Abstract
Several recent studies have identified HIV-infected patients able to produce a broad neutralizing response, and the detailed analyses of their sera have provided valuable information to improve future vaccine design. All these studies have excluded patients on antiretroviral treatment and with undetectable viral loads, who have an improved B cell profile compared to untreated patients. To better understand the induction of neutralizing antibodies in patients on antiretroviral treatment with undetectable viremia, we have screened 508 serum samples from 364 patients (173 treated and 191 untreated) for a broadly neutralizing antibody (bNAb) response using a new strategy based on the use of recombinant viruses. Sera able to neutralize a minipanel of 6 recombinant viruses, including envelopes from 5 different subtypes, were found in both groups. After IgG purification, we were able to confirm the presence of IgG-associated broadly neutralizing activity in 3.7% (7 of 191) of untreated patients with detectable viremia and 1.7% (3 of 174) of aviremic patients receiving antiretroviral treatment. We thus confirm the possibility of induction of a broad IgG-associated neutralizing response in patients on antiretroviral treatment, despite having undetectable viremia. This observation is in stark contrast to the data obtained from long-term nonprogressors, whose little neutralizing activity has been attributed to the low levels of viral replication.
INTRODUCTION
Induction of antibodies that neutralize a broad range of human immunodeficiency virus type 1 (HIV-1) isolates is a major goal in vaccine development. To date, the antibodies elicited by vaccines have had weak activity against a limited spectrum of HIV-1 strains (34, 55, 88). However, many HIV-infected patients produce neutralizing antibodies (NAbs), and a small fraction make extremely potent NAbs with broad cross-reactivity (5, 6, 24, 62, 78, 80). Understanding how a broadly reactive NAb response develops in some HIV-1-infected patients may provide important clues for vaccine design. The clinical parameters associated with broadly reactive NAbs in serum have been the subject of much recent interest (23, 35, 73, 78). A recent comparison of neutralization breadth with clinical and demographic variables in a large cohort of untreated patients has revealed an association between viral load (VL) and neutralization breadth (23). This observation suggests that high levels of viremia increase the exposure to the antigen and may be beneficial for the development of broad NAbs. This correlation has also been observed in some studies with different patient cohorts (73, 78). Consistent with these reports, several laboratories have shown that sera from long-term nonprogressors (LTNPs) with <50 copies of HIV RNA/ml plasma had little neutralizing activity. Compared to patients with higher levels of viremia, LTNPs made weak NAb responses that had been attributed to a reduced antigenic stimulation of B cells (3, 23, 23, 24, 46, 50, 71).
Little is known about the neutralizing activity induced in patients on antiretroviral treatment (ART) with undetectable viral loads. ART patients constitute an interesting group of individuals with improved B cell function compared to untreated individuals who have higher levels of viremia. The changes in the frequency of B cell subpopulations after the administration of ART have already been characterized. Modifications in B cell counts after 12 months of ART have been detected in a group of individuals with chronic HIV infection (59). In these patients, ART leads to a significant increase in B cell numbers and to a normalization of B cell subpopulations, providing a possible explanation for improved B cell responses to both T cell-independent and T cell-dependent immunogens after ART (29, 44, 66). Remarkably, there is little information about the humoral immune response against HIV in ART patients. In these patients, most B cell defects associated with HIV infection can be reversed by ART; however, it is generally believed that the humoral immune response against HIV does not improve due to the reduced antigenic stimulation. To date there is no evidence supporting the induction of an NAb response against HIV in ART patients. Several groups have recently screened sera from cohorts of infected individuals to analyze the neutralizing activity of large groups of patients. However, all the studies had similar sample selection criteria and always excluded patients on antiretroviral treatment (24, 28, 80).
In the present study, we evaluated NAb breadth in a set of 508 serum samples from 364 HIV-1-infected individuals, including 173 patients on antiretroviral treatment. We found a significant broad IgG-associated neutralizing response in ART patients with undetectable viremia. We hypothesize that, in these patients, the lack of antigenic stimulation could have been compensated for by an improved B cell function as a result of the undetectable viremia in response to antiretroviral treatment.
MATERIALS AND METHODS
Study participants and demographics.
In order to better understand the spectrum and breadth of neutralization against HIV-1 in ART patients, we evaluated sera from a large cohort of HIV-infected patients. A total of 508 samples were collected from 364 HIV-infected individuals treated in Hospital Clínic (Barcelona, Spain). Patients in the present study signed informed consent. Overall, 82.1% of participants were male and 17.9% were female. Heterosexual transmission was reported for 24.7% of the patients, while men who have sex with men accounted for 58.5% of the study population. Intravenous drug use accounted for 7.4% of patients, and other constituted 4.5% of the study population and included transmission via blood transfusion or unknown transmission route as self-reported. Data for transmission were not available for 4.9% of samples. All the patients were presumed to be infected with clade B virus on the basis of the locations of residency. Table 1 shows the demographic characteristics of the patients: the median and range for each patient group for CD4+ T cell count, viral load, number of years since HIV diagnosis at the time of screening, characteristics of ART, and number of years on ART. One hundred seventy-three patients were on ART and had undetectable viremia (<50 copies/ml of plasma) and a median CD4+ T cell count of 689 cells/μl. One hundred ninety-one patients were untreated at the time of sampling and had a median number of viral RNA copies/ml in plasma of 10,241 and a median CD4+ T cell count of 567 cells/μl. The median numbers of years since diagnosis were 9 for ART patients and 5 for untreated patients. The median numbers of years on ART were 5 for ART patients and 0 for untreated patients (Table 1).
Table 1.
Clinical characteristics of the patient groups
| Characteristic | Total | ART patients | Untreated patients |
|---|---|---|---|
| Total no. (%) of patients | 364 | 173 (47.5) | 191 (52.5)a |
| No. (%) of patients by gender | |||
| Male | 299 (82.1) | 145 (83.8) | 154 (80.6) |
| Female | 65 (17.9) | 28 (16.2) | 37 (19.4) |
| No. (%) of patients on: | |||
| ART containing EFV | 73 (42.2) | 73 (42.2) | 0 (0) |
| ART containing no EFV | 100 (57.8) | 100 (57.8) | 0 (0) |
| No. (%) of patients by risk group | |||
| Injecting drug user | 27 (7.4) | 15 (8.7) | 12 (6.3) |
| Injecting drug user + homosexual | 8 (2.2) | 6 (3.5) | 2 (1.0) |
| Bisexual | 4 (1.1) | 2 (1.2) | 2 (1.0) |
| Heterosexual contact | 90 (24.7) | 44 (25.4) | 46 (24.1) |
| Hemophiliac | 2 (0.5) | 1 (0.6) | 1 (0.5) |
| Homosexual | 213 (58.5) | 96 (55.5) | 117 (61.3) |
| Transfusion | 2 (0.5) | 2 (1.2) | 0 (0.0) |
| Unknown | 18 (4.9) | 7 (40.5) | 11 (5.8) |
| Median (range) age (yr) | 42 (24–78) | 41.5 (24–78) | 40 (24–68) |
| Median (range) no. of viral RNA copies/ml plasmab | <50 (<50–222,117) | <50 (<50–85,454) | 10,241 (<50–222,117) |
| No. of CD4+ T cells/μl (median/range) | 663 (80–2,154) | 689 (80–1,916) | 567 (184–2,154) |
| Median (range) no. of yr since diagnosisc | 9 (0–24) | 9 (1–24) | 5 (0–24) |
| Median (range) no. of yr on ART | 6 (0.1–17) | 5 (0.1–13) | 0a (0–16) |
Thirty-three of the untreated patients had a history of treatment, and all of them had been off ARVs for at least 3 years. For these patients, the median number of years on ART is 8 and the range is 3 to 16.
The limit of detection was 50 copies/ml. Viremia was undetectable in 167 ART patients and 6 untreated patients.
The date of diagnosis was known in 166 ART patients and 186 untreated patients.
Generation of a minipanel of recombinant viruses.
Full-length infectious molecular clones were constructed by replacing the env sequence of HIVNL4-3 with env sequences from divergent primary isolates to generate a minipanel of recombinant viruses (RVs). The virus strains for the minipanel were selected by the V3C3 (spanning amino acids [aa] 300 to 392 of the gp120 glycoprotein, according to HxB2 numbering) diversity among the env genes, as follows: nucleotide sequences from reference strains were downloaded from the Los Alamos database (http://hiv-web.lanl.gov), and all positions with alignment gaps in at least one sequence were excluded from further analysis; the number of different bases in the pairwise comparisons and phylogenetic analysis was determined by MEGA software; DNA distance matrices were calculated by the Kimura two-parameter method; and a phylogenetic tree was constructed with distance matrices using the neighbor-joining method; finally, the robustness of the tree was evaluated by bootstrap analysis of 1,000 replicas. The selected strains provided by H. Holmes (NIBSC, United Kingdom) through the NeutNet consortium (G. Scarlatti) (28) were the following (clades are given in parentheses): VI191 (A), 92BR025 (C), 92UG024 (D), CM244 (E), and AC10 (B). RVs were obtained by removing the Renilla luciferase gene and restoring the nef gene in a group of Renilla recombinant viruses (30). NL4-3 strain (clade B) was included in the minipanel as a neutralization-sensitive control. An amphotropic vesicular stomatitis virus (VSV) Env pseudotyped on an HIV-1 core was added to the panel as a specificity control virus in IgG neutralization testing. DNA sequence analysis was performed to verify the constructions.
Virus stocks were produced by transfecting the DNA constructions in HEK293T cells using the calcium phosphate method according to the manufacturer's recommended protocol (ProFection mammalian transfection system; Promega, Madison, WI). Virus was quantified by determining the concentration of p24 capsid in the supernatant by an antigen capture assay (Innogenetics, Belgium). VSV pseudotyped virus stocks were produced by cotransfecting HEK293T cells with two plasmids: pNL4-3ΔenvFL (65) and a second plasmid, pVSV-G, that expresses the VSV glycoprotein, using the calcium phosphate method mentioned above.
RVs carrying a Renilla luciferase gene in the position of nef were generated by cloning the full-length envelope from HIV-infected patients in the pNL-lacZ/Env-Ren vector as described previously (33). The envelopes of viral strains NP1525 (E), SF162 (B) QH0692 (B), and MN (B) were amplified from culture supernatants kindly provided by H. Holmes (NIBSC, United Kingdom) through the NeutNet consortium (G. Scarlatti) (28). Envelopes from strains P-1261 (CRF02_AG), 1608-8 (A1), X-845-4 (F1), X-1628-2 (G), X-2118-2 (C), 138.7 (CRF14BG), and 2105 (CRF14BG) from culture supernatants were kindly provided by Lucía Pérez Álvarez, Instituto de Salud Carlos III, Spain. The clade B envelopes of strains 7191, 8439, 37343, 39775, 11525, 14382, 16218, and 22652 were amplified from plasma samples from patients with early infection (kind gift of Jose Maria Miró, Hospital Clinic de Barcelona) and envelopes of strains 1.22, 2.15, 3.18 3.25, and 2.9 from HIV-infected patients with an advanced stage of the disease followed in our department. The BX08 envelope was obtained from a patient with primary infection and has been previously described (61). The resulting constructs were transfected into HEK293T cells to generate chimeric recombinant viruses.
Infectivity assay.
Viral infectivity was measured using TZM-bl indicator cells (expressing CD4 receptor and CCR5/CXCR4 coreceptors with an integrated long terminal repeat-Luc reporter system) (22, 74, 82, 89). Briefly, a 96-well plate was set up, in triplicate, with three sets of 2-fold dilutions of the virus (40 ng to 0.156 ng) and three uninfected controls. To these wells, 104 TZM-bl cells were added, and the plate was transferred to a humidified CO2 incubator at 37°C. After 72 h of incubation at 37°C, supernatants were removed and the cell-associated luciferase activity for each well was determined on a microplate luminometer (Turner Biosystems, Sunnyvale, CA) by using a luciferase assay kit (Biotherma, Sweden).
Neutralization.
Randomly selected serum samples were tested with the six recombinant viruses from the minipanel using a luciferase reporter cell assay. To perform neutralization assays, 96-well plates were set up as follows: to the first three columns, 25 μl of medium (Dulbecco modified Eagle medium [DMEM], 10% fetal bovine serum [FBS]) was added; to each of the other columns (columns 4 through 12), 25-μl aliquots of the corresponding serum dilution (1/200 or 1/2,000) in DMEM–10% FBS were added. All serum samples were heat inactivated at 56°C for 30 min before use in neutralization assays. Each virus in a total volume of 75 μl was then added to each well in columns 3 through 12. Virus-free medium was added to columns 1 and 2 (mock infected). The amount of each virus chosen was the lowest level of viral input sufficient to give a clear luciferase signal within the linear range for each viral strain. The plate was incubated for 1 h at 37°C. After incubation, 104 target cells (TZM-bl) in a volume of 100 μl were added to each well. The plate was then placed into a humidified chamber within a CO2 incubator at 37°C. Luciferase activity was determined as mentioned above. Neutralization activity for all samples was measured in triplicate and reported as the percentage of luciferase activity ± standard deviation.
Neutralization sensitivities of all recombinant viruses from the minipanel were assessed against previously characterized monoclonal antibodies. Neutralization assays were carried out as explained above with eight serial 4-fold dilutions of the following monoclonal antibodies (MAbs): b12, 2G12, 2F5, and 4E10 (4, 8, 11, 12, 19, 27, 54, 75–77, 81, 85). For MAb neutralizing assay, the values are expressed as the concentration of IgG that reduces infectivity by 50% (IC50).
For neutralization assays with Renilla RVs, viruses were preincubated with the IgGs purified from sera at a concentration of 0.2 mg/ml for 1 h at 37°C. The recombinant viruses previously neutralized with IgG were added in a volume of 50 μl/well to cultures of U87.CD4.CCR5 or CXCR4 distributed in 96-well plates (2 × 104 cells/well). Neutralization assays are run in triplicate. Luciferase activity in cell lysates was assessed 48 h after infection using a 96-well plate luminometer (Orion; Berthold). Luciferase activities in cell lysates infected with nonneutralized viruses were considered 100%.
Purification of IgG.
Immunoglobulin G was extracted from selected samples using protein A affinity chromatography. Briefly, 100 μl of heat-inactivated serum was mixed with 500 μl of sterile pH 7.4 phosphate-buffered saline and purified by using a protein A spin purification kit (Pierce, Rockford, IL). Purified IgG was further separated from smaller molecules by extensive dialysis with 50-kDa-cutoff membranes (Spectra/Por; Spectrum Medical Industries, Laguna Hills, CA). The 50-kDa exclusion limit has been previously recommended to eliminate traces of efavirenz (EFV) bound to plasma proteins (9, 10). The immunoglobulin was quantified using a mini-Bradford assay read on a microplate spectrophotometer (Tecan Trading AG, Switzerland). The flowthrough fraction, considered IgG depleted, was further diluted to reproduce the 1:200 serum dilution for neutralization testing. Two concentrations of purified IgG from selected serum samples (0.2 and 0.05 mg/ml) and the corresponding flowthrough fractions (1:200 dilution) were plated in triplicate in a 96-well plate and mixed with all the viruses from the minipanel plus Env-pseudotyped VSV. Neutralization assays were performed as mentioned above.
In vitro assay for detection of traces of EFV antiretroviral activity.
We selected serum samples from one HIV-infected patient who was on antiretroviral treatment that does not include EFV and who has been classified as a broad neutralizer in our screening and one HIV-negative serum sample from a healthy volunteer. EFV was added to both serum samples to a final concentration of 5 μg/ml. EFV-containing sera (and the corresponding controls with no EFV) were incubated at 37°C for 2 h. Immunoglobulin G fractions were purified/dialyzed as mentioned above. The corresponding flowthrough fractions were preserved for further analysis. All fractions were prepared for neutralizing assay as follows: purified/dialyzed IgGs were quantified and diluted to obtain a 0.2-mg/ml concentration; serum samples and flowthrough fractions were diluted to obtain 1:200 dilutions. All samples (with and without EFV) were divided into two aliquots, and one of them was boiled at 100°C for 30 min. All the fractions were subsequently tested against NL4-3, VI191, and pseudotyped VSV in a neutralization assay as mentioned above.
Statistical analysis.
Variables were expressed as the medians and ranges or as proportions, as appropriate. The association of clinical data for all patients with neutralization breadth was tested using the Wilcoxon rank-sum test. Both comparisons were done, by patient, taking the first isolate recovered, and by isolate, taking all isolates available. A stratified analysis by the presence or absence of ART was done by assessing the association of clinical data and neutralization breadth in subjects under ART and in those untreated at the time of sampling. Statistical significance was defined as a P value of <0.05. All statistical analyses were carried out using the SPSS package (version 16; SPSS Inc., Chicago, IL).
RESULTS
Definition of screening panel.
In this screening we used a minipanel of six recombinant viruses with envelopes from different subtypes and tropisms: VI191 subtype A and R5, 92BR025 subtype C and R5, 92UG024 subtype D and X4, AC10 subtype B and R5, CM244 subtype AE and R5, and NL4-3 subtype B and X4 (Table 2). These recombinant viruses were used in a TZM-bl cell line-based HIV neutralization assay. The RV neutralization assay has previously been validated by comparison with the current standardized pseudotype assays, and a good agreement was found (30, 32, 33). In order to determine whether the RVs in the panel adequately represented the global HIV-1 diversity, we have added the RVs in the panel to the phylogenetic analysis of a large group of 135 reference sequences from the different HIV-1 subtypes taken from the Los Alamos database (http://hiv-web.lanl.gov). Phylogenetic analysis of the V3C3 region (aa 300 to 392, according to HxB2 numbering) of the recombinant viruses showed that the sequence diversity covered by the RV in the minipanel was a good representation of the diversity observed among circulating HIV-1 isolates (Fig. 1). We have also determined the overall resistance profile of the RVs in the panel measured against a group of the currently identified broadly neutralizing antibodies (bNAbs): b12, 2G12, 2F5, and 4E10 (Table 2). All the viruses in the panel were sensitive to MAb 4E10. All the viruses except 92BR025 were sensitive to 2F5, and all viruses except AC10 and CM244 were sensitive to b12 and 2G12.
Table 2.
Neutralization profiles of recombinant viruses used in the screening panel
| Virus | Subtype | Tropism | Neutralization profile (IC50 [mg/ml]) |
|||
|---|---|---|---|---|---|---|
| b12 | 2G12 | 2F5 | 4E10 | |||
| VI191 | A | R5 | 0.2 | 5 | 1 | 0.4 |
| NL4-3 | B | X4 | 0.3 | 0.5 | 2 | 2.5 |
| AC10 | B | R5 | >6.25 | >12.5 | 2 | 0.4 |
| 92BR025 | C | R5 | 6 | 2 | >12.5 | 7 |
| 92UG024 | D | X4 | 3 | 1 | 4 | 4 |
| CM224 | AE | R5 | >6.25 | >12.5 | 2 | 2 |
Fig. 1.
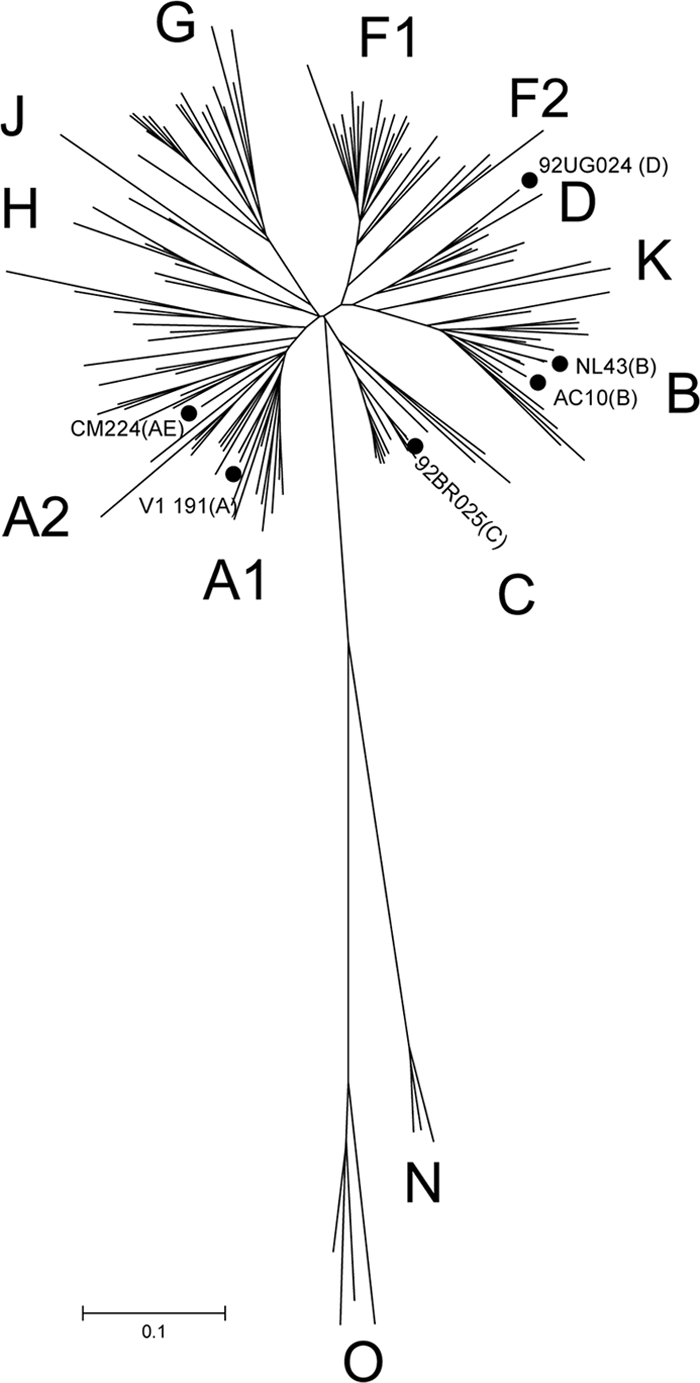
Phylogenetic tree of HIV-1 V3C3 env region by the neighbor-joining method of 135 reference sequences downloaded from the Los Alamos database (http://www.hiv.lanl.gov) and the six recombinant viruses from different subtypes: VI191 subtype A, 92BR025 subtype C, 92UG024 subtype D, AC10 subtype B, CM244 subtype AE, and NL4-3 subtype B. Filled circles indicate the viruses included in the screening panel. The scale bar represents 10% nucleotide genetic distance.
Initial screen for broad cross-neutralizing sera.
We next sought to use the screening panel to survey a group of serum samples for broadly neutralizing activity. Our panel included 508 samples taken from 364 HIV-1-seropositive (HIV+) individuals; for 235 individuals, samples from one time point were analyzed; for 129 individuals, samples from 2 to 4 time points were analyzed. For patients with more than one sample analyzed, time points of sample collection were anywhere from 2 months to 8 years apart. Each virus-serum sample combination was analyzed in triplicate at two dilutions (1/200 and 1/2,000). These dilutions were selected in order to minimize the inhibitory effect of the antiretroviral present in sera (10, 26). Table 3 summarizes the neutralization results for the 508 serum samples. A total of 4.8% of the samples from untreated patients (12 out of 248) achieved 50% neutralization of the 6 recombinant viruses from the panel at a ≥1/200 dilution. Complete neutralization curves were done with these sera, and the reciprocal dilutions of sera that reduce infectivity by 50% (ID50s) calculated from these curves are depicted in Table 4. In patients with samples from more than one time point analyzed, neutralization curves were done only with the serum sample from the first time point. The most notable neutralizing activity detected in this survey was observed in ART patients, in which 112 serum samples (43.1%) achieved 50% neutralization of all the viruses in the panel (33 at a ≥1/200 dilution and 79 at a ≥1/2,000 dilution). A detailed analysis of these samples and the corresponding treatment regimens revealed a strong correlation between EFV-containing treatment and viral inhibition. In fact, 97.3% (108 out of 111) of the serum samples from patients on EFV-containing treatment were able to neutralize all the viruses from the panel (29 at a 1/200 dilution and 79 at a 1/2,000 dilution). What is more, 71.2% (79 out of 111) of the serum samples from patients on treatment containing EFV achieved 50% neutralization at a ≥1/2,000 dilution. This inhibitory activity led us to question whether there was a significant inhibitory activity attributable to the residual antiviral drug (EFV) in sera.
Table 3.
Neutralization screening results for sera and purified IgG
| Patient group | Total no. (%) of serum samples (n = 508) | No. (%) of serum samples with ID50 of: |
No. (%) of purified IgG samples with IC50 of: |
No. of broadly neutralizing serum samples/total no. tested (%) consideringa: |
||||
|---|---|---|---|---|---|---|---|---|
| >2,000 | <2,000 and >200 | <200 | >0.2 mg/ml | <0.2 mg/ml | All samples (n = 508) | One sample per patient (n = 364) | ||
| Untreated patient | 248 (42.8) | 0 (0) | 12 (4.8) | 236 (95.1) | 240 (96.8) | 8 (3.2) | 8/248 (3.2) | 7/191 (3.7) |
| Patients with ART containing: | 6/260 (2.3) | 3/173 (1.7) | ||||||
| EFV | 111 (21.9) | 79 (71.2) | 29 (26.1) | 3 (1.2) | 106 (95.5) | 5 (4.5) | ||
| No EFV | 149 (29.3) | 0 (0) | 4 (2.7) | 145 (97.3) | 148 (99.3) | 1 (0.7) | ||
A serum sample is a broad neutralizer when the corresponding IgG fraction neutralizes all the viruses in the panel at 50% with no significant neutralization of the VSV-pseudotyped control, when a 0.2-mg/ml concentration is used.
Table 4.
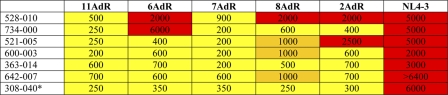
Serum neutralization data (ID50s) for untreated patients capable of neutralizing all the viruses from the panela
*, for patient 308, ID50 values were calculated from the second time point because of the limited amount of serum available from the first time point. Numbers indicate the ID50. A yellow box indicates an ID50 of ≥200 but <1,000, an orange box indicates an ID50 of ≥1,000 but <2,000, and a red box indicates an ID50 of ≥2,000.
IgG purification from sera containing efavirenz.
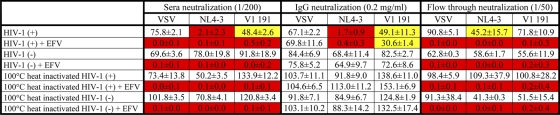
Considering the strong association observed between EFV-containing treatment and viral inhibition, we performed some in vitro assays to evaluate the efficiency of our IgG purification system for removing EFV traces and to rule out the effect of the drug. In these assays, EFV (5 μg/ml) was added to an HIV+ serum sample and an HIV-negative (HIV−) serum sample. The concentration of 5 μg/ml was chosen for being higher than the highest concentration previously reported for plasma samples from EFV-treated patients (52, 53). Subsequently, EFV-containing sera (and the corresponding controls with no antiretroviral) were incubated at 37°C in order to facilitate EFV association with serum proteins. After 2 h of incubation at 37°C, the IgG fraction was purified and extensively dialyzed with a 50-kDa-cutoff pore membrane. The inhibitory activities of each serum sample before and after EFV addition, the purified IgG, and the flowthrough fraction were analyzed using the neutralization assay described above. Each fraction was tested against two viruses from the minipanel (VI191 and NL4-3) and a VSV-pseudotyped virus that was included as a control for nonspecific neutralizing activity. Results are depicted in Table 5. HIV+ and HIV− sera were able to inhibit replication of NL4-3, VI191, and the pseudotyped VSV after EFV addition. The analysis of the IgG fraction isolated from sera showed no viral inhibition attributable to the drug. In addition, the flowthrough fraction showed strong inhibition of the three viruses in the fractions that were preincubated with EFV.
Table 5.
Evaluation of the purification method to isolate the IgG fraction from EFV-containing seraa
Numbers indicate percent luciferase activity (counts per second ± the standard deviation). A white box indicates >50% luciferase activity, a yellow box indicates <50% luciferase activity but >30% and a red box indicates <10% luciferase activity.
EFV activity in sera has previously been proven to be strikingly resistant to temperature compared to a protein-mediated activity (10). In order to determine the effect of EFV in our assay, in the absence of IgG activity, we heated each fraction at 100°C for 30 min and tested the inhibition of NL4-3, VI191, and pseudotyped VSV. The analysis of the data revealed that all the fractions containing EFV retained the inhibitory activity after heat inactivation at 100°C. In addition, there was no inhibitory effect attributable to the drug in the purified IgG fraction (Table 5).
Confirmation that there is a broadly neutralizing activity attributable to the IgG fraction of the sera.
In order to identify sera with IgG-associated broadly neutralizing activity, we isolated the IgG fraction from sera that achieved 50% neutralization of the 6 recombinant viruses from the panel at a 1/200 dilution (sera from untreated patients and patients treated with ART with no EFV) and at a 1/2,000 dilution (patients on EFV-containing treatment). The purified IgGs were tested against the 6 recombinant viruses from the minipanel and against pseudotyped VSV, which was used as a specificity control. With this analysis, we were able to identify 14 IgG fractions purified from 12 patients that were able to achieve 50% neutralization against all the viruses in the panel using a concentration of 0.2 mg/ml, corresponding to a dilution range of 1/40 to 1/80, depending on the serum sample (Table 3). Two patients (521 and 308) showed a good neutralizing response at more than one time point. The neutralization results for these IgGs are depicted in Table 6. In order to rule out the effect of EFV traces in IgGs purified from EFV-containing sera, we heated all the IgG fractions at 100°C for 30 min and tested them against two viruses from the minipanel (NL4-3 and VI191). No neutralizing activity was observed (data not shown). These results indicate that the neutralizing activity observed in IgGs purified from patients with EFV-containing sera was not attributable to the presence of EFV traces in sera.
Table 6.
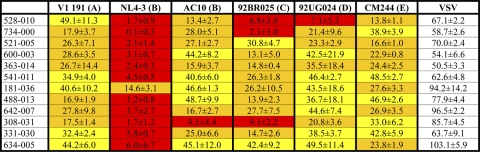
IgG neutralization data (0.2 mg/ml) for patients capable of neutralizing all the viruses from the panela
Numbers indicate percent luciferase activity (counts per second ± standard deviation). A white box indicates >50% luciferase activity, a yellow box indicates <50% luciferase activity but >30%, an orange box indicates <30% luciferase activity but >10%, and a red box indicates <10% luciferase activity. For patients 521 and 308, data from only the first time point are shown.
IgGs isolated from 8 of the best serum samples were tested against a new panel with 25 additional viruses from different subtypes using recombinant viruses that have the Renilla reporter gene in the nef locus. Neutralization results are summarized in Table 7. This new panel confirmed the broadly neutralizing activity of the sera previously selected with the 6-virus panel.
Table 7.
Cross-neutralizing properties of purified IgGs selected from broadly neutralizing seraa
| Virus | No. (%) of purified IgG samples |
|||||||
|---|---|---|---|---|---|---|---|---|
| 528-010 | 734-000 | 521-005 | 600-003 | 363-014 | 642-007 | 308-40 | 331-30 | |
| Clade B (total n = 18) | 17 (94) | 12 (65) | 8 (47) | 13 (71) | 13 (71) | 11 (59) | 14 (76) | 16 (88) |
| Clades no B (total n = 7) | 5 (71) | 2 (29) | 1 (14) | 4 (57) | 3 (43) | 4 (57) | 6 (86) | 1 (14) |
Neutralization was considered positive if 50% neutralization was achieved at an IgG concentration of less than 0.2 mg/ml.
Broadly neutralizing sera were found in both patient groups: untreated and ART patients. The percentage of broadly neutralizing activity in untreated patients was 3.7% (7/191). The percentage of broadly neutralizing activity found in ART patients was similar: 1.7% (3/174; P = 0.34). In this analysis, we have included data for the sample from only the first time point for patients with more than one sample (n = 364). Similar percentages were found when all the serum samples were considered (n = 508), with percentages of broadly neutralizing sera of 2.3% (6/260) for ART patients and 3.2% (8/248) for untreated patients (P = 0.59). Results are depicted in Table 3.
Stability of the IgG-associated broadly neutralizing response.
From seven of the patients that showed a broad neutralizing response, sera from more than one time point were analyzed. For these patients, time points were anywhere from 2 months to 4 years apart. The stabilities of the neutralizing responses for these patients are shown in Table 8. Two of the patients (488 and 331) acquired the neutralizing activity during the study course. In contrast, patients 363, 181, and 634 had a good neutralizing activity in the first sample but showed no activity in the sample from the second time point. Two of the patients (521 and 308) showed a good neutralizing activity that was stable during the study course. Especially significant was the case of patient 308, who showed a good neutralizing response that was stable for a 4-year period.
Table 8.
Clinical characteristics of the patients that showed an IgG-associated broadly neutralizing activity at one time point or more
| Patient | Sample | Time (mo) of study course | bNAb (IgG)a | CD4+ (cells/ml) | CD8+ (cells/ml) | VLb | Peak VL | CD4 nadir (cells/ml) | ART |
|---|---|---|---|---|---|---|---|---|---|
| 528 | 010 | 0 | + | 750 | 1,024 | 26,000 | 104,000 | 354 | |
| 734 | 000 | 0 | + | 500 | 942 | 1,422 | 1,422 | 491 | |
| 521 | 005 | 0 | + | 559 | 2,163 | 2,232 | 1000,000 | 222 | |
| 006 | 15 | + | 637 | 2,240 | 3,455 | 1000,000 | 222 | ||
| 600 | 003 | 0 | + | 1,608 | 1,366 | 2,440 | 297,000 | 1,008 | |
| 363 | 014 | 0 | + | 581 | 664 | 7,367 | 7,367 | 537 | |
| 015 | 12 | − | 686 | 987 | 7,950 | 7,950 | 537 | ||
| 541 | 011 | 0 | + | 499 | 576 | <50 | 78,339 | 286 | Efavirenz + emtricitabine + tenofovir |
| 181 | 036 | 0 | + | 437 | 857 | <50 | 39,947 | 266 | Efavirenz + emtricitabine + tenofovir |
| 488 | 012 | 0 | − | 334 | 998 | 975 | 3,038 | 334 | |
| 013 | 17 | + | 295 | 1,042 | <50 | 5,188 | 291 | Efavirenz + emtricitabine + tenofovir | |
| 642 | 007 | 0 | + | 272 | 799 | 89,356 | 107,300 | 272 | |
| 308 | 031 | 0 | + | 583 | 2,435 | 9,003 | 12,069 | 460 | |
| 040 | 35 | + | 448 | 2,304 | 19,005 | 19,005 | 448 | ||
| 041 | 49 | + | 530 | 1,698 | 49 | 27,462 | 410 | Efavirenz + emtricitabine + tenofovir | |
| 331 | 029 | 0 | − | 80 | 770 | 85,454 | 460,000 | 80 | Zidovudine + lopinavir + ritonavir + emtricitabine + tenofovir |
| 030 | 2 | + | 75 | 1,101 | 509 | 85,454 | 75 | Zidovudine + lopinavir + ritonavir + emtricitabine + tenofovir | |
| 634 | 005 | 0 | + | 686 | 1,291 | <50 | 134,500 | 378 | Efavirenz + emtricitabine + tenofovir |
| 006 | 4 | − | 587 | 1,522 | − | 134,500 | 378 | Efavirenz + emtricitabine + tenofovir |
+, the corresponding IgG fraction neutralizes all the viruses in the panel at 50% with no significant neutralization of the VSV-pseudotyped control, when a 0.2-mg/ml concentration is used; −, the corresponding IgG fraction does not neutralize all the viruses in the panel at 50%.
The limit of detection was 50 copies/ml.
The induction of a broad neutralizing activity does not correlate with viral load when ART patients are included in the study.
Clinical and demographic data for all patients were compared to the presence of neutralization breadth. Some clinical data corresponding to the 12 patients that showed a broad IgG-mediated neutralizing activity at one time point or more are depicted in Table 6. For this analysis we have considered that a serum sample is a broad neutralizer when the corresponding IgG fraction neutralizes all the viruses in the panel at 50% with no significant neutralization of the VSV-pseudotyped control, when a concentration of 0.2 mg/ml is used. The associations between breadth and clinical covariants were tested using nonparametric methods (Wilcoxon rank-sum test). In our panel of sera, no association between viral load and neutralization breadth was observed. We also found no association between CD8+ counts, number of years on ART, number of years since diagnosis, risk group, gender, and age and neutralization breadth. Next we did the same analysis considering only one sample per patient (n = 364). In this analysis we also found a lack of association between CD4+ T cell counts, CD8+ counts, number of years on ART, number of years since diagnosis, risk group, gender, and age and neutralization breadth for both patient groups.
DISCUSSION
The induction of cross-reactive broadly neutralizing antibodies is a critical line of research for HIV vaccine development. Our study represents the first large screening and evaluation of neutralization to date in individuals infected with HIV-1 including a large group of patients on aggressive antiretroviral treatment. Before the era of effective ART, it was widely reported that HIV infection leads to extensive defects in the humoral immune system (1, 16, 41, 47, 67) and that most of these B cell defects can be reversed by ART (13, 20, 58, 63). In the current study, we showed that patients on antiretroviral treatment are capable of inducing a broad and potent humoral immune response against HIV, despite having undetectable levels of viremia.
In the current study, a group of envelope sequences were phylogenetically analyzed, and 6 envelopes from 5 different subtypes were selected for the construction of the recombinant viruses for the minipanel. The phylogenetic analysis showed that this group of envelopes was a good representation of global HIV variation, including a diversity similar to the diversity of the screening panels used in previous studies (24, 28, 80). Different groups have tested the neutralizing activity in plasma or serum samples from a large group of individuals on different pseudovirus panels and reported that screening for a reduced panel (5 or less) of selected viruses provided similar information on the presence of cross-reactive neutralizing activity as screening for a large pseudovirus panel (24, 28, 80). In our study, the prevalence of patients with broadly cross-neutralizing antibodies was lower than that observed in prior studies. A total of 2.7% of the patients showed IgG-mediated neutralizing activity against all the viruses from a panel consisting of 6 viruses with envelopes from 5 different subtypes. The differences in the prevalence of cross-reactive neutralizing activity observed in this study compared to previous studies are probably due to the usage of a more restrictive selection criterion. All these studies use an ID50 of >100 as a cutoff for neutralization, and the cross-reactive sera are supposed to neutralize viruses from 2, 3, 4, or 5 subtypes, depending on the study. In this study the initial cutoff for untreated and no-EFV-treated patients was higher than the one used previously (ID50 > 200). The selection criterion for patients on an EFV-containing treatment was even more restrictive (ID50 > 2,000) due to the nonspecific inhibition observed in these patient serum samples. The neutralization sensitivities of the purified IgGs from the selected sera were determined and used to define the cutoff for cross-reactive neutralization. We consider strong cross-reactive neutralizing activity in serum to be the ability to neutralize the 6 viruses of our minipanel that contain envelopes from 5 different subtypes at an IC50 titer of <0.2 mg/ml of purified IgG. Our definition of cross-reactive neutralizing activity is closer to the definition used by Simek et al. (80) for elite neutralizing activity, which considers elite activity to be “the ability to neutralize, on average, more than one pseudovirus at an IC50 titer of 300 within a clade group and across at least four clade groups.”
A small number of patients with a broad neutralizing response have been previously described. However, most of the LTNP patients show a poor humoral immune response (39, 87). Several studies have compared the induction of broadly neutralizing antibodies in LTNPs to that in progressor patients with detectable levels of viremia (3, 23, 24, 46, 50, 71). Among progressor patients, a significant group of sera with broad and potent neutralizing activity was observed. In contrast, significantly lower titers of NAbs to laboratory isolates as well as lower levels of broadly cross-neutralizing antibodies were found in LTNP patients. This inefficient humoral immune response has been attributed to the low levels of antigenic stimulation. In fact, several studies have shown a positive correlation between plasma viral load and titers of neutralizing antibodies to heterologous virus (21, 23, 73, 78), suggesting that the development of these antibodies was a consequence of viral replication. In the present study, we examined the breadth of neutralization in a large cohort of patients, including patients on highly active antiretroviral therapy (HAART) with persistent undetectable viremia. Remarkably, we found a similar percentage of broadly neutralizing sera in ART patients with VLs of <50 (1.7%) and untreated patients with detectable viremia (3.7%). These results indicate that the level of antigen required for the development of a broad neutralizing response in ART patients is very low, below the limit of detection with the currently used assays. We hypothesize that the good humoral immune response induced in these patients, despite the low levels of antigenic stimulation, could be a consequence of the improved B cell function associated with antiretroviral treatment. Our results are in stark contrast to the data obtained from long-term nonprogressors, whose little neutralizing activity has been attributed to the low levels of viral replication. Our data show that ART patients are capable of inducing a good antibody response; however, LTNP patients have a poor humoral immune response against HIV-1, according to previous reports (3, 23, 24, 46, 50, 71). One possible explanation is that this response has persisted from the pre-ART time. In the longitudinal study, we have two patients whose sera were analyzed before and after treatment. Patient 308 had preexisting broadly neutralizing activity. However, patient 488 showed no significant neutralizing activity before treatment. The longitudinal data obtained in the present study are not complete enough to support or discard this hypothesis. Another possible explanation for these data could be based on differences in the B cell status between ART patients and LTNPs. There is little information about the status of B cells in LTNPs. However, Titanji et al. (83) investigated the serologic memory to non-HIV antigens in different groups of HIV-infected patients. They found that while progressor patients had severe defects in serologic memory, long-term nonprogressors had memory B cell frequencies and levels of antigen-specific antibodies comparable to those of noninfected individuals. According to this study, LTNPs showed no B cell defect that can explain the deficient humoral immune response against HIV. A third possible explanation for the different induction of neutralizing antibodies between ART patients and LTNPs is that the levels of viral replication may be higher in ART patients than LTNPs, even though both groups of patients have levels of viremia below the detection limit (<50 copies/ml) of clinically used viral load assays (70). In fact, the presence of low levels of viremia detectable by more sensitive assays (25, 31, 37, 51, 68, 71) has been demonstrated in most patients receiving standard HAART, but the source of this remains unclear. In addition, ART patients had high levels of viremia prior to the initiation of treatment that contributed to the establishment of cellular reservoirs. Low-level viremia may arise from viral expression in the cellular reservoirs established before HAART (2, 36–38, 42, 64, 68, 72, 84) or from low levels of complete cycles of virus replication (17, 18, 84). The observation that the HIV-1 envelope induces predominantly short-lived memory B cell-dependent plasma Abs (7) supports the idea that low levels of viremia could be required for the induction of bNAbs in ART patients. The presence of ongoing virus replication during long-term suppressive HAART is supported by findings of sequence evolution with the appearance of new drug-resistant variants in a subset of patients during seemingly effective HAART (17, 84) and decreases in plasma viremia with treatment intensification (37). In contrast, little is known about viral replication in LTNPs with levels of viremia below 50 copies/ml. Several studies suggest that infection with replication-defective virus explains the absence of detectable circulating virus in some LTNPs (14, 43, 48, 49, 60, 86). These patients could have inefficient antigen stimulation of their B cells caused by a deficient viral replication. However, this cannot be the explanation in the case of LTNPs in which no defect in viral replication has been observed and the control of viral replication has been attributed to host genetic or immune mechanisms (3, 15, 40, 45, 56, 57).
In this study we have also identified two patients that made a broad neutralizing response that was stable over long periods of time, up to 4 years. The identification and detailed analysis of these patients may provide valuable information to improve the induction of an effective and stable humoral immune response.
In contrast to previous studies (73, 78), we observed no correlation between neutralization breadth and viral load. The previously reported correlation between viral load and neutralization breadth suggested that a vaccine may need to supply viral antigen for long periods of time to allow antibody maturation and development of a broad neutralizing response. This suggestion was supported by the observation that HIV-1 bNAbs often show significant degrees of affinity maturation (69, 79). Whether the extensive maturation requires several years of antigen exposure or high levels of replication needs to be determined. To date, no bNAb has been isolated from ART patients. Therefore, the level of maturation of these antibodies is unknown but may be lower, consistent with the low viral loads. The results of the present study suggest that the requirement of high levels of viral replication for the induction of bNAbs may be true only for untreated HIV-infected individuals, which have several B cell abnormalities associated with HIV replication-induced immune cell activation, and not for uninfected individuals or ART patients (in therapeutic vaccine approaches).
In this study we have also observed that traces of antiretroviral drug present in sera from ART patients showed a strong inhibition of the virus. Specifically, we observed a potent inhibitory effect of traces of efavirenz in sera. In order to separate the effect of traces of antiretroviral drugs or any other nonspecific factor present in sera from the immunoglobulin-mediated effect, we decided to purify IgGs from all the sera that showed good viral inhibition and analyzed their neutralizing activity. We were able to confirm the presence of IgG-associated neutralizing activity in 5 serum samples from patients on EFV-containing treatment. We were able to rule out the effect of EFV traces in these IgGs after observing that this activity was not effective against the VSV specificity control (Table 5) and proving that it was sensitive to heat inactivation at 100°C. The observation that soluble factors present in sera can have an inhibitory viral effect suggests that measurements of HIV-1 neutralization by crude serum and plasma samples must be carried out with some caution.
In summary, the present study demonstrated an induction of a broad humoral immune response in patients on antiretroviral treatment that have undetectable viremia. In these patients, the low level of antigenic stimulation may be compensated for by an improved B cell function induced by antiretroviral treatment. We hypothesize that local residual viral replication, together with an improved B cell function, in ART patients is capable of inducing a broad antibody response that is similar to the response induced in untreated progressor patients with detectable viremia.
ACKNOWLEDGMENTS
We thank Ana García and María T. García for technical assistance and Jacqueline Bixby for assistance in preparing the manuscript. The following reagents were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: TZM-bl from John C. Kappes, Xiaoyun Wu, and Tranzyme Inc.; HIV-1 gp120 monoclonal antibody (IgG1 b12) from Dennis Burton and Carlos Barbas; and HIV-1 gp120 monoclonal antibody (2G12) and HIV-1 gp41 monoclonal antibodies (2F5 and 4E10) from Hermann Katinger.
This work was supported by the Ministerio de Ciencia y Tecnología (RYC-2007-00788), the Instituto de Salud Carlos III (FIS PS09/01459, FIS PS09/01297, FIS PI09/01459, RETIC RD06/0006), the FP6 and FP7 Networks of Excellence from the EU (EUROPRISE and CHAARM), and FIPSE (36630/07, 36780/08), Ayuda para el fomento de la traslación de la aplicación terapéutica de medicamentos de uso humano, huérfanos y terapias avanzadas (TRA-094). M.M.-R. is supported by a Consejo de Ciencia y Tecnología de México fellowship.
Footnotes
Published ahead of print on 6 April 2011.
REFERENCES
- 1. Ammann A. J., et al. 1984. B-cell immunodeficiency in acquired immune deficiency syndrome. JAMA 251:1447–1449 [PubMed] [Google Scholar]
- 2. Bailey J. R., et al. 2006. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J. Virol. 80:6441–6457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bailey J. R., Williams T. M., Siliciano R. F., Blankson J. N. 2006. Maintenance of viral suppression in HIV-1-infected HLA-B*57+ elite suppressors despite CTL escape mutations. J. Exp. Med. 203:1357–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barbas C. F., et al. 1992. Recombinant human Fab fragments neutralize human type 1 immunodeficiency virus in vitro. Proc. Natl. Acad. Sci. U. S. A. 89:9339–9343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beirnaert E., et al. 2000. Identification and characterization of sera from HIV-infected individuals with broad cross-neutralizing activity against group M (env clade A-H) and group O primary HIV-1 isolates. J. Med. Virol. 62:14–24 [PubMed] [Google Scholar]
- 6. Binley J. M., et al. 2008. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J. Virol. 82:11651–11668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bonsignori M., et al. 2009. HIV-1 envelope induces memory B cell responses that correlate with plasma antibody levels after envelope gp120 protein vaccination or HIV-1 infection. J. Immunol. 183:2708–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buchacher A., et al. 1994. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res. Hum. Retroviruses 10:359–369 [DOI] [PubMed] [Google Scholar]
- 9. Burrer R., et al. 2001. Immunoglobulin G (IgG) and IgA, but also nonantibody factors, account for in vitro neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by serum and plasma of HIV-infected patients. J. Virol. 75:5421–5424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burrer R., et al. 2006. Efavirenz in plasma from HIV-infected patients does not directly block reverse transcriptase activity in cell-free assays but inhibits HIV replication in cellular assays. AIDS Res. Hum. Retroviruses 22:865–869 [DOI] [PubMed] [Google Scholar]
- 11. Burton D. R., et al. 1991. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc. Natl. Acad. Sci. U. S. A. 88:10134–10137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burton D. R., et al. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024–1027 [DOI] [PubMed] [Google Scholar]
- 13. Cagigi A., Nilsson A., De Milito A., Chiodi F. 2008. B cell immunopathology during HIV-1 infection: lessons to learn for HIV-1 vaccine design. Vaccine 26:3016–3025 [DOI] [PubMed] [Google Scholar]
- 14. Candotti D., et al. 1999. Status of long-term asymptomatic HIV-1 infection correlates with viral load but not with virus replication properties and cell tropism. French ALT Study Group. J. Med. Virol. 58:256–263 [PubMed] [Google Scholar]
- 15. Cao Y., Qin L., Zhang L., Safrit J., Ho D. D. 1995. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N. Engl. J. Med. 332:201–208 [DOI] [PubMed] [Google Scholar]
- 16. Carbone A., et al. 1985. Lymph node immunohistology in intravenous drug abusers with persistent generalized lymphadenopathy. Arch. Pathol. Lab. Med. 109:1007–1012 [PubMed] [Google Scholar]
- 17. Cohen Stuart J. W., et al. 2001. Transient relapses (“blips”) of plasma HIV RNA levels during HAART are associated with drug resistance. J. Acquir. Immune Defic. Syndr. 28:105–113 [DOI] [PubMed] [Google Scholar]
- 18. Coiras M., Lopez-Huertas M. R., Perez-Olmeda M., Alcami J. 2009. Understanding HIV-1 latency provides clues for the eradication of long-term reservoirs. Nat. Rev. Microbiol. 7:798–812 [DOI] [PubMed] [Google Scholar]
- 19. Crawford J. M., et al. 1999. Characterization of primary isolate-like variants of simian-human immunodeficiency virus. J. Virol. 73:10199–10207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deeks S. G., et al. 2006. Neutralizing antibody responses against autologous and heterologous viruses in acute versus chronic human immunodeficiency virus (HIV) infection: evidence for a constraint on the ability of HIV to completely evade neutralizing antibody responses. J. Virol. 80:6155–6164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Milito A., et al. 2004. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood 103:2180–2186 [DOI] [PubMed] [Google Scholar]
- 22. Derdeyn C. A., et al. 2000. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J. Virol. 74:8358–8367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Doria-Rose N. A., et al. 2010. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J. Virol. 84:1631–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Doria-Rose N. A., et al. 2009. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J. Virol. 83:188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dornadula G., et al. 1999. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA 282:1627–1632 [DOI] [PubMed] [Google Scholar]
- 26. Dreyer K., et al. 1999. Primary isolate neutralization by HIV type 1-infected patient sera in the era of highly active antiretroviral therapy. AIDS Res. Hum. Retroviruses 15:1563–1571 [DOI] [PubMed] [Google Scholar]
- 27. Etemad-Moghadam B., et al. 1999. Determinants of neutralization resistance in the envelope glycoproteins of a simian-human immunodeficiency virus passaged in vivo. J. Virol. 73:8873–8879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Euler Z., et al. 2010. Cross-reactive neutralizing humoral immunity does not protect from HIV type 1 disease progression. J. Infect. Dis. 201:1045–1053 [DOI] [PubMed] [Google Scholar]
- 29. Feikin D. R., Feldman C., Schuchat A., Janoff E. N. 2004. Global strategies to prevent bacterial pneumonia in adults with HIV disease. Lancet Infect. Dis. 4:445–455 [DOI] [PubMed] [Google Scholar]
- 30. Fenyo E. M., et al. 2009. International network for comparison of HIV neutralization assays: the NeutNet report. PLoS One 4:e4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Furtado M. R., et al. 1999. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N. Engl. J. Med. 340:1614–1622 [DOI] [PubMed] [Google Scholar]
- 32. Garcia-Perez J., Sanchez-Palomino S., Perez-Olmeda M., Fernandez B., Alcami J. 2007. A new strategy based on recombinant viruses as a tool for assessing drug susceptibility of human immunodeficiency virus type 1. J. Med. Virol. 79:127–137 [DOI] [PubMed] [Google Scholar]
- 33. Gonzalez N., et al. 2010. A sensitive phenotypic assay for the determination of human immunodeficiency virus type 1 tropism. J. Antimicrob. Chemother. 65:2493–2501 [DOI] [PubMed] [Google Scholar]
- 34. Graham B. S. 2002. Clinical trials of HIV vaccines. Annu. Rev. Med. 53:207–221 [DOI] [PubMed] [Google Scholar]
- 35. Gray E. S., et al. 2009. Antibody specificities associated with neutralization breadth in plasma from human immunodeficiency virus type 1 subtype C-infected blood donors. J. Virol. 83:8925–8937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Havlir D. V., et al. 2001. Prevalence and predictive value of intermittent viremia with combination HIV therapy. JAMA 286:171–179 [DOI] [PubMed] [Google Scholar]
- 37. Havlir D. V., et al. 2003. Productive infection maintains a dynamic steady state of residual viremia in human immunodeficiency virus type 1-infected persons treated with suppressive antiretroviral therapy for five years. J. Virol. 77:11212–11219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hermankova M., et al. 2001. HIV-1 drug resistance profiles in children and adults with viral load of <50 copies/ml receiving combination therapy. JAMA 286:196–207 [DOI] [PubMed] [Google Scholar]
- 39. Humbert M., et al. 2007. Mimotopes selected with antibodies from HIV-1-neutralizing long-term non-progressor plasma. Eur. J. Immunol. 37:501–515 [DOI] [PubMed] [Google Scholar]
- 40. Hunt P. W., et al. 2008. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J. Infect. Dis. 197:126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kekow J., Kern P., Schmitz H., Gross W. L. 1986. Abnormal B-cell response to T-cell-independent polyclonal B-cell activators in homosexuals presenting persistent generalized lymph node enlargement and HTLV-III antibodies. Diagn. Immunol. 4:107–111 [PubMed] [Google Scholar]
- 42. Kieffer T. L., et al. 2004. Genotypic analysis of HIV-1 drug resistance at the limit of detection: virus production without evolution in treated adults with undetectable HIV loads. J. Infect. Dis. 189:1452–1465 [DOI] [PubMed] [Google Scholar]
- 43. Kloosterboer N., et al. 2005. Natural controlled HIV infection: preserved HIV-specific immunity despite undetectable replication competent virus. Virology 339:70–80 [DOI] [PubMed] [Google Scholar]
- 44. Kroon F. P., et al. 1998. Restored humoral immune response to influenza vaccination in HIV-infected adults treated with highly active antiretroviral therapy. AIDS 12:F217–F223 [DOI] [PubMed] [Google Scholar]
- 45. Lambotte O., et al. 2005. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin. Infect. Dis. 41:1053–1056 [DOI] [PubMed] [Google Scholar]
- 46. Lambotte O., et al. 2009. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS 23:897–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lane H. C., et al. 1983. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 309:453–458 [DOI] [PubMed] [Google Scholar]
- 48. Learmont J. C., et al. 1999. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N. Engl. J. Med. 340:1715–1722 [DOI] [PubMed] [Google Scholar]
- 49. Lum J. J., et al. 2003. Vpr R77Q is associated with long-term nonprogressive HIV infection and impaired induction of apoptosis. J. Clin. Invest. 111:1547–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mahalanabis M., et al. 2009. Continuous viral escape and selection by autologous neutralizing antibodies in drug-naive human immunodeficiency virus controllers. J. Virol. 83:662–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Maldarelli F., et al. 2007. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 3:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mallon P. W., Ray J., Cooper D. A. 2003. Effect of therapeutic drug monitoring on outcome in antiretroviral experienced HIV-infected individuals. J. Clin. Virol. 26:223–227 [DOI] [PubMed] [Google Scholar]
- 53. Marzolini C., et al. 2001. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS 15:71–75 [DOI] [PubMed] [Google Scholar]
- 54. Mascola J. R., et al. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mascola J. R., et al. 1996. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. J. Infect. Dis. 173:340–348 [DOI] [PubMed] [Google Scholar]
- 56. Migueles S. A., et al. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061–1068 [DOI] [PubMed] [Google Scholar]
- 57. Migueles S. A., et al. 2000. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. U. S. A. 97:2709–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Moir S., Fauci A. S. 2009. B cells in HIV infection and disease. Nat. Rev. Immunol. 9:235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Moir S., et al. 2008. Normalization of B cell counts and subpopulations after antiretroviral therapy in chronic HIV disease. J. Infect. Dis. 197:572–579 [DOI] [PubMed] [Google Scholar]
- 60. Mologni D., et al. 2006. Vpr and HIV-1 disease progression: R77Q mutation is associated with long-term control of HIV-1 infection in different groups of patients. AIDS 20:567–574 [DOI] [PubMed] [Google Scholar]
- 61. Moog C., Fleury H. J., Pellegrin I., Kirn A., Aubertin A. M. 1997. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J. Virol. 71:3734–3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Moore J. P., et al. 1996. Inter- and intraclade neutralization of human immunodeficiency virus type 1: genetic clades do not correspond to neutralization serotypes but partially correspond to gp120 antigenic serotypes. J. Virol. 70:427–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Morris L., et al. 1998. HIV-1 antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. J. Exp. Med. 188:233–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nettles R. E., et al. 2005. Intermittent HIV-1 viremia (blips) and drug resistance in patients receiving HAART. JAMA 293:817–829 [DOI] [PubMed] [Google Scholar]
- 65. Newman R. M., et al. 2006. Balancing selection and the evolution of functional polymorphism in Old World monkey TRIM5alpha. Proc. Natl. Acad. Sci. U. S. A. 103:19134–19139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Overton E. T., et al. 2005. Undetectable plasma HIV RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clin. Infect. Dis. 41:1045–1048 [DOI] [PubMed] [Google Scholar]
- 67. Pahwa S. G., Quilop M. T., Lange M., Pahwa R. N., Grieco M. H. 1984. Defective B-lymphocyte function in homosexual men in relation to the acquired immunodeficiency syndrome. Ann. Intern. Med. 101:757–763 [DOI] [PubMed] [Google Scholar]
- 68. Palmer S., et al. 2008. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc. Natl. Acad. Sci. U. S. A. 105:3879–3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pancera M., et al. 2010. Crystal structure of PG16 and chimeric dissection with somatically related PG9: structure-function analysis of two quaternary-specific antibodies that effectively neutralize HIV-1. J. Virol. 84:8098–8110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Perelson A. S., et al. 1997. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387:188–191 [DOI] [PubMed] [Google Scholar]
- 71. Pereyra F., et al. 2008. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J. Infect. Dis. 197:563–571 [DOI] [PubMed] [Google Scholar]
- 72. Persaud D., et al. 2004. Continued production of drug-sensitive human immunodeficiency virus type 1 in children on combination antiretroviral therapy who have undetectable viral loads. J. Virol. 78:968–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Piantadosi A., et al. 2009. Breadth of neutralizing antibody response to human immunodeficiency virus type 1 is affected by factors early in infection but does not influence disease progression. J. Virol. 83:10269–10274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Platt E. J., Wehrly K., Kuhmann S. E., Chesebro B., Kabat D. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 72:2855–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Purtscher M., et al. 1996. Restricted antigenic variability of the epitope recognized by the neutralizing gp41 antibody 2F5. AIDS 10:587–593 [DOI] [PubMed] [Google Scholar]
- 76. Purtscher M., et al. 1994. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses 10:1651–1658 [DOI] [PubMed] [Google Scholar]
- 77. Roben P., et al. 1994. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J. Virol. 68:4821–4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sather D. N., et al. 2009. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J. Virol. 83:757–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Scheid J. F., et al. 2009. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458:636–640 [DOI] [PubMed] [Google Scholar]
- 80. Simek M. D., et al. 2009. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J. Virol. 83:7337–7348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Stiegler G., et al. 2001. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses 17:1757–1765 [DOI] [PubMed] [Google Scholar]
- 82. Takeuchi Y., McClure M. O., Pizzato M. 2008. Identification of gammaretroviruses constitutively released from cell lines used for human immunodeficiency virus research. J. Virol. 82:12585–12588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Titanji K., et al. 2006. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood 108:1580–1587 [DOI] [PubMed] [Google Scholar]
- 84. Tobin N. H., et al. 2005. Evidence that low-level viremias during effective highly active antiretroviral therapy result from two processes: expression of archival virus and replication of virus. J. Virol. 79:9625–9634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Trkola A., et al. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70:1100–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang B., et al. 2003. First demonstration of a lack of viral sequence evolution in a nonprogressor, defining replication-incompetent HIV-1 infection. Virology 312:135–150 [DOI] [PubMed] [Google Scholar]
- 87. Wang Q., et al. 2008. High level serum neutralizing antibody against HIV-1 in Chinese long-term non-progressors. Microbiol. Immunol. 52:209–215 [DOI] [PubMed] [Google Scholar]
- 88. Wang S., et al. 2008. Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers. Vaccine 26:1098–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wei X., et al. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46:1896–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]