mutant strain, we infected NIH 3T3 fibroblasts in parallel at an MOI of 1 and harvested total RNA for microarray analysis at 0.5, 6.5, and 48 hpi, along with that of mock-infected cells.
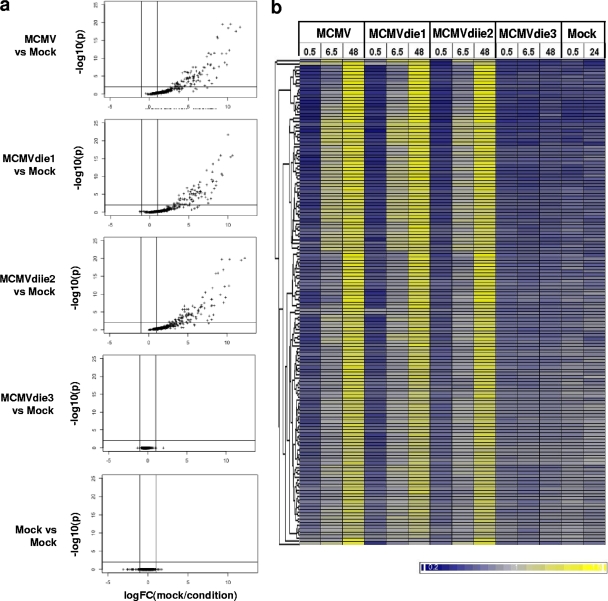
Figure 2 shows the comparative activation of viral transcriptomes among the four MCMV strains at 48 hpi and indicates that the transcriptomes of MCMVdie1 and MCMVdiie2 are activated with profiles of viral gene expression very similar to that of the parental MCMV strain over the 48-h period (Fig. 2a). Similar numbers (within 10%) of MCMV probes were detectable from MCMVdie1 (103 ORFs), MCMVdiie2 (114 ORFs), and the parental strain (113 ORFs) at 48 hpi. The degree of similarity in expression profiles among the MCMVdie1, MCMVdiie2, and parental MCMV strains suggests that ie1 and ie2 have a redundant or negligible transcriptional regulatory role in controlling downstream MCMV gene expression during fibroblast infection. Hierarchical clustering (Fig. 2b) further indicates few, if any, differences in the global gene expression profiles of MCMVdie1 and MCMVdiie2 compared to that of the parental MCMV strain. These results could point to a redundant role for the ie1 and ie2 genes in controlling downstream viral gene expression or, alternatively, a lack of sensitivity in controlling the viral genomic program in fully permissive fibroblasts.
Fig. 2.
Gene expression program of MCMV, MCMVdie1, MCMVdiie2, and MCMVdie3. (a) Volcano plots comparing microarray signals from mock-infected and infected samples at 48 hpi, using P value (y axis) and fold change (FC) (x axis) comparisons derived from empirical Bayes testing, with two biological replicates per group. (b) Hierarchical clustering of high-confidence MCMV probes, with each row representing a single probe normalized to its mean value across the data set to show relative expression. Yellow indicates increased expression and blue indicates decreased expression relative to the mean. Genes clustered based on the similarity of their expression profiles across the data set, with similar genes connected at the hierarchical tree on the left. Data represent mean values from two biological replicates. Numerical values at top indicate hours postinfection.
In marked contrast to the ie1 and ie2 mutant strains, MCMVdie3 exhibited an undetectable level of viral gene expression, suggesting that ie3 acts as a global trans-activator of downstream MCMV gene expression, as indicated by previous studies (5). To further examine the transcriptional status of MCMVdie3 using a more sensitive approach, IE and downstream MCMV genes were measured using qRT-PCR in MCMVdie3-infected cells both in the presence and in the absence of 50 μg/ml cycloheximide (C7698; Sigma, United Kingdom) at 2.5 hpi. These experiments confirmed that IE kinetic class genes were expressed in MCMVdie3 but that genes beyond the IE region were not (see Fig. S5 in the supplemental material).
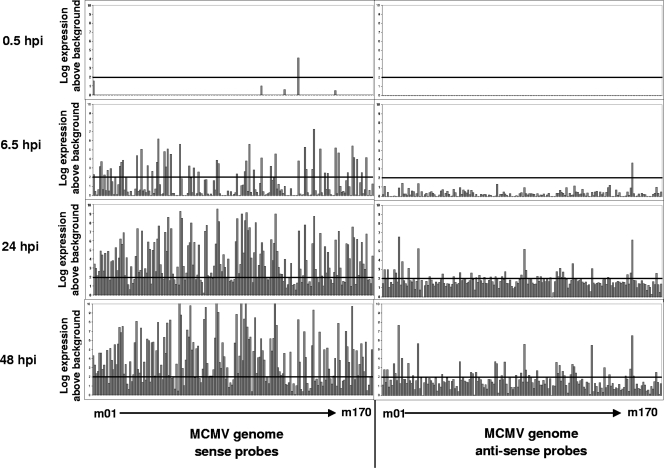
The design of the MCMV microarray platform enables selective detection of transcripts originating from both strands of the viral genome by having probes designed in sense (S) and antisense (AS) orientations to each MCMV ORF. At 24 hpi, we detected antisense transcripts from 23 AS loci, five of which (m104as, M113as, m147as, m163as, and m168as) have overlapping ORFs on the opposite strand of the genome, indicating known or predicted regions of bidirectional transcription based on prior annotation (105). Three other loci were found to have neighboring but nonoverlapping ORFs in their vicinity (M57as, m74as, and M88as). An additional 15 MCMV AS probes detected at 24 hpi were found to have no overlapping or nearby ORFs located on the opposite strand, indicating previously unknown noncoding transcripts derived from regions outside MCMV ORFs (m04as, m05as, m06as, m07as, m09as, m13as, m41as, M47as, M50as, M87as, M102as, M115as, m119.2as, m124as, and m145as). At 48 hpi, an additional eight antisense probes were detectable, but all have overlapping or nearby ORFs on the opposite strand of the genome (M48as, m69.1as, M89as, M94as, m108as, m119.3as, m132as, and m144as). In total, evidence of antisense transcription was detected from 35 loci over the four time points as measured by microarray analysis (Table 2 and Fig. 3). Twenty-six of these loci were also found to have significant signal from the corresponding sense (S) probe, indicating a potential site of bidirectional transcription. A trend toward antisense transcription occurring more frequently at the terminal ends of the MCMV genome is also noted (Fig. 3).
Table 2.
High-confidence MCMV microarray antisense probesa
| Unique probe IDb | ORF | Time onc (hpi) | Details of cDNA cloning validation | Sense probed |
|---|---|---|---|---|
| vMC336 | m148as | 6.5 | Validated with AS cDNA clone L151 at nt 208012 to 207467 (5′ to 3′), overlapping m148 (AS) and m149 (S); clone length, 545 nt | S |
| vMC357 | m169as | 6.5 | S | |
| vMC175 | m05as | 24 | S | |
| vMC180 | m163as | 24 | S | |
| vMC188 | m04as | 24 | Validated with AS cDNA clone IE150 at nt 4043 to 3943 (5′ to 3′), overlapping m04 (AS); clone length, 384 nt | S |
| vMC189 | m05as | 24 | S | |
| vMC190 | m06as | 24 | S | |
| vMC191 | m07as | 24 | ||
| vMC193 | m09as | 24 | ||
| vMC197 | m13as | 24 | S | |
| vMC229 | m41as | 24 | S | |
| vMC236 | M47as | 24 | ||
| vMC241 | M50as | 24 | S | |
| vMC248 | M57as | 24 | S | |
| vMC257 | m74as | 24 | Validated with AS cDNA clone L147 at nt 104825 to 105449 (5′ to 3′), overlapping m74 (AS); clone length, 624 nt | S |
| vMC269 | M87as | 24 | ||
| vMC270 | M88as | 24 | S | |
| vMC283 | M102as | 24 | S | |
| vMC285 | M104as | 24 | ||
| vMC291 | M113as | 24 | S | |
| vMC293 | M115as | 24 | S | |
| vMC299 | m119.2as | 24 | S | |
| vMC311 | m124as | 24 | S | |
| vMC333 | m145as | 24 | S | |
| vMC335 | m147as | 24 | ||
| vMC351 | m163as | 24 | S | |
| vMC356 | m168as | 24 | S | |
| vMC239 | M48as | 48 | S | |
| vMC251 | m69.1as | 48 | ||
| vMC272 | M89 Ex2as | 48 | ||
| vMC277 | M94as | 48 | Validated with AS cDNA clone L164 at nt 137299 to 137096 (5′ to 3′), overlapping m94 (AS); clone length, 203 nt | S |
| vMC289 | m108as | 48 | S | |
| vMC300 | m119.3as | 48 | S | |
| vMC319 | m132 Ex2as | 48 | S | |
| vMC332 | m144 | 48 |
Microarray probes for antisense transcripts found to be significantly upregulated in MCMV-infected cells versus mock-infected cells to a high confidence level (P ≤ 0.05 by empirical Bayes analysis).
Probe ID, probe identifier.
Time on, time detected.
AS probes with significant signal also found from the corresponding sense probes are marked with an “S.”
Fig. 3.
MCMV genome activation measured by microarray analysis and qRT-PCR. Transcript abundance of ORFs expressed from the parental MCMV strain was measured using oligonucleotide microarrays at 0.5, 6.5, 24, and 48 hpi in NIH 3T3 cells at an MOI of 1. Histograms represent mean values from two replicate samples after background (mock infection) subtraction. Transcripts are arranged in order from left to right according to ORF names ranging from m01 to m170, with sense probes shown on the left and antisense probes on the right. All raw data are available in the supplemental material.
In order to independently validate AS transcripts identified by microarray analysis, we generated cDNA libraries from MCMV-infected fibroblasts pooled from 4, 8, and 12 hpi (IE library), 16, 24, and 32 hpi (E library), or 40, 60, 80, and 100 hpi (L library). cDNA libraries were generated as described previously for HCMV (120). cDNA clones overlapping AS microarray regions were found at m04as, m74as, M94as (none of which have overlapping ORFs on the opposite strand of the genome), and m148as (which has the m147 ORF on the opposite strand of the genome) (for validated AS transcripts, see Table 2). We also found one large cDNA clone that overlapped three MCMV ORFs (m119.2, m119.3, and m119.4), two of which had potential AS regions identified by microarray probes (m119.2 and m119.3). Four additional AS cDNA clones were found to overlap AS regions not identified by microarray analysis (m19as, M72as, m149as, and m151as). These experiments thus reveal for the first time that antisense transcription occurs frequently throughout the MCMV genome, an observation that will likely seed further studies.
Supplementary Material
Acknowledgments
This work was supported by Wellcome Trust Programme grant no. WT 066784/Z/02/Z (to P.G.), Unity through Knowledge Fund grant no. UKF 08/07 (to J.T.), and Ministerio de Educación y Ciencia grant no. SAF 2008 00382 (to A.A.). qPCR data were obtained in the Kinetic Parameter Facility of The Centre for Systems Biology at Edinburgh, which is a Centre for Integrative Systems Biology (CISB) funded by BBSRC and EPSRC BB/D019621/1 (P.G.).
We kindly thank Ulrich Koszinowski for his comments and input on the manuscript. We also thank Daniel Foster and Fátima García del Rey for their technical input, Stipan Jonjic for his support in generating cDNA libraries, and Chitrangada Das Mukhopdhyay for bioinformatics analysis of MCMV cDNA clones.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 6 April 2011.
REFERENCES
- 1. Abate D. A., Watanabe S., Mocarski E. S. 2004. Major human cytomegalovirus structural protein pp65 (ppUL83) prevents interferon response factor 3 activation in the interferon response. J. Virol. 78:10995–11006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abenes G., et al. 2001. Murine cytomegalovirus open reading frame M27 plays an important role in growth and virulence in mice. J. Virol. 75:1697–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anders D. G. 1990. Nucleotide sequence of a cytomegalovirus single-stranded DNA-binding protein gene: comparison with alpha- and gammaherpesvirus counterparts reveals conserved segments. J. Gen. Virol. 71(Pt. 10):2451–2456 [DOI] [PubMed] [Google Scholar]
- 4. Anders D. G., Gibson W. 1988. Location, transcript analysis, and partial nucleotide sequence of the cytomegalovirus gene encoding an early DNA-binding protein with similarities to ICP8 of herpes simplex virus type 1. J. Virol. 62:1364–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Angulo A., Ghazal P., Messerle M. 2000. The major immediate-early gene ie3 of mouse cytomegalovirus is essential for viral growth. J. Virol. 74:11129–11136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Angulo A., Messerle M., Koszinowski U. H., Ghazal P. 1998. Enhancer requirement for murine cytomegalovirus growth and genetic complementation by the human cytomegalovirus enhancer. J. Virol. 72:8502–8509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arai Y., et al. 2003. Neuron-specific activation of murine cytomegalovirus early gene e1 promoter in transgenic mice. Am. J. Pathol. 163:643–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arapovic J., Lenac Rovis T., Reddy A. B., Krmpotic A., Jonjic S. 2009. Promiscuity of MCMV immunoevasin of NKG2D: m138/fcr-1 down-modulates RAE-1epsilon in addition to MULT-1 and H60. Mol. Immunol. 47:114–122 [DOI] [PubMed] [Google Scholar]
- 9. Baldick C. J., Jr., Shenk T. 1996. Proteins associated with purified human cytomegalovirus particles. J. Virol. 70:6097–6105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baluchova K., Kirby M., Ahasan M. M., Sweet C. 2008. Preliminary characterization of murine cytomegaloviruses with insertional and deletional mutations in the M34 open reading frame. J. Med. Virol. 80:1233–1242 [DOI] [PubMed] [Google Scholar]
- 11. Baum E. Z., et al. 1993. Expression and analysis of the human cytomegalovirus UL80-encoded protease: identification of autoproteolytic sites. J. Virol. 67:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bilban M., Buehler L. K., Head S., Desoye G., Quaranta V. 2002. Defining signal thresholds in DNA microarrays: exemplary application for invasive cancer. BMC Genomics 3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bogner E., Reschke M., Reis B., Mockenhaupt T., Radsak K. 1993. Identification of the gene product encoded by ORF UL56 of the human cytomegalovirus genome. Virology 196:290–293 [DOI] [PubMed] [Google Scholar]
- 14. Borst E. M., Mathys S., Wagner M., Muranyi W., Messerle M. 2001. Genetic evidence of an essential role for cytomegalovirus small capsid protein in viral growth. J. Virol. 75:1450–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brocchieri L., Kledal T. N., Karlin S., Mocarski E. S. 2005. Predicting coding potential from genome sequence: application to betaherpesviruses infecting rats and mice. J. Virol. 79:7570–7596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Browne E. P., Shenk T. 2003. Human cytomegalovirus UL83-coded pp65 virion protein inhibits antiviral gene expression in infected cells. Proc. Natl. Acad. Sci. U. S. A. 100:11439–11444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brune W., Menard C., Heesemann J., Koszinowski U. H. 2001. A ribonucleotide reductase homolog of cytomegalovirus and endothelial cell tropism. Science 291:303–305 [DOI] [PubMed] [Google Scholar]
- 18. Bubeck A., et al. 2002. The glycoprotein gp48 of murine cytomegalovirus: proteasome-dependent cytosolic dislocation and degradation. J. Biol. Chem. 277:2216–2224 [DOI] [PubMed] [Google Scholar]
- 19. Buck A. H., et al. 2010. Post-transcriptional regulation of miR-27 in murine cytomegalovirus infection. RNA 16:307–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buck A. H., et al. 2007. Discrete clusters of virus-encoded microRNAs are associated with complementary strands of the genome and the 7.2-kilobase stable intron in murine cytomegalovirus. J. Virol. 81:13761–13770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Budt M., Niederstadt L., Valchanova R. S., Jonjic S., Brune W. 2009. Specific inhibition of the PKR-mediated antiviral response by the murine cytomegalovirus proteins m142 and m143. J. Virol. 83:1260–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buhler B., Keil G. M., Weiland F., Koszinowski U. H. 1990. Characterization of the murine cytomegalovirus early transcription unit e1 that is induced by immediate-early proteins. J. Virol. 64:1907–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Busche A., Angulo A., Kay-Jackson P., Ghazal P., Messerle M. 2008. Phenotypes of major immediate-early gene mutants of mouse cytomegalovirus. Med. Microbiol. Immunol. 197:233–240 [DOI] [PubMed] [Google Scholar]
- 24. Cardin R. D., Abenes G. B., Stoddart C. A., Mocarski E. S. 1995. Murine cytomegalovirus IE2, an activator of gene expression, is dispensable for growth and latency in mice. Virology 209:236–241 [DOI] [PubMed] [Google Scholar]
- 25. Chee M., Rudolph S. A., Plachter B., Barrell B., Jahn G. 1989. Identification of the major capsid protein gene of human cytomegalovirus. J. Virol. 63:1345–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chee M. S., et al. 1990. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol. 154:125–169 [DOI] [PubMed] [Google Scholar]
- 27. Cicin-Sain L., et al. 2008. Dominant-negative FADD rescues the in vivo fitness of a cytomegalovirus lacking an antiapoptotic viral gene. J. Virol. 82:2056–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Corbett A. J., Forbes C. A., Moro D., Scalzo A. A. 2007. Extensive sequence variation exists among isolates of murine cytomegalovirus within members of the m02 family of genes. J. Gen. Virol. 88:758–769 [DOI] [PubMed] [Google Scholar]
- 29. Cranage M. P., et al. 1986. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 5:3057–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cranmer L. D., Clark C., Spector D. H. 1994. Cloning, characterization, and expression of the murine cytomegalovirus homologue of the human cytomegalovirus 28-kDa matrix phosphoprotein (UL99). Virology 205:417–429 [DOI] [PubMed] [Google Scholar]
- 31. Crnkovic-Mertens I., et al. 1998. Virus attenuation after deletion of the cytomegalovirus Fc receptor gene is not due to antibody control. J. Virol. 72:1377–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dallas P. B., Lyons P. A., Hudson J. B., Scalzo A. A., Shellam G. R. 1994. Identification and characterization of a murine cytomegalovirus gene with homology to the UL25 open reading frame of human cytomegalovirus. Virology 200:643–650 [DOI] [PubMed] [Google Scholar]
- 33. Dolken L., et al. 2007. Mouse cytomegalovirus microRNAs dominate the cellular small RNA profile during lytic infection and show features of posttranscriptional regulation. J. Virol. 81:13771–13782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ertl P. F., Powell K. L. 1992. Physical and functional interaction of human cytomegalovirus DNA polymerase and its accessory protein (ICP36) expressed in insect cells. J. Virol. 66:4126–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fleming P., et al. 1999. The murine cytomegalovirus chemokine homolog, m131/129, is a determinant of viral pathogenicity. J. Virol. 73:6800–6809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ghazal P., et al. 2005. Elimination of ie1 significantly attenuates murine cytomegalovirus virulence but does not alter replicative capacity in cell culture. J. Virol. 79:7182–7194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goldmacher V. S., et al. 1999. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc. Natl. Acad. Sci. U. S. A. 96:12536–12541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gompels U. A., et al. 1995. The DNA sequence of human herpesvirus-6: structure, coding content, and genome evolution. Virology 209:29–51 [DOI] [PubMed] [Google Scholar]
- 39. Gribaudo G., et al. 1996. The murine cytomegalovirus immediate-early 1 protein stimulates NF-kappa B activity by transactivating the NF-kappa B p105/p50 promoter. Virus Res. 45:15–27 [DOI] [PubMed] [Google Scholar]
- 40. Gutermann A., et al. 2002. Strategies for the identification and analysis of viral immune-evasive genes—cytomegalovirus as an example. Curr. Top. Microbiol. Immunol. 269:1–22 [DOI] [PubMed] [Google Scholar]
- 41. Hasan M., et al. 2005. Selective down-regulation of the NKG2D ligand H60 by mouse cytomegalovirus m155 glycoprotein. J. Virol. 79:2920–2930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hayashi M. L., Blankenship C., Shenk T. 2000. Human cytomegalovirus UL69 protein is required for efficient accumulation of infected cells in the G1 phase of the cell cycle. Proc. Natl. Acad. Sci. U. S. A. 97:2692–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hengel H., et al. 1999. Cytomegaloviral control of MHC class I function in the mouse. Immunol. Rev. 168:167–176 [DOI] [PubMed] [Google Scholar]
- 44. Holtappels R., Grzimek N. K., Thomas D., Reddehase M. J. 2002. Early gene m18, a novel player in the immune response to murine cytomegalovirus. J. Gen. Virol. 83:311–316 [DOI] [PubMed] [Google Scholar]
- 45. Holtappels R., et al. 2008. Subdominant CD8 T-cell epitopes account for protection against cytomegalovirus independent of immunodomination. J. Virol. 82:5781–5796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Holtappels R., et al. 2000. The putative natural killer decoy early gene m04 (gp34) of murine cytomegalovirus encodes an antigenic peptide recognized by protective antiviral CD8 T cells. J. Virol. 74:1871–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jahn G., et al. 1987. Map position and nucleotide sequence of the gene for the large structural phosphoprotein of human cytomegalovirus. J. Virol. 61:1358–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jahn G., Scholl B. C., Traupe B., Fleckenstein B. 1987. The two major structural phosphoproteins (pp65 and pp150) of human cytomegalovirus and their antigenic properties. J. Gen. Virol. 68(Pt. 5):1327–1337 [DOI] [PubMed] [Google Scholar]
- 49. Kattenhorn L. M., et al. 2004. Identification of proteins associated with murine cytomegalovirus virions. J. Virol. 78:11187–11197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kavanagh D. G., Hill A. B. 2001. Evasion of cytotoxic T lymphocytes by murine cytomegalovirus. Semin. Immunol. 13:19–26 [DOI] [PubMed] [Google Scholar]
- 51. Kavanagh D. G., Koszinowski U. H., Hill A. B. 2001. The murine cytomegalovirus immune evasion protein m4/gp34 forms biochemically distinct complexes with class I MHC at the cell surface and in a pre-Golgi compartment. J. Immunol. 167:3894–3902 [DOI] [PubMed] [Google Scholar]
- 52. Kaye J. F., Gompels U. A., Minson A. C. 1992. Glycoprotein H of human cytomegalovirus (HCMV) forms a stable complex with the HCMV UL115 gene product. J. Gen. Virol. 73(Pt. 10):2693–2698 [DOI] [PubMed] [Google Scholar]
- 53. Keil G. M., Ebeling-Keil A., Koszinowski U. H. 1987. Immediate-early genes of murine cytomegalovirus: location, transcripts, and translation products. J. Virol. 61:526–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Keil G. M., Ebeling-Keil A., Koszinowski U. H. 1987. Sequence and structural organization of murine cytomegalovirus immediate-early gene 1. J. Virol. 61:1901–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Keil G. M., Ebeling-Keil A., Koszinowski U. H. 1984. Temporal regulation of murine cytomegalovirus transcription and mapping of viral RNA synthesized at immediate early times after infection. J. Virol. 50:784–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Khan S., Zimmermann A., Basler M., Groettrup M., Hengel H. 2004. A cytomegalovirus inhibitor of gamma interferon signaling controls immunoproteasome induction. J. Virol. 78:1831–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kielczewska A., et al. 2009. Ly49P recognition of cytomegalovirus-infected cells expressing H2-Dk and CMV-encoded m04 correlates with the NK cell antiviral response. J. Exp. Med. 206:515–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kouzarides T., Bankier A. T., Satchwell S. C., Preddy E., Barrell B. G. 1988. An immediate early gene of human cytomegalovirus encodes a potential membrane glycoprotein. Virology 165:151–164 [DOI] [PubMed] [Google Scholar]
- 59. Kouzarides T., et al. 1987. Sequence and transcription analysis of the human cytomegalovirus DNA polymerase gene. J. Virol. 61:125–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Krmpotic A., et al. 2005. NK cell activation through the NKG2D ligand MULT-1 is selectively prevented by the glycoprotein encoded by mouse cytomegalovirus gene m145. J. Exp. Med. 201:211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lagenaur L. A., Manning W. C., Vieira J., Martens C. L., Mocarski E. S. 1994. Structure and function of the murine cytomegalovirus sgg1 gene: a determinant of viral growth in salivary gland acinar cells. J. Virol. 68:7717–7727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Leatham M. P., Witte P. R., Stinski M. F. 1991. Alternate promoter selection within a human cytomegalovirus immediate-early and early transcription unit (UL119-115) defines true late transcripts containing open reading frames for putative viral glycoproteins. J. Virol. 65:6144–6153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lee M., et al. 2000. Murine cytomegalovirus containing a mutation at open reading frame M37 is severely attenuated in growth and virulence in vivo. J. Virol. 74:11099–11107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lemmermann N. A., et al. 2010. Immune evasion proteins of murine cytomegalovirus preferentially affect cell surface display of recently generated peptide presentation complexes. J. Virol. 84:1221–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lenac T., et al. 2006. The herpesviral Fc receptor fcr-1 down-regulates the NKG2D ligands MULT-1 and H60. J. Exp. Med. 203:1843–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li W., Eidman K., Gehrz R. C., Kari B. 1995. Identification and molecular characterization of the murine cytomegalovirus homolog of the human cytomegalovirus UL100 gene. Virus Res. 36:163–175 [DOI] [PubMed] [Google Scholar]
- 67. Littler E., Stuart A. D., Chee M. S. 1992. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature 358:160–162 [DOI] [PubMed] [Google Scholar]
- 68. Loewendorf A., et al. 2004. Identification of a mouse cytomegalovirus gene selectively targeting CD86 expression on antigen-presenting cells. J. Virol. 78:13062–13071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Loutsch J. M., Galvin N. J., Bryant M. L., Holwerda B. C. 1994. Cloning and sequence analysis of murine cytomegalovirus protease and capsid assembly protein genes. Biochem. Biophys. Res. Commun. 203:472–478 [DOI] [PubMed] [Google Scholar]
- 70. Lyons P. A., Dallas P. B., Carrello C., Shellam G. R., Scalzo A. A. 1994. Mapping and transcriptional analysis of the murine cytomegalovirus homologue of the human cytomegalovirus UL103 open reading frame. Virology 204:835–839 [DOI] [PubMed] [Google Scholar]
- 71. Mack C., Sickmann A., Lembo D., Brune W. 2008. Inhibition of proinflammatory and innate immune signaling pathways by a cytomegalovirus RIP1-interacting protein. Proc. Natl. Acad. Sci. U. S. A. 105:3094–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Manning W. C., Mocarski E. S. 1988. Insertional mutagenesis of the murine cytomegalovirus genome: one prominent alpha gene (ie2) is dispensable for growth. Virology 167:477–484 [PubMed] [Google Scholar]
- 73. Manning W. C., Stoddart C. A., Lagenaur L. A., Abenes G. B., Mocarski E. S. 1992. Cytomegalovirus determinant of replication in salivary glands. J. Virol. 66:3794–3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Marshall E. E., Geballe A. P. 2009. Multifaceted evasion of the interferon response by cytomegalovirus. J. Interferon Cytokine Res. 29:609–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Menard C., et al. 2003. Role of murine cytomegalovirus US22 gene family members in replication in macrophages. J. Virol. 77:5557–5570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Messerle M., Buhler B., Keil G. M., Koszinowski U. H. 1992. Structural organization, expression, and functional characterization of the murine cytomegalovirus immediate-early gene 3. J. Virol. 66:27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Messerle M., Keil G. M., Koszinowski U. H. 1991. Structure and expression of murine cytomegalovirus immediate-early gene 2. J. Virol. 65:1638–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Meyer H., et al. 1988. Identification and procaryotic expression of the gene coding for the highly immunogenic 28-kilodalton structural phosphoprotein (pp28) of human cytomegalovirus. J. Virol. 62:2243–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mocarski E. S., Jr 2004. Immune escape and exploitation strategies of cytomegaloviruses: impact on and imitation of the major histocompatibility system. Cell. Microbiol. 6:707–717 [DOI] [PubMed] [Google Scholar]
- 80. Mocarski E. S., Jr 2002. Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends Microbiol. 10:332–339 [DOI] [PubMed] [Google Scholar]
- 81. Munch K., Messerle M., Plachter B., Koszinowski U. H. 1992. An acidic region of the 89K murine cytomegalovirus immediate early protein interacts with DNA. J. Gen. Virol. 73(Pt. 3):499–506 [DOI] [PubMed] [Google Scholar]
- 82. Muranyi W., Haas J., Wagner M., Krohne G., Koszinowski U. H. 2002. Cytomegalovirus recruitment of cellular kinases to dissolve the nuclear lamina. Science 297:854–857 [DOI] [PubMed] [Google Scholar]
- 83. Oliveira S. A., Shenk T. E. 2001. Murine cytomegalovirus M78 protein, a G protein-coupled receptor homologue, is a constituent of the virion and facilitates accumulation of immediate-early viral mRNA. Proc. Natl. Acad. Sci. U. S. A. 98:3237–3242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pinto A. K., Jamieson A. M., Raulet D. H., Hill A. B. 2007. The role of NKG2D signaling in inhibition of cytotoxic T-lymphocyte lysis by the murine cytomegalovirus immunoevasin m152/gp40. J. Virol. 81:12564–12571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Preston V. G., Palfreyman J. W., Dutia B. M. 1984. Identification of a herpes simplex virus type 1 polypeptide which is a component of the virus-induced ribonucleotide reductase. J. Gen. Virol. 65(Pt. 9):1457–1466 [DOI] [PubMed] [Google Scholar]
- 86. Pyles R. B., Sawtell N. M., Thompson R. L. 1992. Herpes simplex virus type 1 dUTPase mutants are attenuated for neurovirulence, neuroinvasiveness, and reactivation from latency. J. Virol. 66:6706–6713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rapp M., et al. 1992. Identification of the murine cytomegalovirus glycoprotein B gene and its expression by recombinant vaccinia virus. J. Virol. 66:4399–4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rawlinson W. D., Barrell B. G. 1993. Spliced transcripts of human cytomegalovirus. J. Virol. 67:5502–5513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rawlinson W. D., Farrell H. E., Barrell B. G. 1996. Analysis of the complete DNA sequence of murine cytomegalovirus. J. Virol. 70:8833–8849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Reusch U., et al. 1999. A cytomegalovirus glycoprotein re-routes MHC class I complexes to lysosomes for degradation. EMBO J. 18:1081–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ruger B., et al. 1987. Primary structure and transcription of the genes coding for the two virion phosphoproteins pp65 and pp71 of human cytomegalovirus. J. Virol. 61:446–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Saederup N., Mocarski E. S., Jr 2002. Fatal attraction: cytomegalovirus-encoded chemokine homologs. Curr. Top. Microbiol. Immunol. 269:235–256 [DOI] [PubMed] [Google Scholar]
- 93. Scalzo A. A., Forbes C. A., Davis-Poynter N. J., Farrell H. E., Lyons P. A. 1995. DNA sequence and transcriptional analysis of the glycoprotein M gene of murine cytomegalovirus. J. Gen. Virol. 76(Pt. 11):2895–2901 [DOI] [PubMed] [Google Scholar]
- 94. Schnee M., Ruzsics Z., Bubeck A., Koszinowski U. H. 2006. Common and specific properties of herpesvirus UL34/UL31 protein family members revealed by protein complementation assay. J. Virol. 80:11658–11666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Scrivano L., et al. 2010. The m74 gene product of murine cytomegalovirus (MCMV) is a functional homolog of human CMV gO and determines the entry pathway of MCMV. J. Virol. 84:4469–4480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sharp E. L., Davis-Poynter N. J., Farrell H. E. 2009. Analysis of the subcellular trafficking properties of murine cytomegalovirus M78, a 7 transmembrane receptor homologue. J. Gen. Virol. 90:59–68 [DOI] [PubMed] [Google Scholar]
- 97. Sherman G., Gottlieb J., Challberg M. D. 1992. The UL8 subunit of the herpes simplex virus helicase-primase complex is required for efficient primer utilization. J. Virol. 66:4884–4892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Simon C. O., Seckert C. K., Dreis D., Reddehase M. J., Grzimek N. K. 2005. Role for tumor necrosis factor alpha in murine cytomegalovirus transcriptional reactivation in latently infected lungs. J. Virol. 79:326–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Singh R., Haghjoo E., Liu F. 2003. Cytomegalovirus M43 gene modulates T helper cell response. Immunol. Lett. 88:31–35 [DOI] [PubMed] [Google Scholar]
- 100. Skaletskaya A., et al. 2001. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc. Natl. Acad. Sci. U. S. A. 98:7829–7834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Spaete R. R., Gehrz R. C., Landini M. P. 1994. Human cytomegalovirus structural proteins. J. Gen. Virol. 75(Pt. 12):3287–3308 [DOI] [PubMed] [Google Scholar]
- 102. Sullivan V., et al. 1992. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature 358:162–164 [DOI] [PubMed] [Google Scholar]
- 103. Tam A., et al. 2003. Murine cytomegalovirus with a transposon insertional mutation at open reading frame M35 is defective in growth in vivo. J. Virol. 77:7746–7755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tang Q., Maul G. G. 2003. Mouse cytomegalovirus immediate-early protein 1 binds with host cell repressors to relieve suppressive effects on viral transcription and replication during lytic infection. J. Virol. 77:1357–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tang Q., Murphy E. A., Maul G. G. 2006. Experimental confirmation of global murine cytomegalovirus open reading frames by transcriptional detection and partial characterization of newly described gene products. J. Virol. 80:6873–6882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Telford E. A., Watson M. S., McBride K., Davison A. J. 1992. The DNA sequence of equine herpesvirus-1. Virology 189:304–316 [DOI] [PubMed] [Google Scholar]
- 107. Thale R., Lucin P., Schneider K., Eggers M., Koszinowski U. H. 1994. Identification and expression of a murine cytomegalovirus early gene coding for an Fc receptor. J. Virol. 68:7757–7765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Thale R., et al. 1995. Identification of the mouse cytomegalovirus genomic region affecting major histocompatibility complex class I molecule transport. J. Virol. 69:6098–6105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Upton J. W., Kaiser W. J., Mocarski E. S. 2008. Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1. J. Biol. Chem. 283:16966–16970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Valchanova R. S., Picard-Maureau M., Budt M., Brune W. 2006. Murine cytomegalovirus m142 and m143 are both required to block protein kinase R-mediated shutdown of protein synthesis. J. Virol. 80:10181–10190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wagner M., Jonjic S., Koszinowski U. H., Messerle M. 1999. Systematic excision of vector sequences from the BAC-cloned herpesvirus genome during virus reconstitution. J. Virol. 73:7056–7060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wing B. A., Lee G. C., Huang E. S. 1996. The human cytomegalovirus UL94 open reading frame encodes a conserved herpesvirus capsid/tegument-associated virion protein that is expressed with true late kinetics. J. Virol. 70:3339–3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Winkler M., Rice S. A., Stamminger T. 1994. UL69 of human cytomegalovirus, an open reading frame with homology to ICP27 of herpes simplex virus, encodes a transactivator of gene expression. J. Virol. 68:3943–3954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Worrad D. M., Caradonna S. 1988. Identification of the coding sequence for herpes simplex virus uracil-DNA glycosylase. J. Virol. 62:4774–4777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wright D. A., Staprans S. I., Spector D. H. 1988. Four phosphoproteins with common amino termini are encoded by human cytomegalovirus AD169. J. Virol. 62:331–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wu C. A., Carlson M. E., Henry S. C., Shanley J. D. 1999. The murine cytomegalovirus M25 open reading frame encodes a component of the tegument. Virology 262:265–276 [DOI] [PubMed] [Google Scholar]
- 117. Xiao J., Tong T., Zhan X., Haghjoo E., Liu F. 2000. In vitro and in vivo characterization of a murine cytomegalovirus with a transposon insertional mutation at open reading frame M43. J. Virol. 74:9488–9497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Xu J., et al. 1994. Identification, sequencing and expression of the glycoprotein L gene of murine cytomegalovirus. J. Gen. Virol. 75(Pt. 11):3235–3240 [DOI] [PubMed] [Google Scholar]
- 119. Zhan X., Lee M., Xiao J., Liu F. 2000. Construction and characterization of murine cytomegaloviruses that contain transposon insertions at open reading frames m09 and M83. J. Virol. 74:7411–7421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zhang G., et al. 2007. Antisense transcription in the human cytomegalovirus transcriptome. J. Virol. 81:11267–11281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhu J., et al. 2003. In vitro and in vivo characterization of a murine cytomegalovirus with a mutation at open reading frame m166. J. Virol. 77:2882–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ziegler H., Muranyi W., Burgert H. G., Kremmer E., Koszinowski U. H. 2000. The luminal part of the murine cytomegalovirus glycoprotein gp40 catalyzes the retention of MHC class I molecules. EMBO J. 19:870–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zimmermann A., et al. 2005. A cytomegaloviral protein reveals a dual role for STAT2 in IFN-{gamma} signaling and antiviral responses. J. Exp. Med. 201:1543–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.