Abstract
To reveal the extent of domain-wide epigenetic features at imprinted gene clusters, we performed a high-resolution allele-specific chromatin analysis of over 100 megabases along the maternally or paternally duplicated distal chromosome 7 (Chr7) and Chr15 in mouse embryo fibroblasts (MEFs). We found that reciprocal allele-specific features are limited to imprinted genes and their differentially methylated regions (DMRs), whereas broad local enrichment of H3K27me3 (BLOC) is a domain-wide feature at imprinted clusters. We uncovered novel allele-specific features of BLOCs. A maternally biased BLOC was found along the H19-Igf2 domain. A paternal allele-specific gap was found along Kcnq1ot1, interrupting a biallelic BLOC in the Kcnq1-Cdkn1c domain. We report novel allele-specific chromatin marks at the Peg13 and Slc38a4 DMRs, Cdkn1c upstream region, and Inpp5f_v2 DMR and paternal allele-specific CTCF binding at the Peg13 DMR. Additionally, we derived an imprinted gene predictor algorithm based on our allele-specific chromatin mapping data. The binary predictor H3K9ac and CTCF or H3K4me3 in one allele and H3K9me3 in the reciprocal allele, using a sliding-window approach, recognized with precision the parental allele specificity of known imprinted genes, H19, Igf2, Igf2as, Cdkn1c, Kcnq1ot1, and Inpp5f_v2 on Chr7 and Peg13 and Slc38a4 on Chr15. Chromatin features, therefore, can unequivocally identify genes with imprinted expression.
INTRODUCTION
Imprinted genes are monoallelically expressed according to parental inheritance. The maternally and paternally inherited alleles are distinguished epigenetically by DNA methylation and histone modifications. Parental allele-specific DNA methylation at germ line differentially methylated regions (DMRs) is established in the male and female germ lines. The hyper- and hypomethylated alleles of DMRs largely coincide with repressive and active histone covalent modifications, respectively. DNA methylation (7, 33, 39, 48, 66) and chromatin differences (59, 65, 81, 95, 98, 100) are important for parental allele-specific gene expression. Chromatin is usually probed with allele-specific chromatin immunoprecipitation (ChIP) assays at discrete genomic positions at the transcription start sites of imprinted genes or at DMRs. The extent of the allele-specific chromatin at imprinted domains is not known except for the Igf2r-Airn imprinted locus on chromosome 17 (Chr17), where the two alleles were investigated using ChIP-on-chip methodology in hemizygous mouse embryo fibroblasts (MEFs) carrying the 250-kb hairpin tail (Thp) deletion in one allele (77).
To assess the chromatin of the parental alleles separately, we used MEFs that carried maternal and paternal duplication of distal Chr7, MatDup.dist7 and PatDup.dist7 (10, 11, 23, 55, 78), respectively (Fig. 1B and C). These resulted from the intercrosses of mice heterozygous for a reciprocal translocation at the T(7;15)9H (T9H) breakpoint (11). MatDup.dist7 MEFs carry maternal duplication and paternal deficiency for the translocated distal Chr7, as well as paternal duplication and maternal deficiency of distal Chr15 (31, 91). PatDup.dist7 MEFs carry paternal duplication and maternal deficiency for the distal Chr7 regions, as well as maternal duplication and paternal deficiency of distal Chr15. The MatDup.dist7 and PatDup.dist7 genotypes are associated with fetal and embryonic lethality, respectively (10, 22, 31, 55), due to misexpression of imprinted genes. To obtain MEFs at 13.5 days postcoitus (dpc), the PatDup.dist7 embryos had been rescued by an Ascl2 transgene (78, 91). MEFs provided a sufficient number of cells for ChIP-on-chip experiments.
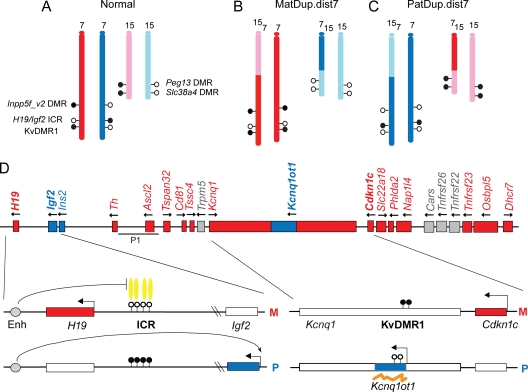
Fig. 1.
Uniparental duplication of distal chromosome 7 allows chromosome-wide analysis of allele-specific epigenetic features of two imprinted domains. (A) Normal embryos inherit one set of chromosomes from each parent. Maternally and paternally inherited alleles of chromosomes 7 (red and blue) and Chr15 (pink and light blue) are shown. (B) In MatDup.dist7 embryos, two copies of the distal Chr7 segment, telomeric to the T9H translocation breakpoint, are inherited from the mother, and two copies of distal Chr15 are inherited from the father. (C) In PatDup.dist7 embryos, two copies of distal Chr7 are inherited from the father, and two copies of distal Chr15 are inherited from the mother. Hyper- and hypomethylated alleles of known DMRs are marked by closed and open circles, respectively. (D) Two clusters of imprinted genes in distal chromosome 7 are regulated by reciprocal germ line methylation and different imprinting mechanisms. Imprinted expression of the H19-Igf2 imprinted domain (to the left) is regulated by a paternally (P) methylated (closed circles) DMR. The unmethylated (open circles) allele is specifically bound by a CTCF insulator protein (yellow ovals) that blocks Igf2 activation by the shared enhancers (gray circles) in the maternal chromosome (M). H19 is expressed from the maternal chromosome (red). Igf2as and Ins2 are paternally expressed (blue), the latter exhibiting imprinted expression in the yolk sac. (B) The Cdkn1c1-Kcnq1 imprinted domain (to the right) is telomeric to the H19-Igf2 domain. It is under the control of the maternally methylated KvDMR1, which overlaps the promoter of the noncoding RNA, Kcnq1ot1 (orange line). Cdkn1c is expressed from the maternal chromosome. Kcnq1ot1 is expressed from the paternal chromosome. Transcription of a set of imprinted genes, Th, Ascl2, Tspan32, Cd81, Tssc4, Slc22a18, Phlda2, Napl14, Tnfrsf23, Osbpl5, and Dhcr7, in the placenta is repressed in the paternal chromosome by Kcnq1ot1 expression from the paternal allele. Imprinted expression of Kcnq1 and Slc22a18 is developmentally regulated. The embryonic lethality phenotype of PatDup.dist7 embryos was rescued by an Ascl2 transgene, integrated at another chromosome. The overlap of the P1 clone with the endogenous locus is indicated. Map is not to scale.
The duplicated chromosome segments in MatDup.dist7 and PatDup.dist7 MEFs harbor five germ line DMRs (Fig. 1A to C): the paternally methylated H19-Igf2 imprinting control region (ICR) (23, 67, 96, 97), the maternally methylated KvDMR1 (19, 25, 105), the maternally methylated Inpp5f_v2 DMR (104) in distal Chr7, the maternally methylated Slc38a4, and Peg13 DMRs in distal Chr15 (79, 89).
The distal portion of mouse chromosome 7 contains two imprinted domains representing two major mechanisms known to regulate imprinted gene expression (Fig. 1D). The H19-Ig2 region is controlled by allele-specific enhancer blocking due to CTCF insulator protein binding in the ICR (5, 32, 36, 38, 69, 82, 90, 91, 93). H19 and Igf2 are expressed from the maternal and paternal chromosomes, respectively (4, 16, 17, 23). CTCF binds in the unmethylated ICR allele and elicits insulation between the shared enhancers and Igf2 in the maternal chromosome. The Igf2as noncoding RNA (ncRNA) is also likely regulated similarly to Igf2 (62). Ins2 is paternally expressed but only in the yolk sac (26). The Cdkn1c-Kcnq1 domain (Fig. 1D) is controlled by the maternally methylated ICR, the Kv differentially methylated region (KvDMR1) (19, 25, 105). The unmethylated paternal allele produces a noncoding RNA, Kcnq1ot1 (88). Transcription of this RNA is required for repressing the paternal allele of an array of maternally expressed imprinted genes in the placenta (25, 53, 68).
The duplicated chromosomes in MatDup.dist7 and PatDup.dist7 MEFs additionally harbor the paternally expressed Ampd3 (83), Inpp5f_v2, and Inpp5f_v3 (12, 104) on Chr7, the maternally expressed Kcnk9 (79) and Trappc9 (101), and the paternally expressed Peg13 (89) and Slc38a4 (57, 89) imprinted genes on distal Chr15.
The parental allele-specific imprinted expression of the H19, Igf2, Cdkn1c, Kcnq1ot1, Peg13, and Slc38a4 transcripts is ubiquitous. Phlda2 is maternally biased in most fetal organs (74). Other imprinted genes exhibit parental allele-specific expression in specific tissues (Ins2 in the yolk sac [26], Inpp5f_v2 [12], Trappc9 in the brain [101], and Tspan32, Cd81, Tssc4, Nap1l4, Tnfrsf23, Osbpl5, and Dhcr7 in the placenta [9, 13, 15, 19, 29, 34, 72, 83]) and are either not expressed or biallelically expressed in other organs (https://atlas.genetics.kcl.ac.uk/) (84). Allele-specific gene expression can also be developmentally regulated: Kcnq1 is maternally expressed in the embryo at 9.5 dpc (47) but becomes biallelic at later fetal stages in a tissue-specific manner (9, 27, 71) and is silent later in development (52). Slc22a18 is maternally biased in the embryo and fetus but not in the adult (15).
We performed ChIP-on-chip and methylated-CpG island recovery assay (MIRA)-on-chip analyses in combination with hybridization to NimbleGen tiling arrays to map the chromatin features and DNA methylation status along the duplicated chromosomal segments and provide a panoramic map of the allele-specific epigenetic features, chromatin and DNA methylation, at the major imprinted domains and scattered imprinted genes on mouse chromosomes 7 and 15.
MATERIALS AND METHODS
MatDup.dist7 and PatDup.dist7 MEFs.
MEFs were derived from 13.5-dpc embryos. PatDup.dist7 embryos that would otherwise die at 10.5 dpc were rescued for this purpose with an Ascl2 transgene (78). The injected P1 clone spanned 84 kb (Fig. 1D).
Chromatin immunoprecipitation.
Chromatin was prepared from MatDup.dist7 and PatDup.dist7 primary MEFs (91) as described earlier (30). The chromatin was cross-linked for 2 min (N-ChIP) or 10 min (X-ChIP) with formaldehyde and sonicated in lysis buffer (87). An aliquot of the chromatin was reverse cross-linked and quantified by optical density (OD) measurement, and the efficiency of sonication was assessed on an agarose gel. Sonicated chromatin was then diluted to a concentration of 0.4 mg/ml and snap-frozen in small aliquots. One aliquot was thawed on the day of ChIP. The chromatin immunoprecipitation was performed as described previously (30) with minor modifications. Preblocked A/G beads from Santa Cruz (catalog number sc-2003) were used for capturing the precipitated chromatin. The antibodies used in the ChIP assays are listed in Table S1 in the supplemental material.
MIRA.
The methylated fraction of sonicated genomic DNA was captured using recombinant MBD3L1 and MBD2b proteins as described earlier (75, 76).
LM-PCR amplification.
Ligation-mediated PCR (LM-PCR) was done to amplify ChIP- and MIRA-enriched DNA as previously described (41) with minor modifications.
ChIP-on-chip analysis.
Custom-designed NimbleGen tiling arrays covering the distal arm of mouse chromosome 7 (080121_Szabó_CoH_mm8_chr7_chip), central chromosome 7 (C4527-18-01 2006-07-17_mm8 tiling set 18), and distal chromosome 15 (C4527-31-01_mm8 tiling set 31) were used for the histone modification profile analysis. The array covers the regions 98000020 to 145134052 for distal Chr7 and 42240000 to 103480000 for distal Chr15. Amplified ChIP DNA fractions were compared with amplified input DNA. The labeling of DNA, microarray hybridization, and scanning were performed by the NimbleGen Service Group (Reykjavik, Iceland). Data were extracted from scanned images by using NimbleScan 2.3 extraction software (NimbleGen Systems).
RNA isolation and cDNA preparation.
RNA was isolated from MatDup.dist7 and PatDup.dist7 MEFs using RNA-Bee according to the manufacturer's instructions (Tel-Test). The pellet was dissolved in diethyl pyrocarbonate (DEPC) water containing RNasin (Promega) and 10 mM dithiothreitol (DTT). Contaminating DNA was removed with the DNA-free kit (Ambion). Double-stranded (ds) cDNA was prepared from 10 μg of MatDup.dist7 and PatDup.dist7 MEF total RNA with oligo(dT) primers using the SuperScript double-stranded cDNA synthesis kit (Invitrogen) according to the NimbleGen Array user's guide (http://www.nimblegen.com/products/lit/exp_userguide_v3p2.pdf). The ds cDNA was purified using the QIAquick PCR purification kit (Qiagen) and was hybridized to custom NimbleGen tiling arrays (080121_Szabó_CoH_mm8_chr7_chip) at the City of Hope Functional Genomics core facility.
Allele-specific peak identification.
We first performed quantile normalization on the distal Chr7 NimbleGen log2 ratio data. Next, peaks were defined in each sample as four consecutive probes with log2 ratios above the 95th percentiles on each array, allowing one probe gap. For each peak present in MatDup.dist7 samples, the median log2 ratios of probes falling into the peak region in the corresponding PatDup.dist7 sample were calculated and compared to the median log2 ratios of these probes in MatDup.dist7 samples. The peaks with signal differences of more than 3-fold between MatDupd.dist7 and PatDup.dist7 samples were designated allele-specific peaks.
Microarray data accession number.
Microarray data can be accessed in the GEO database under superseries GSE26947.
RESULTS
Measuring allele-specific histone chromatin on a chromosome-wide scale.
To reveal the domain-wide allele-specific epigenetic features of imprinted genes with high resolution along Chr7 and Chr15, we used MatDup.dist7 and PatDup.dist7 MEFs (Fig. 1B and C). These cells allowed us to separately assess the maternal and paternal alleles along the duplicated chromosomal segments. We performed ChIP-on-chip and MIRA-on-chip analyses in combination with LM-PCR amplification and hybridization to NimbleGen tiling arrays to map the chromatin features and DNA methylation. Antibodies for well-known active (H3K9ac, H3K4me2, H3K4me3) and repressive (H3K9me3 and H3K27me3) histone tail modifications and also for histone globular domain marks (H4K91ac, H3K79me2, and H3K79me3) were used in ChIP to map chromatin. We recently showed that H4K91ac and H3K79me2 marks are typically found in the unmethylated allele of DMRs, whereas H3K79me3 is predominantly found in the methylated alleles (87). CTCF ChIP was used to map insulators/chromatin barriers. Chromatin and methylation data were cross-referenced with gene expression patterns. Affymetrix RNA microarrays confirmed the allele-specific expression of known imprinted genes along the duplicated chromosome segments (see Fig. S1 in the supplemental material). ChIP with the elongation-type RNA polymerase II (PolII) antibody and double-stranded (ds) cDNA hybridization on the NimbleGen arrays were also used to visualize biallelic and allele-specific gene expression along the duplicated chromosome regions and potentially uncover novel unannotated ncRNAs.
Analysis of the H19-Igf2 imprinted domain.
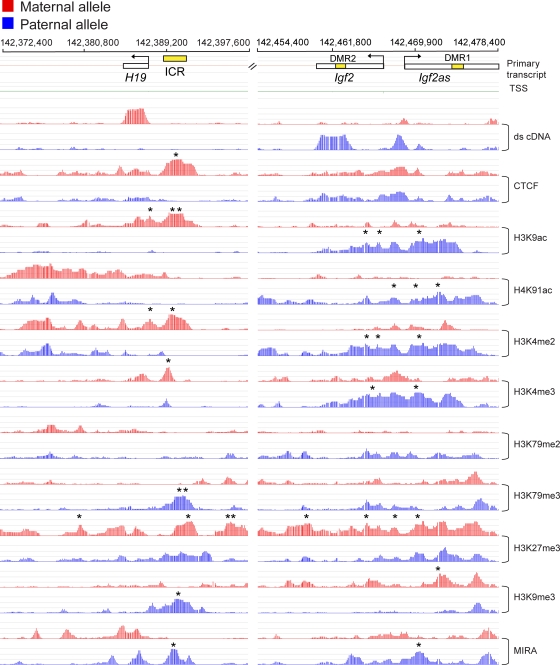
The reciprocal maternal and paternal allele-specific expression of H19 and Igf2 transcripts was confirmed in RNA microarray (see Fig. S1 in the supplemental material) and was visualized by ds cDNA hybridization to custom NimbleGen arrays containing a 47-Mb region of distal Chr7. MatDup.dist7 MEFs exhibited a strong signal for H19 but no signal for Igf2, whereas PatDup.dist.7 MEFs exhibited a strong signal for Igf2 and Igf2as but not for H19 (Fig. 2). No transcript signal was present for Ins2. The PolII signal was very clear along the Igf2 transcript but was hard to detect along H19 (not shown). As expected, DNA methylation was found at the ICR in PatDup.dist7 but not in MatDup.dist7 MEFs, and DNA methylation was biased toward the paternal allele at the Igf2 locus (14). The robust CTCF peak at the ICR in MatDup.dist7 MEFs was in agreement with our in vivo footprinting data obtained using the same cells (91) and ChIP analysis in normal MEFs (30, 99). The MIRA peak coincided with strong H3K9me3 and H3K79me3 peaks at the ICR in PatDup.dist7 MEFs, as in ChIP-single-nucleotide primer extension (SNuPE) results using normal cells (87). The CTCF peak coincided with H3K9ac, H3K4me2, and H3K4me3 peaks. The previously reported maternal allele-specific CTCF peak in neonatal liver chromatin at the Igf2 DMR1 (42) was not apparent in MatDup.dist7 MEFs (Fig. 2), most likely due to cell type-specific differences in CTCF binding. This peak was absent in normal MEFs (30). The expressed allele of imprinted genes, i.e., the maternal allele for H19 and paternal allele for Igf2 and Igf2as, was rich in H3K9ac, H4K91ac, H3K4me2, and H3K4me3 and was slightly enriched in H3K79me2 in MatDup.dist7 and PatDup.dist7 MEFs, respectively. The silent alleles of imprinted genes, i.e., the paternal allele for H19 and maternal allele for Igf2 and Igf2as, lacked all these active chromatin peaks but exhibited H3K9me3 marks in PatDup.dist7 and MatDup.dist7 MEFs, respectively. H3K27me3 showed maternal allele specificity at the Igf2 promoter/gene body. H3K27me3 was biallelic in normal MEFs at the ICR (30, 99) and in MatDup.dist7-PatDup.dist7 MEFs (Fig. 2), except for one maternal allele-specific peak at the telomeric end of the ICR. The ChIP-on-chip data were verified by real-time PCR at the H19-Igf2 ICR, the H19 promoter, and the Igf2 DMR2 regions (see Fig. S2A and B in the supplemental material).
Fig. 2.
High-resolution allele-specific analysis of the H19, Igf2, and Igf2as imprinted genes. The chromatin (with the antibodies indicated to the right) and methylation (MIRA) signals are plotted along the chromosome as −log10 P value scores for the maternal allele in MatDup.dist7 (red bars) and for the paternal allele in PatDup.dist7 (blue bars) MEFs. The P value score was obtained by NimbleScan software and is derived from the Kolmogorov-Smirnov test comparing the log2 ratios (ChIP or MIRA versus input) within a 750-bp window centered at each probe and the rest of the data on the array. Transcripts are marked by rectangles, with arrows indicating the direction of transcription. The H19-Igf2 ICR and the Igf2 DMRs are labeled with yellow rectangles. Significant allele-specific peaks located at −5 kb to +2 kb from the transcription start sites (TSS) are marked by asterisks. Additionally, significant maternal H3K27me3 peaks are marked along the imprinted domain in this figure and in Fig. S3B in the supplemental material. Genomic coordinates are indicated on the top according to UCSC Genome Browser mouse genome version mm8.
When we looked at the panoramic picture of the domain, we observed a novel feature, a stretch of a more-than-100-kb-long maternally biased H3K27me3 enrichment encompassing the entire imprinted domain (see Fig. S3B in the supplemental material). Extended regions of H3K27me3 enrichment were identified by an algorithm, broad local enrichment (BLOC) in MEFs at imprinted and nonimprinted regions (70, 77), but allele-specific features of BLOCs have not been reported. We confirmed the H3K27me3 BLOC at two positions in the H19-Igf2 intergenic region (see Fig. S2C in the supplemental material). This marking excluded the active H19 promoter, confirming the data obtained in normal MEFs (Fig. 2) (30). A panoramic view of the region centromeric to H19 (see Fig. S3A in the supplemental material) shows that the maternally biased BLOC extends beyond the neighboring nonimprinted gene, Mrpl23, and stops before Tnnt3, but the Mrpl23 gene itself is excluded.
Analysis of the Kcnq1-Cdkn1c imprinted domain.
We confirmed the allele-specific expression of Cdkn1c by RNA microarray (see Fig. S1 in the supplemental material). Additionally, the maternally expressed Cdkn1c and paternally expressed Kcnq1ot1 transcripts were visualized by ds cDNA hybridization (Fig. 3). We found that the ds cDNA occupies a window of about 67 kb at the Kcnq1ot1 transcript. This is very close to the previously reported 64 kb (53) but was shorter than the recently reported 91 kb (68). The longer transcript was mapped in 14.5-dpc placenta. It is possible that the different-sized Kcnq1ot1 transcripts have different regulatory potentials. Contrary to previous findings of monoallelic Kcnq1 expression in late mouse embryos (27), we did not detect Kcnq1 expression in MEFs (see Fig. S1 and S3E in the supplemental material). The Ascl2 gene was not interpreted, because an Ascl2 transgene was used to rescue the lethality of PatDup.dist7 MEFs and the hybridization signals may come from the P1 clone integrated elsewhere (78). The other imprinted genes of this domain were not expressed in MatDup.dist7 and PatDup.dist7 MEFs (Th, Tspan32, Kcnq1, and Slc22a18) or were expressed equally in these cells (Cd81, Tssc4, Phld2a, Napl14, Tnfrs23, Osbpl15, and Dhcr7) (see Fig. S3D to F in the supplemental material). We verified the expression of selected genes using reverse transcription (RT)-PCR (see Fig. S4 in the supplemental material). Cd81 and Osbpl5 were both highly expressed in MatDup.dist7 and PatDup.dist7 MEFs and in the embryo, whereas Phlda2 and Slc22a18 expression was very low in MEFs and embryos compared to that in placentas. When allele specificity of the low-level Slc22a18 expression was examined in F1 embryos and placentas, a maternal allele-specific bias (15, 47) was apparent. Osbpl15 was expressed maternal allele specifically in the F1 placenta but biallelically in the F1 embryo. In our experience, very little RNA is sufficient to measure allele-specific gene expression. Mapping transcription by microarray-based approaches requires a higher overall level of each particular transcript.
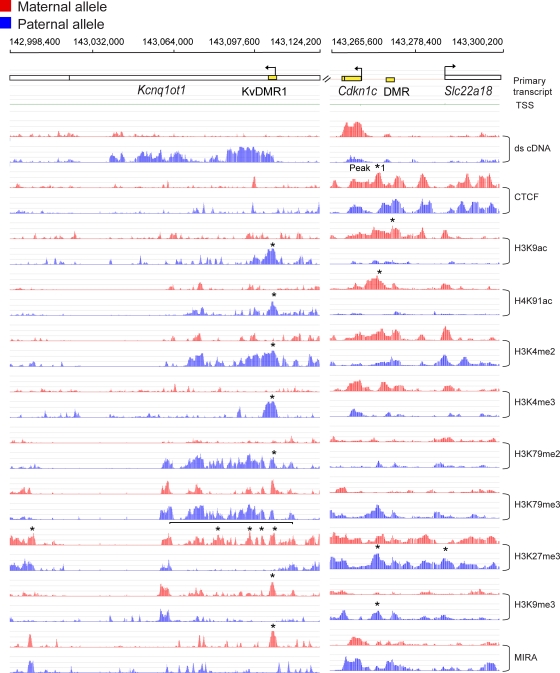
Fig. 3.
High-resolution allele-specific chromatin analysis along the Kcnq1ot1 and Cdkn1c-Slc22a18 imprinted genes. Kcnq1ot1 ncRNA and Cdkn1c are paternally and maternally expressed in MEFs, respectively. The KvDMR1, the Cdkn1c DMR, and the Slc22a18 DMR (47) (yellow rectangles) are very clearly marked by allele-specific chromatin. The H3K27me3 peaks in the bracketed area and peak 1 in the Cdkn1c upstream area were confirmed using ChIP real-time PCR (see Fig. S5 in the supplemental material). Slc22a18 exhibited some allele-specific chromatin marks in the absence of high-level transcription in MEFs (see Fig. S4 in the supplemental material). Other details are as described in Fig. 2.
The KvDMR1 displayed a strong peak of CpG methylation in the maternal allele (Fig. 3). This coincided with a strong H3K9me3 peak and strong H3K27me3 signals in MatDup.dist7 MEFs. H3K79me3 was present in both MatDup.dist7 and PatDup.dist7 MEFs. Active chromatin marks, H3K9ac, H4K91ac, H3K4me2, H3K4me3, and H3K79me2, were strongly enriched at the KvDMR1 in PatDup.dist7 MEFs. Our results confirmed and expanded previous observations that maternal allele-specific H3K9/14ac and H3K4me and paternal allele-specific H3K9me and H3K27me mark the KvDMR1 (98). Additionally, we revealed that H3K4me2, H3K79me2, and H3K79me3 showed enrichment not only at the KvDMR1 but also over a larger region along the Kcnq1ot1 transcript in PatDup.dist7 MEFs. The histone globular domain marks were interesting: H3K79me2 and H3K79me3 were both enriched along the active allele of the Kcnq1ot1 transcript, but in MatDup.dist7 cells only H3K79me3 but not H3K79me2 existed along the silent maternal allele of this gene. We did not detect reproducible CTCF peaks in the KvDMR1 MEFs, contrary to ChIP results in normal MEFs (24). The difference can be due to different specificities of the antibodies. In the paper above, a mixture of nine anti-CTCF antibodies was used. Alternatively, the amplification step in the ChIP-on-chip method may introduce a level of uncertainty at low-occupancy sites (20).
An H3K27me3 BLOC stretched along the Cdkn1c-Kcnq1 imprinted domain between Trpm5 and Phlda2 in both MatDup.dist7 and PatDup.dist7 cells (see Fig. S3D and E in the supplemental material). In PatDup.dist7 cells, this stretch was interrupted by a long gap along the transcription of the Kcnq1ot1 noncoding RNA (Fig. 3). The extent of this paternal allele-specific gap in the H3K27me3 BLOC was verified by ChIP real-time PCR (see Fig. S5A in the supplemental material). H3K27me3 enrichment exhibited a reciprocal pattern with gene expression; it biallelically covered the silent genes but was absent at the expressed genes along this domain. H3K27me3 repressive marks were biallelic at the Kcnq1 transcript, in agreement with the shift from monoallelic expression toward biallelic repression at 12.5 dpc (52).
MIRA peaks in PatDup.dist7 MEFs indicated paternally biased CpG methylation at the Cdkn1c promoter and upstream Cdkn1c DMR (Fig. 3) in accordance with the existence of two paternally methylated somatic DMRs (6, 47, 105). The Cdkn1c promoter exhibits maternal allele-specific H3K9/14ac and H3K4me and paternal allele-specific H3K27me marks in the 9.5-dpc embryo chromatin (98). We found that the Cdkn1c upstream region exhibited more robust chromatin differences than the promoter. These included maternal allele-specific H3K9ac and H4K91ac and paternal allele-specific H3K79me3, H3K27me3, and H3K9me3 marks. We verified these peaks using ChIP real-time PCR (see Fig. S5B in the supplemental material). The repressive H3K27me3 signal covered both alleles of the Cdkn1c gene, but the promoter was free of H3K27me3 in MatDup.dist7 MEFs (Fig. 3). We found that CTCF binding was biased to the maternal allele (Fig. 3; see also Fig. S5B in the supplemental material) at a previously verified CTCF binding consensus binding site (40). Maternal bias of CTCF is likely due to partial paternal-allelic methylation of the region, also seen by others (58).
Those imprinted genes of the cluster that were expressed in both MatDup.dist7 and PatDup.dist7 MEFs, Cd81, Tssc4, Phlda2, Nap1l4, Tnfrs23, Osbpl5, and Dhcr7, showed biallelic active histone modification marks (see Fig. S3D to F in the supplemental material): MatDup.dist7 and PatDup.dist7 MEFs were equally enriched in H3K9ac, H3K4me2, H3K4me3, and H3K79me2, whereas repressive marks were absent at these genes in both cell types. These results are in agreement with observations in 9.5-dpc embryos regarding the biallelic active H3K9ac and H3K4me marks at the Osbl5, Napl14, Phlda2, Tssc4, and Cd81 genes (98), but we did not confirm biallelic repressive marks, except biallelic H3K27me3 at Phlda2. The difference was likely due to the difference in epigenetic status of these genes between 13.5-dpc MEFs and 9.5-dpc embryos. Tspan32, which was not expressed in MEFs, exhibited biallelic active marks H3K9ac, H4K91ac, and H3K4me2 but not H3K4me3 and did not exhibit repressive marks. Slc22a18, which had a very low level of expression in MEFs, exhibited allele-specific chromatin marks in MatDup.dist7 and PatDup.dist7 MEFs. The low level of expression was maternally biased in normal F1 embryos at 13.5 dpc (see Fig. S4 in the supplemental material). It is likely that the proximity to the ubiquitously maternally expressed Cdkn1c may influence the active chromatin marks at the maternal allele of this transcript in MatDup.dist7 MEFs. Th, which was not expressed in MatDup.dist7 and PatDup.dist7 MEFs, did not exhibit active chromatin marks in either cell type but was marked by repressive H3K27me3 in both MEFs. The long stretch of biallelic H3K27me3 along Th coincided with biallelic H3K9me3 and H3K79me3 enrichment. This has to be interpreted with caution, however, because the P1 clone included the first exon of Th and most of the very long first intron (78). H3K79me3 was biallelic at Cd81 but coincided with biallelic H3K79me2 enrichment, not with H3K27me3 enrichment (see Fig. S3D in the supplemental material).
Analysis of scattered imprinted genes along distal Chr7.
Ampd3 is maternally expressed in the 13.5-dpc placenta but biallelically exprressed in the 13.5-dpc embryo (83). We found a low level of biallelic expression using ds cDNA hybridization (see Fig. S6 in the supplemental material). The chromatin composition was largely biallelic except for stronger H3K79me2 signals along the gene body and a stronger H3K4me3 peak downstream of the transcription start site (TSS) in MatDup.dist7 MEFs than in PatDup.dist7 MEFs. No significant repressive chromatin marks were present.
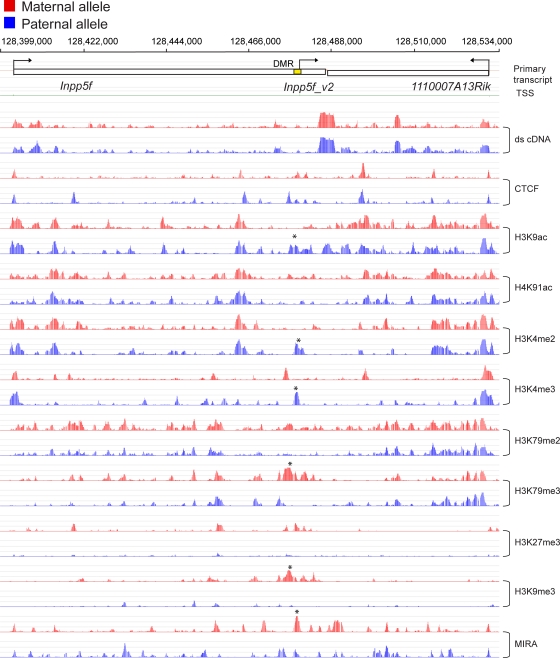
The Inpp5f_v2 transcript is paternally expressed in the brain (12) but is not expressed in the embryo. Allele-specific chromatin features have not been reported at this imprinted gene before. We did not find expression from the Inpp5f_v2 promoter in MEFs based on ds cDNA hybridization (Fig. 4). DNA methylation and H3K9me3, H3K27me3, and H3K79me3 enrichment, however, were apparent in the maternal allele in MatDup.dist7 MEFs, whereas H3K4me3, H3K4me2, and H3K9ac enrichment marked the paternal allele in PatDup.dist7 MEFs (Fig. 4). The nonimprinted variant, Inpp5f, was expressed in MatDup.dist7 and PatDup.dist7 MEFs equally as measured in RNA microarray and ds cDNA hybridization. This variant exhibited biallelic active chromatin marks but lacked repressive marks. The biallelic peaks at the 5′ end and the strong biallelic ds cDNA peaks at the 3′ end of the Inpp5f gene likely represent transcription from the Inpp5f promoter.
Fig. 4.
Chromatin analysis of Inpp5f_v2. Inpp5f_v2 is paternally expressed in the brain but is not expressed in MEFs. Allele-specific marks can be discerned at Inpp5f_v2 but not at the nonimprinted Inpp5f in MEFs, including maternal DNA methylation in the Inpp5f_v2 DMR. Other details are as described in Fig. 2.
Global features along distal chromosome 7.
After analyzing known imprinted loci along distal Chr7, we compared the global chromatin features along this 47-Mb-long chromosome segment. Unsupervised hierarchical clustering was used to reveal the degree of similarity between the ChIP-on-chip samples. Pearson correlation and average linkage analysis found that, in general, the MatDup.dist7 and PatDup.dist7 MEF samples clustered together according to the epigenetic mark analyzed (see Fig. S7 in the supplemental material); for example, CTCF in MatDup.dist7 showed the closest relationship with CTCF in PatDup.dist7. The exception to this rule was that the distribution of H4K91ac and H3K4me2 samples clustered closer by cell type rather than by chromatin marks. Importantly, the repressive histone tail modifications H3K27me3 and H3K9me3 were clustered together and also with the globular domain mark H3K79me3. Active histone tail marks clustered with PolII signals and also with the globular mark H3K79me2. H3K9me3, H3K79me3, and H3K27me3 were significantly enriched at gene deserts, whereas CpG-methylated regions were associated with gene-rich regions.
To determine the pattern of each chromatin mark in relation to the transcription start site, we generated composite profiles (see Fig. S8 in the supplemental material). Average signals (log2 ratios) of each chromatin mark were plotted between −5 kb and +2 kb of the TSS for five equal-sized groups of transcripts, classified according to their expression levels. The composite profiles of each epigenetic mark were very similar between MatDup.dist7 and PatDup.dist7 MEFs (see Fig. S8A and B in the supplemental material) and were largely expected (2, 102). One interesting finding was that whereas both H3K79me2 and H3K79me3 were present downstream of the TSS of highly expressed genes, H3K79me2 exhibited stronger enrichment. Also, interestingly, a peak of H3K79me3 but not H3K79me2 was present downstream of the TSS of genes displaying low expression or no expression.
Generating an imprinted gene chromatin signature consensus algorithm.
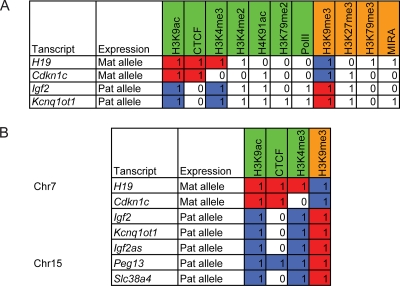
The allele-specific chromatin differences between MatDup.dist7 and PatDup.dist7 MEFs were very clear at each known imprinted gene that exhibited imprinted expression in the embryo. We decided to generate a consensus “imprinted gene chromatin signature” (Fig. 5) from the allele-specific epigenetic features at these transcripts and test whether this consensus can be used to identify imprinted genes along the duplicated chromosomal regions. The first step was to identify allele-specific (MatDup.dist7- or PatDup.dist7-specific) peaks (see Materials and Methods for details) for each modification mark that falls into the promoter region (defined as −5 kb to +2 kb of the TSS) of known imprinted transcripts, H19, Igf2, Cdkn1c, and Kcnq1ot1, on distal Chr7 (see Table S3 in the supplemental material). The second step was to summarize the allele-specific peaks as binary indicators (Fig. 5A). The 11 examined modification marks were divided into activation marks (CTCF, H3K4me2, H3K4me3, H3K9ac, H4K91ac, H3K79me2, and PolII) and inhibition marks (H3K27me3, H3K79me3, H3K9me3, and DNA methylation). The binary indicator was defined as follows: for the H19 and Cdkn1c transcripts, “1” or “0” in the activation mark category codes “true” or “false” for the following statement: MatDup.dist7-specific peak exists in the promoter; “1” or “0” in the inhibition mark category codes “true” or “false” for the following statement: PatDup.dist7-specific peak exists in the promoter. For the Igf2 and Kcnq1ot1 transcripts, the reciprocal parameters were applied. Based on the modification table, an “imprinted gene chromatin signature” algorithm was derived. It is a binary predictor: genes that possess chromatin composition containing activation mark H3K9ac and activation mark CTCF or H3K4me3 and inhibition mark H3K9me3 in their promoter regions are imprinted and expressed from the allele associated with the activation marks.
Fig. 5.
Derivation and testing an imprinted gene predictor algorithm. (A) Allele-specific peaks of epigenetic marks were tabulated at four annotated transcripts according to the allele-specific expression profile of the transcript. Allele-specific peaks were identified for activation and repressive epigenetic marks in the promoter region (−5 kb to +2 kb from the TSS) of four known imprinted genes, located on distal Chr7, in MatDup.dist7 and PatDup.dist7 MEFs (see Table S3 in the supplemental material). From these, the activation mark peaks in the expressed allele (green) and the repression mark peaks in the silent allele (orange) were considered for maternally expressed and paternally expressed transcripts. From this table, a consensus imprinted gene signature was derived: H3K9ac and H3K4me3 or CTCF in the expressed allele and H3K9me3 in the silent allele. These are colored: maternal allele (red) and paternal allele (blue). (B) The consensus was then tested as a bimodal predictor using chromatin data of annotated transcripts along distal Chr7, central Chr7, and distal Chr15 (see Table S4 and Fig. S9 in the supplemental material). The predictor was further tested by a sliding-window approach, which did not depend on transcript annotations (see Table S5 in the supplemental material).
Transcript-based prediction.
The performance of the consensus imprinted gene chromatin signature was tested along the entire duplicated segment of the genome in MatDup.dist7 and PatDup.dist7 MEFs. For this analysis, we generated additional ChIP-on-chip data along distal and central Chr7 and along distal Chr15. Each gene in a given genomic segment was considered, and its imprinted status in either allele was tested. The results are shown in a heat map (see Fig. S9 in the supplemental material). Out of 811 genes between 98000020 and 145134052 along distal Chr7, the five known imprinted genes, H19, Igf2, Igf2as, Cdkn1c, and Kcnq1ot1, were predicted (Fig. 5B). In the region between 62.3 Mb and 98 Mb on central Chr7, 647 genes were tested, and none was recognized as being imprinted. Along distal Chr15, 806 genes were tested, and two genes, the paternally expressed Peg13 and Slc38a4, were recognized by the algorithm (Fig. 5B). Apart from the four imprinted genes that were considered in deriving the algorithm, we predicted an additional three imprinted genes in Chr7 and Chr15 using the algorithm, all with correct allele specificity. The predictive peaks are provided in Table S4 in the supplemental material.
Sliding-window approach.
In the previous prediction analysis, the detection was limited to annotated genes. We then also performed an unbiased analysis along the entire central and distal Chr7 and distal Chr15 regions using the algorithm and the sliding-window approach. The bimodal predictor criteria were applied to each 2-kb window tiled on the arrays with a 1-kb step size. The hypothesis was that a window containing H3K9ac and CTCF or H3K4me3 in one allele and H3K9me3 in the other allele will be associated with an imprinted gene. We identified 9 such windows and called transcripts in a distance equal or less than 3 kb from the peak. Eighteen out of 19 windows were associated with known transcripts and were located upstream or within but not downstream of a transcript (see Table S5 in the supplemental material). The sliding-window approach recognized and correctly predicted the parental expression of each transcript predicted with the gene-based approach and additionally called the TSS of the known paternally expressed transcript variant, Inpp5f_v2, within Inpp5f (Fig. 4). Inpp5f_v2 was not recognized by the transcript-based approach, because it is not annotated in RefSeq. The windows reported within Trappc9 were located at the TSS of Peg13, in a Trappc9 intron on Chr15. No novel imprinted genes were found on distal Chr7 and Chr15.
Allele-specific chromatin at imprinted genes on distal chromosome 15.
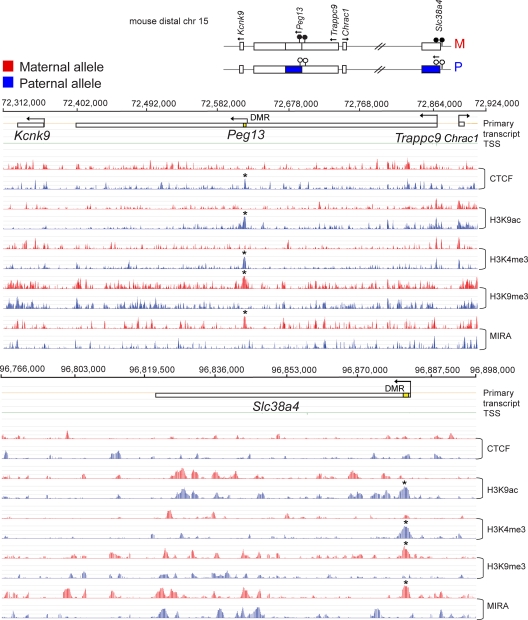
Allele-specific chromatin features along distal Chr15 have not been reported. We characterized the allele-specific chromatin features of the Peg13 and Slc38a4 DMRs (89). At both of these DMRs CpG methylation and H3K9me3 enrichment were observed in the maternal allele, displayed in PatDup.dist7 cells, whereas H3K9ac and H4K4me2 enrichment was found in the paternal allele, displayed in MatDup.dist7 cells (Fig. 6). The Peg13 DMR also had a very clear CTCF peak in the paternal allele. Kcnk9 is maternally expressed in 11.5-dpc embryos and in adult brain (79), and Trappc9 exhibits imprinted expression in the neonatal brain (101). Kcnk9 and Trappc9 were not differentially expressed in MatDup.dist7 versus PatDup.dist7 MEFs, and this was in agreement with the absence of an allele-specific chromatin pattern.
Fig. 6.
Chromatin analysis of imprinted genes on distal Chr15. A map depicting the imprinting status of the Peg13 and the Slc38a4 imprinted regions along distal Chr15 is shown at the top. ChIP-on-chip results are shown for the maternal allele in PatDup.dist7 (red bars) and for the paternal allele in MatDup.dist7 (blue bars) MEFs, with the antibodies indicated to the right. The last two rows depict MIRA analysis.
DISCUSSION
In this study, we provide a panoramic map of the allele-specific epigenetic features along distal chromosomes 7 and 15 with high resolution. This is the first study to systematically analyze chromatin along a more-than-100-Mb-long genomic region in an allele-specific fashion, providing a unique opportunity to determine the extent of allele-specific marking at individual imprinted genes and the extent of imprinted domains. This analysis also allows us to experimentally derive a chromatin-based imprinted gene signature algorithm.
Reciprocal allele-specific chromatin marks are focused at DMRs and imprinted genes.
In MatDup.dist7-PatDup.dist7 MEFs, five known germ line DMRs, the paternally methylated H19-Igf2 ICR and the maternally methylated KvDMR1, Slc38a4, Peg13, and Inpp5f_v5 DMRs, can be investigated along the duplicated part of the genome (Fig. 1). We found that each of these DMRs exhibited reciprocal allele-specific focal chromatin marks, an H3K9me3 peak at the methylated allele and H3K9ac and at least one more active mark, H3K4m3 and/or CTCF insulator peaks, at the unmethylated allele.
Allele-specific chromatin was present at genes that were expressed differentially in MatDup.dist7 and PatDup.dist7 MEFs. Promoters of genes exhibiting imprinted expression were always allele specifically marked by H3K9ac, H3K4me3, and H3K4me2 and sometimes also by H4K91ac and H3K79me2. Marking of imprinted domains by histone acetylation showed differences between the globular domain residue H4K91ac and the tail domain residue H3K9ac. H3K9ac enrichment was more focused at the TSS, similarly to H3K4me3, whereas H4K91ac was more spread out along the transcript, closely mimicking H3K4me2, supporting the idea that acetylation at different lysine residues may have different meanings for imprinted gene expression (86). The silent promoter allele always contained a distinct H3K9me3 peak and sometimes an H3K79me3 peak. This result is similar to recent allele-specific chromatin mapping results along the 250-kb Thp deletion on mouse chromosome 17 that show sharp active and repressive chromatin marks in a mutually exclusive fashion in the opposite alleles of the Igf2r-Airn imprinted domain (77). Allele-specific expression was the prerequisite for exhibiting allele-specific reciprocal active and repressive chromatin composition, except at the Inpp5f_v2 and Slc22a18 transcripts. Imprinted genes with placenta-specific imprinted expression generally did not exhibit allele-specific chromatin in MEFs. This is expected, because the allele-specific expression of imprinted genes and their epigenetic features in MEFs is highly similar to those of the embryo at 13.5 dpc (30, 87).
Broad enrichment of H3K27me3 along imprinted domains has an allele-specific component.
Apart from discrete enrichment foci for reciprocal chromatin marks, we also found extended biallelic H3K27me3 BLOCs (70, 77) along two imprinted domains. In the present study, we discovered the allele-specific nature of H3K27me3 BLOCs. At the H19-Igf2 domain, H3K27me3 spread over a 100-kb-long region encompassing H19, Igf2, Igf2as, and Ins2 imprinted genes and was dominant in the maternal allele. This allele-specific BLOC may facilitate the CTCF protein's insulation over Igf2. Indeed, chromatin organization at the Igf2 gene depends on CTCF binding in the ICR (30) and is mediated by Polycomb protein, Suz12, binding in the ICR (49). The other allele-specific feature of BLOCs was that broad biallelic H3K27me3 enrichment was interrupted by allele-specific gaps at transcripts. In MatDup.dist7 MEFs, the H19 promoter region resided in an H3K27me3-free gap. Reciprocally, the ds cDNA hybridization signal occupied a window of about 67 kb at the Kcnq1ot1 transcript, and the H3K27me3 signal exhibited a sharp gap around this transcript in PatDup.dist7 MEFs (Fig. 3). The paternal allele-specific H3K27me3 gap in the biallelic H3K27me3 BLOC is a novel feature of the Kcnq1-Cdkn1c imprinted domain. Previous chromatin analysis by Umlauf (98) showed that in 9.5-dpc embryos, H3K27me3 is maternal allele specific at one single-nucleotide polymorphism (SNP) located at the 5′ end of the Kcnq1ot1 RNA and biallelic at another SNP about 37 kb downstream. Pandey and colleagues (68) mapped the chromatin along this imprinted domain in placenta and liver in a non-allele-specific fashion and found that H3K27me3 levels are relatively low along the Kcnq1ot1 transcript. They also reported that H3K27me3 is paternal allele specific at four SNPs (Cd81, Tspan32, Kcnq1, and Slc22a18) in the placenta, but they did not investigate allele-specific H3K27me3 in the embryo. It will be interesting to find out whether this Polycomb-dependent repressing mark is required for monoallelic silencing of the Kcnq1ot1 gene or if the Kcnq1ot1 RNA directly excludes H3K27me3 from the paternal allele in the embryo. Interestingly, Kcnq1ot1 has an opposite role in the placenta: it recruits PRC2 complex members to the paternal allele along the domain (68).
CTCF and DMRs.
A robust CTCF peak in the H19-Igf2 ICR in the present study confirmed in vivo CTCF footprints in MatDup.dist7 versus PatDup.dist7 MEFs (91) and ChIP-SNuPE results of maternal allele-specific CTCF binding in normal MEFs (30). The role of CTCF-mediated insulation at this locus in MEFs has been confirmed genetically (30, 93). A CTCF-mediated insulation-based mechanism has been debated with respect to the KvDMR1 (37, 52). Although we didn't find reproducible CTCF binding in PatDup.dist7 MEFs at the KvDMR1 using ChIP-on-chip analysis, paternal allele-specific in vivo CTCF binding had been detected there previously by ChIP (24), suggesting functional importance for these sites. Further genetic analysis will be required to test whether the KvDMR1 regulates imprinted gene expression by an enhancer-blocking mechanism (24) in specific organs (85), perhaps at low-occupancy CTCF sites (20). We found that CTCF binding is maternal allele specific in the paternally methylated upstream somatic DMR of Cdkn1c (6, 47, 105) at a consensus binding site (40) (Fig. 3). In addition, we identified a very clear novel CTCF peak in the Peg13 DMR in the paternal allele. Allele-specific CTCF binding may thus play a role in regulating imprinted expression of the Cdkn1c and the Peg13 genes. Indeed, CTCF may be a more common theme in genomic imprinting than previously appreciated.
Allele specificity of histone globular domain modifications.
We reported recently that the methylated alleles of DMRs are distinguished by H3K79me3, whereas the unmethylated alleles are enriched in H3K79me2 (87). The present panoramic analyses confirmed that H3K79me3 is paternal allele specific at the H19-Igf2 ICR, whereas H3K79me2 is paternal allele specific at the Igf2 gene, and also that H3K79me2 is paternal allele specific, whereas H3K79me3 was biallelic at the KvDMR1 (87). We now reveal that H3K79me3 is also maternal allele specific at the maternally methylated Inpp5f_v2 DMR and paternal allele specific at the paternally methylated Cdkn1c upstream somatic DMR. The allele-specific difference between H3K79me2 and H3K79me3 was not always present at regions outside DMRs (H3K79me3 overlapped sometimes with either H3K79me2 or H3K27me3 at specific regions). Globally, however, cluster analysis placed H3K79me2 together with active chromatin marks, histone acetylation, H3K4 methylation, CTCF, and gene density, whereas H3K79me3 clustered with gene-poor regions and repressive histone marks, H3K9me3 and H3K27me3. Additionally, in the composite profile of genes displaying low expression or no expression, a peak of H3K79me3 but not H3K79me2 was present downstream of the TSS (see Fig. S8 in the supplemental material). It will be interesting to find out the role of the different methylated forms of H3K79 in imprinted gene regulation.
Highly efficient prediction of imprinted genes based on allele-specific chromatin signature.
Recently, large-scale studies aimed at detecting novel imprinted genes (73). The predictions were based on RNA expression differences (1, 28, 57, 83, 101), DNA sequence-based computational predictions (50, 51, 79), DNA methylation differences (35, 89), and epigenetic signature (8, 18, 54, 56, 103). The chromatin-based studies successfully predicted and identified novel imprinted genes but with variable specificity. RNA polymerase II (PolII) binding was allele specifically measured in a high-throughput ChIP-SNP assay (54), but the predicted imprinted genes have not been verified. Using ChIP with deep sequencing, 13/20 of the top sites enriched in H3K4me3 and H3K9me3 marks in the oppositely methylated alleles identified imprinted regions in embryonic stem (ES) cells (56). Overlapping patterns of H3K4me3 and H3K9me3 in somatic cells together with a sperm DMR verified known DMRs in a high-density custom imprinting array and predicted novel imprinted features, but these haven't been confirmed (18). Overlapping H3K4me2 and DNA methylation “double hits” were enriched at imprinted regions, and overlapping H3K4me2, DNA methylation, and CTCF binding “triple hits” were even more enriched (103), but the prediction was not absolutely precise.
The comprehensive allele-specific chromatin data obtained from our study allowed us to generate and test a consensus imprinted gene chromatin signature. This empirically derived definition, H3K9ac and CTCF or H3K4me3 in one allele and H3K9me3 in the other allele, with the sliding-window approach, recognized imprinted genes with an unprecedented 100% precision. It identified only imprinted genes along the duplicated genomic segments, H19, Igf2, Igf2as, Cdkn1c, Kcnq1ot1, and Inpp5f_v2 on distal Chr7 and Peg13 and Slc38a4 on distal Chr15. The proof for the algorithm's utility is that, based on the chromatin of distal Chr7 imprinted genes, it correctly predicted imprinted genes using a similar data set from an independent duplicated chromosome, distal Chr15. Additionally, the approach correctly determined the expressed allele for each gene. The regions having predictive power were located at the proximity of imprinted genes themselves and at their DMRs. A limitation of this method seems to be that it did not predict novel imprinted genes in the genomic region analyzed in the given cell type. The likely explanation can be that there are no more differentially expressed genes in MEFs along the duplicated genomic region. Our study can be considered proof-of-principle analysis, based on two well-studied chromosome regions in one cell type. Indeed, the predictive power of the algorithm for the given genomic region was better than if predictions were based on DNA methylation only or on monoallelic gene expression in the given cell type. The predictor recognized imprinted genes in the absence of a DNA methylation mark and in the absence of expression. Igf2 and Igf2as are not associated with a typical DMR. Methylation of the Igf2 DMR1 is biased toward the expressed paternal allele (14, 63). It would not be possible to predict their imprinting status based on DNA methylation, but the chromatin-based predictor algorithm correctly identified Igf2 and Igf2as as paternally expressed imprinted genes. Inpp5f_v2 is imprinted in brain with paternal allele-specific expression but is not expressed in other organs (12). The imprinting signature algorithm in combination with the sliding-window approach recognized Inpp5f_v2 as imprinted based on the allele-specific chromatin profile even though it was not annotated and did not show expression in MEFs according to ds cDNA hybridization (Fig. 4). Because the prediction of Inpp5f_v2 was an exception, our results suggest that imprinted gene predictions that rely on epigenetic features should be done in a cell-type/organ-specific fashion. Our analysis provides evidence that reciprocal translocations along the mouse genome (http://har.mrc.ac.uk/research/genomic_imprinting/) may aid the discovery of novel imprinted genes with high specificity. Our predictor algorithm can be also applied to deep sequencing of ChIP-enriched DNA fractions from the F1 of different mouse strains/subspecies. The advantage of this method is that duplication of mouse chromosomes is not required, and the two parental alleles can be directly compared in a single sample along the entire genome (56). The disadvantage will be that allele-specific information will be limited to peaks where an SNP is available between the mouse strains and the analysis will not allow a panoramic display of chromatin composition.
Allele-specific chromatin is autonomous to the maternally and paternally inherited chromosomes.
The role of allelic trans-sensing (94) between parental chromosomes has been suggested in genomic imprinting (3, 43–46, 60, 61, 64, 80). Using pronuclear transplantation experiments, we showed earlier that allelic trans-sensing and counting are not required for allele-specific expression of imprinted genes (92). At least at the U2af1-rs1 imprinted locus, trans-sensing is not required for allele-specific chromatin configuration, assessed by nuclease hypersensitivity (21). In MatDup.dist7 and PatDup.dist7 MEFs, the paternally and maternally inherited chromosomes along the distal Chr7 regions never had a chance to physically interact. Yet, the paternally inherited Chr7 correctly established and maintained paternal allele-specific chromatin features in the absence of maternally inherited Chr7 in PatDup.dist7 MEFs. The same was true for the maternally inherited Chr7 in MatDup.dist7 MEFs and for the paternally or maternally inherited alleles of distal Chr15 in MatDup.dist7 and PatDup.dist7 MEFs, respectively. The chromatin compositions along the entire H19-Igf2 imprinted domain were identical between MatDup.dist7 MEFs and the maternal allele of normal MEFs. Similarly, the chromatin was identical between PatDup.dist7 MEFs and the paternal allele of normal MEFs along this domain (30, 87).
Supplementary Material
ACKNOWLEDGMENTS
We thank Diana Tran and Guillermo Rivas for performing the allele-specific RNA quantitation and Mai Dang, summer student, for technical assistance.
This work was supported by a Public Health Service grant (GM064378) from the National Institute of General Medicine to P.E.S.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
Published ahead of print on 14 February 2011.
REFERENCES
- 1. Babak T., et al. 2008. Global survey of genomic imprinting by transcriptome sequencing. Curr. Biol. 18:1735–1741 [DOI] [PubMed] [Google Scholar]
- 2. Barski A., et al. 2007. High-resolution profiling of histone methylations in the human genome. Cell 129:823–837 [DOI] [PubMed] [Google Scholar]
- 3. Bartolomei M. S., Tilghman S. M. 1992. Parental imprinting of mouse chromosome 7. Semin. Dev. Biol. 3:107–117 [Google Scholar]
- 4. Bartolomei M. S., Zemel S., Tilghman S. M. 1991. Parental imprinting of the mouse H19 gene. Nature 351:153–155 [DOI] [PubMed] [Google Scholar]
- 5. Bell A. C., Felsenfeld G. 2000. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature 405:482–485 [DOI] [PubMed] [Google Scholar]
- 6. Bhogal B., Arnaudo A., Dymkowski A., Best A., Davis T. L. 2004. Methylation at mouse Cdkn1c is acquired during postimplantation development and functions to maintain imprinted expression. Genomics 84:961–970 [DOI] [PubMed] [Google Scholar]
- 7. Bourc'his D., Xu G. L., Lin C. S., Bollman B., Bestor T. H. 2001. Dnmt3L and the establishment of maternal genomic imprints. Science 294:2536–2539 [DOI] [PubMed] [Google Scholar]
- 8. Brideau C. M., Eilertson K. E., Hagarman J. A., Bustamante C. D., Soloway P. D. 2010. Successful computational prediction of novel imprinted genes from epigenomic features. Mol. Cell. Biol. 30:3357–3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caspary T., Cleary M. A., Baker C. C., Guan X. J., Tilghman S. M. 1998. Multiple mechanisms regulate imprinting of the mouse distal chromosome 7 gene cluster. Mol. Cell. Biol. 18:3466–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cattanach B. M., et al. 1997. A candidate model for Angelman syndrome in the mouse. Mamm. Genome 8:472–478 [DOI] [PubMed] [Google Scholar]
- 11. Cattanach B. M., et al. 1992. A candidate mouse model for Prader-Willi syndrome which shows an absence of Snrpn expression. Nat. Genet. 2:270–274 [DOI] [PubMed] [Google Scholar]
- 12. Choi J. D., et al. 2005. A novel variant of Inpp5f is imprinted in brain, and its expression is correlated with differential methylation of an internal CpG island. Mol. Cell. Biol. 25:5514–5522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clark L., Wei M., Cattoretti G., Mendelsohn C., Tycko B. 2002. The Tnfrh1 (Tnfrsf23) gene is weakly imprinted in several organs and expressed at the trophoblast-decidua interface. BMC Genet. 3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Constancia M., et al. 2000. Deletion of a silencer element in Igf2 results in loss of imprinting independent of H19. Nat. Genet. 26:203–206 [DOI] [PubMed] [Google Scholar]
- 15. Dao D., et al. 1998. IMPT1, an imprinted gene similar to polyspecific transporter and multi-drug resistance genes. Hum. Mol. Genet. 7:597–608 [DOI] [PubMed] [Google Scholar]
- 16. Davis T. L., Tremblay K. D., Bartolomei M. S. 1998. Imprinted expression and methylation of the mouse H19 gene are conserved in extraembryonic lineages. Dev. Genet. 23:111–118 [DOI] [PubMed] [Google Scholar]
- 17. DeChiara T. M., Robertson E. J., Efstratiadis A. 1991. Parental imprinting of the mouse insulin-like growth factor II gene. Cell 64:849–859 [DOI] [PubMed] [Google Scholar]
- 18. Dindot S. V., Person R., Strivens M., Garcia R., Beaudet A. L. 2009. Epigenetic profiling at mouse imprinted gene clusters reveals novel epigenetic and genetic features at differentially methylated regions. Genome Res. 19:1374–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Engemann S., et al. 2000. Sequence and functional comparison in the Beckwith-Wiedemann region: implications for a novel imprinting centre and extended imprinting. Hum. Mol. Genet. 9:2691–2706 [DOI] [PubMed] [Google Scholar]
- 20. Essien K., et al. 2009. CTCF binding site classes exhibit distinct evolutionary, genomic, epigenomic and transcriptomic features. Genome Biol. 10:R131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feil R., Boyano M. D., Allen N. D., Kelsey G. 1997. Parental chromosome-specific chromatin conformation in the imprinted U2af1-rs1 gene in the mouse. J. Biol. Chem. 272:20893–20900 [DOI] [PubMed] [Google Scholar]
- 22. Ferguson-Smith A. C., Cattanach B. M., Barton S. C., Beechey C. V., Surani M. A. 1991. Embryological and molecular investigations of parental imprinting on mouse chromosome 7. Nature 351:667–670 [DOI] [PubMed] [Google Scholar]
- 23. Ferguson-Smith A. C., Sasaki H., Cattanach B. M., Surani M. A. 1993. Parental-origin-specific epigenetic modification of the mouse H19 gene. Nature 362:751–755 [DOI] [PubMed] [Google Scholar]
- 24. Fitzpatrick G. V., et al. 2007. Allele-specific binding of CTCF to the multipartite imprinting control region KvDMR1. Mol. Cell. Biol. 27:2636–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fitzpatrick G. V., Soloway P. D., Higgins M. J. 2002. Regional loss of imprinting and growth deficiency in mice with a targeted deletion of KvDMR1. Nat. Genet. 32:426–431 [DOI] [PubMed] [Google Scholar]
- 26. Giddings S. J., King C. D., Harman K. W., Flood J. F., Carnaghi L. R. 1994. Allele specific inactivation of insulin 1 and 2, in the mouse yolk sac, indicates imprinting. Nat. Genet. 6:310–313 [DOI] [PubMed] [Google Scholar]
- 27. Gould T. D., Pfeifer K. 1998. Imprinting of mouse Kvlqt1 is developmentally regulated. Hum. Mol. Genet. 7:483–487 [DOI] [PubMed] [Google Scholar]
- 28. Gregg C., et al. 2010. High-resolution analysis of parent-of-origin allelic expression in the mouse brain. Science 329:643–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guillemot F., et al. 1995. Genomic imprinting of Mash2, a mouse gene required for trophoblast development. Nat. Genet. 9:235–242 [DOI] [PubMed] [Google Scholar]
- 30. Han L., Lee D. H., Szabó P. E. 2008. CTCF is the master organizer of domain-wide allele-specific chromatin at the H19/Igf2 imprinted region. Mol. Cell. Biol. 28:1124–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Han L., Szabó P. E., Mann J. R. 2010. Postnatal survival of mice with maternal duplication of distal chromosome 7 induced by a Igf2/H19 imprinting control region lacking insulator function. PLoS Genet. 6:e1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hark A. T., et al. 2000. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature 405:486–489 [DOI] [PubMed] [Google Scholar]
- 33. Hata K., Okano M., Lei H., Li E. 2002. Dnmt3L cooperates with the Dnmt3 family of de novo DNA methyltransferases to establish maternal imprints in mice. Development 129:1983–1993 [DOI] [PubMed] [Google Scholar]
- 34. Higashimoto K., et al. 2002. Characterization and imprinting status of OBPH1/Obph1 gene: implications for an extended imprinting domain in human and mouse. Genomics 80:575–584 [DOI] [PubMed] [Google Scholar]
- 35. Hiura H., et al. 2010. A tripartite paternally methylated region within the Gpr1-Zdbf2 imprinted domain on mouse chromosome 1 identified by meDIP-on-chip. Nucleic Acids Res. 38:4929–4945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaffer C. R., et al. 2000. A transcriptional insulator at the imprinted H19/Igf2 locus. Genes Dev. 14:1908–1919 [PMC free article] [PubMed] [Google Scholar]
- 37. Kanduri C., et al. 2002. A differentially methylated imprinting control region within the Kcnq1 locus harbors a methylation-sensitive chromatin insulator. J. Biol. Chem. 277:18106–18110 [DOI] [PubMed] [Google Scholar]
- 38. Kanduri C., et al. 2000. Functional association of CTCF with the insulator upstream of the H19 gene is parent of origin-specific and methylation-sensitive. Curr. Biol. 10:853–856 [DOI] [PubMed] [Google Scholar]
- 39. Kaneda M., et al. 2004. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature 429:900–903 [DOI] [PubMed] [Google Scholar]
- 40. Kang K., Chung J. H., Kim J. 2009. Evolutionary conserved motif finder (ECMFinder) for genome-wide identification of clustered YY1- and CTCF-binding sites. Nucleic Acids Res. 37:2003–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim T. H., Barrera L. O., Ren B. 2007. ChIP-chip for genome-wide analysis of protein binding in mammalian cells. Curr. Protoc. Mol. Biol. 21:Unit 21.13 [DOI] [PubMed] [Google Scholar]
- 42. Kurukuti S., et al. 2006. CTCF binding at the H19 imprinting control region mediates maternally inherited higher-order chromatin conformation to restrict enhancer access to Igf2. Proc. Natl. Acad. Sci. U. S. A. 103:10684–10689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. LaSalle J. M., Lalande M. 1995. Domain organization of allele-specific replication within the GABR3 gene cluster requires a biparental 15q11-13 contribution. Nat. Genet. 9:386–394 [DOI] [PubMed] [Google Scholar]
- 44. LaSalle J. M., Lalande M. 1996. Homologous association of oppositely imprinted chromosomal domains. Science 272:725–728 [DOI] [PubMed] [Google Scholar]
- 45. Latham K. E., Doherty A. S., Scott C. D., Schultz R. M. 1994. Igf2r and Igf2 gene expression in androgenetic, gynogenetic, and parthenogenetic preimplantation mouse embryos: absence of regulation by genomic imprinting. Genes Dev. 8:290–299 [DOI] [PubMed] [Google Scholar]
- 46. Latham K. E., Rambhatla L., Hayashizaki Y., Chapman V. M. 1995. Stage-specific induction and regulation by genomic imprinting of the mouse U2afbp-rs gene during preimplantation development. Dev. Biol. 168:670–676 [DOI] [PubMed] [Google Scholar]
- 47. Lewis A., et al. 2004. Imprinting on distal chromosome 7 in the placenta involves repressive histone methylation independent of DNA methylation. Nat. Genet. 36:1291–1295 [DOI] [PubMed] [Google Scholar]
- 48. Li E., Beard C., Jaenisch R. 1993. Role for DNA methylation in genomic imprinting. Nature 366:362–365 [DOI] [PubMed] [Google Scholar]
- 49. Li T., et al. 2008. CTCF regulates allelic expression of Igf2 by orchestrating a promoter-polycomb repressive complex 2 intrachromosomal loop. Mol. Cell. Biol. 28:6473–6482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Luedi P. P., et al. 2007. Computational and experimental identification of novel human imprinted genes. Genome Res. 17:1723–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Luedi P. P., Hartemink A. J., Jirtle R. L. 2005. Genome-wide prediction of imprinted murine genes. Genome Res. 15:875–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mancini-DiNardo D., Steele S. J., Ingram R. S., Tilghman S. M. 2003. A differentially methylated region within the gene Kcnq1 functions as an imprinted promoter and silencer. Hum. Mol. Genet. 12:283–294 [DOI] [PubMed] [Google Scholar]
- 53. Mancini-Dinardo D., Steele S. J., Levorse J. M., Ingram R. S., Tilghman S. M. 2006. Elongation of the Kcnq1ot1 transcript is required for genomic imprinting of neighboring genes. Genes Dev. 20:1268–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Maynard N. D., Chen J., Stuart R. K., Fan J. B., Ren B. 2008. Genome-wide mapping of allele-specific protein-DNA interactions in human cells. Nat. Methods 5:307–309 [DOI] [PubMed] [Google Scholar]
- 55. McLaughlin K. J., Szabó P., Haegel H., Mann J. R. 1996. Mouse embryos with paternal duplication of an imprinted chromosome 7 region die at midgestation and lack placental spongiotrophoblast. Development 122:265–270 [DOI] [PubMed] [Google Scholar]
- 56. Mikkelsen T. S., et al. 2007. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448:553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mizuno Y., et al. 2002. Asb4, Ata3, and Dcn are novel imprinted genes identified by high-throughput screening using RIKEN cDNA microarray. Biochem. Biophys. Res. Commun. 290:1499–1505 [DOI] [PubMed] [Google Scholar]
- 58. Mohammad F., Mondal T., Guseva N., Pandey G. K., Kanduri C. 2010. Kcnq1ot1 noncoding RNA mediates transcriptional gene silencing by interacting with Dnmt1. Development 137:2493–2499 [DOI] [PubMed] [Google Scholar]
- 59. Monk D., et al. 2008. Comparative analysis of human chromosome 7q21 and mouse proximal chromosome 6 reveals a placental-specific imprinted gene, TFPI2/Tfpi2, which requires EHMT2 and EED for allelic-silencing. Genome Res. 18:1270–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Monk M. 1995. Epigenetic reprogramming of differential gene expression in development and evolution. Dev. Genet. 17:188–197 [DOI] [PubMed] [Google Scholar]
- 61. Monk M. 1990. Variation in epigenetic inheritance. Trends Genet. 6:110–114 [DOI] [PubMed] [Google Scholar]
- 62. Moore T., et al. 1997. Multiple imprinted sense and antisense transcripts, differential methylation and tandem repeats in a putative imprinting control region upstream of mouse Igf2. Proc. Natl. Acad. Sci. U. S. A. 94:12509–12514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Murrell A., et al. 2001. An intragenic methylated region in the imprinted Igf2 gene augments transcription. EMBO Rep. 2:1101–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mutter G. L., Stewart C. L., Chaponot M. L., Pomponio R. J. 1993. Oppositely imprinted genes H19 and Igf2 are coexpressed in human androgenetic placenta. Am. J. Hum. Genet. 53:1096–1102 [PMC free article] [PubMed] [Google Scholar]
- 65. Nagano T., et al. 2008. The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science 322:1717–1720 [DOI] [PubMed] [Google Scholar]
- 66. Okano M., Bell D. W., Haber D. A., Li E. 1999. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99:247–257 [DOI] [PubMed] [Google Scholar]
- 67. Olek A., Walter J. 1997. The pre-implantation ontogeny of the H19 methylation imprint. Nat. Genet. 17:275–276 [DOI] [PubMed] [Google Scholar]
- 68. Pandey R. R., et al. 2008. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol. Cell 32:232–246 [DOI] [PubMed] [Google Scholar]
- 69. Pant V., et al. 2004. Mutation of a single CTCF target site within the H19 imprinting control region leads to loss of Igf2 imprinting and complex patterns of de novo methylation upon maternal inheritance. Mol. Cell. Biol. 24:3497–3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pauler F. M., et al. 2009. H3K27me3 forms BLOCs over silent genes and intergenic regions and specifies a histone banding pattern on a mouse autosomal chromosome. Genome Res. 19:221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Paulsen M., et al. 1998. Syntenic organization of the mouse distal chromosome 7 imprinting cluster and the Beckwith-Wiedemann syndrome region in chromosome 11p15.5. Hum. Mol. Genet. 7:1149–1159 [DOI] [PubMed] [Google Scholar]
- 72. Paulsen M., et al. 2000. Sequence conservation and variability of imprinting in the Beckwith-Wiedemann syndrome gene cluster in human and mouse. Hum. Mol. Genet. 9:1829–1841 [DOI] [PubMed] [Google Scholar]
- 73. Peters J., Beechey C. 2004. Identification and characterisation of imprinted genes in the mouse. Brief. Funct. Genomic. Proteomic. 2:320–333 [DOI] [PubMed] [Google Scholar]
- 74. Qian N., et al. 1997. The IPL gene on chromosome 11p15.5 is imprinted in humans and mice and is similar to TDAG51, implicated in Fas expression and apoptosis. Hum. Mol. Genet. 6:2021–2029 [DOI] [PubMed] [Google Scholar]
- 75. Rauch T., Pfeifer G. P. 2005. Methylated-CpG island recovery assay: a new technique for the rapid detection of methylated-CpG islands in cancer. Lab. Invest. 85:1172–1180 [DOI] [PubMed] [Google Scholar]
- 76. Rauch T. A., Pfeifer G. P. 2010. DNA methylation profiling using the methylated-CpG island recovery assay (MIRA). Methods 52:213–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Regha K., et al. 2007. Active and repressive chromatin are interspersed without spreading in an imprinted gene cluster in the mammalian genome. Mol. Cell 27:353–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rentsendorj A., Mohan S., Szabó P., Mann J. R. 2010. A genomic imprinting defect in mice traced to a single gene. Genetics 186:917–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ruf N., et al. 2007. Sequence-based bioinformatic prediction and QUASEP identify genomic imprinting of the KCNK9 potassium channel gene in mouse and human. Hum. Mol. Genet. 16:2591–2599 [DOI] [PubMed] [Google Scholar]
- 80. Sandhu K. S., et al. 2009. Nonallelic transvection of multiple imprinted loci is organized by the H19 imprinting control region during germline development. Genes Dev. 23:2598–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sanz L. A., et al. 2008. A mono-allelic bivalent chromatin domain controls tissue-specific imprinting at Grb10. EMBO J. 27:2523–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Schoenherr C. J., Levorse J. M., Tilghman S. M. 2003. CTCF maintains differential methylation at the Igf2/H19 locus. Nat. Genet. 33:66–69 [DOI] [PubMed] [Google Scholar]
- 83. Schulz R., et al. 2006. Chromosome-wide identification of novel imprinted genes using microarrays and uniparental disomies. Nucleic Acids Res. 34:e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Schulz R., et al. 2008. WAMIDEX: a Web atlas of murine genomic imprinting and differential expression. Epigenetics 3:89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Shin J. Y., Fitzpatrick G. V., Higgins M. J. 2008. Two distinct mechanisms of silencing by the KvDMR1 imprinting control region. EMBO J. 27:168–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Singh P., et al. 2010. Coordinated allele-specific histone acetylation at the differentially methylated regions of imprinted genes. Nucleic Acids Res. 38:7974–7990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Singh P., et al. 2010. Allele-specific H3K79 di- versus trimethylation distinguishes opposite parental alleles at imprinted regions. Mol. Cell. Biol. 30:2693–2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Smilinich N. J., et al. 1999. A maternally methylated CpG island in KvLQT1 is associated with an antisense paternal transcript and loss of imprinting in Beckwith-Wiedemann syndrome. Proc. Natl. Acad. Sci. U. S. A. 96:8064–8069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Smith R. J., Dean W., Konfortova G., Kelsey G. 2003. Identification of novel imprinted genes in a genome-wide screen for maternal methylation. Genome Res. 13:558–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Srivastava M., et al. 2000. H19 and Igf2 monoallelic expression is regulated in two distinct ways by a shared cis acting regulatory region upstream of H19. Genes Dev. 14:1186–1195 [PMC free article] [PubMed] [Google Scholar]
- 91. Szabó P., Tang S. H., Rentsendorj A., Pfeifer G. P., Mann J. R. 2000. Maternal-specific footprints at putative CTCF sites in the H19 imprinting control region give evidence for insulator function. Curr. Biol. 10:607–610 [DOI] [PubMed] [Google Scholar]
- 92. Szabó P. E., Mann J. R. 1996. Maternal and paternal genomes function independently in mouse ova in establishing expression of the imprinted genes Snrpn and Igf2r: no evidence for allelic trans-sensing and counting mechanisms. EMBO J. 15:6018–6025 [PMC free article] [PubMed] [Google Scholar]
- 93. Szabó P. E., Tang S. H., Silva F. J., Tsark W. M., Mann J. R. 2004. Role of CTCF binding sites in the Igf2/H19 imprinting control region. Mol. Cell. Biol. 24:4791–4800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tartof K. D., Henikoff S. 1991. Trans-sensing effects from Drosophila to humans. Cell 65:201–203 [DOI] [PubMed] [Google Scholar]
- 95. Terranova R., et al. 2008. Polycomb group proteins Ezh2 and Rnf2 direct genomic contraction and imprinted repression in early mouse embryos. Dev. Cell 15:668–679 [DOI] [PubMed] [Google Scholar]
- 96. Thorvaldsen J. L., Duran K. L., Bartolomei M. S. 1998. Deletion of the H19 differentially methylated domain results in loss of imprinted expression of H19 and Igf2. Genes Dev. 12:3693–3702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tremblay K. D., Saam J. R., Ingram R. S., Tilghman S. M., Bartolomei M. S. 1995. A paternal-specific methylation imprint marks the alleles of the mouse H19 gene. Nat. Genet. 9:407–413 [DOI] [PubMed] [Google Scholar]
- 98. Umlauf D., et al. 2004. Imprinting along the Kcnq1 domain on mouse chromosome 7 involves repressive histone methylation and recruitment of Polycomb group complexes. Nat. Genet. 36:1296–1300 [DOI] [PubMed] [Google Scholar]
- 99. Verona R. I., Thorvaldsen J. L., Reese K. J., Bartolomei M. S. 2008. The transcriptional status but not the imprinting control region determines allele-specific histone modifications at the imprinted H19 locus. Mol. Cell. Biol. 28:71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wagschal A., et al. 2008. G9a histone methyltransferase contributes to imprinting in the mouse placenta. Mol. Cell. Biol. 28:1104–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wang X., et al. 2008. Transcriptome-wide identification of novel imprinted genes in neonatal mouse brain. PLoS One 3:e3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wang Z., et al. 2008. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat. Genet. 40:897–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wen B., et al. 2008. Overlapping euchromatin/heterochromatin-associated marks are enriched in imprinted gene regions and predict allele-specific modification. Genome Res. 18:1806–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wood A. J., et al. 2007. A screen for retrotransposed imprinted genes reveals an association between X chromosome homology and maternal germ-line methylation. PLoS Genet. 3:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Yatsuki H., et al. 2002. Domain regulation of imprinting cluster in Kip2/Lit1 subdomain on mouse chromosome 7F4/F5: large-scale DNA methylation analysis reveals that DMR-Lit1 is a putative imprinting control region. Genome Res. 12:1860–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.