Abstract
Complement is an innate immune response system that most animal viruses encounter during natural infections. We have tested the role of human complement in the neutralization of virus particles harboring the Nipah virus (NiV) glycoproteins. A luciferase-expressing vesicular stomatitis virus (VSV) pseudotype that contained the NiV fusion (F) and attachment (G) glycoproteins (NiVpp) showed dose- and time-dependent activation of human complement through the alternative pathway. In contrast to our findings with other paramyxoviruses, normal human serum (NHS) alone did not neutralize NiVpp infectivity in vitro, and electron microscopy demonstrated no significant deposition of complement component C3 on particles. This lack of NiVpp neutralization by NHS was not due to a global inhibition of complement pathways, since complement was found to significantly enhance neutralization by antibodies specific for the NiV F and G glycoproteins. Complement components C4 and C1q were necessary but not sufficient by themselves for the enhancement of antibody neutralization. Human complement also enhanced NiVpp neutralization by a soluble version of the NiV receptor EphrinB2, and this depended on components in the classical pathway. The ability of complement to enhance neutralization fell into one of two profiles: (i) anti-F monoclonal antibodies showed enhancement only at high and not low antibody concentrations, and (ii) anti-G monoclonal antibodies and EphrinB2 showed enhancement at both high and very low levels of antibody (e.g., 3.1 ng) or EphrinB2 (e.g., 2.5 ng). Together, these data establish the importance of human complement in the neutralization of particles containing the NiV glycoproteins and will help guide the design of more effective therapeutics that harness the potency of complement pathways.
INTRODUCTION
The complement system constitutes a complex group of both soluble and cell-associated proteins that together form an integral part of the innate host defense against pathogens (reviewed in reference 18). Complement can serve to link innate and adaptive immunity to viruses through a large number of activities, including recognition of virions, direct neutralization of infectivity, recruitment and stimulation of leukocytes, opsonization by immune cells, and activation of T and B cell responses (4, 9). Complement activation can play important roles in viral pathogenesis (e.g., see reference 25) and has been the focus of efforts to improve the effectiveness of vaccines and therapeutic vectors (31). The goal of the work described here was to determine the role of human complement in neutralization of the paramyxovirus Nipah virus (NiV), an emerging human pathogen with no currently available vaccines or therapeutics.
The complement cascade can be initiated through three main pathways: the classical pathway, the lectin pathway, and the alternative pathway (9, 30). These three pathways converge on a central component, C3, which is activated by cleavage into the anaphylatoxin C3a and C3b, which can bind covalently to viral components to aid in opsonization and phagocytosis. The association of C3b with further downstream components, such as C6 through C9, can lead to the formation of the membrane attack complex (MAC), which is capable of lysing virus particles or infected cells (reviewed in references 4 and 9). The classical pathway, which is activated by antibodies, and the lectin pathway, which is activated by carbohydrate structures, converge on C4, which is cleaved into the anaphylatoxin C4a and the C4b fragment. Together with C3b, this can also lead to downstream activation of MAC.
We and others have previously shown that the paramyxoviruses parainfluenza virus 5 (PIV5) and mumps virus (MuV) activate the alternative complement pathway and that the activation of complement component C3 is essential for effective in vitro neutralization by normal human serum (NHS) (16, 17, 21). These findings contrast with previous results showing that human parainfluenza type 3 (HPIV3) and Newcastle disease virus (NDV) are neutralized through the classical pathway (33, 36). Thus, it appears that individual members of the paramyxovirus family may activate different arms of the complement pathway and may differ in their sensitivity to complement-mediated neutralization.
Nipah virus is a biosafety level 4 (BSL4) emerging viral pathogen. NiV has been designated a priority pathogen for study due to the high mortality rates following human infections (∼45 to 70%), the potential impact on farm economies, and the potential for use as a bioterrorism agent (11, 19, 20). There are currently no approved vaccines or therapeutics for NiV (7). NiV is a prototype member of the Henipavirus genus of the paramyxovirus family of negative-strand RNA viruses, which also includes the highly pathogenic Hendra virus (11, 34). NiV is thought to spread from fruit bats to humans (8), but person-to-person transmission has become of increasing concern (14). NiV encodes two surface glycoproteins, the fusion protein F, which promotes membrane fusion, and the glycoprotein G, which serves as the attachment protein through binding to the highly conserved cellular receptors EphrinB2 and -B3 (6, 28). The NiV F and G proteins also serve as targets for neutralizing antibodies that can be detected in convalescent-phase sera (32) and can be elicited through experimental vaccine approaches (e.g., see references 13 and 26). To date, the ability of human complement pathways to be activated by particles containing the NiV glycoproteins and to function in promoting neutralization has not been examined.
Given the differing role of complement in the neutralization of different paramyxoviruses and the potential strong impact of complement on antiviral immunity, we have addressed the contribution that human complement makes to NiV neutralization. Pseudotyped particles containing the NiV F and G glycoproteins activated the alternative complement pathway, but in contrast to many other paramyxoviruses, NHS alone was not sufficient to neutralize infectivity in vitro. This lack of neutralization by activated complement responses was not due to a complete inhibition of all complement pathways, since the presence of antibodies to the viral glycoproteins or a soluble version of the EphrinB2 receptor resulted in activation of the classical complement pathway and increased potency of neutralization. Our results have implications for how complement could be harnessed to improve the potency of therapies targeting NiV glycoproteins.
MATERIALS AND METHODS
Cells and viruses.
Vero cell monolayer cultures were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS). NiV envelope-pseudotyped reporter virus (NiVpp) were generated in 293T cells as described earlier (1). The NiVpp consisted of the vesicular stomatitis virus (VSV)-deltaG-luc virus in which the gene for the G attachment protein was replaced with the gene for Renilla luciferase. NiVpp were purified by centrifugation through a sucrose cushion and resuspended in phosphate-buffered saline (PBS) as described previously (1). Genome copy numbers were determined by reverse transcription-PCR. To generate pseudotypes containing the PIV5 glycoproteins, 15-cm dishes of 293T cells were transfected with 25 μg of pCAGGS-F and pCAGGS-HN (kindly provided by Tony Schmitt, Penn State University). After 24 h, cells were infected with the VSV-deltaG-luc virus and then left for another 24 h. Virions were purified as described for NiVpp particles above. Dilutions of the PIV5 pseudotypes (samples from a 1:100 dilution contained 4.8 × 107 genome copies) were used in neutralization assays.
Complement reagents, proteins, and antibodies.
Normal human serum (NHS) was collected from healthy donors, processed, and divided into small aliquots before being frozen at −80°C as published previously (16). Purified complement components human C4, cobra venom factor (CVF), C1q, C4- and C1q-depleted human serum, goat anti-human C1q, and rabbit anti-human C3a were procured from Complement Technologies (Tyler, Texas). Rabbit polyclonal antibodies specific for Nipah virus F (806) and G (834) have been described previously (28). Rabbit monoclonal antibodies against Nipah virus F (clones 92 and 66) and G (clones 26 and 45) have been described previously (2, 3). Monoclonal antibodies 322 (anti-F) and 213 (anti-G) have not been described previously. The recombinant mouse EphrinB2-Fc chimera was purchased from R&D systems.
ELISA and Western blotting.
Two-fold dilutions of NiVpp starting at 1.5 × 105 genome copies in phosphate-buffered saline (PBS, pH 7.4) were mixed with a 1:10 dilution of NHS (assay volume, 20 μl) and incubated for 1 h at 37°C. The samples were further diluted 1:500 and used in an enzyme-linked immunosorbent assay (ELISA) specific for C3a or Bb. In the case of the C4a ELISA, samples included NiVpp alone or samples that had been treated with either anti-Nipah virus F and G antibodies (1:1,600) or mouse EphrinB2 (5 ng). Treatment conditions and volumes were similar to those of the C3a ELISA. ELISA was performed as described by the manufacturer, using a MicroVue Bb kit from Quidel (San Diego, CA) or an OptEIA human C3a and C4a kit from BD Biosciences (San Jose, CA). Statistical significance was determined using Student's t test.
Time- or concentration-dependent activation of C3 by NiVpp was assayed by Western blot analysis. For the concentration experiment, NiVpp was diluted 2-fold, with the highest concentration being 1.5 × 105 genome copies, and incubated for 1 h with NHS (1:10) at 37°C. For the time course experiment, 7.5 × 103 genome copies were incubated for various times with NHS (1:10) at 37°C. The samples were separated on a 15% SDS–PAGE gel and analyzed by Western blotting using rabbit anti-human C3a (1:2,000 dilution) followed by anti-rabbit horseradish peroxidase (Bio-Rad). Blots were visualized by horseradish peroxidase-conjugated antibodies and enhanced chemiluminescence (Pierce Chemicals).
Electron microscopy.
C3 deposition on NiVpp was analyzed by adsorbing 1.5 × 105 genome copies of purified particles on carbon-coated 200-mesh gold grids (Electron Microscopy Sciences, PA), followed by a 5-min incubation at room temperature in a humidified chamber. The particles were either treated with polyclonal anti-Nipah virus F and G antibodies at a dilution of 1:3,200 or left untreated. After treatment with NHS (1:10 dilution), samples were blocked with 1% bovine serum albumin in PBS and then probed with anti-C3 monoclonal antibody (Cell Sciences) at a dilution of 1 μg in 10 μl. Deposition was detected with 12-nm gold bead-labeled goat anti-rabbit antibody and 6-nm gold bead-labeled goat anti-mouse antibodies (Jackson Immunoresearch Laboratories, PA). C1q association was analyzed by using primary goat anti-C1q antibody (1:25) and 6-nm-gold bead-labeled donkey anti-goat secondary antibody. After labeling, particles were subjected to negative staining with 2% phosphotungstic acid (pH 6.6) and analyzed using a Philips TEM400 transmission electron microscope as previously described (16).
NiVpp neutralization assay.
As indicated in the figure legends, NiVpp particles in a total reaction mixture volume of 100 μl were treated for 1 h at 37°C with various concentrations of NHS alone (see Fig. 2) or a combination of either polyclonal anti-Nipah virus F and G antibodies or individual monoclonal antibodies specific for F or G. Concentrations of polyclonal and monoclonal antibodies are indicated in the figures. In some experiments, NHS depleted of either C1q or C4 was utilized along with a control sample in which the physiological concentration of the depleted factor was reconstituted as described previously (17). Samples were diluted to 250 μl in PBS with 1% FBS and used to infect Vero cell monolayers in 24-well plates for 2 h at 37°C. Cells were then rinsed with PBS and incubated for 24 h before analysis using a Renilla luciferase assay system (Promega). In some experiments, NiVpp was incubated with anti-G and anti-F antibodies or with EphrinB2-Fc in the presence or absence of NHS for 1 h prior to assay for infectivity as described in the figure legends. Statistical significance was determined using Student's t test.
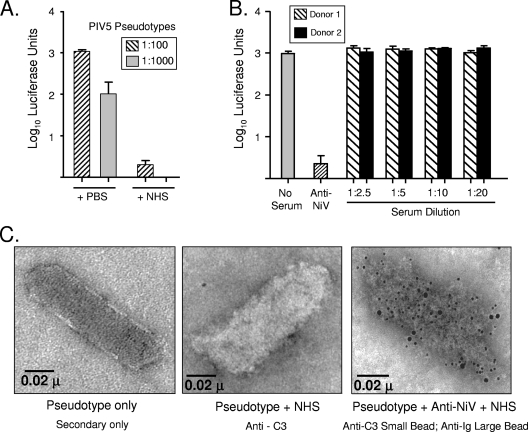
Fig. 2.
NiVpp are not neutralized by NHS in vitro. (A) Two dilutions of PIV5 pseudotypes were incubated with PBS as a control or with a 1:20 dilution of NHS for 1 h at 37°C. The remaining infectivity was determined by infecting Vero cells and assaying luciferase activity. (B) NiVpp were incubated for 1 h alone (No Serum), with polyclonal antibodies specific for the NiV F and G proteins as a positive control (Anti-NiV), or with the indicated dilutions of NHS from two separate donors (black and hatched bars). The remaining infectivity was determined by infecting Vero cells and assaying luciferase activity. Error bars show standard deviations. (C) NiVpp were applied to carbon grids and treated with NHS or a combination of NHS and polyclonal antibody specific for NiV F and G proteins as described in Materials and Methods. Samples were subsequently treated with gold-conjugated anti-C3 (6-nm beads) or anti-IgG (12-nm beads) before they were examined by electron microscopy.
RESULTS
Pseudotypes containing the NiV glycoproteins activate the alternative arm of the complement pathway.
Due to the constraints of working with BSL4 live NiV, pseudotyped reporter viruses (NiVpp) were generated to contain the NiV glycoproteins F and G in an envelope surrounding a VSV genome that encodes the Renilla luciferase gene in place of the VSV G glycoprotein gene (1). These single-cycle pseudotypes were purified by centrifugation and used in assays for interactions with complement factors. To determine whether the NiVpp activated human complement pathways, increasing levels of NiVpp were incubated with NHS and then assayed by Western blotting for levels of the C3 cleavage product C3a. As shown by the results of the titration experiment shown in Fig. 1A, incubation of increasing levels of NiVpp with NHS led to increased appearance of the C3a product. Quantitation of C3a levels by ELISA showed an increase in C3 activation by exposure to increasing amounts of NiVpp, starting with ∼38,000 genome copies (Fig. 1C). In time course experiments (Fig. 1B), complement activation by NiVpp was rapid, with the appearance of C3a cleavage products beginning within 5 min of incubation with NHS. C3a levels plateaued within 15 to 30 min of incubation at levels which approached that seen following treatment of NHS with the positive-control cobra venom factor (CVF).
Fig. 1.
Activation of human complement pathways by NiVpp. (A and C) NHS (1:20 dilution) was incubated for 1 h alone, with CVF as a positive control, or with dilutions of purified NiVpp. Samples were analyzed by Western blotting (A) or by ELISA (C) for the presence of the C3 cleavage product C3a. Error bars show standard deviations. (B) NiVpp were incubated for the indicated times with a 1:10 dilution of NHS before analysis by Western blotting for the presence of C3a. Control samples were incubated for 60 min. (D) Activation of alternative pathway. NiVpp (1.5 × 105 genome copies) were treated as described for panel A, and samples were assayed for the Bb cleavage product by ELISA. *, P < 0.002.
The activation of classical and lectin pathways results in the appearance of the C4a cleavage product, whereas the activation of the alternative pathway results in cleavage of factor B into Ba and Bb (9, 30). Using Western blotting and ELISAs, we found that exposure of NiVpp to NHS did not result in the appearance of C4a cleavage products (data not shown), indicating that the classical/lectin arms were not activated under these in vitro conditions. In contrast, NHS treatment of NiVpp resulted in the appearance of the factor B cleavage product Bb (Fig. 1D), consistent with the activation of the alternative pathway. Together, these data indicate that NiVpp show a dose-dependent and rapid activation of the alternative complement pathway.
Activated alternative complement pathway does not neutralize NiVpp infectivity.
For many paramyxoviruses, complement activation results in very efficient neutralization in vitro, and C3 cleavage products are deposited on virion particles (4, 16, 17). For example, we have previously shown that treatment of PIV5 with NHS results in neutralization (16, 17). As shown in Fig. 2A, VSV-based pseudotypes that contained the PIV5 glycoproteins HN and F produced high levels of luciferase activity on infected Vero cells (+PBS bars). Treatment with NHS prior to infection of cells led to a dramatic loss of infectivity (+NHS bars). Similar results were seen with NHS from a total of five separate donors (not shown). Thus, the VSV-based pseudotypes containing PIV5 glycoproteins have sensitivity to NHS similar to that seen with bone fide PIV5 particles (16, 17).
To determine if activation of human complement by the NiVpp also resulted in neutralization, NiVpp were incubated with dilutions of NHS or with purified antibodies specific for the NiV F and G proteins as a positive control. Cells were then infected with the treated NiVpp, and infectivity was measured at 24 h postinfection (p.i.) by assaying luciferase levels. As shown in Fig. 2B, polyclonal antibodies to F and G effectively reduced luciferase levels by ∼500-fold compared to the levels in the untreated control sample. In sharp contrast, the results for NHS from two donors showed that there was no loss of infectivity when NiVpp were incubated with NHS even at a 1:2.5 dilution. These data indicate that, in contrast to results with PIV5 and mumps virus (16, 17), complement activation does not result in neutralization of NiVpp infectivity in vitro.
To determine if the activation of complement by NiVpp resulted in C3 deposition, NiVpp were treated with NHS and examined for the presence of C3 by immunogold electron microscopy. As shown in Fig. 2C, the levels of C3 on NiVpp treated with NHS (middle panel) were similar to the low level seen with untreated NiVpp (left panel). As a positive control, NiVpp were treated first with purified anti-F and anti-G antibodies and then with NHS. As expected, particles in the positive-control sample were difficult to distinguish due to the substantial deposition of IgG (large beads) and C3 (small beads) on NiVpp (Fig. 2C, right panel). Taken together, these data indicate that while complement is activated by NiVpp, this does not result in the deposition of C3 on particles in the absence of anti-NiV antibodies.
Complement enhances the potency of neutralizing anti-NiV antibodies.
The above finding that C3 is deposited on antibody-treated NiVpp suggested that while activated complement in NHS alone was insufficient for neutralization, complement could enhance the neutralizing capacity of antibodies specific for the viral glycoproteins. To test this hypothesis, NiVpp were treated for 1 h with dilutions of a mixture of purified anti-F and anti-G polyclonal antibodies alone or in combination with NHS and the remaining infectivity was determined by assaying luciferase activity from infected Vero cells. As shown in Fig. 3A, as the dilution of antibodies used to treat NiVpp was increased, there was a corresponding increase in luciferase activity, indicating reduced neutralization capacity. In contrast, in the presence of NHS, no luciferase activity was detected until the antibody dilution reached 1:3,200. In time course experiments, treatment of NiVpp with F and G antibodies alone or in combination with NHS resulted in a rapid, ∼10-fold reduction in infectivity as soon as 1 min after incubation (Fig. 3B). Over time, antibody alone gave only a marginal further reduction in infectivity. In contrast, samples that also contained NHS showed a continuous time-dependent loss of infectivity to zero.
Fig. 3.
Complement enhances the potency of NiVpp neutralization by antibodies to the viral glycoproteins. (A) Titration. NiVpp were incubated alone (PBS) or with the indicated dilutions of a combination of polyclonal anti-F and anti-G antibodies with or without a 1:20 dilution of NHS. The remaining infectivity was determined by infecting Vero cells and assaying luciferase activity. (B) Time course. NiVpp were incubated with a 1:20 dilution of NHS with or without anti-F and anti-G antibodies. At the indicated times p.i., the remaining infectivity was determined by infecting Vero cells and assaying luciferase activity. (C) Activation of classical/lectin pathway. NiVpp were treated with anti-F and anti-G antibody (Anti-FG; 1:1,600) plus NHS as described for panel A, and samples were assayed for the C4a cleavage product by ELISA. (D) NHS-mediated enhancement of neutralization requires C4. NiVpp were incubated for 1 h with the indicated combinations of antibody against F and G, C4-depleted serum (C4 depl), and physiological levels of C4. The remaining infectivity was determined by infecting Vero cells and assaying luciferase activity. Error bars show standard deviations. #, P < 0.001; *, P < 0.004, ^, P < 0.01.
Activation of the classical pathway results in C4 cleavage. While NiVpp alone activated the alternative pathway (Fig. 1), treatment of NiVpp with both antibody and NHS resulted in the appearance of the C4a cleavage product (Fig. 3C). To determine whether C4 was necessary for NHS-enhanced neutralization, the infectivity of NiVpp was determined after treatment with combinations of C4-depleted serum, purified polyclonal anti-F and anti-G antibodies, and C4-depleted serum that was reconstituted with physiological levels of C4. As shown in Fig. 3D, treatment of NiVpp with C4-depleted serum (sample 2) or depleted serum supplemented with C4 (sample 4) had only a minor effect on infectivity (Fig. 3D). Antibodies to the NiV glycoproteins partially neutralized NiVpp (sample 1), but this was only minimally enhanced by the addition of C4-depleted serum (sample 3). The highest level of neutralization was seen using the combined treatment with antibodies, C4-depleted serum, and physiological levels of supplemented C4 (sample 5). These data indicate that C4 is activated when NiVpp are exposed to antibodies to the viral glycoproteins and that C4 is necessary for maximal enhancement of neutralization.
In the case of influenza virus and West Nile virus (WNV) (12, 23), C1q has been shown to be both necessary and sufficient for complement-mediated enhancement of antibody neutralization. To determine the role of C1q in NiV neutralization, NiVpp were treated with purified C1q in the presence or absence of anti-F and anti-G antibodies and then analyzed by immunogold labeling for C1q deposition. As shown in Fig. 4A, C1q was not associated with NiVpp (middle panel) except when antibodies to the viral glycoproteins were included along with C1q (right panel).
Fig. 4.
C1q is required for NHS-mediated enhancement of neutralization of NiVpp by anti-NiV antibodies. (A) NiVpp were applied to carbon-coated grids and treated with combinations of purified C1q or polyclonal antibody specific for NiV F and G proteins. Samples were subsequently treated with gold-conjugated anti-C1q (6-nm beads) or anti-IgG (12-nm beads) before they were examined by electron microscopy. (B) NHS-mediated enhancement of neutralization requires C1q. NiVpp were incubated for 1 h with the indicated combinations of polyclonal antibody against F and G, C1q-depleted serum, and physiological levels of C1q. The remaining infectivity was determined by infecting Vero cells and assaying luciferase activity. Error bars show standard deviations. #, P < 0.001.
To determine if C1q was sufficient for enhancing antibody neutralization, infectivity was determined after treatment of NiVpp with combinations of C1q-depleted serum, purified polyclonal anti-F and anti-G antibodies, and physiological levels of C1q. As shown in Fig. 4B, treatment of NiVpp with C1q alone (sample 6), C1q-depleted serum (sample 2), or depleted serum supplemented with C1q (sample 4) had only a minor effect on infectivity. Treatment with antibodies to the NiV glycoproteins partially neutralized NiVpp (sample 1), but this was not enhanced by C1q alone (sample 7) or C1q-depleted serum (sample 3). The highest level of neutralization was seen in the case of NiVpp treated with antibody and C1q-containing serum (sample 5). These data demonstrate that neutralization of NiVpp differs from neutralization of influenza virus and West Nile virus (12, 23) in that C1q is necessary but is not by itself sufficient for enhanced neutralization by NHS.
Complement enhances neutralization of NiVpp by monoclonal antibodies targeting both F and G proteins.
The above-described results were obtained using a mixture of polyclonal rabbit antibodies specific for both NiV F and G proteins. To test complement enhancement of neutralization by individual antibodies targeting F or G, NiVpp were treated with dilutions of purified anti-F and anti-G monoclonal antibodies (2, 3) with or without NHS. The remaining infectivity was determined by infecting Vero cells and assaying luciferase activity. Representative results from two antibodies are shown in Fig. 5. In the case of anti-F monoclonal 322 (Fig. 5A), NHS enhanced neutralization at the highest concentration of antibody tested (e.g., 50 to 200 ng) but not at lower antibody concentrations. In contrast, NHS enhanced neutralization by anti-G monoclonal antibody 213 across the entire range of concentrations tested (Fig. 5B). As shown in Table 1, three individual anti-F antibodies displayed an NHS enhancement profile similar to that shown in Fig. 5A, in which enhancement was evident only at the highest antibody concentrations. Thus, with 12.5 ng of the anti-F antibodies, there was no enhancement of neutralization by NHS, regardless of their values for the concentration of antibody needed to reduce infectivity by 95% (IC95). The anti-G antibodies behaved differently, as shown in Table 1. Three anti-G antibodies had the profile shown in Fig. 5B, with enhancement at 12.5 ng of anti-G antibody ranging from 2- to 7.5-fold. With these three anti-G antibodies, the level of enhancement appeared to correlate with the IC95 values. NHS-mediated enhancement of neutralization by anti-F and anti-G antibodies depended on both C1q and C4 (data not shown). These data indicate that NHS can enhance neutralization by antibodies targeting either F or G but that the extent of enhancement of an individual monoclonal antibody may depend on both the target protein (e.g., F or G) and the IC95 of the particular antibody.
Fig. 5.
NHS enhances NiVpp neutralization by monoclonal antibodies against NiV F or G proteins. NiVpp were incubated alone (PBS) or with the indicated concentrations of a monoclonal anti-F (A) or anti-G (B) antibody in the absence (hatched bars) or presence (black bars) of a 1:20 dilution of NHS. The remaining infectivity was determined by infecting Vero cells and assaying luciferase activity. Error bars show standard deviations. #, P < 0.001; ^, P < 0.015; *, P < 0.004.
Table 1.
Properties of NHS enhancement of antibody-mediated NiVpp neutralizationa
| Antibody | Target | IC95b | Enhancement profilec | Fold enhancementd |
|---|---|---|---|---|
| 322 | F | 0.6 | A | 1.1 |
| 66 | F | 3.98 | A | 1.4 |
| 92 | F | 5.25 | A | 1.4 |
| 213 | G | 0.03 | B | 7.5 |
| 26 | G | 1.0 | B | 7 |
| 45 | G | 7.0 | B | 2 |
| EphrinB2-Fc | G | B | 4.3 |
NiVpp were mixed with dilutions of the indicated antibodies or soluble EphrinB2-Fc with or without a 1:20 dilution of NHS. After 1 h at 37°C, the remaining infectivity was determined as described in Materials and Methods.
IC95, concentration of antibody needed to reduce infectivity by 95% as reported in references 2 and 3.
Letters indicate titration results with or without NHS grouped as having neutralization profiles similar to those depicted in either Fig. 5A or B.
The NHS-mediated fold enhancement in neutralization was calculated as the ratio of the luciferase level remaining after treatment with 12.5 ng of antibody or 10 ng of EphrinB2-Fc without NHS divided by the value with NHS.
Complement enhances NiVpp neutralization by a soluble EphrinB2 receptor.
Previous results have shown that a soluble version of the cellular receptor EphrinB2 can block fusion and entry of particles containing the NiV G protein (28). We tested the ability of complement components to enhance NiVpp neutralization by a soluble version of EphrinB2. This commercially available soluble EphrinB2 consists of the extracellular domain of murine EphrinB2 linked to the Fc region from human IgG1. As shown in Fig. 6A for NHS from two donors, there was a dose-dependent neutralization of NiVpp with soluble EphrinB2, but importantly, this was significantly enhanced in the presence of NHS. It is noteworthy that the effect of NHS on neutralization by various concentrations of EphrinB2 had a profile similar to that seen with anti-G antibodies (Table 1). Neutralization by soluble EphrinB2 alone was very rapid, but the loss of infectivity did not change significantly with further incubation (Fig. 6B, hatched bars). In contrast, soluble EphrinB2 along with NHS showed a rapid initial neutralization, within 1 min, followed by a time-dependent increased neutralization (black bars) that was not seen with soluble EphrinB2 alone.
Fig. 6.
Complement enhances neutralization of NiVpp by soluble EphrinB2 receptor through a C4-dependent pathway. (A) Titration. NiVpp were incubated alone (PBS) or with the indicated concentrations of soluble EphrinB2 receptor in the absence (hatched bars) or presence (black bars) of a 1:20 dilution of NHS from two different donors. The remaining infectivity was determined by infecting Vero cells and assaying luciferase activity. (B) Time course. NiVpp were incubated with a 1:20 dilution of NHS. At the indicated times p.i., the remaining infectivity was determined by infecting Vero cells and assaying luciferase activity. (C) Activation of classical pathway. NiVpp were treated as described for panel A, and samples were assayed for the C4a cleavage product by ELISA. Error bars show standard deviations. #, P < 0.001; α, P < 0.04; *, P < 0.004.
Complement-mediated enhancement of EphrinB2 neutralization proceeded through the classical/lectin pathway and required C4. This is evident from the results shown in Fig. 6C, where the C4a cleavage product resulting from C4 activation was increased by the exposure of NiVpp to soluble EphrinB2 and NHS. To determine the role of C4 and C1q in neutralization by soluble EphrinB2, NiVpp were incubated with combinations of NHS, C4- or C1q-depleted serum, and physiological levels of C4 and C1q. Neutralization was not enhanced by C4-depleted serum (Fig. 7, compare samples 2 and 5), but the combination of C4-depleted serum plus reconstituted C4 was equally as effective as treatment with NHS (Fig. 7, compare samples 3, 5, and 7). Likewise, C1q-depleted serum did not enhance ephrin-mediated neutralization unless C1q was present (Fig. 7, compare samples 9 and 11). Together, these data indicate that the potency of soluble EphrinB2 in neutralizing NiVpp is greatly enhanced by complement components within the C1q- and C4-dependent pathway.
Fig. 7.
EphrinB2-mediated enhancement of neutralization requires C4 and C1q. NiVpp were incubated for 1 h with the indicated combinations of NHS, EphrinB2, C4- or C1q-depleted serum, and physiological levels of C4 or C1q. The remaining infectivity was determined by infecting Vero cells and assaying luciferase. Error bars show standard deviations. *, P < 0.004.
DISCUSSION
The high mortality rate of NiV infections and the potential use of the virus as an agent of bioterrorism have raised intense interest in developing vaccines or therapeutic approaches targeting the NiV glycoproteins (7, 8, 19, 29). In view of the potent role that complement can play in paramyxovirus neutralization (16, 17, 21, 36), the goal of the work described here was to determine the role of complement in the neutralization of particles containing the NiV glycoproteins. Our work demonstrates that exposure of the NiVpp particles to NHS results in activation of the alternative complement pathway, but in contrast to findings with a range of other paramyxoviruses, the activation of this innate immune pathway did not result in substantial neutralizing capacity. This lack of complement-mediated neutralization was not due to a global inhibition of complement pathways, since the addition of NHS to anti-F or anti-G antibodies increased the rate and extent of neutralization by activation of the classical pathway. Our most striking finding was that complement had a strong enhancing effect on neutralization by a soluble version of the EphrinB2 receptor protein, resulting in very rapid and complete neutralization through activation of the classical pathway. Together, these data establish the importance of complement in the neutralization of particles containing the NiV glycoproteins and will help guide the design of more effective therapeutics that harness the potency of complement pathways.
Why would complement be an important factor in innate responses to NiV infections? NiV infection in humans is characterized by systemic infections, where viral antigen can be detected in neurons, in parenchymal cells of the central nervous system and lymphoid tissue, and in endothelial cells lining blood vessels (reviewed in references 7 and 19). Thus, in view of targeted infections of blood vessel endothelium, it is reasonable to assume that NiV encounters high levels of serum complement factors during natural human infections. In addition to serum, it is also clear that complement factors are produced locally during infections of organ systems, such as the respiratory tract. This synthesis of complement components can be from cells that are part of the organ structure (e.g., epithelial cells), as well as immune cells recruited to the site of infection (e.g., macrophages) (5). Thus, the sites of infection, which include the respiratory tract, lymphoid tissue, and endothelial cells, suggest that complement is a highly relevant innate immune pathway to consider during NiV infections.
Previous results have shown that paramyxoviruses activate complement pathways involving either alternative (16, 21) or classical pathways (33, 36) and that NHS has a strong neutralizing capacity. Similar to our results for PIV5 and MuV (16), we have found that NiVpp are strong activators of the alternative pathway. This contrasts with the activation of the classical pathway by VSV (24), which forms the core particles used to generate the pseudotypes, consistent with the idea that the NiV glycoproteins F and G are major contributors to complement activation. In the case of PIV5 and MuV, neuraminidase activity associated with the HN protein contributes to complement activation through the exposure of surfaces lacking sialic acid (21). As NiV glycoproteins lack neuraminidase activity, it is currently unclear what signals in F or G contribute to complement activation.
An unexpected finding was that while PIV5 pseudotypes were neutralized by NHS, as shown previously for bone fide virus (Fig. 2), the strong activation of complement by NiVpp in vitro did not result in NiVpp neutralization, even in the presence of high concentrations of NHS. A similar finding using normal rabbit serum was reported previously but without data being provided (6). The fact that C3 was not stably deposited on NiVpp suggests that after activation, complement pathways are rapidly inactivated at an upstream point and C3 products are prevented from forming a stable complex with particles. This finding raises the possibility that NiV glycoproteins recruit host cell regulators of complement activation (RCA) during the budding process (e.g., CD46) or from the fluid phase (e.g., factor H) in order to inactivate the complement cascade. While there are clear examples of these two mechanisms for PIV5 (17) and for the flavivirus West Nile virus (WNV) (10), the analysis of whether NiV uses similar mechanisms to resist neutralization will require BSL4-level experiments with bone fide NiV particles and infected cells.
The lack of NiVpp neutralization by NHS was not due to inhibition of all complement pathways, as evidence by the finding that the classical pathway was activated when NHS was combined with antibody specific for F or G. It is thought that C1q acts to lower the threshold for the number of antibodies needed for neutralization (23). Our results with depleted serum demonstrate that both C4 and C1q are necessary but that neither component is sufficient for complement-mediated enhancement of neutralization with anti-F or anti-G antibodies. This insufficiency for C1q in neutralization of NiVpp contrasts with findings for both influenza virus (12) and WNV (23), where C1q alone was sufficient to enhance antibody-mediated neutralization. In the case of WNV, C1q-mediated complement fixation by neutralizing antibodies does not require the MAC or virolysis (22). Thus, this difference in C1q sufficiency may reflect that NiVpp neutralization requires downstream factors and formation of the MAC, a proposal supported by the requirement for C4 for NHS to enhance neutralization. Alternatively, this may reflect a difference in the use of pseudotypes versus bone fide particles or differences in the cellular membranes from which the viral envelope is derived.
Our results have implications for the design of more effective antibodies targeting the NiV glycoproteins. Vaccination experiments have shown that protection can be conferred by anti-NiV antibodies that are specific for either F or G (13, 35). In addition, the glycosylation status of an NiV glycoprotein can substantially influence antibody neutralization (1). Importantly, however, to date it has not been demonstrated whether complement enhances the potency of anti-F or anti-G neutralization capacity. Here, titration experiments with monoclonal antibodies and soluble EphrinB2 demonstrated that the ability of complement to enhance neutralization fell into one of two general profiles: (i) that of anti-F antibodies, which showed enhancement only at higher (e.g., 50 ng) but not lower antibody concentrations, or (ii) that of anti-G antibodies and EphrinB2, which showed enhancement at all concentrations tested, including very low levels of antibody (e.g., 3.1 ng) or EphrinB2 (e.g., 2.5 ng). Although only a limited number of monoclonal antibodies were analyzed here, the available data suggest that the ability of complement to enhance anti-F activity was relatively independent of the IC95, whereas the anti-G antibodies with the lowest IC95 values showed the highest level of enhancement (Table 1). As antibodies that show NHS-enhanced neutralization at all concentrations would be the most promising agents to pursue therapeutically, future work will be aimed at identifying key determinants of these antibodies (e.g., affinity, epitopes, etc) that confer the highest level of interactions with complement components. For example, it may be possible to select anti-NiV molecules from human antibody libraries that show the highest levels of enhancement by complement components (37).
The identification of EphrinB2 as the NiV attachment receptor (6, 28) opened the way for the use of soluble EphrinB2 as a novel receptor decoy inhibitor of NiV infections. Here, we show that complement components greatly enhance the potency of neutralization by a chimeric EphrinB2 molecule that is fused to an IgG1 Fc domain. While soluble EphrinB2 alone produced a rapid initial neutralization of NiVpp similar to that seen with antibody alone, this was followed by a further time-dependent extension of neutralizing activity in the presence of complement components that acted through the classical pathway. Soluble receptor decoys, such as the EphrinB2-Fc chimera, have several advantages over conventional antibody approaches, including high-affinity interactions between the attachment protein and receptor and the low probability that virus will mutate to escape interactions with its receptor. It may be possible in the future to engineer the EphrinB2-Fc chimera to increase its activity in the recruitment of complement factors, as shown previously for antibody therapies (15, 27).
ACKNOWLEDGMENTS
We thank members of the Parks and Barton laboratories for helpful comments on the manuscript and Ellen Young and Ken Grant for excellent technical assistance.
The project described was supported by NIH grants AI081022 and #U54 AI057157 from Southeastern Regional Center of Excellence for Emerging Infections and Biodefense.
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Published ahead of print on 30 March 2011.
REFERENCES
- 1. Aguilar H. C., et al. 2006. N-Glycans on Nipah virus fusion protein protect against neutralization but reduce membrane fusion and viral entry. J. Virol. 80:4878–4889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aguilar H. C., et al. 2007. Polybasic KKR motif in the cytoplasmic tail of Nipah virus fusion protein modulates membrane fusion by inside-out signaling. 81:4520–4532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aguilar H. C., et al. 2009. A novel receptor-induced activation site in the Nipah virus attachment glycoprotein (G) involved in triggering the fusion glycoprotein (F). J. Biol. Chem. 284:1628–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blue C. E., Spiller O. B., Blackbourn D. J. 2004. The relevance of complement to virus biology. Virology 319:176–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bolger M. S., et al. 2007. Complement levels and activity in the normal and LPS-injured lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 292:L748–L759 [DOI] [PubMed] [Google Scholar]
- 6. Bonaparte M. I., et al. 2005. Ephrin-B2 ligand is a functional receptor for Hendra virus and Nipah virus. Proc. Natl. Acad. Sci. U. S. A. 102:10652–10657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bossart K. N., Broder C. C. 2006. Developments towards effective treatments for Nipah and Hendra virus infection. Expert Rev. Anti Infect. Ther. 4:43–55 [DOI] [PubMed] [Google Scholar]
- 8. Bossart K. N., et al. 2009. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute Nipah virus infection. PLoS Pathog. 5:e1000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carroll M. C. 2004. The complement system in regulation of adaptive immunity. Nat. Immunol. 5:981–986 [DOI] [PubMed] [Google Scholar]
- 10. Chung K. M., et al. 2006. West Nile virus nonstructural protein NS1 inhibits complement activation by binding the regulatory factor H. Proc. Natl. Acad. Sci. U. S. A. 103:19111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eaton B. T., Broder C. C., Middleton D., Wang L. F. 2006. Hendra and Nipah viruses: different and dangerous. Nat. Rev. Microbiol. 4:23–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feng J. O., Mozdzanowska K., Gerhard W. 2002. Complement component C1q enhances the biological activity of influenza virus hemagglutinin-specific antibodies depending on their fine antigen specificity and heavy-chain isotype. J. Virol. 76:1369–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guillaume V., et al. 2004. Nipah virus: vaccination and passive protection studies in a hamster model. J. Virol. 78:834–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Homaira N., et al. 2010. Nipah virus outbreak with person-to-person transmission in a district of Bangladesh. Epidemiol. Infect. 138:1630–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Idusogie E. E., et al. 2001. Engineered antibodies with increased activity to recruit complement. J. Immunol. 166:2571–2575 [DOI] [PubMed] [Google Scholar]
- 16. Johnson J. B., Capraro G. A., Parks G. D. 2008. Differential mechanisms of complement-mediated neutralization of the closely related paramyxoviruses simian virus 5 and mumps virus. Virology 376:112–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnson J. B., Grant K., Parks G. D. 2009. The paramyxoviruses simian virus 5 and mumps virus recruit host cell CD46 to evade complement-mediated neutralization. J. Virol. 83:7602–7611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lambris J. D., Ricklin D., Geisbrecht B. V. 2008. Complement evasion of human pathogens. Nat. Rev. Microbiol. 6:132–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee B. 2007. Envelope-receptor interactions in Nipah virus pathobiology. Ann. N. Y. Acad. Sci. 1102:51–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lo M. K., Rota P. A. 2008. The emergence of Nipah virus, a highly pathogenic paramyxovirus. J. Clin. Virol. 43:396–400 [DOI] [PubMed] [Google Scholar]
- 21. McSharry J. J., Pickering R. J., Caliguiri L. A. 1981. Activation of the alternative complement pathway by enveloped viruses containing limited amounts of sialic acid. Virology 114:507–515 [DOI] [PubMed] [Google Scholar]
- 22. Mehlhop E., Fuchs A., Engle M., Diamond M. S. 2009. Complement modulates pathogenesis and antibody-dependent neutralization of West Nile virus infection through a C5-independent mechanism. Virology 383:11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mehlhop E., et al. 2009. Complement protein C1q reduces the stoichiometric threshold for antibody-mediated neutralization of West Nile virus. Cell Host Microbe 6:381–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mills B. J., Cooper N. R. 1978. Antibody-independent neutralization of vesicular stomatitis virus by human complement. I. Complement requirements. J. Immunol. 121:1549–1557 [PubMed] [Google Scholar]
- 25. Morrison T. E., Fraser R. J., Smith P. N., Mahalingam S., Heise M. T. 2007. Complement contributes to inflammatory tissue destruction in a mouse model of Ross River virus-induced disease. J. Virol. 81:5132–5143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mungall B. A., et al. 2006. Feline model of acute Nipah virus infection and protection with a soluble glycoprotein-based subunit vaccine. J. Virol. 80:12293–12302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Natsume A. M., et al. 2008. Engineered antibodies of IgG1/IgG3 mixed isotype with enhanced cytotoxic activities. Cancer Res. 68:3863–3872 [DOI] [PubMed] [Google Scholar]
- 28. Negrete O. A., et al. 2005. EphrinB2 is the entry receptor for Nipah virus, an emergent deadly paramyxovirus. Nature 436:401–405 [DOI] [PubMed] [Google Scholar]
- 29. Porotto M., et al. 2010. Inhibition of Nipah virus infection in vivo: targeting an early stage of paramyxovirus fusion activation during viral entry. 6:e1001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roozendaal R., Carroll M. C. 2006. Emerging patterns in complement-mediated pathogen recognition. Cell 125:29–32 [DOI] [PubMed] [Google Scholar]
- 31. Schauber-Plewa C., Simmons A., Tuerk M. J., Pacheco C. D., Veres G. 2005. Complement regulatory proteins are incorporated into lentiviral vectors and protect particles against complement inactivation. Gene Ther. 12:238–245 [DOI] [PubMed] [Google Scholar]
- 32. Tamin A., et al. 2009. Development of a neutralization assay for Nipah virus using pseudotype particles. J. Virol. Methods 160:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vasantha S., et al. 1988. Interactions of a nonneutralizing IgM antibody and complement in parainfluenza virus neutralization. Virology 167:433–441 [PubMed] [Google Scholar]
- 34. Wang L., et al. 2001. Molecular biology of Hendra and Nipah viruses. Microbes Infect. 3:279–287 [DOI] [PubMed] [Google Scholar]
- 35. Weingartl H. M., et al. 2006. Recombinant Nipah virus vaccines protect pigs against challenge. J. Virol. 80:7929–7938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Welsh R. M., Jr. 1977. Host cell modification of lymphocytic choriomeningitis virus and Newcastle disease virus altering viral inactivation by human complement. J. Immunol. 118:348–354 [PubMed] [Google Scholar]
- 37. Zhu Z., et al. 2008. Exceptionally potent cross-reactive neutralization of Nipah and Hendra viruses by a human monoclonal antibody. J. Infect. Dis. 197:846–853 [DOI] [PMC free article] [PubMed] [Google Scholar]