Abstract
Subtilase cytotoxin (SubAB) that selectively cleaves BiP/GRP78 triggers the unfolded protein response (UPR) and protects mice from endotoxic lethality and collagen arthritis. We found that pretreatment of cells with SubAB suppressed tumor necrosis alpha (TNF-α)-induced activation of NF-κB and NF-κB-dependent chemokine expression. To elucidate underlying mechanisms, the involvement of C/EBP and Akt, putative regulators of NF-κB, was investigated. Among members of the C/EBP family, SubAB preferentially induced C/EBPβ. Overexpression of C/EBPβ suppressed TNF-α-induced NF-κB activation, and knockdown of C/EBPβ attenuated the suppressive effect of SubAB on NF-κB. We identified that the ATF6 branch of the UPR plays a crucial role in the induction of C/EBPβ. In addition to this effect, SubAB depressed basal and TNF-α-induced phosphorylation of Akt via the UPR. It was mediated by the induction of ATF6 and consequent activation of mTOR that dephosphorylated Akt. Inhibition of Akt attenuated activation of NF-κB by TNF-α, suggesting that the mTOR-Akt pathway is another target for SubAB-initiated, UPR-mediated NF-κB suppression. These results elucidated that SubAB blunts activation of NF-κB through ATF6-dependent mechanisms, i.e., preferential induction of C/EBPβ and mTOR-dependent dephosphorylation of Akt.
INTRODUCTION
We previously reported that subtilase cytotoxin (SubAB), the prototype of a distinct AB5 cytotoxin family, is a serine protease that selectively cleaves endoplasmic reticulum (ER) chaperone BiP/78-kDa glucose-regulated protein (GRP78) (40). In mice, preadministration with SubAB triggers the unfolded protein response (UPR) and protects mice from endotoxic lethality and collagen arthritis (13). It is associated with suppression of nuclear factor κB (NF-κB) and NF-κB-dependent gene expression (13). These results raise a possibility that pre-exposure of cells to SubAB suppresses subsequent responses to inflammatory stimuli. Currently, however, mechanisms underlying the anti-inflammatory potential of SubAB are largely unknown.
SubAB causes the degradation of BiP/GRP78 and the consequent activation of three major branches of the UPR. These include pathways mediated by RNA-dependent protein kinase-like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring ER-to-nucleus signal kinase 1 (IRE1) (51). Activation of PERK leads to phosphorylation of eukaryotic translation initiation factor 2α (eIF2α), which triggers general inhibition of translation. In response to ER stress, p90ATF6 transits to the Golgi complex, where it is cleaved by the proteases S1P and S2P, yielding an active transcription factor, p50ATF6. Similarly, activated IRE1 catalyzes removal of a small intron from the mRNA of X-box-binding protein 1 (XBP1). This splicing event creates a translational frameshift in XBP1 mRNA to produce an active transcription factor. Active p50ATF6 and XBP1 subsequently bind to the ER stress response element (ERSE) and the UPR element (UPRE), leading to expression of target genes, including ER chaperones and genes involved in ER-associated degradation (46). Several previous reports suggested that the IRE1/tumor necrosis factor (TNF) receptor-associated factor 2 (TRAF2) pathway and the PERK-eIF2α pathway cause activation of NF-κB and thereby contribute to development of inflammation (59). In contrast, information is very limited regarding negative regulation of NF-κB by the UPR.
CCAAT/enhancer-binding protein (C/EBP) is a family of transcription factors that contain a highly conserved, basic-leucine zipper domain at the C terminus. It is required for dimerization and DNA binding (44). In general, C/EBP plays important roles in the control of cell proliferation, differentiation, and metabolism (44), and some C/EBP may be induced under stress conditions (28). A previous report indicated that C/EBP interfered with phosphorylation of the p65 NF-κB subunit and thereby inhibited NF-κB-mediated transcription in TNF-tolerant monocytes (60). ER stress caused by SubAB could induce C/EBPs and thereby suppress activation of NF-κB and NF-κB-dependent gene expression.
The phosphatidylinositol 3-kinase (PI3K)-Akt pathway regulates activity of NF-κB positively or negatively, depending on cellular contexts (15). A previous report indicated cross talk among GRP78, Akt, and NF-κB in prostate cancer cells (33). Ozes et al. reported that kinase-dead Akt inhibited TNF-α-induced NF-κB activation in embryonic kidney cells (37). Recently, we found that SubAB caused modest, transient activation of NF-κB in the early phase. In this acute phase response, phosphorylation of Akt is an event upstream of NF-κB activation (55).
Activated Akt phosphorylates particular target proteins, including mammalian target of rapamycin (mTOR). mTOR is an evolutionarily conserved serine/threonine kinase that regulates various cell functions, cell cycle progression and cell proliferation (10). mTOR is involved in distinct complexes mTORC1 and mTORC2 that exert different signaling functions. mTORC1 phosphorylated by Akt forms a negative feedback loop that limits Akt activation (38, 50). In contrast, mTORC2 induces activation of Akt. The mTORC1-mediated suppression of Akt is sensitive to rapamycin (mTORC1 inhibitor), but mTORC2-mediated Akt activation is not affected by this drug (8). Based on this current knowledge, it is probable that transient phosphorylation of Akt by SubAB may activate mTORC1 in the early phase and consequently inhibit phosphorylation of Akt in the later phase.
In the present report, we investigate molecular mechanisms underlying the suppressive effect of SubAB on NF-κB, especially focusing on C/EBP, Akt, and mTOR. We also examine UPR branches responsible for the regulation of these molecules under the SubAB-triggered ER stress condition. Our current results demonstrate that selective abrogation of BiP/GRP78 blunts activation of NF-κB through ATF6-dependent mechanisms, i.e., preferential induction of C/EBPβ and mTOR-dependent dephosphorylation of Akt.
MATERIALS AND METHODS
Reagents.
SubAB was purified by Ni-nitrilotriacetic acid (NTA) chromatography from recombinant Escherichia coli, as we described previously (40, 41). A subtoxic concentration of 10 ng/ml was generally used for studies. Cleavage of BiP/GRP78 by SubAB was confirmed by Western blot analysis (55). Tunicamycin, thapsigargin, A23187, 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF), rapamycin, and SP600125 were purchased from Sigma-Aldrich Japan (Tokyo, Japan). Human recombinant TNF-α was obtained from R&D Systems (Minneapolis, MN), Akti-1/2, 4-phenylbutyrate (4-PBA), tauroursodeoxycholic acid (TUDCA), SB203580, and PD98059 were from Calbiochem (San Diego, CA), and salubrinal was from Tocris Bioscience (Ellisville, MO).
Cells.
The rat renal tubular epithelial cell line NRK-52E was purchased from American Type Culture Collection (Manassas, VA). SM43 rat mesangial cells were established as described previously (23). Murine podocytes were kindly provided by Karlhans Endlich (University of Heidelberg, Heidelberg, Germany) and cultured as described previously (54). Wild-type and iATF6 mouse embryonic fibroblasts (MEF) that stably express ATF6α small interfering RNA (siRNA) were provided by Laurie Glimcher (Harvard Medical School, Boston, MA) (26), and PERK−/− MEF were provided by David Ron (New York University School of Medicine, New York, NY). All cells were maintained in Dulbecco's modified Eagle's medium/Ham's F-12 (DMEM/F-12) (Gibco-BRL, Gaithersburg, MD) supplemented with 5% fetal bovine serum (FBS). Experiments were performed in the presence of 1% FBS.
Establishment of stable transfectants.
Using electroporation, NRK-52E cells were stably transfected with pNFκB-Luc (Panomics, Fremont, CA), which introduces a luciferase gene under the control of the κB site, and NRK/NFκB-Luc cells were established. NRK/siC/EBPβ cells were established by stable transfection of NRK-52E cells with pRNA-U6.1-siC/EBPβ, which introduces siRNA-targeting rat C/EBPβ. Mock-transfected control cells (NRK/siControl) were also established by stable transfection with pRNA-U6.1-Neo (GenScript, Piscataway, NJ). NRK/ATF6-DN cells stably expressing a dominant-negative (DN) mutant of ATF6 (ATF6-DN) were established by transfection of NRK-52E cells with pcDNA3.1-ATF6α(171-373)ΔAD (provided by Kazutoshi Mori, Kyoto University, Kyoto, Japan) (58). NRK/HA-TRAF2 cells were established by transfection with pcDNA3-HA-TRAF2 (provided by Antonio Leonardi, University of Naples, Naples, Italy) (27). NRK/ORP150 cells that constitutively express 150-kDa oxygen-regulated protein (ORP150) were established by using pCIneo-ORP150 (provided by Satoshi Ogawa, Kanazawa University, Kanazawa, Japan) (36). Mock-transfected NRK/Neo cells were also established by transfection with pcDNA3.1 (Invitrogen, Carlsbad, CA).
Transient transfection.
Using electroporation, NRK-52E cells were transiently cotransfected with pNFκB-Luc together with pcDNA3.1, pCMV-C/EBPβ (provided by Ez-Zoubir Amri, CNRS, Nice, France) (2), pcDNA-LAP, or pcDNA-LIP (provided by Jacob Friedman, University of Colorado, Denver, CO) (48) at a 1:4 ratio. After 48 h, cells were pretreated with or without SubAB for 24 h, exposed to TNF-α for 6 h, and subjected to a luciferase assay, as described later. NRK-52E cells were also transiently cotransfected with pC/EBP-Luc (provided by Yoshihiko Nishio, Shiga University of Medical Science, Shiga, Japan) (24) together with pcDNA3.1, pCAG-hIRE1α-K599A (provided by Masayuki Miura, University of Tokyo, Tokyo, Japan) encoding IRE1-DN (20), pcDNA3.1-dnXBP1 (provided by Laurie H. Glimcher) encoding XBP1-DN (25), pcDNA3-hPERK-K621M (provided by Ronald C. Wek, Indiana University School of Medicine, Indianapolis, IN) encoding PERK-DN (9), pcDNA3.CD2-heIF2α-S51A (provided by David Ron) encoding eIF2α-DN, or pcDNA3.1-ATF6α(171-373)ΔAD at a 1:3 ratio. pC/EBP-Luc contains 3 tandem copies of the consensus sequence of the C/EBP binding site upstream of a luciferase gene (24). After 48 h, cells were treated with or without SubAB for 12 to 24 h and subjected to a luciferase assay. NRK-52E cells were also transiently transfected with pcDNA3-myrHA-Akt1 encoding constitutively active Akt (Akt-CA) (provided by Kenneth Walsh, Boston University School of Medicine, Boston, MA) (12), treated with SubAB, and subjected to Western blot analysis. In some experiments, NRK/NFκB-Luc cells were transiently cotransfected with pCMV4 or pCMV4-p65 (Addgene, Cambridge, MA) encoding the p65 NF-κB subunit together with pcDNA3.1 or pCMV-C/EBPβ. After 48 h, cells were subjected to a luciferase assay. To evaluate activation of individual UPR pathways, cells were also transfected with pUPRE-Luc (provided by Laurie H. Glimcher) (26), pERSE-Luc (provided by Laurie H. Glimcher) (26), pCHOP-Luc (provided by Pierre Fafournoux, INRA de Theix, Saint Genes Champanelle, France) (4), or pCAX-F-XBP1ΔDBD-Luc (provided by Takao Iwawaki, RIKEN, Wako, Japan) (19) and subjected to a luciferase assay. pCHOP-Luc introduces a luciferase gene under the control of the CHOP gene promoter [originally designated pCHOP(−954 − +91)-Luc] (4).
Using GeneJuice (Novagen, Madison, WI), NRK-52E cells were transfected with tuberous sclerosis complex 2 (TSC2) siRNA (sense, 5′-GGCCCUCACAGACAAUGGA-3′; antisense, 3′-CCGGGAGUGUCUGUUACCU-5′) (Takara, Shiga, Japan) and subjected to Western blot analysis. A control siRNA (sense, 5′-GCUGCAAUCGAUUGAUAGC-3′; antisense, 3′-CGACGUUAGCUAACUAUCG-5′) was used for control experiments. NRK-52E cells were also transiently cotransfected with pNFκB-Luc together with TSC2 siRNA or control siRNA, treated with or without TNF-α, and subjected to a luciferase assay.
Luciferase assay.
The activity of luciferase was evaluated by the luciferase assay system (Promega, Madison, WI) according to the manufacturer's protocol (16).
Northern blot analysis.
Total RNA was extracted by a single-step method, and Northern blot analysis was performed as described previously (22). cDNAs for C/EBPα, C/EBPβ, C/EBPδ (provided by Ez-Zoubir Amri) (2), firefly luciferase, monocyte chemoattractant protein 1 (MCP-1) (45), ORP150 (provided by Satoshi Ogawa) (36), and BiP/GRP78 (provided by Kazunori Imaizumi, University of Miyazaki, Miyazaki, Japan) (21) were used to prepare radiolabeled probes. Expression of the glyceraldehyde-3-phosphate dehydrogenase gene (GAPDH) was used as a loading control. Densitometric analysis was performed using Scion Image (Scion Corporation; Frederick, MO).
Western blot analysis.
Western blot analysis was performed by the enhanced chemiluminescence system (Amersham Biosciences, Buckinghamshire, United Kingdom), as described previously (57). Levels of phosphorylated Akt and total Akt protein were evaluated using the PhosphoPlus Akt (Ser473) antibody kit (Cell Signaling Technology, Beverly, MA). Other antibodies used were anti-C/EBPβ, anti-eIF2α, anti-IκBα, and anti-TRAF2 from Santa Cruz Biotechnology (Santa Cruz, CA), antihemagglutinin (anti-HA) and anti-β-actin from Sigma-Aldrich Japan, anti-mTOR, anti-phospho-mTOR (Ser2448), anti-phospho-p70S6K (Thr389), anti-TSC2, and anti-phospho-eIF2α (Ser51) from Cell Signaling Technology, and anti-KDEL from Stressgen (Victoria, Canada).
Formazan assay.
The number of viable cells was assessed by a formazan assay using Cell Counting Kit-8 (Dojindo Laboratory; Kumamoto, Japan) (54).
Statistical analysis.
In reporter assays and the formazan assay, experiments were performed in quadruplicate, and data were expressed as means ± the standard error (SE). Statistical analysis was performed using the nonparametric Mann-Whitney U test to compare data in different groups. A P value of <0.05 was considered an indication of a statistically significant difference.
RESULTS
Suppression of TNF-α-triggered NF-κB activation by SubAB.
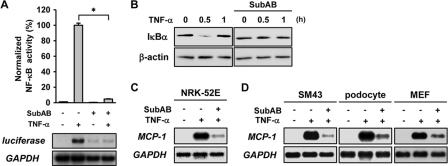
We previously reported that in mice, preadministration with SubAB triggered the UPR and protected mice from endotoxic lethality and collagen arthritis. It was associated with suppression of NF-κB and NF-κB-dependent gene expression (13). This result raised a possibility that pre-exposure of cells to SubAB suppresses subsequent responses to inflammatory stimuli. To examine this possibility, NRK/NFκB-Luc cells were pretreated with or without SubAB for 24 h, exposed to TNF-α for 6 h, and subjected to a luciferase assay to evaluate the activity of NF-κB. As shown in Fig. 1A (top), TNF-α induced activation of NF-κB, and it was abrogated by the pretreatment with SubAB. This inhibitory effect was not due to ER stress-initiated translational suppression, because induction of luciferase mRNA was also suppressed by the pretreatment with SubAB (Fig. 1A, bottom). Consistent with this result, degradation of IκBα, another marker of NF-κB activation, was detected following the treatment with TNF-α, whereas the degradation was not observed with SubAB-pre-exposed cells (Fig. 1B). The suppression of NF-κB was also confirmed by blunted induction of a NF-κB-dependent gene MCP-1 in SubAB-pretreated cells (Fig. 1C). This suppressive effect was not specific to NRK-52E cells, and similar results were also observed with glomerular mesangial cells (SM43), podocytes, and MEF (Fig. 1D).
Fig. 1.
Suppression of TNF-α-induced activation of NF-κB by SubAB. (A) NRK/NFκB-Luc cells were pretreated with or without 10 ng/ml SubAB for 24 h, exposed to 10 ng/ml TNF-α for 6 h, and subjected to a luciferase assay (top) and Northern blot analysis of luciferase mRNA (bottom). Activity of luciferase was normalized by the number of viable cells estimated by formazan assay, and relative values (%) are shown. Reporter assay and formazan assay were performed in quadruplicate. Data are presented as means ± SE. Statistical analysis was performed using the nonparametric Mann-Whitney U test to compare data in different groups. An asterisk indicates a statistically significant difference (P < 0.05). (B) NRK-52E cells were pretreated with or without SubAB, exposed to TNF-α for up to 1 h, and subjected to Western blot analysis of IκBα. The level of β-actin is shown at the bottom as a loading control. (C and D) NRK-52E cells, SM43 cells, podocytes, and MEF were pretreated with or without SubAB, exposed to TNF-α for 6 h, and subjected to Northern blot analysis of MCP-1. Expression of GAPDH is shown at the bottom as a loading control.
Suppression of NF-κB by SubAB through induction of C/EBPβ.
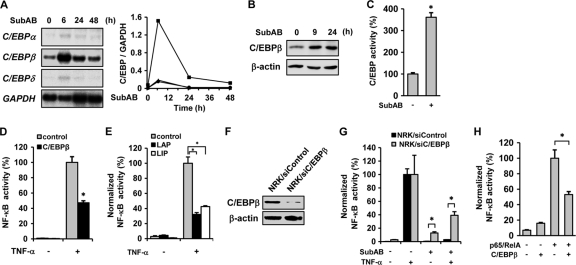
A previous report indicated that in TNF-tolerant monocytic cells, C/EBP blocked phosphorylation of p65 and thereby attenuated NF-κB-mediated transcription (60). Because some C/EBP may be induced under stress conditions, we tested whether SubAB has the potential to induce C/EBP family members. Northern blot analysis showed that SubAB upregulated expression of C/EBPβ without substantial induction of C/EBPα and C/EBPδ (Fig. 2A). The induction of C/EBPβ was correlated with an increase in the level of the C/EBPβ protein (Fig. 2B) and enhancement of the transacting potential of C/EBP (Fig. 2C).
Fig. 2.
Involvement of C/EBPβ in the suppression of NF-κB by SubAB. (A) NRK-52E cells were treated with SubAB for up to 48 h, and expression of C/EBPα, C/EBPβ, and C/EBPδ was examined by Northern blot analysis (left). Densitometric analysis of Northern blot data (C/EBP genes/GAPDH) is shown (right). •, C/EBPα; ▪, C/EBPβ; ▴, C/EBPδ. (B) Cells were treated with SubAB for up to 24 h and subjected to Western blot analysis of C/EBPβ. (C) Cells were transiently transfected with pC/EBP-Luc, treated with SubAB for 12 h, and subjected to luciferase assay to evaluate the transacting potential of C/EBP. (D and E) Cells were transiently cotransfected with empty vector (control), pCMV-C/EBPβ (D), pcDNA-LAP, or pcDNA-LIP (E) together with pNFκB-Luc, treated with or without TNF-α for 6 h, and subjected to luciferase assay. (F) Mock-transfected NRK/siControl cells and NRK/siC/EBPβ cells were subjected to Western blot analysis of C/EBPβ. (G) Control cells and NRK/siC/EBPβ cells were transiently transfected with pNFκB-Luc, pretreated with or without SubAB, exposed to TNF-α, and subjected to luciferase assay. The values were normalized by the number of viable cells. (H) NRK/NFκB-Luc cells were cotransfected with pCMV4 or pCMV4-p65 together with pcDNA3.1 or pCMV-C/EBPβ. After 48 h, cells were subjected to luciferase assay.
To examine involvement of C/EBPβ in the suppression of NF-κB by SubAB, NRK-52E cells were transiently cotransfected with pNFκB-Luc together with C/EBPβ and stimulated by TNF-α. As shown in Fig. 2D, overexpression of C/EBPβ significantly inhibited TNF-α-induced activation of NF-κB. There are two translation products from C/EBPβ mRNA, liver activating protein (LAP) and liver inhibitory protein (LIP), the latter of which functions as a dominant-negative inhibitor of C/EBPβ-mediated transcription (7). Interestingly, overexpression of LIP, as well as LAP, also suppressed TNF-α-induced activation of NF-κB (Fig. 2E), suggesting that the suppression of NF-κB by C/EBPβ is independent of its transacting potential. Of note, like LAP, LIP was also induced by SubAB (data available on request). To further confirm involvement of C/EBPβ, NRK/siC/EBPβ cells stably expressing siRNA for C/EBPβ were established. Western blot analysis showed substantial reduction in the level of the C/EBPβ protein in NRK/siC/EBPβ cells (Fig. 2F). Control cells and NRK/siC/EBPβ cells were then transfected with pNFκB-Luc, pretreated with or without SubAB, and exposed to TNF-α. The chemiluminescent assay revealed that, compared with the control, knockdown of C/EBPβ partially reversed the suppressive effect of SubAB on NF-κB (Fig. 2H). A reporter assay showed that activation of NF-κB by overexpression of p65/RelA was also suppressed significantly by C/EBPβ (Fig. 2H). This result indicates that C/EBPβ interferes with NF-κB signaling, at least in part, downstream of IκB degradation.
Induction of C/EBPβ by SubAB via the ATF6 branch of the UPR.
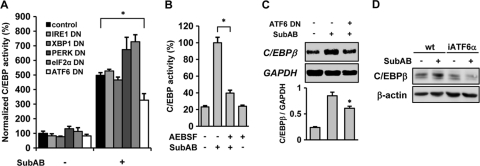
SubAB selectively cleaves BiP/GRP78, resulting in the induction of the UPR (51). We examined the involvement of individual branches of the UPR in the induction of C/EBPβ by SubAB. NRK-52E cells were transiently cotransfected with pC/EBP-Luc together with a dominant-negative (DN) mutant of IRE1α, XBP1, PERK, eIF2α, or ATF6, stimulated by SubAB, and subjected to a luciferase assay. Efficient suppression of the UPR by dominant-negative mutants was confirmed by reporter assays (data not shown). A chemiluminescent assay revealed that, among the dominant-negative mutants tested, only ATF6-DN significantly attenuated activation of C/EBP by SubAB. None of the other mutants, including IRE1-DN, XBP1-DN, PERK-DN, and eIF2α-DN suppressed SubAB-triggered induction of C/EBP (Fig. 3 A). Of note, downregulation of the PERK-eIF2α pathway by either PERK-DN or eIF2α-DN rather enhanced the activity of C/EBP. It is possibly caused by downregulation of CHOP, a C/EBP family member that is induced by the PERK-eIF2α pathway and inhibits the transacting potential of other C/EBPs (44). To confirm the crucial involvement of the ATF6 pathway, an ATF6 inhibitor AEBSF was used. AEBSF is an inhibitor of S1P and S2P and prevents ER stress-induced cleavage of p90ATF6 (34). The efficient blockade of ATF6 by AEBSF was confirmed by a reporter assay (data not shown). NRK-52E cells were transiently transfected with pC/EBP-Luc and treated with SubAB in the absence or presence of AEBSF. Consistent with the results shown in Fig. 3A, blockade of ATF6 decreased SubAB-triggered induction of C/EBP (Fig. 3B). To further confirm this result, we generated NRK/ATF6-DN cells stably expressing ATF6-DN. Mock-transfected cells and NRK/ATF6-DN cells were treated with SubAB, and induction of C/EBPβ was evaluated. Northern blot analysis revealed that induction of C/EBPβ was alleviated in NRK/ATF6-DN cells, compared with mock-transfected cells (Fig. 3C). Furthermore, in MEF stably transfected with ATF6 siRNA (iATF6α), induction of the C/EBPβ protein by SubAB was blunted (Fig. 3D), confirming involvement of the ATF6 pathway. In contrast, even if the eIF2α pathway was activated by salubrinal, an inhibitor of eIF2α dephosphorylation (3), expression of C/EBPβ was not induced. Consistently, salubrinal did not enhance the transacting potential of C/EBP (data not shown), confirming the lack of involvement of the PERK-eIF2α pathway. Taken together, these results suggest a crucial role for the ATF6 branch of the UPR in the induction of C/EBPβ by SubAB.
Fig. 3.
Roles of individual branches of the UPR in the induction of C/EBPβ by SubAB. (A) NRK-52E cells were transiently cotransfected with pC/EBP-Luc and empty vector (control), IRE1-DN, XBP1-DN, PERK-DN, eIF2α-DN, or ATF6-DN, treated with or without SubAB, and subjected to luciferase assay. The values were normalized by the number of viable cells. (B) Cells were transiently transfected with pC/EBP-Luc, treated with SubAB in the absence or presence of 200 μM AEBSF, and subjected to luciferase assay. (C) NRK/Neo cells (ATF6 DN [−]) and NRK/ATF6-DN cells (ATF6 DN [+]) were treated with SubAB for 6 h, and expression of C/EBPβ was examined by Northern blot analysis. Densitometric analysis of individual bands (C/EBPβ/GAPDH) is shown at the bottom (n = 4). (D) Wild-type (wt) MEF and iATF6α MEF were treated with SubAB and subjected to Western blot analysis of C/EBPβ.
Suppression of NF-κB by SubAB through UPR-mediated dephosphorylation of Akt.
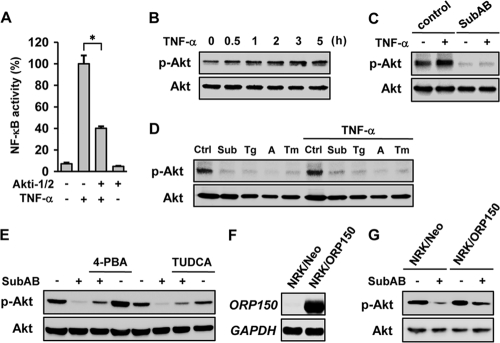
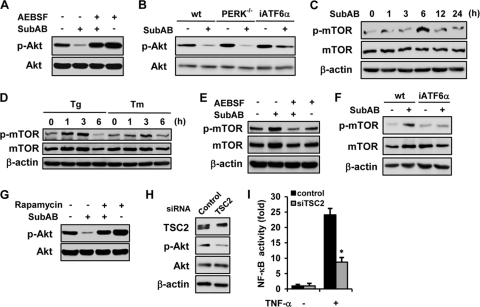
Although pretreatment with SubAB abrogated activation of NF-κB by TNF-α (Fig. 1A), overexpression of C/EBPβ only partially suppressed NF-κB activation (Fig. 2D). Similarly, knockdown of C/EBPβ reversed the suppressive effect of SubAB only partially (Fig. 2G). Furthermore, C/EBPβ interfered with the NF-κB signaling downstream of IκB degradation (Fig. 2H), whereas SubAB also inhibited degradation of IκBα (Fig. 1B). These results suggest that additional mechanisms should be involved in the suppression of NF-κB by SubAB. Previous reports suggested that the PI3K-Akt pathway has the potential to regulate NF-κB positively or negatively, depending on cellular contexts (15). We also demonstrated recently that, in the acute phase, Akt phosphorylation is an event upstream of NF-κB activation in SubAB-exposed cells (55). We therefore investigated whether Akt is another target for SubAB to downregulate NF-κB. For this purpose, NRK/NFκB-Luc cells were stimulated by TNF-α in the absence or presence of a selective inhibitor of Akt, Akti-1/2. Effective inhibition of Akt by Akti-1/2 was confirmed by Western blot analysis (data not shown). A reporter assay showed that activation of NF-κB by TNF-α was significantly suppressed by the treatment with Akti-1/2 (Fig. 4 A). Western blot analysis showed that NRK-52E cells have basal activity of Akt, and it was further enhanced by the treatment with TNF-α (Fig. 4B). When cells were exposed to SubAB for 24 h, both basal and inducible phosphorylation of Akt was suppressed (Fig. 4C). This suppressive effect was mimicked by the treatment with other UPR inducers, including thapsigargin, A23187, and tunicamycin (Fig. 4D).
Fig. 4.
Suppression of Akt and consequent inhibition of NF-κB activation by SubAB. (A) NRK/NFκB-Luc cells were treated with or without TNF-α in the absence or presence of 10 μM Akti-1/2 and subjected to luciferase assay. (B) NRK-52E cells were treated with TNF-α for up to 5 h and subjected to Western blot analysis of phosphorylated Akt (p-Akt) and total Akt protein (Akt). (C) Cells were pretreated with or without SubAB, exposed to TNF-α, and subjected to Western blot analysis. (D) Cells were treated with SubAB (Sub), thapsigargin (Tg; 500 nM), A23187 (A; 2 μM), or tunicamycin (Tm; 1 μg/ml) for 24 h, treated with or without TNF-α for 5 h, and subjected to Western blot analysis. Ctrl, untreated control. (E) Cells were treated with SubAB in the absence or presence of chemical chaperone 4-PBA (1 mM) or TUDCA (1 mM), and the level of phosphorylated Akt was evaluated. (F) Cells were stably transfected with ORP150. Mock transfected cells (NRK/Neo) and established transfectants (NRK/ORP150) were subjected to Northern blot analysis of ORP150. (G) NRK/Neo cells and NRK/ORP150 cells were treated with SubAB, and phosphorylated Akt was examined.
To confirm that the suppression of Akt by SubAB is, indeed, attributable to the UPR, NRK-52E cells were treated with SubAB in the presence of chemical chaperone 4-PBA or TUDCA, and the level of phosphorylated Akt was evaluated. Effective suppression of the UPR by these agents was confirmed by Western blot analysis and a reporter assay (data not shown). As shown in Fig. 4E, both chemical chaperones reversed the suppressive effect of SubAB on Akt phosphorylation (Fig. 4E). To further confirm the conclusion, cells were stably transfected with ORP150, an ER chaperone, and ER stress-resistant NRK/ORP150 cells were created. The established cells expressed ORP150 abundantly (Fig. 4F), which was correlated with blunted activation of the UPR in response to SubAB (our unpublished data). When the established cells were treated with SubAB, dephosphorylation of Akt was attenuated compared with mock-transfected cells (Fig. 4G). These results suggest that suppression of Akt by SubAB is through the induction of the UPR, which is in part responsible for the suppressive effect of SubAB on NF-κB.
Dephosphorylation of Akt by SubAB via ATF6-mediated activation of mTOR.
We reported recently that in the acute phase, SubAB causes transient activation of Akt through the ATF6 branch of the UPR (55). However, once activated, Akt may exert a negative feedback loop to dephosphorylate Akt. If so, the ATF6 pathway may also be involved in the suppression of Akt by SubAB in the late phase. To examine this possibility, NRK-52E cells were treated with or without SubAB in the absence or presence of AEBSF, and the level of phosphorylated Akt was evaluated. Western blot analysis revealed that suppression of the ATF6 pathway abrogated the SubAB-triggered dephosphorylation of Akt (Fig. 5 A). Consistent with this result, iATF6α MEF were resistant to the suppressive effect of SubAB on Akt (Fig. 5B, right), suggesting involvement of the ATF6 pathway. Of note, with PERK-null mutant MEF, suppression of Akt by SubAB was observed to be similar to that of wild-type MEF (Fig. 5B, middle).
Fig. 5.
Suppression of Akt phosphorylation by SubAB through the ATF6-Akt-mTOR pathway. (A) NRK-52E cells were treated with or without SubAB for 24 h in the absence or presence of AEBSF and subjected to Western blot analysis of phosphorylated Akt. (B) Wild-type MEF (wt), PERK-knockout MEF (PERK−/−), and iATF6α MEF were treated with or without SubAB and subjected to Western blot analysis. NRK-52E cells were treated with SubAB (C) or thapsigargin or tunicamycin (D) for indicated time periods and subjected to Western blot analysis of phosphorylated mTOR. Levels of total mTOR and β-actin were used as loading controls. (E) Cells were treated with or without SubAB for 3 h in the absence or presence of AEBSF and subjected to Western blot analysis. (F) Wild-type MEF and iATF6α MEF were treated with or without SubAB and subjected to Western blot analysis of phosphorylated mTOR. (G) NRK-52E cells were pretreated with or without 100 nM rapamycin for 30 min, exposed to SubAB for 24 h, and subjected to Western blot analysis of phosphorylated Akt. (H) Western blot analysis of TSC2 and phosphorylated Akt in cells transiently transfected with control siRNA or TSC2 siRNA. (I) Cells were transiently cotransfected with pNFκB-Luc together with control siRNA or TSC2 siRNA. After 48 h, cells were treated with or without TNF-α for 6 h and subjected to luciferase assay.
It is known that mTOR is located downstream of Akt and may form a negative feedback loop that limits Akt phosphorylation. Previous reports suggested that phosphorylation of mTORC1 by Akt in turn negatively regulated the PI3K-Akt pathway (38, 50). To examine involvement of mTOR in the suppression of Akt by SubAB, phosphorylation of mTOR was evaluated. Following exposure of NRK-52E cells to SubAB, transient phosphorylation of mTOR was observed (Fig. 5C). The phosphorylation of mTOR was also caused by other UPR inducers, including thapsigargin and tunicamycin (Fig. 5D). Consistent with the result shown in Fig. 5A, inhibition of ATF6 by AEBSF attenuated SubAB-induced phosphorylation of mTOR (Fig. 5E). Furthermore, treatment with SubAB caused phosphorylation of mTOR in wild-type MEF, whereas it was blunted in iATF6α MEF (Fig. 5F), confirming involvement of the ATF6 pathway in the regulation of Akt-mTOR signaling.
Function of mTORC1 is inhibited by rapamycin (8). Indeed, thapsigargin-induced activation of mTORC1 was abrogated by the treatment with rapamycin in NRK-52E cells (data not shown). We examined an effect of rapamycin on the suppression of Akt by SubAB. As shown in Fig. 5G, inhibition of mTOR by rapamycin abolished dephosphorylation of Akt by SubAB. On the other hand, activation of mTOR by knockdown of TSC2, an endogenous inhibitor of mTORC1, caused dephosphorylation of Akt (Fig. 5H), and it was correlated with blunted activation of NF-κB in response to TNF-α (Fig. 5I). These results suggest that SubAB dephosphorylates Akt via ATF6-dependent activation of mTOR.
Relationship between induction of C/EBPβ and suppression of Akt in response to SubAB.
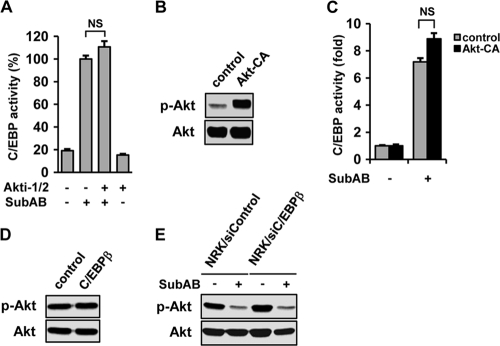
To examine the link between induction of C/EBPβ and suppression of Akt by SubAB, we first tested an effect of Akt on the induction of C/EBPβ. NRK-52E cells were transiently transfected with pC/EBP-Luc and treated with SubAB in the absence or presence of Akti-1/2. A reporter assay showed that inhibition of Akt did not affect induction of C/EBP by SubAB (Fig. 6 A). To confirm this result, NRK-52E cells were transiently cotransfected with pC/EBP-Luc and pcDNA3-myrHA-Akt1 encoding constitutively active Akt (Akt-CA). Western blot analysis confirmed substantial enhancement of phosphorylated Akt in Akt-CA-transfected cells (Fig. 6B). Mock-transfected cells and Akt-CA-transfected cells were then treated with SubAB and subjected to analysis. A chemiluminescent assay showed that enhanced phosphorylation of Akt did not attenuate induction of C/EBP by SubAB (Fig. 6C). These results suggest that downregulation of Akt is not responsible for the induction of C/EBP by SubAB.
Fig. 6.
Independent regulation of C/EBPβ and Akt by SubAB. (A) NRK-52E cells were transiently transfected with pC/EBP-Luc, treated with SubAB for 12 h in the absence or presence of Akti-1/2, and subjected to luciferase assay. NS, not statistically significant. (B) Cells were transfected with empty vector (control) or Akt-CA and subjected to Western blot analysis of Akt. (C) Cells were cotransfected with pC/EBP-Luc together with empty vector or Akt-CA, treated with or without SubAB, and subjected to luciferase assay. (D) Cells were transfected with empty vector or C/EBPβ. After 48 h, the level of Akt phosphorylation was evaluated. (E) Mock-transfected NRK/siControl cells and NRK/siC/EBPβ cells were treated with or without SubAB and subjected to Western blot analysis of phosphorylated Akt.
We next examined an effect of C/EBPβ on the suppression of Akt by SubAB. For this purpose, cells were transiently transfected with C/EBPβ and subjected to Western blot analysis of Akt. As shown in Fig. 6D, overexpression of C/EBPβ did not influence the basal level of Akt phosphorylation. Furthermore, downregulation of C/EBPβ by siRNA did not affect the suppressive effect of SubAB on Akt phosphorylation (Fig. 6E).
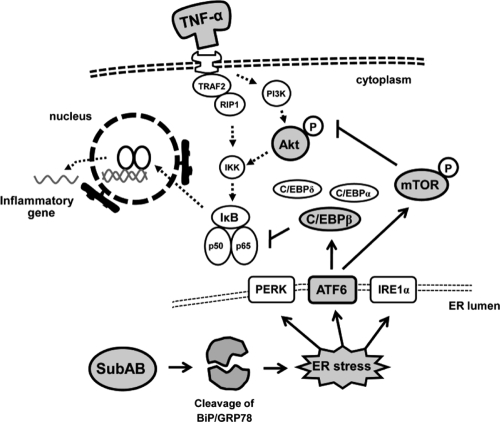
Taken together, these results suggest dual mechanisms underlying the suppressive effect of SubAB on NF-κB, i.e., preferential induction of C/EBPβ and mTOR-dependent dephosphorylation of Akt, both of which are regulated independently by SubAB (Fig. 7).
Fig. 7.
Putative mechanisms underlying suppression of NF-κB by SubAB. SubAB causes ER stress via selective cleavage of BiP/GRP78 and preferential induction of C/EBPβ through the ATF6 branch of the UPR. C/EBPβ physically interacts with NF-κB subunits, especially p65, and thereby inhibits the transacting potential of NF-κB downstream of IκB. SubAB also transiently induces phosphorylation of mTOR via the ATF6 pathway and thereby downregulates Akt phosphorylation, leading to blockade of the TNF-α-Akt-NF-κB pathway upstream of IκB.
DISCUSSION
Preadministration with SubAB protects mice from LPS-induced endotoxic lethality and experimental arthritis. It is associated with inhibition of NF-κB and blunted induction of NF-κB-dependent molecules, including MCP-1 and TNF-α (13). In the present study, we disclosed mechanisms underlying this anti-inflammatory potential of SubAB. Our results showed crucial roles of C/EBPβ upregulation and mTOR-dependent dephosphorylation of Akt, both of which are caused by SubAB via the ATF6 pathway.
In the present report, we first showed that C/EBPβ is preferentially upregulated by SubAB through the ATF6 branch of the UPR. A previous report indicated that the human C/EBPβ gene has a UPRE at the 3′ end and that it could be responsible for induction of C/EBPβ by ER stress (5). However, the UPRE is regulated mainly by the IRE1-XBP1 pathway. Because dominant-negative inhibition of IRE1 or XBP1 did not affect induction of C/EBP by SubAB, contribution of the IRE1-XBP1 pathway is unlikely. We previously reported that in the acute phase, SubAB transiently activates NF-κB through the ATF6 pathway (55), which could be involved in the induction of C/EBPβ. However, our preliminary results showed that none of the NF-κB inhibitors affected induction of C/EBPβ by SubAB (data not shown), excluding this possibility. Some previous reports indicated roles of mitogen-activated protein (MAP) kinases and its downstream target activator protein 1 (AP-1) in the induction of C/EBPβ (29, 39, 43). We found recently that in the early phase, SubAB transiently phosphorylated c-Jun N-terminal kinase (JNK), p38 MAP kinase, and extracellular signal-regulated kinase (ERK). The phosphorylation of MAP kinases was associated with activation of AP-1 (our unpublished data). The transient activation of the MAP kinase-AP-1 pathway might be responsible for the induction of C/EBPβ by SubAB. Indeed, our preliminary data showed that inhibition of the p38 MAP kinase by SB203580 or ERK by PD98059, but not inhibition of JNK by SP600125, modestly suppressed induction of C/EBPβ by SubAB (data not shown), implying this possibility. Currently, it is unclear whether and how the ATF6 pathway regulates the MAP kinase-AP-1 pathway. However, a recent report demonstrated that ATF6 induced expression of Rheb (Ras homolog enriched in brain) (47), a member of the Ras family. Rheb was originally identified as an immediate-early gene in the brain, but now, it is known that this molecule is expressed in various tissues (52). Rheb interacts with and activates Raf-1 kinase (56), leading to phosphorylation of MEK and ERK. This mechanism might underlie induction of C/EBPβ by the ATF6 pathway. Similarly, Rheb is known as a critical activator of mTOR (1). Rheb can bind directly to the mTOR kinase domain, leading to activation of mTORC1. Rheb might also be involved in the regulation of mTOR by ATF6.
Physical interaction occurs between the bZIP region of C/EBPβ and the Rel homology domain of the NF-κB subunit (49). The inhibitory effect of C/EBP on NF-κB activation may be independent of its transacting potential. Indeed, Prösch et al. reported that C/EBPα and -β interacted with p65 and suppressed NF-κB activation even in the absence of direct binding of p65-C/EBP complexes to the κB site (42). We found in this report that, like that of LAP, overexpression of LIP also suppressed TNF-α-induced activation of NF-κB, supporting the idea that the suppression of NF-κB by C/EBPβ is independent of its transacting potential.
We showed that SubAB suppressed basal and TNF-α-inducible phosphorylation of Akt. The similar effect was observed with other UPR inducers, and chemical and genetic chaperoning abrogated this effect, suggesting that ER stress is responsible for depression of Akt. In contrast, however, a previous report indicated that ER stress induced by glucosamine rather reinforced Akt phosphorylation in astroglia (32). We reported previously that in the early phase, SubAB, as well as tunicamycin and thapsigargin, caused activation of Akt in NRK-52E cells. The phosphorylation of Akt was, however, only transient, with a peak at 3 to 9 h (55). Consistent with this result, Hosoi et al. reported that, in glial cells, Akt phosphorylation was increased by short-term exposure to ER stress but was downregulated by long-term exposure to ER stress inducers (17). These results strongly suggest the potential of ER stress for biphasic, bidirectional regulation of Akt. In the early phase, SubAB triggers phosphorylation of Akt, whereas in the later phase, it depresses both basal and inducible Akt activity. The downregulation of NF-κB by SubAB demonstrated here is caused, at least in part, by suppression of Akt in the late phase.
We suggested in the present report that early activation of the Akt-mTORC1 pathway induced by the UPR in turn downregulates Akt in the late phase. It is supported by the following findings: (i) SubAB and other UPR inducers caused acute, transient activation of Akt and mTOR; (ii) suppression of Akt by SubAB was attenuated by treatment with chemical chaperones or overexpression of ER chaperone; (iii) inhibition of mTORC1 by rapamycin reversed the suppressive effect of SubAB on Akt; and (iv) activation of mTORC1 by siTSC2 mimicked the suppressive effect of SubAB on NF-κB. To our knowledge, this is the first demonstration that the UPR causes phosphorylation of mTOR and activation of mTORC1, leading to negative feedback on Akt phosphorylation. Of note, consistent with our findings, recent reports also suggested that the Akt pathway has the potential to phosphorylate mTORC1 (30) and that phosphorylation of mTORC1 by Akt may in turn negatively regulate the PI3K-Akt pathway (38). These results suggest that SubAB causes activation of mTOR through the ATF6-Akt pathway in the early phase, which in turn inhibits the Akt pathway in the later phase.
Recently, we reported that preceding ER stress caused the insensitivity of glomerular cells to TNF-α-induced activation of NF-κB via suppression of TRAF2, an essential upstream component in TNF-α signaling (14, 35). This repression was not at the transcriptional level but was caused by accelerated degradation of the TRAF2 protein, possibly via ER-associated degradation (18). In the present study, we also found that SubAB causes downregulation of the TRAF2 protein in NRK-52E cells (data available on request). The targets for SubAB to suppress NF-κB seem to be multiple: not only C/EBPβ and Akt, but also other molecules, including TRAF2.
In the present report, we disclosed that selective abrogation of BiP/GRP78 leads to severe impairment in NF-κB and Akt pathways. As is well known, NF-κB is a central coordinator for innate and adaptive immune responses, inflammation, and cancer development. Akt also coordinates embryonic development, adipocyte differentiation, glucose homeostasis, and tumorigenesis. Based on this current knowledge, BiP/GRP78 should play critical roles in the NF-κB- and Akt-related, diverse range of pathophysiologies. Indeed, Luo et al. reported that GRP78-null mutant mice were embryonically lethal. Cells from GRP78−/− embryos exhibited a defect in cell proliferation and massive apoptosis in the inner cell mass (31). It is possibly caused by impairment of protein quality control in the ER, but it might also be ascribed to the impaired function of NF-κB and Akt, the key regulators for cell survival and cell proliferation. Of note, some investigators reported that targeted knockout of BiP/GRP78 in the prostate epithelium suppressed Akt activation and tumorigenesis (11). The biological function of BiP/GRP78 seems to be not so simple as previously predicted. It may be one of the master regulators for various biological processes and could be a therapeutic target for immune abnormality, inflammation, metabolic disorders, and cancer development.
In the present repot, we demonstrated the anti-inflammatory aspect of SubAB to attenuate NF-κB. The ATF6 branch of the UPR and consequent induction of C/EBPβ and suppression of Akt underlie its anti-inflammatory potential. NF-κB serves as a crucial regulator for immune responses and is involved in the pathogenesis of a variety of inflammatory diseases as well as atherosclerosis, neurodegenerative disorders, and malignant diseases (53). ER stress and the consequent UPR are often observed under these pathological conditions. From this point of view, the UPR may function as a self-defense mechanism against NF-κB-related pathologies. Like inhibitors of Akt (6), selective manipulation of the ATF6 pathway or downstream C/EBPβ might open a new window toward efficient treatment of NF-κB-related disorders. Elucidation of the therapeutic utility of SubAB and other selective UPR inducers will be our next lines of investigation.
ACKNOWLEDGMENTS
We thank Masayuki Miura (University of Tokyo), Ronald C. Wek (Indiana University School of Medicine), Kazutoshi Mori (Kyoto University), Antonio Leonardi (University of Naples), Ez-Zoubir Amri (CNRS), Yoshihiko Nishio (Shiga University of Medical Science), Laurie H. Glimcher (Harvard Medical School), David Ron (New York University School of Medicine), Kenneth Walsh (Boston University School of Medicine), Satoshi Ogawa (Kanazawa University), Kazunori Imaizumi (University of Miyazaki), Pierre Fafournoux (INRA de Theix), Jacob Friedman (University of Colorado), and Takao Iwawaki (RIKEN) for providing us with expression plasmids.
This work was supported by Grant-in-Aids for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (no. 20390235) to M. Kitamura.
Footnotes
Published ahead of print on 7 February 2011.
REFERENCES
- 1. Avruch J., et al. 2009. Activation of mTORC1 in two steps: Rheb-GTP activation of catalytic function and increased binding of substrates to raptor. Biochem. Soc. Trans. 37:223–226 [DOI] [PubMed] [Google Scholar]
- 2. Bezy O., et al. 2005. Delta-interacting protein A, a new inhibitory partner of CCAAT/enhancer-binding protein β, implicated in adipocyte differentiation. J. Biol. Chem. 280:11432–11438 [DOI] [PubMed] [Google Scholar]
- 3. Boyce M., et al. 2005. A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science 307:935–939 [DOI] [PubMed] [Google Scholar]
- 4. Bruhat A., et al. 2000. Amino acids control mammalian gene transcription: activating transcription factor 2 is essential for the amino acid responsiveness of the CHOP promoter. Mol. Cell. Biol. 19:7192–7204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen C., Dudenhausen E. E., Pan Y. X., Zhong C., Kilberg M. S. 2004. Human CCAAT/enhancer-binding protein β gene expression is activated by endoplasmic reticulum stress through an unfolded protein response element downstream of the protein coding sequence. J. Biol. Chem. 279:27948–27956 [DOI] [PubMed] [Google Scholar]
- 6. Cheng G. Z., et al. 2008. Advances of AKT pathway in human oncogenesis and as a target for anti-cancer drug discovery. Curr. Cancer Drug Targets 8:2–6 [PubMed] [Google Scholar]
- 7. Descombes P., Schibler U. 1991. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell 67:569–579 [DOI] [PubMed] [Google Scholar]
- 8. Ehninger D., de Vries P. J., Silva A. J. 2009. From mTOR to cognition: molecular and cellular mechanisms of cognitive impairments in tuberous sclerosis. J. Intellect. Disabil. Res. 53:838–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fernandez J., Yaman I., Sarnow P., Snider M. D., Glou M. 2002. Regulation of internal ribosomal entry site-mediated translation by phosphorylation of the translation initiation factor eIF2α. J. Biol. Chem. 277:19198–19205 [DOI] [PubMed] [Google Scholar]
- 10. Fingar D. C., Blenis J. 2004. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 23:3151–3171 [DOI] [PubMed] [Google Scholar]
- 11. Fu Y., et al. 2008. Pten null prostate tumorigenesis and AKT activation are blocked by targeted knockout of ER chaperone GRP78/BiP in prostate epithelium. Proc. Natl. Acad. Sci. U. S. A. 105:19444–19449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fujio Y., et al. 1999. Cell cycle withdrawal promotes myogenic induction of Akt, a positive modulator of myocyte survival. Mol. Cell. Biol. 19:5073–5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harama D., et al. 2009. A sub-cytotoxic dose of subtilase cytotoxin prevents LPS-induced inflammatory responses, depending on its capacity to induce the unfolded protein response. J. Immunol. 183:1368–1374 [DOI] [PubMed] [Google Scholar]
- 14. Hayakawa K., et al. 2009. Acquisition of anergy to proinflammatory cytokines in nonimmune cells through endoplasmic reticulum stress response: a mechanism for subsidence of inflammation. J. Immunol. 182:1182–1191 [DOI] [PubMed] [Google Scholar]
- 15. Hazeki K., Nigorikawa K., Hazeki O. 2007. Role of phosphoinositide 3-kinase in innate immunity. Biol. Pharm. Bull. 30:1617–1623 [DOI] [PubMed] [Google Scholar]
- 16. Hiramatsu N., Kasai A., Hayakawa K., Yao J., Kitamura M. 2006. Real-time detection and continuous monitoring of ER stress in vitro and in vivo by ES-TRAP: evidence for systemic, transient ER stress during endotoxemia. Nucleic Acids Res. 34(13):e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hosoi T., Hyoda K., Okuma Y., Nomura Y., Ozawa K. 2007. Akt up- and down-regulation in response to endoplasmic reticulum stress. Brain Res. 1152:27–31 [DOI] [PubMed] [Google Scholar]
- 18. Hu P., Han Z., Couvillon A. D., Kaufman R. J., Exton J. H. 2006. Autocrine tumor necrosis factor α links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1α-mediated NF-κB activation and down-regulation of TRAF2 expression. Mol. Cell. Biol. 26:3071–3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iwawaki T., Akai R. 2006. Analysis of the XBP1 splicing mechanism using endoplasmic reticulum stress-indicators. Biochem. Biophys. Res. Commun. 350:709–715 [DOI] [PubMed] [Google Scholar]
- 20. Iwawaki T., Akai R., Kohno K., Miura M. 2004. A transgenic mouse model for monitoring endoplasmic reticulum stress. Nat. Med. 10:98–102 [DOI] [PubMed] [Google Scholar]
- 21. Katayama T., et al. 2001. Disturbed activation of endoplasmic reticulum stress transducers by familial Alzheimer's disease-linked presenilin-1 mutations. J. Biol. Chem. 276:43446–43454 [DOI] [PubMed] [Google Scholar]
- 22. Kitamura M. 1997. Identification of an inhibitor targeting macrophage production of monocyte chemoattractant protein-1 as TGF-β1. J. Immunol. 159:1404–1411 [PubMed] [Google Scholar]
- 23. Kitamura M., et al. 1994. Gene transfer into the rat renal glomerulus via a mesangial cell vector: site-specific delivery, in situ amplification, and sustained expression of an exogenous gene in vivo. J. Clin. Invest. 94:497–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kodama K., et al. 2005. Bidirectional regulation of monocyte chemoattractant protein-1 gene at distinct sites of its promoter by nitric oxide in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 289:C582–C590 [DOI] [PubMed] [Google Scholar]
- 25. Lee A. H., Iwakoshi N. N., Anderson K. C., Glimcher L. H. 2003. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc. Natl. Acad. Sci. U. S. A. 100:9946–9951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee A. H., Iwakoshi N. N., Glimcher L. H. 2003. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 23:7448–7459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leonardi A., Ellinger-Ziegelbauer H., Franzoso G., Brown K., Siebenlist U. 2000. Physical and functional interaction of filamin (actin-binding protein-280) and tumor necrosis factor receptor-associated factor 2. J. Biol. Chem. 275:271–278 [DOI] [PubMed] [Google Scholar]
- 28. Li Y., et al. 2008. Differential control of the CCAAT/enhancer-binding protein β (C/EBPβ) products liver-enriched transcriptional activating protein (LAP) and liver-enriched transcriptional inhibitory protein (LIP) and the regulation of gene expression during the response to endoplasmic reticulum stress. J. Biol. Chem. 283:22443–22456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin W. C., et al. 2002. Transcriptional activation of C/EBPβ gene by c-Jun and ATF2. DNA Cell Biol. 21:551–560 [DOI] [PubMed] [Google Scholar]
- 30. Liu F. Y., et al. 2010. Mammalian target of rapamycin (mTOR) is involved in the survival of cells mediated by chemokine receptor 7 through PI3K/Akt in metastatic squamous cell carcinoma of the head and neck. Br. J. Oral Maxillofac. Surg. 48:291–296 [DOI] [PubMed] [Google Scholar]
- 31. Luo S., Mao C., Lee B., Lee A. S. 2006. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol. Cell. Biol. 26:5688–5697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matthews J. A., Belof J. L., Acevedo-Duncan M., Potter R. L. 2007. Glucosamine-induced increase in Akt phosphorylation corresponds to increased endoplasmic reticulum stress in astroglial cells. Mol. Cell. Biochem. 298:109–123 [DOI] [PubMed] [Google Scholar]
- 33. Misra U. K., Deedwania R., Pizzo S. V. 2006. Activation and cross-talk between Akt, NF-κB, and unfolded protein response signaling in 1-LN prostate cancer cells consequent to ligation of cell surface-associated GRP78. J. Biol. Chem. 281:13694–13707 [DOI] [PubMed] [Google Scholar]
- 34. Okada T., et al. 2003. A serine protease inhibitor prevents endoplasmic reticulum stress-induced cleavage but not transport of the membrane-bound transcription factor ATF6. J. Biol. Chem. 278:31024–31032 [DOI] [PubMed] [Google Scholar]
- 35. Okamura M., et al. 2008. Suppression of cytokine responses by indomethacin in podocytes: a mechanism through induction of unfolded protein response. Am. J. Physiol. Renal Physiol. 295:F1495–F1503 [DOI] [PubMed] [Google Scholar]
- 36. Ozawa K., et al. 1999. 150-kDa oxygen-regulated protein suppresses hypoxia-induced apoptotic cell death. J. Biol. Chem. 274:6397–6404 [DOI] [PubMed] [Google Scholar]
- 37. Ozes O. N., et al. 1999. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 401:82–85 [DOI] [PubMed] [Google Scholar]
- 38. Park S., et al. 2009. RIP1 activates PI3K-Akt via a dual mechanism involving NF-κB-mediated inhibition of the mTOR-S6K-IRS1 negative feedback loop and down-regulation of PTEN. Cancer Res. 69:4107–4111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Patel D. N., et al. 2007. Interleukin-17 stimulates C-reactive protein expression in hepatocytes and smooth muscle cells via p38 MAPK and ERK1/2-dependent NF-κB and C/EBPβ activation. J. Biol. Chem. 282:27229–27238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Paton A. W., et al. 2006. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature 443:548–552 [DOI] [PubMed] [Google Scholar]
- 41. Paton A. W., Srimanote P., Talbot U. M., Wang H., Paton J. C. 2004. A new family of potent AB5 cytotoxins produced by Shiga toxigenic Escherichia coli. J. Exp. Med. 200:35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Prösch S., Heine A. K., Volk H. D., Krüger D. H. 2001. CCAAT/enhancer-binding proteins α and β negatively influence the capacity of tumor necrosis factor α to up-regulate the human cytomegalovirus IE1/2 enhancer/promoter by NF-κB during monocyte differentiation. J. Biol. Chem. 276:40712–40720 [DOI] [PubMed] [Google Scholar]
- 43. Qiao L., et al. 2003. Bile acid regulation of C/EBPβ, CREB, and c-Jun function, via the extracellular signal-regulated kinase and c-Jun NH2-terminal kinase pathways, modulates the apoptotic response of hepatocytes. Mol. Cell. Biol. 23:3052–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ramji D. P., Foka P. 2002. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem. J. 365:561–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rollins B. J., Morrison E. D., Stiles C. D. 1988. Cloning and expression of JE, a gene inducible by platelet-derived growth factor and whose product has cytokine-like properties. Proc. Natl. Acad. Sci. U. S. A. 85:3738–3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rutkowski D. T., Kaufman R. J. 2004. A trip to the ER: coping with stress. Trends Cell Biol. 14:20–28 [DOI] [PubMed] [Google Scholar]
- 47. Schewe D. M., Aguirre-Ghiso J. A. 2008. ATF6α-Rheb-mTOR signaling promotes survival of dormant tumor cells in vivo. Proc. Natl. Acad. Sci. U. S. A. 105:10519–10524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shao J., Qiao L., Janssen R. C., Pagliassotti M., Friedman J. E. 2005. Chronic hyperglycemia enhances PEPCK gene expression and hepatocellular glucose production via elevated liver activating protein/liver inhibitory protein ratio. Diabetes 54:976–984 [DOI] [PubMed] [Google Scholar]
- 49. Stein B., Cogswell P. C., Baldwin A. S., Jr 1993. Functional and physical associations between NF-κB and C/EBP family members: a Rel domain-bZIP interaction. Mol. Cell. Biol. 13:3964–3974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tremblay F., Marette A. 2001. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J. Biol. Chem. 276:38052–38060 [DOI] [PubMed] [Google Scholar]
- 51. Wolfson J. J., et al. 2008. Subtilase cytotoxin activates PERK, IRE1 and ATF6 endoplasmic reticulum stress-signalling pathways. Cell. Microbiol. 10:1775–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yamagata K., et al. 1994. Rheb, a growth factor- and synaptic activity-regulated gene, encodes a novel Ras-related protein. J. Biol. Chem. 269:16333–16369 [PubMed] [Google Scholar]
- 53. Yamamoto Y., Gaynor R. B. 2001. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J. Clin. Invest. 107:135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yamauchi K., et al. 2006. Screening and identification of substances that regulate nephrin gene expression using engineered reporter podocytes. Kidney Int. 70:892–900 [DOI] [PubMed] [Google Scholar]
- 55. Yamazaki H., et al. 2009. Activation of the Akt-NF-κB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J. Immunol. 183:1480–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yee W. M., Worley P. F. 1997. Rheb interacts with Raf-1 kinase and may function to integrate growth factor- and protein kinase A-dependent signals. Mol. Cell. Biol. 17:921–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yokouchi M., et al. 2008. Involvement of selective reactive oxygen species upstream of proapoptotic branches of unfolded protein response. J. Biol. Chem. 283:4252–4260 [DOI] [PubMed] [Google Scholar]
- 58. Yoshida H., et al. 2000. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol. Cell. Biol. 20:6755–6767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang K., Kaufman R. J. 2008. From endoplasmic-reticulum stress to the inflammatory response. Nature 454:455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zwergal A., et al. 2006. C/EBPβ blocks p65 phosphorylation and thereby NF-κB-mediated transcription in TNF-tolerant cells. J. Immunol. 177:665–672 [DOI] [PubMed] [Google Scholar]