Abstract
Highly active antiretroviral therapy (HAART) can reduce plasma HIV-1 levels to below the detection limit. However, due to the latent reservoir in resting CD4+ cells, HAART is not curative. Elimination of this reservoir is critical to curing HIV-1 infection. Agents that reactivate latent HIV-1 through nonspecific T cell activation are toxic. Here we demonstrate in a primary CD4+ T cell model that the FDA-approved drug disulfiram reactivates latent HIV-1 without global T cell activation. The extent to which disulfiram reactivates latent HIV-1 in patient cells is unclear, but the drug alone or in combination may be useful in future eradication strategies.
TEXT
Highly active antiretroviral therapy (HAART) can reduce plasma HIV-1 levels to below the detection limit of clinical assays and reverse disease progression. However, HIV-1 persists in latently infected resting CD4+ T cells carrying an integrated HIV-1 genome (3). In these cells, the provirus remains transcriptionally silent as long as the host cell remains quiescent, allowing evasion of immune surveillance as well as of HAART, which targets replicating viruses (4, 7, 16). Following cellular activation, latent viral genomes are transcribed and virus is produced, contributing to rebound viremia upon discontinuation of HAART. The estimated size of this latent reservoir is 105 to 106 cells/patient, and the estimated half-life is 44 months (12), resulting in an elimination time of >60 years. This stable reservoir makes life-long HAART necessary. Given the potentials for toxicity and resistance and the expense of HAART, elimination of the latent reservoir is an important goal (14).
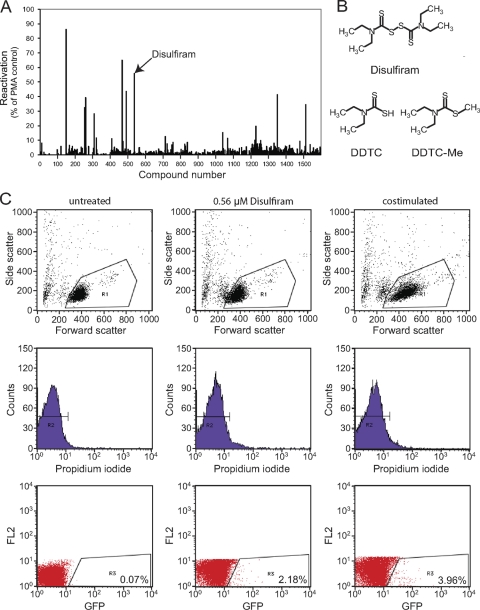
The reactivation of latent HIV-1 in patients on HAART is an approach for elimination of the latent reservoir (2, 5, 14). It is assumed that following reactivation infected cells will die from viral cytopathic effects or host cytolytic mechanisms. If this assumption is incorrect, reactivation strategies must be coupled with strategies that kill productively infected cells (2). In any event, the first step is to reactivate HIV-1 gene expression. We developed a primary cell model of HIV-1 latency to search for compounds that reverse latency. Primary human CD4+ T cells were transduced with the prosurvival gene bcl-2 to allow long-term in vitro culture. Latency was established by infecting transduced CD4+ lymphoblasts and allowing them to return to a resting state that recapitulated the state of resting CD4+ T cells in vivo (17). The virus used contains destabilized green fluorescent protein (GFP) in the env open reading frame, allowing detection of virus gene expression. Using this system, we screened the Johns Hopkins Drug Library and the MicroSource Spectrum Library (Fig. 1) and identified 8 hits in addition to the previously described hit 5-hydroxynaphthoquinone (17). Of these, 6 were confirmed with fresh drug samples. One of the confirmed hits, disulfiram, is an FDA-approved drug.
Fig. 1.
Screening of small-molecule libraries identifies disulfiram as an agent that reactivates latent HIV-1. (A) Summary of screening results from the MicroSource Spectrum library. The results were normalized to the response to 10 ng/ml phorbol myristate acetate. (B) Chemical structures of disulfiram and its metabolites, DDTC and DDTC-Me. (C) Representative flow cytometry data sets for untreated, disulfiram-treated, and anti-CD3 plus anti-CD28 antibody-treated cells. The dot plots in the first row are forward scatter-side scatter (FSC-SSC) plots. Histographs in the second row show cells gated in R1 in FSC-SSC plots. The GFP-FL2 dot plots in the third row show viable cells gated in R2 in propidium iodide histographs. Cells that appear in R3 (GFP+) are reactivated latently infected cells. The percentage of GFP+ cells was calculated based on the number of cells in R3 divided by the number of cells in R2.
Disulfiram [bis(diethylthiocarbamoyl) disulfide] is used to treat alcoholism. It inhibits aldehyde dehydrogenase, leading to increased acetaldehyde levels in patients who drink alcohol while on disulfiram. Acetaldehyde has aversive effects that deter alcohol consumption. Disulfiram chelates copper (13) and inhibits the copper-containing enzyme dopamine β-hydroxylase (DβH) (15). Disulfiram also inhibits plasma carboxylesterases and cholinesterase, blocking cocaine and dopamine metabolism. Increased blood levels of these compounds lead to unpleasant anxiety, making disulfiram useful in treating cocaine dependence (10). Disulfiram has little toxicity in the absence of alcohol or cocaine, and it is used to treat both these addictions in HIV-1-infected individuals. Disulfiram also inhibits angiogenesis (11).
We examined the dose-response relationship and the kinetics of disulfiram reactivation of latent HIV-1 by using latently infected, Bcl-2-transduced CD4+ T cells. Latently infected cells were treated with serial dilutions of disulfiram for various times, and reactivation was measured by flow cytometry as the percentage of GFP+ cells, normalized to the percentage of GFP+ cells following costimulation with anti-CD3 plus anti-CD28 antibodies. Cells from different donors showed similar dose-response curves (Fig. 2A). For most donors, peak activation occurred at ∼0.3 μM, below the reported peak plasma concentration of disulfiram (1.4 μM) (6).
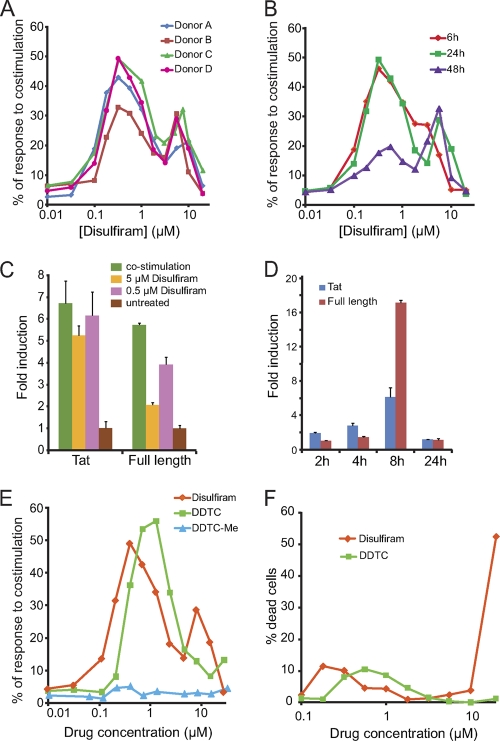
Fig. 2.
(A) Effects of disulfiram on latently infected Bcl-2-transduced CD4+ T cells from different donors. (B) Kinetic profile for the reactivation of latent HIV-1 by different concentrations of disulfiram. (C) Effects of disulfiram and costimulation on levels of HIV-1 transcripts in latently infected Bcl-2-transduced cells after 16 h of treatment. The fold change is shown relative to levels in untreated cells. Data are means plus standard deviations of triplicate samples from 1 of 2 independent experiments, both of which produced similar results. (D) Kinetic profile of the effect of 0.5 μM disulfiram on levels of HIV-1 transcripts in latently infected Bcl-2-transduced cells. The fold change is shown relative to levels in untreated cells. Data are means plus standard deviations of triplicate samples from 1 of 2 independent experiments, both of which produced similar results. (E) Comparison of disulfiram, DDTC, and DDTC-Me for reactivating latent HIV-1. (F) Toxicity of disulfiram and DDTC treatment, measured in a propidium iodide exclusion assay.
The dose-response curves for HIV-1 reactivation by disulfiram have two peaks. The first peak appears around 0.3 to 0.5 μM disulfiram, and the second appears around 5 to 10 μM. The decrease in GFP+ cells over the range of 0.5 to 2 μM may reflect activation of inhibitory pathways. The first peak decays with time, showing much lower reactivation after 48 h. The second peak appears later than the first (24 h versus 6 h) and does not decay (Fig. 2B). The kinetic differences between the peaks indicate that multiple mechanisms may be involved in the reactivation of latent HIV-1 by disulfiram.
To confirm the effect of disulfiram on latent HIV-1, we examined levels of HIV-1 transcripts before and after disulfiram stimulation. Multiply spliced transcripts encoding Tat and full-length HIV-1 transcripts were measured. Latently infected Bcl-2-transduced cells were treated for various times with 0.5 or 5 μM disulfiram or with anti-CD3 plus anti-CD28 antibodies or were left untreated. Total RNA was isolated using RNeasy (Qiagen). Reverse transcription was performed using SuperScript III reverse transcriptase (RT; Invitrogen) with random hexamers, and transcript levels were measured by real-time RT-PCR with SYBR Green PCR master mix (Applied Biosystems). Results were normalized to 18S rRNA. At 16 h, 0.5 μM disulfiram increased Tat transcripts by >6-fold and full-length transcripts by ∼4-fold relative to untreated samples (Fig. 2C). The increase in HIV-1 transcripts was lower with 5 μM disulfiram, consistent with the lower reactivation levels observed by fluorescence-activated cell sorting. We also observed a time dependence of transcriptional induction by 0.5 μM disulfiram (Fig. 2D). The induction of multiply spliced Tat transcripts was observed as early as 2 h after treatment, followed by the full-length transcripts. The induction increased with time during the first 8 h, then fell to initial transcript levels at 24 h. This may have caused the lower percentage of GFP+ cells at 48 h. Together these results show that disulfiram increases HIV-1 gene expression in latently infected cells in this primary cell model.
In this system, peak GFP expression occurred 24 h postactivation, with the number of GFP+ cells approaching 50% of that seen with anti-CD3 plus anti-CD28 treatment (Fig. 2B). Although disulfiram cannot reactivate the entire latent reservoir in a single treatment, higher efficiency can be achieved by multiple rounds of treatment. After treating the latently infected cells with 0.5 μM disulfiram, we sorted GFP− cells and treated them again with 0.5 μM disulfiram, and we observed GFP+ cells at a level that was 35% of similarly treated cultures activated with anti-CD3 plus anti-CD28 (data not shown).
In vivo, disulfiram is rapidly converted to diethyldithiocarbamic acid (DDTC) (Fig. 1B) (8). In our system, DDTC also reactivates latent HIV-1 (Fig. 2E; see also Fig. S1 in the supplemental material). However, the next metabolite, diethyldithiocarbamate methyl ester (DDTC-Me), does not reactive latent HIV-1, suggesting that the free thiol is critical for reactivation. In plasma, disulfiram is ∼95% protein bound (8). To assess the effects of protein binding, reactivation experiments were carried out in 50% human serum. The dose-response curves for reactivation of latent HIV-1 shifted to the right by ∼10-fold (data not shown). Neither disulfiram nor DDTC caused significant toxicity below 10 μM (Fig. 2F).
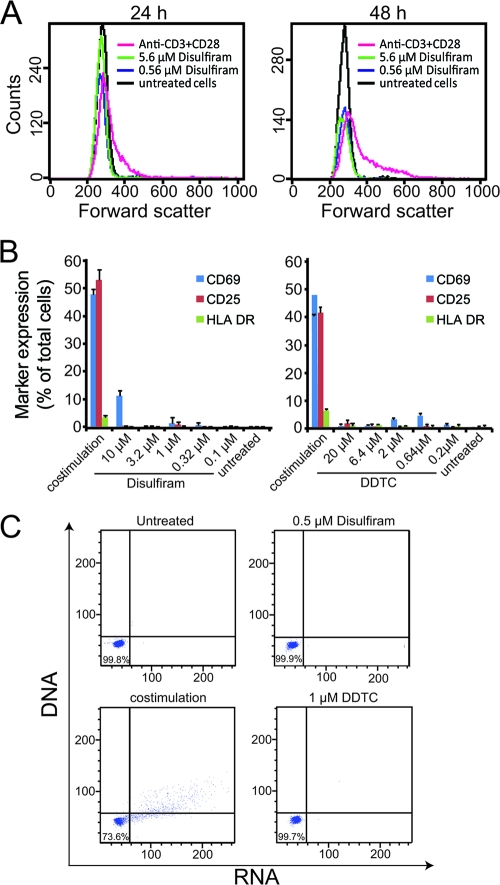
Since agents that induce latent HIV-1 through global T cell activation are toxic, we confirmed that disulfiram does not cause global T cell activation. Increased cell size is a direct indicator of T cell activation. Unlike cells stimulated with anti-CD3 plus anti-CD28 antibodies, latently infected cells treated with disulfiram retained the same small size as untreated cells (Fig. 3A). By staining for well-characterized activation markers, we confirmed that neither disulfiram nor DDTC induced T cell activation. Freshly isolated primary resting CD4+ T cells were treated with different concentrations of disulfiram, DDTC, or anti-CD3 plus anti-CD28 for 24 h and then stained for surface expression of CD69, CD25, and HLA-DR. Neither disulfiram nor DDTC upregulated surface expression of these markers, except for a minor induction of CD69 (Fig. 3B). In addition, no change was observed in surface expression of CCR7 (data not shown). Neither disulfiram nor DDTC induced changes in global DNA or RNA levels as measured by Hoechst 33342/pyronin Y staining, indicating that disulfiram treatment does not stimulate proliferation in primary resting CD4+ T cells (Fig. 3C).
Fig. 3.
Disulfiram does not cause CD4+ T cell activation. (A) Effects of disulfiram and anti-CD3 plus anti-CD28 treatment on the sizes of resting CD4+ T cells. Cell size was measured by flow cytometry based on forward scatter at 24 h and 48 h. (B) Effects of disulfiram and DDTC on the expression of activation markers in primary resting CD4+ T cells, compared to the effect of anti-CD3 plus anti-CD28 costimulation. Data are means plus standard deviations of triplicate samples from 1 of 2 independent experiments. (C) Effects of disulfiram and DDTC on global DNA and RNA levels in primary resting CD4+ T cells, compared to the effect of anti-CD3 plus anti-CD28 costimulation. Hoechst 33342 and pyronin Y were used to stain DNA and RNA, respectively, and results were read using a flow cytometer.
Clinical toxicity caused by global T cell activation is most likely due to cytokine release. Therefore, we determined whether disulfiram induces production of cytokines by using real-time RT-PCR and cytokine arrays. Freshly isolated primary resting CD4+ T cells were treated for 24 h with disulfiram, DDTC, or anti-CD3 plus anti-CD28 antibodies or were left untreated. Total RNA was isolated and reverse transcribed as described above. Gamma interferon (IFN-γ), interleukin-2 (IL-2), and tumor necrosis factor alpha (TNF-α) transcripts were measured in TaqMan gene expression assays. Results were normalized to 18S rRNA. None of the three cyto-kines were induced by disulfiram or DDTC (Table 1). In addition, cytokine secretion by primary resting CD4+ T cells treated with medium alone, disulfiram, or DDTC or costimulated for 48 h was measured using the Meso Scale human TH1/TH2 10-plex cytokine array. Neither disulfiram nor DDTC induced significant cytokine secretion compared to costimulation (Table 1).
Table 1.
Cytokine profiles of in vitro-stimulated primary human resting CD4+ T cells
| Cytokine | Mean (± SD) secreted cytokine concn (pg/ml)a |
mRNA fold inductionb |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Medium alone | DSF, 0.5 μM | DSF, 5 μM | DDTC, 1 μM | DDTC, 10 μM | Anti-CD3 + anti-CD28 | DSF, 0.5 μM | DDTC, 1 μM | Anti-CD3 + anti-CD28 | |
| TNF-α | 0.0 | 0.8 ± 0.0 | 1.4 ± 0.1 | 0.8 ± 0.2 | 1.5 ± 0.1 | 12,814.7 ± 300.9 | 0.7 ± 0.3 | 1.1 ± 0.2 | 148.0 ± 3.5 |
| IFN-γ | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 15,765.4 ± 839.2 | 0.8 ± 0.2 | 0.6 ± 0.3 | 67,400 ± 2.7 |
| IL-2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 13,063.5 ± 1.1 | 0.2 ± 0.03 | 0.1 ± 0.03 | 63.4 ± 1.8 |
| IL-1β | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 13.4 ± 5.6 | NA | NA | NA |
| IL-4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | NA | NA | NA |
| IL-5 | 0.0 | 0.3 ± 0.4 | 0.5 ± 0.1 | 0.2 ± 0.2 | 0.1 ± 0.2 | 186.9 ± 1.7 | NA | NA | NA |
| IL-8 | 33.9 ± 0.5 | 41.9 ± 4.3 | 54.5 ± 4.2 | 44.2 ± 0.1 | 52.8 ± 0.5 | 934.8 ± 67.3 | NA | NA | NA |
| IL-10 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2,072.4 ± 144.4 | NA | NA | NA |
| IL-12p70 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | NA | NA | NA |
| IL-13 | 0.0 | 0.0 | 2.4 ± 1.3 | 0.0 | 0.0 | 357.7 ± 15.1 | NA | NA | NA |
Resting CD4+ T cells from healthy donors were stimulated for 48 h with the indicated stimulus. Data are the means of duplicate samples from 1 of 3 independent experiments, all of which produced similar results. A 72-h treatment produced similar results.
Resting CD4+ T cells from healthy donors were stimulated for 24 h with the indicated stimulus. Induction is shown relative to unstimulated samples. Data are means of triplicate samples from 1 of 2 independent experiments that produced similar results. NA, not applicable.
To gain insight into the potential clinical efficacy of disulfiram, we measured virus release by primary resting CD4+ T cells from patients on suppressive HAART following disulfiram stimulation. Levels of HIV-1 RNA in the supernatants of disulfiram-treated cells were low compared to those seen with costimulation (see Table S1 in the supplemental material). In addition, a previously described virus culture assay (1) was used to determine whether disulfiram induced release of replication-competent virus from CD4+ T cells of patients on suppressive HAART. Disulfiram-induced virus production was not detected (data not shown). These in vitro results could reflect additional mechanisms that enforce latency in vivo but which were not captured in our primary cell model. Alternatively, disulfiram may upregulate virus gene expression in patient cells, but restrictions on virus assembly and/or budding may interfere with detection of this reactivation. Disulfiram-treated resting CD4+ T cells remain quiescent and are thus fundamentally different than cells activated by costimulation. The limitations inherent in in vitro assays with patient cells suggest that in vivo studies in macaque models or in patients will be required to determine the efficacy of latency-reversing drugs like disulfiram.
In conclusion, disulfiram can induce HIV-1 gene expression in latently infected cells in a primary cell model without causing a substantial degree of T cell activation. This work expands the number of latency-reversing agents and illustrates the possibility of less-toxic approaches to reversing latency. Unlike agents that cause global T cell activation, disulfiram is a relatively nontoxic drug that has been taken daily by some patients for decades. Although it is not yet clear that disulfiram can reactivate latent HIV-1 in vivo, it is possible that this drug, either alone or in combination with other agents, may be useful in future eradication strategies.
Supplementary Material
Acknowledgments
We thank Shunyou Long and the Johns Hopkins University High-Throughput Center for helping us with the screening. We thank Jun O. Liu for provision of the Johns Hopkins Drug Library for the screening. We also thank Nancy Archin for outgrowth assays using patients' cells.
This work was supported by NIH grants AI43222 and DA030156, by the Division of Intramural Research, NIAID, NIH, and by an ARCHE grant from the American Foundation for AIDS Research.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 6 April 2011.
REFERENCES
- 1. Archin N. A., et al. 2008. Standard ART and valproic acid have limited impact on the persistence of HIV infection in resting CD4+ T cells. AIDS 22:1131–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brooks D. G., et al. 2003. Molecular characterization, reactivation, and depletion of latent HIV. Immunity 19:413–423 [DOI] [PubMed] [Google Scholar]
- 3. Chun T. W., et al. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387:183–188 [DOI] [PubMed] [Google Scholar]
- 4. Chun T. W., et al. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. U. S. A. 94:13193–13197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Contreras X., et al. 2009. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J. Biol. Chem. 284:6782–6789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Faiman M. D., Jensen J. C., Lacoursiere R. B. 1984. Elimination kinetics of disulfiram in alcoholics after single and repeated doses. Clin. Pharmacol. Ther. 36:520–526 [DOI] [PubMed] [Google Scholar]
- 7. Finzi D., et al. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295–1300 [DOI] [PubMed] [Google Scholar]
- 8. Johansson B. 1992. A review of the pharmacokinetics and pharmacodynamics of disulfiram and its metabolites. Acta Psychiatr. Scand. Suppl. 369:15–26 [DOI] [PubMed] [Google Scholar]
- 9. Reference deleted.
- 10. McCance-Katz E. F., Kosten T. R., Jatlow P. 1998. Disulfiram effects on acute cocaine administration. Drug Alcohol Depend. 52:27–39 [DOI] [PubMed] [Google Scholar]
- 11. Sauna Z. E., Shukla S., Ambudkar S. V. 2005. Disulfiram, an old drug with new potential therapeutic uses for human cancers and fungal infections. Mol. BioSyst. 1:127–134 [DOI] [PubMed] [Google Scholar]
- 12. Siliciano J. D., et al. 2003. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat. Med. 9:727–728 [DOI] [PubMed] [Google Scholar]
- 13. Towell J. F., III, Cho J. K., Roh B. L., Wang R. I. 1983. Disulfiram and erythrocyte aldehyde dehydrogenase inhibition. Clin. Pharmacol. Ther. 33:517–521 [DOI] [PubMed] [Google Scholar]
- 14. Trono D., et al. 2010. HIV persistence and the prospect of long-term drug-free remissions for HIV-infected individuals. Science 329:174–180 [DOI] [PubMed] [Google Scholar]
- 15. Vaccari A., Saba P. L., Ruiu S., Collu M., Devoto P. 1996. Disulfiram and diethyldithiocarbamate intoxication affects the storage and release of striatal dopamine. Toxicol. Appl. Pharmacol. 139:102–108 [DOI] [PubMed] [Google Scholar]
- 16. Wong J. K., et al. 1997. Recovery of replication competent HIV despite prolonged suppression of plasma viremia. Science 278:1291–1295 [DOI] [PubMed] [Google Scholar]
- 17. Yang H. C., et al. 2009. Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation. J. Clin. Invest. 19:3473–3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.