Abstract
Vaccine-induced memory is necessary for protective immunity to pathogens, but many viruses induce a state of transient immune suppression that might contribute to the inability of a vaccine to elicit immunity. We evaluated here the fate of bystander T cells activated by third party cognate antigens during acute viral infections in vivo, using distinct models to track and specifically activate HY and P14 transgenic bystander CD8 T cells in vivo during acute arenavirus infections of mice. Viral infections acted as stimulatory adjuvants when bystander T cells were exposed to an inflammatory milieu and cognate antigens at the beginning of infections, but bystander CD8 T cell proliferation in response to cognate antigen was inhibited 3 to 9 days after virus infection. Reduced proliferation was not dependent on Fas-FasL- or tumor necrosis factor (TNF)-induced activation-induced cell death or on deficiencies of antigen presentation. Instead, reduced proliferation was associated with a delayed onset of division that was an intrinsic defect of T cells. Inhibition of proliferation could be simulated by exposure of T cells to the Toll-like receptor agonist and type I interferon (IFN) inducer poly(I · C). T cells lacking IFN-α/β receptors resisted both the suppressive effects of preexposure to poly(I · C) and the stimulatory effects of type I IFN, indicating that the timing of exposure to IFN can have negative or positive effects on T cell proliferation. Inhibition of T cell receptor-stimulated bystander CD8 T cell proliferation during acute viral infections may reflect the reduced ability of vaccines to elicit protective immunity when administered during an acute illness.
INTRODUCTION
Many virus infections induce a transient state of immune suppression that can be measured by reduced T cell responses in vitro to mitogens or specific recall antigens and by diminished delayed-type hypersensitivity (DTH) responses in vivo to antigens such as tuberculin (17, 24, 47). Although some viruses encode proteins that inhibit various phases of the immune response, many do not. The diverse natures of the many viruses that induce immune suppression suggest broad and possibly host-regulated mechanisms. In several acute and persistent viral infections, the failure of T cells to proliferate in response to these mitogens or antigens in vitro has been linked to the Fas-FasL-dependent activation-induced cell death (AICD) of these cell populations (1, 11, 50). Studies of other virus systems, however, have indicated that the failure to proliferate in vitro is linked to deficient dendritic cell (DC) function and/or T cell costimulation (2, 3, 8, 26, 38–40). Other reports indicate that production of virus-induced anti-inflammatory cytokines, such as interleukin 10 (IL-10) or transforming growth factor β (TGF-β) (5, 8, 32, 43), or reduced production of inflammatory cytokines, such as IL-12 (26, 38, 40), may suppress T cell outgrowth. Another possibility is that cytokine receptors on virus-specific T cells may bind limiting amounts of growth factors, such as IL-2 or IL-15, and compete with newly developing T cell responses. This lack of consensus using in vitro assays suggests that an in-depth analysis of virus-induced transient immune deficiency should be studied in vivo, using tractable models in which T cells clearly defined as not specific or cross-reactive for the virus can be examined in the milieu of a viral infection when they encounter their cognate ligand.
Vaccination efficacy can be compromised by an ongoing viral infection, and this may be linked to transient virus-induced immune suppression. A moderate-to-severe illness is a U.S. Centers for Disease Control-recommended precaution against vaccination, but even a mild illness might reduce vaccine efficacy. This reduced efficacy is not simply due to an infection-induced interferon (IFN) response restricting the replication of a live virus vaccine, as the immunity induced by both infectious and noninfectious vaccines can be compromised when administered during an ongoing infection. For example, hepatitis C virus (HCV)-infected patients elicited reduced antibody responses to a recombinant HBV protein vaccine (HB-Vax) compared to those of healthy controls (48). Nematode and malaria infections have also been shown to reduce the protective efficacy of heterologous vaccines, whether they are live (e.g., Mycobacterium bovis BCG [BCG]) or subunit (e.g., meningococcal polysaccharide) vaccines (9, 14, 41).
Naïve bystander T cells not specific for a virus do not proliferate during an infection, nor do they upregulate expression of the activation marker CD44 or downregulate expression of CD62L, both indicators of T cell receptor (TCR) stimulation (7). This does not mean, however, that they are unchanged within the inflammatory milieu of an infection. Some naïve T cells will upregulate expression of CD69 and granzyme B, and we have recently reported that a subpopulation of those cells may respond to self-major histocompatibility complex (MHC) in the presence of type I IFN and upregulate expression of Eomesodermin (Eomes), a transcription factor important for T cell effector functions and memory (20). In this study, we sought to investigate whether bystander naïve CD8 T cells would be susceptible to immune suppression if the T cells were activated by cognate antigen during an acute viral infection in vivo. Consistent with the enhanced susceptibility to cell death noted in vitro, we found that bystander CD8 T cells when activated with cognate antigen in vivo during acute viral infections underwent markedly reduced proliferation. Despite the reduced proliferation of TCR-stimulated bystander CD8 T cells, susceptibility to Fas-FasL and tumor necrosis factor (TNF)-TNF receptor (TNFR) AICD was not required. This virus-induced transient immune suppression in vivo was instead associated with a delayed onset of division that was not due to an antigen presentation defect but rather to a T cell-intrinsic defect. This suppression could be simulated with the IFN inducer poly(I · C), indicating that inflammation could mediate suppression. Finally, CD8 T cells lacking receptors for IFN-α/β could proliferate normally, demonstrating that direct IFN-α/β signaling on T cells was required for suppression.
MATERIALS AND METHODS
Mice.
C57BL/6J (Ly5.2+) male mice and B6.Smm.C3H-Tnfsf6gld (gld) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). B6.SJL (Ly5.1+) male and female mice were purchased from Taconic Farms (Germantown, NY) or bred within the Department of Animal Medicine at the University of Massachusetts Medical School (UMMS). P14 (33) and HY (18) TCR-transgenic mice, B6.MRL-Tnfrsf6lpr (lpr) mice (34), and B6 IFN-α/β receptor knockout (B6.IFNαβR KO) mice (29) were bred at the UMMS. The P14 transgenic mice were bred onto the Thy1.1 and Ly5.1 C57BL/6 backgrounds in order to distinguish the transgenic cells from each other and from wild-type (WT) cells in C57BL/6 (Thy1.2+ Ly5.2+) mice. The P14 transgenic mice were also bred onto the B6.IFNαβR KO background in order to generate P14 CD8 T cells lacking IFN-α/βR; we screened for such cells by determining the surface expression of Vα2+ TCR on CD8 T cells and by performing genomic PCR to detect the IFN-α/βR KO locus. The HY transgenic mice were crossed to lpr mice to generate HY transgenic CD8 T cells expressing a mutated Fas protein. B6.Tg(HY)-Tnfrsf6lprF2 mice (here referred to as HY/lpr mice) were screened via surface expression of the HY TCR and Fas after a 5-h in vitro anti-CD3 stimulation. All mice were maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee of the UMMS.
Virus stocks and inoculations.
Lymphocytic choriomeningitis virus (LCMV), strain Armstrong, and Pichinde virus (PV), strain AN3739, were propagated in baby hamster kidney (BHK21) cells, as previously described (46, 49). Mice were injected intraperitoneally (i.p.) with 5 × 104 PFU of LCMV or 1.5 × 107 PFU of PV. To activate P14 TCR-transgenic CD8 T cells in vivo, mice were inoculated with 5 μg of a 13-mer peptide of the LCMV glycoprotein from amino acids 33 to 45 (GP33–45) (KAVYNFATCGIFA), which is more effective than the minimal epitope (GP33–41) for T cell activation in vivo (KAVYNFATC) (6). Alternatively, mice were inoculated with 107 GP33–41-labeled DC2.4 cells intravenously (i.v.). Both peptides were purchased from 21st Century Biochemicals (Marlborough, MA). DC2.4 cells were incubated with 1 μM GP33–41 for 45 min at 37°C and washed 4 times in Hanks balanced salt solution (HBSS) to label. To block TNF, mice were inoculated with 100 μg etanercept (Enbrel) i.p. (25).
Adoptive transfers.
Spleens were harvested from TCR-transgenic mice (P14 or HY), and single-cell suspensions were prepared. Red blood cells were lysed with a 0.84% NH4Cl solution, and lymphocytes were washed with HBSS. Where described below, cells were labeled with the fluorescent dye carboxyfluorescein succinimidyl ester (CFSE) by incubation in 2 μM CFSE in HBSS (Invitrogen, Carlsbad, CA) at 37°C for 15 min. TCR-transgenic T cells (5 × 105 to 20 × 105) were injected into congenic recipient mice i.v.
Flow cytometry.
Spleen leukocytes were stained with a combination of fluorescently labeled monoclonal antibodies (MAb) specific for CD8 (53-6.7; BD Pharmingen), Ly5.2/CD45.2 (104; BD Pharmingen), Ly5.1/CD45.1 (A20; eBioscience, San Diego, CA, or BioLegend, San Diego, CA), Thy1.2/CD90.2 (53-2.1; BD Pharmingen), Thy1.1/CD90.1 (H1S51; eBioscience), Vα2 TCR (B20.1; eBioscience), HY TCR (T3.70; eBioscience), CD44 (IM7; BD Pharmingen), CD62L (MEL-14; BD Pharmingen), CD43 (1B11; BioLegend), and IFNAR-1 (MAR1-5A3; BioLegend) for 20 min at 4°C. To stain for intracellular Ki-67, spleen leukocytes were fixed and permeabilized with Cytofix/Cytoperm (BD Pharmingen) for 20 min at 4°C. Following permeabilization, cells were stained with anti-human Ki-67 (B56; BD Pharmingen).
Freshly stained and previously fixed samples were acquired using a BD Biosciences LSR II flow cytometer with FACS Diva software and analyzed with FlowJo software (TreeStar Inc., Ashland, OR).
Poly(I · C) experiments.
Poly(I · C) was purchased from InvivoGen (San Diego, CA) and used as a nonspecific stimulator for IFN-α/β and other cytokines. Splenocytes (1 × 107), representing 1.2 × 106 to 1.8 × 106 transgenic CD8 T cells, from P14 transgenic mice bred onto the Ly5.1+ or Thy1.1+ background were transferred i.v. into congenic C57BL/6 (Ly5.2+ Thy1.2+) mice. One day after transfer, mice were either untreated, mock treated with HBSS, or treated with 200 μg poly(I · C) i.p. Spleens were harvested 18 to 20 h later, and single-cell suspensions were prepared. The percentage of P14 cells in each group [mock and poly(I · C) treated] was determined via flow cytometry, and a total of 10,000 P14 cells (5,000 from each environment) were cotransferred into congenic mice i.v. Competition groups included (i) Ly5.1+ P14 cells from mock-treated mice mixed 1:1 with Thy1.1+ P14 cells from poly(I · C)-treated mice and (ii) Thy1.1+ P14 cells from mock-treated mice mixed 1:1 with Ly5.1+ P14 cells from poly(I · C)-treated mice. Mice were infected with 5 × 104 PFU LCMV Armstrong i.p. and peritoneal exudate cells (PECs), and spleens were harvested 7 days postinfection.
Statistical analyses.
Where appropriate, Student's t tests were calculated using GraphPad InStat software. Significance was set at a P of <0.05; * indicates a P of <0.05, ** a P of <0.005, and *** a P of <0.0005. All results are expressed as means ± standard deviations.
RESULTS
Transient inhibition of TCR-stimulated bystander CD8 T cell proliferation during acute viral infections.
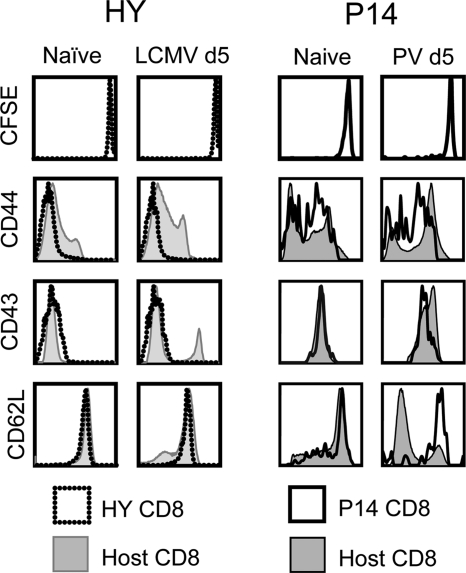
In order to study the susceptibility of bystander CD8 T cells to transient immune suppression in a physiological setting, we developed in vivo models to track and to specifically activate bystander CD8 T cells. We tested HY (male-antigen-specific) and P14 (LCMV-specific) transgenic CD8 T cells for this purpose and defined bystander as a transgenic CD8 T cell population that did not divide (dilute CFSE), proliferate (increase in cell number), or regulate activation markers during the viral infection. As shown in Fig. 1 (left), in the LCMV-infected female mice, HY transgenic CD8 T cells did not divide (with a high concentration of CFSE [CFSEhi]) and remained phenotypically naïve (with low expression of CD44 and CD43 [CD44lo and CD43lo, respectively] and high expression of CD62L [CD62Lhi]), as opposed to the polyclonal host CD8 T cells, which included LCMV-specific CD8 T cells. LCMV-specific P14 transgenic CD8 T cells similarly did not divide and remained phenotypically naïve during PV infection (Fig. 1, right). Additionally, the HY and P14 CD8 T cells did not proliferate, in terms of frequency (data not shown) or cell number (Fig. 2A and B), during the respective viral infections. Of note is that naïve HY T cells, whether in the HY transgenic mouse or after transfer into a female mouse, were always lower in CD44 expression than P14 T cells. This might reflect differences in endogenous antigen stimulation of these transgenic cells. Nevertheless, CD44 was substantially still upregulated in the P14 cells after antigen stimulation.
Fig. 1.
HY and P14 CD8 T cells remain phenotypically naïve during LCMV and PV infections, respectively. CFSE-labeled HY transgenic CD8 T cells were adoptively transferred into naïve congenic female recipients, which were infected with 5 × 104 PFU LCMV i.p. the following day. Similarly, CFSE-labeled P14 transgenic CD8 T cells were adoptively transferred into naïve congenic male recipients, which were infected with 1.5 × 107 PFU PV i.p. the following day. At days 0 (naïve) and 5 (d5) postinfection, splenocytes were stained for the activation markers shown. Host polyclonal CD8 T cells and TCR-transgenic CD8 T cells were gated, and representative histograms from the same individual mice are overlaid.
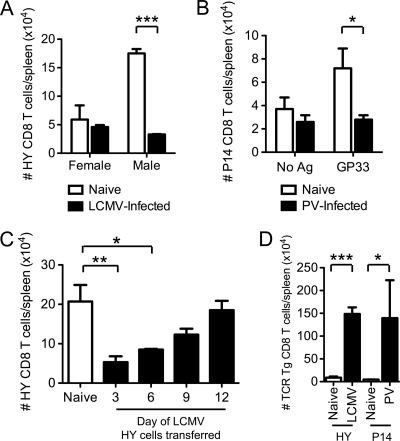
Fig. 2.
Transient susceptibility to proliferative inhibition of TCR-stimulated bystander CD8 T cells during acute viral infections. (A) HY CD8 T cells were adoptively transferred into naïve or day 5 LCMV-infected female and male congenic recipients. Two days after cell transfer, the number of HY CD8 T cells in the spleen was determined. (B) P14 CD8 T cells were adoptively transferred into naïve congenic recipients, followed by infection with 1.5 × 107 PFU PV the following day. At day 5 of PV infection, mice were inoculated i.v. with 5 μg GP33–45 peptide i.v. Two days after peptide inoculation, the number of P14 CD8 T cells in the spleen was determined. (C) HY CD8 T cells were adoptively transferred into naïve or LCMV-infected male mice at day 3, 6, 9, or 12 postinfection. Three days after cell transfer, the number of HY CD8 T cells in the spleen was determined. (D) HY CD8 T cells were transferred into naïve male congenic recipients, followed immediately by infection with 5 × 104 PFU LCMV i.p. P14 CD8 T cells were transferred into naïve congenic recipients. The following day, mice were inoculated with 5 μg GP33–45 peptide i.v. immediately prior to infection with 1.5 × 107 PFU PV. Four days after activation, the number of transgenic CD8 T cells in the spleen was determined. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
To determine if bystander CD8 T cells were susceptible to immune suppression when activated by cognate antigen during an acute viral infection in vivo, we adoptively transferred HY transgenic CD8 T cells into naïve or day 5 LCMV-infected congenic female or male recipients (Fig. 2A). Because of their male antigen specificity, HY CD8 T cells are reported to become activated in male but not female C57BL/6 WT mice (36). Two days later, proliferation was measured by calculating the number of HY CD8 T cells in the spleens of each group of mice. There was a slight reduction in the number of HY CD8 T cells recovered from the LCMV-infected female mice compared to the number in the naïve female mice (Fig. 2A), and the HY CD8 T cells that remained in either female host had a naïve phenotype (data not shown). However, the HY CD8 T cells became activated and proliferated in the naïve male mice, resulting in an increase in their overall cell number, but this proliferation was inhibited in the LCMV-infected male mice (Fig. 2A).
As a second model for testing virus-induced immune suppression, P14 CD8 T cells were adoptively transferred into naïve congenic recipients, followed by infection with PV. At day 5 of PV infection, mice were inoculated with the highly immunogenic GP33–45 13-mer peptide i.v. to specifically activate the P14 CD8 T cells (6). Two days later, the same time after activation as in the HY experiment, the numbers of P14 CD8 T cells in the spleens of each group were determined. Without antigen, there was a slight decrease in the number of P14 CD8 T cells recovered from the PV-infected mice, possibly due to IFN-α/β-induced T cell attrition (21) (Fig. 2B). After infusion of GP33–45 peptide, the P14 CD8 T cells in the naïve mice proliferated, but this proliferation was inhibited in the PV-infected mice (Fig. 2B), much like the inhibition of proliferation of HY cells in LCMV-infected male mice. These data therefore demonstrate, in two independent experimental models, that acute arenavirus infections inhibited the proliferation of TCR-stimulated bystander CD8 T cells in vivo.
We next addressed whether the virus-induced inhibition of proliferation was a transient event by transferring HY CD8 T cells into naïve or LCMV-infected male mice at 3, 6, 9, and 12 days postinfection and examining the proliferation of HY CD8 T cells 3 days later. The proliferation of HY CD8 T cells activated on days 3 to 9 of LCMV infection was inhibited compared to the proliferation in naïve mice (Fig. 2C). HY CD8 T cells activated on day 3 of LCMV infection had the most pronounced defect in proliferation, followed by cells activated on day 6 and day 9, but the proliferation was back to normal naïve levels at day 12 postinfection (Fig. 2C). In contrast, antigen-mediated activation of CD8 T cells at the onset of virus infection, either involving HY cell transfer immediately prior to the time of LCMV infection or GP33–45 inoculation immediately prior to the time of PV infection, markedly enhanced the proliferation of the bystander CD8 T cells (note differences in y axes) (Fig. 2D). These data indicated that viral infections acted as powerful stimulatory adjuvants when bystander T cells were exposed to the inflammatory milieu and cognate antigens at the beginning of infections but were immunosuppressive when bystander T cells engaged antigen after the infection had progressed.
Reduced proliferation of TCR-stimulated bystander CD8 T cells in vivo is not exclusively mediated by Fas-FasL- or TNF-induced AICD.
The reduced proliferation of TCR-stimulated bystander CD8 T cells during acute viral infections was predicted by numerous in vitro studies showing that T cells derived from virus-infected hosts are often more susceptible to AICD on TCR stimulation with antigen or T cell mitogens (1, 11, 50). We therefore tested whether the reduced proliferation of defined bystander T cell populations was a consequence of AICD in vivo by testing the influence of Fas-FasL and TNF in this process. To investigate the role of Fas-FasL, we studied the fate of HY transgenic T cells bred onto the B6.MRL-Tnfrsf6lpr (lpr, Fas mutant) background and the fate of HY or P14 CD8 T cells in B6.Smm.C3H-Tnfsf6gld (gld, FasL-deficient) recipient mice. The HY/lpr CD8 cells lack a functional Fas surface receptor and cannot, therefore, undergo apoptosis initiated by FasL (31). gld mice lack a functional FasL and cannot, therefore, initiate apoptosis in Fas-expressing T cells (31). To investigate the role of TNF, we treated mice with the human TNF receptor fusion protein etanercept to neutralize serum TNF (25). Using these systems, we found that HY and P14 CD8 T cell proliferation was still inhibited in virus-infected mice, whether Fas-FasL, TNF, or both pathways were blocked (Table 1), indicating that Fas-FasL and TNF are not required for the reduced proliferation of TCR-stimulated bystander CD8 T cells. Further, the overall transgenic T cell numbers in these mice were not statistically different from those of wild-type controls.
Table 1.
Fas-FasL and TNF are not required for the inhibition of TCR-stimulated bystander CD8 T cell proliferationa
| No. of expts | Source of TCR transgene | Host mice | Treatment | Functional |
Avg fold inhibition ± SD | |
|---|---|---|---|---|---|---|
| Fas-FasL | TNF/TNFR | |||||
| 7 | HY | WT | + | + | 4.2 ± 1.4 | |
| 8 | P14 | WT | + | + | 4.4 ± 1.9 | |
| 2 | HY | gld | − | + | 4.9 ± 1.9 | |
| 4 | HY/lpr | WT | − | + | 4.4 ± 1.1 | |
| 4 | P14 | gld | − | + | 4.5 ± 3.7 | |
| 3 | HY | WT | Etanercept | + | − | 4.0 ± 1.6 |
| 3 | P14 | WT | Etanercept | + | − | 5.1 ± 4.5 |
| 1 | HY | gld | Etanercept | − | − | 4.3 ± 0.0 |
| 1 | HY/lpr | WT | Etanercept | − | − | 4.1 ± 0.0 |
| 2 | P14 | gld | Etanercept | − | − | 3.8 ± 0.9 |
HY or HY/lpr-transgenic CD8 T cells were adoptively transferred into naïve or day 5 LCMV-infected WT or gld recipient male mice. Two days after adoptive transfer, the numbers of HY CD8 T cells in the spleens of individual mice were determined. P14 transgenic CD8 T cells were adoptively transferred into naïve WT or gld recipient mice, followed by infection with 1.5 × 107 PFU PV i.p. At day 5 of PV infection, mice were inoculated with GP33-45 i.v. Two days after peptide treatment, the numbers of P14 CD8 T cells in the spleens of individual mice were determined. Etanercept-treated mice were inoculated with 100 μg etanercept i.p. at day 4 of virus infection (1 day prior to transgenic T cell activation). Fold inhibition was calculated as the number of transgenic CD8 T cells in naïve mice over the number of transgenic CD8 T cells in virus-infected mice. The numbers of independent experiments are indicated, and the average levels of inhibition (n-fold) for all experiments performed ± standard deviations are shown.
Despite not finding a role for Fas-FasL or TNF in mediating AICD, we further explored the possibility that TCR-stimulated bystander CD8 T cells underwent cell death in vivo through another apoptotic pathway. In several experiments, we assessed apoptosis by caspase activation, phosphatidyl serine exposure via annexin-V reactivity, and DNA fragmentation via terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) stain on TCR-stimulated HY and P14 CD8 T cells, but the TCR-stimulated bystander CD8 T cells did not consistently show an apoptotic phenotype compared to unstimulated T cells (data not shown). This was investigated at various time points between 1 h and 8 days after T cell activation and in different tissues (spleen, lymph nodes, bone marrow, peritoneum, lung, liver, and blood) for both transgenic models, indicating that the reduced expansion of TCR-stimulated bystander CD8 T cells may only minimally be due to enhanced cell death. We also found that the reduced number of TCR-stimulated transgenic T cells in the spleen was not due to sequestration or specific trafficking to peripheral tissues, such as lymph nodes, bone marrow, peritoneum, lung, and liver, or in the circulating blood, as we could not recover them in those compartments (data not shown).
Reduced proliferation of TCR-stimulated bystander CD8 T cells in vivo is associated with delayed division.
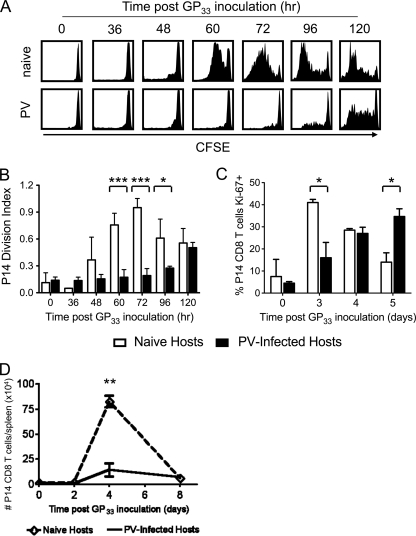
Because the reduced proliferation of TCR-stimulated bystander CD8 T cells could not be exclusively explained by their death, we questioned whether a defect in division accounted for their diminished expansion. Thus, CFSE-labeled P14 CD8 T cells were assessed for dilution of CFSE at various time points between 0 and 5 days after GP33–45 peptide inoculation. As shown in Fig. 3A, P14 CD8 T cells activated by GP33–45 in naïve mice started to divide approximately 48 h after peptide treatment, and by 120 h, many of the P14 CD8 T cells in the naïve mice had fully diluted the CFSE. In contrast, only 23% ± 8% (n = 12) of the P14 CD8 T cells activated during the acute PV infection had divided at least once by 72 h after peptide treatment, compared to 87% ± 6.4% (n = 10) of P14 CD8 T cells activated in naïve mice at the same 72-h time point. It was only at 96 h and later that a larger proportion of P14 cells activated in the PV-infected mice had divided.
Fig. 3.
Delayed division of TCR-stimulated bystander P14 CD8 T cells during acute PV infection. CFSE-labeled P14 CD8 T cells were adoptively transferred into naïve congenic recipients, followed by infection with 1.5 × 107 PFU PV the following day. At day 5 of PV infection, mice were inoculated i.v. with 5 μg GP33–45 peptide and at 0, 36, 48, 60, 72, 96, and 120 h after peptide inoculation, the dilution of CFSE in P14 CD8 T cells was assessed. (A) CFSE profiles from representative naïve and PV-infected mice at each time point are shown. (B) The division index of P14 CD8 T cells was calculated and graphed. (C) At days 0, 3, 4, and 5 after peptide inoculation, the intracellular expression of Ki-67 antigen was assessed. (D) The numbers of P14 CD8 T cells in the spleen at 2, 4, and 8 days after GP33–45 peptide treatment are shown. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
The kinetics of CFSE dilution were also analyzed by calculating the division index, a measure of the average number of divisions that any cell in the starting population underwent. The division index for P14 CD8 T cells activated by GP33–45 peptide in naïve mice peaked at 72 h after peptide treatment, while it was still increasing at 120 h for the P14 CD8 T cells activated in PV-infected mice (Fig. 3B). Additionally, the expression of Ki-67 is often used as a marker of cell division (12), and P14 CD8 T cells activated with GP33–45 in naïve mice upregulated Ki-67 within 3 days after peptide treatment, but the upregulation of Ki-67 was delayed in the P14 CD8 T cells activated in the PV-infected mice (Fig. 3C). These data suggest that the reduced proliferation of TCR-stimulated bystander P14 CD8 T cells during an acute viral infection was a consequence of delayed onset of division. Despite the delayed onset of division of the TCR-stimulated bystander CD8 T cells during acute viral infection, the overall kinetics of T cell proliferation were similar regardless of whether the CD8 T cells were stimulated in a naïve or virus-infected environment (Fig. 3D).
Reduced proliferation of TCR-stimulated bystander CD8 T cells is not due to a defect in antigen presentation during acute viral infection.
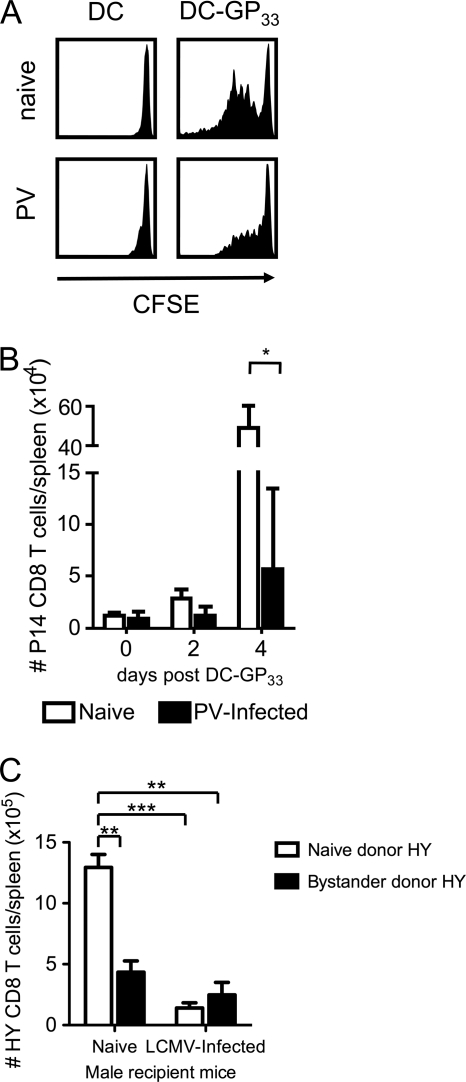
The delayed onset of TCR-stimulated bystander CD8 T cell division could have been due to a T cell-intrinsic defect or could have been a consequence of aberrant antigen presentation during the acute viral infection. However, the frequency of total CD11c+ DCs in the spleen at day 5 of PV infection (1.7% ± 0.17%, n = 8) was not different than that found in naïve mice (1.5% ± 0.08%, n = 6). Nevertheless, since our experiments relied on host antigen-presenting cells (APC) to present the endogenous male Smcy epitope to HY CD8 T cells or the inoculated GP33–45 peptide to P14 CD8 T cells, we asked if the proliferation of bystander CD8 T cells could be rescued if exogenous GP33–41-pulsed DCs were administered to PV-infected mice instead of only the GP33–45 peptide. We inoculated P14-implanted naïve and PV-infected mice with 107 unlabeled DCs or peptide-labeled DCs-GP33 i.v. and measured the division and proliferation of the P14 CD8 T cells 2 to 4 days later. This administration of DCs-GP33 activated the P14 CD8 T cells in naïve mice to divide by day 2 (Fig. 4A) and substantially proliferate by day 4 (Fig. 4B). However, both the division and proliferation of P14 CD8 T cells in response to DCs-GP33 were inhibited in the PV-infected mice, much like that in response to peptide infusion only. This suggests that the reduced proliferation of P14 CD8 T cells in PV-infected mice was not due to a defect in the processing or presentation of the GP33–45 peptide but was seemingly a T cell-intrinsic defect.
Fig. 4.
Proliferative inhibition of TCR-stimulated bystander CD8 T cells still occurs if P14 CD8 T cells are activated with GP33–41-labeled DCs or if bystander HY CD8 T cells are activated in naïve male mice. (A and B) CFSE-labeled P14 CD8 T cells were adoptively transferred into naïve congenic recipients followed by infection with 1.5 × 107 PFU PV the following day. At day 5 postinfection, mice were inoculated i.v. with 107 GP33–45 peptide-pulsed DC2.4 cells, and 2 and 4 days later, the CFSE profiles (day 2) (A) and the numbers of P14 CD8 T cells (B) were analyzed. (C) HY transgenic mice were infected with 5 × 104 PFU LCMV. At day 5 postinfection, splenocytes from naïve and LCMV-infected HY transgenic female mice were normalized to obtain equal numbers of naïve donor HY or bystander donor HY CD8 T cells, which were transferred into naïve or day 5 LCMV-infected male recipient mice. Two days later, the number of HY CD8 T cells in the spleen was determined. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
Nevertheless, it was still possible that the milieu of the viral infection inhibited the functions of the inoculated GP33-pulsed DCs, or alternatively, the developing host T cell response may have competed against the P14 response by consuming growth factors, so we used an alternative approach to test the intrinsic sensitivity of virus infection-sensitized T cells on exposure to normal antigen-presenting cells. We asked whether bystander HY CD8 T cells from LCMV-infected female mice could proliferate normally if transferred into uninfected male mice displaying the cognate male antigen ligand. If the reduced proliferation of HY CD8 T cells in LCMV-infected male mice were due only to a defect in antigen presentation during the virus infection, then we would predict that HY CD8 T cells from LCMV-infected female mice would be able to proliferate normally when transferred into naïve uninfected male mice, which should present antigen normally. To do this, we harvested splenocytes from naïve (naïve donor HY) and day 5 LCMV-infected (bystander donor HY) HY transgenic female mice and transferred equal numbers of naïve donor HY or bystander donor HY CD8 T cells into naïve or infection-matched day 5 LCMV-infected male recipient C57BL/6 WT mice. Proliferation of either donor cell population, as expected, was inhibited in the infected male HY mice (Fig. 4C). Notably, however, the proliferation of bystander-sensitized donor HY cells was also inhibited in the uninfected naïve male mice (Fig. 4C). This result indicates that the defect in proliferation of HY CD8 T cells in LCMV-infected male mice was not due to a defect in antigen presentation of the recipient host or competition with another T cell response in the recipient host but instead may have been due to a T cell-intrinsic inhibition developed in the infected donor. It was possible that this experimental setup resulted in the transfer of virus from the LCMV-infected HY female mice to the naïve male mice, but we noted that the host CD8 T cells in the naïve male mice receiving LCMV-infected HY T cells remained phenotypically naïve (CD44lo and CD62Lhi) during this time period, suggesting that little-to-no virus was transferred. Taken together, these data demonstrate that the inhibition of TCR-stimulated bystander CD8 T cell proliferation was not due to a defect in antigen presentation or to a competition with expanded numbers of virus-specific T cells during the acute viral infections but instead was probably due to a T cell-intrinsic defect caused by the milieu of viral infection.
Impaired proliferation of poly(I · C)-stimulated CD8 T cells.
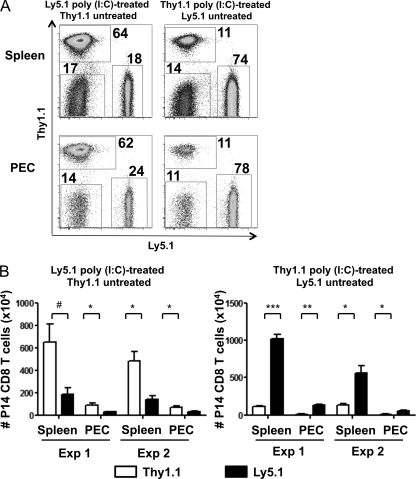
In a parallel study using similar transgenic and viral systems, we reported that naïve bystander CD8 T cells behaved like memory cells with regard to rapidly becoming activated to produce IFN-γ upon encountering their cognate ligand (20). This sensitization was associated with a type I IFN-dependent upregulation of the transcription factor Eomes, which transcriptionally activates the IFN-γ gene and other T cell effector genes, such as perforin. Of note is that the simple exposure of mice to the type I IFN-inducer poly(I · C) would upregulate Eomes in naïve T cells and sensitize them to rapidly become effector cells when encountering their cognate ligand (20). In the present study, we noted that the peak inhibition of bystander T cell proliferation occurred at day 3 after viral infection (Fig. 2C), near the published peak of the type I IFN and innate cytokine responses (22, 45). We therefore questioned whether the virus-induced impairment in cell proliferation could be mimicked by this IFN inducer. To that end, Thy1.1+ Ly5.2+ or Thy1.2+ Ly5.1+ P14 transgenic CD8 T cells were transferred into C57BL/6 mice (Thy1.2+ and Ly5.2+), some of which were treated with poly(I · C). After 1 day of stimulation in vivo, splenocytes were removed from poly(I · C) and control treatment groups and cotransferred at equal numbers of P14 cells into uninfected C57BL/6 mice, which were then subsequently infected with LCMV. The CD8 T cells exposed to the poly(I · C)-treated environment underwent substantially reduced expansion compared to the control P14 CD8 T cells from the nonstimulated mice (Fig. 5). Figure 5A shows representative plots of donor P14 CD8 T cells from the spleen and peritoneum (PECs). The poly(I · C)-treated samples are on the Ly5.1+ background in the plots on the left and on the Thy1.1+ background in the plots on the right; we deemed it important to test these experiments on different congenic backgrounds to rule out any effects of the backgrounds versus the treatments on proliferation. The averages of total P14 CD8 T cells in the spleen and PECs from two independent experiments revealed that the poly(I · C)-sensitized P14 CD8 T cells underwent reduced proliferation in response to LCMV infection, regardless of the congenic background (Fig. 5B). Therefore, exposure to poly(I · C) 1 day prior to infection can simulate the type of immune suppression induced by many viruses, indicating that exposure to innate factors before antigen stimulation is sufficient to inhibit the proliferation of T cells receiving signals from their cognate ligands during the context of a virus infection.
Fig. 5.
Poly(I · C)-induced sensitized P14 CD8 T cells have an impaired ability to proliferate compared to untreated P14 cells. P14 CD8 T cells (Thy1.1+ or Ly5.1+) were transferred into naïve congenic C57BL/6 mice that were untreated or treated with poly(I · C). One day later, P14 CD8 T cells were isolated from poly(I · C)- or control vehicle-treated mice and cotransferred at equal numbers i.v. into the same naïve mice. Recipient mice were infected with 5 × 104 PFU LCMV i.p., and at day 7 postinfection, splenocytes and PECs were harvested. (A) Representative plots gated on CD8+ Vα2+ cells; on the left are plots using poly(I · C)-treated Ly5.1+ P14 cells, and on the right are poly(I · C)-treated Thy1.1+ P14 cells. (B) Total numbers of P14 CD8 T cells from spleens and PECs, with 3 mice per group in two independent experiments. #, P = 0.05; *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
Poly(I · C) is a potent type I IFN inducer, but it also induces other cytokines, directly and indirectly, so we sought to determine if IFN played a role in this system. Our recently published report on poly(I · C)-dependent sensitization of naïve T cells to rapid effector function on exposure to cognate ligand showed that this sensitization occurred whether or not P14 T cells expressed receptors for type I IFN, indicating that this sensitization was an indirect effect of IFN, possibly acting on antigen-presenting cells (20). It was unclear whether the poly(I · C)-induced suppressed proliferation of P14 cells observed herein was due to type I IFN and whether it was a direct or indirect effect of IFN on the T cells. To design an experiment to test this, we could not put IFN-α/βR-expressing T cells in competition with IFN-α/βR KO T cells, because exposure to IFN greatly enhances the proliferation of P14 T cells if it occurs after ligand stimulation (19). We therefore asked if the reduced proliferation of T cells sensitized in the poly(I · C)-treated environment could be seen in mice receiving only one donor population and challenged with LCMV. When poly(I · C)-sensitized versus untreated WT P14 cells were separately transferred into hosts that were infected with LCMV and tested 7 days later, statistically higher numbers of P14 cells were derived from the untreated control populations than from the poly(I · C)-sensitized populations [control = 5.1 ± 0.9 × 106 P14 cells; poly(I · C)-sensitized = 1.7 ± 0.2 × 106 P14 cells; n = 4, P = 0.009], consistent with the data for which combined transfers were used (Fig. 5). We hypothesized that if a direct stimulation of IFN-α/βR on P14 cells was responsible for this impaired proliferation, there would be no differences in expansion of the IFN-α/βR KO P14 cells under these similar conditions. Our data first showed that the expansion of IFN-α/βR KO P14 cells sensitized in either environment was substantially (∼200-fold) lower than that of wild-type P14 cells sensitized in either environment (e.g., after being in a control environment, WT P14 cells numbered 5.1 × 106 ± 0.9 × 106 versus IFN-α/βR KO P14 cells, which numbered 2.3 × 104 ± 0.3 × 104 [n = 4 per group, P = 0.001]), reflective of the augmentation effect that type I IFN can have when exposed to T cells after TCR ligand stimulation, such as under normal conditions of a virus infection (19). Nevertheless, IFN-α/βR KO P14 populations still expanded 20-fold during the LCMV infection (Fig. 6A and B), but there were no significant differences in the percentages or total numbers of those sensitized with poly(I · C) versus those that were not treated (Fig. 6B) (P > 0.9). This pattern of no inhibition of the IFN-α/βR KO P14 cells after poly(I · C) pretreatment was seen in five separate day 7 experiments. It could be argued that, with the IFN-α/βR KO P14 cell experiment, there were not enough cell divisions for us to be able to detect a difference in division rate. We therefore examined cells at days 8 and 9 after infection. At day 8, the cells had doubled in number over day 7 numbers, but again no differences were seen in numbers of poly(I · C)-treated (8.7 × 104 ± 1.7 × 104) versus control vehicle-treated (8.3 × 104 ± 1.1 × 104) IFN-α/βR KO P14 T cell per spleen. By day 9, the numbers of IFN-α/βR KO P14 cells were in decline. To further evaluate the degree of cell division, sensitized CFSE-labeled versus control IFN-α/βR KO P14 cells were transferred into mice and examined at 7 days postinfection. Both cell groups lost all CFSE staining, indicating that they had undergone at least 8 divisions. Kolumam et al. had previously reported that the yield of IFN-α/βR KO P14 cells was less than that expected for their number of division because of decreased cell survival during proliferation (19). Thus, there should have been enough divisions to see differences in proliferation between the poly(I · C)-sensitized versus control-sensitized IFN-α/βR KO P14 cells, had there been any, and we conclude that IFN-α/βR on the T cells was needed for the inhibitory effects of IFN on proliferation.
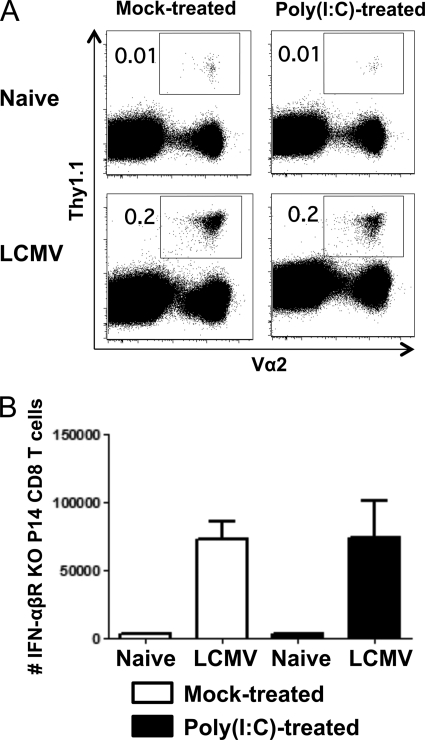
Fig. 6.
IFN-α/βR KO P14 cells treated with poly(I · C) are not impaired in proliferation compared to control vehicle-treated IFN-α/βR KO P14 cells. IFN-α/βR KO P14 cells were transferred into naïve congenic mice that were mock treated or treated with poly(I · C). One day later, these IFN-α/βR KO P14 cells were isolated from poly(I · C)- or control vehicle-treated mice and transferred in equal numbers into separate naïve mice. Recipient mice were then infected with 5 × 104 PFU LCMV i.p., and at day 7 postinfection, spleens were harvested. (A) Representative results from each group are shown; plots are gated on CD8+ cells. (B) Total numbers of IFN-α/βR KO P14 CD8 T cells ± standard deviations (4 mice per group) are calculated.
When we began these experiments, we could not put IFN-α/βR KO P14 cells exposed to different environments in direct competition with each other in the same host, as we did for the experiments whose results are shown in Fig. 5, because we had these IFN-α/βR KO P14 cells on only one congenic background. However, enough Thy1.1+ Thy1.2+ IFN-α/βR KO P14 cells became available to compare them with Thy1.1+ Thy1.2− cells in a mixed experiment within a Thy1.1− Thy1.2+ congenic C57BL/6 host background in an experiment designed like that for Fig. 5. Four mice received poly(I · C)-sensitized Thy1.1+ Thy1.2+ cells combined with control vehicle-treated Thy1.1+ Thy1.2− cells, whereas four mice received the reciprocal, i.e., poly(I · C)-sensitized Thy1.1+ Thy1.2− cells combined with control vehicle-treated Thy1.1+ Thy1.2+ cells, all followed by a 7-day LCMV infection. The average proliferation of the Thy1.1+ Thy1.2− cells was about half (52%, 54%) of that of the Thy1.1+ Thy1.2+ cells, regardless of the pretreatment, indicating a need to be careful about subtle variations in cell proliferation between different strains of congenic mice. However, when all 16 poly(I · C)-treated versus control vehicle-treated cell populations were analyzed, regardless of congenic markers, their proliferations in spleens were very similar: the percentage of poly(I · C)-treated cells was 2.8% ± 0.4%, whereas the percentage of control vehicle-treated cells was 2.5% ± 0.5% (P = 0.7, n = 8 per group); the total number of poly(I · C)-treated cells was 3.5 × 104 ± 0.6 × 104, whereas the total number of control vehicle-treated cells was 3.0 × 104 ± 0.5 × 104 (P = 0.5, n = 8). Because these several experiments indicated that impaired proliferation after poly(I · C) pretreatment was not observed with IFN-α/βR KO P14 cells, we conclude that direct IFN signaling was involved in this impaired proliferation seen in wild-type P14 cells. Hence, the recently published IFN-induced sensitization to acquisition of effector functions of bystander T cells (20) probably occurred by a mechanism distinct from the IFN-induced suppression of cell proliferation.
DISCUSSION
Many viruses induce a transient state of immune suppression, and there is in vitro evidence linking immune suppression to T cell AICD, aberrant costimulation, and DC dysfunction (1–3, 5, 8, 11, 23, 26, 32, 38–40, 43, 50). Due to the lack of consensus from in vitro systems, we sought to investigate the transient immune suppression induced by acute infections with the arenaviruses LCMV and PV using adoptive-transfer models to track and specifically activate TCR-transgenic bystander CD8 T cells in vivo (Fig. 1). We demonstrated here that the proliferation of bystander CD8 T cells activated by cognate antigen during the acute phase of virus infection in vivo was inhibited (Fig. 2A and B). Susceptibility to immune suppression was transient, as by day 12 postinfection, TCR-stimulated bystander CD8 T cells underwent normal proliferation (Fig. 2C), and the proliferation of TCR-stimulated bystander CD8 T cells was actually dramatically enhanced if the T cells were activated with antigen immediately prior to the viral infection (Fig. 2D). Thus, while there was a strong adjuvant effect of viral infections on T cell proliferation at the beginning of infection, there was a strong suppression of T cell proliferation as early as 3 days into the infection. Our studies focused on T cells with specificities for ligands other than those encoded by the infecting virus, but we presume that these findings are also relevant to latecomer virus-specific T cells whose TCR does not encounter viral antigen until later in infection.
Despite the reported susceptibility of bystander CD8 T cells to AICD as measured in vitro (1, 11, 50), the reduced proliferation of bystander CD8 T cells in our models was not exclusively mediated by or dependent on Fas-FasL- and/or TNF-induced AICD (Table 1). In addition, we could not convincingly show enhanced apoptosis in vivo, though it should be noted that apoptotic cells can sometimes be cleared in vivo before they are readily detectable. Nevertheless, most of the inhibition in antigen-induced proliferation could be explained by a delay in the onset of division (Fig. 3).
Infections of human or mouse DCs with human cytomegalovirus, murine cytomegalovirus, measles virus, adenovirus, respiratory syncytial virus, vaccinia virus, or LCMV clone 13 can interfere with DC maturation and inhibit MHC and costimulatory-molecule expression (2, 10, 26, 30, 32, 38–40), and measles virus, vaccinia virus, and herpes simplex virus (HSV) have all been shown to induce apoptosis of DCs (4, 10, 11, 15, 28). These events are proposed to lead to reduced T cell responses. However, we found that administration of peptide-labeled uninfected DCs could not rescue the inhibition of bystander CD8 T cell proliferation (Fig. 4A and B), suggesting that DC dysfunction in vivo was not the exclusive cause of immune suppression during acute infections of mice with PV. Reduced expression of the homeostatic chemokines CCL21 and CXCL13 in lymphoid organs during acute viral infections have also been postulated to inhibit the development of new T cell responses by hindering the localization of naïve T cells with DCs during ongoing antiviral immune responses (27). Again, this does not appear to be the cause of immune suppression in our models, because the bystander donor HY CD8 T cells sensitized by virus infection in female mice could not proliferate to the extent of naïve HY CD8 T cells when transferred into uninfected male mice (Fig. 4C), where there should be normal APC and normal gradients of chemokines expressed.
Type I IFN seems to be at least one of the factors inducing the immune suppression, as impaired proliferation correlated with the peak of IFN production during viral infection, virus-induced suppression of proliferation could be simulated by exposure to the IFN inducer poly(I · C) (Fig. 5), and no poly(I · C)-induced impairment in proliferation could be seen in cells lacking IFN-α/βR (Fig. 6). The IFN-associated impaired proliferation may occur through a mechanism distinct from that of the IFN-induced upregulation of Eomes and sensitization to effector cell activation, as studies with IFN-α/βR KO P14 cells indicated that Eomes expression was due to an indirect effect of IFN, perhaps acting on antigen-presenting cells (20), but that the suppression of proliferation may be a direct effect. Analyses of these experiments is challenging, as type I IFN can also be a potent augmenter of CD8 T cell proliferation in the LCMV system (19). This means that the timing of IFN exposure is of utmost importance in regard to whether IFN inhibits or enhances T cell proliferation. Note that, as shown in Fig. 2D, the simultaneous stimulation of transgenic cells with virus infection and antigen greatly augmented proliferative responses to either HY or P14 transgenic T cells.
That type I IFN can either enhance or inhibit T cell proliferation in vitro has been known since the 1970s (44). Here we report that the timing of the IFN response in vivo is fundamental in determining the inhibitory or stimulatory properties of IFN. How IFN causes this suppression is at the moment unclear. Our unpublished microarray analyses of poly(I · C)- or LCMV-induced naïve-phenotype CD8 T cells show many changes in gene expression in addition to the augmentation of Eomes mRNA that we recently reported (20). Further, the cell growth-inhibitory properties of IFN, particularly in tumor models, have been known for years and are well described (35). IFN has also been shown to cause a hyporesponsiveness of cells to IFN inducers, and some studies have shown that natural killer cells eventually become hyporesponsive to IFN-mediated activation after they have previously been treated with IFN (37, 42). Exposure to IFN at the time of or soon after TCR stimulation dramatically enhances T cell proliferation (Fig. 2D and reference 19), and it is possible that the suppressive effect of IFN exposure prior to TCR stimulation may create a state hyporesponsive to the IFN signal that occurs after TCR stimulation. It has been suggested that changes in STAT1 expression by CD8 T cells under different conditions of activation may modulate their sensitivity to the growth-inhibitory effects of IFN, and this could be a factor influencing how the timing of IFN exposure could cause different outcomes in proliferation (13). A hyporesponsive state might also be caused by IFN downregulating the expression of its receptor. Indeed, we found an approximately 25% loss in receptor expression on P14 T cells as detected by MAb to the IFNA1 receptor {MFI = 372 ± 4.6 [control] versus 277 ± 6.7 [poly(I · C)}. We do not know at this time whether this represents a true loss of receptor expression or a blockade of MAb binding by the bound IFN, and this is under investigation. Nevertheless, most of the IFN receptors are still present. Finally, we showed in a previous publication that IFN-sensitized CD8 T cells can rapidly manifest effector function upon exposure to cognate antigen (20). Thus, it is possible that a rapid cytolytic elimination of APC may limit the ability of the APC to provide good stimulation for T cell proliferation. Whatever the mechanism, the timing of inflammatory signals likely has an impact on vaccine efficacy, and immunization of a host harboring a viral infection would likely lead to a reduced proliferation of vaccine-specific T cells. This mechanism would also limit the proliferation and expansion of latecomer virus-specific T cells that initially encounter their ligand after the first 3 days of infection. Given that the proliferative expansion of T cells is a programmed event (16), this would likely allow for a more discrete and synchronized T cell contraction as the viral infection resolves.
ACKNOWLEDGMENTS
This work was supported by NIH, U.S. Public Health Service, research grants R37AI-017672 and AI-081675 and training grant T32AIO7349-16.
We thank Keith Daniels for technical assistance.
Footnotes
Published ahead of print on 6 April 2011.
REFERENCES
- 1. Akbar A. N., et al. 1993. The significance of low bcl-2 expression by CD45RO T cells in normal individuals and patients with acute viral infections. The role of apoptosis in T cell memory. J. Exp. Med. 178:427–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andrews D. M., Andoniou C. E., Granucci F., Ricciardi-Castagnoli P., Degli-Esposti M. A. 2001. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat. Immunol. 2:1077–1084 [DOI] [PubMed] [Google Scholar]
- 3. Averill L., Lee W. M., Karandikar N. J. 2007. Differential dysfunction in dendritic cell subsets during chronic HCV infection. Clin. Immunol. 123:40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bosnjak L., et al. 2005. Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J. Immunol. 174:2220–2227 [DOI] [PubMed] [Google Scholar]
- 5. Brockman M. A., et al. 2009. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 114:346–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ciupitu A. M., et al. 1998. Immunization with a lymphocytic choriomeningitis virus peptide mixed with heat shock protein 70 results in protective antiviral immunity and specific cytotoxic T lymphocytes. J. Exp. Med. 187:685–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dailey M. O. 1998. Expression of T lymphocyte adhesion molecules: regulation during antigen-induced T cell activation and differentiation. Crit. Rev. Immunol. 18:153–184 [DOI] [PubMed] [Google Scholar]
- 8. Diaz-San Segundo F., Rodriguez-Calvo T., de Avila A., Sevilla N. 2009. Immunosuppression during acute infection with foot-and-mouth disease virus in swine is mediated by IL-10. PLoS One 4:e5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elias D., et al. 2005. Schistosoma mansoni infection reduces the protective efficacy of BCG vaccination against virulent Mycobacterium tuberculosis. Vaccine 23:1326–1334 [DOI] [PubMed] [Google Scholar]
- 10. Engelmayer J., et al. 1999. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J. Immunol. 163:6762–6768 [PubMed] [Google Scholar]
- 11. Fugier-Vivier I., et al. 1997. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J. Exp. Med. 186:813–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gerdes J., Schwab U., Lemke H., Stein H. 1983. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int. J. Cancer 31:13–20 [DOI] [PubMed] [Google Scholar]
- 13. Gil M. P., Salomon R., Louten J., Biron C. A. 2006. Modulation of STAT1 protein levels: a mechanism shaping CD8 T-cell responses in vivo. Blood 107:987–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greenwood B. M., et al. 1980. The immune response to a meningococcal polysaccharide vaccine in an African village. Trans. R. Soc. Trop. Med. Hyg. 74:340–346 [DOI] [PubMed] [Google Scholar]
- 15. Jones C. A., et al. 2003. Herpes simplex virus type 2 induces rapid cell death and functional impairment of murine dendritic cells in vitro. J. Virol. 77:11139–11149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaech S. M., Ahmed R. 2001. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2:415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kantzler G. B., et al. 1974. Immunosuppression during influenza virus infection. Infect. Immun. 10:996–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kisielow P., Bluthmann H., Staerz U. D., Steinmetz M., von Boehmer H. 1988. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature 333:742–746 [DOI] [PubMed] [Google Scholar]
- 19. Kolumam G. A., Thomas S., Thompson L. J., Sprent J., Murali-Krishna K. 2005. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202:637–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marshall H. D., Prince A. L., Berg L. J., Welsh R. M. 2010. IFN-alpha beta and self-MHC divert CD8 T cells into a distinct differentiation pathway characterized by rapid acquisition of effector functions. J. Immunol. 185:1419–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McNally J. M., et al. 2001. Attrition of bystander CD8 T cells during virus-induced T-cell and interferon responses. J. Virol. 75:5965–5976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Merigan T. C., Oldstone M. B., Welsh R. M. 1977. Interferon production during lymphocytic choriomeningitis virus infection of nude and normal mice. Nature 268:67–68 [DOI] [PubMed] [Google Scholar]
- 23. Meyaard L., et al. 1992. Programmed death of T cells in HIV-1 infection. Science 257:217–219 [DOI] [PubMed] [Google Scholar]
- 24. Mims C. A., Wainwright S. 1968. The immunodepressive action of lymphocytic choriomeningitis virus in mice. J. Immunol. 101:717–724 [PubMed] [Google Scholar]
- 25. Moreland L. W. 1998. Soluble tumor necrosis factor receptor (p75) fusion protein (ENBREL) as a therapy for rheumatoid arthritis. Rheum. Dis. Clin. North Am. 24:579–591 [DOI] [PubMed] [Google Scholar]
- 26. Moutaftsi M., Mehl A. M., Borysiewicz L. K., Tabi Z. 2002. Human cytomegalovirus inhibits maturation and impairs function of monocyte-derived dendritic cells. Blood 99:2913–2921 [DOI] [PubMed] [Google Scholar]
- 27. Mueller S. N., et al. 2007. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science 317:670–674 [DOI] [PubMed] [Google Scholar]
- 28. Muller D. B., Raftery M. J., Kather A., Giese T., Schonrich G. 2004. Frontline: induction of apoptosis and modulation of c-FLIPL and p53 in immature dendritic cells infected with herpes simplex virus. Eur. J. Immunol. 34:941–951 [DOI] [PubMed] [Google Scholar]
- 29. Muller U., et al. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918–1921 [DOI] [PubMed] [Google Scholar]
- 30. Munir S., et al. 2008. Nonstructural proteins 1 and 2 of respiratory syncytial virus suppress maturation of human dendritic cells. J. Virol. 82:8780–8796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nagata S., Suda T. 1995. Fas and Fas ligand: lpr and gld mutations. Immunol. Today 16:39–43 [DOI] [PubMed] [Google Scholar]
- 32. Newton K. R., et al. 2008. Human dendritic cells infected with an adenoviral vector suppress proliferation of autologous and allogeneic T cells. Immunology 125:469–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pircher H., Burki K., Lang R., Hengartner H., Zinkernagel R. M. 1989. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature 342:559–561 [DOI] [PubMed] [Google Scholar]
- 34. Razvi E. S., Jiang Z., Woda B. A., Welsh R. M. 1995. Lymphocyte apoptosis during the silencing of the immune response to acute viral infections in normal, lpr, and Bcl-2-transgenic mice. Am. J. Pathol. 147:79–91 [PMC free article] [PubMed] [Google Scholar]
- 35. Rizza P., Moretti F., Belardelli F. 2010. Recent advances on the immunomodulatory effects of IFN-alpha: implications for cancer immunotherapy and autoimmunity. Autoimmunity 43:204–209 [DOI] [PubMed] [Google Scholar]
- 36. Rocha B., von Boehmer H. 1991. Peripheral selection of the T cell repertoire. Science 251:1225–1228 [DOI] [PubMed] [Google Scholar]
- 37. Saito T., Ruffman R., Welker R. D., Herberman R. B., Chirigos M. A. 1985. Development of hyporesponsiveness of natural killer cells to augmentation of activity after multiple treatments with biological response modifiers. Cancer Immunol. Immunother. 19:130–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Servet-Delprat C., et al. 2000. Measles virus induces abnormal differentiation of CD40 ligand-activated human dendritic cells. J. Immunol. 164:1753–1760 [DOI] [PubMed] [Google Scholar]
- 39. Sevilla N., McGavern D. B., Teng C., Kunz S., Oldstone M. B. 2004. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. J. Clin. Invest. 113:737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smith A. P., et al. 2005. Viral replication-independent blockade of dendritic cell maturation and interleukin-12 production by human herpesvirus 6. J. Virol. 79:2807–2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Su Z., Segura M., Stevenson M. M. 2006. Reduced protective efficacy of a blood-stage malaria vaccine by concurrent nematode infection. Infect. Immun. 74:2138–2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Talmadge J. E., et al. 1985. Hyporesponsiveness to augmentation of murine natural killer cell activity in different anatomical compartments by multiple injections of various immunomodulators including recombinant interferons and interleukin 2. J. Immunol. 135:2483–2491 [PubMed] [Google Scholar]
- 43. Tinoco R., Alcalde V., Yang Y., Sauer K., Zuniga E. I. 2009. Cell-intrinsic transforming growth factor-beta signaling mediates virus-specific CD8+ T cell deletion and viral persistence in vivo. Immunity 31:145–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Welsh R. M. 1984. Natural killer cells and interferon. Crit. Rev. Immunol. 5:55–93 [PubMed] [Google Scholar]
- 45. Welsh R. M., Jr 1978. Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. I. Characterization of natural killer cell induction. J. Exp. Med. 148:163–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Welsh R. M., Seedhom M. O. 2008. Lymphocytic choriomeningitis virus (LCMV): propagation, quantitation, and storage. Curr. Protoc. Microbiol. 8:15A.1.1-15A.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Welsh R. M., Selin L. K., Razvi E. S. 1995. Role of apoptosis in the regulation of virus-induced T cell responses, immune suppression, and memory. J. Cell. Biochem. 59:135–142 [DOI] [PubMed] [Google Scholar]
- 48. Wiedmann M., et al. 2000. Decreased immunogenicity of recombinant hepatitis B vaccine in chronic hepatitis C. Hepatology 31:230–234 [DOI] [PubMed] [Google Scholar]
- 49. Yang H. Y., Dundon P. L., Nahill S. R., Welsh R. M. 1989. Virus-induced polyclonal cytotoxic T lymphocyte stimulation. J. Immunol. 142:1710–1718 [PubMed] [Google Scholar]
- 50. Zarozinski C. C., McNally J. M., Lohman B. L., Daniels K. A., Welsh R. M. 2000. Bystander sensitization to activation-induced cell death as a mechanism of virus-induced immune suppression. J. Virol. 74:3650–3658 [DOI] [PMC free article] [PubMed] [Google Scholar]